Abstract
Background:
Anastomotic leakage (AL) is a serious clinical complication after anterior resection for rectal cancer and will lead to an increase in postoperative mortality. However, the effect on long-term oncology outcomes remains controversial.
Methods:
We searched the PubMed, Embase, and Cochrane library databases for related articles. The included studies assessed local recurrence, distant recurrence, overall survival, cancer-specific survival and disease-free survival. The systematic reviews and meta-analyses was conducted in accordance with the PRISMA guidelines. The combined RRs with 95% CI were then calculated using a fixed effects model or a randomized effect model.
Results:
A total of 18 cohort studies included 34,487 patients who met the inclusion criteria. The meta-analysis demonstrated that AL was associated with increased local recurrence (RR 1.47, 95% CI 1.14–1.90, I2 = 57.8%). Anastomotic leakage decreased overall survival (RR 0.92, 95% CI 0.88–0.96, I2 = 58.1%), cancer-specific survival (RR 0.96, 95% CI 0.92–1.00, I2 = 30.4%), and disease-free survival (RR 0.85, 95% CI 0.77–0.94, I2 = 80.4%). Distant recurrence may had no significant effects of AL (RR 1.16, 95% CI 0.91–1.46, I2 = 58.4%).
Conclusion:
AL has a negative effect on local recurrence and long-term survival (including overall survival, cancer-specific survival, and disease-free survival) after anterior resection for rectal cancer, but not related to distant recurrence.
Keywords: anastomotic leak, anterior resection, rectal cancer, recurrence, survival
1. Introduction
Anastomotic leakage (AL) is a serious complication after rectal cancer surgery. AL can lead to increased postoperative mortality, decreased quality of life, and increased hospitalization costs.[1–3] There are reports of AL incidence ranging from 3% to 23%.[4–6] Differences may be due to the lack of a well-established explanation of the ALs definitions and the grade.[7] With advancements in operative techniques, the lower-or ultra-low-position sphincter preserving rectal resections are increasing, however this has consequently increased the likelihood of AL.[8,9]
Some previous studies have shown that the long-term prognosis after rectal cancer surgery is associated with a number of factors, including age, tumor stage, tumor pathological type, obstruction, perforation, the presence of lymphovascular invasion, and so on.[10,11] However, the effect of AL on the long-term prognosis of tumor remains controversia. Some studies have shown that anastomotic fistula has a negative effect on tumor recurrence and survival rate,[12–14] but some views are just the opposite.[15] A previous meta-analysis by Zheqin et al[16] indicated that anastomotic leakage after anterior resection for rectal cancer adversely affected cancer-specific survival and local recurrence, but not distant recurrence. However, with the improvement of diagnosis and treatment, the long-term prognosis of the disease has changed. Around the effect of AL on recurrence and survival, we summarize the relevant articles in the last 10 years and conduct a meta-analysis from 5 aspects: local recurrence, distant recurrence, overall survival, cancer-specific survival, and disease-free survival.
2. Materials and methods
This meta-analysis followed the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.[17] Ethical approval was not necessary and all analyses in this study were based on previously published studies and therefore do not require ethical approval and patient consent.
2.1. Search strategy
PubMed, EMBASE, and Cochrane Library databases were systematically searched for eligible studies by 2 independent reviewers (Ma and Pang) from January 2009 to January 2020. Search terms included the following keywords and freewords in various combinations: “rectal neoplasms”, “anastomotic leak”, “recurrence”, “survival”. A reference list of qualified studies was also reviewed to identify more articles. Two authors independently reviewed the title and abstract of each paper. After excluding significantly irrelevant articles, the authors reviewed the full text of the selected article and decided that the exact list of literature to be included in this meta-analysis. The authors resolved their differences through discussion. Only studies published in English were included in the present meta-analysis.
2.2. Study selection
The included study criteria are as follows:
-
1.
Patients underwent a curative anterior resection for rectal cancer.
-
2.
Articles to study the long-term tumor outcomes of AL, including local recurrence, distant recurrence, overall survival, cancer-specific survival, and disease-free survival.
-
3.
The study was limited to case-control or cohort trials.
The following studies would be excluded:
-
1.
Patients underwent non-curative anterior procedures such as Hartmanns operation or abdominoperineal resection.
-
2.
Study of postoperative sepsis rather than AL itself.
-
3.
Colorectal cancer data were mixed and rectal cancer data could not be extracted separately.
-
4.
Letters, summaries, meta-analyses, and abstracts were also excluded.
AL is defined as the connection between the cavity and the outside of the cavity caused by defects in the integrity of the intestinal wall at the anastomosis, which can be confirmed by the evidence of enteroscopy, extravasation of endoluminally administered water-soluble contrast at radiography or computed tomography, or reoperation. In addition, the presence of pelvic abscess near the anastomosis is also considered to be AL.[18]
2.3. Data extraction
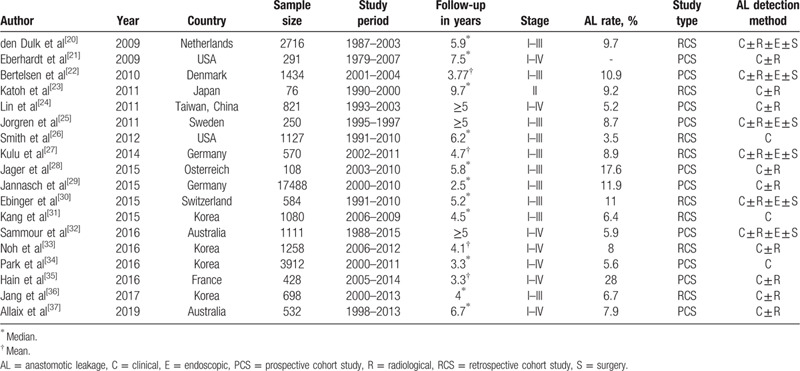
We refined the essential characteristics of these included studies, including author names, publication date, country, diagnostic criteria, number of patients, incidence of all. We also extracted detailed information such as follow-up time, results, etc. This information is reflected in Table 1. Two investigators (Ma and Pang) independently extracted data from eligible articles, and any inconsistent judgments were resolved through joint discussions.
Table 1.
General characteristics of included studies.
2.4. Quality assessment
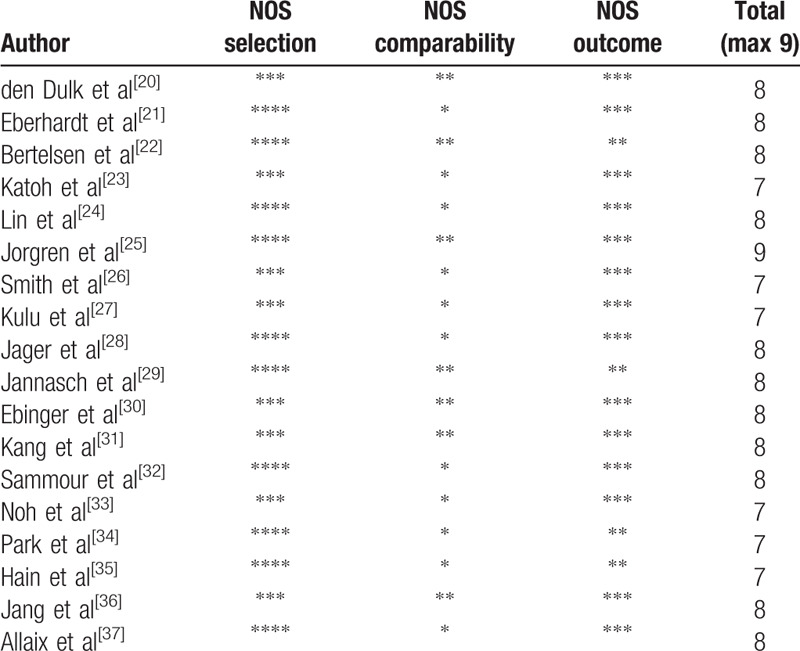
The quality of each qualified study was evaluated according to the Newcastle-Ottawa scale (NOS). A study with a score of 6 or more was of better quality. Studies with scores between 0 and 5 were considered low-quality. The 2 evaluators independently assessed the quality of the included studies.
2.5. Statistical analysis
The association of AL with long-term oncology outcomes was evaluated by calculating the relative risk (RR) and 95% confidence interval (CI). We used Cochrans Q statistic and the I2 statistic to assess heterogeneity across studies. P < .1 or I2 > 50% indicated significant heterogeneity. We used a randomized effects model to pool the results for significant heterogeneity; otherwise, a fixed effects model was applied in our meta-analysis. To explore the potential source of heterogeneity, sensitivity analysis was conducted to test the stability of the results by removing 1 study at a time. We performed Beggs test to assess publication bias.[19] All statistical analyses were performed using STATA12.0 software. A P value of <.05 was considered statistical significance.
3. Results
3.1. Study selection
According to the search strategy and manual search supplement, 1528 possible related articles were obtained. Of these, 392 articles were deleted for repetition. The remaining 1136 articles, after reading the title and abstract, excluded 1080 unrelated articles. Then, the full text of the 47 articles was evaluated. Finally, 18 compliant articles were included in our meta-analysis after full text assessment.[20–37] A flow chart of the screening process is shown in Figure 1.
Figure 1.
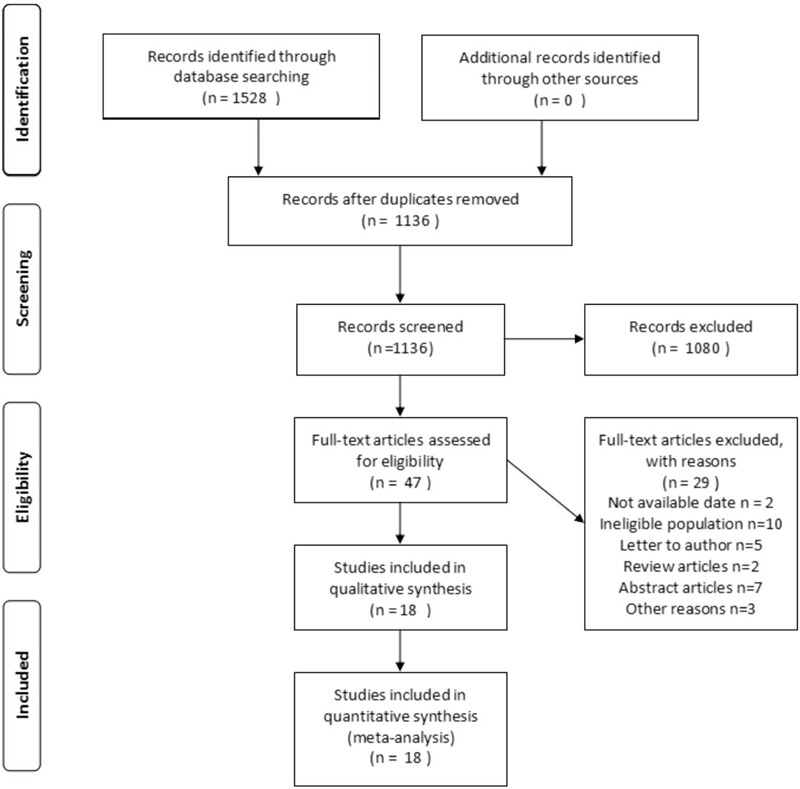
The process of study identification.
3.2. Characteristics of the studies and quality assessment
The 18 studies encompass a total of 3480 patients with AL and 31,004 patients without AL which included 10 prospective cohort studies[21,22,24,25,28,29,32,34,35,37] and 8 retrospective cohort studies.[20,23,26,27,30,31,33,36] The quality of 18 trials were evaluated using the Newcastle-Ottawa scale (NOS) which classifies individual studies as having low or high risk of bias across 3 domains: selection, comparability, and outcome. All articles scored more than 6 points, indicating that the article quality were good. The clinical characteristics and quality assessments of the included publications are summarized in Tables 1 and 2.
Table 2.
Assessment of study quality using the Newcastle-Ottawa scale for cohort studies.
3.3. Anastomotic leakage and local recurrence
Sixteen studies reported on local recurrence presenting a total sample size of 34,300 patients. Combined results indicated that AL patients had significantly increased local recurrence with compared controls (RR 1.47, 95% CI 1.14–1.90, I2 = 58%), as shown in Figure 2. Because of heterogeneity, we used a randomized effects model. We conducted a sensitivity analysis to assess the stability of the results, and the overall results did not change significantly after excluding each study.
Figure 2.
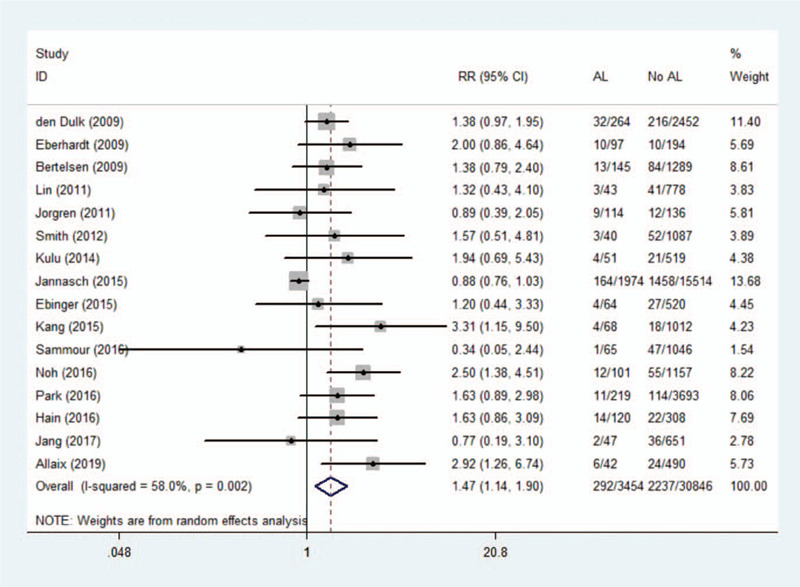
Effect of anastomotic leakage on the risk of local recurrence.
3.4. Anastomotic leakage and distant recurrence
Eight studies reported overall survival, showing a total sample size of 10,937 patients. A meta-analysis using a random effects model showed no significant association between AL and distant recurrence (RR 1.16, 95% CI 0.91–1.46, I2 = 58.4%). As shown in Figure 3, we observed that the findings of Allaix et al were significantly beyond the range established by others, which may be one of the reason for this heterogeneity. After excluding this study, the remaining 10,405 patients were analyzed, and the results showed that there was still no statistical significance (RR 1.05, 95% CI 0.90–1.20). There was no significant heterogeneity in remaining studies (I2 = 0.00%).
Figure 3.
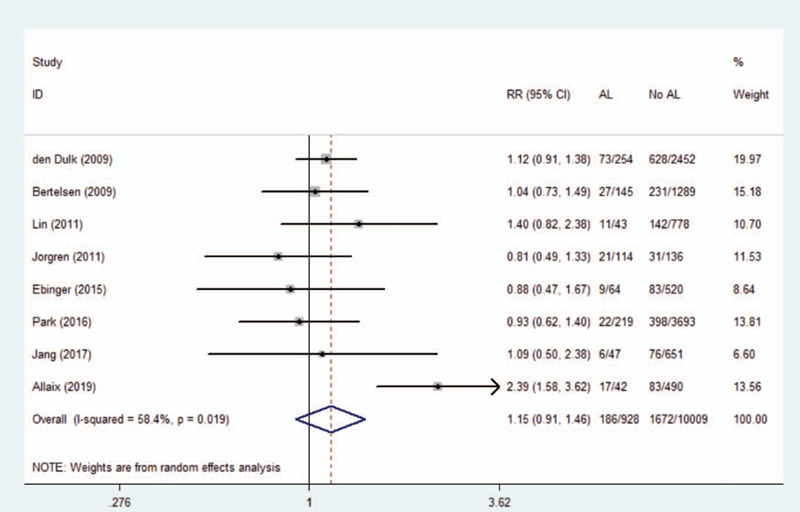
Effect of anastomotic leakage on the risk of distant recurrence.
3.5. Anastomotic leakage and overall survival
A total of 17 studies (34,105 patients) investigated the long-term overall survival after AL. Original data in mortality figures were converted to survival. Outcome demonstrated a significantly reduced overall survival after AL (RR 0.92, 95% CI 0.88–0.96, I2 = 57.5%).A summary of data and forest plots were shown in Figure 4. We performed a sensitivity analysis without significant differences in heterogeneity after excluding each study. Therefore, the results are generally constant and stable.
Figure 4.
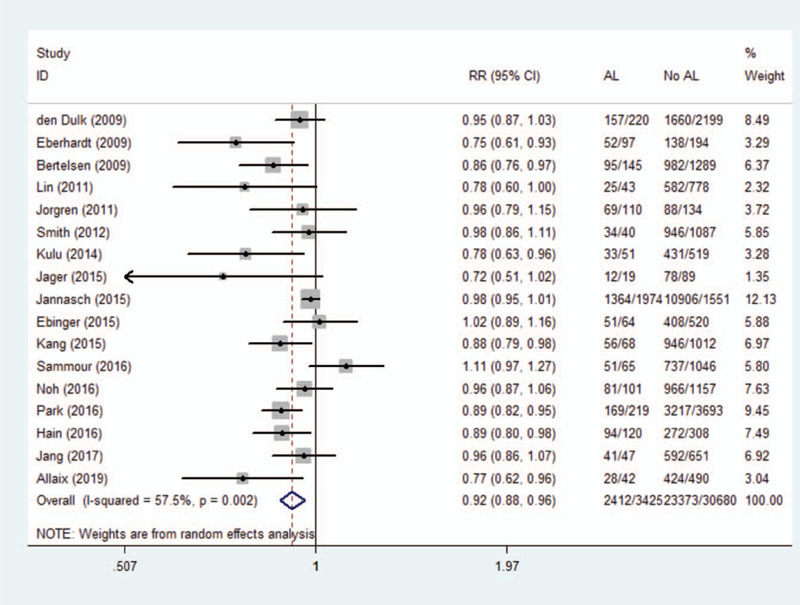
Effect of anastomotic leakage on overall survival.
3.6. Anastomotic leakage and cancer-specific survival
Nine studies investigated long-term cancer-specific survival in 7057 patients after rectal cancer surgery. Our meta-analysis confirmed a negative correlation between AL and increased long-term cancer-specific survival (RR 0.96, 95% CI 0.92–1.00, I2 = 30.4%). Data and forest plots are shown in Figure 5.
Figure 5.
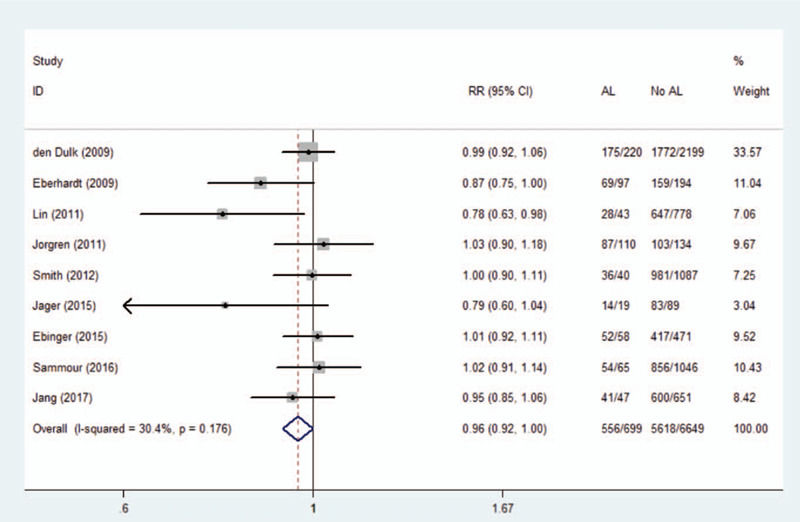
Effect of anastomotic leakage on cancer-specific survival.
3.7. Anastomotic leakage and disease-free survival
Ten studies involving a total of 28,392 patients reported the disease-free survival rate after rectal cancer resection. The results showed AL decrease in disease-free survival (RR 0.85, 95% CI 0.77–0.94, I2 = 80.0%), as shown in Figure 6. A random-effects model was applied due to the high heterogeneity. The outcome was not changed after serial exclusion of studies.
Figure 6.

Effect of anastomotic leakage on disease-free survival.
3.8. Publication bias
We used Beggs tests to assess publication bias and no potential publication bias was observed (P > .05).
4. Discussion
AL has been a serious postoperative complication of rectal cancer. Our meta-analysis assessed the relationship between AL and long-term oncological outcomes after curative anterior resection for rectal cancer. Findings suggest that postoperative AL leads to increased local recurrence of the tumor and to worse long-term survival. But it does not seem to affect the distant recurrence of cancer. Mirnezami et al[38] and Zheqin et al[16] have done similar studies before, but the description of long-term survival is mainly cancer-specific survival. But cancer-specific survival could result in inaccurate classification of the underlying causes of death.[39,40] Furthermore, AL patients are more likely to receive long-term follow-up. Compared with their studies, this study compared the survival rate from overall survival, cancer-specific survival, disease-free survival 3 aspects, and described the long-term survival rate of patients after operation more comprehensively. Secondly, with the improvement of diagnosis and treatment, the treatment of rectal cancer has undergone major changes in surgical techniques and adjuvant treatment, and the change of perioperative management may affect the prognosis of patients. This study incorporated articles published in nearly 10 years, especially 7 recently published articles never included in similar studies, with a total of 25,430 patients. And this will more accurately reflect AL true impact on recurrence and long-term survival.
According to the articles we included, the conclusions of different studies are widely divergent. For example, adverse effects of AL on local recurrence and long-term survival were reported in a multicenter analysis of Kang et al.[31] On the contrary, Ebinger et al[30] concluded in a propensity score analysis that the patients oncological findings were not impaired by the development of AL. Although the long-term oncological effects of AL on anterior resection for rectal cancer have been debated until now. But we found that there may be a variety of factors contributing to the differences in the results. Above all, AL effects on short-term mortality may interfere with the observation of long-term outcomes. Bertelsen et al[22] found a 4-fold increase in 30-day mortality in AL patients in a multicenter study. This suggests that in the analysis of long-term oncological effects, 30-day of mortality should be eliminated to reduce the impact on the correct outcome. However, in the article we included, only 7 studies clearly indicated the results of the data after excluding 30 days of mortality.[20,22,25,29–31,36] Besides, there is debate about the effect of temporary stoma on recurrence and survival in AL patients. In a study of 3912 patients, Park et al[34] stated that rectal AL is a risk factor associated with poor overall survival in patients without diverting stoma. However, Lin et al[24] believe that although diverting stomas can largely alleviate AL-induced abdominal sepsis and possibly reduce the occurrence of systemic inflammatory reactions, the large amount of leakage caused by AL will offset Beneficial effects of relapse and survival. Similarly, Kulu et al[27] reported that in their study, no temporary stoma was found to be associated with anastomotic leakage or oncological outcomes such as local recurrence or overall survival. Research in this area has not been analyzed and reported in all articles, which may lead to differences in oncology. In addition, because preoperative chemoradiotherapy (CRT) or radiotherapy (RT) has a certain positive effect on local tumor control and prolonged survival, the adverse effects of AL may be offset by preoperative CRT or RT.[24,41–43] Therefore, patients undergoing preoperative CRT and RT may not have significant differences in oncological outcomes with or without AL after surgery.[36] However, factors for preoperative CRT and RT have not been included in all standardized analyses. The proportions of such patients in the study varied, which could lead to differences in results. The above confounding factors, different recruitment criteria, and many other factors may cause inconsistent research conclusions.
There are several explanations for the mechanism by which AL has an adverse effect on long-term oncology outcomes. First, despite rectal lavage during surgery, live tumor cells can be implanted intraoperatively in the intestine, as confirmed in experiments by Gertsch et al and van den Tol et al.[44,45] AL may make it easier for exfoliated cancer cells remaining in the intestinal lumen to grow outside the lumen, resulting in an increase in local recurrence and affecting long-term survival.[46] On the other side, AL can cause abdominal infections, secondary to systemic inflammatory response syndrome. This can lead to the release of interleukin-6, endotoxin, vascular endothelial growth factor, and other pro-inflammatory cytokines and growth factors, which seems to promote the growth of residual cancer cells, thereby reducing the long-term survival rate of patients.[47–49] Moreover, abdominal inflammation can directly promote the adhesion, migration, and invasion of cancer cells.[50,51] And rectal leaks are often confined to the pelvis, which increases this chance. Other studies have pointed out that abdominal sepsis can cause immune suppression in the body, which can lead to the suppression of the neutrophil-proportion associated with the risk of tumor recurrence and a reduction in the prognostic nutrition index (PNI), which increases the risk of tumor recurrence.[33] Adjuvant chemotherapy has a positive effect on the recurrence and metastasis of patients with non-metastatic rectal cancer after surgery, which has been confirmed,[52] and widely recognized. The most effective results obtained by adjuvant chemotherapy within 8 weeks after surgery can extend overall survival and disease-free survival.[53,54] However, in clinical practice, more than half of patients with AL often have to delay the use of adjuvant chemotherapy or no longer receive adjuvant chemotherapy, increasing the risk of recurrence and metastasis and long-term mortality.[37,55] It is worth mentioning that although the distant recurrence has not been confirmed to be related to AL, some studies suggest that AL delays mucosal healing, which may provide conditions for tumor cells to implant on the surface of blood vessels, leading to tumor growth and distant spread. In our analysis, the number of patients studying the distant recurrence is relatively small, and further conclusions need to be confirmed in future studies.
The present meta-analysis has several limitations. First of all, in the included study, there were mixed factors such as 30-day postoperative mortality, the use of neoadjuvant therapy, and the application of diverting stoma. The data for these factors were incomplete, so that these factors could not be included in the meta-analysis. Second, the definition and diagnostic criteria of AL have not been unified in the study. Some studies only cover clinical AL, while others include AL found through radiological examination, endoscopy, and reoperation. This may lead to significant differences in the incidence of AL and affect the evaluation of long-term oncological outcomes. Third, despite our sensitivity analysis and other analysis of the results, the potential heterogeneity among studies has not been fully resolved. Finally, some studies included in this meta-analysis have shorter follow-up times and may not be sufficient to observe exact long-term oncology results.
5. Conclusion
This study demonstrates that AL is associated with poor oncology outcomes after Curative Anterior Resection for Rectal Cancer. AL increases local recurrence and decreases overall survival, cancer-specific survival, disease-free survival, but has no statistically significant effect on distant recurrence. Future studies need to further confirm the mechanism of ALs poor prognosis in order to guide patients treatment and long-term follow-up.
Author contributions
Conceptualization: Lushun Ma, Xinyuan Pang, Guofeng Ji, Haojie Sun, Qihao Fan, Chong Ma.
Data curation: Lushun Ma, Xinyuan Pang.
Formal analysis: Lushun Ma.
Methodology: Lushun Ma, Xinyuan Pang.
Project administration: Lushun Ma, Guofeng Ji, Haojie Sun.
Supervision: Lushun Ma, Qihao Fan.
Writing – original draft: Lushun Ma, Xinyuan Pang, Chong Ma.
Writing – review & editing: Lushun Ma, Xinyuan Pang, Guofeng Ji, Haojie Sun, Chong Ma.
Footnotes
Abbreviations: AL = anastomotic leakage, CI = confidence interval, CRT = chemoradiotherapy, RR = relative risk, RT = radiotherapy.
How to cite this article: Ma L, Pang X, Ji G, Sun H, Fan Q, Ma C. The impact of anastomotic leakage on oncology after curative anterior resection for rectal cancer: A systematic review and meta-analysis. Medicine. 2020;99:37(e22139).
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
The author(s) of this work have nothing to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
The datasets generated during and/or analyzed during the current study are publicly available.
References
- [1].Gong J, Yang L, Huang X, et al. Outcomes based on risk assessment of anastomotic leakage after rectal cancer surgery. Asian Pac J Cancer Prev 2014;15:707–12.. [DOI] [PubMed] [Google Scholar]
- [2].Brown SR, Mathew R, Keding A, et al. The impact of postoperative complications on long-term quality of life after curative colorectal cancer surgery. Ann Surg 2014;259:916–23.. [DOI] [PubMed] [Google Scholar]
- [3].Eriksen MT, Wibe A, Norstein J, et al. Anastomotic leakage following routine mesorectal excision for rectal cancer in a national cohort of patients. Colorectal Dis 2005;7:51–7.. [DOI] [PubMed] [Google Scholar]
- [4].Branagan G, Finnis D. Prognosis after anastomotic leakage in colorectal surgery. Dis Colon Rectum 2005;48:1021–6.. [DOI] [PubMed] [Google Scholar]
- [5].Lee W, Yun SH, Roh Y, et al. Risk factors and clinical outcome for anastomotic leakage after total mesorectal excision for rectal cancer. World J Surg 2008;32:1124–9.. [DOI] [PubMed] [Google Scholar]
- [6].Chang JS, Keum KC, Kim NK, et al. Preoperative chemoradiotherapy effects on anastomotic leakage after rectal cancer resection: a propensity score matching analysis. Ann Surg 2014;259:516–21.. [DOI] [PubMed] [Google Scholar]
- [7].Bruce J, Krukowski ZH, Al-Khairy G, et al. Systematic review of the definition and measurement of anastomotic leak after gastrointestinal surgery. Br J Surg 2001;88:1157–68.. [DOI] [PubMed] [Google Scholar]
- [8].Chau A, Maggiori L, Debove C, et al. Toward the end of abdominoperineal resection for rectal cancer? an 8-year experience in 189 consecutive patients with low rectal cancer. Ann Surg 2014;260:801–6.. [DOI] [PubMed] [Google Scholar]
- [9].Vignali A, Fazio VW, Lavery IC, et al. Factors associated with the occurrence of leaks in stapled rectal anastomoses: a review of 1,014 patients. J Am Coll Surg 1997;185:105–13.. [DOI] [PubMed] [Google Scholar]
- [10].Reshef A, Lavery I, Kiran RP. Factors associated with oncologic outcomes after abdominoperineal resection compared with restorative resection for low rectal cancer. Dis Colon Rectum 2012;55:51–8.. [DOI] [PubMed] [Google Scholar]
- [11].Heald RJ, Moran BJ, Ryall RDH, et al. Rectal cancer: the basing stoke experience of total mesorectal excision, 1978-1997. Arch Surg 1998;133:894–8.. [DOI] [PubMed] [Google Scholar]
- [12].Bell SW, Walker KG, Rickard MJ, et al. Anastomotic leakage after curative anterior resection results in a higher prevalence of local recurrence. Br J Surg 2003;90:1261–6.. [DOI] [PubMed] [Google Scholar]
- [13].Gunkova P, Gunka I, Martinek L, et al. [Impact of anastomotic leakage on oncological outcomes after rectal cancer resection]. Rozhl Chir 2013;92:244–9.. [PubMed] [Google Scholar]
- [14].Merkel S, Wang WY, Schmidt O, et al. Locoregional recurrence in patients with anastomotic leakage after anterior resection for rectal carcinoma. Colorectal Dis 2001;3:154–60.. [DOI] [PubMed] [Google Scholar]
- [15].Amato A, Pescatori M, Butti A. Local recurrence following abdominoperineal excision and anterior resection for rectal carcinoma. Dis Colon Rectum 1991;34:317–22.. [DOI] [PubMed] [Google Scholar]
- [16].Lu ZR, Rajendran N, Lynch AC, et al. Anastomotic leaks after restorative resections for rectal cancer compromise cancer outcomes and survival. Dis Colon Rectum 2016;59:236–44.. [DOI] [PubMed] [Google Scholar]
- [17].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006–12.. [DOI] [PubMed] [Google Scholar]
- [18].Rahbari NN, Weitz J, Hohenberger W, et al. Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the International Study Group of Rectal Cancer. Surgery 2010;147:339–51.. [DOI] [PubMed] [Google Scholar]
- [19].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101.. [PubMed] [Google Scholar]
- [20].den Dulk M, Marijnen CA, Collette L, et al. Multicentre analysis of oncological and survival outcomes following anastomotic leakage after rectal cancer surgery. Br J Surg 2009;96:1066–75.. [DOI] [PubMed] [Google Scholar]
- [21].Eberhardt JM, Kiran RP, Lavery IC. The impact of anastomotic leak and intra-abdominal abscess on cancer-related outcomes after resection for colorectal cancer: a case control study. Dis Colon Rectum 2009;52:380–6.. [DOI] [PubMed] [Google Scholar]
- [22].Bertelsen CA, Andreasen AH, Jorgensen T, et al. Anastomotic leakage after curative anterior resection for rectal cancer: short and long-term outcome. Colorectal Dis 2010;12:e76–81.. [DOI] [PubMed] [Google Scholar]
- [23].Katoh H, Yamashita K, Wang G, et al. Anastomotic leakage contributes to the risk for systemic recurrence in stage II colorectal cancer. J Gastrointest Surg 2011;15:120–9.. [DOI] [PubMed] [Google Scholar]
- [24].Lin JK, Yueh TC, Chang SC, et al. The influence of fecal diversion and anastomotic leakage on survival after resection of rectal cancer. J Gastrointest Surg 2011;15:2251–61.. [DOI] [PubMed] [Google Scholar]
- [25].Jorgren F, Johansson R, Damber L, et al. Anastomotic leakage after surgery for rectal cancer: a risk factor for local recurrence, distant metastasis and reduced cancer-specific survival? Colorectal Dis 2011;13:272–83.. [DOI] [PubMed] [Google Scholar]
- [26].Smith JD, Paty PB, Guillem JG, et al. Anastomotic leak is not associated with oncologic outcome in patients undergoing low anterior resection for rectal cancer. Ann Surg 2012;256:1034–8.. [DOI] [PubMed] [Google Scholar]
- [27].Kulu Y, Tarantio I, Warschkow R, et al. Anastomotic leakage is associated with impaired overall and disease-free survival after curative rectal cancer resection: a propensity score analysis. Ann Surg Oncol 2015;22:2059–67.. [DOI] [PubMed] [Google Scholar]
- [28].Jager T, Nawara C, Neureiter D, et al. [Impact of anastomotic leakage on long-term survival in mid-to-low rectal cancer]. Chirurg 2015;86:1072–82.. [DOI] [PubMed] [Google Scholar]
- [29].Jannasch O, Klinge T, Otto R, et al. Risk factors, short and long term outcome of anastomotic leaks in rectal cancer. Oncotarget 2015;6:36884–93.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ebinger SM, Warschkow R, Tarantino I, et al. Anastomotic leakage after curative rectal cancer resection has no impact on long-term survival: a propensity score analysis. Int J Colorectal Dis 2015;30:1667–75.. [DOI] [PubMed] [Google Scholar]
- [31].Kang J, Choi GS, Oh JH, et al. Multicenter analysis of long-term oncologic impact of anastomotic leakage after laparoscopic total mesorectal excision: the korean laparoscopic colorectal surgery study group. Medicine (Baltimore) 2015;94:e1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sammour T, Hayes IP, Jones IT, et al. Impact of anastomotic leak on recurrence and survival after colorectal cancer surgery: a BioGrid Australia analysis. ANZ J Surg 2018;88:E6–10.. [DOI] [PubMed] [Google Scholar]
- [33].Noh GT, Ann YS, Cheong C, et al. Impact of anastomotic leakage on long-term oncologic outcome and its related factors in rectal cancer. Medicine (Baltimore) 2016;95:e4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Park JS, Huh JW, Park YA, et al. Risk factors of anastomotic leakage and long-term survival after colorectal surgery. Medicine (Baltimore) 2016;95:e2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hain E, Maggiori L, Manceau G, et al. Oncological impact of anastomotic leakage after laparoscopic mesorectal excision. Br J Surg 2017;104:288–95.. [DOI] [PubMed] [Google Scholar]
- [36].Jang JH, Kim HC, Huh JW, et al. Anastomotic leak does not impact oncologic outcomes after preoperative chemoradiotherapy and resection for rectal cancer. Ann Surg 2019;269:678–85.. [DOI] [PubMed] [Google Scholar]
- [37].Allaix ME, Rebecchi F, Famiglietti F, et al. Long-term oncologic outcomes following anastomotic leak after anterior resection for rectal cancer: does the leak severity matter? Surg Endosc 2019. [DOI] [PubMed] [Google Scholar]
- [38].Mirnezami A, Mirnezami R, Chandrakumaran K, et al. Increased local recurrence and reduced survival from colorectal cancer following anastomotic leak. Ann Sur 2011;253:890–9.. [DOI] [PubMed] [Google Scholar]
- [39].Sarfati D, Blakely T, Pearce N. Measuring cancer survival in populations: relative survival vs cancer-specific survival. Int J Epidemiol 2010;39:598–610.. [DOI] [PubMed] [Google Scholar]
- [40].Dickman PW, Adami HO. Interpreting trends in cancer patient survival. J Intern Med 2006;260:103–17.. [DOI] [PubMed] [Google Scholar]
- [41].Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731–40.. [DOI] [PubMed] [Google Scholar]
- [42].Hartley A, Ho KF, McConkey C, et al. Pathological complete response following pre-operative chemoradiotherapy in rectal cancer: analysis of phase II/III trials. Br J Radiol 2005;78:934–8.. [DOI] [PubMed] [Google Scholar]
- [43].Roh MS, Colangelo LH, O’Connell MJ, et al. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol 2009;27:5124–30.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Gertsch P, Baer HU, Kraft R, et al. Malignant cells are collected on circular staplers. Dis Colon Rectum 1992;35:238–41.. [DOI] [PubMed] [Google Scholar]
- [45].van den Tol PM, van Rossen EE, van Eijck CH, et al. Reduction of peritoneal trauma by using nonsurgical gauze leads to less implantation metastasis of spilled tumor cells. Ann Surg 1998;227:242–8.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ptok H, Marusch F, Meyer F, et al. Impact of anastomotic leakage on oncological outcome after rectal cancer resection. Br J Surg 2007;94:1548–54.. [DOI] [PubMed] [Google Scholar]
- [47].Abramovitch R, Marikovsky M, Meir G, et al. Stimulation of tumour growth by wound-derived growth factors. Br J Cancer 1999;79:1392–8.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001;357:539–45.. [DOI] [PubMed] [Google Scholar]
- [49].Alonso S, Pascual M, Salvans S, et al. Postoperative intra-abdominal infection and colorectal cancer recurrence: a prospective matched cohort study of inflammatory and angiogenic responses as mechanisms involved in this association. Eur J Surg Oncol 2015;41:208–14.. [DOI] [PubMed] [Google Scholar]
- [50].Salvans S, Mayol X, Alonso S, et al. Postoperative peritoneal infection enhances migration and invasion capacities of tumor cells in vitro: an insight into the association between anastomotic leak and recurrence after surgery for colorectal cancer. Ann Surg 2014;260:939–43.. 943-944. [DOI] [PubMed] [Google Scholar]
- [51].Oosterling SJ, van der Bij GJ, Bogels M, et al. Anti-beta1 integrin antibody reduces surgery-induced adhesion of colon carcinoma cells to traumatized peritoneal surfaces. Ann Surg 2008;247:85–94.. [DOI] [PubMed] [Google Scholar]
- [52].Petersen SH, Harling H, Kirkeby LT, et al. Postoperative adjuvant chemotherapy in rectal cancer operated for cure. Cochrane Database Syst Rev 2012;D4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Gray R, Barnwell J, McConkey C, et al. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet 2007;370:2020–9.. [DOI] [PubMed] [Google Scholar]
- [54].Des Guetz G, Nicolas P, Perret GY, et al. Does delaying adjuvant chemotherapy after curative surgery for colorectal cancer impair survival? A meta-analysis. Eur J Cancer 2010;46:1049–55.. [DOI] [PubMed] [Google Scholar]
- [55].Tevis SE, Kohlnhofer BM, Stringfield S, et al. Postoperative complications in patients with rectal cancer are associated with delays in chemotherapy that lead to worse disease-free and overall survival. Dis Colon Rectum 2013;56:1339–48.. [DOI] [PMC free article] [PubMed] [Google Scholar]


