Abstract
We aim to summarize the evidence focusing on the effects of various doses of human milk on the risk of neonatal necrotizing enterocolitis (NEC). The eligible articles in the study were those investigating the association between human milk and NEC published before June 26, 2019, in the PubMed, EMBASE, the Cochrane Library, VIP database, CNKI database, and Wangfang database. The included criteria were as follows: premature infants of <37 weeks; randomly controlled trials (RCTs); those fed by mother's own milk or donor human milk; studies focused on the comparison of human milk and formula milk, involving various breast milk doses; and NEC-related studies. Compared with the exclusive formula, the incidence of NEC in the infants fed by exclusive human milk was significantly lower. The incidence of NEC in the infants fed by exclusive human milk was significantly lower than that of partial human milk [risk ratio (RR) = 0.54, 95% confidence interval (95% CI): 0.36–0.79, P < .05]. The incidence of NEC in the infants fed mainly by human milk was significantly lower than that of mainly fed by formula. Incidence of NEC in the infants fed by exclusive human milk was significantly lower than that of any formula (RR = 0.49, 95% CI: 0.34–0.71, P < .05). In summary, this meta-analysis was based on the RCTs involving the prevention of NEC using human milk. Exclusive human milk and partial human milk reduced the incidence of NEC in premature infants, especially in the those fed by high proportion of human milk. In addition, more RCTs are needed to further validate such conclusion.
Keywords: formula milk, human milk, neonatal necrotizing enterocolitis, systematic review
1. Introduction
The survival of premature infants, especially the very low birth weight infant, increases with the advances of reproductive techniques and perinatology, as well as the extensive establishing of the neonatal intensive care unit. On this basis, the neonatal necrotizing enterocolitis (NEC) associated with neonatal feeding and infection is on an increasing trend, which is one of the major causes for neonatal death and disability.[1] The incidence of NEC among the premature infants is high, resulting in an extremely high mortality. To our best knowledge, NEC has been related to various factors, including premature labor, low-body weight, infection, posthypoxic and postischemic reperfusion injury in intestinal tract, as well as improper feeding.[2,3]
NEC was closely related to the gestational age. In the previous studies, the majority of the NEC cases (85%) were premature infants with a gestational age of less than 35 weeks, while the incidence of NEC in the infants with a body weight of less than 1.5 kg increased from 0.7% to 6.6%.[4–6] Nowadays, the incidence of NEC among the very low birth weight infants was in a range of 5% to 12% in the developed countries. For example, about 5.1% of the infants with a gestational age of less than 33 weeks in Canada were affected by NEC.[4,7,8]
Most of the patients (>90%) presented NEC after enteral feeding. Therefore, enteral feeding is a pacing factor for the pathogenesis of NEC.[2,9] Infection or improper feeding may induce local immunity in intestinal tracts, which then resulted in massive release of inflammatory factors and injury of intestinal mucosa. Meanwhile, under the stress states, the blood would flow into most of the crucial organs such as heart, brain, and kidneys, leading to reduction of blood supply in the intestinal tract, together with local ischemia in intestinal mucosa and immunologic barrier damages. In addition, the peristalsis of the intestinal tract showed reduction and the bacterial proliferation in the intestinal tract was more obvious. All these may trigger the pathogenesis of NEC.[10,11]
For the patients with severe NEC, wide excision is usually required due to massive intestinal tract necrosis. The survived infants are apt to develop complications such as intestinal dysfunction, growth delay, and nervous system disorders. Therefore, the mortality of NEC is very high and the long-term prognosis is usually poor, which severely affects the life quality of these survivors. In the past 2 decades, the NEC is still not well controlled despite the advances in the management of such condition, including application of antibiotics, probiotics, and novel feeding strategies.[12–14] Therefore, it is urgent to develop new methods for the prevention of NEC.
Human milk contains many nutritive materials and bioactive substances, which can contribute to the neonatal growth and enhance the innate immunity and antibiosis. Thus, it contributes to the prevention of NEC.[15,16] Nevertheless, a part of the neonates are partially fed by human milk, and even some others fed by formula milk. In this meta-analysis, we aim to investigate the protective effects of various doses of human milk on the NEC, as well as the relationship between human milk and NEC.
2. Materials and methods
2.1. Literature search
A comprehensive literature search was performed to the Chinese and English articles on human milk and NEC. The publication time was before June 26, 2019. The articles were obtained from PubMed, EMBASE, Cochrane Library, CNKI database, VIP database, and Wanfang database. The key words used in the literature search were as follows: “breast feeding” or “breastfeeding” or “breast milk” or “breastmilk” or “humanmilk” or “human milk” or “donor milk” or “donormilk” or “maternal milk” or “maternalmilk” or “mother milk” or “mothermilk” or “breast feed” or “breastfeed” or “Breast-fed” or “formula milk” AND “Necrotizing enterocolitis” or “NEC” or “premature infant” or “very low birth weight infants” or “VLBW” or “extremely low birth weight infants” or “ELBW.” In addition, manual search was given to the articles obtained from these databases focusing on the relationship between human milk and NEC.
2.2. Eligible studies
The inclusion criteria were as follows: premature infants with a gestational age of less than 37 weeks old; randomly controlled trials (RCTs); those fed by mother's own milk or donor human milk; studies focused on the comparison of human milk and formula milk, involving various human milk doses; NEC-related studies. The exclusion criteria were as follows: studies performed in special population other than premature infants, such as those with a certain disease; studies with no adequate information or errors; duplicated publication; cohort studies, case–control studies, review articles, case report, correspondence or animal studies.
2.3. Data extraction
Two experienced researchers read the whole texture of each article and did the data extraction independently. In cases of any disputes, a comprehensive discussion was given until consensus. Epidata 3.02 software was used for the data entry. The extracted information included title, authors, year of publication, region and duration of the research, study protocols, sample size, gestational age, weight at birth, feeding type, NEC diagnostic standards, case number, as well as NEC staging.
2.4. Evaluation of article quality
Cochrane Risk of Bias Tool 19 was utilized to assess the risk of bias in the eligible trials. The trials were considered as high risk of bias, low risk of bias, or unclear risk of bias according to the sequence generation, allocation concealment, blinding of outcome assessment, blinding of participants and personnel, reporting bias, and other possible sources of bias.
2.5. Statistical analysis
Cochrane Collaboration's Review Manager V 5.3 was used for the data analysis. The relative risk (RR) for dichotomous outcomes and weighted mean difference (WMD)/mean difference (MD) for continuous outcomes was evaluated, by using a 95% confidence interval (95% CI). Heterogeneity was determined using the P value and I2 statistic. In the presence of no significant heterogeneity in the analysis (P > .10 or I2 < 50%), a fixed-effect model was adopted. In cases of significant heterogeneity (P < .10 or I2 ≥ 50%), a random-effect model was adopted. Subgroup analysis was conducted according to the dose of human milk. Sensitivity analysis was utilized to evaluate the influence of every single study on the pooled effects. The results were reported using the Preferred Reporting Items for Systematic Reviews (PRISMA) guidelines. P < .05 was considered to be statistically significant.
3. Results
3.1. Features of the eligible studies
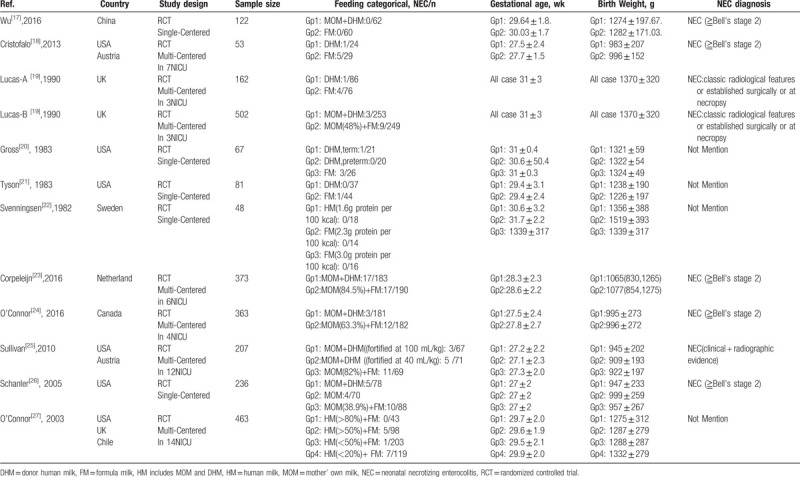
In total, 3642 citations were identified after electronic search and other sources. Upon screening the titles, abstracts, and full-text, 3631 studies were excluded after excluding duplication, letters, reviews, none-RCTs, or reporting no NEC outcomes. Finally, there were 12 RCTs that met the inclusion criteria involving a total of 2677 infants. Flow diagram of studies identified in this meta-analysis is shown in Fig. 1. The mean gestational age and birth weight were in a range of 24.8 to 33.8 weeks and 690 to 1912 g, respectively. The feeding strategy and NEC were variable in different studies. Among the included patients, 131 infants showed NEC. For the data entry, the feeding types of the infants were divided into 6 categories: exclusive human milk (100% human milk feeding), partial human milk (0<human milk feeding<100%), mainly human milk (50%<human milk feeding<100%), exclusive formula (100% formula feeding), mainly formula (50%<formula feeding<100%), and any formula (0<formula feeding≤100%). Patients with any stage of NEC were defined as NEC (Table 1).
Figure 1.
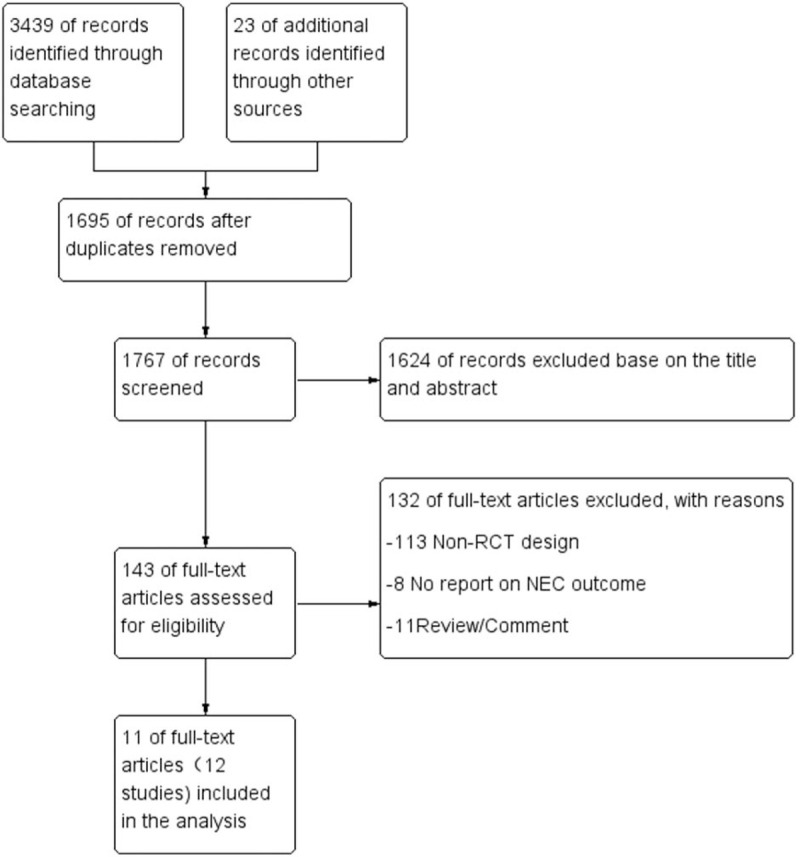
The flow diagram of selected studies.
Table 1.
The characteristics of the 12 studies included in the meta-analysis.
3.2. Sensitivity analysis and publication bias
Sensitivity analysis was performed through gradual exclusion. No obvious changes were noticed after incorporation of OR values. Sensitivity analysis indicated that all the studies showed satisfactory homogeneity. The results of the meta-analysis were reliable. The risk of bias is shown in Fig. 2. The funnel plot was symmetric (Fig. 3), which indicated a small publication bias. This implied that the included studies showed satisfactory representativeness.
Figure 2.
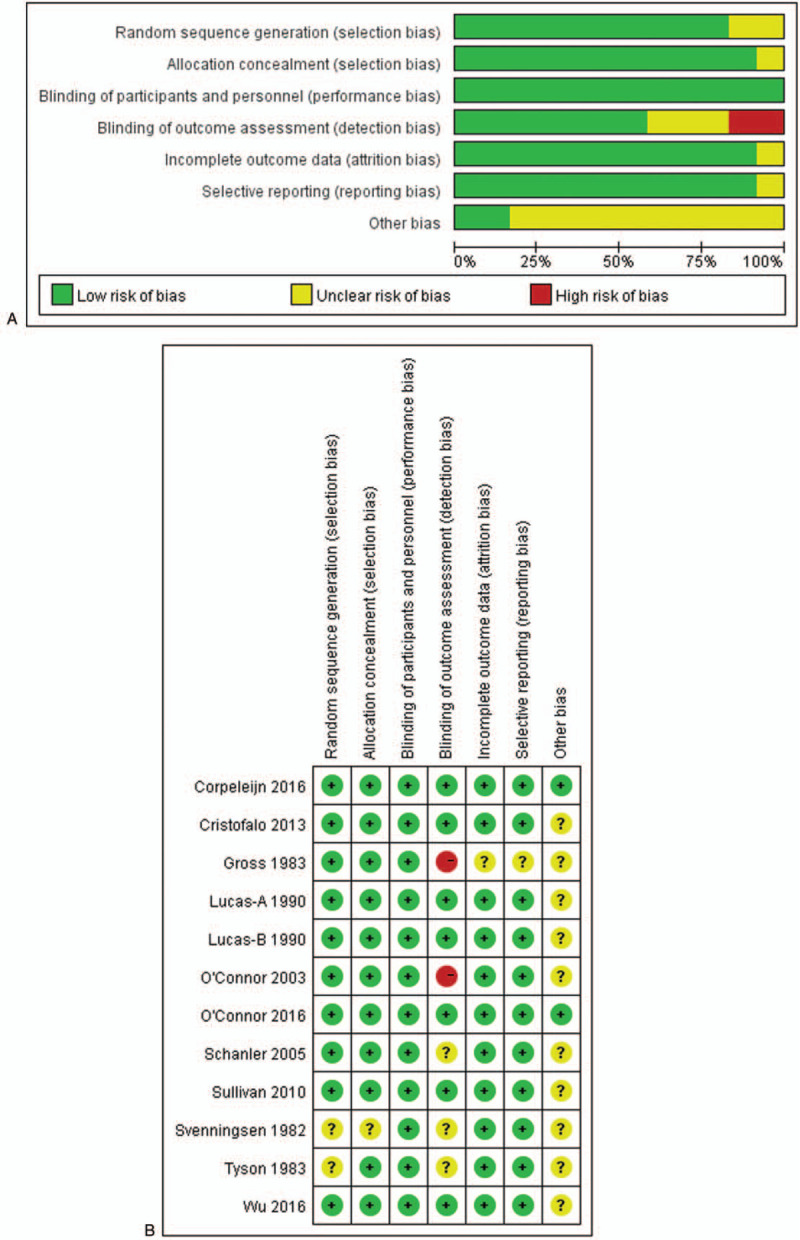
Quality evaluation data of RCTs.
Figure 3.
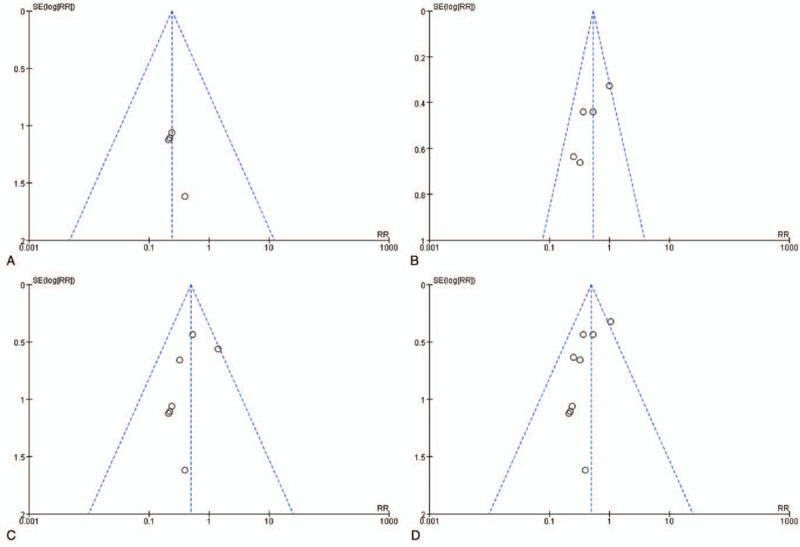
Funnel plot of human milk and formula. (A) Funnel plot of exclusive human milk versus exclusive formula. (B) Funnel plot of exclusive human milk versus partial human milk. (C) Funnel plot of mainly human milk versus mainly formula. (D) Funnel plot of exclusive human milk versus any formula.
3.3. Feeding and NEC
For the further analysis, meta-analysis was performed after dividing the feeding strategies into 4 groups (e.g., exclusive human milk vs exclusive formula; exclusive human milk vs partial human milk; mainly human milk vs mainly formula; and exclusive human milk vs any formula). Heterogeneity analysis demonstrated that there were no heterogeneities in the subgroup analysis. On this basis, fixed effect model was utilized for the incorporation of effects. In total, 6 studies[17–22] compared the incidence of NEC between infants received exclusive human milk or exclusive formula. Meta-analysis showed that compared with the exclusive formula, the incidence of NEC in the infants fed by exclusive human milk was significantly lower (RR = 0.24, 95% CI: 0.08–0.77, P < .05). Five studies[19,23–26] compared the incidence of NEC among the infants fed by exclusive human milk or partial human milk. Meta-analysis showed that the incidence of NEC in the infants fed by exclusive human milk was significantly lower than that of partial human milk (RR = 0.54, 95% CI: 0.36–0.79, P < .05). Nine studies[17–22,26,27] focused on the comparison of NEC incidences between the infants fed by mainly human milk or mainly formula. The data indicated that the incidence of NEC in the infants fed by mainly human milk was significantly lower than that of mainly formula (RR = 0.50, 95% CI: 0.30–0.83, P < .05). Eleven studies[17–26] focused on the comparison of NEC incidence between the infants fed by exclusive human milk and any formula, which indicated that the incidence of NEC in the infants fed by exclusive human milk was significantly lower than that of any formula (RR = 0.49, 95% CI: 0.34–0.71, P < .05, Fig. 4).
Figure 4.
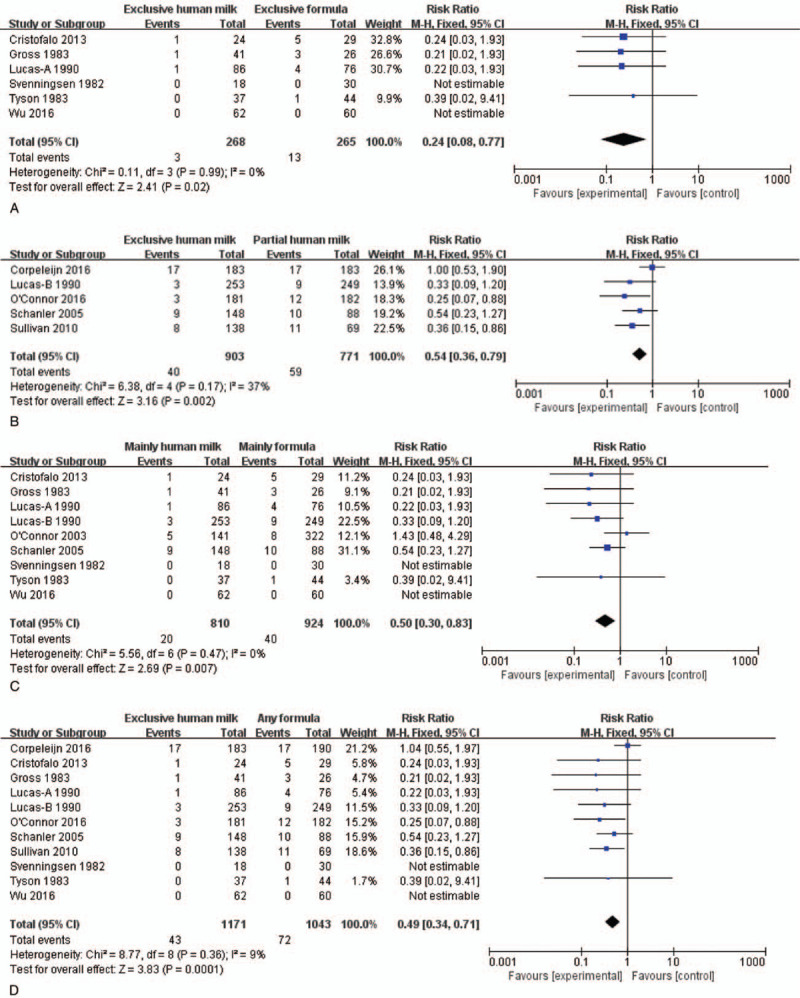
Forest plots of human milk versus formula. (A) Forest plots of exclusive human milk versus exclusive formula; The fix effects model was selected in the presence of I2 value <50% and P > .1; RR = 0.24 indicates that the RR of NEC in preterm infants with exclusive human milk was 0.24-fold, compared with exclusive formula. (B) Forest plots of exclusive human milk versus partial human milk; The fixed effects model was selected in the presence of I2 value <50% and P > .1; RR = 0.54 indicates that the RR of NEC in preterm infant with the exclusive human milk was 0.54-fold, compared with partial human milk. (C) Forest plots of mainly human milk versus mainly formula; The fixed effects model was selected in the presence of I2 value <50% and P > .1; RR = 0.50 indicated that the RR of NEC in preterm infant with mainly human milk is 0.50-fold, compared with mainly formula. (D) Forest plots of exclusive human milk versus any formula; The fix effects model was selected because the I2 value <50% and P > .1; RR = 0.49 indicates that the RR of NEC in preterm infant with the exclusive human milk was 0.49-fold, compared with any formula.
4. Discussion
In this study, we first investigated the effects of various doses of human milk on the prevention of NEC through a meta-analysis based on RCTs. Our data indicated that the incidence of NEC showed significant decline in the premature infants fed mainly by human milk (P < .05). In addition, the incidence of NEC showed a tendency of decline with the increase of human milk proportion. In contrast, with the increase of formula milk proportion, the incidence of NEC showed a tendency of increase. Such correlation between the NEC incidence and proportion of human milk has never been reported before. In the previous studies,[28–30] there was a negative correlation between the proportion of human milk uptake and complications, hospital stay, and the financial expenditure in the premature infants. Moreover, human milk contributed to the feeding of the premature infants, as well as the development of the physical and nervous behaviors. In some studies, in the premature infants with NEC, the conditions in the infants fed by formula milk were more severe than those fed by human milk, leading to a higher requirements for the surgical intervention.[31–33] This implied that feeding strategy was associated with the severity of NEC. However, in this meta-analysis, we did not divide the NEC into different groups based on the severity. Therefore, no subgroup analysis was performed to the NEC severity in our study. Interestingly, in a previous study, Cristofalo et al[18] demonstrated that premature infants fed by human milk showed a lower incidence of severe NEC and requirement of surgical intervention compared with the counterparts fed by formula milk. Besides, the long- and short-term outcomes after treatment were much better, which implied that premature infants fed by human milk showed mild severity and good outcome compared with the others. To date, no RCTs have been conducted to investigate the effects of human milk or formula milk on the mortality of NEC.
Human milk could prevent the onset of NEC, as the nutrients in the human milk could be easily absorbed by the premature gastrointestinal tracts. Besides, there are many biologically active components in the human milk that can regulate the host immune system to inhibit the activation of local immunity and decrease the injury of intestinal mucosa. In addition, epidermal growth factors in the human milk can directly repair the injury in the intestinal mucosa. Furthermore, the human milk could lower the potential of hydrogen value in the intestinal tract, leading to formation of acidic environments in the intestinal tract that inhibited the growth of inimical bacteria. This would finally enhance the natural barrier of the intestinal mucosa.[15,34–36]
As far as we know, this is the first study to investigate the protective effects of various doses of human milk on NEC. We only collect RCTs that were rigorously designed with adequate cases and strong representativeness. This study showed strong evidence. Finally, all the comparison analyses showed a strong consensus. This implied that both exclusive human milk and partial human milk could reduce the incidence of NEC. Sensitivity analysis revealed that our findings were not affected by the fluctuation of certain studies.
Indeed, there are some limitations in this study. For example, we only focused on the English and Chinese articles, and not included the articles published in other languages. In addition, most of the included studies did not divide the NEC severity. Therefore, we cannot perform the subgroup analysis based on the NEC severity, which may lead to possibility of heterogeneity. Finally, human milk included mother’ own milk and donor human milk, and may present differences in the prevention of NEC for different types of human milk. Nevertheless, as most of the studies did not categorize the human milk, we did not perform the subgroup analysis to investigate the efficiency of various types of human milk on NEC prevention. This may lead to heterogeneity.
5. Conclusion
This meta-analysis was based on the RCTs involving the prevention of NEC using human milk. It indicated that human milk was effective for preventing NEC, and there was a negative correlation between uptake of human milk and incidence of NEC. Meta-analysis demonstrated that the premature infants fed by human milk showed a lower incidence of NEC than those fed by formula milk, especially those fed mainly or completely by human milk. In future, more RCTs are required to provide conclusions in strength and capacity.
Author contributions
Authorship: ZBQ wrote the manuscript; YCY revised the manuscript; XWL did the data analysis; DY did the data collection.
Footnotes
Abbreviations: 95% CI = 95% confidence interval, DHM = donor human milk, ELBW = extremely low birth weight infants, FM = formula milk, HM = human milk, MD = mean difference, MOM = mother’ own milk, NEC = neonatal necrotizing enterocolitis, OR = odds ratio, PH = potential of hydrogen, PRISMA = Preferred Reporting Items for Systematic Reviews, RCT = randomized controlled trial, RR = risk ratio, VLB = very low birth weight infants, WMD = weighted mean difference.
How to cite this article: Zhang B, Xiu W, Dai Y, Yang C. Protective effects of different doses of human milk on neonatal necrotizing enterocolitis. Medicine. 2020;99:37(e22166).
This study was supported by the Young Talent Program of Fujian Health Bureau (No. 2019-1-16) and the Social Developmental Induction Program of Fujian Province (No. 2018Y0006).
This study was approved by the Fujian Maternity and Child Health Hospital, Affiliated Hospital of Fujian Medical University.
The authors declare that they have no conflicts of interest.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Knell J, Han SM, Jaksic T, et al. Current status of necrotizing enterocolitis. Curr Probl Surg 2019;56:11–38.. [DOI] [PubMed] [Google Scholar]
- [2].Isani MA, Delaplain PT, Grishin A, et al. Evolving understanding of neonatal necrotizing enterocolitis. Curr Opin Pediatr 2018;30:417–23.. [DOI] [PubMed] [Google Scholar]
- [3].Caplan MS. Improving outcomes due to neonatal necrotizing enterocolitis. Clin Perinatol 2019;46:xvii-i. [DOI] [PubMed] [Google Scholar]
- [4].Yajamanyam PKRS, Ewer AK. Necrotizing enterocolitis: current perspectives. Res Rep Neonatol 2014;4:31–42.. [Google Scholar]
- [5].Sharma R, Hudak ML. A clinical perspective of necrotizing enterocolitis: past, present, and future. Clin Perinatol 2013;40:27–51.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Edstedt Bonamy AK, Zeitlin J. Wide variation in severe neonatal morbidity among very preterm infants in European regions. Arch Dis Child Fetal Neonatal Ed 2019;104:F36–45.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Johnson TJ, Patel AL, Bigger HR, et al. Cost savings of human milk as a strategy to reduce the incidence of necrotizing enterocolitis in very low birth weight infants. Neonatology 2015;107:271–6.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ghoneim N, Bauchart-Thevret C, Oosterloo B, et al. Delayed initiation but not gradual advancement of enteral formula feeding reduces the incidence of necrotizing enterocolitis (NEC) in preterm pigs. PLoS One 2014;9:e106888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Battersby C, Modi N. Challenges in advancing necrotizing enterocolitis research. Clin Perinatol 2019;46:19–27.. [DOI] [PubMed] [Google Scholar]
- [10].Torrazza RM, Neu J. The altered gut microbiome and necrotizing enterocolitis. Clin Perinatol 2013;40:93–108.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Markel TA, Engelstad H, Poindexter BB. Predicting disease severity of necrotizing enterocolitis: how to identify infants for future novel therapies. J Clin Neonatol 2014;3:1–9.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Neu J, Walker WA. Probiotics and the prevention of necrotizing enterocolitis. J Pediatr Surg 2019;54:405–12.. [DOI] [PubMed] [Google Scholar]
- [13].Maffei D, Schanler RJ. Human milk is the feeding strategy to prevent necrotizing enterocolitis!. Semin Perinatol 2017;41:36–40.. [DOI] [PubMed] [Google Scholar]
- [14].Foster JP, Seth R, Cole MJ. Oral immunoglobulin for preventing necrotizing enterocolitis in preterm and low birth weight neonates. Cochrane Database Syst Rev 2016;4:Cd001816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gebauer C, Klotz D, Springer S. The value of human milk for preterm infants-overview and practical aspects. BUNDESGESUNDHEITSBLA 2018;61:952–9.. [DOI] [PubMed] [Google Scholar]
- [16].Garofalo NA, Caplan MS. Oropharyngeal mother's milk: state of the science and influence on necrotizing enterocolitis. Clin Perinatol 2019;46:77–88.. [DOI] [PubMed] [Google Scholar]
- [17].Wu Y, Zhong XY, Jiang J, et al. Prospective and controlled study on effect of fortified human milk feeding on infants with extremely and very low birth weight during hospital stay. Beijing Da Xue Xue Bao Yi Xue Ban 2016;48:143–8.. [PubMed] [Google Scholar]
- [18].Cristofalo EA, Schanler RJ, Blanco CL, et al. Randomized trial of exclusive human milk versus preterm formula diets in extremely premature infants. J Pediatr 2013;163:1592–5.. e1. [DOI] [PubMed] [Google Scholar]
- [19].Lucas A, Cole TJ. Breast milk and neonatal necrotising enterocolitis. Lancet 1990;336:1519–23.. [DOI] [PubMed] [Google Scholar]
- [20].Gross SJ. Growth and biochemical response of preterm infants fed human milk or modified infant formula. N Eng J Med 1983;308:237–41.. [DOI] [PubMed] [Google Scholar]
- [21].Tyson JE, Lasky RE, Mize CE, et al. Growth, metabolic response, and development in very-low-birth-weight infants fed banked human milk or enriched formula. I. Neonatal findings. J Pediatr 1983;103:95–104.. [DOI] [PubMed] [Google Scholar]
- [22].Svenningsen NW, Lindroth M, Lindquist B. A comparative study of varying protein intake in low birthweight infant feeding. Acta Paediatr Scand Suppl 1982;296:28–31.. [DOI] [PubMed] [Google Scholar]
- [23].Corpeleijn WE, de Waard M, Christmann V, et al. Effect of donor milk on severe infections and mortality in very low-birth-weight infants: the early nutrition study randomized clinical trial. JAMA Pediatr 2016;170:654–61.. [DOI] [PubMed] [Google Scholar]
- [24].O’Connor DL, Gibbins S, Kiss A, et al. Effect of supplemental donor human milk compared with preterm formula on neurodevelopment of very low-birth-weight infants at 18 months: a randomized clinical trial. JAMA 2016;316:1897–905.. [DOI] [PubMed] [Google Scholar]
- [25].Sullivan S, Schanler RJ, Kim JH, et al. An exclusively human milk-based diet is associated with a lower rate of necrotizing enterocolitis than a diet of human milk and bovine milk-based products. J Pediatr 2010;156:562–7.. e1. [DOI] [PubMed] [Google Scholar]
- [26].Schanler RJ, Lau C, Hurst NM, et al. Randomized trial of donor human milk versus preterm formula as substitutes for mothers’ own milk in the feeding of extremely premature infants. Pediatr 2005;116:400–6.. [DOI] [PubMed] [Google Scholar]
- [27].O’Connor DL, Jacobs J, Hall R, et al. Growth and development of premature infants fed predominantly human milk, predominantly premature infant formula, or a combination of human milk and premature formula. J Pediatr Gastroenterol Nutr 2003;37:437–46.. [DOI] [PubMed] [Google Scholar]
- [28].Lechner BE, Vohr BR. Neurodevelopmental outcomes of preterm infants fed human milk: a systematic review. Clin Perinatol 2017;44:69–83.. [DOI] [PubMed] [Google Scholar]
- [29].Lewis ED, Richard C, Larsen BM, et al. The importance of human milk for immunity in preterm infants. Clin Perinatol 2017;44:23–47.. [DOI] [PubMed] [Google Scholar]
- [30].Lonnerdal B. Bioactive proteins in human milk: potential benefits for preterm infants. Clin Perinatol 2017;44:179–91.. [DOI] [PubMed] [Google Scholar]
- [31].Raval MV, Hall NJ, Pierro A, et al. Evidence-based prevention and surgical treatment of necrotizing enterocolitis: a review of randomized controlled trials. Semin Pediatr Surg 2013;22:117–21.. [DOI] [PubMed] [Google Scholar]
- [32].Pierro A. The surgical management of necrotising enterocolitis. Early Hum Dev 2005;81:79–85.. [DOI] [PubMed] [Google Scholar]
- [33].Linder N, Hammel N, Hernandez A, et al. Intestinal perforation in very-low-birth-weight infants with necrotizing enterocolitis. J Pediatr Surg 2013;48:562–7.. [DOI] [PubMed] [Google Scholar]
- [34].Bering SB. Human milk oligosaccharides to prevent gut dysfunction and necrotizing enterocolitis in preterm neonates. Nutrients 2018;10:1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Gianni ML, Roggero P, Mosca F. Human milk protein vs. formula protein and their use in preterm infants. Curr Opin Clin Nutr Metab Care 2019;22:76–81.. [DOI] [PubMed] [Google Scholar]
- [36].Patel AL, Kim JH. Human milk and necrotizing enterocolitis. Semin Pediatr Surg 2018;27:34–8.. [DOI] [PubMed] [Google Scholar]


