Supplemental Digital Content is available in the text
Keywords: arterial resection, pancreatectomy, pancreatic cancer
Abstract
Pancreatectomy for pancreatic cancer with arterial invasion is controversial and performed infrequently. As its indication evolves and neoadjuvant chemotherapy also evolves, it is meaningful to identify short- and long-term outcomes of pancreatectomy with arterial resection (AR). This study aimed to retrospectively analyze the clinical outcomes of pancreatectomy with AR for pancreatic ductal adenocarcinoma.
Patients with pancreatic ductal adenocarcinoma treated with pancreatectomy with AR at our institute between January 2000 and April 2017 were retrospectively reviewed. Operative outcome and survival were compared according to the presence of neoadjuvant chemotherapy.
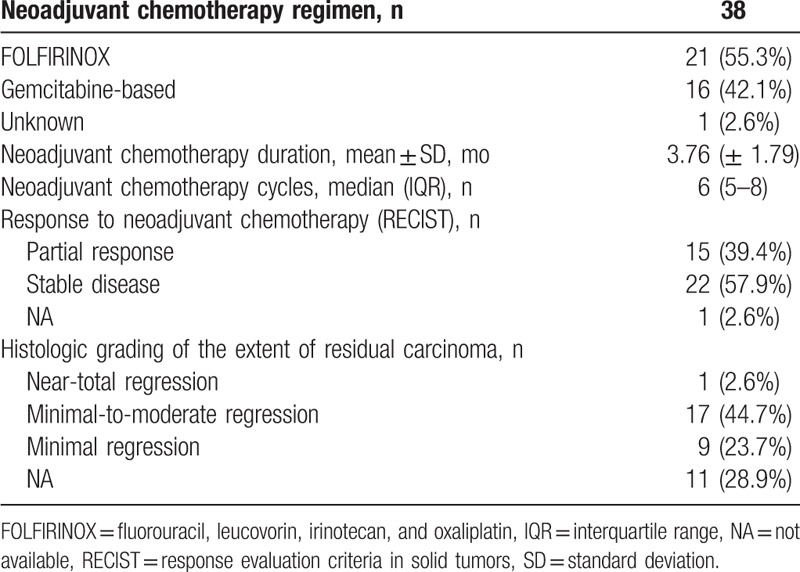
This study included 109 patients (38 underwent surgery after neoadjuvant chemotherapy, 71 underwent upfront surgery). The median hospital stay was 17 (interquartile range, 12–26.5) days. Clinically relevant postoperative pancreatic fistula (grade B or C) occurred in 14 patients (12.8%). The major morbidity (≥grade III) and mortality rates were 26.6% and 0.9%, respectively. R0 resection was achieved in 80 patients (73.4%). Microscopic actual tumor invasion into the arterial wall was identified in 25 patients (22.9%). The median overall survival (OS) of all patients was 18.4 months. The neoadjuvant chemotherapy group showed better OS than the upfront surgery group, without statistical significance (25.3 vs 16.2 months, P = .06). Progression-free survival was better in patients with neoadjuvant chemotherapy (13.2 vs 7.1 months, P = .01). Patients with partial response to neoadjuvant chemotherapy showed better OS than those with stable disease (33.7 vs 17.5 months, P = .04).
Pancreatectomy with AR for advanced pancreatic cancer showed acceptable procedure-related morbidity and mortality. A survival benefit of neoadjuvant chemotherapy was identified, compared to upfront surgery.
1. Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of the most aggressive malignancies. Only 10% to 20% of cases are potentially curable with surgical resection upon diagnosis.[1,2] This notorious disease with dismal prognosis and poor clinical outcome is characterized by aggressive local invasion and early metastases. To define localized progression and the potential involvement of major vessels, PDAC has been subdivided into resectable pancreatic cancer, borderline resectable pancreatic cancer (BRPC), and locally advanced unresectable pancreatic cancer (LAPC). These definitions have been suggested by the National Comprehensive Cancer Network.[3] Resectability is mostly determined using imaging-based assessment of the tumor extent in the context of invasion into the celiac axis (CA), hepatic artery (HA), superior mesenteric artery (SMA), and superior mesenteric vein/portal vein (SMV/PV).
LAPC without metastasis occurs in approximately 30% of newly diagnosed cases.[4] Although the National Comprehensive Cancer Network guidelines recommend neoadjuvant therapy for locally advanced disease, they have not addressed the appropriate timing of conversion surgery after neoadjuvant therapy, especially for cancers invading the major arteries. Furthermore, although neoadjuvant therapy allows conversion of an unresectable tumor to a resectable tumor, only one-third of patients initially determined to have LAPC would be expected to undergo surgery.[5] Ferrone et al[6] reported that determining resectability by means of cross-sectional imaging is no longer appropriate in patients who received the FOLFIRINOX regimen (fluorouracil, leucovorin, irinotecan, and oxaliplatin) with or without radiation therapy as neoadjuvant treatment.
Studies have reported favorable results of planned arterial resection (AR) at the time of pancreatectomy, with acceptable morbidity and mortality.[7,8] However, those studies, which included a small number of patients, have not reported the short- or long-term outcomes of AR stratified according to upfront surgery or surgery following neoadjuvant therapy. Thus, it is meaningful to identify the short- and long-term outcomes of pancreatectomy with AR in the setting of upfront surgery and neoadjuvant therapy.
In the present study, we retrospectively analyzed and evaluated the clinical outcomes of patients who underwent pancreatectomy with AR as upfront surgery or following neoadjuvant treatment.
2. Materials and methods
2.1. Patient database
Between January 2000 and April 2017, a total of 2457 consecutive patients with PDAC underwent surgical resection at Asan Medical Center, Seoul, South Korea. Of these, patients who underwent pancreatectomy combined with major AR for BRPC or LAPC were selected for the present study. Pancreatectomy included distal pancreatectomy (DP), pancreaticoduodenectomy (PD), and total pancreatectomy (TP), and the resected major arteries included the HA, CA, and SMA. Patients presenting with resectable cancer at diagnosis according to the National Comprehensive Cancer Networkguidelines,[3,9,10] those with distant metastasis, or those with other forms of pancreatic cancer (eg, acinous carcinoma, endocrine tumor, or mucinous carcinoma) were excluded. A total of 11 patients (7 cases of HA resection and 4 cases of left gastric artery resection) who underwent unplanned AR owing to incidental arterial injury during the operation were also excluded because the focus of this study included not only short-term postoperative outcomes but also the oncologic outcomes of pancreatectomy with AR for LAPC or BRPC. On the basis of the above inclusion and exclusion criteria, the data of selected patients were obtained from the electronic medical records of our institute and were retrospectively reviewed.
The following clinicopathologic data were collected and analyzed: age, sex, body mass index, presence of neoadjuvant chemotherapy, duration and number of cycles of neoadjuvant chemotherapy, response to neoadjuvant chemotherapy, operative procedures, pathologic findings, tumor size, TNM (tumor-node-metastasis) stage (American Joint Committee on Cancer stage, 8th edition), postoperative pancreatic fistula (POPF), and postoperative complications.
For patients who received neoadjuvant chemotherapy, an experienced oncologist and radiologist reviewed and evaluated the response to neoadjuvant chemotherapy according to the revised Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1.[11] An experienced pathologist evaluated the pathologic regression of residual carcinoma following the schemes of the Histologic Grading of the Extent of Residual Carcinoma guidelines.[12] For postoperative surveillance, all patients underwent contrast-enhanced abdominoperineal computed tomography (CT) and carbohydrate antigen 19-9 (CA 19-9) test every 3 months for the first 2 years following surgery and subsequently every 6 months. The diagnosis of recurrence was based on the detection of new progressive lesions and elevated CA19-9 levels. When lesions of potential recurrent disease were detected, 18F-fluorodeoxyglucose positron emission tomography and/or chest CT were performed. Then, biopsy was performed, if a differential diagnosis is needed. The duration of overall survival (OS) was measured from the time of the first treatment (surgery or neoadjuvant chemotherapy) until death or the last visit to the outpatient department. This retrospective cohort study was approved by the Asan Medical Center Institutional Review Board (Approval number: 2018-1560).
2.2. Neoadjuvant chemotherapy
Because neoadjuvant chemotherapy was not established at our institution until October 2007, upfront pancreatectomy with AR was performed for curative resection in selected patients who had focal involvement of major arteries including the HA, CA, and SMA. After neoadjuvant therapy was established in 2007, patients with PDAC involving major arteries (BRPC or LAPC) as determined during preoperative imaging have been considered candidates for neoadjuvant therapy. After neoadjuvant chemotherapy, if the patients did not show progressive disease without distant metastasis, surgery can be considered. The regimen and management of neoadjuvant chemotherapy are explained in the Results section.
2.3. Surgical technique
Before operation, we routinely evaluate arterial variation and invasion using dynamic helical CT. In principle, when BRPC or LAPC is converted to resectable carcinoma by neoadjuvant therapy, the patient becomes a candidate for curative resection. However, in this study, surgical treatment with planned AR was also performed even when the tumor was not within the resectable range because of persisting focal involvement of the artery or vein, despite neoadjuvant therapy being performed. In cases in which the tumor was incidentally found to be macroscopically adhered to or had invaded an artery, AR was sometimes performed to increase resectability and achieve a margin-negative resection. Four main methods of resection and anastomosis for the artery were used (See Fig. 1 Supplemental content which illustrates 4 major methods of resection and anastomosis for arteries). The first was end-to-end anastomosis in which the segmentally invaded site was resected, and the remaining proximal part and distal portions were sutured. The second was wedge resection followed by primary repair or patching. In the third method, if the affected area was long and could not be directly anastomosed, an autonomous vein, cadaveric vessel, or synthetic graft (polytetrafluoroethylene) was used for end-to-end anastomosis. In the fourth method, resection without anastomosis was applied, especially in cases requiring mainly CA resection.
If only invasion into the right or left HA had occurred, the anastomosis was determined according to the backflow condition after the resection. We assessed arterial blood backflow by identifying blood pumping at the remnant arterial stump or intraoperative ultrasonography. For all surgical procedures, low-molecular-weight heparin was routinely used to prevent thrombosis.
2.4. Statistical analysis
Statistical analyses were performed using SPSS 21.0 (IBM Corp., Armonk, NY). Continuous variables were compared using Student t test. Categorical variables were compared using the Chi-square test or Fisher exact test. All tests were 2-sided, and a P-value of ≤.05 was considered statistically significant. Survival curves were generated using the Kaplan–Meier method. The comparison of survival according to the presence of neoadjuvant chemotherapy, response to neoadjuvant chemotherapy, and location of the resected artery was performed using the log-rank test. Risk factors for OS and progression-free survival (PFS) were tested in a multivariable logistic regression with background elimination model, with results expressed as odds ratios with 95% confidence intervals. The significance level for variable elimination was 0.05.
3. Results
According to the inclusion and exclusion criteria, 109 patients treated with pancreatectomy combined with major AR for PDAC were included. The demographics and perioperative outcomes of the 109 patients (71 men, 38 women) are shown in Table 1. The median age at diagnosis was 59 (interquartile range [IQR], 51–65) years. The median body mass index was 23.00 (IQR, 21.10–25.15) kg/m2. Of the patients, 38 underwent surgery after neoadjuvant chemotherapy and 71 underwent upfront surgery. Patients who underwent upfront surgery were treated between 2000 and 2016 and those who received neoadjuvant treatment underwent surgery between 2007 and 2017. None of the patients received neoadjuvant chemotherapy combined with radiation therapy. The median CA 19-9 level was 68.9 ng/mL (mean, 707.7 ng/mL; range, 0.5–40,700 ng/mL). All 4 laparoscopic operations involved laparoscopic DP with CA resection. Overall, 42 cases of PD, 46 cases of DP, and 21 cases of TP with AR were performed. HA or SMA resection was mainly performed in PD, and CA resection was performed in DP. The types of graft used for anastomosis were as follows: autonomous inferior mesenteric vein (n = 1), cadaveric iliac artery (n = 1), cadaveric iliac vein (n = 5), and synthetic graft (polytetrafluoroethylene) (n = 5). Figure 1 shows TP with CA resection and cadaveric iliac artery interposition. The non-anastomosis cases included 45 patients (41 cases of DP with CA resection, 1 case of DP with left HA resection; normal anatomy, 1 case of PD with left HA resection; right HA from SMA, Michels type III, 1 case of PD with right HA resection; accessory left HA from left gastric artery, Michels type V, and 1 case of TP with left HA resection; common HA from SMA, Michels type IX).[13] Concurrent PV or SMV resection was performed in 62 patients (56.9%). The median length of hospital stay was 17 (IQR, 12–26.5) days. Clinically relevant POPF (grade B or C) occurred in 14 patients (12.8%). The incidence of grade III complications according to the Clavien–Dindo classification was 23.9% and that of grade IV complications was 1.8%. In-hospital 90-day mortality occurred because of hepatic failure in 1 patient. This single case also represents the overall 90-day mortality. Additionally, the incidence of above-grade III complications in 11 cases of unplanned AR, which were excluded from this study, was 18.2%, and mortality was 0%.
Table 1.
Demographics and perioperative outcome of patients who underwent pancreatectomy with arterial resection.
Figure 1.
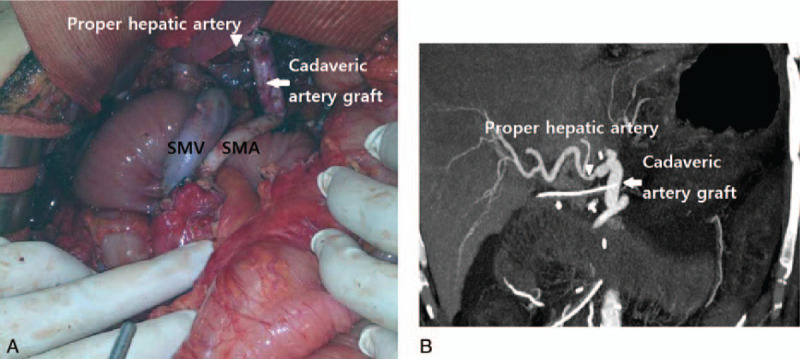
Intraoperative finding of TP with CA resection performed for LAPC (A). After CA resection, the SMA and proper HA were connected with cadaveric iliac artery graft. Dynamic helical CT was performed at 5 d postoperatively (B). Triangle, proper hepatic artery; arrow, cadaveric iliac artery graft. CA = celiac axis, CT = computed tomography, HA = hepatic artery, LAPC = locally advanced unresectable pancreatic cancer, SMA = superior mesenteric artery, TP = total pancreatectomy.
The details of postoperative complications according to the location of the resected artery are described in Table 2. Two cases of hepatic infarction occurred in HA resection. One case was right HA from SMA (Michels type III) and the patient had PD with PV resection and right HA resection with end to side anastomosis to left HA. After surgery, hepatic infarction occurred at segment 4; the patient was discharged postoperative 22 days after ICU care. Anatomy in the other case was normal and the patient had PD with common HA resection and cadaveric vein interposition. Hepatic infarction occurred at segments 1, 4 and the patient was discharged postoperative 17 days with conservative treatment. Three cases of hepatic infarction occurred in CA resection. All of these cases had DP with CA resection. One patient was a 69-year-old woman, who expired postoperative 11 days with left liver infarction and liver failure. The patient showed weak right HA flow and left HA occlusion. The other 2 patients had multifocal hepatic infarction due to PV thrombus. In present study, there were 3 non-anastomosis cases of PD or TP with right or left HA resection. All 3 cases showed elevated serum alanine aminotransferase and aspartate aminotransferase in postoperative 1 day (4 times higher than baseline), but thereafter liver enzyme decreased and normalized before postoperative 7 days. Among them, 2 patients were discharged postoperative 11 and 12 days without complication including hepatic infarction or abscess. Pseudoaneurysm of replaced right HA from SMA occurred at postoperative 22 days in PD with left HA resection, Michels type III. Pseudoaneurysm was treated with embolization, nevertheless, no liver failure or abscess occurred after the procedure. This patient was discharged postoperative 63 days after POPF treatment.
Table 2.
Postoperative complications after pancreatectomy with arterial resection according to the location of arterial resection.
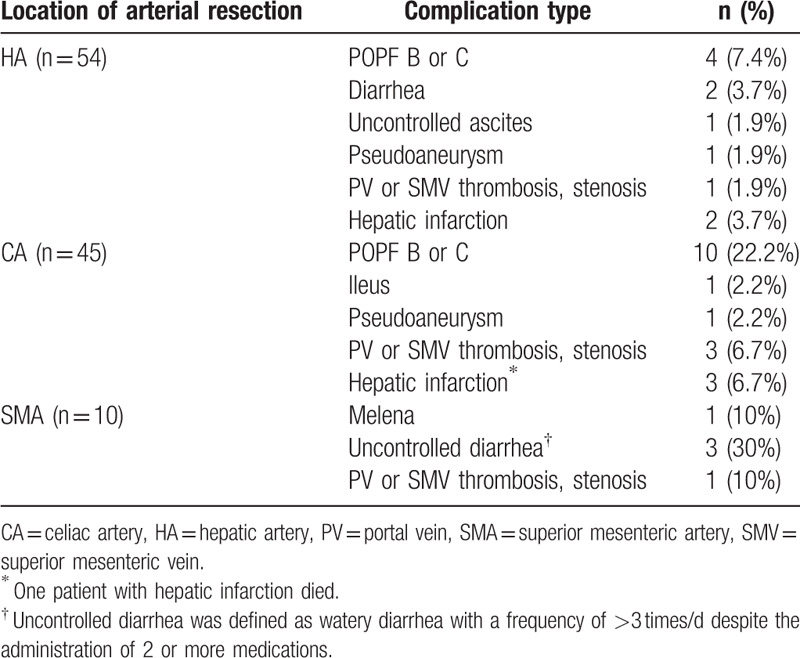
Table 3 shows the pathological outcomes. The median tumor size was 3.50 (IQR, 2.85–4.50) cm. R0 resection was achieved in 80 patients (73.4%), and microscopic actual tumor invasion into the arterial wall was identified in 25 patients (22.9%). Therefore, the actual proportion of pathologic T4 grade cases was 22.9%. One patient with M1 disease underwent combined hepatic resection for an incidentally found single metastatic nodule. Lymphovascular invasion was present in 49 patients (45%), and perineural invasion was identified in 99 patients (90.8%). The mean number of harvested lymph nodes was 19.58, and the mean number of positive lymph nodes was 1.88. The lymph node ratio was 11.14%.
Table 3.
Pathologic outcome of patients undergoing pancreatectomy with arterial resection.
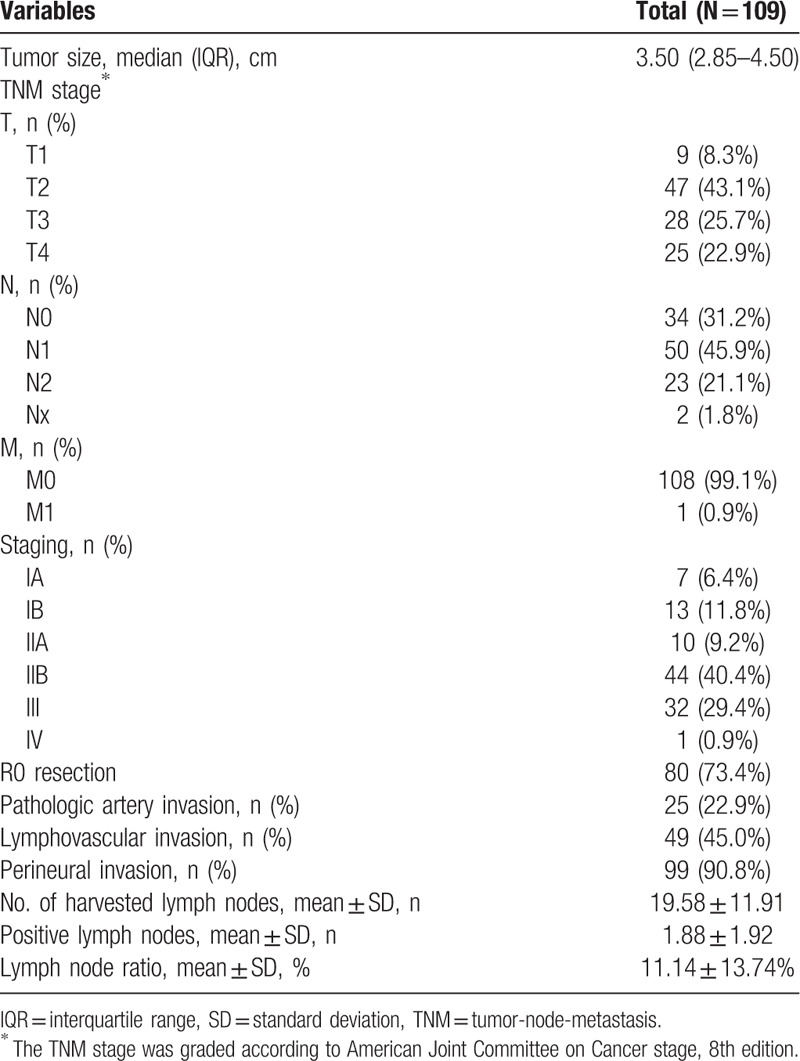
Figure 2 shows the Kaplan–Meier survival curves of the OS and PFS of patients who underwent pancreatectomy with AR. The mean follow up time was 24.5 months (range, 5.0–130 months). The median OS following the first treatment was 18.4 (IQR, 15.7–21.0) months, and the estimated 1-, 2-, and 5-year OS rates were 75.2%, 40.7%, and 10.5%, respectively. The median PFS was 9.8 (IQR, 8.3–11.2) months, and the estimated 1-, 2-, and 5-year PFS rates were 36.7%, 11.8%, and 1.8%, respectively.
Figure 2.
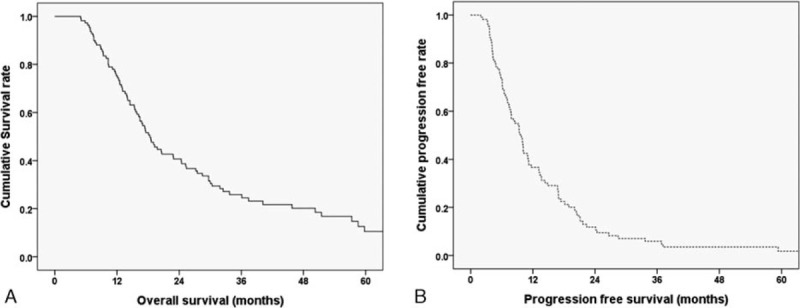
Kaplan–Meier survival curves of patients who underwent pancreatectomy with arterial resection (N = 109). (A) The median overall survival (OS) was 18.4 mo, and the estimated 1-, 2-, and 5-yr OS rates were 75.2%, 40.7%, and 10.5%, respectively. (B) The median progression-free survival (PFS) was 9.8 mo, and the estimated 1-, 2-, and 5-yr PFS rates were 36.7%, 11.8%, and 1.8%, respectively.
We compared the neoadjuvant chemotherapy group with the upfront surgery group in terms of perioperative and pathologic outcomes (Table 4). The operation type, AR type, concurrent vein resection, R0 resection, tumor size, and pathologic arterial invasion did not differ between the 2 groups; however, the proportion of patients according to TNM stage was different between the 2 groups. The incidence of lymphovascular invasion was lower in the neoadjuvant chemotherapy group but that of perineural invasion was similar between the 2 groups. The number of positive lymph nodes and the lymph node ratio were smaller in the neoadjuvant chemotherapy group. The duration of hospital stay and the complication rates were not different between the 2 groups; however, clinically relevant POPF occurred more frequently in the neoadjuvant chemotherapy group. There was no difference in the rate of postoperative adjuvant treatment after surgery between the 2 groups.
Table 4.
Comparison of outcomes between neoadjuvant chemotherapy and upfront surgery.
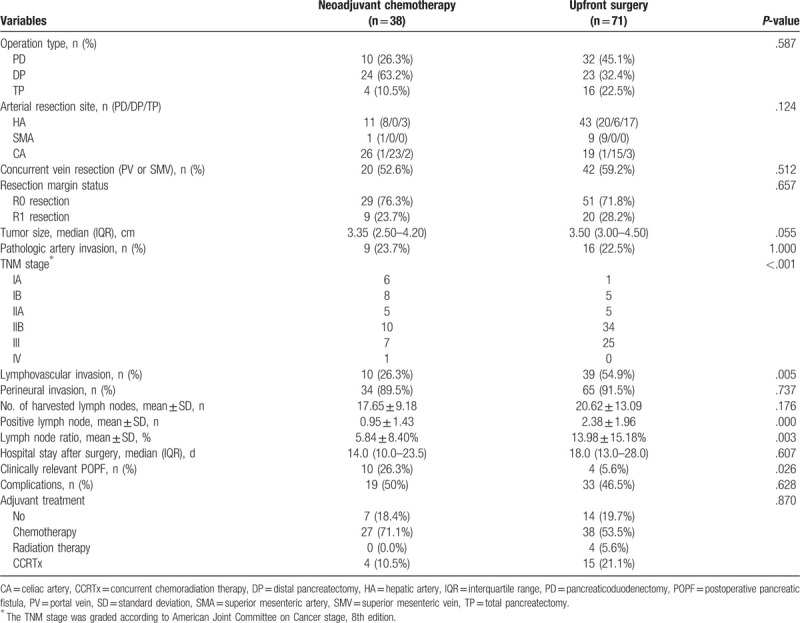
The treatment regimens and treatment process of patients with neoadjuvant chemotherapy are shown in Table 5. Of the 38 patients with neoadjuvant chemotherapy, 21 received FOLFIRINOX and 16 received gemcitabine-based chemotherapy. The mean duration of neoadjuvant treatment was 3.76 ± 1.79 months, and the median number of treatment cycles was 6 (IQR, 5–8). Of the patients who received neoadjuvant chemotherapy, with the exception of 1 patient with missing pre-chemotherapy data, 15 patients (39.5%) showed a partial response and 22 (57.9%) patients showed stable disease according to RECIST 1.1. Of 38 patients who received neoadjuvant chemotherapy, 27 were assessed for histologic grading of the extent of residual carcinoma following neoadjuvant chemotherapy; 1 patient showed grade 1 residual disease (near total regression), 17 patients showed grade 2–3 (minimal to moderate regression), and 9 patients showed grade 3 (minimal regression).
Table 5.
Treatment regimens and management of patients with neoadjuvant chemotherapy.
As shown in Figure 3, the OS was better in the neoadjuvant chemotherapy group than in the upfront surgery group, but the difference was not statistically significant (25.3 vs 16.2 months, P = .06). PFS was better in patients who underwent surgery following neoadjuvant chemotherapy (13.2 vs 7.1 months, P = .01). When the 38 patients with neoadjuvant treatment were classified according to RECIST 1.1, the partial response group showed better survival than the stable disease group (33.7 vs 17.5 months, P = .04); however, PFS was not statistically different between the 2 groups (13.6 vs 11.2 months, P = .20).
Figure 3.
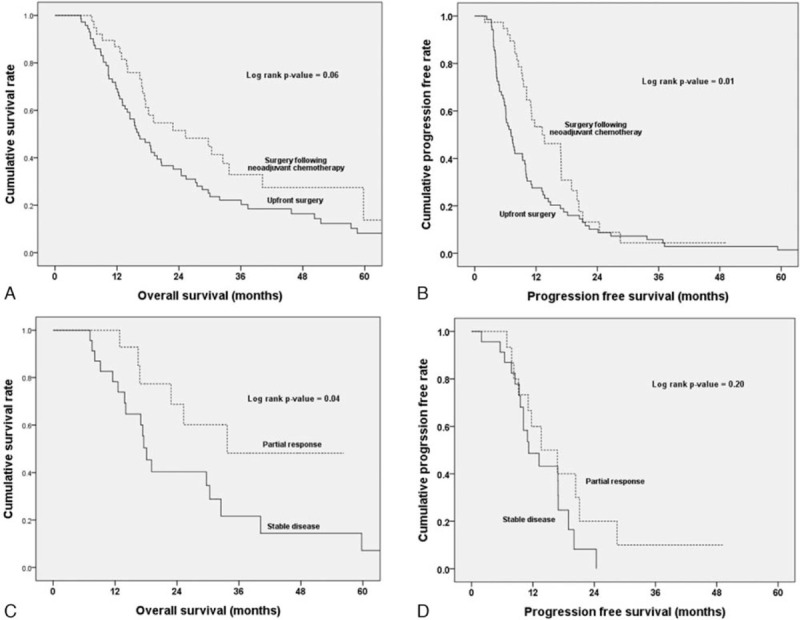
Kaplan–Meier survival curves of patients who underwent upfront surgery (n = 71) and those who underwent surgery following neoadjuvant chemotherapy (n = 38). (A) The median overall survival (OS) and the estimated 1-, 2-, and 5-yr OS rates were 16.2 mo and 69.0%, 35.2%, and 8.2%, respectively, in the upfront surgery group, and 25.3 mo and 86.8%, 51.5%, and 13.7%, respectively, in the neoadjuvant chemotherapy group (P = .06). (B) The median progression-free survival (PFS) and the estimated 1-, 2-, and 5-yr PFS rates were 7.1 mo and 27.5%, 10.1%, and 1.4%, respectively, in the upfront surgery group, and 13.2 mo and 53.4%, 13.2%, and not available, respectively, in the neoadjuvant chemotherapy group (P = .01). (C) Survival comparison of patients with a partial response (n = 15) and those with stable disease (n = 22) in the neoadjuvant chemotherapy group. The median OS and the estimated 1-, 2-, and 5-yr OS rates were 33.7 mo and 92.9%, 68.8%, and not available, respectively, in the partial response group, and 18.1 mo and 78.3%, 40.3%, and 7.2%, respectively, in the stable disease group (P = .04). (D) The median PFS and the estimated 1- and 2-yr PFS rates were 13.6 mo and 60.0% and 20.0%, respectively, in the partial response group, and 11.2 mo and 48.6% and 8.2%, respectively, in the stable disease group (P = .20).
We also compared the neoadjuvant chemotherapy group and the upfront surgery group according to operation type. In PD and TP, the proportion of patients according to TNM stage was different between the 2 groups. The incidence of lymphovascular invasion and the lymph node ratio were smaller in the neoadjuvant chemotherapy group (See Table 1 Supplemental content, which shows comparison of outcomes between neoadjuvant chemotherapy and upfront surgery in PD and TP). In case of DP, HA resection was more frequently performed in the upfront surgery group. The proportion of patients according to TNM stage was also different, and the lymph node ratio was also smaller in the neoadjuvant chemotherapy group (See Table 2 Supplemental content, which shows comparison of outcomes between neoadjuvant chemotherapy and upfront surgery in DP). Clinically relevant POPF occurred more frequently in the neoadjuvant chemotherapy group (33.3% vs 4.3%, P = .023).
In the subgroup analysis according to operation type, OS and PFS were better in the neoadjuvant chemotherapy group after PD and TP (See Fig. 2, Supplemental content which illustrates Kaplan–Meier survival curves of patients who underwent upfront surgery and those who underwent surgery following neoadjuvant chemotherapy according to operation type). In case of PD, the median OS was 14.5 months in the upfront surgery group and 32.5 months in the neoadjuvant chemotherapy group (P = .041). The median PFS was 6.1 months in the upfront surgery group and 16.9 months in the neoadjuvant chemotherapy group (P = .005). In the subgroup analysis in case of DP, there was no statistical difference between the neoadjuvant chemotherapy and upfront surgery groups.
The SMA resection group (n = 10) had a shorter median PFS than the CA resection group or the HA resection group. (See Fig. 3, Supplemental content which illustrates Kaplan–Meier survival curves according to the location of AR).
A multivariable logistic regression model identified upfront surgery (P = .008) as an independent risk factor for OS (See Table 3 Supplemental content, which shows univariate and multivariate models of risk factor for OS), and upfront surgery (P = .011) and SMA resection (P = .023) were identified as risk factors for PFS (See Table 4 Supplemental content, which shows univariate and multivariate models of risk factor for progression free survival).
4. Discussion
PDAC is an aggressive disease for which operative resection is the only curative therapy. However, even if patients with PDAC undergo surgery, a low level of survival is expected. In the United Kingdom, Ravikumar et al reported an OS of 1.5 years after conventional PD and an OS of 1.52 years after PD with vein resection, whereas the OS of patients who underwent bypass operation was 0.67 years.[14] Amano et al reported a median OS of 12 months in 23 cases of pancreatectomy with arterial reconstruction.[15] Recently, Bachellier et al reported a median OS of 15.5 months in 118 cases of pancreatectomy with AR.[16] According to studies from our institution, the median survival of patients with resectable PDAC was 24.6 months[17] and the median survival of patients who underwent pancreatectomy with PV or SMV resection for PDAC was 17.2 months.[18] When compared with the above results, it is meaningful that the median survival was 18.4 months in patients who underwent pancreatectomy with AR for PDAC. It cannot be judged with certainty that operation is better than chemotherapy for patients with LAPC; however, a previous study reported a median survival of 19.9 months in 33 patients with BRPC or LAPC treated with FOLFIRINOX followed by consolidative chemoradation,[19] which is comparable to our result. Among these 33 patients, 5 had R0 resection, and the median OS was 22.0 months. Further, in another study, the median OS was significantly longer in patients with LAPC who underwent resection after FOLFIRINOX therapy than in non-resected patients with LAPC.[20] Therefore, we suggest that even in the presence of arterial invasion, pancreatectomy with AR should be considered.
In this study, 21 patients received FOLFIRINOX and 16 received gemcitabine-based chemotherapy. FOLFIRINOX neoadjuvant chemotherapy started in 2013, but gemciabine-based regimen started in 2007. R0 resection (66.7% vs 87.5%, P = .248), OS (37.9 months vs 27.1 months, P = .068), and PFS (18.7 months vs 13.2 months, P = .138) were not statistically different between the 2 groups. Yoo et al reported that FOLFIRINOX was feasible and effective as neoadjuvant chemotherapy for patients with BRPC and may have improved efficacy compared to a gemcitabine-based regimen.[21] Dhir et al also reported that neoadjuvant FOLFIRINOX was associated with a 4.9-month improvement in survival compared with G-nP after adjustment for covariates for resectable and BRPC.[22] As in previous reports, it has been shown that FOLFIRINOX may yield better results regarding oncological outcome in the current study even though there were no statistical differences in our study.
The rate of invasion into the arterial wall on actual biopsy was 22.9%, which means that the radiologic or macroscopic findings in the operative field do not always coincide with the actual invasion. Because the current imaging modalities have a limited ability to distinguish between vascular abutment, adherence, and invasion,[23] surgeons are unable to identify whether invasion into the arterial wall is present in the preoperative stage. In addition, with the increasing application of neoadjuvant therapy, it is sometimes difficult to determine the timing for surgical resection in patients suspected of having arterial invasion or abutment. If the arterial invasion shows improvement after neoadjuvant chemotherapy, surgery can be decided without hesitation. However, even if the tumor extent is reduced, surgery cannot be easily decided if arterial invasion remains. In our study, we concluded that if pancreatectomy with AR is useful option for survival gain in this situation.
The pathologic outcomes of patients who responded to neoadjuvant chemotherapy showed that subsequent pancreatectomy with AR was associated with improved oncological outcomes in terms of the number of positive lymph nodes, positive lymph node ratio, lymphovascular invasion, and TNM stage. Similar R0 resection, pathologic arterial invasion, and perineural invasion were not expected results, but rate of pathologic arterial invasion after pancreatectomy was reported as 8.3% to 74.2% in previous studies,[7,8,16,24] which means that the pathologic results are not yet clear even if there is radiologic invasion. Currently, there are few cases where pancreatectomy is performed directly on LAPC, and this pancreatectomy with AR itself is a rare operation; further research on these pathological findings is needed. In addition, some pathologic findings did not differ, but positive lymph nodes, positive lymph node ratio, lymphovascular invasion, and TNM stage seemed to be different between the 2 groups. Therefore, neoadjuvant treatment may be considered more oncologically advantageous. The OS of patients who underwent pancreatectomy with AR following neoadjuvant chemotherapy was comparable with the OS reported in previous studies investigating general pancreatectomy without AR.[17,25] In addition, patients with partial response showed better OS than patients with stable disease (33.7 vs 17.5 months, P = .04), and similar results have been previously reported.[26] On the basis of these results, pancreatectomy with AR could be particularly useful in patients with partial response after neoadjuvant chemotherapy. In the subgroup analysis according to operation type, the oncologic outcome was better in the neoadjuvant chemotherapy group after PD and TP, but not after DP. This difference according to operation type could not be definitely explained because the number of patients is smaller in subgroup analyses. However, the proportion of patients with partial response was higher in the PD and TP groups (42.9%) than in the DP group (37.5%). More cases need to be accumulated to explain the discrepancy in these results.
In our study, the SMA resection group showed a shorter PFS than the CA and HA resection groups. There is 1 study that examined the oncological outcome according to the location of pancreatectomy with AR. Tee et al[27] reported no long-term survival detriment based on the arterial invasion type. However, this report also described a small number of cases (15 SMA resection cases). SMA resection is more likely to cause serious complications for patients; however, as data are insufficient, more clinical cases are needed to further analyze oncological survival according to the involved artery.
Most surgeons are reluctant to perform pancreatectomy with AR for PDAC because of the difficulty of the procedure and the high incidence of morbidity and mortality following surgery. In previously, pancreatectomy with AR was associated with increased morbidity and mortality and did not improve long-term survival.[28,29] Mollberg et al reported perioperative morbidity rates ranging from 16.7% to 100% (median, 53.6%) in 14 studies, and the mortality rates ranged from 0% to 45.5% (median, 11.8%) in 22 studies.[30] A recent study also reported somewhat unsatisfactory results in which 111 elective pancreatectomies with AR (from 1990 to 2017) were associated with a high overall 90-day major morbidity (≥grade III) and mortality (54% and 13%, respectively).[27] Comparatively, our study, which included a relatively recent cohort (from 2000 to 2017), demonstrated significantly lower morbidity and mortality rates. Major complications (classified as Clavien–Dindo grades III to V), including complicated fluid collection, POPF, hepaticojejunostomy stricture, venous thrombosis or stenosis, pseudoaneurysm, acute renal failure, hepatic infarction, hepatic failure, and septic shock, occurred in 26.6% of our cohort, whereas 90-day mortality was reported in 1 patient. This patient, who underwent DP with CA resection and PV resection, died of hepatic failure after 11 postoperative days. The reasons why the morbidity and mortality rates in the present study were lower than those in other studies are unclear, but there may be 2 possible explanations. First, the operators or participating vascular surgeons had a wide-ranging experience in organ transplantation and were specialized in with AR with anastomosis. Second, our study cohort included relatively young patients (median age, 59 years) compared with other studies.[27,31,32] More aggressive procedures were performed selectively in younger patients who might have been better able to withstand operative insults than average-aged patients with PDAC.
In present study, there was no liver abscess or liver failure after PD or TP with right or left HA resection and non-anastomosis. Recently, Asano et al reported that liver abscess occurred in 11% of PD with replaced right HA resection and non-anastomosis.[33] Yang et al reported neither liver abscess nor biliary fistula occurred in 11 patients who treated with pancreatectomy with HA resection and non-anastomosis.[34] The reason of hepatic infarction or abscess did not occur can be explained by network between left HA and right HA at hilar plate,[35,36] collateral circulation of liver such as right inferior phrenic artery[37] and assessing adequate hepatic blood flow with identifying blood pumping at the remnant arterial stump or intraoperative ultrasonography. However, because the number of cases are still small, it should not be concluded that resection of left or right HA during pancreatectomy will not cause liver abscess or liver function. When resecting HA, it should be considered that abnormality of liver perfusion can occur, which may lead to liver failure. Differences in the incidence of POPF were found in DP. Our clinically relevant POPF rate after DP in neoadjuvant group was high (33.3%), but there are reports that percentage of clinically relevant POPF after DP with CA resection after neoadjuvant chemotherapy is 32% to 40%, comparable to our reports.[24,38] Clinically relevant POPF after conventional open DP at our institution was also 3.7%,[39] which is comparable to upfront DP in this study (4.3%). Neoadjuvant treatment may have influenced the incidence of POPF.
The present study has some limitations. Data were collected retrospectively, and only highly selected patients were included. In addition, as the data for this study were derived from a large tertiary center, it may be difficult to generalize the results. Although this study included a large number of patients who underwent neoadjuvant therapy or upfront surgery, the patients were selected empirically. Thus, the comparison between surgery following neoadjuvant chemotherapy and upfront surgery may also be statistically biased. Nevertheless, this series demonstrated that pancreatectomy with AR for PDAC could be safely performed in selected patients. Furthermore, extended surgical resection might improve survival in patients with persistent involvement of the arteries despite a response to neoadjuvant chemotherapy. This suggests that more patients with BRPC or LAPC may have the potential for cure with neoadjuvant chemotherapy and extended surgical treatment.
Pancreatectomy with AR for pancreatic cancer with arterial invasion may be an acceptable procedure in terms of procedure-related morbidity and mortality, although a survival benefit may be achieved only in highly selected patients. A survival benefit has been identified in patients who underwent neoadjuvant chemotherapy compared with those who underwent upfront surgery; however, further studies are needed to establish better selection criteria for improving survival through AR.
Author contributions
Conceptualization: Jaewoo Kwon, Sang Hyun Shin, Jae Hoon Lee, Song Cheol Kim.
Data curation: Jaewoo Kwon, Sang Hyun Shin, Daegwang Yoo, Sarang Hong, Jong Woo Lee, Woo Young Youn, Kyungyeon Hwang, Seung Jae Lee, Guisuk Park, Yejong Park.
Formal analysis: Jaewoo Kwon, Daegwang Yoo, Sarang Hong, Guisuk Park, Yejong Park.
Investigation: Sang Hyun Shin, Jong Woo Lee, Kyungyeon Hwang.
Methodology: Jaewoo Kwon, Sang Hyun Shin, Daegwang Yoo, Sarang Hong, Jong Woo Lee, Woo Young Youn, Yejong Park, Ki Byung Song, Jae Hoon Lee, Dae Wook Hwang.
Project administration: Jae Hoon Lee.
Resources: Daegwang Yoo, Ki Byung Song.
Software: Jong Woo Lee, Kyungyeon Hwang, Seung Jae Lee, Ki Byung Song.
Supervision: Woohyung Lee, Dae Wook Hwang.
Validation: Seung Jae Lee, Woohyung Lee, Dae Wook Hwang, Song Cheol Kim.
Visualization: Sarang Hong, Woo Young Youn.
Writing – original draft: Jaewoo Kwon, Sang Hyun Shin.
Writing – review & editing: Song Cheol Kim.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: AR = arterial resection, AR = arterial resection, BRPC = borderline resectable pancreatic cancer, CA = celiac axis, CT = computed tomography, DP = distal pancreatectomy, FOLFIRINOX = fluorouracil, leucovorin, irinotecan, and oxaliplatin, HA = hepatic artery, LAPC = locally advanced unresectable pancreatic cancer, OS = overall survival, PD = pancreaticoduodenectomy, PDAC = pancreatic ductal adenocarcinoma, PFS = progression-free survival, POPF = postoperative pancreatic fistula, PV = portal vein, RECIST = response evaluation criteria in solid tumors, SMA = superior mesenteric artery, SMV = superior mesenteric vein, TP = total pancreatectomy.
How to cite this article: Kwon J, Shin SH, Yoo D, Hong S, Lee JW, Youn WY, Hwang K, Lee SJ, Park G, Park Y, Lee W, Song KB, Lee JH, Hwang DW, Kim SC. Arterial resection during pancreatectomy for pancreatic ductal adenocarcinoma with arterial invasion: a single-center experience with 109 patients. Medicine. 2020;99:37(e22115).
JK and SHS contributed equally to this work as co-first authors.
This study was supported by a grant from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (grant no. HI14C2640).
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].Harrison LE, Klimstra DS, Brennan MF. Isolated portal vein involvement in pancreatic adenocarcinoma. A contraindication for resection? Ann Surg 1996;224:342–7.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bilimoria KY, Bentrem DJ, Ko CY, et al. National failure to operate on early stage pancreatic cancer. Ann Surg 2007;246:173–80.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Tempero MA, Malafa MP, Al-Hawary M, et al. Pancreatic adenocarcinoma, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2017;15:1028–61.. [DOI] [PubMed] [Google Scholar]
- [4].Heestand GM, Murphy JD, Lowy AM. Approach to patients with pancreatic cancer without detectable metastases. J Clin Oncol 2015;33:1770–8.. [DOI] [PubMed] [Google Scholar]
- [5].Gillen S, Schuster T, Meyer Zum Buschenfelde C, et al. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med 2010;7:e1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ferrone CR, Marchegiani G, Hong TS, et al. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann Surg 2015;261:12–7.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Christians KK, Pilgrim CH, Tsai S, et al. Arterial resection at the time of pancreatectomy for cancer. Surgery 2014;155:919–26.. [DOI] [PubMed] [Google Scholar]
- [8].Amano R, Kimura K, Nakata B, et al. Pancreatectomy with major arterial resection after neoadjuvant chemoradiotherapy gemcitabine and S-1 and concurrent radiotherapy for locally advanced unresectable pancreatic cancer. Surgery 2015;158:191–200.. [DOI] [PubMed] [Google Scholar]
- [9].Tempero MA, Arnoletti JP, Behrman S, et al. Pancreatic adenocarcinoma. J Natl Compr Canc Netw 2010;8:972–1017.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Al-Hawary MM, Francis IR, Chari ST, et al. Pancreatic ductal adenocarcinoma radiology reporting template: consensus statement of the society of abdominal radiology and the American pancreatic association. Gastroenterology 2014;146:291–304.e1.. [DOI] [PubMed] [Google Scholar]
- [11].Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47.. [DOI] [PubMed] [Google Scholar]
- [12].Chatterjee D, Katz MH, Rashid A, et al. Histologic grading of the extent of residual carcinoma following neoadjuvant chemoradiation in pancreatic ductal adenocarcinoma: a predictor for patient outcome. Cancer 2012;118:3182–90.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Michels NA. Newer anatomy of the liver and its variant blood supply and collateral circulation. Am J Surg 1966;112:337–47.. [DOI] [PubMed] [Google Scholar]
- [14].Ravikumar R, Sabin C, Abu Hilal M, et al. Portal vein resection in borderline resectable pancreatic cancer: a United Kingdom multicenter study. J Am Coll Surg 2014;218:401–11.. [DOI] [PubMed] [Google Scholar]
- [15].Amano H, Miura F, Toyota N, et al. Is pancreatectomy with arterial reconstruction a safe and useful procedure for locally advanced pancreatic cancer? J Hepatobiliary Pancreat Surg 2009;16:850–7.. [DOI] [PubMed] [Google Scholar]
- [16].Bachellier P, Addeo P, Faitot F, et al. Pancreatectomy with arterial resection for pancreatic adenocarcinoma: how can it be done safely and with which outcomes?: a single institution's experience with 118 patients. Ann Surg 2020;271:932–40.. [DOI] [PubMed] [Google Scholar]
- [17].Shin SH, Kim SC, Song KB, et al. Chronologic changes in clinical and survival features of pancreatic ductal adenocarcinoma since 2000: a single-center experience with 2,029 patients. Surgery 2018;164:432–42.. [DOI] [PubMed] [Google Scholar]
- [18].Hwang JW, Kim SC, Song KB, et al. Significance of radiologic location and extent of portal venous involvement on prognosis after resection for pancreatic adenocarcinoma. Pancreas 2015;44:665–71.. [DOI] [PubMed] [Google Scholar]
- [19].Mancini BR, Stein S, Lloyd S, et al. Chemoradiation after FOLFIRINOX for borderline resectable or locally advanced pancreatic cancer. J Gastrointest Oncol 2018;9:982–8.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lee J, Lee JC, Gromski MA, et al. Clinical outcomes of FOLFIRINOX in locally advanced pancreatic cancer: a single center experience. Medicine (Baltimore) 2018;97:e13592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yoo C, Kang J, Kim KP, et al. Efficacy and safety of neoadjuvant FOLFIRINOX for borderline resectable pancreatic adenocarcinoma: improved efficacy compared with gemcitabine-based regimen. Oncotarget 2017;8:46337–47.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Dhir M, Zenati MS, Hamad A, et al. FOLFIRINOX versus gemcitabine/nab-paclitaxel for neoadjuvant treatment of resectable and borderline resectable pancreatic head adenocarcinoma. Ann Surg Oncol 2018;25:1896–903.. [DOI] [PubMed] [Google Scholar]
- [23].Soriano A, Castells A, Ayuso C, et al. Preoperative staging and tumor resectability assessment of pancreatic cancer: prospective study comparing endoscopic ultrasonography, helical computed tomography, magnetic resonance imaging, and angiography. Am J Gastroenterol 2004;99:492–501.. [DOI] [PubMed] [Google Scholar]
- [24].Yoshitomi H, Sakai N, Kagawa S, et al. Feasibility and safety of distal pancreatectomy with en bloc celiac axis resection (DP-CAR) combined with neoadjuvant therapy for borderline resectable and unresectable pancreatic body/tail cancer. Langenbecks Arch Surg 2019;404:451–8.. [DOI] [PubMed] [Google Scholar]
- [25].Itchins M, Arena J, Nahm CB, et al. Retrospective cohort analysis of neoadjuvant treatment and survival in resectable and borderline resectable pancreatic ductal adenocarcinoma in a high volume referral centre. Eur J Surg Oncol 2017;43:1711–7.. [DOI] [PubMed] [Google Scholar]
- [26].Kim HS, Jang JY, Han Y, et al. Survival outcome and prognostic factors of neoadjuvant treatment followed by resection for borderline resectable pancreatic cancer. Ann Surg Treat Res 2017;93:186–94.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Tee MC, Krajewski AC, Groeschl RT, et al. Indications and perioperative outcomes for pancreatectomy with arterial resection. J Am Coll Surg 2018;227:255–69.. [DOI] [PubMed] [Google Scholar]
- [28].Fortner JG. Regional pancreatectomy for cancer of the pancreas, ampulla, and other related sites. Tumor staging and results. Ann Surg 1984;199:418–25.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sindelar WF. Clinical experience with regional pancreatectomy for adenocarcinoma of the pancreas. Arch Surg 1989;124:127–32.. [DOI] [PubMed] [Google Scholar]
- [30].Mollberg N, Rahbari NN, Koch M, et al. Arterial resection during pancreatectomy for pancreatic cancer: a systematic review and meta-analysis. Ann Surg 2011;254:882–93.. [DOI] [PubMed] [Google Scholar]
- [31].Bockhorn M, Burdelski C, Bogoevski D, et al. Arterial en bloc resection for pancreatic carcinoma. Br J Surg 2011;98:86–92.. [DOI] [PubMed] [Google Scholar]
- [32].Martin RC, 2nd, Scoggins CR, Egnatashvili V, et al. Arterial and venous resection for pancreatic adenocarcinoma: operative and long-term outcomes. Arch Surg 2009;144:154–9.. [DOI] [PubMed] [Google Scholar]
- [33].Asano T, Nakamura T, Noji T, et al. Outcome of concomitant resection of the replaced right hepatic artery in pancreaticoduodenectomy without reconstruction. Langenbecks Arch Surg 2018;403:195–202.. [DOI] [PubMed] [Google Scholar]
- [34].Yang F, Wang X, Jin C, et al. Pancreatectomy with hepatic artery resection for pancreatic head cancer. World J Surg 2019;43:2909–19.. [DOI] [PubMed] [Google Scholar]
- [35].Tohma T, Cho A, Okazumi S, et al. Communicating arcade between the right and left hepatic arteries: evaluation with CT and angiography during temporary balloon occlusion of the right or left hepatic artery. Radiology 2005;237:361–5.. [DOI] [PubMed] [Google Scholar]
- [36].Gunji H, Cho A, Tohma T, et al. The blood supply of the hilar bile duct and its relationship to the communicating arcade located between the right and left hepatic arteries. Am J Surg 2006;192:276–80.. [DOI] [PubMed] [Google Scholar]
- [37].Yoshidome H, Shimizu H, Ohtsuka M, et al. Pancreaticoduodenetomy combined with hepatic artery resection following preoperative hepatic arterial embolization. J Hepatobiliary Pancreat Sci 2014;21:850–5.. [DOI] [PubMed] [Google Scholar]
- [38].Yoshiya S, Fukuzawa K, Inokuchi S, et al. Efficacy of neoadjuvant chemotherapy in distal pancreatectomy with en bloc celiac axis resection (DP-CAR) for locally advanced pancreatic cancer. J Gastrointest Surg 2020;24:1605–11.. [DOI] [PubMed] [Google Scholar]
- [39].Shin SH, Kim SC, Song KB, et al. A comparative study of laparoscopic vs. open distal pancreatectomy for left-sided ductal adenocarcinoma: a propensity score-matched analysis. J Am Coll Surg 2015;220:177–85.. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


