Abstract
Background:
Osteogenesis imperfecta (OI), a heritable connective tissue disorder with wide clinical variability, predisposes to recurrent fractures and bone deformity. Management requires a multidisciplinary approach in which intramedullary rodding plays an important role, especially for moderate and severe forms. We investigated the patterns of surgical procedures in OI in order to establish the benefits of rodding. The main hypothesis that guided this study was that rodded participants with moderate and severe OI would have lower fracture rates and better mobility.
Methods:
With data from the Linked Clinical Research Centers, we analyzed rodding status in 558 individuals. Mobility and fracture data in OI Types III and IV were compared between rodded and non-rodded groups. Univariate regression analyses were used to test the association of mobility outcomes with various covariates pertinent to rodding.
Results:
Of the individuals with OI, 42.1% had undergone rodding (10.7% of those with Type I, 66.4% with Type III, and 67.3% with Type IV). Rodding was performed more frequently and at a younger age in femora compared with tibiae. Expanding intramedullary rods were used more frequently in femora. In Type III, the rate of fractures per year was significantly lower (p ≤ 0.05) for rodded bones. In Type III, the mean scores on the Gillette Functional Assessment Questionnaire (GFAQ) and Brief Assessment of Motor Function (BAMF) were higher in the rodded group. However, Type-IV non-rodded subjects had higher mean scores in nearly all mobility outcomes. OI type, the use of expanding rods in tibiae, and anthropometric measurements were associated with mobility outcomes scores.
Conclusions:
Current practice in 5 orthopaedic centers with extensive experience treating OI demonstrates that most individuals with moderate and severe types of OI undergo rodding procedures. Individuals with severe OI have improved mobility outcomes and lower fracture rates compared with their non-rodded peers, which suggests that early bilateral rodding benefits OI Type III. Our analysis showed a change in practice patterns in the final years of the study in the severe forms, with earlier and more simultaneous rodding procedures performed.
Level of Evidence:
Therapeutic Level III. See Instructions for Authors for a complete description of levels of evidence.
Osteogenesis imperfecta (OI) is a heritable connective tissue disorder, which predisposes patients to recurrent fractures and bone deformity1 and presents with variable phenotypic severity. Although originally classified by Sillence et al. into 4 categories2, new subtypes were later described and expanded to 7 subtypes3-5. The current genetic classification has extended to at least 16 subtypes6. A commonly accepted nomenclature does not yet exist7. However, decisions regarding surgical procedures and other medical decisions are usually made on the basis of the clinical severity of the disease using a modified Sillence classification identifying mild disease (Type I), moderate disease (Type IV), and severe disease (Type III)8. Type-I patients have quantitative deficits in type-I collagen, near-normal stature, and little bone bowing, and patients with moderate and severe types of OI produce a defective type-I collagen and experience multiple long-bone deformities and fractures, with the lower extremities typically involved to a greater extent9. The treatment of OI comprises a multidisciplinary effort that involves genetic evaluation, bisphosphonates, pain management, physical therapy, and orthopaedic care10. Although bisphosphonates may increase bone mineral density and facilitate rodding surgical procedures11-14, these medications have not been shown to have an effect on fractures related to bone bowing14,15.
Intramedullary rodding is indicated in individuals with moderate to severe OI and a subset with mild OI who develop disability from recurrent fractures or progressive deformity. The technique was first proposed by Sofield and Millar in 1959; in 1963, telescoping rods designed to elongate as children grew were introduced10. The typical operative intervention consists of realignment osteotomies and intramedullary fixation. Over the last 2 decades, new rods and modifications of techniques with less extensive surgical incisions have been introduced to decrease morbidity9,14,16.
The determination of the best time to perform rodding depends on the patient’s clinical situation. Some have suggested rodding in patients who are attempting to stand1,17. Others have suggested lower-extremity alignment and protection as important factors to consider in nonambulatory individuals10. Although surgical treatment at younger ages may require earlier revision due to growth, this relative disadvantage may be countered by the benefits of early surgical stabilization9,14.
Many factors impact mobility, including the severity of OI, intramedullary rodding, and anthropometric factors. Engelbert et al. reported intramedullary rodding to be associated with greater OI severity, specifically interference with walking ability18. We previously reported that the phenotypic severity of OI has a significant influence on mobility outcomes19. Additionally, some studies have described the benefits of rodding with respect to mobility14,20. Anthropometric measurements have also been related to the severity of the condition and mobility outcomes19,21,22.
Collaborative multicenter studies in rare diseases can lead to a better understanding of the disease and provide novel therapeutic options1. We investigated the patterns of surgical procedures in OI in order to establish the benefits of rodding. The main hypothesis that guided this study was that rodded participants with moderate and severe OI would have lower fracture rates and better mobility.
Materials and Methods
The Linked Clinical Research Centers (LCRC) consist of 5 clinical sites in North America: Baylor College of Medicine, Kennedy Krieger Institute in collaboration with Nemours/Alfred I. duPont Hospital for Children, Oregon Health & Science University, Shriners Hospital for Children-Chicago, and Shriners Hospital for Children-Montreal14. The OI LCRC conducted a multicenter, longitudinal, observational study that provided data for this work. Protocol approval was obtained from the institutional review board at each participating site and informed consent was obtained from the subjects or their legal guardians.
All individuals with a clinical or molecular diagnosis of OI who attended these 5 clinical centers between 2008 and 2015 were enrolled. Genotypic information was used to classify patients when available. For participants without a molecular diagnosis, the site principal investigator and project principal investigator were required to be in agreement with regard to the subtype of OI based on specific criteria1,23-26. Data were collected uniformly at each site and the quality was assessed at the data entry point. Clinical data were obtained at enrollment, and follow-up assessments were made on a yearly basis.
The following data were collected from the baseline visit: OI type, age at enrollment, sex, height, weight, rodding location (femur or tibia), type of rod (expanding intramedullary rodding: Fassier-Duval or Bailey-Dubow rod; or non-expanding intramedullary rodding: Kirschner wire, Rush rod, or other), and history of bisphosphonate use. The mobility metrics included were age at first walk, Gillette Functional Assessment Questionnaire (GFAQ)27, Functional Mobility Scale (FMS)28, and Brief Assessment of Motor Function (BAMF)29,30. All metrics, except FMS and BAMF, were self-reported. Mobility data were analyzed from individuals ≥2 years of age.
The location and the number of fractures that occurred in the year preceding each visit, starting at enrollment and continuing through annual visits for up to 5 years, were recorded. The number of fractures per year for femora and tibiae was calculated and was compared between rodded and non-rodded bones. When the date of rodding was available, age at rodding, rodding sequence, and simultaneous rodding (>1 bone, femur and/or tibia, within 1 month) for lower limbs were derived.
Statistical Analysis
Rodding data were summarized by OI type. Height, weight, and body mass index (BMI) were converted to age and sex-specific z-scores based on reference data31. Normality was assessed using the Shapiro-Wilk test. Differences in the mean mobility scores between rodded and non-rodded groups were compared using a t test or nonparametric test as appropriate. Differences in proportions for GFAQ tasks between groups were compared using a chi-square test. Univariate linear (for continuous variables) and logistic regressions (for discrete variables) were used to evaluate mobility outcome predictors in the rodded group (α = 0.05).
The analysis of the relations of rodding with mobility outcomes and fractures was focused on Type-III and IV subjects because they underwent rodding more frequently than Type-I subjects. Other OI types were not considered because of low subject numbers. The frequency of simultaneous rodding and the age of rodding in Type-III and IV subjects were analyzed considering the year of rodding, as, during the final years of the study (2003 to 2015), important changes in rodding techniques were made, including the introduction of new telescoping rods.
Results
In the LCRC, 558 individuals were enrolled (44.6% male); the median age was 12.4 years (range, 0.0 to 67.2 years), and the mean follow-up was 3.2 years (range, 1.0 to 5.0 years). From this cohort, 235 individuals who underwent rodding in the femur or tibia were identified (Table I). Of the Type-III and IV subjects, approximately 67% of all subjects had undergone a rodding in the femur or tibia compared with only 10.7% of Type-I subjects. As shown in Table II, for Type-I subjects, unilateral femora were the most common rodding location (Figs. 1-A and 1-B), and bilateral femoral and tibial rodding was more frequent in Type-III subjects (Figs. 2-A, 2-B, and 2-C) and Type-IV subjects.
TABLE I.
Participants with Rodded Bones (Tibiae Only, Femora Only, and Both Femora and Tibiae) per OI Type
| OI Type | No. of Participants | Rodded Participants* | Rodded Bones* | ||
| Femur(s) Only | Tibia(s) Only | Femur(s) and Tibia(s) | |||
| Type I | 244 | 26 (10.7%) | 18 (69.2%) | 2 (7.7%) | 6 (23.1%) |
| Type III | 110 | 73 (66.4%) | 12 (16.4%) | 3 (4.1%) | 58 (79.5%) |
| Type IV | 153 | 103 (67.3%) | 39 (37.9%) | 4 (3.9%) | 60 (58.3%) |
| Type V | 18 | 10 (55.6%) | 3 (30%) | 4 (40%) | 3 (30%) |
| Type VI | 12 | 10 (83.3%) | 6 (60%) | 0 (0%) | 4 (40%) |
| Type VII | 5 | 2 (40%) | 0 (0%) | 0 (0%) | 2 (100%) |
| Unclassified | 16 | 11 (68.8%) | 3 (27.3%) | 1 (9.1%) | 7 (63.6%) |
| Total | 558 | 235 (42.1%) | 81 (34.5%) | 14 (6%) | 140 (59.6%) |
The values are given as the number of patients, with the row percentage in parentheses.
TABLE II.
Participants with OI Who Underwent Bilateral Rodding of the Femora or Tibiae
| OI Type | Femoral Rodding* | Bilateral Femoral Rodding* | Tibial Rodding* | Bilateral Tibial Rodding* |
| Type I | 24 | 9 (37.5%) | 8 | 2 (25.0%) |
| Type III | 70 | 67 (95.7%) | 61 | 56 (91.8%) |
| Type IV | 99 | 83 (83.8%) | 64 | 50 (78.1%) |
| Type V | 6 | 0 (0.0%) | 7 | 6 (85.7%) |
| Type VI | 10 | 8 (80.0%) | 4 | 3 (75.0%) |
| Type VII | 2 | 2 (100.0%) | 2 | 2 (100.0%) |
| Unclassified | 10 | 9 (90.0%) | 8 | 7 (87.5%) |
The values are given as the number of patients, with or without the percentage in parentheses.
Figs. 1-A and 1-B Anteroposterior radiographs of the left femur of an 11-year-old girl with OI Type I.
Fig. 1-A.
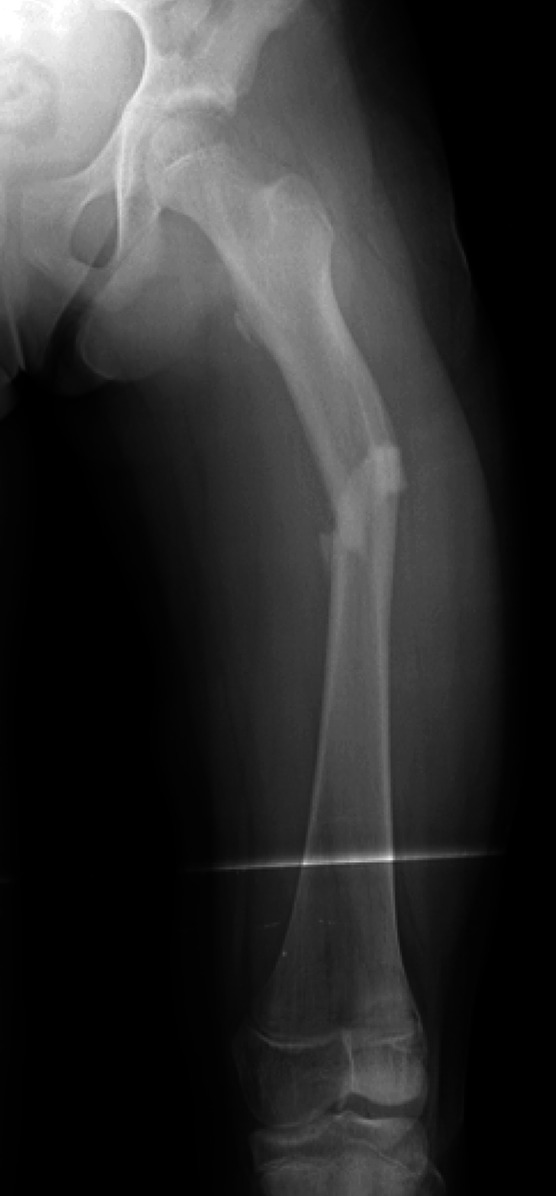
Preoperative radiograph showing the left femur with a mid-diaphyseal femoral fracture sustained while the patient was dancing competitively.
Fig. 1-B.
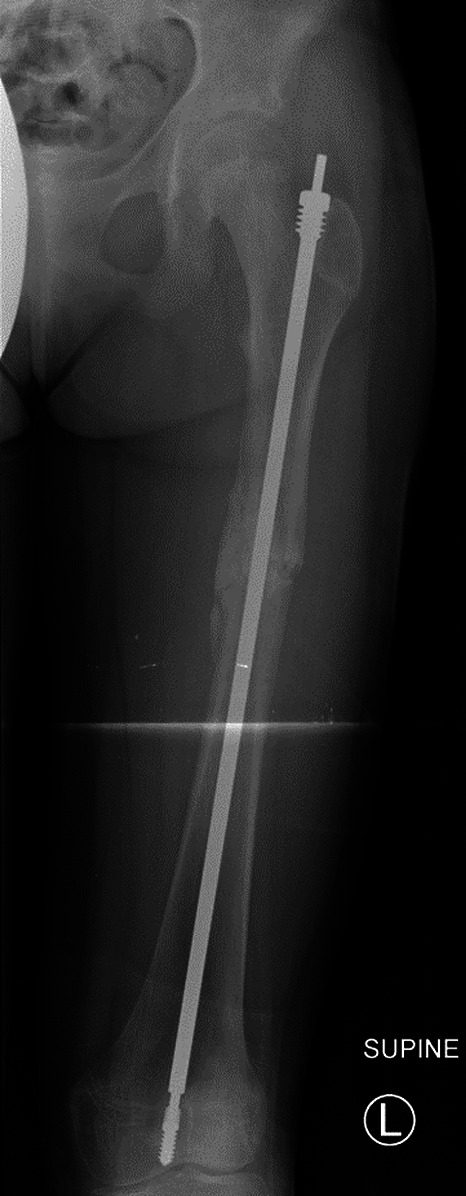
Postoperative radiograph showing a 5.4-mm Fassier-Duval expanding rod.
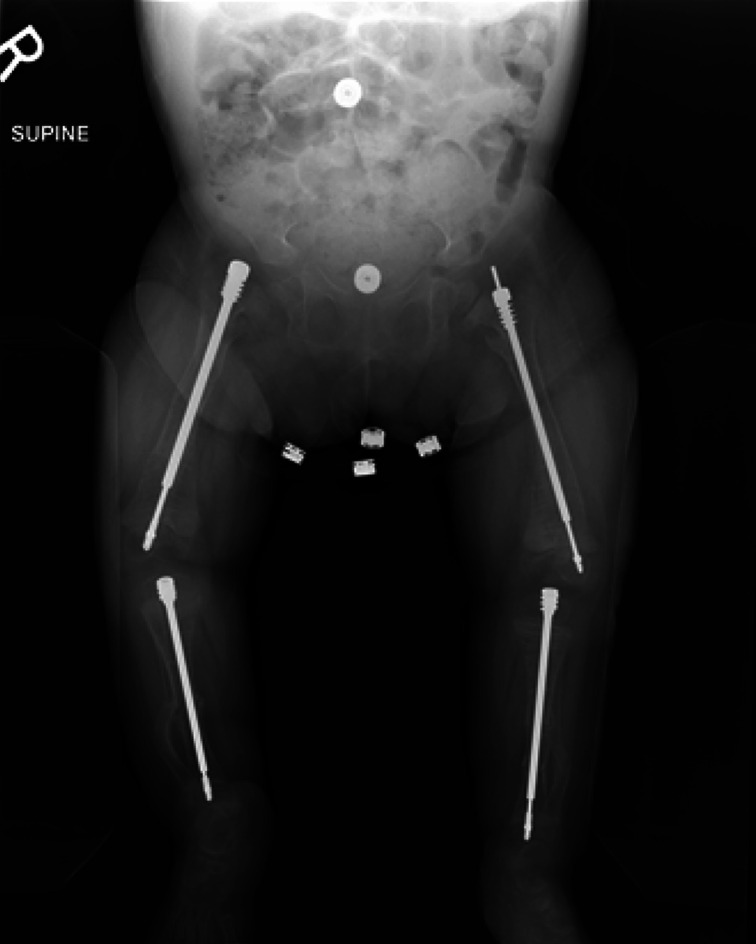
Figs. 2-A, 2-B, and 2-C Radiographs of the lower limbs of a 2-year-old patient with OI Type III who has a severe deformity and has been undergoing cyclic bisphosphonate treatment since infancy. Preoperative anteroposterior (Fig. 2-A) and lateral (Fig. 2-B) radiographs of lower limbs of the patient.
Fig. 2-A.
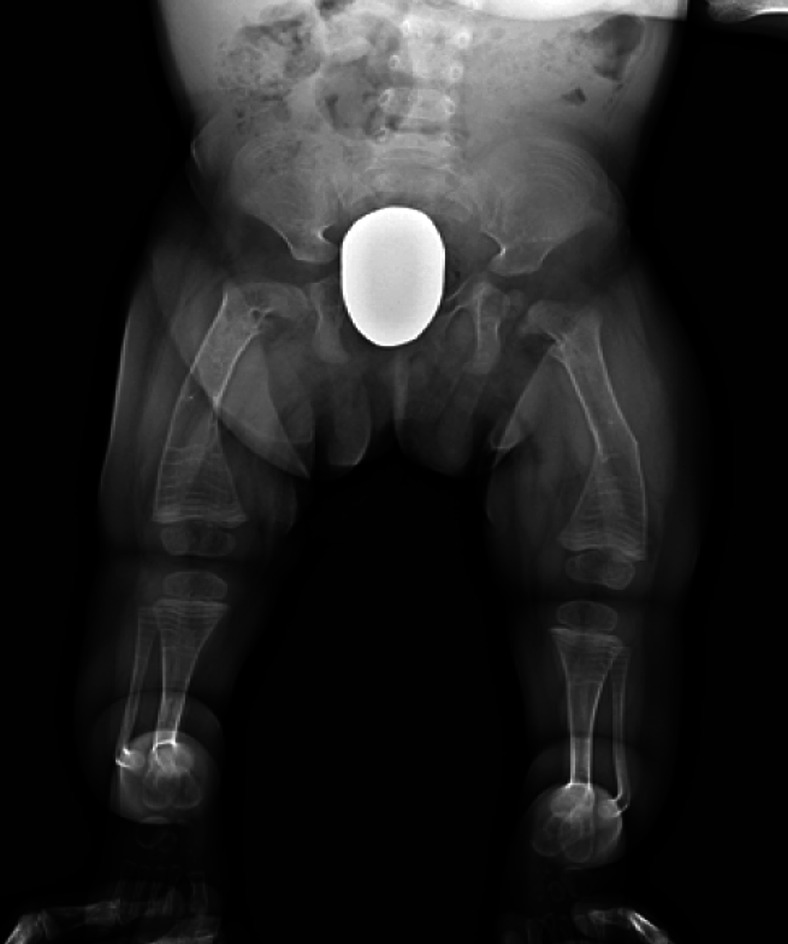
Fig. 2-B.
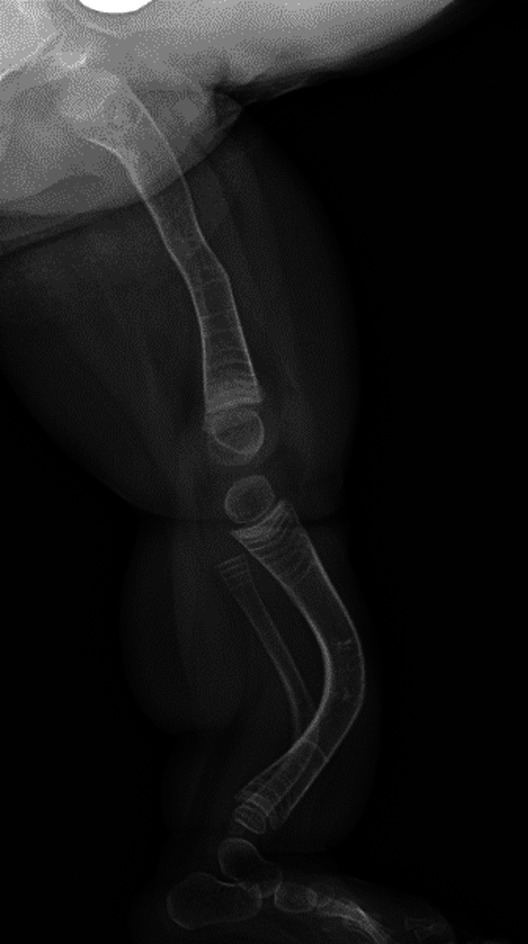
Fig. 2-C.
Postoperative radiograph showing the fragmentation and insertion of expanding 3.2-mm Fassier-Duval rods in both femora and tibiae has been performed in a staged fashion. The surgical procedure was performed when the patient began pulling to stand. The goal of the surgical procedure was to improve alignment and stability, reduce the fracture rate, and increase mobility.
The mean number of bones with rods per patient was significantly higher (p ≤ 0.01) for all other OI types when compared with Type-I subjects. For example, the mean number of rodded bones was 0.2 in Type-I subjects compared with 2.3 in Type-III subjects (Table III). Also, rodding of femora was performed earlier, at a mean age (and standard deviation) of 6.65 ± 2.18 years, than rodding of tibiae, at 8.14 ± 1.56 years. Expanding intramedullary rods were used significantly more frequently in femora (p ≤ 0.01) (Table IV) (Figs. 3-A, 3-B, and 4).
Fig. 4.
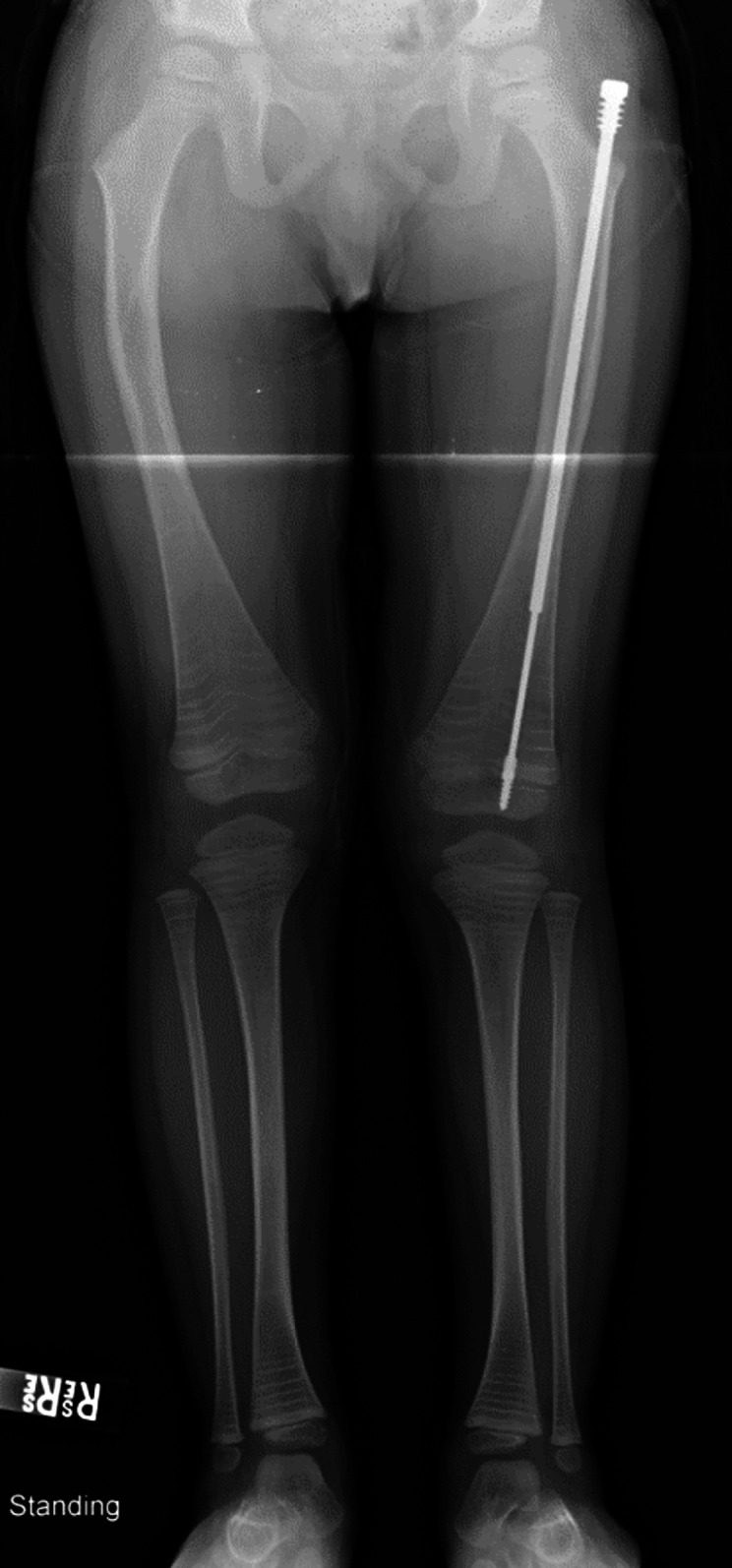
Anteroposterior radiograph of the lower limbs of a 4-year-old boy with OI Type IV who underwent isolated rodding of the left femur with the insertion of a 4.0-mm Fassier-Duval expanding rod after a femoral fracture while attempting to run. He has undergone cyclic bisphosphonate treatment since he was 6 months of age. Currently, he is a community ambulator and demonstrates mild bowing of the contralateral femur.
TABLE III.
Lower-Limb Rodded Bones per OI Type
| OI Type | No. of Rodded Bones | Femora | Tibiae | Mean No. of Rodded Bones per Patient (Range) | ||
| % Rodded | Mean Age of Rodding (yr) | % Rodded | Mean Age of Rodding (yr) | |||
| Type I | 43 | 76.7% | 6.7 | 23.3% | 8.4 | 0.2 (0 to 4) |
| Type III | 254 | 53.9% | 4.1 | 46.1% | 5.5 | 2.3 (0 to 4)* |
| Type IV | 396 | 61.5% | 7.5 | 38.5% | 9.0 | 1.9 (0 to 4)* |
| Type V | 19 | 31.6% | 9.8 | 68.4% | 8.8 | 1.0 (0 to 3)* |
| Type VI | 25 | 72.0% | 4.2 | 28.0% | 8.8 | 2.1 (0 to 4)* |
| Type VII | 8 | 50% | 9.4 | 50% | 10.2 | 1.6 (0 to 4)† |
| Unclassified | 34 | 55.9% | 5.1 | 44.1% | 6.1 | 2.1 (0 to 4)* |
Significant at p ≤ 0.01 (Mann-Whitney test for the mean number of rodded bones per patient compared with OI Type I).
Significant at p ≤ 0.01 (t test for the mean number of rodded bones per patient compared with OI Type I).
TABLE IV.
Types of Intramedullary Rods Used in Femora and Tibiae: Expanding Compared with Non-Expanding Rod Types
| Rod Type | Femora | Tibiae |
| Expanding intramedullary rod | 69.7%* | 36.9%* |
| Non-expanding intramedullary rod | 30.3% | 66.1% |
Significant at p ≤ 0.01 (Mann-Whitney test for expanding rods in femora compared with expanding rods in tibiae).
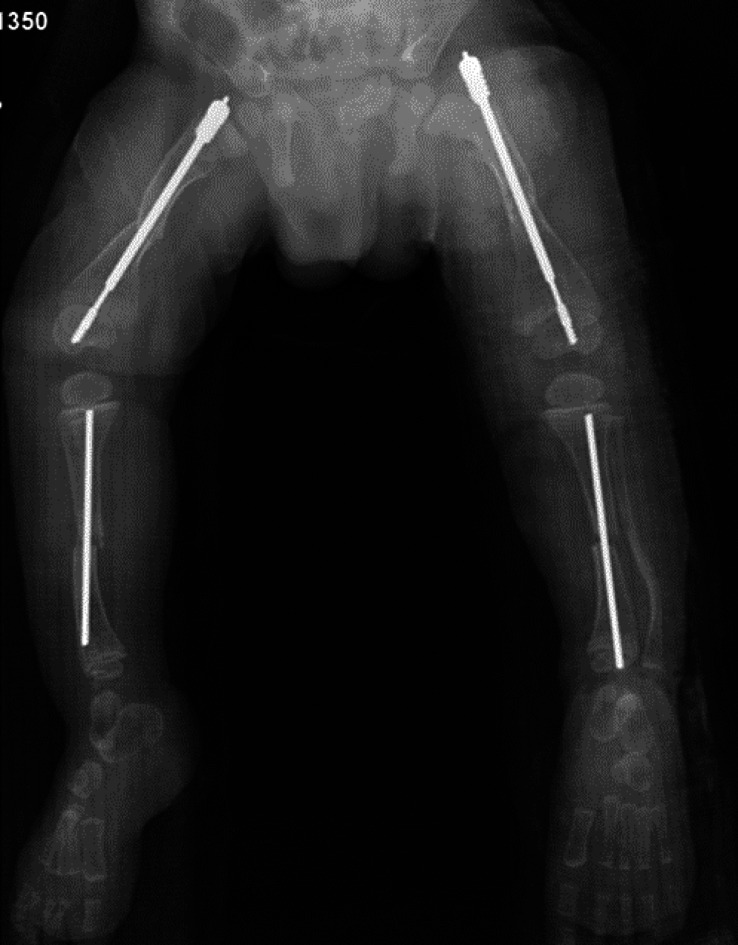
Figs. 3-A and 3-B Anteroposterior radiographs of the lower limbs of a 27-month-old boy with OI Type III and severe deformity of the femora and tibiae. The patient has been undergoing cyclic bisphosphonate treatment since infancy; his weight was 9.5 kg.
Fig. 3-A.
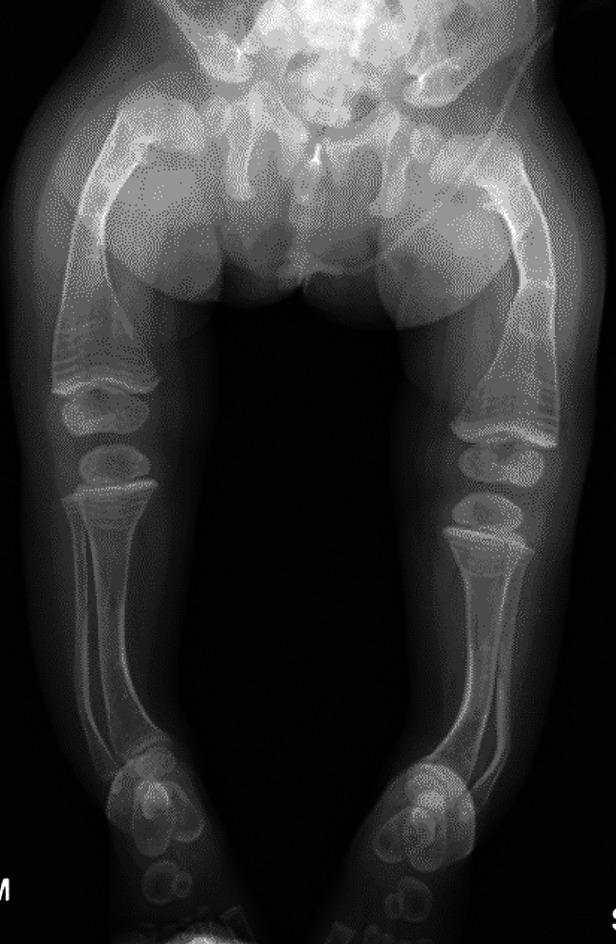
Preoperative radiograph showing the severe deformity of the femora and tibiae.
Fig. 3-B.
Postoperative radiograph. When the patient became more mobile and attempted pulling to stand, he underwent fragmentation and rodding of both femora and tibiae, the 2 legs (the tibia and the femur in each) were staged 2 weeks apart. The diameter of the tibiae precluded the use of expanding rods, so non-expanding rods were used in both tibiae (2.38-mm [3/32-inch] Steinmann pins) and expanding rods (3.2-mm Fassier-Duval) were inserted in the femora.
In individuals with rodding of the femora and tibiae, the sequence of rodding was also analyzed. Femora were rodded first in 70.9% of cases. The simultaneous rodding of lower-limb bones was performed in 45.7% of OI Type-III subjects and 34.3% of OI Type-IV subjects (p < 0.05). The percentage of simultaneous rodding and the age of rodding before and after the beginning of 2003 were evaluated. The number of surgical procedures with simultaneous rodding for Type-III and IV subjects increased from 31.3% to 43.1% (p < 0.05), and for Type-III subjects, the mean age of first rodding decreased from 5.0 to 3.7 years (p = 0.09).
Of 263 Type-III and IV subjects, 45.6% completed the fracture form during the annual follow-up visits (51.5% of these with fracture data from 1 visit, 36% from 2 to 3 visits, and 12.5% from 4 to 5 visits).
The rate of fractures per year in rodded compared with non-rodded femora and tibiae was significantly lower (p ≤ 0.05) for rodded bones in Type-III subjects but not significantly different for Type-IV subjects (Table V).
TABLE V.
Fractures per Year for OI Types III and IV: Rodded Group Compared with Non-Rodded Group
| Variable | OI Type III* | OI Type IV* | ||||||
| Femora | Tibiae | Femora | Tibiae | |||||
| Rodded | Non-Rodded | Rodded | Non-Rodded | Rodded | Non-Rodded | Rodded | Non-Rodded | |
| Fractures per year | 0.79† (0.25 to 2.00) | 1.31† (0.33 to 4.00) | 0.57† (0.25 to 1.00) | 0.84† (0.50 to 1.00) | 0.87‡ (0.25 to 2.00) | 0.79‡ (0.25 to 1.50) | 0.93‡ (0.33 to 2.50) | 0.81‡ (0.25 to 1.50) |
The values are given as the mean, with the range in parentheses.
Significant at p ≤ 0.05 (Mann-Whitney test for the rodded group compared with the non-rodded group per bone type).
Nonsignificant.
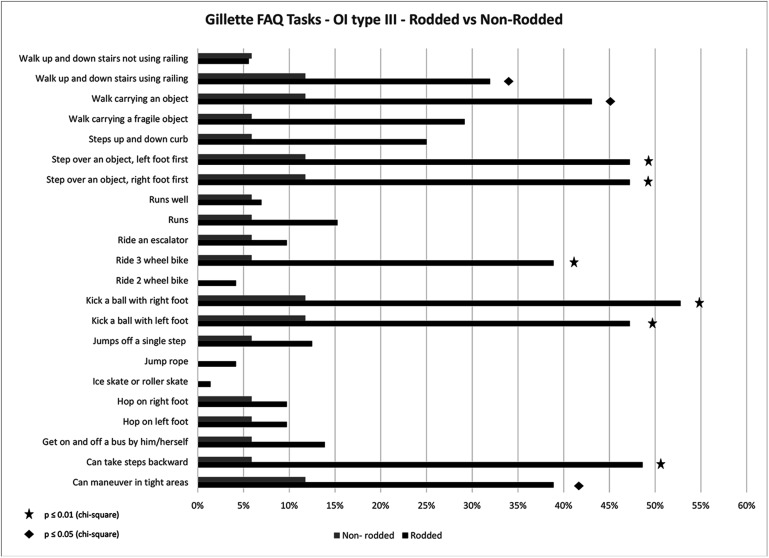
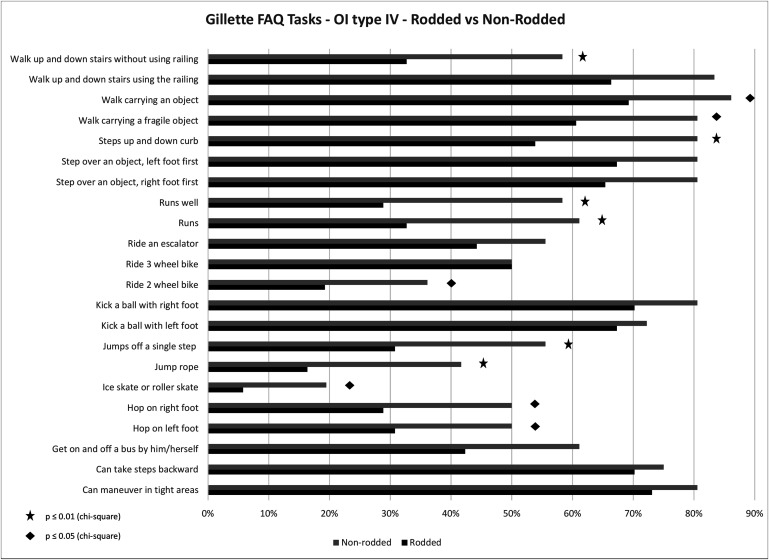
Patients started walking before undergoing rodding in 51.2% of Type-III cases and 86.8% of Type-IV cases. We compared mobility outcomes in Type-III and IV rodded and non-rodded groups (Table VI). In Type-III subjects, significantly better results (p ≤ 0.05) in the GFAQ walking ability score and the BAMF were found for the rodded group, with no significant differences for the FMS 5-m (p = 0.93), FMS 50-m (p = 0.69), and FMS 500-m tasks (p = 0.63). On the contrary, in Type-IV subjects, the non-rodded group showed better results for nearly all mobility outcomes.
TABLE VI.
Mobility Outcomes for OI Types III and IV: Rodded Group Compared with Non-Rodded Group
| Mobility Outcome (Scale Range) | OI Type III* | OI Type IV* | ||
| Rodded | Non-Rodded | Rodded | Non-Rodded | |
| FMS 5 m (1 to 6) | 2.84 ± 2.11† | 2.83 ± 2.41† | 4.20 ± 1.96‡ | 4.94 ± 1.76‡ |
| FMS 50 m (1 to 6) | 2.19 ± 1.86† | 2.00 ± 1.95† | 3.78 ± 2.14‡ | 4.64 ± 2.04‡ |
| FMS 500 m (1 to 6) | 1.65 ± 1.58† | 2.00 ± 1.95† | 3.24 ± 2.29‡ | 4.39 ± 2.20‡ |
| GFAQ walking ability score (1 to 10) | 4.19 ± 3.06§ | 2.40 ± 3.00§ | 6.92 ± 2.97§ | 8.17 ± 2.86§ |
| BAMF lower limbs (1 to 10) | 6.48 ± 2.49§ | 4.13 ± 2.58§ | 8.44 ± 1.83‡ | 9.06 ± 1.84‡ |
The values are given as the mean and standard deviation in points.
Nonsignificant (Mann-Whitney test for rodded bones compared with non-rodded bones for OI Types III and IV).
Significant at p ≤ 0.05.
Significant at p ≤ 0.01.
As the numerical scores for the mobility metrics may not capture aspects required for daily functioning, mobility parameters were assessed using GFAQ tasks, with a percentage of subjects responding “yes” to being able to complete each of the GFAQ tasks (see Figs. 5 and 6). In Type-III subjects, a greater proportion of individuals in the rodded group responded “yes” to specific tasks compared with the non-rodded group. In contrast, the non-rodded group performed better than the rodded group in Type-IV subjects.
Fig. 5.
Percentage of Type-III subjects responding “yes” to being able to complete each task from the GFAQ: the rodded group compared with the non-rodded group.
Fig. 6.
Percentage of Type-IV subjects responding “yes” to being able to complete each task from the GFAQ: the rodded group compared with the non-rodded group.
Table VII shows the results of the univariate logistic and linear regression analysis between different parameters and mobility outcomes in the Type-III and IV rodded groups.
TABLE VII.
Predictors of Mobility Outcomes in OI Types III and IV: Rodded Group
| Predictor | Mobility Outcome* | Conclusions | ||||
| GFAQ Walking Ability Score† | FMS 5 m† | FMS 50 m† | FMS 500 m† | BAMF Lower-Extremity Gross Motor Scale† | ||
| Logistic regressions: ln(P/(1 − P)) = A + B × X | ||||||
| OI Type III or IV vs. OI Type I | 10.2‡ | 2.2‡ | 4.4‡ | 5.7‡ | 5.7‡ | OI Types III and IV showed worse mobility outcome compared with OI Type I |
| Non-expanding vs. expanding rods in tibiae | 3.6§ | NS | 4.4‡ | 3.6§ | 2.9‡ | Subjects with non-expanding rods in tibiae showed worse results than those with expanding rods |
| Non-expanding vs. expanding rods in femora | NS | NS | NS | NS | NS | No significant differences in mobility outcome for subjects with expanding or non-expanding rods in femora |
| Bisphosphonates: no or yes | NS | NS | NS | NS | NS | No significant differences in mobility outcome for rodded subjects with or without treatment with oral or intravenous bisphosphonates |
| Simultaneous rodding of both femora vs. 1 femur | NS | NS | NS | NS | NS | No significant differences in mobility outcome for subjects with simultaneous or non-simultaneous femoral rodding |
| Simultaneous rodding of femur and tibia vs. 1 femur | 6.5‡ | 3.9‡ | 6.4‡ | 4.1‡ | 6.3‡ | Subjects with tibiae and femora rodded simultaneously had significantly worse mobility outcome |
| Sequence of rodding: 2 femora vs. 1 femur first | NS | NS | NS | NS | NS | No significant differences in mobility outcome for subjects with 1 femoral rodding first compared with 2 femora first |
| Linear regressions: Y = A + B × X | ||||||
| Standardized height | 0.22‡ | 0.18‡ | 0.21‡ | 0.13‡ | 0.21‡ | A weak association was found between standard height and all of the mobility outcome (direct relation) |
| Standardized weight | 0.10‡ | 0.07‡ | 0.10‡ | 0.07‡ | 0.08‡ | A weak association was found between standard weight and all of the mobility outcomes (direct relation) |
| Standardized BMI | 0.11‡ | 0.05‡ | 0.06‡ | 0.06‡ | 0.10‡ | A weak association was found between standard BMI and all of the mobility outcomes (direct relation) |
| Age at first rodding | NS | NS | NS | NS | NS | No association was found between the age at the time of the first rodding surgical procedure reported and the mobility outcome |
NS = no significant correlation.
The values are given as the odds ratio for the logistic regression or as the coefficient of determination (R2) for the linear regression.
Significant at p ≤ 0.05.
Significant at p ≤ 0.01.
Discussion
Since its introduction, intramedullary rodding has had an essential role in management of OI with the objective of reducing bone deformity and periods of inactivity due to fractures.
Considering all types of OI, we found that femoral rodding was performed more frequently than tibial rodding, and it was performed first when both bones were rodded non-simultaneously. Most Type-I patients only underwent femoral rodding procedures, and combined femora and tibiae were rodded more frequently in Type-III and IV subjects. Although the reason for the initial rodding was not queried, for most of the Type-IV patients, the surgical procedure occurred after they started walking, which suggests recurrent fractures and progressive bowing of lower limbs related to weight-bearing. For Type-III subjects, about half of the participants required a surgical procedure before being able to walk, probably due to a higher incidence of early bone deformity in this group. In addition, it was found that in Type-III subjects, simultaneous rodding was significantly more frequent than in Type-IV subjects. Interestingly, the percentage of individuals who eventually underwent rodding was similar between Type-III and IV individuals. The relatively high rate of rodding in moderate and severe types of OI in this series likely reflects the familiarity and expertise with OI of the centers involved.
Femora and tibiae are usually rodded bilaterally in moderate and severe forms but unilaterally in mild forms. In agreement with Azzam et al. and Ruck et al.7,14, we found that expanding intramedullary rods were used more often in femora and non-expanding rods were used more often in tibiae. Although this study could not compare the results of surgical procedures over different time periods, it did show that the practice of rodding has shifted toward simultaneous rodding of multiple bones in Type-III and IV subjects and to earlier surgical procedures in Type-III subjects, most likely because of improvements in surgical instrumentation and techniques.
In addition to correcting long-bone deformity, reducing the fracture rate is another goal of rodding9,10,32,33. We found that the Type-III rodded group had a significantly lower fracture rate than the non-rodded group. This improvement was not seen in the Type-IV group, probably because of the wide clinical variability of this group. There exists a subset of individuals in the Type-IV group who have excellent mobility and low fracture rates and have never required rodding procedures.
Little is known about functional outcomes following rodding procedures in OI14. In this study, the Type-III rodded group showed better results in most mobility outcomes than the non-rodded group, except for the FMS. This benefit of a surgical procedure was not observed in Type-IV subjects, most likely because only the patients with more severe OI typically require rodding, so those who do not require a surgical procedure likely have better mobility. Conversely, it is possible that some participants in the OI Type-III non-rodded group had contraindications to rodding because of increased severity.
Functional outcomes and quality of life have been reported to be related to condition severity19,34-36. In agreement with Kruger et al.19, we found that the OI type was associated with mobility outcomes in the rodded group. Additionally, within the rodded group, a height deficit was associated with reduced mobility outcomes, agreeing with the literature in which height has been related to condition severity19,21,22. Although the use of BMI in populations with short stature is controversial because it may overestimate the proportion of obesity24,37, many studies found a high prevalence of elevated BMI, especially in individuals with OI Type III33,38. Our results showed that increased BMI had an association with poor results across mobility outcomes.
When looking at the predictors of mobility outcomes in the rodded group, even though expanding rods were used less frequently in tibiae, they showed better results. However, it is unknown if this is related to the type of rod or the size of the bone, as expanding rods are typically used in larger bones.
Some have postulated that a younger age of rodding could be a positive determinant of results9,14. In our study, we found no relation between the age of rodding and mobility outcomes, in agreement with other studies, which suggests that rodding criteria based on functional outcomes should be employed rather than age32. In future work, gait analysis may play an important role in preoperative planning.
A simultaneous rodding surgical procedure has been associated with benefits including reducing rehabilitation time14, number of anesthesia procedures, postoperative pain management needs, and hospital stay days17. In this study, having 1 or 2 femora rodded at the same time showed no differences in mobility outcomes and less favorable results were found for the case of simultaneous femoral and tibial rodding. However, we must point out that this last finding could be related to the severity of the disease of the participants who needed this type of surgical procedure.
Some studies have postulated that use of bisphosphonates could facilitate a rodding surgical procedure11,13,14,39 and benefit mobility, muscle force, and well-being40,41, and other studies found no changes in mobility19,42,43. A recent systematic review concluded that children given intravenous bisphosphonates have increased mobility44. However, in this study, we found no correlation between the use of bisphosphonates and mobility outcomes in the rodded group.
A limitation of this study was that mobility outcomes before and after the surgical procedures, where benefits in both OI types III and IV have been reported7,14,34, were not compared, and the data were analyzed at variable times after the surgical procedure. Also, fracture information was available for only about half of the population, with data collected in a variable number of visits, and, even though multidisciplinary treatment, which is associated with the best results34,41,45, was offered in all centers, the contributions of the different interventions and their interactions were not evaluated.
In conclusion, the analysis of current practice at 5 orthopaedic centers with extensive experience treating OI demonstrated that most individuals with moderate and severe types of OI undergo rodding procedures and that rodded individuals with severe OI have improved mobility outcomes and lower fracture rates. Not all individuals with moderate OI require rodding; in fact, the non-rodded group performed better, probably representing the variability of function within that group. Future multicenter studies evaluating the outcomes before and after surgical procedures could be helpful to better delineate the effects of rodding in the moderate group.
However, we present evidence that early bilateral rodding is beneficial in OI Type-III subjects. We have shown a change in practice patterns in the past few years in the severe forms of OI, with more simultaneous rodding procedures and earlier rodding, likely as a result of improvements in surgical procedures.
Acknowledgments
Note: The Members of the Brittle Bone Disorders Consortium (BBDC) include Brendan Lee and V. Reid Sutton, Texas Children’s Hospital, Houston, Texas; Frank Rauch and Francis Glorieux, Shriner’s Hospital for Children, Montreal, Quebec, Canada; Jean-Marc Retrouvey, Faculty of Dentistry, McGill University, Montreal, Quebec, Canada; Paul Esposito and Maegen Wallace, University of Nebraska Medical Center, Omaha, Nebraska; Michael Bober, Division of Medical Genetics, Alfred I. duPont Hospital for Children, Wilmington, Delaware; David Eyre, Department of Orthopedic and Sports Medicine, University of Washington, Seattle, Washington; Danielle Gomez, Shriners Hospital for Children, Tampa, Florida; Tracy Hart, Osteogenesis Imperfecta Foundation, Gaithersburg, Maryland; Mahim Jain, Departments of Bone and Osteogenesis Imperfecta, Kennedy Krieger Institute, Baltimore, Maryland; Deborah Krakow, Departments of Orthopedic Surgery and Obstetrics and Gynecology, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, California; Jeffrey Krischer, College of Medicine, University of South Florida, Tampa, Florida; Eric Orwoll and Lindsey Nicol, Division of Endocrinology, Department of Medicine, Oregon Health & Science University, Portland, Oregon; Cathleen Raggio, Hospital for Special Surgery, New York, NY; and Laura Tosi, Bone Health Program, Children’s National Health System, Washington, DC. The Members of the Linked Clinical Research Centers (LCRC) not included in the BBDC include Jay R. Shapiro, MD, Department of Bone and Osteogenesis Imperfecta, Kennedy Krieger Institute, Baltimore, Maryland; Robert D. Steiner, MD, University of Wisconsin School of Medicine and Public Health, Madison, Wisconsin; and Peter H. Byers, MD, Division of Medical Genetics, Department of Medicine, and Department of Pathology, University of Washington, Seattle, Washington.
The authors are very grateful to the participants and their families for their support. They thank Sergey Tarima and Alexis Visotcky, both from the Department of Biostatistics, Medical College of Wisconsin, Milwaukee, Wisconsin, for their statistical consultation. They also thank Kathryn Reiners for her careful reading of the manuscript.
Footnotes
A list of the Brittle Bone Disorders Consortium (BBDC) and Linked Clinical Research Centers (LCRC) members is given as a note at the end of the article.
Disclosure: The study was supported by the BBDC (1U54AR068069-0), a part of the Rare Diseases Clinical Research Network (RDCRN) of the National Center for Advancing Translational Sciences (NCATS); the Clinical Translational Core of the Baylor College of Medicine Intellectual and Developmental Disabilities Research Center (BCM IDDRC) (1U54HD083092) from the Eunice Kennedy Shriver NICHD (National Institute of Child Health and Human Development); U.S. Department of Health and Human Services/National Institute on Disability, Independent Living, and Rehabilitation Research (HHS/NIDILRR) grant 90AR5022-01; and internal support from Shriners Hospitals for Children. The BBDC is funded through the Osteogenesis Imperfecta (OI) Foundation and the Office of Rare Diseases Research (ORDR) of the NCATS, the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Institute of Child Health and Human Development (NICHD), and the National Institute of Dental and Craniofacial Research (NIDCR). On the Disclosure of Potential Conflicts of Interest forms, which are provided with the online version of the article, one or more of the authors checked “yes” to indicate that the author had a relevant financial relationship in the biomedical arena outside the submitted work (http://links.lww.com/JBJSOA/A198).
References
- 1.Patel RM, Nagamani SC, Cuthbertson D, Campeau PM, Krischer JP, Shapiro JR, Steiner RD, Smith PA, Bober MB, Byers PH, Pepin M, Durigova M, Glorieux FH, Rauch F, Lee BH, Hart T, Sutton VR. A cross-sectional multicenter study of osteogenesis imperfecta in North America - results from the linked clinical research centers. Clin Genet. 2015. February;87(2):133-40. Epub 2014 May 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sillence DO, Senn A, Danks DM. Genetic heterogeneity in osteogenesis imperfecta. J Med Genet. 1979. April;16(2):101-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glorieux FH, Rauch F, Plotkin H, Ward L, Travers R, Roughley P, Lalic L, Glorieux DF, Fassier F, Bishop NJ. Type V osteogenesis imperfecta: a new form of brittle bone disease. J Bone Miner Res. 2000. September;15(9):1650-8. [DOI] [PubMed] [Google Scholar]
- 4.Glorieux FH, Ward LM, Rauch F, Lalic L, Roughley PJ, Travers R. Osteogenesis imperfecta type VI: a form of brittle bone disease with a mineralization defect. J Bone Miner Res. 2002. January;17(1):30-8. [DOI] [PubMed] [Google Scholar]
- 5.Ward LM, Rauch F, Travers R, Chabot G, Azouz EM, Lalic L, Roughley PJ, Glorieux FH. Osteogenesis imperfecta type VII: an autosomal recessive form of brittle bone disease. Bone. 2002. July;31(1):12-8. [DOI] [PubMed] [Google Scholar]
- 6.Forlino A, Marini JC. Osteogenesis imperfecta. Lancet. 2016. April 16;387(10028):1657-71. Epub 2015 Nov 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azzam KA, Rush ET, Burke BR, Nabower AM, Esposito PW. Mid-term results of femoral and tibial osteotomies and Fassier-Duval nailing in children with osteogenesis imperfecta. J Pediatr Orthop. 2018. July;38(6):331-6. [DOI] [PubMed] [Google Scholar]
- 8.Van Dijk FS, Sillence DO. Osteogenesis imperfecta: clinical diagnosis, nomenclature and severity assessment. Am J Med Genet A. 2014. June;164A(6):1470-81. Epub 2014 Apr 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esposito P, Plotkin H. Surgical treatment of osteogenesis imperfecta: current concepts. Curr Opin Pediatr. 2008. February;20(1):52-7. [DOI] [PubMed] [Google Scholar]
- 10.Riff A, Smith PA. Transitional care in osteogenesis imperfecta: advances in biology, technology, and clinical practice. Chicago: Shriners Hospital for Children; 2015. [Google Scholar]
- 11.Palomo T, Fassier F, Ouellet J, Sato A, Montpetit K, Glorieux FH, Rauch F. Intravenous bisphosphonate therapy of young children with osteogenesis imperfecta: skeletal findings during follow up throughout the growing years. J Bone Miner Res. 2015. December;30(12):2150-7. Epub 2015 Jun 30. [DOI] [PubMed] [Google Scholar]
- 12.Dwan K, Phillipi CA, Steiner RD, Basel D. Bisphosphonate therapy for osteogenesis imperfecta. Cochrane Database Syst Rev. 2014. July 23;7:CD005088. [DOI] [PubMed] [Google Scholar]
- 13.Biggin A, Munns CF. Long-term bisphosphonate therapy in osteogenesis imperfecta. Curr Osteoporos Rep. 2017. October;15(5):412-8. [DOI] [PubMed] [Google Scholar]
- 14.Ruck J, Dahan-Oliel N, Montpetit K, Rauch F, Fassier F. Fassier-Duval femoral rodding in children with osteogenesis imperfecta receiving bisphosphonates: functional outcomes at one year. J Child Orthop. 2011. June;5(3):217-24. Epub 2011 May 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Letocha AD, Cintas HL, Troendle JF, Reynolds JC, Cann CE, Chernoff EJ, Hill SC, Gerber LH, Marini JC. Controlled trial of pamidronate in children with types III and IV osteogenesis imperfecta confirms vertebral gains but not short-term functional improvement. J Bone Miner Res. 2005. June;20(6):977-86. Epub 2005 Jan 18. [DOI] [PubMed] [Google Scholar]
- 16.Birke O, Davies N, Latimer M, Little DG, Bellemore M. Experience with the Fassier-Duval telescopic rod: first 24 consecutive cases with a minimum of 1-year follow-up. J Pediatr Orthop. 2011. June;31(4):458-64. [DOI] [PubMed] [Google Scholar]
- 17.Fassier F. Fassier-Duval telescopic system: how I do it? J Pediatr Orthop. 2017. September;37(Suppl 2):S48-51. [DOI] [PubMed] [Google Scholar]
- 18.Engelbert RH, Uiterwaal CS, Gulmans VA, Pruijs H, Helders PJ. Osteogenesis imperfecta in childhood: prognosis for walking. J Pediatr. 2000. September;137(3):397-402. [DOI] [PubMed] [Google Scholar]
- 19.Kruger KM, Caudill A, Rodriguez Celin M, Nagamani SCS, Shapiro JR, Steiner RD, Bober MB, Hart T, Cuthbertson D, Krischer J, Byers PH, Durigova M, Glorieux FH, Rauch F, Sutton VR, Lee B, Rush ET, Smith PA, Harris GF. Mobility in osteogenesis imperfecta: a multicenter North American study. Genet Med. 2019. October;21(10):2311-8. Epub 2019 Mar 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilkinson JM, Scott BW, Clarke AM, Bell MJ. Surgical stabilisation of the lower limb in osteogenesis imperfecta using the Sheffield Telescopic Intramedullary Rod System. J Bone Joint Surg Br. 1998. November;80(6):999-1004. [DOI] [PubMed] [Google Scholar]
- 21.Ben Amor IM, Glorieux FH, Rauch F. Genotype-phenotype correlations in autosomal dominant osteogenesis imperfecta. J Osteoporos. 2011;2011:540178. Epub 2011 Sep 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rauch F, Lalic L, Roughley P, Glorieux FH. Genotype-phenotype correlations in nonlethal osteogenesis imperfecta caused by mutations in the helical domain of collagen type I. Eur J Hum Genet. 2010. June;18(6):642-7. Epub 2010 Jan 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bellur S, Jain M, Cuthbertson D, Krakow D, Shapiro JR, Steiner RD, Smith PA, Bober MB, Hart T, Krischer J, Mullins M, Byers PH, Pepin M, Durigova M, Glorieux FH, Rauch F, Sutton VR, Lee B, Nagamani SC; Members of the BBD Consortium. Cesarean delivery is not associated with decreased at-birth fracture rates in osteogenesis imperfecta. Genet Med. 2016. June;18(6):570-6. Epub 2015 Oct 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jain M, Tam A, Shapiro JR, Steiner RD, Smith PA, Bober MB, Hart T, Cuthbertson D, Krischer J, Mullins M, Bellur S, Byers PH, Pepin M, Durigova M, Glorieux FH, Rauch F, Lee B, Sutton VR, Nagamani SCS; Members of the Brittle Bone Disorders Consortium*. Growth characteristics in individuals with osteogenesis imperfecta in North America: results from a multicenter study. Genet Med. 2019. February;21(2):275-83. Epub 2018 Jul 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tam A, Chen S, Schauer E, Grafe I, Bandi V, Shapiro JR, Steiner RD, Smith PA, Bober MB, Hart T, Cuthbertson D, Krischer J, Mullins M, Byers PH, Sandhaus RA, Durigova M, Glorieux FH, Rauch F, Reid Sutton V, Lee B, Rush ET, Nagamani SCS; Members of the Brittle Bone Disorders Consortium. A multicenter study to evaluate pulmonary function in osteogenesis imperfecta. Clin Genet. 2018. December;94(6):502-11. Epub 2018 Sep 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bains JS, Carter EM, Citron KP, Boskey AL, Shapiro JR, Steiner RD, Smith PA, Bober MB, Hart T, Cuthbertson D, Krischer J, Byers PH, Pepin M, Durigova M, Glorieux FH, Rauch F, Sliepka JM, Sutton VR, Lee B, Nagamani SC, Raggio CL; Members of the BBD Consortium. A multicenter observational cohort study to evaluate the effects of bisphosphonate exposure on bone mineral density and other health outcomes in osteogenesis imperfecta. JBMR Plus. 2019. January 7;3(5):e10118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Novacheck TF, Stout JL, Tervo R. Reliability and validity of the Gillette Functional Assessment Questionnaire as an outcome measure in children with walking disabilities. J Pediatr Orthop. 2000. Jan-Feb;20(1):75-81. [PubMed] [Google Scholar]
- 28.Graham HK, Harvey A, Rodda J, Nattrass GR, Pirpiris M. The Functional Mobility Scale (FMS). J Pediatr Orthop. 2004. Sep-Oct;24(5):514-20. [DOI] [PubMed] [Google Scholar]
- 29.Cintas HL, Siegel KL, Furst GP, Gerber LH. Brief Assessment of Motor Function: reliability and concurrent validity of the Gross Motor Scale. Am J Phys Med Rehabil. 2003. January;82(1):33-41. [DOI] [PubMed] [Google Scholar]
- 30.Parks R, Cintas HL, Chaffin MC, Gerber L. Brief Assessment of Motor Function: content validity and reliability of the Fine Motor Scale. Pediatr Phys Ther. 2007. Winter;19(4):315-25. [DOI] [PubMed] [Google Scholar]
- 31.Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, Grummer-Strawn LM, Curtin LR, Roche AF, Johnson CL. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002. January;109(1):45-60. [DOI] [PubMed] [Google Scholar]
- 32.Franzone JM, Shah SA, Wallace MJ, Kruse RW. Osteogenesis imperfecta: a pediatric orthopedic perspective. Orthop Clin North Am. 2019. April;50(2):193-209. [DOI] [PubMed] [Google Scholar]
- 33.Germain-Lee EL, Brennen FS, Stern D, Kantipuly A, Melvin P, Terkowitz MS, Shapiro JR. Cross-sectional and longitudinal growth patterns in osteogenesis imperfecta: implications for clinical care. Pediatr Res. 2016. March;79(3):489-95. Epub 2015 Nov 5. [DOI] [PubMed] [Google Scholar]
- 34.Montpetit K, Palomo T, Glorieux FH, Fassier F, Rauch F. Multidisciplinary treatment of severe osteogenesis imperfecta: functional outcomes at skeletal maturity. Arch Phys Med Rehabil. 2015. October;96(10):1834-9. Epub 2015 Jul 2. [DOI] [PubMed] [Google Scholar]
- 35.Dahan-Oliel N, Oliel S, Tsimicalis A, Montpetit K, Rauch F, Dogba MJ. Quality of life in osteogenesis imperfecta: a mixed-methods systematic review. Am J Med Genet A. 2016. January;170A(1):62-76. Epub 2015 Sep 14. [DOI] [PubMed] [Google Scholar]
- 36.Fano V, del Pino M, Rodríguez Celin M, Buceta S, Obregón MG. [Osteogenesis imperfecta: quality of life in children]. Arch Argent Pediatr. 2013. Jul-Aug;111(4):328-31. Spanish. [DOI] [PubMed] [Google Scholar]
- 37.Navti LK, Samani-Radia D, David McCarthy H. Children’s body fatness and prevalence of obesity in relation to height for age. Ann Hum Biol. 2014. Jan-Feb;41(1):84-90. Epub 2013 Nov 25. [DOI] [PubMed] [Google Scholar]
- 38.Barber LA, Abbott C, Nakhate V, Do AND, Blissett AR, Marini JC. Longitudinal growth curves for children with classical osteogenesis imperfecta (types III and IV) caused by structural pathogenic variants in type I collagen. Genet Med. 2019. May;21(5):1233-9. Epub 2018 Oct 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.el-Sobky MA, Hanna AA, Basha NE, Tarraf YN, Said MH. Surgery versus surgery plus pamidronate in the management of osteogenesis imperfecta patients: a comparative study. J Pediatr Orthop B. 2006. May;15(3):222-8. [DOI] [PubMed] [Google Scholar]
- 40.Seikaly MG, Kopanati S, Salhab N, Waber P, Patterson D, Browne R, Herring JA. Impact of alendronate on quality of life in children with osteogenesis imperfecta. J Pediatr Orthop. 2005. Nov-Dec;25(6):786-91. [DOI] [PubMed] [Google Scholar]
- 41.Land C, Rauch F, Montpetit K, Ruck-Gibis J, Glorieux FH. Effect of intravenous pamidronate therapy on functional abilities and level of ambulation in children with osteogenesis imperfecta. J Pediatr. 2006. April;148(4):456-60. [DOI] [PubMed] [Google Scholar]
- 42.Bishop N, Harrison R, Ahmed F, Shaw N, Eastell R, Campbell M, Knowles E, Hill C, Hall C, Chapman S, Sprigg A, Rigby A. A randomized, controlled dose-ranging study of risedronate in children with moderate and severe osteogenesis imperfecta. J Bone Miner Res. 2010. January;25(1):32-40. [DOI] [PubMed] [Google Scholar]
- 43.Ward LM, Rauch F, Whyte MP, D’Astous J, Gates PE, Grogan D, Lester EL, McCall RE, Pressly TA, Sanders JO, Smith PA, Steiner RD, Sullivan E, Tyerman G, Smith-Wright DL, Verbruggen N, Heyden N, Lombardi A, Glorieux FH. Alendronate for the treatment of pediatric osteogenesis imperfecta: a randomized placebo-controlled study. J Clin Endocrinol Metab. 2011. February;96(2):355-64. Epub 2010 Nov 24. [DOI] [PubMed] [Google Scholar]
- 44.Constantino CS, Krzak JJ, Fial AV, Kruger KM, Rammer JR, Radmanovic K, Smith PA, Harris GF. Effect of bisphosphonates on function and mobility among children with osteogenesis imperfecta: a systematic review. JBMR Plus. 2019. October 18;3(10):e10216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aström E, Söderhäll S. Beneficial effect of long term intravenous bisphosphonate treatment of osteogenesis imperfecta. Arch Dis Child. 2002. May;86(5):356-64. [DOI] [PMC free article] [PubMed] [Google Scholar]