Abstract
~10% of dementia patients have idiopathic normal pressure hydrocephalus (iNPH), an expansion of the cerebrospinal fluid (CSF)-filled brain ventricles. iNPH and Alzheimer’s disease (AD) both exhibit sleep disturbances, build-up of brain metabolic wastes and Aβ plaques, perivascular reactive astrogliosis, and mislocalization of astrocyte Aquaporin-4 (AQP4). The glymphatic system facilitates brain fluid clearance and waste removal during sleep via glia-supported perivascular channels. Human studies have implicated impaired glymphatic function in both AD and iNPH. Continued investigation into the role of glymphatic system biology in AD and iNPH models could lead to new strategies to improve brain health by restoring homeostatic brain metabolism and CSF dynamics.
Keywords: Hydrocephalus, idiopathic Normal Pressure Hydrocephalus, Glial-lymphatic, Glymphatic, Aging, Dementia, Alzheimer’s Disease
Idiopathic normal pressure hydrocephalus and Alzheimer’s disease
~50 million people worldwide currently live with dementia, and this figure is expected to triple by 2050, reflecting our rapidly aging population [1]. Nearly 10% of these 50 million people with dementia suffer from idiopathic normal pressure hydrocephalus (iNPH) (see glossary), a disorder characterized by progressive ventriculomegaly and the clinical triad of gait ataxia, urinary incontinence, and dementia [2]. iNPH therefore affects 1–3% of all medical patients over the age of 65 and between 9–14% of nursing home residents [3–5]. Despite iNPH’s pervasive prevalence in the elderly population, the vast majority of iNPH patients are misdiagnosed with Alzheimer’s disease (AD) or other neurodegenerative disorders, reflecting clinical overlap with other neurodegenerative diseases and the medical community’s widespread unfamiliarity with or skepticism towards iNPH (see Box 1) [2,6].
Text Boxes: Box 1: Diagnosing iNPH.
iNPH presents clinically with gait ataxia, dementia, and urinary incontinence, but there are many untreatable disorders that also present with the same clinical triad (see table I below) [86]. Thus, prompt diagnosis of iNPH is critically important.
iNPH and AD both present clinically with neurodegeneration, progressive cognitive and physical decline, and sleep disturbances that include increased number of awakenings, decreased nightly sleep time, and an overall reduction in sleep quality [7,8]. For these reasons, AD has become the leading differential diagnosis to iNPH [9]. However, given the relative reversibility of iNPH, early diagnostic discrimination between the two conditions is critical [10]. Suspected iNPH is currently assessed by both magnetic resonance imaging (MRI) evidence of disproportionately dilated ventricles and by an invasive in-patient lumbar drain (LD) trial or large-volume lumbar puncture followed by monitoring for clinical improvement (see Clinician’s Corner) [10]. iNPH treatment with permanent CSF diversion via shunting is not without drawbacks, such as variable or only temporary response, infection, shunt failure, and other post-surgical risks associated with surgery in geriatric populations [10,11]. Most notably, CSF diversion confers no symptomatic relief to patients with AD [11,12].
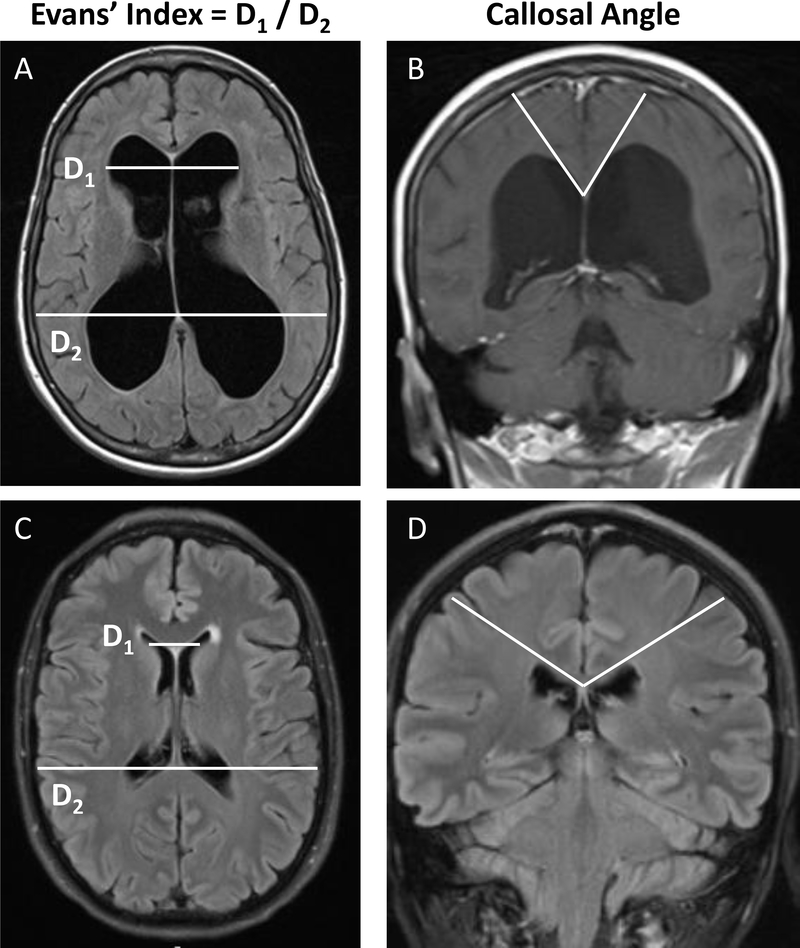
Improved diagnostic, prognostic, and therapeutic strategies for the treatment of iNPH require further characterization of the disease’s molecular pathophysiology. The clinical similarity of iNPH and AD suggests that their careful comparison could yield pathogenic insights. Although less severe in iNPH, accumulation of interstitial amyloid-β (Aβ) plaques are a characteristic feature of both iNPH and AD cortical brain biopsies, a pathological finding that correlates with poor shunt responsiveness in iNPH patients [6,13–16]. Additionally, both AD and iNPH are characterized by reactive astrogliosis surrounding Aβ plaques, resulting in disordered perivascular spaces [17]. iNPH can sometimes be differentiated from AD and other neurodegenerative diseases by the presence of ventriculomegaly, a severely reduced callosal index, and a significantly increased Evan’s index (Figure 1) [16,18,19]. However, among these cardinal features of iNPH, ventricular enlargement can also be seen in AD as a result of severe cerebral atrophy, further complicating radiographic recognition of iNPH [20]. The many clinical and cellular similarities between AD and iNPH warrant further investigation into potential overlapping pathological mechanisms.
Figure 1: T1-Weighted MRIs showing cardinal diagnostic features of iNPH.
Axial and coronal brain MRIs of an individual diagnosed with iNPH (A, B) compared to brain MRIs of an age and sex-matched control (C, D). Note the key diagnostic features of iNPH: ventriculomegaly determined by an increased Evan’s index and a steeper callosal angle.
We argue here that the pathological link between the two diseases could be the glia-lymphatic (glymphatic) system – a sleep assisted highly polarized CSF and interstitial fluid (ISF) transport system that facilitates extracellular waste removal through a network of astroglia-supported perivascular or perineural channels that drain into the cervical and basal meningeal lymphatic networks or the major dural sinuses (Figure 2) [21,22]. Attenuation of glymphatic function has been associated with significant increases in Aβ plaque accumulation, and is therefore implicated in the pathogenesis AD [20]. Despite its critical involvement in CSF homeostasis and its hypothesized involvement in other neurodegenerative diseases [23], glymphatic dysfunction has been understudied in the context of hydrocephalus. In the few studies that do address this relationship, glymphatic impairment is seemingly characteristic of iNPH. Thus, it is posited here that a compromised glymphatic system may be fundamental to the development and progression of dementia-related disorders, and that the recapitulation of studies focused on glymphatics and AD in iNPH models may elucidate the pathogenic mechanism of a currently nebulous disease.
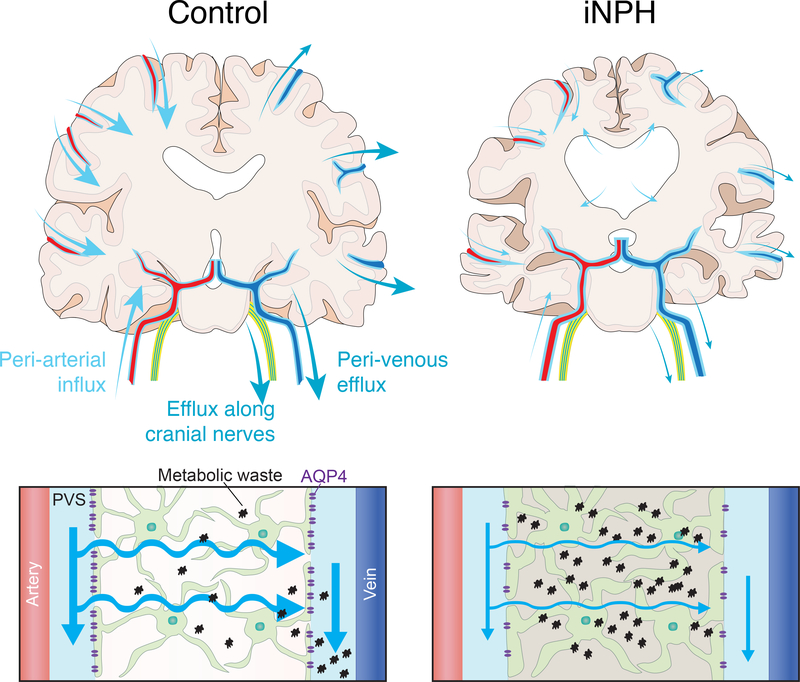
Figure 2: Schematic of potential glymphatic dysfunction in iNPH.
Top Panel – Diagram showing the periarterial CSF influx routes and proposed perivenous and perineural cerebrospinal (CSF) efflux routes of the glymphatic system in a control (left) and iNPH (right) cortical brain section. The less prominent arrows in the iNPH brain denote reduced CSF flux along the perivascular and perineural pathways demonstrated by Ringstad and Per Eide in their human MRI studies. The arrows in the iNPH brain also demonstrate transependymal flow of CSF from the lateral ventricles into the brain parenchyma. Bottom Panel – Schematic demonstrating glymphatic system function in a control (left) brain and iNPH brain (right). Reduced arrow sizes in the iNPH schematic indicate attenuated CSF periarterial inflow, CSF-interstitial fluid (ISF) exchange, and perivenous efflux of CSF mediated by the depolarization of AQP4 that has been observed in human iNPH patients. This reduction in the exchange of CSF and ISF causes increased concentrations of neuronal metabolic waste products in the brain interstitial space.
Note: The periarterial influx routes shown on the left side of the control brain and the perivenous efflux routes shown on the right of the control brain are for illustrative purposes only and do not imply that fluid enters on one side of the brain and leaves on the opposite side.
The glia-lymphatic (glymphatic) system
Extracellular ion concentrations, CSF dynamics, and the clearance of metabolic waste must be tightly regulated to maintain optimal conditions for neuronal functioning and synaptic activity [24]. The glymphatic system helps to maintain this delicate balance by aiding in the delivery of fluid and interstitial solutes to the major CSF egress routes from the brain [21,25,26]. After CSF circulates into the subarachnoid space (SAS) from the ventricular system, arterial pulsation drives the fluid back into the brain parenchyma within the periarterial spaces that surround the penetrating cerebral arteries [22,27–29]. Ensheathing the perivascular spaces, otherwise known as the Virchow-Robin space, are astroglial endfeet processes that are densely packed with aquaporin 4 channels (AQP4) [22,30]. Facilitated by AQP4, CSF flows from the periarterial space into the brain interstitium and mixes with ISF [22,29]. Within the neuropil, the CSF-ISF mixture, along with interstitial solutes, is hypothesized to travel via diffusion or bulk flow into the perivenous or perineuronal spaces, which then direct the fluid and its contents into the deep veins via arachnoid granulations or into the basal meningeal and cervical lymphatic vessels that ultimately drain to peripheral lymph nodes or to the general circulation for clearance by the liver [21,25,26,31]. Perivascular influx and efflux is suggested to be a low-resistance route for CSF-ISF flow as the width of the perivascular spaces is believed to be orders of magnitude larger than the interstitial space or the gaps between the cellular structures of the brain [32]. Despite this, perivenous efflux remains controversial and the most dominant and well characterized exit route is along the olfactory nerve, where CSF-ISF passes along the cribriform plate and enters the cervical lymphatic vasculature through the nasal mucosa [31,33]. Glymphatic dysfunction, characterized by reduced CSF-ISF exchange, leads to an abnormal accumulation of cerebral CSF and impaired interstitial solute clearance, both defining clinical features of iNPH and a likely cause of cognitive deterioration.
Iliff et al. [27] initially proposed that glymphatic flux is driven by cardiac induced arterial pulsations after showing that unilateral internal carotid artery ligation significantly reduced CSF-ISF exchange in murine brains [27]. This notion was supported by more recent results that demonstrated that arterial pulsations from the cardiac cycle drive perivascular CSF flow [28]. However, magnetic resonance encephalography in healthy humans, established that pressure fluctuations generated by respiration and changes in vascular tone also contribute to the convective flow that drives glymphatic flux [34]. These results have also been supported by recent human MRI studies suggesting cardiac and respiratory influence in glymphatic influx and efflux, indicating the importance of cardiovascular health in CSF homeostasis [32,35,36].
Most interestingly, the rate of convective exchange between CSF and ISF fluctuates along with the sleep-wake cycle. Two-photon microscopic assessment of the movement of fluorescent tracers injected into the cisterna magna revealed a ~95% increase in periarterial and parenchymal influx of dye into murine brains during sleep or a sleep-like anesthetized state as compared to awake animals [37]. Using the real-time iontophoretic tetramethyl-ammonium method, the group proposed that this increase in glymphatic function during sleep likely reflects, at least in part, the increase in interstitial volume consistently observed during sleep [37]. Based on correlational evidence, increases in the interstitial space volume are hypothesized to reduce resistance to diffusion or convective flow through the interstitial space, allowing for ISF and soluble metabolites to travel unimpeded through the parenchyma and into perivenous or perineural spaces for subsequent drainage [37].
Glymphatic function and Alzheimer’s disease
Multiple mechanisms contribute to removal of soluble interstitial Aβ from the brain, including degradation by both glial cells and neurons, transport across the BBB, and glymphatic ISF flow along perivascular or perineural tracts to lymphatic vessels or draining veins [20]. AD patients show altered CSF dynamics [38], potentially causing an imbalance in the production and clearance of soluble Aβ, and leading to an accumulation of interstitial Aβ with increased likelihood of plaque formation [39]. While Aβ and later tauopathy are believed to lead to neuronal demise, the relative contributions to neurotoxicity of Aβ oligomers, amyloid plaques, or intraneuronal tau accumulation remain debated [40]. For example, the number of interstitial Aβ plaques in AD does not correlate with the degree of cognitive impairment as well as does the extent of Aβ build-up in the vascular spaces does [41]. This vascular impairment, known as cerebral amyloid angiopathy (CAA), results in increased arterial stiffness, reducing the arterial pulsatility that plays a primary role in driving periarterial CSF flow and CSF-ISF exchange [22,28,42]. Additionally, amyloid build-up along the blood vessels may reduce the large, low-resistance perivascular spaces in which glymphatic flux depend upon [42]. This indicates that AD may generate a vicious cycle in which Aβ accumulation along the blood vessels reduces glymphatic function, promoting even more severe parenchymal build-up of Aβ and neuronal death.
Depolarization of the AQP4 channels that line the basal membranes of the perivascular glial barrier have been suggested to lead to an accelerated progression of AD. Xu et al. [39] showed in APP/PS1 transgenic mice overexpressing Aβ that AQP4 deletion significantly increased interstitial Aβ plaque accumulation, CAA, and astrocytic atrophy, each of which has been implicated in aggravating cognitive and motor impairment in mice. Post-mortem human studies of AD brains have shown markedly reduced polarization of AQP4 to the astrocytic endfeet surrounding the cerebral vasculature as compared to the perivascular spaces of age-matched controls [43]. The relative importance of AQP4 in the transport of CSF and ISF was contested by a study that showed no reduction in solute clearance in AQP4 depleted rodents [44]; however, their findings have more recently been attributed to the use of an anesthetic that is not permissive of glymphatic function [45]. Additionally, multiple groups have since reaffirmed the initial findings reported that CSF clearance depends on astroglial endfeet AQP4 expression, although the mechanism by which AQP4 aids in CSF-ISF flux remains unelucidated (see Outstanding Questions) [11,46–49].
Outstanding Questions.
To what extent are glymphatic CSF efflux pathways impaired in iNPH? What about other forms of hydrocephalus?
How does the timing of glymphatic function reduction relate to potential changes in choroid plexus CSF secretion and the development of ventriculomegaly?
Is the well-established impact of sleep on the glymphatic function affected in hydrocephalus? Do sleep disorders precede iNPH development or arise following iNPH related brain changes?
Can sleep therapy impact the development of iNPH by improving glymphatic function?
What are the associated cellular and molecular changes in the glymphatic system in iNPH? Do they resemble changes seen in post-hemorrhagic hydrocephalus or Alzheimer’s disease (e.g., astrogliosis)?
Can positive pharmacological or genetic modulation of glymphatic system CSF efflux improve iNPH clinical signs and pathology in model systems? Are there any FDA-approved drugs that could be repurposed for this?
What is the mechanism by which AQP4 channels mediate fluid flux from the perivascular space into the interstitial space and vice versa? Is there an unexplored ion transport system associated with glymphatic flux or are arterial pulsations enough to propel fluid through AQP4?
AD is marked by chronic nighttime insomnia and excessive daytime sleep, which positively correlate with the severity of cognitive decline [50]. However, whether disordered sleep represents a contributor to or a symptom of AD remains unclear and requires further study [51]. Levels of interstitial soluble Aβ vary daily along with the sleep-wake cycle, and chronic sleep deprivation greatly increases the likelihood of Aβ plaque formation in mice [52] and in humans [53]. Aβ deposition in a human brain significantly increased after a single night of sleep deprivation, as compared to rested controls [54]. While these studies suggest that sleep problems precede AD development, sleep disturbances do not arise in the APP/PS1 mouse model until plaques begin to form, suggesting a potential bidirectional causal relationship [53]. Further supporting the importance of sleep pathology in AD, are data showing that the clearance of intracortically injected radiolabeled 125I-Aβ1–40 is twofold faster in sleeping mice than awake mice [37]. Perhaps age-related changes to sleep architecture attenuate glymphatic function, which thereby increases interstitial Aβ plaques, leading to an even further reduction of glymphatic flow and Aβ clearance.
Although the literature presents this as a reasonable pathogenic component, it has also been argued that Aβ is physiologically produced more frequently during neuronal activity present only when awake and during the rapid eye movement (REM) sleep states, suggesting any increase in Aβ may be the result of increased production during AD-related increases in wakefulness and changes in sleep cycle, rather than reduced clearance [51]. However, studies have indicated that APP production is driven by the constitutively active prion promoter, thus suggesting impaired clearance of Aβ aggregations as the only pathogenic mechanism [55]. Moreover, data showing mitigated CSF tracer removal in an awakened state compliment the Aβ clearance data and implicate impaired sleep and glymphatic function as a root cause of Aβ accumulation [37].
Evidence for impaired glymphatic function in human iNPH patients
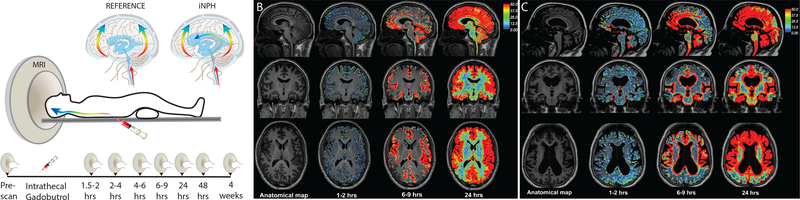
Ringstad et al. [35], injected gadobutrol (contrast agent) via the lumbar spine into 15 confirmed iNPH patients and 8 reference subjects. Influx and subsequent clearance of the tracer was tracked by MRI over 24 hours [35]. The authors noted, in addition to the transependymal flow commonly observed in iNPH, that the influx of gadobutrol into the extra-parenchymal SAS regions around the foramen magnum, pontine cistern, sylvian fissure, and precentral sulci was delayed in iNPH patients, but significantly impeded only near the sylvian fissure and precentral sulci [35]. Following emergence from the SAS, flow into the periarterial regions along the cortical surface was also significantly delayed in all iNPH patients, indicating disturbances in the onset of glymphatic fluid flux [35]. Additionally, CSF enhancement at 24 hours post-injection was greater in all regions of the iNPH brains than in corresponding control brain regions [35], suggesting impairment of both glymphatic influx and efflux in iNPH pathology. A longer term study of 9 iNPH patients and 8 reference subjects, using a similar gadobutrol injection protocol with follow-up imaging out to 4 weeks post-injection suggested similar impairments in glymphatic flux (Figure 3) [32]. Yet, more recent work by Eide and Ringstad demonstrated reduced glymphatic clearance in the entorhinal cortex (ERC), the main interface between the neocortex and hippocampus [56]. These findings are particularly interesting as the ERC plays a major role in memory consolidation and hippocampal function, and is severely degraded early in AD development, suggesting ERC dysfunction may be a hallmark of dementia progression in both iNPH and AD [56,57]. In addition, recent results indicate reduced CSF production from the choroid plexus in both iNPH and AD brains, which may further attenuate glymphatic-mediated CSF-ISF turnover and associated clearance of Aβ [58].
Figure 3: Impaired glymphatic CSF transport in hydrocephalus.
(A) Repeated contrast-enhanced magnetic resonance imaging (MRI) after intrathecal delivery of gadobutrol. The contrast agent distributed into all parts of the brain over 24 hrs in 9 iNPH and 8 reference patients. The diagrams illustrate gadobutrol dispersal in a centripetal pattern along the large cerebral arteries in both patient groups, but enrichment in periventricular white matter only in idiopathic normal pressure hydrocephalus (iNPH) patients, presumably reflecting ventricular CSF reflux. (B) Repeated scans of a reference patient display brain-wide distribution of the contrast agent 24 hrs after intrathecal delivery. (C) An iNPH patient also exhibits brain-wide contrast enrichment at 24 hrs. Enhanced gadobutrol uptake was evident in iNPH patients in periventricular white matter. Gadobutrol clearance was significantly delayed in iNPH compared with reference patients at 24 hrs, but all 17 subjects cleared the contrast agent at 4 weeks (data not shown). Modified from Ringstad et al., 2018, with permission.
While Ringstad and Eide [32,35] were the first to present data suggesting glymphatic dysfunction in human iNPH subjects, verification of their interpretations will require further investigation. In each study, MRI data were collected from control groups that were ~30 years younger than the iNPH patient groups [32,35], presenting a confounding variable as the influx and efflux of both intrathecally injected fluorescent tracer and radiolabeled Aβ is non-pathologically attenuated with age in mice[30]. Therefore, it is difficult to determine to what degree iNPH pathogenesis or normal aging can be attributed to the observed glymphatic impairment. However, the authors contend that the speed of gadobutrol propagation and observed ventricular reflux more likely indicate disease pathology than age-related deficiencies [32]. The difficulty of finding age-matched controls in human MRI experiments is also recognized, emphasizing the importance of developing reliable iNPH animal models.
Suggesting iNPH may reduce glymphatic function beyond the effects of normal aging, Yokota et al. [18] used diffusion tensor imaging (DTI) to track glymphatic flux along the perivascular spaces of periventricular deep white matter vessels in 12 pseudo-iNPH patients, 12 confirmed iNPH patients, and 12 age-match controls [18]. Pseudo-iNPH patients were individuals who presented with ventriculomegaly and the classic iNPH clinical triad, but did not respond symptomatically to an LD trial [18]. The authors measured glymphatic flux using the Analysis Along the Perivascular Space (ALPS) index, a calculated value reflecting water diffusivity alongside blood vessels in the X, Y, and Z planes after correction for increased water diffusivity caused by periventricular white matter change [18]. In this small study, ALPS index scores were dramatically lower in both the pseudo-iNPH and iNPH patients than in healthy controls [18]. ALPS indices of the iNPH patients were also lower than those of the pseudo-iNPH group, again suggesting impaired glymphatics as a fundamental and measurable component specific to iNPH pathology that may contribute to diagnostic criteria and constitute a potential therapeutic target [18]. Most interestingly. the authors found that the ALPS index positively correlated with ventricular volume, suggesting a direct link between glymphatic impairment and ventriculomegaly [18]. In future clinical screenings, this method could be used to non-invasively detect glymphatic deficiency in human subjects, thereby identifying those pre-disposed to or in the early stages of developing iNPH.
Inflammation and reduced perivascular AQP4 density in iNPH
Immune cells do not normally enter brain parenchyma, but instead survey the brain from within perivascular, subarachnoid, and meningeal CSF spaces [59]. Recently developed understandings of the meningeal lymphatic and glymphatic system has led to the hypothesis that glymphatic dysfunction is an early side-effect of neuro-inflammation [60]. Inflammation induced by aging, stroke, and traumatic brain injury (TBI) are all associated with a subsequent reduction in glymphatic fluid flux, possibly due to coincident reactive astrogliosis and associated depolarization of AQP4 [30,61]. Indeed, human [62–64] and animal [62,63,72–74,64–71] studies have shown correlations between perivascular space inflammation and iNPH development, with inflammation subsiding after shunt surgery [64]. Additionally, recent infant animal studies of post-germinal matrix hemorrhage (GMH) hydrocephalus show that inhibition of astrogliosis improved glymphatic function and attenuated hydrocephalus by preserving AQP4 polarization [75].
Also, AQP4 immunogold cytochemistry analysis of cortical brain biopsies from 30 iNPH patients and 12 reference subjects demonstrated reduced AQP4 density in the perivascular astroglial endfeet of iNPH brains as compared to control brains [76]. Astroglial AQP4 density facing the neuropil was unchanged, as was perivascular AQP4 density in brains from patients with aneurysms, epilepsy, and cancer [76]. This correlation may indicate a role of inflammation-induced depolarization of AQP4 in iNPH pathogenesis, such that attenuated perivascular AQP4 expression reduces glymphatic fluid flux, subsequently exacerbating Aβ accumulation and ventriculomegaly. Once again, however, this study failed to control for age differences between the healthy controls (44.0 years) and iNPH patients (70.8 years) [76]. Moreover, as progressive AQP4 depolarization occurs throughout physiological aging in mice [30], further age-matched studies of humans are needed to test the specificity of pathologic AQP4 depolarization specific for iNPH or other neurodegenerative disorders, as opposed to the normal aging process.
Sleep disruption in iNPH
A study that analyzed the sleep patterns of 31 patients with iNPH determined that all subjects were concurrently living with an undiagnosed sleep abnormality [7]. Most prominently, they found that obstructive sleep apnea (OSA), a common sleep disorder associated with AD [77], affected 28 of the 31 (90.3%) iNPH patients [7]. In a larger and more recent study, the authors found OSA to be diagnosed in between 65 to 90% of iNPH patients, a substantially higher proportion than the 44% of AD patients suffering from a sleep related comorbidity [77]. In animal models, glymphatic clearance of Aβ and conjugated tracer during sleep or an anesthetized state is two-fold faster than in an awake state, emphasizing the critical importance of sleep in brain homeostasis [37]. Despite this, OSA is rarely implicated in iNPH pathogenesis, remaining to be considered as only a comorbidity [2,7]. Blockage of the airway in OSA causes increased awakenings and considerably reduced slow wave (non-REM) sleep, resulting in a decreased quality of sleep and increased cerebral Aβ aggregation [78]. Due to intermittent airway obstruction in OSA, patients have reduced oxygen intake, hypoxemia, and respiratory acidosis against a closed airway, causing sufficient intrathoracic negative pressure to cause atrial distortion and reduced venous return to the heart [7]. The resultant increased intracranial venous pressure reduces perivenous CSF egress and CSF-ISF exchange, potentially leading to the accumulation of interstitial proteins and ventriculomegaly observed in iNPH [7]. Furthermore, OSA patients have less neuronally-derived proteins in their CSF with no overall reduction in total protein, supporting the notion that increased venous pressure may affect ISF-CSF turnover [79]. In line with the hydrodynamic concept of hydrocephalus, which states hydrocephalus is caused by a bulk increase in cerebral vasculature pressure, OSA-induced cerebral hypertension may further exacerbate glymphatic impairment by reducing the arterial pulsations that drive periarterial flux [80].
The association between sleep abnormalities and dementia has long been recognized, and classically attributed to the death of neurons in the suprachiasmatic nucleus and other brain regions controlling of these functions [81–83]. Nonetheless, the impact of sleep disturbances on dementia pathogenesis and progression has long been underappreciated. Emerging research emphasizing the pathological impact sleep deprivation has on glymphatic function has begun to highlight the pathogenic potential of sleep disorders in dementia onset and progression. The critical importance of glymphatic function on brain health and its functional dependence on sleep suggests that sleep dysfunction may not only accelerate the progression of dementia, but may itself be a risk factor for dementia development [84]. The occurrence of OSA following surgical shunting or lumbar drainage has shown that CSF diversion does not improve sleep quality, suggesting that iNPH induces irreversible damage in the sleep centers of the brain [69–71]. However this understanding has been challenged by a recent case study of an iNPH patient whose central sleep apnea was ameliorated three-months after VPS placement [85]. Additional retrospective studies investigating iNPH or AD incidence rates in patients previously diagnosed with a sleep disorder may illuminate the cause and effect relationship between sleep and dementia. Whether alterations in sleep precede or proceed iNPH development, the current data suggests that impaired glymphatics is implicated either in the initial pathogenesis, disease progression, or both.
Concluding Remarks
iNPH is an understudied and poorly characterized dementia that, in many ways, reflects the clinical and pathological presentation of AD and many other neurodegenerative disorders. Our limited knowledge of the molecular pathophysiology of iNPH is an obstacle to developing improved diagnostic, prognostic, and therapeutic strategies for patients, resulting in the underdiagnosis of most iNPH patients. Moreover, the few correctly diagnosed patients are offered only surgical interventions plagued by high failure and complication rates. Emerging data suggest that glymphatic dysfunction may be a therapeutically targetable component of iNPH pathogenesis (Figure 2). However, very few published (or to our knowledge unpublished) studies have analyzed glymphatic flux in the context of human iNPH, and animal models remain limited. In addition, much of the existing data demonstrates only correlation between factors that disrupt glymphatic function and the development of iNPH, emphasizing the need for mechanistic studies of glymphatic dysfunction and iNPH. As shown for AD, extensive characterization of the relationship between the glymphatic system and iNPH in larger human and animal-based studies will be essential to identifying the risk factors and underlying causes of an otherwise idiopathic disease, potentially leading to new diagnostic and therapeutic strategies.
In addition, many other disorders mimic one or two of the three key clinical diagnostic criteria associated with iNPH, complicating disease diagnosis. These disorders include other forms of dementia such as AD and frontotemporal dementia; obstructions or closure of the CSF pathway caused by disorders such as lumbosacral stenosis and cervical stenosis; and various additional pathologies such as peripheral neuropathy, myelopathy, and vitamin B12 deficiency [86]. The differential diagnoses are typically evaluated using standard dementia blood work and imaging of the brain. Radiographically, the presence of ventriculomegaly does not always indicate iNPH. Cerebral atrophy in AD and subcortical vascular dementia can also cause ventricular enlargement, and attributing enlarged ventricles to iNPH or to cerebral atrophy remains a difficult diagnostic problem [86,87]. Although non-invasive perfusion-imaging methods are being tested, with some positive correlations noted, for the diagnosis of iNPH and the selection of possible responders to CSF-shunting, research has remained limited [87].
With many disorders sharing the same clinical and radiological features, iNPH is most frequently diagnosed by the elimination of alternative, more common diseases. Better diagnostic strategies are needed as this process of elimination subjects patients to uncertain, arduous, and emotionally stressful stays in hospitals with no effective treatment.
Clinician’s Corner
Hydrocephalus can be broadly classified into two distinct categories: primary and secondary. The majority of chronic adult hydrocephalus cases are secondary, meaning they develop following a primary event such as a trauma, infection, tumor, or hemorrhage. iNPH, however, is distinguished from these alternative hydrocephalic disorders by etiology. In iNPH patients, the pathological cause is unknown and roughly 50% of those diagnosed have no discernable predisposing factors to hydrocephalus or dementia [10]. Diversion of CSF via a ventriculo-peritoneal shunt (VPS) confers limited and temporary symptom-variable relief [10,88]. In one case study of 116 iNPH patients, 64.9% of patients were noted to improve in gait instability following VPS surgery, while only 20.7% and 18% demonstrated improvements in cognition and incontinence, respectively [10]. In addition to variable outcomes following successful shunt placement, a systematic review of 1,573 VPS patients found that 8.2% suffered from complications including subdural hematoma, infection, hemorrhage, and death [10]. The shunts themselves are also plagued by high rates of mechanical and tubing complications. In fact, shunts have the highest rate of medical device failure in the U.S., with 2 and 10-year failure rates of 50% and 70%, respectively [89].
Suspected iNPH patients are scored for the severity of gait ataxia, incontinence, and dementia on a scale from 1–5 (with 1 being severely affected and 5 being asymptomatic) [88]. Patients with ventriculomegaly, as determined by an Evan’s index of >.3, and who score below a certain threshold are most often assessed for symptomatic attenuation following CSF diversion in an invasive LD trail or large volume lumbar tap [2]. Aiming to limit the number of invasive procedures and improve treatment outcomes, studies are searching for correlations between positive shunt responsiveness and other components like the severity of radiographical features (large Evan’s index, small callosal angles, etc.), the existence of comorbidities, age and gender, and duration of symptoms prior to surgery [2,10]. While results have been controversial, it has been consistently reported that comorbidities and increased age both correlate well with poor shunt outcomes [10]. In addition, studies have showed that high pre-operation pulsatile intracranial pressure in iNPH patients may correlate well with positive shunt responsiveness [88]. Further research focused on the etiology and mechanism of iNPH is required before more reliable and less invasive diagnostic and therapeutic strategies are determined.
Table I:
Disorders with same clinical presentation
| Corticobasal degeneration |
| Parkinson’s Disease |
| Neurosyphilis |
| Dementia with Lewy Bodies |
| Progressive supranuclear palsy |
| Subcortical Vascular Dementia |
| Multiple system atrophy |
Highlights.
iNPH constitutes ~10% of the 50 million people currently diagnosed with a dementia-related disorder. This is expected to exceed 150 million by 2050.
iNPH and AD share multiple clinical and pathologic features such as Aβ deposition, cerebrovascular inflammation, impaired localization of perivascular AQP4, and sleep disturbances.
While glymphatic system dysfunction has been extensively studied in AD, it has not yet been thoroughly examined in model systems of iNPH and other types of hydrocephalus.
Several studies analyzing brain MRIs of human iNPH patients have shown reduced perivascular influx and efflux of intrathecally injected contrast agent as when compared to controls, suggesting impairment of glymphatic function iNPH.
The relationship between glymphatic system function and iNPH requires further investigation as it may point toward identifiable risk factors or therapeutic targets.
Acknowledgements
Special thank you to Dan Xue for her expert graphic design and for the use of her figures.
Glossary
- AQP4 Depolarization
an increase in AQP4 expression in the astrocytic cell bodies and fine processes leads to a relative decrease in the relative polarization of AQP4 channels in the vascular endfeet of astrocytes that enclose the perivascular space.
- Callosal index
the angle between the frontal horns of the lateral ventricles when viewed from the coronal plane at the level of the posterior commissure.
- Evan’s index
the maximum width of the ventricular frontal horns divided by the maximum internal diameter of the skull when viewing an axial brain slice. A value greater than 0.3 is considered to be hydrocephalic.
- Glia-lymphatic System
a glia-lymphatic fluid system that, via the perivascular spaces, supports exchange of cerebrospinal and interstitial fluid. The glymphatic system aids with the clearance of waste molecules from the central nervous system by draining via efflux along the meningeal and cervical lymphatic vessels.
- Hydrocephalus
excess accumulation of CSF within the cerebral ventricles causing progressive distention of the brain’s ventricular system.
- iNPH
idiopathic normal pressure hydrocephalus, a reversible neurodegenerative disease characterized by dementia, gait ataxia, urinary incontinence, and progressive enlargement of the cerebral ventricles without associated increases in intracranial pressure.
- Slow Wave Sleep
deep, non-REM, sleep characterized by the presence of EEG delta waves.
- Ventriculomegaly
enlarged ventricles.
References
- 1.International D and Patterson C (2018) World Alzheimer Report 2018 - The state of the art of dementia research: New frontiers. Alzheimer’s Dis. Int DOI: 10.1103/PhysRevLett.78.4414 [DOI] [Google Scholar]
- 2.Nassar BR and Lippa CF (2016) Idiopathic Normal Pressure Hydrocephalus: A Review for General Practitioners. Gerontol. Geriatr. Med 2, 2333721416643702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kiefer M and Unterberg A (2012) The Differential Diagnosis and Treatment of Normal-Pressure Hydrocephalus. Dtsch. Arztebl. Int 109, 15–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wallenstein MB and McKhann GM (2010) Salomón Hakim and the discovery of normal-pressure hydrocephalus. Neurosurgery 67, 155–159 [DOI] [PubMed] [Google Scholar]
- 5.Martín-Láez R et al. (2016) Incidence of Idiopathic Normal-Pressure Hydrocephalus in Northern Spain. World Neurosurg. DOI: 10.1016/j.wneu.2015.10.069 [DOI] [PubMed] [Google Scholar]
- 6.Pomeraniec IJ et al. (2015) Concurrent Alzheimer’s pathology in patients with clinical normal pressure hydrocephalus: correlation of high-volume lumbar puncture results, cortical brain biopsies, and outcomes. J. Neurosurg 124, 382–388 [DOI] [PubMed] [Google Scholar]
- 7.Román GC et al. (2019) Sleep-Disordered Breathing and Idiopathic Normal-Pressure Hydrocephalus: Recent Pathophysiological Advances. Curr. Neurol. Neurosci. Rep 19, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rauchs G et al. (2008) Is there a link between sleep changes and memory in Alzheimer’s disease? Neuroreport 19, 1159–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laiterä T et al. (2015) The expression of transthyretin and amyloid-β protein precursor is altered in the brain of idiopathic normal pressure hydrocephalus patients. J. Alzheimer’s Dis 48, 959–968 [DOI] [PubMed] [Google Scholar]
- 10.Wu EM et al. (2019) Ventriculoperitoneal Shunt Outcomes of Normal Pressure Hydrocephalus: A Case Series of 116 Patients. Cureus DOI: 10.7759/cureus.4170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xia M et al. (2017) Mechanism of depression as a risk factor in the development of Alzheimer’s disease: the function of AQP4 and the glymphatic system. Psychopharmacology (Berl). 234, 365–379 [DOI] [PubMed] [Google Scholar]
- 12.Kang MJ et al. (2018) Hydrocephalus in a Patient with Alzheimer’s Disease. Dement. Neurocognitive Disord 17, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leinonen V et al. (2010) Amyloid and tau proteins in cortical brain biopsy and Alzheimer’s disease. Ann. Neurol 68, 446–453 [DOI] [PubMed] [Google Scholar]
- 14.S., S. et al. (1999) Prevalence of Alzheimer’s disease in patients investigated for presumed normal pressure hydrocephalus: A clinical and neuropathological study. Acta Neurochir. (Wien) 141, 849–853 [DOI] [PubMed] [Google Scholar]
- 15.J., G. et al. (2000) Alzheimer’s disease comorbidity in normal pressure hydrocephalus: Prevalence and shunt response. J. Neurol. Neurosurg. Psychiatry 68, 778–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hiraoka K et al. (2015) Amyloid deposits and response to shunt surgery in idiopathic normal-pressure hydrocephalus. J. Neurol. Sci DOI: 10.1016/j.jns.2015.06.029 [DOI] [PubMed] [Google Scholar]
- 17.V., G.G. (2019) Pathological changes in human brain biopsies from patients with idiopathic normal pressure hydrocephalus. S. S. Korsakova 119, 50–54 [DOI] [PubMed] [Google Scholar]
- 18.Yokota H et al. (2019) Diagnostic Performance of Glymphatic System Evaluation Using Diffusion Tensor Imaging in Idiopathic Normal Pressure Hydrocephalus and Mimickers. Curr. Gerontol. Geriatr. Res 2019, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoshi A et al. (2012) Characteristics of aquaporin expression surrounding senile plaques and cerebral amyloid angiopathy in Alzheimer disease. J. Neuropathol. Exp. Neurol 71, 750–759 [DOI] [PubMed] [Google Scholar]
- 20.Tarasoff-Conway JM et al. Clearance systems in the brain - Implications for Alzheimer disease., Nature Reviews Neurology. (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aspelund A et al. (2015) A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med 212, 991–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iliff JJ et al. (2012) A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med DOI: 10.1126/scitranslmed.3003748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rasmussen MK et al. The glymphatic pathway in neurological disorders., The Lancet Neurology. (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plog BA and Nedergaard M (2018) The Glymphatic System in Central Nervous System Health and Disease: Past, Present, and Future. Annu. Rev. Pathol. Mech. Dis DOI: 10.1146/annurev-pathol-051217-111018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Louveau A et al. (2015) Structural and functional features of central nervous system lymphatic vessels. Nature 523, 337–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahn JH et al. (2019) Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature DOI: 10.1038/s41586-019-1419-5 [DOI] [PubMed] [Google Scholar]
- 27.Iliff JJ et al. (2013) Cerebral Arterial Pulsation Drives Paravascular CSF-Interstitial Fluid Exchange in the Murine Brain. J. Neurosci 33, 18190–18199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mestre H et al. (2018) Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension. Nat. Commun 9, 4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Albargothy NJ et al. (2018) Convective influx/glymphatic system: tracers injected into the CSF enter and leave the brain along separate periarterial basement membrane pathways. Acta Neuropathol. 136, 139–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kress BT et al. (2014) Impairment of paravascular clearance pathways in the aging brain. Ann. Neurol 76, 845–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma Q et al. (2017) Outflow of cerebrospinal fluid is predominantly through lymphatic vessels and is reduced in aged mice. Nat. Commun 8, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ringstad G et al. (2018) Brain-wide glymphatic enhancement and clearance in humans assessed with MRI. JCI Insight 3, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Norwood JN et al. (2019) Anatomical basis and physiological role of cerebrospinal fluid transport through the murine cribriform plate. Elife DOI: 10.7554/eLife.44278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kiviniemi V et al. (2016) Ultra-fast magnetic resonance encephalography of physiological brain activity-Glymphatic pulsation mechanisms? J. Cereb. Blood Flow Metab 36, 1033–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ringstad G et al. (2017) Glymphatic MRI in idiopathic normal pressure hydrocephalus. Brain 140, 2691–2705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bradley WG (2015) CSF Flow in the Brain in the Context of Normal Pressure Hydrocephalus. AJNR. Am. J. Neuroradiol 36, 831–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xie L et al. (2013) Sleep drives metabolite clearance from the adult brain. Science (80-.). DOI: 10.1126/science.1241224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schubert JJ et al. (2019) Dynamic 11 C-PiB PET shows cerebrospinal fluid flow alterations in Alzheimer’s disease and multiple sclerosis. J. Nucl. Med DOI: 10.2967/jnumed.118.223834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu Z et al. (2015) Deletion of aquaporin-4 in APP/PS1 mice exacerbates brain Aβ accumulation and memory deficits. Mol. Neurodegener 10, 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Donev R et al. (2009) Neuronal death in Alzheimer’s disease and therapeutic opportunities. J. Cell. Mol. Med 13, 4329–4348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thai DR et al. (2008) Cerebral amyloid angiopathy and its relationship to Alzheimer’s disease. Acta Neuropathol. 115, 599–609 [DOI] [PubMed] [Google Scholar]
- 42.Hughes TM et al. (2013) Pulse wave velocity is associated with β-amyloid deposition in the brains of very elderly adults. Neurology DOI: 10.1212/01.wnl.0000435301.64776.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zeppenfeld DM et al. (2017) Association of perivascular localization of aquaporin-4 with cognition and Alzheimer disease in aging brains. JAMA Neurol. DOI: 10.1001/jamaneurol.2016.4370 [DOI] [PubMed] [Google Scholar]
- 44.Smith AJ et al. (2017) Test of the “glymphatic” hypothesis demonstrates diffusive and aquaporin-4-independent solute transport in rodent brain parenchyma. Elife 6, 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hablitz LM et al. (2019) Increased glymphatic influx is correlated with high EEG delta power and low heart rate in mice under anesthesia. Sci. Adv 5, eaav5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ren H et al. (2017) Omega-3 polyunsaturated fatty acids promote amyloid-b clearance from the brain through mediating the function of the glymphatic system. FASEB J. 31, 282–293 [DOI] [PubMed] [Google Scholar]
- 47.Lundgaard I et al. (2017) Glymphatic clearance controls state-dependent changes in brain lactate concentration. J. Cereb. Blood Flow Metab 37, 2112–2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Achariyar TM et al. (2016) Glymphatic distribution of CSF-derived apoE into brain is isoform specific and suppressed during sleep deprivation. Mol. Neurodegener 11, 1–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mestre H et al. (2018) Aquaporin-4-dependent glymphatic solute transport in the rodent brain. Elife DOI: 10.7554/eLife.40070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moran M et al. (2005) Sleep disturbance in mild to moderate Alzheimer’s disease. Sleep Med. 6, 347–352 [DOI] [PubMed] [Google Scholar]
- 51.Ju Y-ES et al. (2014) Sleep and Alzheimer disease pathology--a bidirectional relationship. Nat. Rev. Neurol 10, 115–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kang J-E et al. (2009) Orexin and the Sleep-Wake Cycle. Science (80-. ) 326, 1005–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang Y et al. (2012) Disruption of the Sleep-Wake Cycle and Diurnal Fluctuation of - Amyloid in Mice with Alzheimer’s Disease Pathology. Sci. Transl. Med 4, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shokri-Kojori E et al. (2018) β-Amyloid accumulation in the human brain after one night of sleep deprivation. Proc. Natl. Acad. Sci 115, 4483–4488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McHugh PC et al. (2012) Prion protein expression alters APP cleavage without interaction with BACE-1. Neurochem. Int DOI: 10.1016/j.neuint.2012.07.002 [DOI] [PubMed] [Google Scholar]
- 56.Eide PK and Ringstad G (2019) Delayed clearance of cerebrospinal fluid tracer from entorhinal cortex in idiopathic normal pressure hydrocephalus: A glymphatic magnetic resonance imaging study. J. Cereb. Blood Flow Metab 39, 1355–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hyman BT et al. (1984) Alzheimer’s disease: Cell-specific pathology isolates the hippocampal formation. Science (80-.). DOI: 10.1126/science.6474172 [DOI] [PubMed] [Google Scholar]
- 58.Eide PK et al. (2019) Delayed clearance of cerebrospinal fluid tracer from choroid plexus in idiopathic normal pressure hydrocephalus. J. Cereb. Blood Flow Metab DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Louveau A et al. Revisiting the Mechanisms of CNS Immune Privilege., Trends in Immunology. (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Plog BA et al. (2019) When the air hits your brain: decreased arterial pulsatility after craniectomy leading to impaired glymphatic flow. J. Neurosurg DOI: 10.3171/2019.2.jns182675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iliff JJ et al. (2014) Impairment of Glymphatic Pathway Function Promotes Tau Pathology after Traumatic Brain Injury. J. Neurosci DOI: 10.1523/jneurosci.3020-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sosvorova L et al. (2014) Selected pro- and anti-inflammatory cytokines in cerebrospinal fluid in Normal pressure hydrocephalus. Neuroendocrinol. Lett [PubMed] [Google Scholar]
- 63.Sosvorova L et al. (2015) The Impact of selected cytokines in the follow-up of normal pressure hydrocephalus. Physiol. Res [DOI] [PubMed] [Google Scholar]
- 64.Tarkowski E et al. (2003) Normal pressure hydrocephalus triggers intrathecal production of TNF-α. Neurobiol. Aging DOI: 10.1016/S0197-4580(02)00187-2 [DOI] [PubMed] [Google Scholar]
- 65.Lee MJ et al. (2009) Longitudinal evaluation of an N-Ethyl-N-nitrosourea-created murine model with normal pressure hydrocephalus. PLoS One DOI: 10.1371/journal.pone.0007868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mlakar J et al. (2016) Zika Virus Associated with Microcephaly. N. Engl. J. Med DOI: 10.1056/nejmoa1600651 [DOI] [PubMed] [Google Scholar]
- 67.Larroche JC (1972) Post-haemorrhagic hydrocephalus in infancy anatomical study. Neonatology DOI: 10.1159/000240472 [DOI] [PubMed] [Google Scholar]
- 68.Häusler M et al. (2005) Murine gammaherpesvirus-68 infection of mice: A new model for human cerebral Epstein-Barr virus infection. Ann. Neurol. DOI: 10.1002/ana.20440 [DOI] [PubMed] [Google Scholar]
- 69.Zhu W et al. (2014) Mouse models of intracerebral hemorrhage in ventricle, cortex, and hippocampus by injections of autologous blood or collagenase. PLoS One DOI: 10.1371/journal.pone.0097423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Harada T et al. (2013) Young C3H mice infected with Toxoplasma gondii are a novel experimental model of communicating hydrocephalus. Neurol. Res 29, 615–621 [DOI] [PubMed] [Google Scholar]
- 71.Sharma S et al. (2017) Cytokines do play a role in pathogenesis of tuberculous meningitis: A prospective study from a tertiary care center in India. J. Neurol. Sci DOI: 10.1016/j.jns.2017.06.001 [DOI] [PubMed] [Google Scholar]
- 72.Chaudhry SR et al. (2017) Elevated systemic IL-6 levels in patients with aneurysmal subarachnoid hemorrhage is an unspecific marker for post-SAH complications. Int. J. Mol. Sci DOI: 10.3390/ijms18122580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kitazawa K and Tada T (1994) Elevation of transforming growth factor-ßl level in cerebrospinal fluid of patients with communicating hydrocephalus after subarachnoid hemorrhage. Stroke DOI: 10.1161/01.STR.25.7.1400 [DOI] [PubMed] [Google Scholar]
- 74.Whitelaw A et al. (1999) Transforming Growth Factor-β1: A Possible Signal Molecule for Posthemorrhagic Hydrocephalus? Pediatr. Res DOI: 10.1203/00006450-199911000-00014 [DOI] [PubMed] [Google Scholar]
- 75.Ding Y et al. (2019) Astrogliosis inhibition attenuates hydrocephalus by increasing cerebrospinal fluid reabsorption through the glymphatic system after germinal matrix hemorrhage running title: Glymphatic system and hydrocephalus. Exp. Neurol DOI: 10.1016/j.expneurol.2019.113003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hasan-Olive MM et al. (2019) Loss of perivascular aquaporin-4 in idiopathic normal pressure hydrocephalus. Glia 67, 91–100 [DOI] [PubMed] [Google Scholar]
- 77.Ooms S and Ju Y El Treatment of Sleep Disorders in Dementia., Current Treatment Options in Neurology. (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ju YES et al. (2019) Obstructive sleep apnea treatment, slow wave activity, and amyloid-β. Ann. Neurol DOI: 10.1002/ana.25408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ju YES et al. (2016) Obstructive sleep apnea decreases central nervous system–derived proteins in the cerebrospinal fluid. Ann. Neurol DOI: 10.1002/ana.24672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Greitz D (2004) Radiological assessment of hydrocephalus: New theories and implications for therapy. Neurosurg. Rev 27, 145–165 [DOI] [PubMed] [Google Scholar]
- 81.Ohayon MM et al. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: Developing normative sleep values across the human lifespan., Sleep. (2004) [DOI] [PubMed] [Google Scholar]
- 82.Deschenes CL and McCurry SM Current treatments for sleep disturbances in individuals with dementia., Current Psychiatry Reports. (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Swaab DF et al. (1985) The suprachiasmatic nucleus of the human brain in relation to sex, age and senile dementia. Brain Res. DOI: 10.1016/0006-8993(85)91350-2 [DOI] [PubMed] [Google Scholar]
- 84.MacEdo AC et al. Is Sleep Disruption a Risk Factor for Alzheimer’s Disease?, Journal of Alzheimer’s Disease. (2017) [DOI] [PubMed] [Google Scholar]
- 85.Oliveira MF et al. (2015) Improvement of Central Sleep Apneas Following Ventricular Shunt for Normal Pressure Hydrocephalus. J. Neuropsychiatry Clin. Neurosci 27, e206–e208 [DOI] [PubMed] [Google Scholar]
- 86.Williams MA and Malm J (2005) Diagnosis and Treatment of Idiopathic Normal Pressure Hydrocephalus. - PubMed - NCBI. Continuum (Minneap. Minn) 57, 4–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim MJ et al. (2011) Differential diagnosis of idiopathic normal pressure hydrocephalus from other dementias using diffusion tensor imaging. Am. J. Neuroradiol 32, 1496–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Eide PK and Sorteberg W (2016) Outcome of Surgery for Idiopathic Normal Pressure Hydrocephalus: Role of Preoperative Static and Pulsatile Intracranial Pressure. World Neurosurg. DOI: 10.1016/j.wneu.2015.09.067 [DOI] [PubMed] [Google Scholar]
- 89.Kulkarni AV et al. (2013) Outcomes of CSF shunting in children: Comparison of Hydrocephalus Clinical Research Network cohort with historical controls. J. Neurosurg. Pediatr DOI: 10.3171/2013.7.PEDS12637 [DOI] [PubMed] [Google Scholar]