Abstract
Immune-related adverse events (IRAEs) are becoming increasingly common as use of immune checkpoint inhibitors (ICIs) expands into more tumor types and treatment settings. Although the majority of IRAEs are mild and can be managed in the outpatient setting by the medical oncologist, severe IRAEs can be life-threatening and often require complex care coordination among multiple providers. These providers include a variety of non-oncology specialists who have interest and expertise in managing IRAEs. Multiple systems-based solutions have been proposed in the literature, but these need to be tailored to the needs and resources of each practice setting. In this article, we highlight the challenges of IRAE care by presenting an illustrative case from our institution. We then describe the format and structure of the IRAE Tumor Board established at the University of Washington/Seattle Cancer Care Alliance/Fred Hutchinson Cancer Research Center. Finally, we discuss how this tumor board attempts to address clinical issues related to complex IRAE presentations and provide IRAE education.
1. Introduction
Immune checkpoint inhibitors (ICIs) have become standard therapies as well as commonly used investigational agents in a variety of tumor types. ICIs hold the potential for rapid and durable responses through the inhibition of immune checkpoints, such as PD-1/PD-L1 or CTLA-4/CD80, which regulate and prevent excessive immune responses. With the success of these agents in the palliative treatment of several malignancies, there has been a movement towards developing these therapies in the curative-intent setting, which means an increasing number of patients may receive these agents as part of their treatment.
However, the toxicity profile of ICIs is unique and can cause significant morbidity and mortality. Immune-related adverse events (IRAEs) are thought to be due to off-target immune responses against host tissues. These immune responses can affect any organ, but most commonly affect the skin, gut, liver, lungs, and thyroid (1). IRAEs are quite common, affecting 61–86% of patients receiving single agent ICI and up to 96% of patients receiving dual checkpoint inhibition, e.g. with ipilimumab/nivolumab (2). Severe IRAEs (grade 3 or higher) vary in incidence from 6–28% with PD-1 or PD-L1 monotherapy and 46–59% with ipilimumab/nivolumab (2). Treatment is based on IRAE severity, and it can range from symptomatic management for mild IRAEs to holding ICI therapy and starting systemic steroids (and other immunosuppressive agents as needed) for more severe IRAEs.
Choosing an immunotherapy regimen for an individual patient must balance the risks of toxicity with the likelihood of clinical benefit. Dual immunotherapy with ipilimumab 3 mg/kg plus nivolumab 1 mg/kg has both a higher objective response rate and a higher 5-year survival when compared to single-agent immunotherapy (3, 4). However, while permanent discontinuation due to life-threatening toxicity is relatively rare with ICI monotherapy (3–12%), it is more common with ipilimumab/nivolumab (22–39%) (2). Adjusting the dosing of the regimen to ipilimumab 1 mg/kg plus nivolumab 3 mg/kg does mitigate a portion of the toxicity (permanent discontinuation rate 24% vs 33%) with similar treatment outcomes in patients with melanoma, but the study was not designed to assess non-inferiority (5). Other strategies that have been employed to reduce toxicity include increasing the interval between immunotherapy doses and/or limiting the number of anti-CTLA4 doses. To date, there are no predictive biomarkers for severe IRAE toxicity, and we are limited to judging an individual patient’s risk for life-threatening toxicity based on the treatment regimen selected, patient or family history of autoimmune/inflammatory conditions, and history of prior IRAEs (6, 7).
Wider incorporation of ICIs into oncology treatment has resulted in IRAEs becoming increasingly relevant to non-oncology specialties, such as emergency and hospital medicine. In a case series that reviewed the prevalence of IRAEs requiring inpatient admission at a single center over a 6-month period in 2017, 5% of all oncology admissions were related to IRAEs; 13% of these admissions resulted in death, and 91% of these admissions required a medicine subspecialty consultation (8). This data highlights both the potential high acuity of patients who develop severe IRAEs and the need for well-coordinated interdisciplinary care to optimally manage these patients. However, coordinating care for these patients can be challenging in the outpatient setting, where timely access to subspecialists with expertise in IRAE management can be quite limited, especially in non-academic practices. In addition, non-oncology providers are often providing the first-line of care for these patients, making it essential to educate these providers about the diverse and potentially subtle presentations of IRAEs to ensure early diagnosis and appropriate management.
Addressing these new challenges requires a tailored approach for each individual institution or practice depending on the location, infrastructure, and resources of that particular area. The University of Washington/Seattle Cancer Care Alliance/Fred Hutchinson Cancer Research Center (UW/SCCA/FHCRC) is a tertiary care center located in an urban environment that serves a multi-state catchment area. To promote education and interdisciplinary care around IRAEs, we have developed a multispecialty IRAE tumor board with robust participation of both oncology and non-oncology services. In this article, we present an illustrative case to highlight the scope, structure, format, and potential benefits of this IRAE tumor board, then discuss the infrastructure innovations and solutions implemented at other institutions and described in the literature.
2. Case Presentation
A 62-year-old man with HER2-negative gastroesophageal adenocarcinoma had metastases in the mediastinal and retroperitoneal lymph nodes, pleura, liver, and bone. His previous treatments included: 1) FOLFOX x 6 cycles followed by avelumab on a clinical trial, discontinued for disease progression after 3 cycles of avelumab; 2) paclitaxel and ramucirumab, paclitaxel discontinued after 3 cycles due to neuropathy and ramucirumab discontinued after 11 total cycles for disease progression. While on avelumab, he developed subclinical (asymptomatic) hyperthyroidism with a thyroid-stimulating hormone (TSH) of 0.149 μIU/mL and a free thyroxine (T4) 1.1 ng/dL (see Supplementary Table 1 for reference ranges); TSH normalized 4 months after discontinuing avelumab. Other past medical history was notable for chronic musculoskeletal pain related to injuries sustained at work. Medications included pain medications, bowel medications, and a proton pump inhibitor. The patient had a distant history of smoking (had quit 35 years prior to diagnosis) and a prior history of heavy alcohol use (quit at time of cancer diagnosis). His family history was notable for lung cancer in his father and esophageal cancer in his paternal uncle, both of whom were smokers, with no family history of autoimmune disease noted.
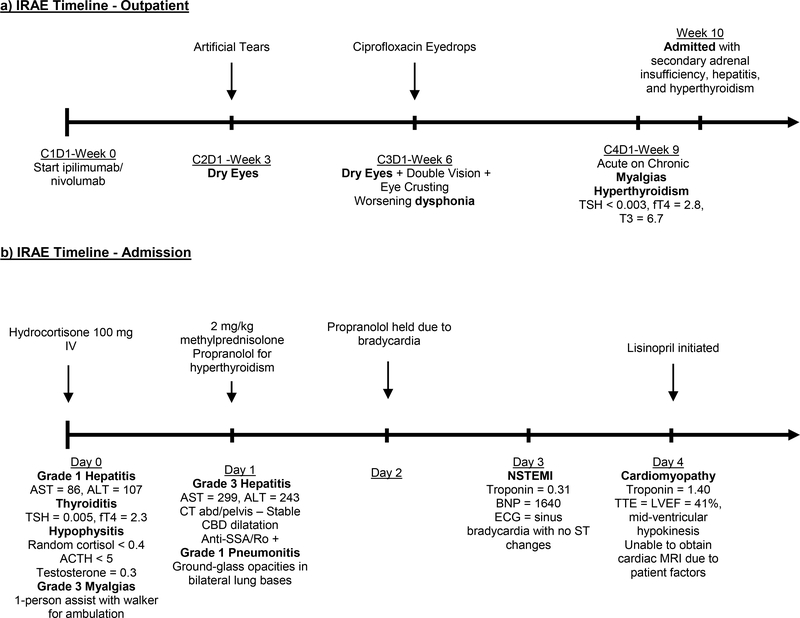
The patient started treatment with ipilimumab (3 mg/kg) plus nivolumab (1 mg/kg) administered every 3 weeks on a clinical trial. His thyroid function testing prior to treatment was normal (TSH = 4.451 μIU/mL, free T4 = 0.8 ng/dL, free triiodothyronine (T3) = 2.9 pg/mL). He noted dry eyes and hoarseness after the first cycle (Figure 1a). He was prescribed artificial tears and later ciprofloxacin ophthalmic solution for his ocular symptoms. Otolaryngology felt his dysphonia was related to a viral illness. After 3 cycles of immunotherapy, restaging imaging showed a partial response. He presented for his fourth cycle of treatment reporting worsening generalized myalgias. A respiratory virus nasal swab was positive for rhinovirus. Laboratory studies were concerning for thyrotoxicosis (low TSH <0.003 μIU/mL, high free T4 2.6 ng/dL, high free T3 6.7 pg/mL), but he was asymptomatic. He continued trial therapy. Three days after cycle #4, he had further worsening of myalgias. He was seen by his primary care provider, who referred him to a local emergency department due to dizziness, generalized weakness, and hypotension. He was diagnosed with a viral illness and mild dehydration, received intravenous fluids, then discharged home. He presented for follow-up with his medical oncologist several days later. The patient noted continued worsening of myalgias to the point that it took about an hour for him to dress himself. He presented to clinic appearing unwell and pale, and he was noted to be hypotensive with a blood pressure of 88/61. Remainder of physical exam was unrevealing.
Figure 1. Immune-related adverse events (IRAE) Timeline.
divided by outpatient (a) and inpatient (b) setting. CxDy = cycle #x, day #y. TSH = thyroid stimulating hormone, fT4 = free thyroxine, IV = intravenous, mg/kg = milligrams per kilogram, AST = aspartate aminotransferase, ALT = alanine aminotransferase, ACTH = adrenocorticotropic hormone, CT = computed tomography, CBD = common bile duct, NSTEMI = non-ST elevation myocardial infarction, BNP = B-type natriuretic peptide, ECG = electrocardiogram, TTE = transthoracic echocardiogram, EF = ejection fraction.
He was transferred to the emergency department (Figure 1b). His vital signs and exam were unchanged from clinic. His laboratory evaluation was notable for normal white blood cell count, anemia (hemoglobin 8.5 g/dL), mild hyponatremia (sodium 133 mEq/L), hypercalcemia (corrected calcium = 11.5 mg/dL), newly noted transaminitis with aspartate aminotransferase (AST) 86 U/L, alanine aminotransferase (ALT) 107 U/L, and normal total bilirubin 0.5 mg/dL. Lactate and creatine kinase levels were normal. A random cortisol and adrenocorticotrophic hormone (ACTH) levels were undetectable. Testosterone levels were low, and prolactin, follicle stimulating hormone, and luteinizing hormone levels were at the lower end of normal range. Hypophysitis was suspected. He received 1.5 liters of hydration with no improvement in blood pressure. He received 100 mg of hydrocortisone in the emergency department and was admitted to the hospital. Blood pressure normalized after administration of hydrocortisone. Overnight, his transaminases rose to AST 299 U/L, ALT 243 U/L and total bilirubin rose to 1.1 mg/dL. Methylprednisolone 2 mg/kg was initiated for treatment of suspected grade 3 immune-mediated hepatitis.
He was evaluated by hepatology, endocrinology, ophthalmology, and physical therapy on hospital day 1. Imaging to evaluate his hepatitis showed stable biliary dilatation, no new biliary obstruction, and an incidental finding of ground-glass opacities in the bilateral lung bases that were favored to be infectious or inflammatory. Sites of cancer showed a continued partial response with no significant change from restaging imaging a month prior. Hepatitis B and C virus serologies were negative and iron studies were within normal limits. An anti-nuclear antibody test was positive (speckled pattern, 1:80 titer, anti-SSA/Ro). The patient required a one-person assist and a walker to ambulate 20 feet. Endocrinology recommended a trial of propranolol due to the patient experiencing palpitations; a thyroid autoantibody panel to evaluate for Graves’ disease was negative. MRI pituitary protocol was recommended but deferred as it would not change management. Ophthalmology recommended continued artificial tear use for meibomian gland dysfunction. His transaminases and bilirubin were rapidly trending downward on hospital day 2.
On hospital day 3, he developed bradycardia with heart rates in the 50s and propranolol was held. Bradycardia persisted despite holding the beta-blocker, and an ECG, troponin, and b-type natriuretic peptide (BNP) were checked. ECG showed sinus bradycardia with no ST changes, troponin-I was 0.31 ng/ml (normal <0.04 ng/mL), and BNP was 1360 pg/mL (normal <101 pg/mL). The patient endorsed new pleuritic chest pain with associated shortness of breath that improved when leaning forward. Chest x-ray showed mild pulmonary edema. Cardiology was consulted. Echocardiogram revealed a decreased left ventricular ejection fraction of 41% with a pattern of mid-ventricular hypokinesis/akinesis and no pericardial effusion. This was felt to be most consistent with stress-induced cardiomyopathy, though an atypical presentation of ICI-mediated myocarditis could not be excluded. His troponin peaked at 1.40 ng/ml within 12 hours of his first troponin and then trended downward; the chest pain self-resolved. Cardiac MRI was attempted to evaluate for ICI-mediated myocarditis, but the patient was not able to tolerate the exam. A coronary angiogram to rule out ischemic heart disease was done and negative. Based on the characteristic appearance of the echocardiogram, his clinical picture was felt to be most consistent with stress-induced cardiomyopathy rather than ICI-mediated myocarditis. It was recommended that he have a follow-up echocardiogram in 4 weeks to reevaluate as stress-induced cardiomyopathy would be expected to self-resolve with no intervention. He was discharged on hospital day 8 with a planned prednisone taper over 6 weeks for his grade 3 immune-related hepatitis, sulfamethoxazole-trimethoprim for Pneumocystis jiroveci prophylaxis, and continuation of a proton pump inhibitor. Hepatitis had improved to grade 1 by day of discharge. He remained free of chest pain. His weakness and myalgias were significantly improved, and he was able to ambulate independently without assistive device.
Six weeks later, he had successfully weaned the prednisone down to a dose of 7.5 mg/day and transaminases had completely normalized. A repeat echocardiogram showed resolution of the mid-ventricular hypokinesis and a normal left ventricular ejection fraction, thus supporting the prior working diagnosis of stress-induced cardiomyopathy. However, as expected after ICI-induced thyrotoxicosis, he developed laboratory evidence of hypothyroidism with a free T4 0.2 ng/dL and TSH 3.365 μIU/mL. He started levothyroxine replacement for hypothyroidism. Recent body imaging had shown a sustained partial response in his malignancy as well as resolution of the ground-glass opacities in his lung bases.
Because he had developed hepatitis on dual immunotherapy, which has a higher rate of immunotherapy-induced hepatitis compared to PD-1 inhibition alone(9, 10), he restarted nivolumab monotherapy after an 8-week immunotherapy hold with careful monitoring. At time of resuming ICI treatment, he was on prednisone 7.5 mg daily. He continued to taper steroids and transitioned to hydrocortisone with no increase in his transaminases. Restaging imaging 9 weeks after nivolumab resumption showed progression in his retroperitoneal lymph nodes but no change in his other metastatic sites; the patient elected to continue nivolumab. He did have an increase in his baseline myalgias, and he requested to transition back to prednisone 7.5 mg daily from the hydrocortisone with improvement in his pain.
Five months past his initial progression, he had imaging that revealed disease progression at all sites and was subsequently transitioned to chemotherapy with irinotecan. About 6 months after starting chemotherapy, he was hospitalized with transaminitis, bradycardia, hypotension, hypoglycemia, and profound fatigue; imaging showed cancer progression in all sites of disease. Laboratory evaluation included a morning cortisol that was low at 1.7 mcg/dL, a TSH that was high at 9.807 mIU/mL, and a free thyroxine was low at 0.5 ng/dL. The patient had read about the side effects of long-term steroids and self-discontinued his hormone replacement due to concerns about the side effects. He had clinical improvement with resumption of his hormone replacement, and he was subsequently discharged from the hospital. He unfortunately continued to have symptomatic cancer progression with a repeat admission and transitioned to hospice care.
3. Discussion
The use of ICI is becoming increasingly common, and it is estimated that 44% of all oncology patients were eligible to receive these agents in 2018 (11). As the number of patients treated with ICIs increases, IRAEs are also becoming more prevalent. Here, we presented a case of a patient treated with dual ICI blockade with nivolumab 1 mg/kg and ipilimumab 3 mg/kg who subsequently developed up to six IRAEs (thyrotoxicosis, hepatitis, hypophysitis, pneumonitis, sicca syndrome, and myalgias) as well as a presumed stress-induced cardiomyopathy. During his IRAE course, he was seen by his medical oncologist, primary care provider, emergency medicine, and the inpatient oncology service. He was also evaluated by four subspecialty services (endocrinology, hepatology, ophthalmology, cardiology) during his hospitalization and after discharge had appointments with Medical Oncology, Endocrinology, and Cardiology for follow-up care. After the diagnosis of his IRAEs, the patient was subsequently hospitalized when he became concerned about the hormone replacement for hypophysitis and self-discontinued it. This case highlights several practical and logistical challenges of managing these complex patients: 1) communication between oncology, non-oncology, and external providers that a patient is at risk for IRAEs, 2) early consideration of IRAEs as a potential cause of a patient’s symptoms, 3) coordination of care between multiple subspecialists and the medical oncologist during the process of IRAE diagnosis and treatment, 4) balanced discussion of the benefits vs risks of ICI re-challenge after significant improvement or resolution of IRAE, and 5) need for early, rigorous, and continued patient education around IRAEs, even after the acute toxicity has subsided.
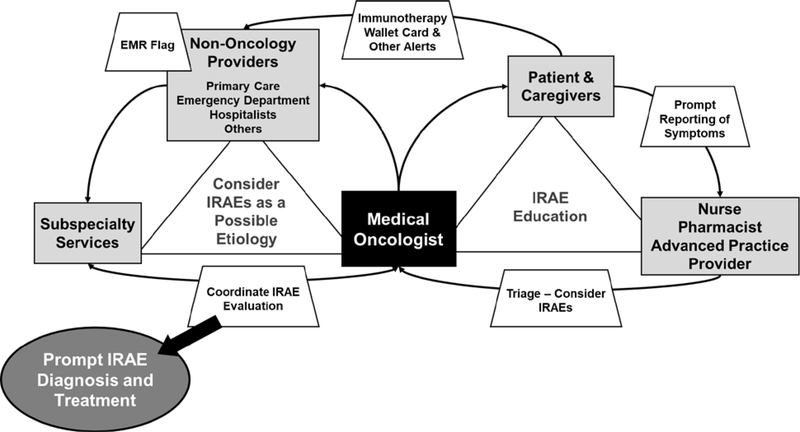
Figure 2 illustrates the communication complexities of diagnosing and managing IRAEs. The primary medical oncologist serves as a central hub to educate, communicate, and coordinate care for a patient regarding IRAE recognition, diagnosis, and management. Ancillary providers such as nursing, pharmacy, and advanced practice practitioners can also play a key role in patient teaching, particularly around the patient notifying the oncology team of new or worsening symptoms and in symptom triage (12, 13). In the case presented, the patient received treatment with combination ipilimumab/nivolumab, which is associated with a significantly higher risk of moderate to severe toxicity. When the patient had worsening myalgias, he presented to his primary care doctor for evaluation, but he did not notify his oncology team until he presented to the oncology clinic several days later. This highlights the importance of teaching patients receiving immunotherapy to call with any changes in symptoms, even symptoms that mirror a patient’s pre-existing musculoskeletal issues. In addition, nursing and pharmacy can provide education and reinforcement regarding the importance of medication compliance, particularly for medications like levothyroxine and hydrocortisone, which are chronic (and likely life-long) medications that should not be discontinued without the recommendation of a medical provider. Further education on timely symptom reporting and medication compliance may shorten or prevent hospital admissions, decrease morbidity, and decrease healthcare utilization.
Figure 2. Communication schema.
for effective immune-related adverse event (IRAE) diagnosis and management. Education around IRAEs for both the patient and care providers as well as a high degree of suspicion for IRAEs are the essential tenets to effective IRAE management. The medical oncologist is a central figure in both the recognition and evaluation of IRAEs as the common point of contact for the patient and the other care team members. Additional strategies, such as patient wallet cards and electronic medical record (EMR) flags, can be helpful for notifying non-oncology providers that the patient is receiving or has received immunotherapy and IRAEs should be considered in the differential diagnosis.
Communication and education of non-oncology providers around IRAEs is also very important. It is difficult to suspect an IRAE if the provider is unaware that the patient has received or is receiving immunotherapy. As in this patient case, the first presentation of an IRAE may be to an external facility, such as a primary care clinic or emergency department rather than the oncology clinic. Patients and caregivers need to alert any provider they may see about a history of immunotherapy receipt, history of IRAEs, and history of current or recent steroid use. Providing patients with a wallet card, such as the card developed by the Oncology Nursing Society, can be critical for expediting care (14). A number of health care systems have also implemented a flag in the electronic medical record (EMR) that will notify providers opening the chart that the patient has previously received an immunotherapy agent (14). All of these approaches are designed to notify the current provider of immunotherapy exposure to optimize recognition of a potential IRAE.
Once the potential for an IRAE is established, the diagnosis and treatment of IRAEs requires consideration of the full differential diagnosis. This may require the expertise of multiple subspecialty providers to ensure that the appropriate laboratory and imaging evaluation is obtained. In the presented case, the patient required additional laboratory and imaging evaluation during his admission to diagnose an IRAE in the case of hypophysitis, thyrotoxicosis, and hepatitis, but also an additional evaluation for a non-ST elevation myocardial infarction and cardiomyopathy. Obtaining multiple opinions can be more straightforward in the inpatient setting, but many patients that develop IRAEs are managed in the ambulatory setting. Direct and rapid communication between the oncology team and the subspecialists can be helpful to facilitate an effective evaluation plan, particularly if the subspecialists are less familiar with IRAEs. This type of complex care coordination can be time-consuming and inefficient, however, and pre-identifying a designated group of subspecialists with expertise and interest in IRAEs can streamline care.
There are multiple potential strategies to addressing this difficulty. A number of institutions have developed specialized IRAE clinics and inpatient consultation services. These services and clinics have designated “physician champions” from the subspecialty services to promote prompt evaluation and initiation of treatment. At Johns Hopkins University, the immunotherapy rapid response team is an electronic consult service that can quickly provide expert recommendations, while at Massachusetts General Hospital there is a specific inpatient consult service for severe IRAEs (14, 15). These types of services can also assist in identifying patients with IRAEs for research studies with the appropriate institutional regulatory approvals. In addition, professional societies have created published guidelines for IRAE education, diagnosis, and treatment through expert and evidence-based recommendations (16–19).
At our institution, we have developed an IRAE Tumor Board to address a number of these issues. Our tumor board is similar to other IRAE tumor boards that have been developed at institutions like Cleveland Clinic (20). The primary missions of this tumor board are to a) discuss complex and challenging IRAE cases in the context of the literature with a forum of oncologists and subspecialty experts, b) provide educational material on timely work up, accurate diagnosis, and management of IRAEs to both oncology and non-oncology providers, and c) develop and discuss ideas for quality improvement initiatives and projects to improve outcomes for patients treated with ICI and those who develop IRAEs. The tumor board also presents opportunities to develop basic science, translational, and clinical research projects focused on understanding the mechanism of IRAEs, identifying risk factors for IRAEs, as well as creating databases and biorepositories for IRAE-related research. We present as many as three cases every month at our IRAE tumor board. We have both in-person and remote attendance by oncologists, advanced practice practitioners, nurses, pharmacists, community affiliate oncology providers, and relevant non-oncology subspecialists. Two members of the administrative team, a Hematology-Oncology fellow, and a Medical Oncology faculty member are responsible for 1) requesting, collecting, and reviewing IRAE cases, 2) identifying speakers and discussants, 3) reviewing presentation slides, and 4) directing the flow of each Tumor Board to ensure regulatory compliance and appropriate time allocation for adequate multispecialty discussion.
This one-hour live interactive venue allows direct discussion and interaction between oncologists and non-oncology subspecialists about a given case. There is a wide range of IRAE cases that have been presented by providers at our institution, including, but not limited to, dermatitis, hepatitis, hypophysitis, myocarditis, and neurological complications. We also discuss challenging cases in terms of steroid-refractory IRAEs, decisions about ICI re-challenge, and initiation of ICI therapy in a patient that would have been excluded from clinical trials (e.g. patient with a solid organ allograft or autoimmune conditions (2, 21)). For each case, we review the applicable expert recommendations and guidelines by National Cancer Care Network (NCCN) and other professional societies to reinforce evidence-based care in the diagnosis, treatment, and follow-up of IRAEs. We allow remote/virtual participation and de-identify the cases so the tumor board can serve as a resource for the broader oncology community in the Northwest region (Washington, Idaho, Montana, and Alaska), while still preserving patient privacy and confidentiality.
A key component of this tumor board has been the persistent and ongoing engagement of our subspecialty providers, including cardiology, pulmonology, dermatology, endocrinology, gastroenterology, hepatology, neurology, nephrology, ophthalmology, infectious diseases, rheumatology, radiology, and others. These subspecialists provide recommendations around appropriate diagnostic work-up for potential IRAEs, recommendations for the management of IRAEs based on the current guidelines, and expertise in selecting additional immunosuppressive agents for steroid-refractory IRAEs. These subspecialty providers are our institution’s designated IRAE experts, and they may serve as a resource when an immediate clinical question arises in the care of a patient. This allows our institution to facilitate prompt diagnosis, treatment, and follow-up appointments for patients experiencing IRAEs.
The tumor board also provides an educational forum for trainees from all specialties to learn about IRAEs. The tumor board was initially implemented with a hematology-oncology fellow as a co-leader, and it remains co-led and operationally co-organized by a hematology-oncology fellow. As a result of the tumor board, trainees have participated in quality improvement and research projects focused on IRAEs.
The patient case included in this review was presented at the IRAE tumor board after the patient was discharged from the hospital. Most of the discussion focused on strategies to improve early recognition of IRAEs, such as endocrine dysfunction and myocarditis, strategies to facilitate proper IRAE management, and strategies to reduce potentially preventable hospital admissions. Endocrinology provided valuable input on the benefits versus disadvantages of random cortisol level monitoring in patients starting ICI therapy. This prompted a broader discussion in our institution about harmonization of ICI electronic order-sets. Cardiology also discussed the work-up for ICI-induced myocarditis, the benefits of cardiac MRI for the diagnosis, and why this patient’s echocardiogram findings and reduced left ventricular ejection fraction were ultimately most consistent with stress-induced cardiomyopathy.
There was also discussion around if this patient should be re-challenged with immunotherapy after he had recovered sufficiently from his IRAEs. During his hospitalization, the patient had both grade 3 hypophysitis and grade 3 hepatitis. Although his hypophysitis was severe and required hospitalization, there were less concerns that immunotherapy re-initiation would cause notable recurrent symptoms because the patient was taking hormone replacement. As noted in the case presentation above, dual immune checkpoint blockade is associated with a higher rate of IRAE hepatitis(9, 10), but it may be reasonable to resume ICI monotherapy after the hepatitis has improved to a grade 1 or lower toxicity. In the case of this patient, who had had a partial response after 4 cycles of therapy that was maintained during the period off immunotherapy, it is unclear based on current evidence if additional anti-PD1 treatment provides additional anti-cancer benefit. The general consensus amongst the group was that either active surveillance or ICI re-challenge would be reasonable, and the patient was later re-challenged successfully with no recurrence of the hepatitis.
Optimizing the care of patients who develop severe IRAEs is a significant logistical challenge that must be tailored to the practice setting and resources available for a given institution or facility. For our institution, the IRAE tumor board has been a valuable tool to facilitate IRAE care, provide IRAE education, and generate discussion around IRAE research questions and quality improvement issues. A key first step in creating the tumor board was the identification of a team of physician champions from within both the oncology group and the relevant non-oncology subspecialties. The consistent engagement of these IRAE experts has promoted excellent discussion, suggestions, and research questions at the tumor board. Active participation by trainees in the tumor board has also contributed to its success. Fellows and residents contribute and present cases for discussion with active mentorship from an attending oncologist. Moreover, advanced practice providers, nurses, pharmacists, research staff, and other team members also can be both active presenters and participants. Finally, the de-identification of cases and the inclusion of a virtual/remote option for participation allows the tumor board to serve as a resource to other oncology providers in the UW/SCCA/FHCRC community network. The main limitation of this format is the monthly frequency; clinical questions of immediate need are better routed directly to the relevant IRAE expert subspecialists. However, despite the latency in feedback, discussion of the case at the next tumor board still has value from a clinical, quality improvement, educational, and research standpoint for both the presenter and attendees.
4. Conclusions
Immuno-oncology drugs are increasingly being used and may cause isolated or multisystem IRAEs with either early or delayed onset. Early recognition is a key aspect in the management of IRAEs as timely treatment may reduce the risk of subsequent complications. Patients who develop IRAEs benefit from collaborative and multidisciplinary care for the timely diagnosis and the optimal management of these toxicities. IRAE tumor boards provide an educational forum for multidisciplinary interaction that can lead to more effective and evidence-based care as well as the development of collaborative research and quality improvement initiatives among oncology and non-oncology experts.
Supplementary Material
Key Points.
Severe IRAEs present challenges in provider awareness, patient education, and care coordination
Systems-based solutions are critical to ensuring optimal care for patients experiencing severe IRAEs
An IRAE Tumor Board has provided significant value for our institution in managing IRAEs, educating providers and trainees around IRAEs, and providing a forum for multidisciplinary IRAE discussion
Acknowledgments
Funding: This manuscript required no funding support.
Training support for LC Kennedy, N Kamat, and AR Khaki was provided by the NCI (T32 CA009515).
Conflicts of interest/Competing Interest: LC Kennedy, K Wong, N Kamat, AR Khaki, and JA Thompson have no conflict of interest disclosures. S Bhatia reports advisory board participation (with honorarium) from Genentech, EMD-Serono, Bristol-Myers-Squibb (BMS), and Sanofi Genzyme and research funding to his institution (University of Washington) from Oncosec, EMD-Serono, Merck, BMS, NantKwest, Immune Design, Novartis, Nektar, and Exicure. P Grivas has done unrelated consulting with AstraZeneca; Bayer; Biocept; Bristol-Myers Squibb; Clovis Oncology; Driver; EMD Serono; Exelixis; Foundation Medicine; Genentech; Genzyme; GlaxoSmithKline; Heron Therapeutics; Janssen; Merck; Mirati Therapeutics; Pfizer; Seattle Genetics; QED Therapeutics; Roche; and has received institutional research funding from AstraZeneca, Bayer; Genentech/Roche; Merck; Mirati Therapeutics; Oncogenex; Pfizer, Clovis Oncology, Bavarian Nordic, Immunomedics, Debiopharm, Bristol-Myers Squibb, Kure It Cancer Research, QED Therapeutics (in the last 36 months).
Footnotes
Declarations
Ethics approval: STUDY00008393
Consent to participate: N/A
Consent to publication: N/A
Availability of Data and Material: N/A
Code Availability: N/A
6. References
- 1.Michot JM, Bigenwald C, Champiat S, Collins M, Carbonnel F, Postel-Vinay S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139–48. [DOI] [PubMed] [Google Scholar]
- 2.Kennedy LC, Bhatia S, Thompson JA, Grivas P. Preexisting Autoimmune Disease: Implications for Immune Checkpoint Inhibitor Therapy in Solid Tumors. J Natl Compr Canc Netw. 2019;17(6):750–7. [DOI] [PubMed] [Google Scholar]
- 3.Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 2017;377(14):1345–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med. 2019;381(16):1535–46. [DOI] [PubMed] [Google Scholar]
- 5.Lebbé C, Meyer N, Mortier L, Marquez-Rodas I, Robert C, Rutkowski P, et al. Evaluation of Two Dosing Regimens for Nivolumab in Combination With Ipilimumab in Patients With Advanced Melanoma: Results From the Phase IIIb/IV CheckMate 511 Trial. J Clin Oncol. 2019;37(11):867–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tison A, Quéré G, Misery L, Funck-Brentano E, Danlos FX, Routier E, et al. Safety and Efficacy of Immune Checkpoint Inhibitors in Patients With Cancer and Preexisting Autoimmune Disease: A Nationwide, Multicenter Cohort Study. Arthritis Rheumatol. 2019;71(12):2100–11. [DOI] [PubMed] [Google Scholar]
- 7.Haanen J, Ernstoff MS, Wang Y, Menzies AM, Puzanov I, Grivas P, et al. Autoimmune diseases and immune-checkpoint inhibitors for cancer therapy: Review of the literature and personalized risk-based prevention strategy. Ann Oncol. 2020. [DOI] [PubMed] [Google Scholar]
- 8.Balaji A, Zhang J, Wills B, Marrone KA, Elmariah H, Yarchoan M, et al. Immune-Related Adverse Events Requiring Hospitalization: Spectrum of Toxicity, Treatment, and Outcomes. J Oncol Pract. 2019:JOP1800703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reynolds K, Thomas M, Dougan M. Diagnosis and Management of Hepatitis in Patients on Checkpoint Blockade. Oncologist. 2018;23(9):991–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larkin J, Hodi FS, Wolchok JD. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373(13):1270–1. [DOI] [PubMed] [Google Scholar]
- 11.Haslam A, Prasad V. Estimation of the Percentage of US Patients With Cancer Who Are Eligible for and Respond to Checkpoint Inhibitor Immunotherapy Drugs. JAMA Netw Open. 2019;2(5):e192535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lasa-Blandon M, Stasi K, Hehir A, Fischer-Cartlidge E. Patient Education Issues and Strategies Associated With Immunotherapy. Semin Oncol Nurs. 2019;35(5):150933. [DOI] [PubMed] [Google Scholar]
- 13.Renna CE, Dow EN, Bergsbaken JJ, Leal TA. Expansion of pharmacist clinical services to optimize the management of immune checkpoint inhibitor toxicities. J Oncol Pharm Pract. 2019;25(4):954–60. [DOI] [PubMed] [Google Scholar]
- 14.Cole S, Zibelman M, Bertino E, Yucebay F, Reynolds K. Managing Immuno-Oncology Toxicity: Top 10 Innovative Institutional Solutions. Am Soc Clin Oncol Educ Book. 2019;39:96–104. [DOI] [PubMed] [Google Scholar]
- 15.Naidoo J, Zhang J, Lipson EJ, Forde PM, Suresh K, Moseley KF, et al. A Multidisciplinary Toxicity Team for Cancer Immunotherapy-Related Adverse Events. J Natl Compr Canc Netw. 2019;17(6):712–20. [DOI] [PubMed] [Google Scholar]
- 16.Thompson JA, Schneider BJ, Brahmer J, Andrews S, Armand P, Bhatia S, et al. Management of Immunotherapy-Related Toxicities, Version 1.2019. J Natl Compr Canc Netw. 2019;17(3):255–89. [DOI] [PubMed] [Google Scholar]
- 17.Haanen JBAG, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl_4):iv119–iv42. [DOI] [PubMed] [Google Scholar]
- 18.Puzanov I, Diab A, Abdallah K, Bingham CO, Brogdon C, Dadu R, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017;5(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36(17):1714–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patil PD, Fernandez AP, Velcheti V, Tarhini A, Funchain P, Rini B, et al. Cases from the irAE Tumor Board: A Multidisciplinary Approach to a Patient Treated with Immune Checkpoint Blockade Who Presented with a New Rash. Oncologist. 2019;24(1):4–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richter MD, Pinkston O, Kottschade LA, Finnes HD, Markovic SN, Thanarajasingam U. Brief Report: Cancer Immunotherapy in Patients With Preexisting Rheumatic Disease: The Mayo Clinic Experience. Arthritis Rheumatol. 2018;70(3):356–60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.