Abstract
Colorectal cancer (CRC) is one of the most common cancers worldwide. Epidemiological studies indicate that consumption of fruits and vegetables containing procyanidins is associated with lower CRC risk. This study investigated the capacity of two dimeric procyanidins composed of epicatechin gallate (ECG) or epigallocatechin gallate (EGCG) isolated from persimmons, to inhibit CRC cell growth and promote apoptosis, characterizing the underlying mechanisms. ECG and EGCG dimers reduced the growth of five human CRC cell lines in a concentration (10–60 μM)- and time (24–72 h)- dependent manner, with a 72h-IC50 value in Caco-2 cells of 10 and 30 μM, respectively. ECG and EGCG dimers inhibited Caco-2 cell proliferation by arresting the cell cycle in G2/M phase and by inducing apoptosis via the mitochondrial pathway. In addition, ECG and EGCG dimers inhibited cell migration, invasion, and adhesion, decreasing the activity of matrix metalloproteinases (MMP-2/9). Mechanistically, ECG and EGCG dimers inhibited the activation of lipid raft-associated epidermal growth factor (EGF) receptor (EGFR), without affecting its localization at lipid rafts. In particular, ECG and EGCG dimers reduced EGFR phosphorylation at Tyr1068 residue, prevented EGFR dimerization and activation upon (EGF) stimulation, and induced EGFR internalization both in the absence and presence of EGF. Furthermore, ECG and EGCG dimers increased EGFR phosphorylation at Tyr1045 residue, providing a docking site for ubiquitin ligase c-Cbl and induced EGFR degradation by the proteasome. Downstream of EGFR, ECG and EGCG dimers inhibited the activation of the MEK/ERK1/2 and PI3K/Akt signaling pathways, downregulating proteins involved in the modulation of cell survival. In conclusion, ECG and EGCG dimers reduced CRC cell growth, by inhibiting EGFR activation at multiple steps, including the disruption of lipid rafts integrity and promoting EGFR degradation. These results shed light on a potential molecular mechanism on how procyanidins-rich diets may lower CRC risk.
Keywords: ECG and EGCG dimers, Colorectal cancer, Apoptosis, Lipid rafts, EGFR signaling
1. Introduction
Colorectal cancer (CRC) is the third most common cancer diagnosed and the second-highest cause of cancer-related deaths in the US, accounting for over 1 million new cases and about 0.5 million deaths each year based on data from the American Cancer Society’s Cancer Statistics Center (https://cancerstatisticscenter.cancer.org). The global burden of CRC is expected to increase by 60% to more than 2.2 million new cases and 1.1 million deaths by 2030 (Arnold, Sierra et al. 2017). Surgery remains the most effective curative treatment for CRC, but the risk of recurrence is high. Although the development of new therapeutic approaches and the implementation of early detection tests have lowered the incidence and improved the prognosis of CRC, still about 40% of diagnosed patients die from metastasis (Siegel, Miller et al. 2017).
While several risk factors have been identified for CRC, no single one can account for their substantial extrinsic risk proportions, suggesting complex mechanisms for CRC etiologies (Wu, Powers et al. 2016). In this regard, CRC is widely considered to be an environmental disease (Baena and Salinas 2015), with smoking, alcohol consumption, obesity, unhealthy diets and less physical activity constituting important environmental factors linked to increased CRC risk. In particular, diet is the most important risk factor apart from age and hereditary factors, being responsible for 35% of CRC (Baena and Salinas 2015). Thus, the search for potential bioactives present in human diet for the prevention of CRC is highly relevant.
Epidermal growth factor (EGF) and Insulin-like growth factor 1 (IGF1) receptors (EGFR, IGF1R) are members of the receptor tyrosine kinases (RTKs) family. Although these receptors are essential for normal cellular functions, they are both overexpressed and hyperactivated in many types of cancers, including CRC (Guo, Cai et al. 2011). They promote cancer cell proliferation, differentiation, survival, motility, invasion, adhesion, DNA repair, and metastasis (Sigismund, Avanzato et al. 2018). The activation of EGFR/IGF1R triggers a chain of downstream signaling cascades including PI3K/AKT and MEK/ERK1/2 pathways which mediate cell proliferation and survival (Seshacharyulu, Ponnusamy et al. 2012). Thus, blocking of EGFR or IGF1R signaling can abrogate anchorage-independent growth, survival, oncogenic transformation and metastasis (Oberthür, Seemann et al. 2017, Khan, Valeri et al. 2019).
An important characteristic of the EGFR and IGF1R is their enrichment in certain areas of the cell membrane. In particular, EGFR and IGF1R are located in lipid rafts domains, which play a critical role as signal processing hubs (Shimizu, Adachi et al. 2011). Human CRC cells often display overexpression of EGFR and IGF1R in lipid rafts. The constitutive activation of these receptors and related downstream signaling pathways occurs in the early stages of human colorectal carcinogenesis (Wee and Wang 2017). Therefore, targeting these RTKs and their downstream pathways or lipid rafts domains may be a potentially effective strategy for the prevention and, in certain cases, treatment of CRC.
Epidemiological and clinical studies provide strong evidence that diets rich in fruits, vegetables, and fiber lower the risk of CRC (Terry, Giovannucci et al. 2001, Lin, Zhang et al. 2005, Aune, Lau et al. 2011, Ben, Sun et al. 2014, Reddy 2018). Procyanidins, which are abundantly present in fruits and vegetables such as berries, apples, persimmons, and tea, have been shown to have health beneficial effects at the gastrointestinal tract (Oteiza, Fraga et al. 2018). A higher dietary procyanidins consumption has been associated with a lower risk of CRC in human populations (Rossi, Negri et al. 2010). In vitro, cocoa hexameric procyanidins promote CRC cell apoptosis and inhibit cell cycle progression (Choy, Fraga et al. 2016). Procyanidin extracts from grape seed, apples and cocoa also inhibit both in vitro and in vivo the growth of human CRC cells (Carnesecchi, Schneider et al. 2002, Gosse, Guyot et al. 2005, Kaur, Singh et al. 2006). Hexameric procyanidins interact with lipid rafts, inhibit ERK1/2 and exert anti-CRC actions (Da Silva, Jaggers et al. 2012, Verstraeten, Jaggers et al. 2013, Choy, Fraga et al. 2016). Moreover, recent studies have revealed that several phytochemicals exert antitumor activity by suppressing the activation of the EGFR and their downstream effectors in cancer cells (Shimizu, Adachi et al. 2011, Ma, Li et al. 2014).
Our group has recently isolated and purified dimeric procyanidins from persimmons, consisting of epicatechin-3-gallate (ECG) and epigallocatechin-3-gallate (EGCG) subunits, linked by 4β→8 and 2β→O→7 bonds (Fig. 1A). Previously, we have shown that ECG and EGCG dimers interact with lipid rafts, affecting their physical properties and structural integrity in 3T3-L1 preadipocytes, thus exerting anti-differentiation effect (Zhu, Zou et al. 2015, Zhu, Deng et al. 2017). Given the above, in this work, we investigated the anticancer effect of ECG and EGCG dimers in human CRC cell lines, characterizing the involvement of lipid rafts-associated EGFR and IGF1R and downstream signaling cascades. The results suggest that ECG and EGCG dimers inhibit CRC cell growth and induce cell apoptosis, which may be associated with their actions at lipid rafts and the inhibition of the EGFR signaling.
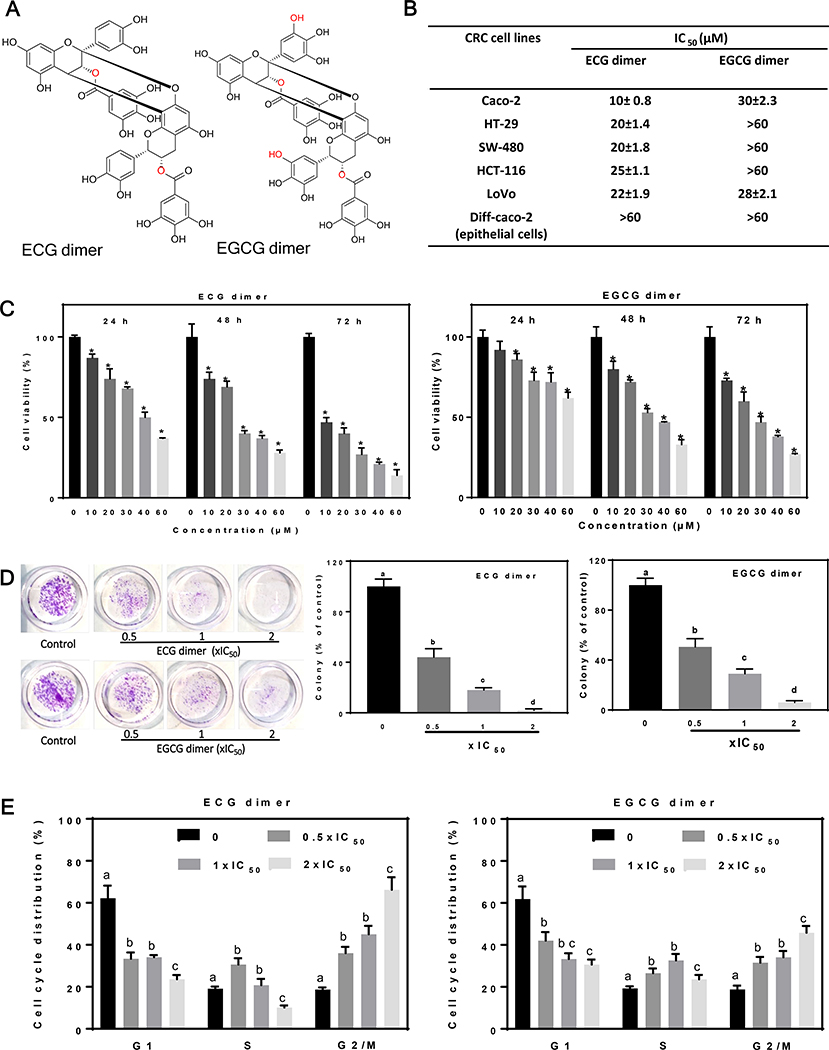
Fig. 1.
ECG and EGCG dimers decreased cell viability of human CRC cells and arrested the Caco-2 cell cycle in G2/M phase. (A) Chemical structures of ECG and EGCG dimers. (B) IC50 for ECG and EGCG dimers to inhibit, after 72h incubation, the growth of five different human CRC cell lines and Caco-2 cells differentiated into intestinal epithelial cells. Results are expressed as the mean ± SEM. (C) ECG and EGCG dimers concentration- and time-dependently decreased Caco-2 cell viability. (D) ECG and EGCG dimers inhibited Caco-2 cell colony formation measured at 24 h. (E) ECG and EGCG dimers blocked Caco-2 cell cycle progression after 72 h incubation. Values are shown as means ± SEM of 3–5 independent experiments. *indicated significantly different from untreated cells. Values having different superscripts are significantly different (p< 0.05, One-way ANOVA-test).
2. Materials and methods
2.1. Materials
The human CRC Caco-2, HCT-116, SW-480, HT-29, and LoVo cell lines were purchased from the American Type Culture Collection (ATCC). All cell culture media and FBS were obtained from Gibco (Waltham, MA). The CellTiter-Glo Luminescent Cell Viability and Caspase-Glo 3/8/9 Apoptotic Kits were obtained from Enzo Life Sciences (Farmingdale, NY). Annexin V-FITC, propidium iodide (PI) and Alexa Fluor 488-EGF were from Thermo Fisher Scientific (Waltham, MA). EGF of high purity was from PeproTech (Rocky Hill, NJ). Hoechst, OptiPrep™ medium and transwell PET membrane cell culture inserts (diam. 8.0 μm) and all other reagents were from Sigma-Aldrich (St. Louis, MO).
Primary antibodies for p(Tyr458)-PI3K (#4228), PI3K (#4292), p(Ser473)-Akt (#4060), Akt (#4691), p(Ser136)-Bad (#4366), Bad (#9239), Cleaved PARP (#5625), Cleaved caspase 3 (#9661), and p(Ser217, 221)-MEK (#9154), MEK (#4694), p(Thr202, Tyr204)-ERK (#4370), ERK (#9102), p(Ser1068)-EGFR (#3777), EGFR (#4267), IGF1R (#9750), c-Cbl (#2179) and ubiquitin (#3936), β-actin (#12620), anti-rabbit and anti-mouse secondary antibodies were all from Cell Signaling Technology (Danvers, MA); p(Ser1162, 1163)-IGF1R (#44–804G) was from Thermo Fisher Scientific (Waltham, MA). Flotillin (#25506) was from Santa Cruz Biotechnology (Dallas, TX). Secondary antibodies biotinylated and streptavidin were from Pierce Biotechnology (Waltham, MA).
2.2. Methods
2.2.1. Isolation of ECG and EGCG dimers
ECG and EGCG dimers were extracted and purified as previously described with small modifications (Dong, Zou et al. 2013). Instead of applying samples onto a Toyopearl HW-50F column (3.0 × 50 cm) equilibrated with methanol, the 30% ethanol elute lyophilized residue was dissolved in methanol (100 mg/ml) and applied onto a medium pressure-LC to be purified and then subjected to preparative-HPLC for further purification. The purity of ECG and EGCG dimers was 95.72 and 95.56%, respectively.
2.2.2. Cell culture
Caco-2 cells were cultured in MEM medium supplemented with 10% (v/v) FBS, 1% (v/v) NEAA, 1% (v/v) sodium pyruvate and 0.5% (v/v) penicillin-streptomycin. HCT-116 and HT-29 cells were cultured in McCoy’s 5A, SW-480 and HCT-15 cells in RPMI and LoVo cells in DMEM medium, which were all supplemented with 10% (v/v) FBS and 0.5% (v/v) penicillin-streptomycin.
2.2.3. Cell viability assay
The effect of ECG and EGCG dimers on cell viability was determined with the CellTiter-Glo Luminescent Cell Viability Assay Kit. Undifferentiated or differentiated cells were treated with 10–60 μM ECG or EGCG dimers for 24, 48, and 72 h. Cell viability was subsequently measured following the manufacturer’s protocol. The half-maximal inhibitory concentration (IC50) of ECG and EGCG dimers were calculated for each proliferating CRC cell line.
2.2.4. Clonogenic assay
Caco-2 cells were seeded (5×102 cells/dish) in 35 mm2 dishes and incubated overnight. Subsequently, cells were treated with 0.5x, 1x, and 2xIC50 of ECG or EGCG dimers for 24 h and incubated for further 6 days with complete growth medium. Cells were then rinsed with PBS, fixed with cold 4% (w/v) paraformaldehyde in PBS for 30 min, and stained with 0.1% (w/v) crystal violet for 1 h. Images were recorded, and the number of colonies was counted.
2.2.5. Transwell migration and invasion assays
Caco-2 cells were re-suspended at the concentration of 1×106 cells/ml in MEM medium containing 0.1% (v/v) FBS. 100 μl of the Caco-2 cell suspension were added to the upper chamber and 600 μl of MEM medium containing 10% (v/v) FBS were added to the lower chamber. After 12 h, ECG or EGCG dimers at 0.5x, 1x, and 2xIC50 concentrations were added to the upper chamber. After 24 h incubation, transwell inserts were removed and cells were fixed with 70% (v/v) ethanol and stained with 0.1% (w/v) crystal violet. Cells that had migrated through the membrane and attached on the underside of the membrane were counted. A similar protocol was followed to assess cell invasion, except that transwell inserts were coated with extracellular matrix (ECM) material prior to plate cells.
2.2.6. Adhesion assay
Caco-2 cells were first treated with or without 0.5x, 1x, and 2xIC50 ECG or EGCG dimers for 30 min, then cells were seeded in 96-well plates for further 24 h. The medium was removed, cells were washed three times with PBS, and adhesive cells were measured using the 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay. Briefly, MTT (0.5 mg/ml) was added and cells were incubated for further 4 h, subsequently, MTT was removed, 200 μl DMSO were added and cells were incubated for 30 min. The absorbance at 570 nm was read.
2.2.7. Determination of matrix metalloproteinases (MMPs) activity by gelatin zymography analysis
Because MMP-2 and −9 proteins have gelatinase activity, their activity can be assessed by the degree of gelatin degradation using zymography (Kupai, Szucs et al. 2010). Analysis of gelatinolytic activity was performed by using 10% (w/v) polyacrylamide gel impregnated with 0.1% (w/v) gelatin. Caco-2 cells were seeded in 60 mm2 dishes (1×106 cells/dish). After reaching 80% confluency, cells were washed three times with PBS and then treated with 0.5x, 1x, and 2xIC50 ECG or EGCG dimers for 24 h in FBS-free MEM medium. Same volumes of medium were collected from all wells, samples were centrifuged at 800 x g for 8 min to eliminate cell debris, and concentrated 7X using a Vacufuge concentrator (Eppendorf, Hamburg, Germany). Non-reducing sample buffer was added to 75 μl of the concentrated medium, and proteins were separated by SDS-PAGE. Gels were washed twice in washing buffer (2.5% (v/v) Triton X-100, 50 mM Tris-HCl pH 7.5, 5 mM CaCl2, 1 μM ZnCl2) for 20 min at room temperature to remove the SDS, then equilibrated with developing buffer (50 mM Tris-HCl, 5 mM CaCl2, 1 μM ZnCl2, pH 7.5) for 30 min at room temperature and incubated for another 24 h at 37 °C. Subsequently, gels were stained with Coomassie Brilliant Blue R-250 (Bio-Rad Laboratories, Hercules, CA) overnight, and destained in a solution containing 5% (v/v) acetic acid and 10% (v/v) methanol until the gelatinase activity could be seen. Bands were visualized in a MyECL Imager (Thermo Scientific, Waltham, MA).
2.2.8. Caspase-3, −8 and −9 activities
Caspase-3, −8 and −9 activities were determined by colorimetric assays as previously described (Kumar, Inigo et al. 2018). Briefly, cells were seeded in 60 mm2 dishes and treated with ECG or EGCG dimers at 0.5x, 1x, and 2xIC50 for 24, 48 and 72 h, respectively. DEVD-AFC, IETD-AFC, and LEHD-AFC were used as substrates to assess caspase-3, −8 and −9 activity, respectively. Fluorescence was measured at λEx/Em=405/510 nm using a fluorescence plate reader (Bio-Tek, Winooski, VT), and values were normalized by their respective protein concentration.
2.2.9. Evaluation of apoptosis by immunocytochemistry
Caco-2 cells were seeded on coverslips in 24-well plates (5×104 cells/coverslip). After 24 h treatment with 0.5x, 1x and 2xIC50 ECG or EGCG dimers, cells were rinsed with PBS and nuclei were stained by incubation with Hoechst 33342 (0.5 μg/ml) for 15 min, followed by incubation with PI (5 μg/ml) for further 5 min. Cells were washed with PBS, mounted onto glass slides, and visualized using a fluorescence microscope (Leica DMI3000B, Wetzlar, Germany).
2.2.10. Flow cytometry analysis
To evaluate cell cycle progression, cells were treated with 0.5x, 1x, and 2xIC50 ECG or EGCG dimers for 24–72 h. Then cells were collected and fixed with 70% (v/v) cold ethanol for 24 h, and stained with PI (50 μg/ml) containing 100 μg/ml RNase A. To evaluate cell apoptosis, after the corresponding treatments, cells were collected and stained with the Annexin-V FITC/PI Kit following the manufacturer’s protocol. Stained cells were immediately subjected to flow cytometry (LSRIIA, BD Biosciences, San Jose, CA), collecting 20,000 events. Data were analyzed using FlowJo software (Ver.10.6.1, FlowJo, LLC., Ashland, OR).
2.2.11. Western blot
Caco-2 cells were correspondingly treated, and total cell fractions were obtained as previously described (Da Silva, Jaggers et al. 2012). Aliquots of total fractions containing 25–50 μg protein were separated by reducing 7.5–12.5% (w/v) polyacrylamide gel electrophoresis and electroblotted to Polyvinylidene fluoride (PVDF) membranes. The membranes were probed overnight with the primary antibodies against the protein of interest. Following incubation, for 90 min at room temperature, in the presence of the corresponding secondary antibody (HRP-conjugated; 1:5,000 dilution), the conjugates were visualized by chemiluminescence.
2.2.12. Lipid rafts isolation
Lipid rafts were isolated with the OptiPrep™ density gradient medium as reported previously with some modifications (Macdonald and Pike 2005). Cells were seeded in 150 mm2 dishes (4×106 cells/dish) and incubated for 2 days. Cells were then incubated in the absence or the presence of 1xIC50 ECG or EGCG dimer for 30 min and then with or without EGF (10 ng/ml) for 15 min. Cells were washed, scraped, collected in cold PBS and then lysed in 100 μl cold OptiPrep™ base buffer (20 mM Tris-HCl, 250 mM sucrose, 6 mM EDTA, 1mM Cacl2, phosphor-protease and protease inhibitors, pH=7.8). The whole cell lysate was passed 20 times through a 1 ml syringe with an λ/22G x 1–1/2-gauge needle and centrifuged at 1000 x g for 10 min at 4 °C. The supernatant was collected, and the pellet re-suspended in 100 μl of lysis buffer, and lysis and centrifugation steps were repeated as described before. The supernatants were joined and 200 μl supernatant were mixed with 600 μl cold Optiprep medium (60% w/v) to have a final concentration of 40% (w/v). 800 μl of this mix was placed at the bottom of an ultracentrifuge tube and sequentially layered with 300 μl 35% (w/v) Opti-prep and 300 μl 5% (w/v) Opti-prep prepared in lysis buffer. Cell lysates were centrifuged at 140,000xg for 2.5 h at 4 °C in an RC-M120GX centrifuge using an SS55 rotor (Beckman, Brea, CA). 200 μl fractions were collected starting from the top of the gradient, the protein content measured, and samples analyzed by Western blot.
2.2.13. Lipid rafts disruption
The effects of ECG and EGCG dimers on lipid raft integrity were measured by combining fluorescence staining and cold 1% (v/v) Triton X-100 solubility assay (Adachi, Nagao et al. 2007). Lipids with long and saturated acyl chains, such as the synthetic fluorescent lipid analogue 1,1’-dihexadecyl-3,3,3’,3’-tetramethylin-docarbocyanine perchlorate (DiIC16), preferentially partition into the ordered domains of the membrane (Mukherjee, Soe et al. 1999). Caco-2 cells were first stained with DiIC16 for 3 min at 37 °C and then exposed to solvent (DMSO) or 0.5x, 1x, and 2xIC50 ECG or EGCG dimers for 30 min. After washing with cold PBS, the attached cells were extracted with 0.5% (v/v) cold Triton X-100 for 30 min on ice. Cells were then fixed with 2% (w/v) paraformaldehyde for 15 min and examined by confocal microscopy. At least, 3 fields were quantified for each sample. Fluorescence was normalized to control values.
2.2.14. Evaluation of EGFR dimerization
EGFR receptor dimerization was measured as previously described (Turk and Chapkin 2015). Briefly, cells were seeded in 100 mm2 dishes and after the corresponding treatments were washed twice with PBS, added with 3 mM bis(sulfosuccinimidyl)suberate (BS3) prepared in ice-cold Ca+2- and Mg+2-free PBS and incubated on ice for 20 min. The reaction was then quenched by the addition of 250 mM glycine in PBS. Cells were washed with PBS and total cell fractions prepared as described before. Protein concentration was measured, and EGFR levels assessed by Western blot.
2.2.15. EGFR internalization analysis
The ability of ECG and EGCG dimers to induce the internalization of EGFR was assessed by a cell-ELISA assay. Caco-2 cells were cultured in 96-well plates at a density of 5×103 cells/well, and after 24 h starvation, cells were incubated with or without 0.5x, 1x, and 2xIC50 of ECG or EGCG dimers for 30 min and subsequently with or without 10 ng/mL EGF for 15 min on ice to allow the ligand-receptor binding. Cells were then incubated for 15 min at 37 °C to allow EGFR internalization. After washing twice with PBS, cells were fixed with 2% (w/v) paraformaldehyde for 20 min at room temperature and blocked with 1% (w/v) BSA in PBS for 1 h at room temperature. Cells were then added with the EGFR primary antibody (1:200, v/v) in 1% (w/v) BSA in PBS for 2 h at room temperature. Plates were then washed and incubated with an HRP-conjugated secondary antibody (1:10.000 v/v) in 1% (v/v) BSA for 90 min at room temperature. Cells were rinsed with PBS, incubated with 100 μl of 3,3′,5,5′-tetramethylbenzidine (TMB) solution at room temperature in the dark, and after 10 min the enzymatic reaction was stopped by addition of 100 μl 1 M H2SO4. The absorbance at 450 nm was measured with a microplate reader (Bio-Tek, Winooski, VT).
2.2.16. EGF-EGFR binding assay
Before treatment, cells were starved in serum-free MEM medium for 24 h. After pretreatment with 0.5x, 1x, and 2xIC50 dimers for 30 min in serum-free MEM medium, cells were washed with PBS and incubated with 10 ng/ml Alexa Fluor 488-EGF complex for 1 h on ice to allow EGF binding to the EGFR, and then fixed with 2% (v/v) paraformaldehyde for 30 min. Cells were then examined using fluorescent confocal microscopy (LSM510, Carl Zeiss, Germany). Software ImageJ (Ver. 1.8.0–172, NIH) was used to quantify the fluorescence. Average fluorescence intensity =(Integrated density)/(Cell area). At least, 3 fields were quantified for each sample. Fluorescence was normalized to control values.
2.2.17. Statistical analysis
All experiments were performed in triplicates and are the average of 3–6 independent experiments. Results are shown as means ± SEM. Data were analyzed by one-way analysis of variance (ANOVA) using SPSS Ver. 19.0. (IBM Inc., Armonk, NY). Fisher least significant difference test was used to examine differences between group means. P<0.05 was considered statistically significant.
3. Results
3.1. ECG and EGCG dimers decreased cell viability of human CRC cells and arrested the cell cycle in G2/M phase
To study the effects of ECG and EGCG dimers on cell growth, we determined the IC50 for their capacity to inhibit human CRC cell growth at 72 h incubation in various human colon cancer cell lines (Fig. 1B). Both dimers significantly decreased the viability of the five tested human CRC cell lines in a concentration-dependent manner. The 72 h-IC50 values for the ECG dimer on the five CRC cells were between 10–25 μM (Fig. 1B). Caco-2 cells were the most sensitive to ECG dimer cytotoxicity, with an IC50 value of 10 μM. The 72 h-IC50 values for the EGCG dimer in Caco-2 and LoVo cells were about 30 μM, while for the other CRC cell lines they were higher than 60 μM. Importantly, both ECG and EGCG dimers did not affect the cell growth of differentiated Caco-2 intestinal epithelial cells within the tested range of concentrations (10–60 μM). Considering the higher sensitivity of Caco-2 cells compared to the other CRC cell lines, we decided to conduct the subsequent mechanistic studies using undifferentiated Caco-2 cells. ECG and EGCG dimers both decreased Caco-2 cell viability in a concentration- and time-dependent manner (Fig. 1C). We next investigated the impact of ECG and EGCG dimers at 0.5x, 1x, and 2xIC50 on Caco-2 colony formation. Generally, a clonogenic assay tests the survival capacity of single cells according to their ability to form colonies after drug treatment (Munshi, Hobbs et al. 2005). ECG and EGCG dimers at 0.5xIC50 inhibited colony formation by 60 and 50%, respectively, while at 2xIC50 concentration, they fully prevented colony formation when compared to controls (Fig. 1D). These results suggest that ECG and EGCG dimers can impair the long-term survival of Caco-2 cancer cells. Subsequently, we evaluated the effects of ECG and EGCG dimers on Caco-2 cell cycle progression using PI staining and flow cytometry analysis. Cells were incubated for 24, 48, and 72 h in the absence or presence of ECG or EGCG dimers. As shown in Fig. 1E, ECG and EGCG dimers concentration-dependently arrested the cell cycle in G2/M phase after 72 h exposure (data for 24 and 48 h were not shown). The above results show that ECG and EGCG dimers can suppress CRC cell growth and proliferation partly by arresting Caco-2 cells cell cycle progression.
3.2. ECG and EGCG dimers inhibited cell migration and invasion and decreased matrix metalloproteinases (MMPs ) activity
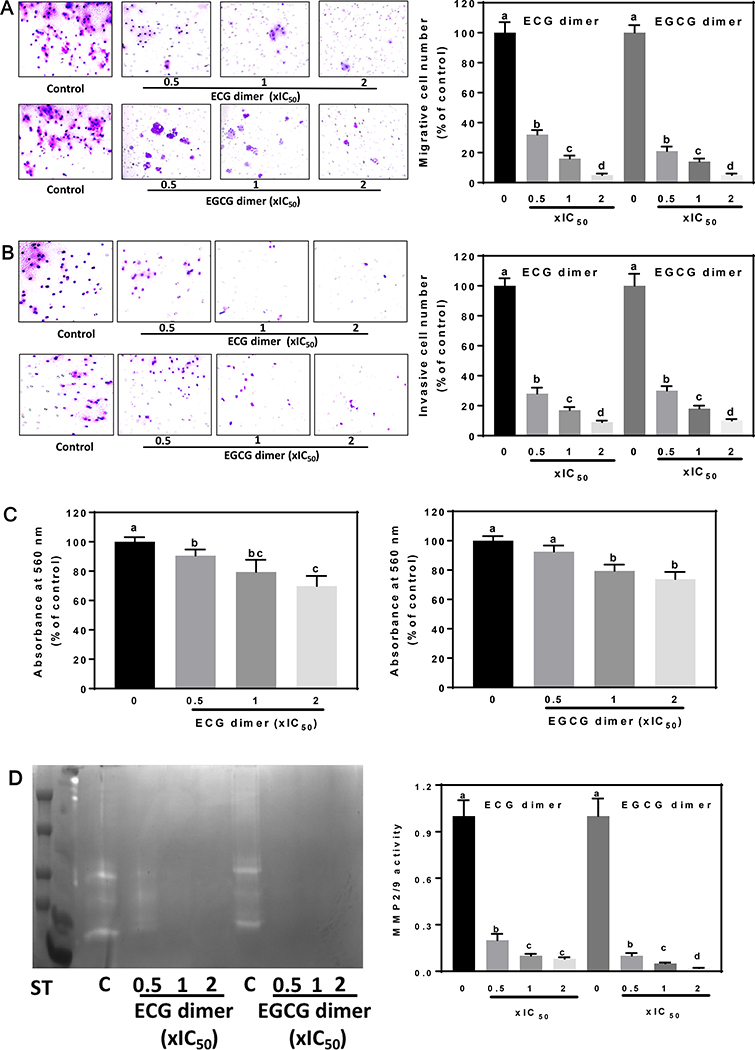
Migration and invasion are the initial and critical events in cancer metastasis (Chaffer and Weinberg 2011). Using a transwell assay, we observed that ECG and EGCG dimers caused a concentration-dependent inhibition of Caco-2 cell migration. At 0.5xIC50, ECG and EGCG dimers inhibited cell migration by 70 and 75%, respectively, while at 2xIC50, the inhibition by both dimers was almost complete (Fig. 2A). Similar results were observed for the cell invasion inhibition by both dimers (Fig. 2B). Additionally, ECG and EGCG dimers at 1x and 2xIC50 significantly inhibited cell adhesion (Fig. 2C). MMPs activity is highly related to cell invasion and tumor metastasis since degradation of the ECM caused by proteinases are required for cancer cell metastasis (Kleiner and Stetler-Stevenson 1999). ECG and EGCG dimers decreased MMP2/9 concentration/activity in the cell medium concentration-dependently (Fig. 2D). At 0.5xIC50, ECG and EGCG dimers inhibited MMP2/9 activity by 90 and 95%, respectively. This finding is consistent with the important inhibitory effects of the dimers on cell invasion, but not with their mild effects on cell adhesion. The latter may be due to the involvement of additional biological events also contributing to cell adhesion, e.g. specific receptors and signaling pathways.
Fig. 2.
ECG and EGCG dimers inhibited the Caco-2 cell migration, invasion, and adhesion. The effects of ECG and EGCG dimers on Caco-2 cell migration, invasion, and MMPs activation were evaluated after 24 h incubation as described in the methods section. (A) ECG and EGCG dimers concentration-dependently decreased Caco-2 cell migration evaluated after 24 h treatment. (B) ECG and EGCG dimers concentration-dependently decreased Caco-2 cell invasion after 24 h treatment. (C) ECG and EGCG dimers concentration-dependently decreased Caco-2 cell adhesion. (D) ECG and EGCG dimers concentration-dependently decreased MMP2/9 activity measured in the cell culture medium after 24 h treatment. Results are shown as means ± SEM of 3–5 independent experiments. Values having different superscripts are significantly different (p< 0.05, One-way ANOVA-test).
3.3. ECG and EGCG dimers induced apoptotic cell death
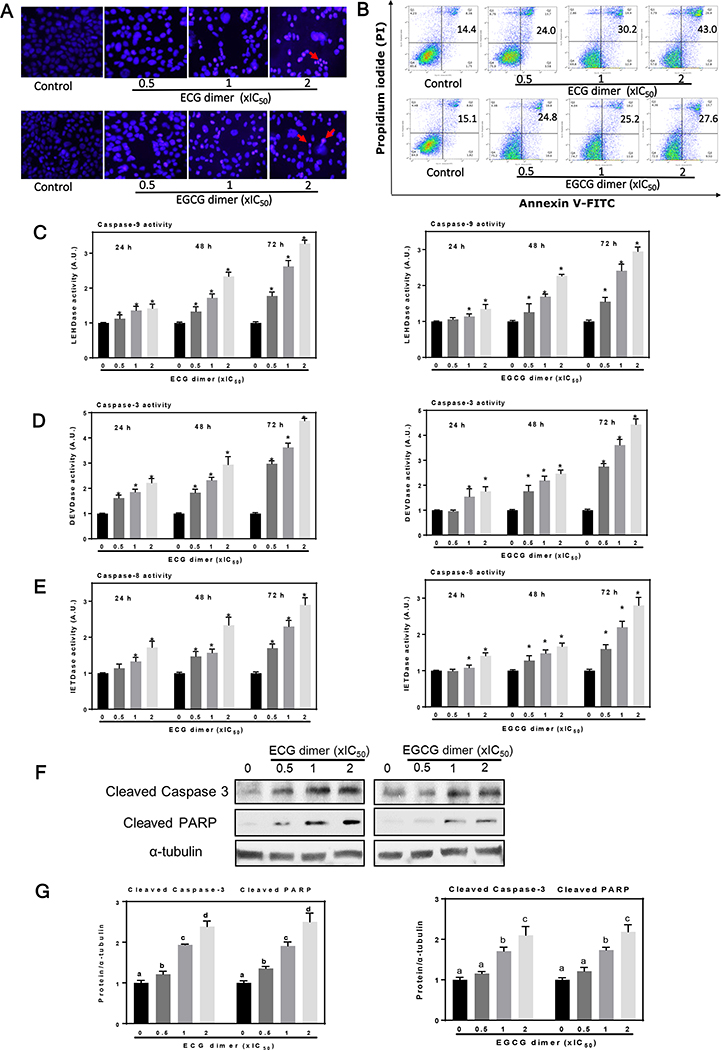
We next investigated whether ECG and EGCG dimers can induce Caco-2 cell apoptosis. Firstly, we examined the occurrence of apoptotic cell death by evaluating nuclear morphology (chromatin condensation) upon Hoechst and propidium iodide (PI) dual staining. After 48 h treatment, both dimers triggered apoptotic cell death as evidenced by an increase in the number of cells with rounded, small nuclei and bright condensed chromatin. The presence of cells in late apoptosis/necrosis was also evidenced by observation of cells permeable to PI (Fig. 3A). Next, we evaluated the capacity of ECG and EGCG dimers to induce apoptosis by staining cells with Annexin V-FITC and PI and subsequent FACS. Treatment with ECG or EGCG dimers for 48 h both increased the proportion of apoptotic cells (Fig. 3B). For instance, ECG and EGCG dimers at 2xIC50 increased about 3- and 2- fold the percentage of apoptotic cells compared to controls, respectively.
Fig. 3.
ECG and EGCG dimers induced apoptotic cell death in Caco-2 cells. Cells were incubated for 48 h in the presence of the ECG and EGCG dimers. (A) Cell apoptosis was evaluated by staining with Hoechst (blue fluorescence)/PI (red fluorescence) and subsequent immunocytochemistry. The red arrows indicated apoptotic cells. (B) Early and late apoptosis was quantified by Annexin-V/PI dual staining and subsequent flow cytometry analysis (numbers in the top right quadrants indicated the percentage of cells undergoing apoptosis). (C-E) ECG and EGCG dimers promoted caspase 8, 9, and 3 activation in Caco-2 cells after 24, 48 and 72 h incubation. (F-G) ECG or EGCG dimers exposure for 48 h dose-dependently increased levels of cleaved caspase 3 and cleaved PARP as measured by Western blot. Results are shown as means ± SEM of 3–5 independent experiments. *indicated significantly different from untreated cells. Values having different superscripts are significantly different (p< 0.05, One-way ANOVA-test).
Meanwhile, we evaluated the effects of ECG and EGCG dimers on the cascade of caspases which are crucial initiators or effectors in the cell death pathways (Porter and Jänicke 1999). Caspase-3, 8 and 9 activities were measured after 24, 48 and 72 h treatment with ECG dimer or EGCG dimer at 0.5x, 1x, and 2xIC50 concentrations. Both dimers promoted caspase activation in a concentration- and time-dependent manner (Fig. 3C–E). Compared to controls, ECG and EGCG dimers both induced about 4.5- and 3-fold increase in caspase-3 and 8/9 activity after 72 h treatment, respectively. Moreover, ECG and EGCG dimers at 0.5x, 1x, and 2xIC50 concentrations increased the levels of cleaved caspase-3 and cleaved PARP about 1.5 to 3-fold over control values, as evaluated by western blot (Fig. 3F–G). These results indicate that ECG and EGCG dimers induce early as well as late apoptosis in Caco-2 cells.
3.4. ECG and EGCG dimers inhibited EGFR phosphorylation (Tyr1068) and dimerization, without affecting EGFR localization in lipid rafts domains
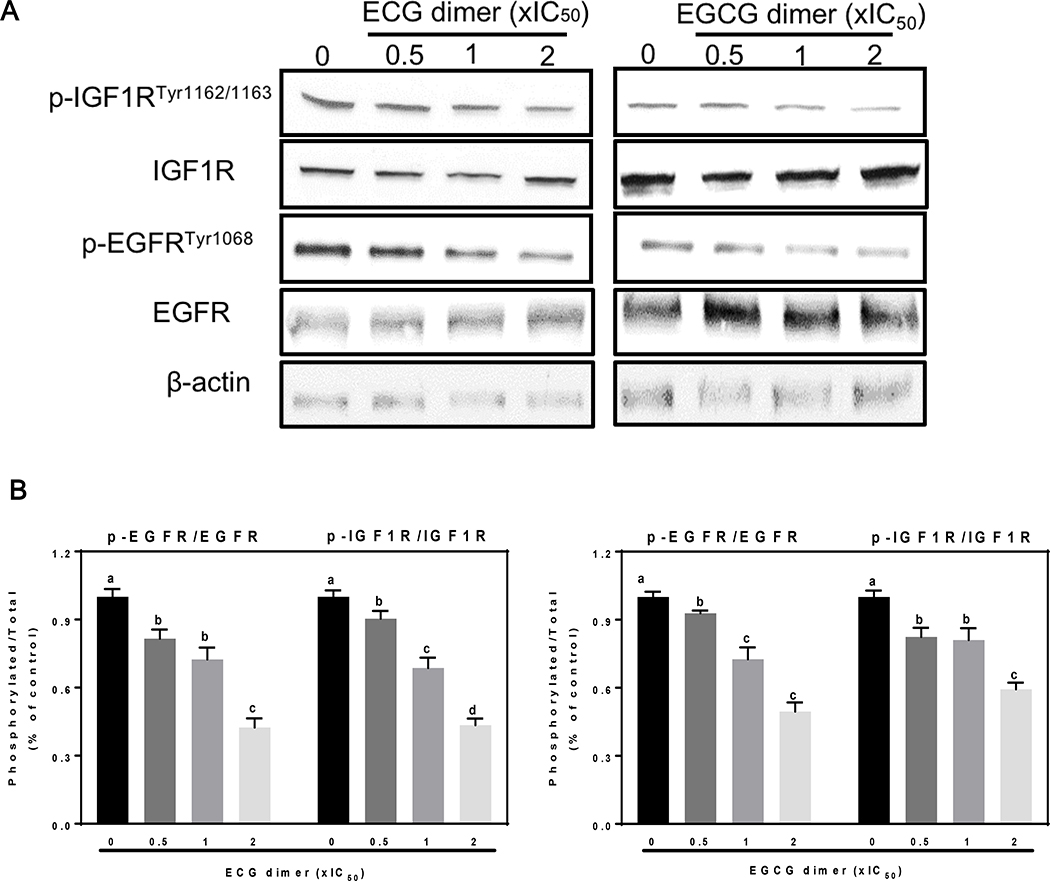
Overexpression and activation of receptor tyrosine kinases (RTKs), such as EGFR and IGF1R, are frequent phenomena in CRC (Nicholson, Gee et al. 2001). We next evaluated the effects of the simultaneous inhibition of these two RTKs by ECG and EGCG dimers. ECG and EGCG dimers inhibited the activation of EGFR and IGF1R concentration-dependently (Fig. 4A, B). At 2xIC50 concentration, ECG and EGCG dimers inhibited about 50–60% the phosphorylation of both receptors.
Fig. 4.
ECG and EGCG dimers inhibited both the EGFR and IGF1R activation in human Caco-2 cancer cells. Cells were incubated for 24 h in the absence or presence of 0.5X, 1X and 2XIC50 concentrations of ECG and EGCG dimers. EGFR phosphorylation (Tyr1068), or IGF1R phosphorylation (Tyr1162/1163) were evaluated by Western blot. Bands were quantified and values for phosphorylated proteins were referred to the respective total protein content. Results are shown as means ± SEM of 3–5 independent experiments. Values having different superscripts are significantly different (p< 0.05, One-way ANOVA-test).
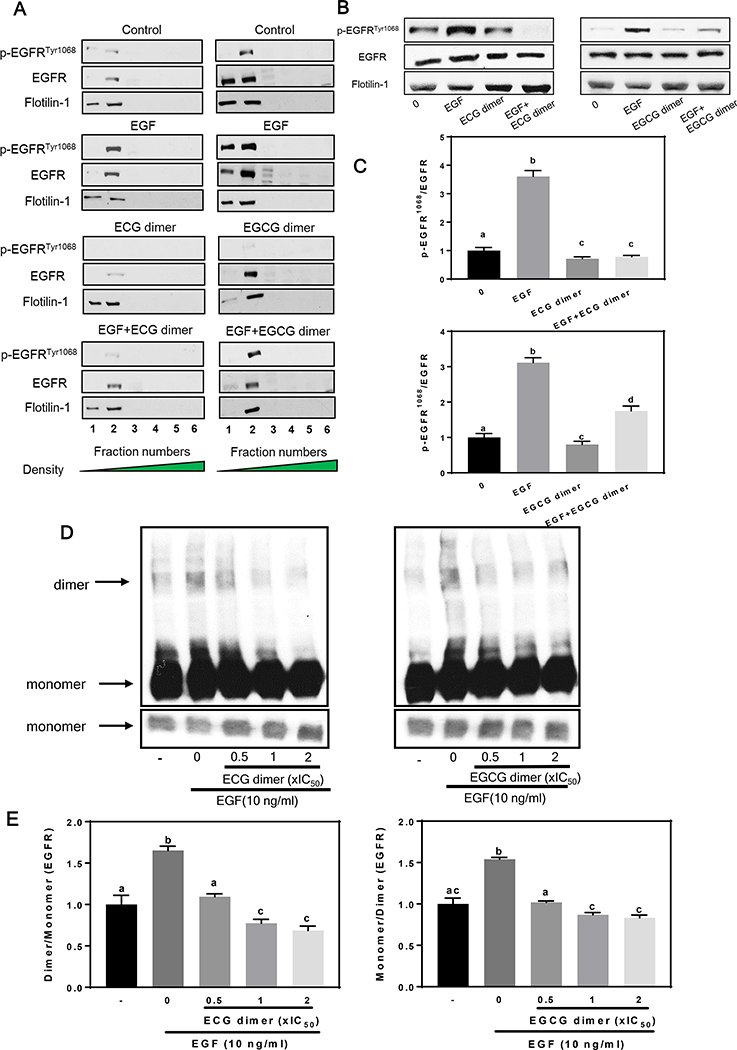
At the cell membrane, EGFR and IGF1R are localized in lipid raft domains. Then, we examined whether the inhibitory effects of ECG and EGCG dimers on the activation of the EGFR/IGF1R were associated with an effect of these compounds on lipid rafts. After gradient ultracentrifugation in Optiprep, 6 fractions of the cell total lysis were isolated. As indicated by the lipid raft marker protein Flotillin, both Tyr1068 phosphorylated (active) EGFR and total EGFR were located in the lipid rafts fraction (fraction 1 and 2), while pre-treatment with ECG dimer or EGCG dimer at 1xIC50 concentration did not affect the location of the EGFR in lipid rafts (Fig. 5A). However, pre-treatment with ECG or EGCG dimer inhibited EGF-induced Tyr1068 EGFR phosphorylation in lipid rafts. Treatment of the cells with 10 ng/ml EGF for 15 min resulted in a 4-times increase in the amount of lipid rafts p-EGFRTyr1068, while pre-treatment of the cells with 1xIC50 of ECG dimer or EGCG dimer for 30 min caused a marked decrease in EGFR phosphorylation (Fig. 5B–C). The binding of EGF to the extracellular domain of the EGFR induces a dramatic conformational change in the EGFR protein, exposing a dimerization site in the cytoplasmic domain that is normally occluded in the inactivated conformation, thus promoting dimer formation (Ferguson 2004). We next investigated whether ECG and EGCG dimers could affect EGFR dimerization. As shown in Fig. 5D–E, pre-treatment of the cells with 1xIC50 of ECG or EGCG dimer for 30 min significantly inhibited the EGFR dimerization (Fig. 5D–E). These results provide evidence that ECG and EGCG dimers in part inhibit EGFR activation by inhibiting its dimerization.
Fig. 5.
ECG and EGCG dimers did not displace the EGFR from lipid rafts but inhibited EGF-induced phosphorylation and dimerization of the EGFR at lipid rafts. After 24 h starvation, cells were incubated in the absence (control) or presence of ECG or EGCG dimers for 30 min at 37°C, subsequently, cells were incubated without or with EGF (10 ng/ml) for further 15 min. Cells were harvested, and lipid rafts fractions were isolated as described in Methods. (A-C) EGFR phosphorylation in Tyr1068 was evaluated by Western blot. Bands were quantified and values for phosphorylated proteins were referred to the respective total protein content. (D-E) EGFR dimerization was measured as by crosslinking with (BS3) and subsequent Western blot, as described in Methods. Results are shown as means ± SEM of 3–5 independent experiments. Values having different superscripts are significantly different (p< 0.05, One-way ANOVA-test).
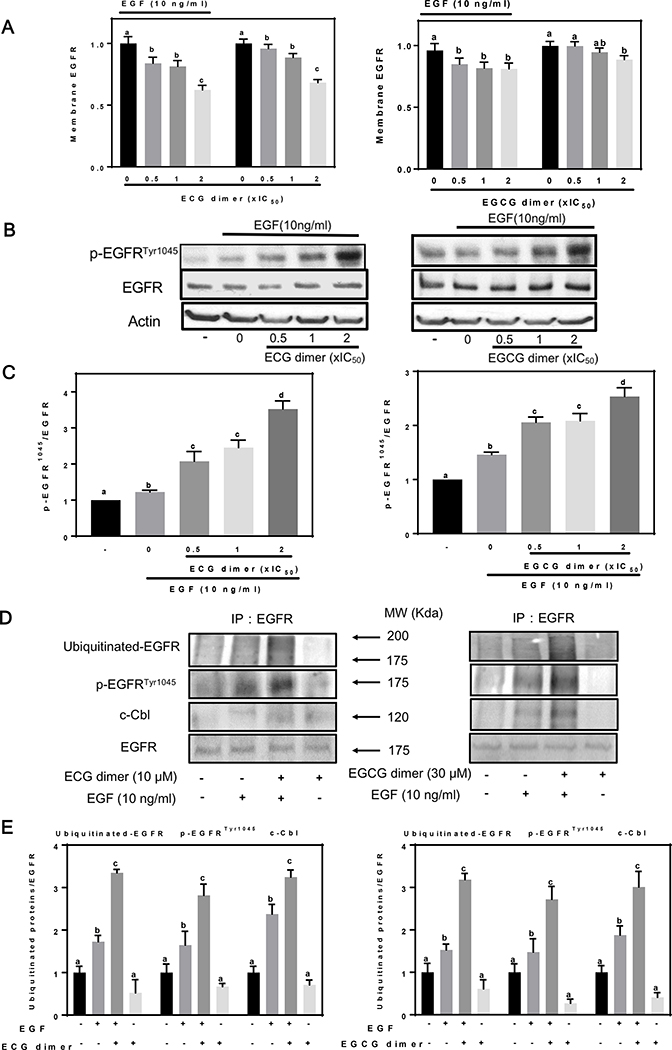
3.5. ECG and EGCG dimers promoted the internalization and degradation of the EGFR
EGF induces internalization of the EGFR via endocytosis which is associated with the subsequent ubiquitin-mediated degradation of the EGFR. Therefore, we next examined whether ECG and EGCG dimers could induce changes in the cellular localization of the EGFR using an ELISA method. ECG and EGCG dimers promoted EGFR internalization concentration-dependently both in the presence and absence of EGF (Fig. 6A). Exposure of cells to EGF resulted in a rapid EGFR autophosphorylation, at the Tyr1045 site, which provides a docking site for the ubiquitin ligase c-Cbl, resulting in ubiquitination of the EGFR and removal of the EGFR via endocytosis from the cell surface into an early endosomal compartment (Massie and Mills 2006). In the presence of EGF, ECG and EGCG dimers caused Tyr1045 phosphorylation of the EGFR in a concentration-dependent manner (Fig. 6B, C). To assess the effects of ECG and EGCG dimers on EGFR ubiquitination, after immunoprecipitation of the EGFR, both p-EGFRTyr1045 levels, c-Cbl, and ubiquitinated-EGFR were assessed by Western blot. EGF (10 ng/ml) caused EGFR Tyr1045 phosphorylation, c-Cbl binding and EGFR ubiquitination of the EGFR, while ECG and EGCG dimers per se did not affect these events. Pretreatment with ECG or EGCG dimers for 30 min enhanced EGF-induced EGFR ubiquitination (Fig. 6D, E), presumably because ECG and EGCG dimers promoted EGF-mediated Tyr1045 activation and consequent c-Cbl binding to initiate this process. These results are consistent with those obtained for EGFR degradation (Fig. 6B). Taken together, these findings support the concept that, similar to the effects seen with EGF, the internalization of EGFR by endocytosis is induced by ECG and EGCG dimers and is associated with the ubiquitin-mediated degradation of the EGFR, at least within the first hour.
Fig. 6.
ECG and EGCG dimers promoted EGFR internalization and degradation. After 24 h starvation, cells were treated in the absence or presence of ECG or EGCG dimers for 30 min at 37 °C, and subsequently incubated without or with EGF (10 ng/ml) for 15 min. (A) The internalization of the EGFR was evaluated by measuring membrane EGFR content by cell-Elisa assay as described in Methods. (B-C) The effects of ECG and EGCG dimers on the phosphorylation of the EGFR at Tyr1045 was evaluated by Western blot. (D-E) ECG and EGCG dimers promoted degradation of the EGFR via the ubiquitin pathway. After immunoprecipitation of the EGFR, Western blots were done for (D) ubiquitinated-EGFR, p-EGFRTyr1045 and the ubiquitin ligase c-Cbl and (E) bands were quantified and values for phosphorylated proteins were referred to the respective total protein content. Results are shown as means ± SEM of 3–5 independent experiments. Values having different superscripts are significantly different (p< 0.05, One-way ANOVA-test).
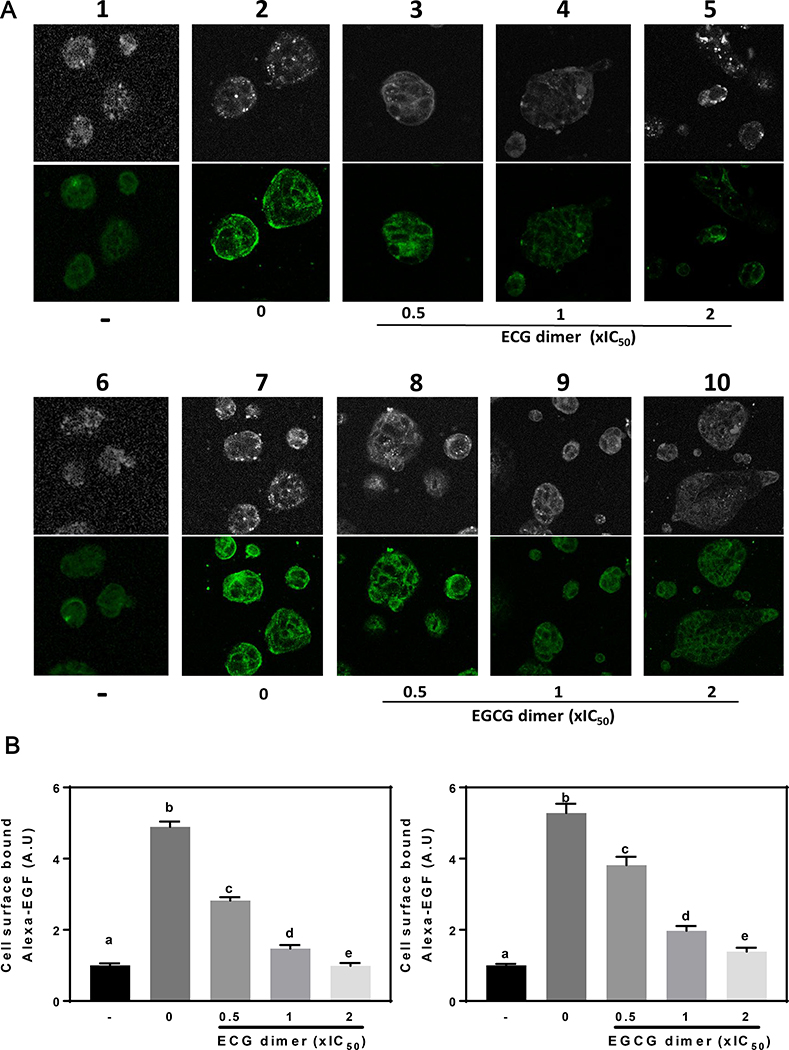
3.6. ECG and EGCG dimers prevented EGF binding to the EGFR
To further investigate how ECG and EGCG dimers inhibited the activation of EGFR, we examined their effects on EGF binding to Caco-2 cells using Alexa Fluor 488–labeled EGF and fluorescence confocal microscopy. Caco-2 cells incubated with Alexa-EGF for 1 h on ice, the cell membrane displayed strong fluorescence (Fig. 7A, lane 2, 7). Compared to the control group(Fig. 7A, lane 1, 6), the fluorescence intensity increased 4 times (Fig. 7B). However, pretreatment of cells with 0.5x to 2xIC50 ECG dimer or EGCG dimer at 37 °C for 30 min before the addition of Alexa-EGF markedly prevented the binding of Alexa-EGF to the cell membrane in a concentration-dependent manner, thus showing lower fluorescence intensity (Fig. 7A, lane 3–5, 8–10, 7B). These data suggest that ECG and EGCG dimers inhibit EGFR phosphorylation and dimerization in part through their ability to prevent the binding of EGF to the EGFR.
Fig. 7.
ECG and EGCG dimers inhibited EGF binding to the EGFR. Caco-2 cells were treated with/without ECG or EGCG dimers of 0.5x, 1x, 2xIC50 for 30 min at 37 ° C. Then, cells were exposed to Alexa-EGF (10 ng/ml) for 1 h on ice. (A) Representative confocal microscopy images. (B) Images were quantified using Image J, and values referred to the total cell area that was positive for Alexa-EGF staining. Results are shown as means ± SEM of 3–5 independent experiments. Values having different superscripts are significantly different (p< 0.05, One-way ANOVA-test).
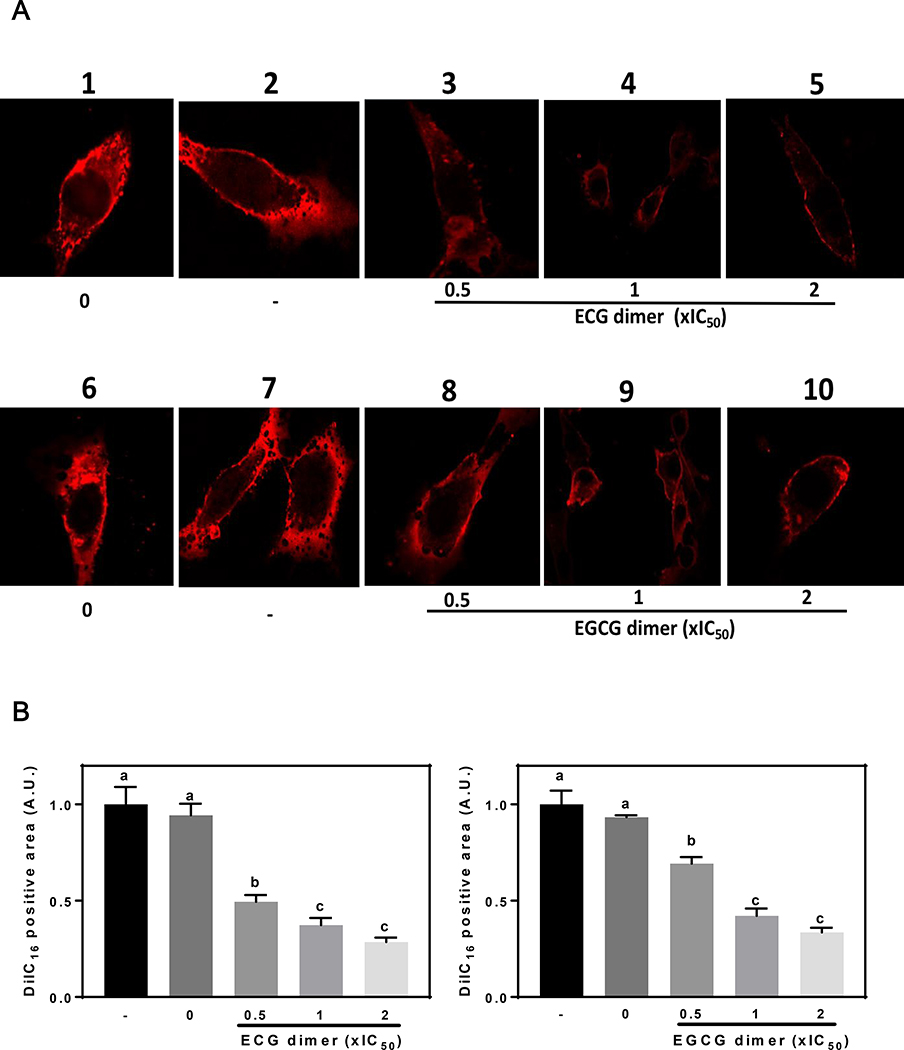
3.7. ECG and EGCG dimers caused a reduction of lipid rafts domains of the cell membrane
There is strong evidence that lipid organization in membranes can affect the activation and functions of the EGFR and other membrane-associated receptors. We next evaluated the potential effects of ECG and EGCG dimers on lipid rafts structure. We stained cells with the compound DiIC16 which preferentially incorporates into lipid rafts domains and then exposed cells to Triton X-100, which preferentially extracts the membrane non-lipid rafts domains. Caco-2 cells were labeled with DiIC16 for 3 min and then exposed to the solvent (DMSO, control) or to ECG or EGCG dimers at 0.5x, 1x, and 2xIC50 concentrations for 30 min at 37 °C before extraction with cold Triton X-100. Without Triton X-100 extraction, most areas of the cell membrane were stained by DiIC16 (Fig. 8A, lane 1, 6). After Triton X-100 extraction, as it has been reported for other cells, a large fraction of the plasma membrane remains unextracted, and the remaining plasma membrane shows many small holes (Fig. 8A, lane 2, 7). Treatment with ECG dimer or EGCG dimer led to extensive loss of DiIC16-labeled membrane after cold Triton X-100 extraction (Fig. 8A, lane 3–5, 8–10). The percentage of unextracted cell area was quantified and shown in Fig. 8B. There was a concentration-dependent decrease in the amount of unextracted DiIC16-labeled membrane when the cells were treated with ECG dimer or EGCG dimer, suggesting that dimers caused a reduction in membrane lipid rafts domains.
Fig. 8.
ECG and EGCG dimers decreased lipid rafts domains surface area. The lipid raft relative surface area was evaluated using the DiIC16 staining and cold 1% (v/v) Triton X-100 solubility assay as described in Methods. (A) representative confocal microscopy images of cells before (images 1, 6) or after (images 2–5, 7–10) cold 1% (v/v) Triton X-100 extraction. (B) Fluorescence intensity was quantified using ImageJ and was expressed as the percentage of the total cell area that was positive for DiIC16 staining. Results are shown as means ± SEM of 3–5 independent experiments. Values having different superscripts are significantly different (p< 0.05, One-way ANOVA-test).
3.8. ECG and EGCG dimers inhibited the PI3K/AKT and MEK/ERK1/2 pathways
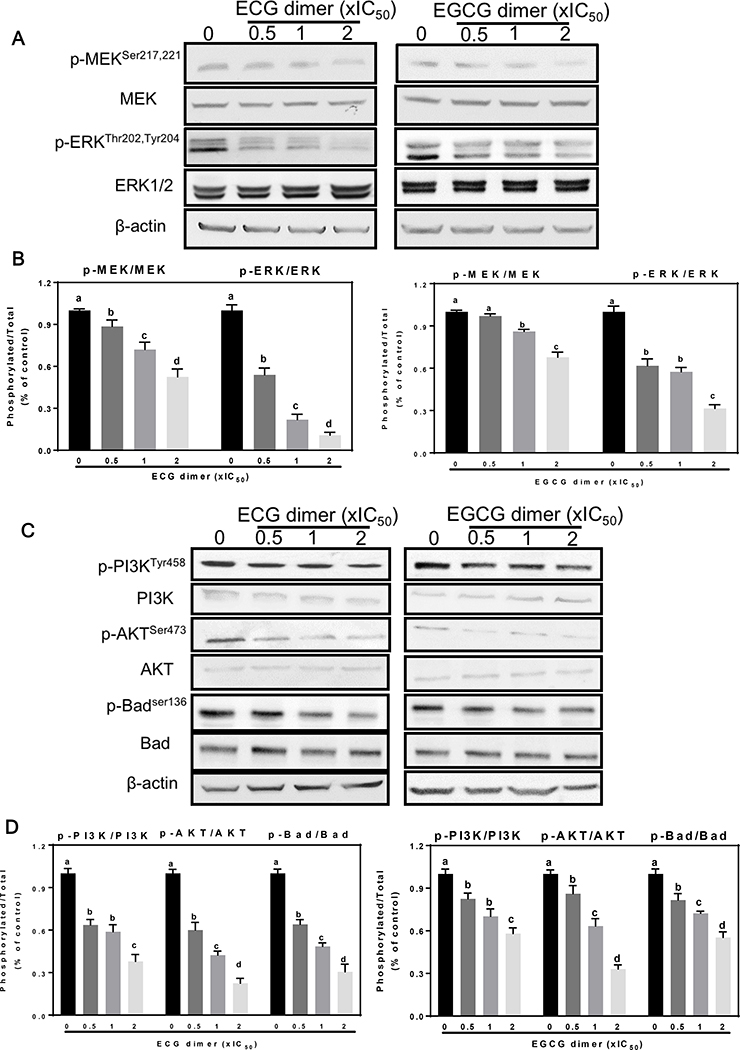
EGFR regulate downstream signaling cascades which mediate cancer cell fate. Since the MEK/ERK1/2 pathway is critical in the regulation of cell proliferation and survival, we next measured the effects of ECG and EGCG dimers on the MEK and ERK1/2 activation (phosphorylation). As shown in Fig. 9A, B, ECG and EGCG dimers both inhibited the phosphorylation of MEK and ERK concentration-dependently. Inhibition of MEK and ERK1/2 phosphorylation by the ECG dimer were between 12–48% and 47–89%, respectively, and 3–33% and 39–59%, respectively, for the EGCG dimer. These results support the concept that ECG and EGCG dimers could interrupt the cell cycle and reduce cell proliferation by inhibiting the MEK/ERK pathway.
Fig. 9.
ECG and EGCG dimers inhibited both the EGFR and IGF1R-associated activation of the MEK/ERK1/2 and PI3K/AKT pathways in human Caco-2 cancer cells. Cells were incubated for 24 h in the absence or presence of 0.5X, 1X and 2XIC50 concentrations of ECG or EGCG dimers. (A, B) MEK and ERK1/2 phosphorylation and (C, D) PI3K, AKT and Bad phosphorylation evaluated by Western blot. Bands were quantified and values for phosphorylated proteins were referred to the respective total protein content. Results are shown as means ± SEM of 3–5 independent experiments. Values having different superscripts are significantly different (p< 0.05, One-way ANOVA-test).
The PI3K/AKT pathway is regulated by a variety of growth factors, and its activation is strongly implicated in the regulation of survival or protection of cells from apoptosis (Vara, Casado et al. 2004). We next measured the effect of ECG and EGCG dimers on PI3K and AKT activation. As shown in Fig. 9C, D, ECG and EGCG dimers both inhibited PI3K and AKT phosphorylation concentration-dependently. At 2xIC50 concentration, the ECG dimer significantly reduced both PI3K and AKT phosphorylation about 70% with respect to controls, while the EGCG dimer inhibited PI3K and AKT phosphorylation about 40 and 70%, respectively, compared to controls. Downstream, AKT inhibits the intrinsic mitochondrial pathway by phosphorylating Bad at Ser136, which prevents Bad translocation to the mitochondria and the initiation of apoptosis. ECG and EGCG dimers both decreased Bad phosphorylation concentration-dependently. Results suggest that ECG and EGCG dimers can suppress cell proliferation and induce apoptosis via the mitochondrial pathway via inhibition of the EGFR/IGF1R-associated activation of the MEK/ERK1/2 and PI3K/AKT pathways in human Caco-2 cells.
4. Discussion
The present study showed that ECG and EGCG dimers obtained from persimmon fruits inhibited human CRC cell proliferation, migration, and invasion as well as induced apoptosis via the mitochondrial pathway in human Caco-2 cells. These effects were associated with the capacity of ECG and EGCG dimers to promote changes in lipid rafts organization and inhibit the lipid raft-associated EGFR signaling pathway.
We observed that ECG and EGCG dimers decreased the viability of five human CRC cell lines. This can be due to a decrease in cell proliferation and/or an increase in apoptotic cell death. In Caco-2 cells, the most susceptible among the tested CRC cells, ECG and EGCG dimers caused a cell cycle arrest at G2/M phase, thus inhibiting cell proliferation. Cell migration and invasion are of particular interest in cancer research given that they participate in the metastatic progression of the disease. The activation of MMPs is involved in tumor growth, cell invasion and tumor metastasis (Shay, Lynch et al. 2015). Both dimers markedly inhibited Caco-2 cell migration and invasion as well as MMPs activity/expression. ECG and EGCG dimers also promoted Caco-2 cell apoptosis, an important anticancer mechanism. Dimer-mediated induction of apoptosis occurred via the mitochondrial pathway as evidenced by the translocation of Bad to the mitochondria and the activation of caspase 9 and 3. Accordingly, procyanidins extracted from apples and cocoa decreased cell viability by arresting the cell cycle in G2/M phase and inducing apoptosis via the mitochondrial pathway, in melanoma/mammary tumor cells and CRC cells, respectively (Miura, Chiba et al. 2007, Choy, Fraga et al. 2016). It is important to note that, although procyanidins that are absorbed minimally or are not absorbed into circulation, they still have key effects at the gastrointestinal tract and may present potential agents for CRC prevention/treatment.
Overexpression and activation of RTKs, such as the IGF1R and the EGFR, are frequent phenomena in many types of cancers, including CRC. EGFR is overexpressed up to 82% in CRC patients (Goldstein and Armin 2001, Spano, Lagorce et al. 2005). Several studies have also shown that IGF1R is frequently overexpressed in CRC patients (Hakam, Yeatman et al. 1999, Weber, Fottner et al. 2002). These receptors mediate downstream mitogen-activated protein kinase (MAPK) and PI3K/AKT pathways, which regulate cancer cell growth and apoptosis (Reinmuth, Fan et al. 2002). Recently, EGFR/IGF1R has been associated to more aggressive histological and clinical CRC (Srivatsa, Paul et al. 2017). Therefore, targeting EGFR/IGF1R is desirable in CRC patients. Indeed, inhibition of EGFR/IGF1R signaling significantly inhibits tumor growth in numerous preclinical models, including CRC models (Chan, Segelov et al. 2017, Garcia-Aranda and Redondo 2019). Simultaneous inhibition of IGF1R and EGFR enhances the efficacy of standard drug treatment for CRC (Peters, Dicker et al. 2013, Oberthur, Seemann et al. 2017). In the present study, we observed that either ECG or EGCG dimers significantly inhibited EGFR and IGF1R expression and the downstream signaling cascades, suggesting that ECG and EGCG dimers could be a promising inhibitor of EGFR and IGF1R.
Containing numerous receptors and being a platform for signal transduction (Simons and Toomre 2000), lipid rafts are a novel and promising target of phytochemicals to exert their ubiquitous beneficial effects (Shimizu, Adachi et al. 2011). EGCG was proposed to exert its anti-allergic and anti-CRC effects through lipid rafts-linked mechanisms (Tachibana, Fujimura et al. 2004, Adachi, Nagao et al. 2007). EGCG could prevent the activation of the c-Met receptor by altering the structure and/or function of lipid rafts in prostate cancer cells (Duhon, Bigelow et al. 2010). Lipid rafts were also proposed for the anti-CRC effect of resveratrol and quercetin (Delmas, Rebe et al. 2004, Psahoulia, Drosopoulos et al. 2007, Colin, Limagne et al. 2011). Importantly, cocoa hexameric procyanidins that have the capacity to interact with lipid rafts (Verstraeten, Jaggers et al. 2013), also inhibit the EGFR/ERK1/2-AKT signaling pathway and promote Caco-2 cell apoptosis and cell cycle progression (Da Silva, Jaggers et al. 2012, Choy, Fraga et al. 2016) These studies support the concept that procyanidins, even when they may not be taken up by cells, could target lipid rafts and alter the activity of resident receptors and downstream pathways. We observed that ECG and EGCG dimers did not act displacing the EGFR from lipid rafts, but they inhibited EGF binding to the EGFR and the subsequent EGFR activation. Both dimers also promoted EGFR internalization and degradation. Our previous studies showed that ECG and EGCG dimers could be incorporated into cell membrane or into synthetic liposomes, and affect their biophysical properties (Zhu, Zou et al. 2015, Zhu, Xiong et al. 2016). We observed that ECG and EGCG dimers also caused a decrease in the area of lipid raft domains, which was paralleled by an increase in EGFR internalization. An increased rate of EGFR endocytosis can in part explain ECG/EGCG dimers-mediated decrease in lipid raft area. Thus, ECG and EGCG dimers interact with CRC cell membranes and affect the structure/function of lipid rafts leading to a decreased activation of the EGFR through a reduction of membrane EGFR that is in part mediated by increased receptor endocytosis.
Extensive epidemiological evidence supports the notion that consumption of foods high in procyanidins is associated with a lower CRC risk. In particular, the capacity of reducing CRC risk is related to the structure/polymerization degree of procyanidins. For example, the viability of colorectal and gastric cancer cells decreases with increasing procyanidin polymerization degree and galloylation percentage (Lizarraga, Lozano et al. 2007, Pierini, Kroon et al. 2008). In the present study, based on IC50 values of cell viability on multiple colon cancer cell lines, we observed that the ECG dimer is more potent than the EGCG dimer in inhibiting the growth of CRC cells (Fig. 1B). When comparing the growth inhibition of 20 μM ECG and EGCG dimers on these CRC cell lines after 72 h incubation, we found that ECG dimer inhibited 62, 57, 55, 31, 58% cell growth of Caco-2, HT-29, SW-480, HCT-116, LoVo cell lines, respectively, while EGCG dimer inhibited 41, 22, 24, 10, 39%, respectively, in these cell lines. Consistently, in 3T3-L1 preadipocytes and L02 cells, we previously observed that the ECG dimer is more effective than the EGCG dimer inhibiting cell differentiation and alleviating hepatic steatosis (Zou, Nie et al. 2014, Zhu, Zou et al. 2015). Although similar in structure, it should be considered that the tridimensional structure of dimers can significantly differ based on the type of monomeric subunit (Mackenzie, Delfino et al. 2009). Moreover, as suggested before, the interactions of polyphenols with cell membrane which can be determined by a set of structural characteristics would mediate, in part, their biological effects (Verstraeten, Fraga et al. 2015). The interaction of polyphenol with lipid headgroups positively correlates with the number of hydroxyl groups, and inversely with the hydrophobicity of the molecule (Ollila, Halling et al. 2002, Erlejman, Verstraeten et al. 2004). However, large amounts of hydroxyl moieties in small-size flavonoids can prevent their membrane adsorption by causing a large decrease in their hydrophobicity (Ratty, Sunamoto et al. 1988). The calculated LogP (a parameter of hydrophobicity) was ECG dimer (5.24) > EGCG dimer (4.17) (Zhu, Zou et al. 2015), suggesting that the ECG dimer have higher affinity and can more easily absorb into membranes than the EGCG dimer. Consistently, Caturla et al (2003) reported that compared to EGCG and other green tea catechins, ECG which has the highest partition coefficient for phospholipid membranes, significantly affected the physical properties of lipid bilayers (Caturla, Vera-Samper et al. 2003). These studies support the concept that the ECG dimer, possessing higher hydrophobicity, can be more easily absorbed into the cell membrane and exert stronger disrupting membrane effects than the EGCG dimer. These can in part explain the observed ECG dimer’s stronger disrupting effect on lipid rafts domains and displaying more potential anti-CRC effects (with lower IC50 value than EGCG dimer).
In summary, the obtained results indicate that ECG and ECG dimers isolated from persimmons present anticancer effects in CRC cells by arresting cell cycle, inhibiting proliferation, preventing cell invasion and promoting apoptosis. These beneficial effects can be in part explained by their capacity to inhibit the EGFR signaling pathway. This inhibition is associated to the capacity of ECG and EGCG dimers to interact with lipid rafts domains of the cell membranes, inhibiting EGF binding to the EGFR receptor, and promoting EGFR internalization and degradation.
Acknowledgments
Funding
This study was supported by the funds from the University of California, Davis and NIFA-USDA CA-D-NTR-7244-H to P.I.O. and (CA-D-NTR-2397-H) to G.G.M., and the Fundamental Research Funds for the Central Universities (2662018PY058) to M.C.L.. W.Z. was sponsored by a China Scholarship Council fellowship. Flow cytometry experiments were funded in part by the UC Davis Comprehensive Cancer Center Support Grant (CCSG) (NCI P30CA093373).
Footnotes
Conflicts of interest
The authors declare no conflict of interest.
References
- Adachi S, et al. (2007). “The inhibitory effect of (−)-epigallocatechin gallate on activation of the epidermal growth factor receptor is associated with altered lipid order in HT29 colon cancer cells.” Cancer Research 67(13): 6493–6501. [DOI] [PubMed] [Google Scholar]
- Arnold M, et al. (2017). “Global patterns and trends in colorectal cancer incidence and mortality.” Gut 66(4): 683–691. [DOI] [PubMed] [Google Scholar]
- Aune D, et al. (2011). “Nonlinear Reduction in Risk for Colorectal Cancer by Fruit and Vegetable Intake Based on Meta-analysis of Prospective Studies.” Gastroenterology 141(1): 106–118. [DOI] [PubMed] [Google Scholar]
- Baena R and Salinas P (2015). “Diet and colorectal cancer.” Maturitas 80(3): 258–264. [DOI] [PubMed] [Google Scholar]
- Ben QW, et al. (2014). “Dietary Fiber Intake Reduces Risk for Colorectal Adenoma: A Meta-analysis.” Gastroenterology 146(3): 689–+. [DOI] [PubMed] [Google Scholar]
- Carnesecchi S, et al. (2002). “Flavanols and procyanidins of cocoa and chocolate inhibit growth and polyamine biosynthesis of human colonic cancer cells.” Cancer Letters 175(2): 147–155. [DOI] [PubMed] [Google Scholar]
- Caturla N, et al. (2003). “The relationship between the antioxidant and the antibacterial properties of galloylated catechins and the structure of phospholipid model membranes.” Free Radical Biology and Medicine 34(6): 648–662. [DOI] [PubMed] [Google Scholar]
- Chaffer CL and Weinberg RA (2011). “A perspective on cancer cell metastasis.” Science 331(6024): 1559–1564. [DOI] [PubMed] [Google Scholar]
- Chan DLH, et al. (2017). “Epidermal growth factor receptor (EGFR) inhibitors for metastatic colorectal cancer.” Cochrane Database of Systematic Reviews(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy YY, et al. (2016). “The PI3K/Akt pathway is involved in procyanidin-mediated suppression of human colorectal cancer cell growth.” Molecular Carcinogenesis 55(12): 2196–2209. [DOI] [PubMed] [Google Scholar]
- Colin D, et al. (2011). “Endocytosis of resveratrol via lipid rafts and activation of downstream signaling pathways in cancer cells.” Cancer Prevention Research 4(7): 1095–1106. [DOI] [PubMed] [Google Scholar]
- Da Silva M, et al. (2012). “Large procyanidins prevent bile-acid-induced oxidant production and membrane-initiated ERK1/2, p38, and Akt activation in Caco-2 cells.” Free Radical Biology and Medicine 52(1): 151–159. [DOI] [PubMed] [Google Scholar]
- Delmas D, et al. (2004). “Redistribution of CD95, DR4 and DR5 in rafts accounts for the synergistic toxicity of resveratrol and death receptor ligands in colon carcinoma cells.” Oncogene 23(55): 8979. [DOI] [PubMed] [Google Scholar]
- Dong X. q., et al. (2013). “Preparation of A-type proanthocyanidin dimers from peanut skins and persimmon pulp and comparison of the antioxidant activity of A-type and B-type dimers.” Fitoterapia 91: 128–139. [DOI] [PubMed] [Google Scholar]
- Duhon D, et al. (2010). “The polyphenol epigallocatechin-3-gallate affects lipid rafts to block activation of the c-Met receptor in prostate cancer cells.” Molecular carcinogenesis 49(8): 739–749. [DOI] [PubMed] [Google Scholar]
- Erlejman AG, et al. (2004). “The interaction of flavonoids with membranes: Potential determinant of flavonoid antioxidant effects.” Free Radical Research 38(12): 1311–1320. [DOI] [PubMed] [Google Scholar]
- Ferguson K (2004). Active and inactive conformations of the epidermal growth factor receptor, Portland Press Limited. [DOI] [PubMed] [Google Scholar]
- Garcia-Aranda M and Redondo M (2019). “Targeting Receptor Kinases in Colorectal Cancer.” Cancers 11(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein NS and Armin M (2001). “Epidermal growth factor receptor immunohistochemical reactivity in patients with American Joint Committee on Cancer Stage IV colon adenocarcinoma - Implications for a standardized scoring system.” Cancer 92(5): 1331–1346. [DOI] [PubMed] [Google Scholar]
- Gosse F, et al. (2005). “Chemopreventive properties of apple procyanidins on human colon cancer-derived metastatic SW620 cells and in a rat model of colon carcinogenesis.” Carcinogenesis 26(7): 1291–1295. [DOI] [PubMed] [Google Scholar]
- Guo GF, et al. (2011). “Overexpression of SGLT1 and EGFR in colorectal cancer showing a correlation with the prognosis.” Medical Oncology 28: S197–S203. [DOI] [PubMed] [Google Scholar]
- Hakam A, et al. (1999). “Expression of insulin-like growth factor-1 receptor in human colorectal cancer.” Human Pathology 30(10): 1128–1133. [DOI] [PubMed] [Google Scholar]
- Kaur M, et al. (2006). “Grape seed extract inhibits in vitro and in vivo growth of human colorectal carcinoma cells.” Clinical Cancer Research 12(20): 6194–6202. [DOI] [PubMed] [Google Scholar]
- Khan K, et al. (2019). “TARGETING EGFR PATHWAY IN METASTATIC COLORECTAL CANCER-TUMOUR HETEROGENIETY AND CONVERGENT EVOLUTION.” Critical Reviews in Oncology/Hematology. [DOI] [PubMed] [Google Scholar]
- Kleiner DE and Stetler-Stevenson WG (1999). “Matrix metalloproteinases and metastasis.” Cancer Chemotherapy and Pharmacology 43(1): S42–S51. [DOI] [PubMed] [Google Scholar]
- Kumar S, et al. (2018). “Nimbolide reduces CD44 positive cell population and induces mitochondrial apoptosis in pancreatic cancer cells.” Cancer Letters 413: 82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupai K, et al. (2010). “Matrix metalloproteinase activity assays: Importance of zymography.” Journal of Pharmacological and Toxicological Methods 61(2): 205–209. [DOI] [PubMed] [Google Scholar]
- Lin J, et al. (2005). “Dietary intakes of fruit, vegetables, and fiber, and risk of colorectal cancer in a prospective cohort of women (United States).” Cancer Causes & Control 16(3): 225–233. [DOI] [PubMed] [Google Scholar]
- Lizarraga D, et al. (2007). “The importance of polymerization and galloylation for the antiproliferative properties of procyanidin-rich natural extracts.” Febs Journal 274(18): 4802–4811. [DOI] [PubMed] [Google Scholar]
- Ma Y-C, et al. (2014). “Epigallocatechin gallate inhibits the growth of human lung cancer by directly targeting the EGFR signaling pathway.” Oncology Reports 31(3): 1343–1349. [DOI] [PubMed] [Google Scholar]
- Macdonald JL and Pike LJ (2005). “A simplified method for the preparation of detergent-free lipid rafts.” Journal of Lipid Research 46(5): 1061–1067. [DOI] [PubMed] [Google Scholar]
- Mackenzie GG, et al. (2009). “Dimeric procyanidins are inhibitors of NF-κB–DNA binding.” Biochemical Pharmacology 78(9): 1252–1262. [DOI] [PubMed] [Google Scholar]
- Massie C and Mills IG (2006). “The developing role of receptors and adaptors.” Nature Reviews Cancer 6(5): 403. [DOI] [PubMed] [Google Scholar]
- Miura T, et al. (2007). “Apple procyanidins induce tumor cell apoptosis through mitochondrial pathway activation of caspase-3.” Carcinogenesis 29(3): 585–593. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, et al. (1999). “Endocytic sorting of lipid analogues differing solely in the chemistry of their hydrophobic tails.” Journal of Cell Biology 144(6): 1271–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munshi A, et al. (2005). Clonogenic cell survival assay Chemosensitivity, Springer: 21–28. [DOI] [PubMed] [Google Scholar]
- Nicholson R, et al. (2001). “EGFR and cancer prognosis.” European Journal of Cancer 37: 9–15. [DOI] [PubMed] [Google Scholar]
- Oberthür R, et al. (2017). “Simultaneous inhibition of IGF1R and EGFR enhances the efficacy of standard treatment for colorectal cancer by the impairment of DNA repair and the induction of cell death.” Cancer Letters 407: 93–105. [DOI] [PubMed] [Google Scholar]
- Oberthur R, et al. (2017). “Simultaneous inhibition of IGF1R and EGFR enhances the efficacy of standard treatment for colorectal cancer by the impairment of DNA repair and the induction of cell death.” Cancer Letters 407: 93–105. [DOI] [PubMed] [Google Scholar]
- Ollila F, et al. (2002). “Characterization of flavonoid–biomembrane interactions.” Archives of Biochemistry and Biophysics 399(1): 103–108. [DOI] [PubMed] [Google Scholar]
- Oteiza PI, et al. (2018). “Flavonoids and the gastrointestinal tract: Local and systemic effects.” Molecular Aspects of Medicine 61: 41–49. [DOI] [PubMed] [Google Scholar]
- Peters KL, et al. (2013). “The IGF1 receptor/insulin receptor dual kinase inhibitor BMS-754807 targets the cancer stem cell population in addition to its synergism with Lapatinib in colorectal cancer.” Cancer Research 73(8). [Google Scholar]
- Pierini R, et al. (2008). “Procyanidin effects on oesophageal adenocarcinoma cells strongly depend on flavan-3-ol degree of polymerization.” Molecular Nutrition & Food Research 52(12): 1399–1407. [DOI] [PubMed] [Google Scholar]
- Porter AG and Jänicke RU (1999). “Emerging roles of caspase-3 in apoptosis.” Cell Death and Differentiation 6(2): 99. [DOI] [PubMed] [Google Scholar]
- Psahoulia FH, et al. (2007). “Quercetin enhances TRAIL-mediated apoptosis in colon cancer cells by inducing the accumulation of death receptors in lipid rafts.” Molecular Cancer Therapeutics 6(9): 2591–2599. [DOI] [PubMed] [Google Scholar]
- Ratty A, et al. (1988). “Interaction of flavonoids with 1, 1-diphenyl-2-picrylhydrazyl free radical, liposomal membranes and soybean lipoxygenase-1.” Biochemical Pharmacology 37(6): 989–995. [DOI] [PubMed] [Google Scholar]
- Reddy BS (2018). “Diet and Colon Cancer: Evidence from Human and Animal Model Studies” Diet, nutrition and cancer: a critical evaluation. CRC Press: 47–66. [Google Scholar]
- Reinmuth N, et al. (2002). “Impact of insulin-like growth factor receptor-I function on angiogenesis, growth, and metastasis of colon cancer.” Laboratory Investigation 82(10): 1377–1389. [DOI] [PubMed] [Google Scholar]
- Rossi M, et al. (2010). “Proanthocyanidins and the risk of colorectal cancer in Italy.” Cancer Causes Control 21(2): 243–250. [DOI] [PubMed] [Google Scholar]
- Seshacharyulu P, et al. (2012). “Targeting the EGFR signaling pathway in cancer therapy.” Expert Opinion on Therapeutic Targets 16(1): 15–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shay G, et al. (2015). “Moving targets: Emerging roles for MMPs in cancer progression and metastasis.” Matrix Biology 44–46: 200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu M, et al. (2011). “Cancer chemoprevention with green tea catechins by targeting receptor tyrosine kinases.” Molecular Nutrition & Food Research 55(6): 832–843. [DOI] [PubMed] [Google Scholar]
- Siegel RL, et al. (2017). “Colorectal cancer statistics, 2017.” Ca-a Cancer Journal for Clinicians 67(3): 177–193. [DOI] [PubMed] [Google Scholar]
- Sigismund S, et al. (2018). “Emerging functions of the EGFR in cancer.” Molecular Oncology 12(1): 3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K and Toomre D (2000). “Lipid rafts and signal transduction.” Nature Reviews Molecular Cell Biology 1(1): 31–39. [DOI] [PubMed] [Google Scholar]
- Spano JP, et al. (2005). “Impact of EGFR expression on colorectal cancer patient prognosis and survival.” Annals of Oncology 16(1): 102–108. [DOI] [PubMed] [Google Scholar]
- Srivatsa S, et al. (2017). “EGFR in Tumor-Associated Myeloid Cells Promotes Development of Colorectal Cancer in Mice and Associates With Outcomes of Patients.” Gastroenterology 153(1): 178–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana H, et al. (2004). “Tea polyphenol epigallocatechin-3-gallate associates with plasma membrane lipid rafts: Lipid rafts mediate anti-allergic action of the catechin.” Biofactors 21(1-4): 383–385. [DOI] [PubMed] [Google Scholar]
- Terry P, et al. (2001). “Fruit, vegetables, dietary fiber, and risk of colorectal cancer.” Journal of the National Cancer Institute 93(7): 525–533. [DOI] [PubMed] [Google Scholar]
- Turk HF and Chapkin RS (2015). Analysis of Epidermal Growth Factor Receptor Dimerization by BS 3 Cross-Linking Receptor Tyrosine Kinases, Springer: 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vara JÁF, et al. (2004). “PI3K/Akt signalling pathway and cancer.” Cancer Treatment Reviews 30(2): 193–204. [DOI] [PubMed] [Google Scholar]
- Verstraeten SV, et al. (2015). “Interactions of flavan-3-ols and procyanidins with membranes: mechanisms and the physiological relevance.” Food & Function 6(1): 32–40. [DOI] [PubMed] [Google Scholar]
- Verstraeten SV, et al. (2013). “Procyanidins can interact with Caco-2 cell membrane lipid rafts: involvement of cholesterol.” Biochim Biophys Acta 1828(11): 2646–2653. [DOI] [PubMed] [Google Scholar]
- Weber MM, et al. (2002). “Overexpression of the insulin-like growth factor I receptor in human colon carcinomas.” Cancer: Interdisciplinary International Journal of the American Cancer Society 95(10): 2086–2095. [DOI] [PubMed] [Google Scholar]
- Wee P and Wang Z (2017). “Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways.” Cancers (Basel) 9(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, et al. (2016). “Substantial contribution of extrinsic risk factors to cancer development.” Nature 529(7584): 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, et al. (2017). “A-type ECG and EGCG dimers inhibit 3T3-L1 differentiation by binding to cholesterol in lipid rafts.” The Journal of nutritional biochemistry 48: 62–73. [DOI] [PubMed] [Google Scholar]
- Zhu W, et al. (2016). “Structure-Dependent Membrane-Perturbing Potency of Four Proanthocyanidin Dimers on 3T3-L1 Preadipocytes.” Journal of Agricultural and Food Chemistry 64(37): 7022–7032. [DOI] [PubMed] [Google Scholar]
- Zhu W, et al. (2015). “A-type ECG and EGCG dimers disturb the structure of 3T3-L1 cell membrane and strongly inhibit its differentiation by targeting peroxisome proliferator-activated receptor gamma with miR-27 involved mechanism.” J Nutr Biochem 26(11): 1124–1135. [DOI] [PubMed] [Google Scholar]
- Zou B, et al. (2014). “Persimmon tannin alleviates hepatic steatosis in L02 cells by targeting miR-122 and miR-33b and its effects closely associated with the A type ECG dimer and EGCG dimer structural units.” Journal of Functional Foods 11: 330–341. [Google Scholar]