Key Points
Question
What is the risk of developing cardiomyopathy among patients with the acute phase of Chagas infection or the indeterminate chronic form of Chagas disease?
Findings
In this systematic review and meta-analysis of 32 studies of patients with Chagas disease, the pooled estimated annual rate of cardiomyopathy was 4.6% among patients with acute Chagas infection and 1.9% among patients with indeterminate chronic Chagas disease.
Meaning
The findings indicate that asymptomatic individuals with indeterminate chronic Chagas disease without cardiac injury and individuals with acute Chagas infection may have a significant risk of developing chronic cardiomyopathy.
Abstract
Importance
Chagas cardiomyopathy is associated with substantial morbidity and mortality. Precise estimates of the risk of developing cardiomyopathy among patients with the acute or indeterminate chronic forms of Chagas disease are lacking.
Objective
To estimate the risk of developing chronic cardiomyopathy in patients with acute and indeterminate chronic forms of Chagas disease.
Data Sources
A systematic search in the Cochrane Library, Embase, Latin American and Caribbean Health Sciences Literature (LILACS), Medline, and Web of Science Core Collection databases was conducted from October 8 to October 24, 2018. Studies published between January 1, 1946, and October 24, 2018, that were written in the English, Spanish, and Portuguese languages were included. Search terms included Chagas disease; development of cardiomyopathy; latency duration; and determinants of the Chagas latency period.
Study Selection
Longitudinal observational studies of participants diagnosed with the acute phase of Chagas infection or the indeterminate chronic form of Chagas disease who were followed up until the development of cardiomyopathy were included. Studies were excluded if they did not provide sufficient outcome data. Of 10 761 records initially screened, 32 studies met the criteria for analysis.
Data Extraction and Synthesis
Critical appraisals of studies were performed using checklists from the Joanna Briggs Institute Reviewer’s Manual, and data were collected from published studies. A random-effects meta-analysis was used to obtain pooled estimated annual rates. Data were analyzed from September 11 to December 4, 2019. This study followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline for the registration of the protocol, data collection and integrity, assessment of bias, and sensitivity analyses.
Main Outcomes and Measures
Main outcomes were defined as the composite of the development of any new arrhythmias or changes in electrocardiogram results, dilated cardiomyopathy and segmental wall motion abnormalities in echocardiogram results, and mortality associated with Chagas disease.
Results
A total of 5005 records were screened for eligibility. Of those, 298 full-text articles were reviewed, and 178 of those articles were considered for inclusion in the quantitative synthesis. After exclusions, 32 studies that included longitudinal observational outcomes were selected for the analysis; 23 of those studies comprised patients with the indeterminate chronic form of Chagas disease, and 9 of those studies comprised patients in the acute phase of Chagas infection. The analysis indicated that the pooled estimated annual rate of cardiomyopathy development was 1.9% (95% CI, 1.3%-3.0%; I2 = 98.0%; τ2 [ln scale] = 0.9992) in patients with indeterminate chronic Chagas disease and 4.6% (95% CI, 2.7%-7.9%; I2 = 86.6%; τ2 [ln scale] = 0.4946) in patients with acute Chagas infection.
Conclusions and Relevance
Patients with the indeterminate chronic form of Chagas disease had a significant annual risk of developing cardiomyopathy. The annual risk was more than double among patients in the acute phase of Chagas infection.
This systematic review and meta-analysis assesses the risk of developing chronic cardiomyopathy among patients with acute and indeterminate chronic forms of Chagas disease.
Introduction
Approximately 6 million people in Latin America have Chagas disease, and more than 70 million people are at risk of developing the infection.1 The hemoflagellate protozoan Trypanosoma cruzi is the infectious agent. Transmission is most commonly vectorial through a bite from the triatomine insect (commonly known as the kissing bug). Other important routes of T cruzi transmission are oral (through food or drink contaminated with triatomine feces or secretions from infected mammals), vertical (or congenital, from mother to child during pregnancy), organ transplantation, blood transfusion, and unintentional laboratory exposure.
Chagas disease has 2 phases, acute and chronic. In the acute phase, which occurs during the first few weeks or months after infection, the disease is often subclinical and undiagnosed, as patients are asymptomatic or present with nonspecific symptoms, such as fever, malaise, hepatosplenomegaly, skin edema (termed chagoma, an inflammatory nodule at the site of the triatomine bite), or edema of 1 or both eyelids (termed the Romaña sign, a sensitization response to the triatomine bite). However, cardiac involvement may also occur and is generally indicative of a worse prognosis.2 The chronic phase of Chagas disease has 2 forms: indeterminate and determinate. The indeterminate form occurs after the acute phase and may last for decades (or even a lifetime) without symptoms. The indeterminate form can progress to the determinate form, which includes the development of cardiomyopathy, digestive disease, or cardiodigestive disease.3 The primary factor associated with morbidity in patients with Chagas disease is the development of chronic Chagas cardiomyopathy, which is manifested in heart failure, systemic and pulmonary embolism, arrhythmia, and sudden cardiac death. Diagnosis and treatment of patients with acute Chagas infection or the indeterminate chronic form of Chagas disease may decrease the risk of cardiomyopathy, vertical transmission, and death.4,5,6,7
The Chagas latency period encompasses the asymptomatic period from the end of the acute phase until the development of a determinate form of the disease. Early studies and clinical observations have estimated the latency period to be approximately 10 to 30 years. A longitudinal study in Brazil conducted from 1974 to 1984 found a progression rate of 38% from the indeterminate chronic form to the development of chagasic cardiomyopathy over the 10-year follow-up period.8 A cohort study in Argentina found a more rapid progression rate of 21% in less than 3 years.9 Oral transmission is also associated with a higher incidence of myocarditis and mortality.10
The risk of developing cardiomyopathy in patients with latent Chagas disease has prompted clinicians and public health agencies to design strategies to optimize Chagas screening programs and improve the treatment of asymptomatic individuals. However, as reported in several longitudinal studies, data regarding the risk of cardiomyopathy and its factors are inconsistent. A more informative and detailed analysis of contemporary data can be important to achieving a better understanding of the natural progression of Chagas disease. Through a systematic review and meta-analysis, we aimed to estimate the progression rates to cardiomyopathy among patients with the acute or the indeterminate chronic forms of Chagas disease.
Methods
Search Strategy and Selection Criteria
We performed a systematic review and meta-analysis of studies of cardiomyopathy development in patients with Chagas disease. The systematic review considered studies that explored the rate of progression to cardiomyopathy among patients with acute or indeterminate chronic Chagas disease. Longitudinal observational studies of participants diagnosed with the acute phase of Chagas infection or the indeterminate chronic form of Chagas disease who were followed up until the development of cardiomyopathy were included. Studies were excluded if they did not include sufficient outcome data. The detailed methods and Medline search strategy are available in eMethods in the Supplement. Additional details on the systematic review protocol have been published previously (PROSPERO CRD42019118019).11 This study followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline for the registration of the protocol, data collection and integrity, assessment of bias, and sensitivity analyses.
A systematic search in the Cochrane Library, Embase, Latin American and Caribbean Health Sciences Literature (LILACS), Medline, and Web of Science Core Collection databases was conducted from October 8 to October 24, 2018. Studies published between January 1, 1946, and October 24, 2018, that were written in the English, Spanish, and Portuguese languages were included. Search terms included Chagas disease; development of cardiomyopathy; latency duration; and determinants of the Chagas latency period.
Data Analysis and Quality Assessment
Study data were collected and managed using REDCap electronic data capture tools hosted at the University of Colorado Denver. Extracted data included the type of study, the study’s country of origin, the number of participants, the duration of follow-up, the demographic characteristics of the study population, and the study’s methods, interventions (diagnostic and therapeutic), and primary outcomes of interest. The following primary cardiac outcomes were extracted: (1) the development of cardiac or heart failure symptoms, including shortness of breath, dyspnea on exertion, lower extremity edema, paroxysmal nocturnal dyspnea, and orthopnea; (2) the development of structural cardiomyopathy or cardiac arrhythmias, as observed in abnormal echocardiogram and/or abnormal electrocardiogram (ECG) results; and (3) the presence of complications associated with advanced cardiomyopathy, including mortality associated with advanced heart failure, sudden death, pulmonary embolism, or stroke. Secondary outcomes, such as hospitalization rates, heart transplant receipt status, and implantable cardioverter-defibrillator or pacemaker requirements, which were outlined in the initial protocol, were not analyzed owing to insufficient data.
Two reviewers with expertise in Chagas disease (A.H.M. and C.F.P.) independently reviewed the selected studies for methodological quality and performed quality standard measures using the System for the Unified Management, Assessment and Review of Information (SUMARI; Joanna Briggs Institute) software. A third reviewer (S.C.) was asked to reconcile any disagreements between the 2 reviewers. Critical appraisals were performed using checklists from the Joanna Briggs Institute Reviewer’s Manual for cohort studies, case-control studies, case-series studies, case reports, and cross-sectional studies.12 All studies that had positive answers of more than 60% for the critical appraisal questions were subject to data extraction and synthesis.
Statistical Analysis
Cardiac events were defined as the composite of the development of any new arrhythmias or changes in ECG results, dilated cardiomyopathy or segmental wall motion abnormalities in echocardiogram, and mortality associated with Chagas disease. Event rates were extracted by calculating the cumulative percentage of cardiac events among the participants over the study duration, assuming a constant exponential hazard time to event distribution and solving for the rate. The extracted ratio was log transformed, and SEs were obtained from the cumulative percentage of cardiac events, the number of participants, and the duration of the study using the delta method. Because the studies were conducted over varying lengths of time, and we could only obtain the proportion of events that occurred at the studies’ end points, a constant rate had to be assumed. Log transformation of the estimated rates reduced the skew of their distributions.
A random-effects meta-analysis was then performed to combine the estimated log rates from the different studies into a single estimated log rate, which was back-transformed for interpretation. Between-study heterogeneity was estimated using the I2 statistic. Subgroup and meta-regression analyses were conducted to examine other factors of cardiomyopathy development and sources of heterogeneity; these analyses included the study’s country of origin, the participants’ ages, and the percentages of men vs women. A post hoc analysis was performed to examine antiparasitic treatments received (per study arm), year of study, study size and duration, and route of disease transmission. Continuous variables were dichotomized using the mean. When high heterogeneity was obtained, we performed a subgroup analysis or removed selected studies to assess the source of the high heterogeneity. Contour-enhanced funnel plots were constructed to assess publication bias. Statistical analyses were performed using Stata software, version 16.0 (StataCorp). Data were analyzed from September 11 to December 4, 2019.
Results
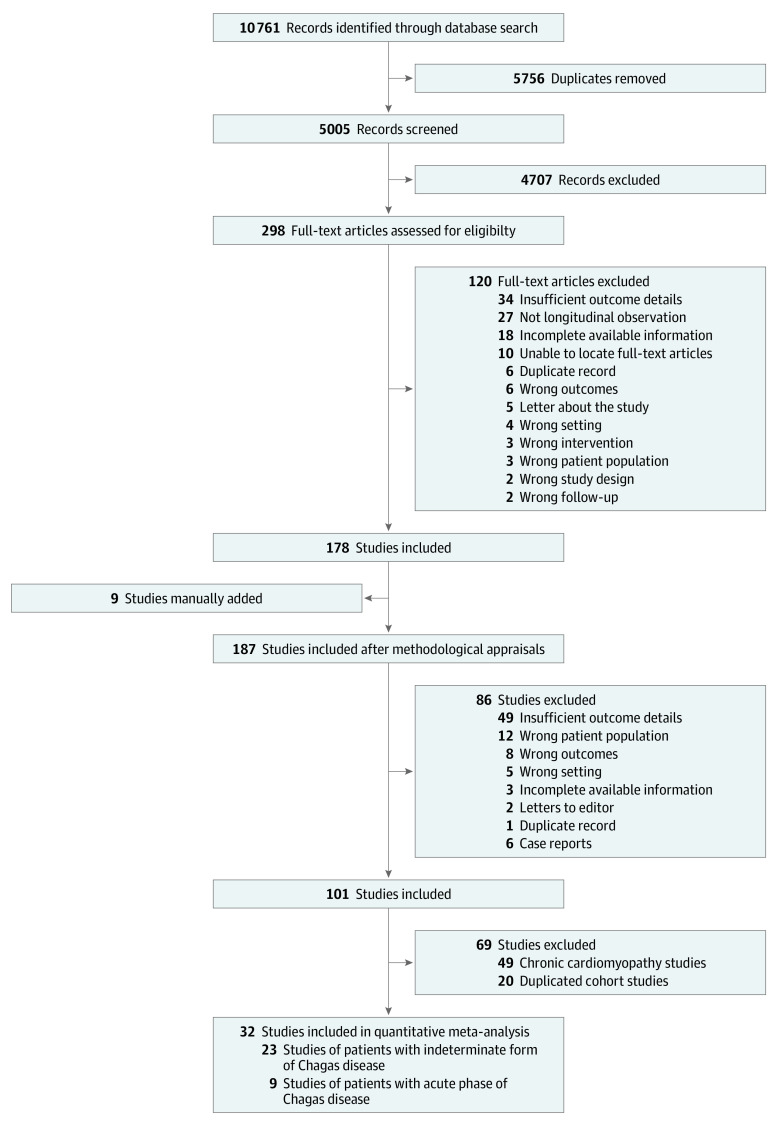
A total of 10 761 records were identified through database searches. Of those, 5005 records were screened for eligibility based on titles and abstracts; 298 full-text articles were reviewed, and 178 of those articles were considered for inclusion in the quantitative synthesis (Figure 1). Nine articles were then manually added, for a total of 187 studies. Of those, 101 studies passed the initial methodological appraisal. We then excluded 20 studies because they originated from identical cohorts and 49 studies because they included patients who had already received a diagnosis of chronic cardiomyopathy. After all exclusions, 32 studies were included in the meta-analysis, 23 of which comprised patients with the indeterminate chronic form of Chagas disease and 9 of which comprised patients with acute Chagas infection at the onset of observation.
Figure 1. PRISMA Flow Diagram .
Indeterminate Chronic Chagas Infection
Twenty-three studies8,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34 had longitudinal observational outcomes for patients with the indeterminate chronic form of Chagas disease (Table 1). Most of these studies were of prospective cohorts and were conducted in either Brazil or Argentina between 1960 and 2005. Samples ranged from 9 to 3336 participants, with a mean of 345 participants per study. Distribution among sexes was equal, with a mean of 46% men per study. Not all studies had age data available. Among studies that included age data, the ages ranged from 10 to 44 years, with a mean age of 31 years. The mean follow-up duration was 8.5 years, with a range of 3 to 18 years. The pooled estimated annual rate of the development of chronic Chagas cardiomyopathy was 1.9% (95% CI, 1.3%-3.0%; I2 = 98.0%; τ2 [ln scale] = 0.9992) (Figure 2). The cumulative probability of experiencing a cardiac event was approximately 17% at 10 years and 31% at 20 years (eFigure 1 in the Supplement).
Table 1. Baseline Characteristics and Clinical Outcomes of Patients With the Indeterminate Chronic Form of Chagas Disease.
| Source | Study design | Country | Participants, No. | Male sex, No. (%) | Age, mean, y | Interventiona | Study duration, y | Cardiac events, No. (%)b | Estimated rate (95% CI)c | Weight, % |
|---|---|---|---|---|---|---|---|---|---|---|
| Viotti et al,13 2004 | Cross-sectional | Argentina | 505 | 333 (65.9) | 40.5 | None | 9.9 | 139 (27.5) | 3.3 (2.8-3.8) | 4.9 |
| Machado-de-Assis et al,14 2013 | Prospective cohort | Brazil | 23 | 8 (34.8) | 26.7 | Benznidazole therapy | 13.0 | 4 (17.4) | 1.5 (0.6-3.9) | 4.0 |
| Zulantay et al,15 2005 | Prospective cohort | Chile | 10 | NA | NA | Other antiparasitic therapyd | 7.0 | 1 (10.0) | 1.5 (0.2-10.7) | 2.5 |
| Mota et al,16 1990 | Prospective cohort | Brazil | 248 | 108 (43.5) | NA | None | 5.8 | 90 (36.3) | 7.8 (6.3-9.6) | 4.9 |
| Coura et al,8 1985 | Prospective cohort | Brazil | 60 | NA | NA | None | 10.0 | 23 (38.3) | 4.8 (3.2-7.3) | 4.7 |
| Pereira et al,17 1985 | Case-control | Brazil | 77 | NA | 31.2 | None | 6.0 | 33 (42.9) | 9.3 (6.6-13.2) | 4.8 |
| Espinosa et al,18 1985 | Prospective cohort | Venezuela | 18 | 9 (50.0) | 37.0 | None | 9.4 | 1 (5.6) | 0.6 (0.1-4.3) | 2.5 |
| Apt et al,19 2003 | Randomized clinical trial | Chile | 202 | NA | NA | Other antiparasitic therapyd | 9.0 | 30 (14.9) | 0.2 (0.1-0.6) | 4.1 |
| Fabbro De Suasnabar et al,20 2000 | Prospective cohort | Argentina | 179 | NA | NA | Benznidazole or nifurtimox therapye | 14.0 | 10 (5.6) | 0.4 (0.2-0.8) | 4.5 |
| Colantonio et al,21 2016 | Retrospective cohort | Argentina | 86 | NA | 10.0 | Benznidazole therapy or placebof | 13.0 | 16 (18.6) | 1.6 (1.0-2.6) | 4.7 |
| de Andrade et al,22 1998 | Prospective cohort | Brazil | 125 | NA | 10.4 | None | 3.0 | 5 (4.0) | 1.4 (0.6-3.3) | 4.1 |
| Andrade et al,23 2016 | Prospective cohort | Brazil | 9 | NA | NA | Benznidazole therapy | 5.0 | 2 (22.2) | 5.0 (1.3-20.2) | 3.3 |
| Fragata-Filho et al,24 2016 | Prospective cohort | Brazil | 310 | 107 (34.5) | 34.5 | Benznidazole therapyg | 18.0 | 80 (25.8) | 1.7 (1.3-2.1) | 4.9 |
| Pereira et al,25 1990 | Prospective cohort | Brazil | 92 | NA | 39.6 | None | 4.5 | 12 (13.0) | 3.1 (1.8-5.5) | 4.6 |
| Viotti et al,26 2005 | Prospective cohort | Argentina | 731 | 355 (48.6) | 43.7 | None | 8.0 | 34 (4.7) | 0.6 (0.4-0.8) | 4.8 |
| Ianni et al,27 1998 | Prospective cohort | Brazil | 160 | 62 (38.8) | 36.5 | None | 8.2 | 4 (2.5) | 0.3 (0.1-0.8) | 4.0 |
| da Silva et al,28 1994 | Prospective cohort | Brazil | 73 | NA | NA | None | 7.8 | 8 (11.0) | 1.5 (0.7-3.0) | 4.4 |
| Castro et al,29 2001 | Prospective cohort | Brazil | 120 | NA | NA | None | 13.0 | 19 (15.8) | 1.3 (0.8-2.1) | 4.7 |
| Macedo,30 1976 | Prospective cohort | Brazil | 471 | NA | NA | None | 5.0 | 190 (40.3) | 10.3 (8.9-11.9) | 4.9 |
| Manzullo et al,31 1982 | Prospective cohort | Multicenterh | 3336 | 1944 (58.3) | NA | None | 3.0 | 771 (23.1) | 8.8 (8.2-9.4) | 5.0 |
| Storino,32 1993 | Prospective cohort | Argentina | 78 | 35 (44.9) | 36.1 | None | 5.0 | 16 (20.5) | 4.6 (2.8-7.5) | 4.7 |
| Brasil,33 1965 | Prospective cohort | Brazil | 43 | NA | NA | None | 9.1 | 8 (18.6) | 2.3 (1.1-4.5) | 4.4 |
| Forichon,34 1975 | Prospective cohort | Brazil | 885 | 373 (42.1) | NA | None | 10.0 | 32 (3.6) | 0.4 (0.3-0.5) | 4.8 |
Abbreviation: NA, not available or not applicable.
Antiparasitic treatment.
Cardiac events include the development of any new changes in electrocardiogram results, arrhythmias, dilated cardiomyopathy, segmental motion abnormality, or mortality associated with Chagas disease.
Estimated rate was calculated using the exponential survival method (1 divided by the number of years multiplied by the − logarithmic function of [100 minus the number of cardiac events divided by the total number of participants]).
Allopurinol and itraconazole therapy.
Two cardiac events occurred in 63 participants in the treatment arm, and 8 cardiac events occurred in 116 participants in the control arm.
Eight cardiac events occurred in 48 participants in the treatment arm, and 8 cardiac events occurred in 38 participants in the control arm.
A total of 55 cardiac events occurred in 263 participants in the treatment arm, and 25 cardiac events occurred in 47 participants in the control arm.
Centers included Argentina, Bolivia, Brazil, Chile, Paraguay, Uruguay, and Peru.
Figure 2. Forest Plot of Cardiomyopathy Risk in Studies of Patients With the Indeterminate Chronic Form of Chagas Disease.
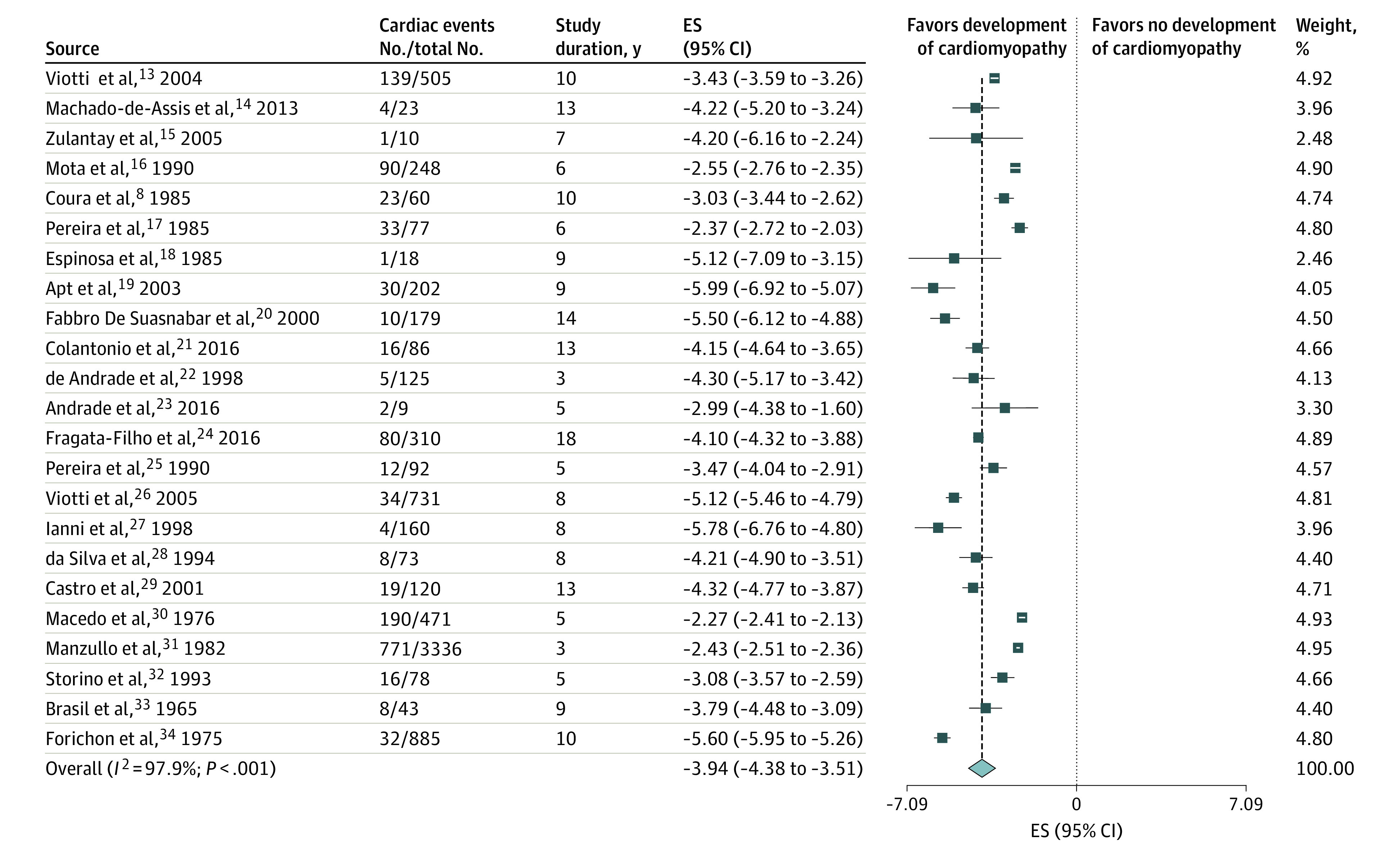
A greater negative logarithmic estimated rate converts to a lower back-transformed rate. Weights are from random-effects analysis. ES indicates effect size.
Subgroup and post hoc analyses indicated no difference between studies based on year of study (2.8% for studies before 1985 vs 1.4% for studies after 1985; P = .10), study size (1.9% for studies with <200 participants vs 2.1% for studies with >200 participants; P = .83), mean age of participants (2.4% for studies with a mean participant age of <32 years vs 1.6% for studies with a mean participant age of >32 years; P = .84), and percentage of men (1.0% for studies comprising <40% men vs 2.2% for studies comprising >40% men; P = .46) (eTable 1, eFigure 3, eFigure 4, eFigure 5, and eFigure 6 in the Supplement). Among studies originating in Brazil, participants had a significantly higher annual rate of cardiomyopathy development (2.3%; 95% CI, 1.2%-4.3%) compared with studies of patients from other South American countries (1.1%; 95% CI, 0.5%-2.4%; P = .05; 1 study was omitted because it was conducted in both Brazil and Peru) (eTable 1 and eFigure 7 in the Supplement).
The post hoc subgroup of participants who received antiparasitic treatment had a significantly lower pooled estimated annual rate of cardiomyopathy development (1.0%; 95% CI, 0.5%-1.9%) compared with the subgroup who did not receive treatment (2.3%; 95% CI, 1.5%-3.5%; P = .03) (eTable 1 and eFigure 8 in the Supplement). Studies of longer duration (≥9 years) also had a significantly lower pooled estimated annual rate than those of shorter duration (<9 years; 1.2% vs 3.2%, respectively; P = .001) (eTable 1 and eFigure 9 in the Supplement). Heterogeneity remained high after we individually removed specific studies; however, the pooled estimated rates were consistent at a range of 1.6% to 1.9%.
Acute Chagas Infection
Nine studies35,36,37,38,39,40,41,42,43 included longitudinal observational outcomes for patients with acute Chagas infection (Table 2). These studies were primarily case series or prospective cohorts performed between 1940 and 2006 in Brazil, Venezuela, and El Salvador. The main routes of transmission in these studies were vectorial followed by oral. The mean number of participants per study was 50, with an even distribution of men and women and a mean age of 26 years. The duration of longitudinal observation ranged from 6 months to 27 years. The pooled estimated rate of the development of chronic Chagas cardiomyopathy was 6.8% (95% CI, 3.3%-14.2%; I2 = 93.3%; τ2 [ln scale] = 1.1347) (eFigure 10 in the Supplement). The cumulative probability of a cardiac event was approximately 49% at 10 years. After removal of 1 study that had a follow-up period of only 6 months,37 we found a pooled estimated annual rate of 4.6% (95% CI, 2.7%-7.9% per year; I2 = 86.6%; τ2 [ln scale] = 0.4946), with a modest decrease in heterogeneity (Figure 3), and a cumulative probability of experiencing a cardiac event of approximately 40% at 10 years (eFigure 2 in the Supplement).
Table 2. Baseline Characteristics and Clinical Outcomes of Patients With the Acute Form of Chagas Disease.
| Source | Study design | Country | Type of transmission | Participants, No. | Male sex, No. (%) | Age, mean, y | Study duration, y | Cardiac events, No. (%)a | Estimated rate (95% CI)b | Weight, % |
|---|---|---|---|---|---|---|---|---|---|---|
| Pedrosa et al,35 1993 | Case series | Brazil | Vectorial | 40 | 24 (60.0) | NA | 9.0 | 14 (35.0) | 4.8 (2.8-8.1) | 11.6 |
| Inglessis,361998 | Case series | Venezuela | Vectorial | 10 | 6 (60.0) | 23.0 | 5.5 | 6 (60.0) | 16.8 (7.3-38.4) | 10.7 |
| Bastos et al,37 2010 | Case series | Brazil | Oral | 11 | 8 (72.7) | 24.6 | 0.5 | 6 (54.5) | 157.5 (69.1-359.3) | 10.7 |
| Pinto et al,38 2013 | Prospective cohort | Brazil | Oral | 179 | NA | NA | 5.5 | 52 (29.1) | 6.1 (4.7-8.1) | 12.1 |
| Gus et al,39 1993 | Case series | Brazil | Oral | 17 | 8 (47.1) | 30.4 | 26.0 | 6 (35.3) | 1.7 (0.7-3.8) | 10.7 |
| Urrutia,40 1976 | Case series | El Salvador | Vectorial | 40 | 14 (35.0) | NA | 5.0 | 6 (15.0) | 3.3 (1.5-7.2) | 10.7 |
| Ortiz et al,41 2019 | Prospective cohort | Brazil | Oral | 25 | NA | NA | 1.3 | 4 (16.0) | 13.5 (5.1-36.1) | 10.1 |
| Dias et al,42 1956 | Prospective cohort | Brazil | Vectorial | 40 | NA | NA | 10.0 | 17 (42.5) | 5.5 (3.4-9.0) | 11.7 |
| Pinto Dias,43 2015 | Prospective cohort | Brazil | Vectorial | 59 | NA | NA | 27.0 | 18 (30.5) | 1.3 (0.8-2.1) | 11.7 |
Abbreviation: NA, not available or not applicable.
Cardiac events include the development of any new arrhythmias, changes in electrocardiogram results, or sudden death.
Estimated rate was calculated using the exponential survival method (1 divided by the number of years multiplied by the − logarithmic function of [100 minus the number of cardiac events divided by the total number of participants]).
Figure 3. Forest Plot of Cardiomyopathy Risk in Studies of Patients With the Acute Form of Chagas Disease.

A greater negative logarithmic estimated rate converts to a lower back-transformed rate. Weights are from random-effects analysis. ES indicates effect size.
After exclusion of the study with a follow-up period of 6 months, the subgroup post hoc analysis based on the year of study onset (before or after 1975, with 1975 representing the mean of all years of study onset) indicated statistically higher pooled estimated annual rates of cardiomyopathy development for studies after 1975 compared with those before 1975 (10.1% vs 2.9%, respectively; P = .007) (eTable 2 and eFigure 11 in the Supplement). A meta-regression analysis with an indicator variable revealed an estimated rate ratio for pre-1975 studies compared with post-1975 studies of 3.49 (95% CI, 1.09–11.19; P = .04; I2 = 79.7; τ2 [ln scale] = 0.3094), which represented an increase of 249%. By modeling the year of study onset as a continuous variable in the meta-regression analysis, we estimated a statistically significant rate increase of 3.32% per year (estimated rate ratio, 1.03; 95% CI, 1.01-1.06; P = .02; I2 = 68.2; τ2 [ln scale] = 0.1922).
In our subgroup and post hoc analyses, the results were not substantially changed by the study’s size (7.2% for studies with <40 participants vs 3.8% for studies with >40 participants; P = .42), the study’s country of origin (4.0% for studies performed in Brazil vs 7.4% for studies performed in other countries; P = .50), the percentage of men (8.6% for studies with <50% men vs 2.3% for studies with >50% men; P = .27), or the route of disease transmission (4.4% for studies of vectorial transmission vs 5.1% for studies of oral transmission; P = .84) (eTable 1, eFigure 12, eFigure 13, and eFigure 14 in the Supplement). There were not enough individual data to perform subgroup analyses by age, type of study, or receipt of antitrypanosomal treatment. A model that included the percentage of men as a continuous variable estimated a rate increase of 5.6% per year (estimated rate ratio, 1.06; 95% CI, 0.87-1.33; P = .36; I2 = 80.1; τ2 [ln scale] = 0.6266) (eTable 1 and eFigure 15 in the Supplement).
A funnel plot for publication bias indicated missing studies in the middle and right side of the plot, suggesting that publication bias was plausible (eFigure 16 in the Supplement). Possible explanations for a noninterventional outcome measure included inadequate analysis, different effect sizes, sampling variation, and chance.44
Discussion
We found a pooled estimated annual rate of 1.9% for the development of cardiomyopathy among patients with indeterminate chronic Chagas disease. This rate increased to 4.6% annually among patients diagnosed with acute Chagas infection. Among studies of patients with indeterminate chronic Chagas disease, we found an increased rate of cardiomyopathy development in studies that were conducted in Brazil, studies with shorter durations, and studies that did not include antiparasitic treatment. Researchers from Brazil have been pioneers in not only the discovery of Chagas disease but also in the performance of initial longitudinal studies of the disease. We hypothesize that earlier studies, which were mainly conducted in Brazil, had fewer treatment options available and were performed during a time when vectorial control measures were not as strict compared with more contemporary studies.
In studies in which patients received treatment with an antiparasitic drug, the progression to cardiomyopathy was significantly reduced compared with studies in which patients did not receive antiparasitic treatment. Longer observation periods in studies can introduce survival or disease-free biases. Male sex has also been associated with an increase in cardiomyopathy progression in several studies.45,46 Possible explanations include occupational differences that may be associated with an increased risk of continued exposure to vectors or intrinsic host-mediated factors. However, we could not assess the differences between sexes in our meta-analyses, probably owing to a lack of statistical power. Although we did not find an association between age and cardiomyopathy progression among patients with the indeterminate chronic form of Chagas disease, in many countries in which the disease is endemic, heart disease associated with Chagas infection is most frequently observed in older adults.
We also found an increase in the progression from acute infection to cardiomyopathy among studies conducted after 1975, likely because of the use and availability of more sensitive methods for the detection of cardiomyopathy. Most acute Chagas infections remain unnoticed because symptoms are mild, nonspecific, or even absent.47 Recognition of acute infection, although clinically challenging, becomes more important in endemic regions.
Our pooled estimates were consistent with those of previously published studies.46,48 One cohort study sponsored by the National Institutes of Health found an annual cardiomyopathy progression rate of 1.85% at 10 years of follow-up among patients with asymptomatic Chagas disease, which had been detected because the individuals had donated blood.46 A more recently published Brazilian cohort study also found a similar annual progression rate of 1.48% at 22 years of follow-up.48 Guidelines from the American Heart Association cite an annual rate of progression from the indeterminate chronic form of Chagas disease to cardiomyopathy of 1.85% to 7.0%.49
The process of cardiac injury during indeterminate chronic Chagas disease is multifactorial. The T cruzi genotype, persistent exposure to vectors, detection of parasitemia through polymerase chain reaction testing, oral acquisition, and recurrent infections have all been associated with cardiomyopathy onset and severity.50,51,52 The cumulative burden of disease observed in endemic areas suggest that persistent exposure to vectors is one of the main factors in the progression to cardiomyopathy. There is consensus that persistent tissue parasitism is associated with myocardial injury.50 Recurrent infections and substantial exposure to T cruzi, as observed with oral transmission, may be associated with more severe lesions.51
The higher risk of progression to cardiomyopathy that is observed in patients with acute Chagas disease suggests the need for rapid assessments of this population for the initiation of treatment to decrease disease morbidity. This higher risk also highlights the importance of ensuring that populations living in areas with high transmission rates have access to regular disease detection and treatment. This approach was instrumental in a Trypanosoma brucei elimination strategy that was implemented in Africa.53 However, only 1% to 2% (or less) of people with Chagas disease currently benefit from diagnosis and treatment.54,55
Oral transmission has also emerged as an important source of infection and warrants the implementation of public health measures to prevent contamination of food and drink. Annual cardiomyopathy progression rates of approximately 2% to 5% are substantial enough to explore the benefits of antitrypanosomal therapy at diagnosis (with either the indeterminate or acute form) coupled with strict vector control in endemic areas. Previous epidemiologic statements of indeterminate periods of 10 to 30 years may distract clinicians and public health authorities from the importance of exploring treatment options for patients with acute or indeterminate chronic forms of the disease. Annual estimates are a more accurate way to assess the short- and long-term risks of disease progression and provide real-time updated information to clinicians managing this condition when they are evaluating patients for treatment.
Limitations
This study has several limitations. A substantial number of the observational studies included in our analysis had different epidemiologic settings and study designs, which translated to high heterogeneity. Given the relatively small samples, we could not be highly selective with the studies we used. The longitudinal studies on Chagas disease differed in their study populations, case selection processes, and follow-up durations.
The small samples were also a limitation for our subgroup analyses. Most studies used only changes in ECG results to determine the onset of chronic Chagas cardiomyopathy, which can decrease the sensitivity of cardiac injury detection and underestimate the risk of cardiomyopathy; however, the common use of ECG results added uniformity to the outcome’s definition. In contrast, the annual risk of cardiomyopathy development is typically estimated from the diagnosis of indeterminate chronic Chagas disease; however, patients may enter the latent phase of the disease several years before they receive a diagnosis.
The progression rate to cardiomyopathy is also nonlinear, as we assumed for this type of analysis. The rates of progression in patients with the early indeterminate form of Chagas disease are probably lower than those of patients with late indeterminate form. Few studies have included samples of younger patients with indeterminate chronic Chagas disease because most patients remain asymptomatic for 10 to 30 years after the initial infection (most often acquired during childhood through vector transmission), which is supported by the limited number of samples comprising younger patients with the indeterminate form that were included in this meta-analysis. Thus, the cardiomyopathy progression rate might be overestimated.
In this population, the mean follow-up duration was 8.5 years, with a maximum duration of 18 years. We were unable to identify whether the rate of cardiomyopathy development increased, remained stable, or decreased over time. We were also unable to extrapolate these data to patients with Chagas infection in nonendemic countries. It is possible that in nonendemic settings, which present little or no risk of reinfection, the progression to cardiomyopathy is delayed.
Conclusions
To our knowledge, this systematic review addressed, for the first time in a scientific framework, the compiled data on factors associated with progression from the acute phase or the indeterminate chronic form of Chagas disease to the chronic cardiac form of the disease as well as the estimated annual rate of cardiomyopathy development. Based on our findings, a substantial cumulative risk of cardiomyopathy development may exist among those populations. Implementation and improvement of screening programs for Chagas disease that include assessment for antitrypanosomal treatment at the time of diagnosis are needed.
eMethods. Search Strategy, Selection Criteria, and Database Search Strategy
eTable 1. Subgroup Analysis of Patients With the Indeterminate Chronic Form of Chagas Disease
eTable 2. Subgroup Analysis of Patients With the Acute Form of Chagas Disease
eFigure 1. Cumulative Risk of a Cardiac Event in Studies of Patients With the Indeterminate Chronic Form of Chagas Disease
eFigure 2. Cumulative Risk of a Cardiac Event in Studies of Patients With the Acute Form of Chagas Disease
eFigure 3. Subgroup Analysis by Year of Study for Studies of Patients With the Indeterminate Chronic Form of Chagas Disease
eFigure 4. Subgroup Analysis by Study Size for Studies of Patients With the Indeterminate Chronic Form of Chagas Disease
eFigure 5. Subgroup Analysis by Mean Age of Participants for Studies of Patients With the Indeterminate Chronic Form of Chagas Disease
eFigure 6. Subgroup Analysis by Percentage of Men for Studies of Patients With the Indeterminate Chronic Form of Chagas Disease
eFigure 7. Subgroup Analysis by Study’s Country of Origin for Studies of Patients With the Indeterminate Chronic Form of Chagas Disease
eFigure 8. Subgroup Analysis by Use of Antitrypanosomal Treatment Intervention for Studies of Patients With the Indeterminate Chronic Form of Chagas Disease
eFigure 9. Subgroup Analysis by Study Duration (in Years) for Studies of Patients With the Indeterminate Chronic Form of Chagas Disease
eFigure 10. Forest Plot of Cardiomyopathy Risk in Studies of Patients With the Acute Form of Chagas Disease (Including the 6-Month Follow-Up Study)
eFigure 11. Subgroup Analysis by Year of Study for Studies of Patients With the Acute Form of Chagas Disease
eFigure 12. Subgroup Analysis by Study Size for Studies of Patients With the Acute Form of Chagas Disease
eFigure 13. Subgroup Analysis by Study’s Country of Origin for Studies of Patients With the Acute Form of Chagas Disease
eFigure 14. Subgroup Analysis by Route of Transmission for Studies of Patients With the Acute Form of Chagas Disease
eFigure 15. Subgroup Analysis by Percentage of Men for Studies of Patients With the Acute Form of Chagas Disease
eFigure 16. Funnel Plot for Publication Bias
eReferences
References
- 1.Chagas disease in Latin America: an epidemiological update based on 2010 estimates. News release. World Health Organization; February 6, 2015. Accessed December 7, 2019. https://www.who.int/wer/2015/wer9006.pdf?ua=1 [PubMed]
- 2.das Neves Pinto AY, da Silva Valente SA, da Costa Valente V. Emerging acute Chagas disease in Amazonian Brazil: case reports with serious cardiac involvement. Braz J Infect Dis. 2004;8(6):454-460. [DOI] [PubMed] [Google Scholar]
- 3.Rassi A Jr, Rassi A, Marin-Neto JA. Chagas disease. Lancet. 2010;375(9723):1388-1402. doi: 10.1016/S0140-6736(10)60061-X [DOI] [PubMed] [Google Scholar]
- 4.Sguassero Y, Cuesta CB, Roberts KN, et al. . Course of chronic Trypanosoma cruzi infection after treatment based on parasitological and serological tests: a systematic review of follow-up studies. PLoS One. 2015;10(10):e0139363. doi: 10.1371/journal.pone.0139363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Viotti R, Vigliano C, Lococo B, et al. . Long-term cardiac outcomes of treating chronic Chagas disease with benznidazole versus no treatment: a nonrandomized trial. Ann Intern Med. 2006;144(10):724-734. doi: 10.7326/0003-4819-144-10-200605160-00006 [DOI] [PubMed] [Google Scholar]
- 6.Alvarez MG, Vigliano C, Lococo B, Bertocchi G, Viotti R. Prevention of congenital Chagas disease by benznidazole treatment in reproductive-age women. an observational study. Acta Trop. 2017;174:149-152. doi: 10.1016/j.actatropica.2017.07.004 [DOI] [PubMed] [Google Scholar]
- 7.Fabbro DL, Danesi E, Olivera V, et al. . Trypanocide treatment of women infected with Trypanosoma cruzi and its effect on preventing congenital Chagas. PLoS Negl Trop Dis. 2014;8(11):e3312. doi: 10.1371/journal.pntd.0003312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coura JR, de Abreu LL, Pereira JB, Willcox HP. Morbidity in Chagas’ disease. IV. longitudinal study of 10 years in Pains and Iguatama, Minas Gerais, Brazil. Article in Portuguese. Mem Inst Oswaldo Cruz. 1985;80(1):73-80. doi: 10.1590/S0074-02761985000100011 [DOI] [PubMed] [Google Scholar]
- 9.Basquiera AL, Sembaj A, Aguerri AM, et al. . Risk progression to chronic Chagas cardiomyopathy: influence of male sex and of parasitaemia detected by polymerase chain reaction. Heart. 2003;89(10):1186-1190. doi: 10.1136/heart.89.10.1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henao-Martinez AF. Rapid progressive chagasic myocarditis: a multifactorial condition. Am J Trop Med Hyg. 2016;95(1):247. doi: 10.4269/ajtmh.16-0119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henao-Martinez AF, Chadalawada S, Villamil-Gomez WE, DeSanto K, Rassi A Jr, Franco-Paredes C Duration and determinants of Chagas latency: an etiology and risk systematic review protocol. JBI Database System Rev Implement Rep 2019;17(10):2122-2128. [DOI] [PubMed]
- 12.Aromataris E, Munn Z, eds. Joanna Briggs Institute Reviewer’s Manual. Joanna Briggs Institute; 2017. [Google Scholar]
- 13.Viotti RJ, Vigliano C, Laucella S, et al. . Value of echocardiography for diagnosis and prognosis of chronic Chagas disease cardiomyopathy without heart failure. Heart. 2004;90(6):655-660. doi: 10.1136/hrt.2003.018960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Machado-de-Assis GF, Diniz GA, Montoya RA, et al. . A serological, parasitological and clinical evaluation of untreated Chagas disease patients and those treated with benznidazole before and thirteen years after intervention. Mem Inst Oswaldo Cruz. 2013;108(7):873-880. doi: 10.1590/0074-0276130122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zulantay I, Arribada A, Honores P, et al. . No association between persistence of the parasite and electrocardiographic evolution in treated patients with Chagas disease. Article in Spanish. Rev Med Chil. 2005;133(10):1153-1160. [DOI] [PubMed] [Google Scholar]
- 16.Mota EA, Guimaraes AC, Santana OO, Sherlock I, Hoff R, Weller TH. A nine year prospective study of Chagas’ disease in a defined rural population in northeast Brazil. Am J Trop Med Hyg. 1990;42(5):429-440. doi: 10.4269/ajtmh.1990.42.429 [DOI] [PubMed] [Google Scholar]
- 17.Pereira JB, Willcox HP, Coura JR. Morbidity in Chagas’ disease. III. longitudinal study of 6 years, in Virgem da Lapa, MG, Brazil. Article in Portuguese. Mem Inst Oswaldo Cruz. 1985;80(1):63-71. doi: 10.1590/S0074-02761985000100010 [DOI] [PubMed] [Google Scholar]
- 18.Espinosa R, Carrasco HA, Belandria F, et al. . Life expectancy analysis in patients with Chagas’ disease: prognosis after one decade (1973-1983). Int J Cardiol. 1985;8(1):45-56. doi: 10.1016/0167-5273(85)90262-1 [DOI] [PubMed] [Google Scholar]
- 19.Apt W, Arribada A, Zulantay I, Sanchez G, Vargas SL, Rodriguez J. Itraconazole or allopurinol in the treatment of chronic American trypanosomiasis: the regression and prevention of electrocardiographic abnormalities during 9 years of follow-up. Ann Trop Med Parasitol. 2003;97(1):23-29. doi: 10.1179/000349803125002751 [DOI] [PubMed] [Google Scholar]
- 20.Fabbro De Suasnabar D, Arias E, Streiger M, et al. . Evolutive behavior towards cardiomyopathy of treated (nifurtimox or benznidazole) and untreated chronic chagasic patients. Rev Inst Med Trop Sao Paulo. 2000;42(2):99-109. doi: 10.1590/S0036-46652000000200007 [DOI] [PubMed] [Google Scholar]
- 21.Colantonio LD, Prado N, Segura EL, Sosa-Estani S. Electrocardiographic abnormalities and treatment with benznidazole among children with chronic infection by Trypanosoma cruzi: a retrospective cohort study. PLoS Negl Trop Dis. 2016;10(5):e0004651. doi: 10.1371/journal.pntd.0004651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Andrade AL, Zicker F, Rassi A, et al. . Early electrocardiographic abnormalities in Trypanosoma cruzi–seropositive children. Am J Trop Med Hyg. 1998;59(4):530-534. doi: 10.4269/ajtmh.1998.59.530 [DOI] [PubMed] [Google Scholar]
- 23.Andrade MC, Rocha EA, da Costa AC, et al. . Electrocardiographic and serological evolution of patients with chronic Chagas disease followed-up for six years after treatment with benzonidazole. Article in Portuguese. RELAMPA:Revista Latino-Americana de Marcapasso e Arritmia. 2016;29(4):141-149. [Google Scholar]
- 24.Fragata-Filho AA, Franca FF, da Silva Fragata C, Lourenco AM, Faccini CC, de Jesus Costa CA. Evaluation of parasiticide treatment with benznidazol in the electrocardiographic, clinical, and serological evolution of Chagas disease. PLoS Negl Trop Dis. 2016;10(3):e0004508. doi: 10.1371/journal.pntd.0004508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pereira JB, da Cunha RV, Willcox HP, Coura JR. Development of chronic human Chagas cardiopathy in the hinterland of the Paraíba State, Brazil, in a 4.5 year period. Article in Portuguese. Rev Soc Bras Med Trop. 1990;23(3):141-147. doi: 10.1590/S0037-86821990000300002 [DOI] [PubMed] [Google Scholar]
- 26.Viotti R, Vigliano C, Lococo B, et al. . Clinical predictors of chronic chagasic myocarditis progression. Rev Esp Cardiol. Article in Spanish. 2005;58(9):1037-1044. doi: 10.1157/13078551 [DOI] [PubMed] [Google Scholar]
- 27.Ianni BM, Mady C, Arteaga E, Fernandes F. Cardiovascular diseases observed during follow-up of a group of patients with undetermined form of Chagas’ disease. Article in Portuguese. Arq Bras Cardiol. 1998;71(1):21-24. doi: 10.1590/S0066-782X1998000700005 [DOI] [PubMed] [Google Scholar]
- 28.da Silva MA, Costa JM, Barbosa JM, et al. . Chronic phase of Chagas disease. clinical aspects and course of the disease. Article in Portuguese. Arq Bras Cardiol. 1994;63(4):281-285. [PubMed] [Google Scholar]
- 29.Castro C, Prata A, Macedo V. A 13-year clinical study on 190 chronic chagasic patients from Mambaí, Goiás, Brazil. Article in Portuguese. Rev Soc Bras Med Trop. 2001;34(4):309-318. doi: 10.1590/S0037-86822001000400001 [DOI] [PubMed] [Google Scholar]
- 30.Macedo V. Influence of exposure to reinfection in the development of Chagas disease. longitudinal study of five years. Article in Spanish. Rev Patol Trop. 1976;5:33-116. [Google Scholar]
- 31.Manzullo ECD, Miguel A, Libonatti O, Rozlosnik J. Estudio Longitudinal de la Cardiopatia Chagasica Cronica. Centro de Chagas de la Catedra de Enfermedades Infecciosas de la Facultad de Ciencias Medicas de Buenos Aires, Universidad de Buenos Aires; 1982. [Google Scholar]
- 32.Storino RMJ. Estudios de seguimiento evolutivo de la enfermedad de Chagas In: Madoery RJ, Madoery C, Cámera MI, eds. Actualizaciones en la Enfermedad de Chagas. Organismo Oficial del Congreso Nacional de Medicina;1993:67-77. [Google Scholar]
- 33.Brasil A. Development and prognosis of Chagas’ disease. Article in Portuguese. Arq Bras Cardiol. 1965;18(5):365-380. [PubMed] [Google Scholar]
- 34.Forichon E. Contribution aux estimations de morbidite et de mortalite dans la maladie de Chagas. Rev Pat Trop. 1975;4(1):57-78. [Google Scholar]
- 35.Pedrosa RC, Cancado JR, Decache W. A longitudinal electrocardiogram study of Chagas’ disease from the acute phase. Article in Portuguese. Rev Soc Bras Med Trop. 1993;26(3):163-174. doi: 10.1590/S0037-86821993000300006 [DOI] [PubMed] [Google Scholar]
- 36.Inglessis I, Carrasco HA, Anez N, et al. . Clinical, parasitological and histopathologic follow-up studies of acute Chagas patients treated with benznidazole. Article in Spanish. Arch Inst Cardiol Mex. 1998;68(5):405-410. [PubMed] [Google Scholar]
- 37.Bastos CJC, Aras R, Mota G, et al. . Clinical outcomes of thirteen patients with acute Chagas disease acquired through oral transmission from two urban outbreaks in northeastern Brazil. PLoS Negl Trop Dis. 2010;4(6):e711. doi: 10.1371/journal.pntd.0000711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pinto AYN, da Costa Valente VC, Coura JR, et al. . Clinical follow-up of responses to treatment with benznidazol in Amazon: a cohort study of acute Chagas disease. PLoS One. 2013;8(5):e64450. doi: 10.1371/journal.pone.0064450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gus I, Molon ME, Bueno AP. Chagas disease—review of 8 simultaneous cases of acute Chagas myocarditis: 25 years later. Article in Portuguese. Arq Bras Cardiol. 1993;60(2):99-101. [PubMed] [Google Scholar]
- 40.Urrutia LE. Acute chagasic myocardiopathy in children. five years evolution. Arch Inst Cardiol Mex. 1976;46(4):396-413. [Google Scholar]
- 41.Ortiz JV, Pereira BVM, Couceiro KDN, et al. . Cardiac evaluation in the acute phase of Chagas’ disease with post-treatment evolution in patients attended in the state of Amazonas, Brazil. Arq Bras Cardiol. 2019;112(3):240-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dias E, Laranja FS, Miranda A, Nobrega G. Chagas’ disease; a clinical, epidemiologic, and pathologic study. Circulation. 1956;14(6):1035-1060. doi: 10.1161/01.CIR.14.6.1035 [DOI] [PubMed] [Google Scholar]
- 43.Pinto Dias JC, Dias E, da Cunha Nobrega GC. Long-term follow-up of a patient since the acute phase of Chagas disease (South American trypanosomiasis): further treatment and cure of the infection. Rev Soc Bras Med Trop. 2015;48(5):629-632. doi: 10.1590/0037-8682-0073-2015 [DOI] [PubMed] [Google Scholar]
- 44.Sterne JAC, Sutton AJ, Ioannidis JPA, et al. . Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002 [DOI] [PubMed] [Google Scholar]
- 45.de Araujo Silva S, Gontijo ED, Pinto Dias JC, de Souza Andrade CG, Amaral CFS. Predictive factors for the progression of chronic Chagas cardiomyopathy in patients without left ventricular dysfunction. Rev Inst Med Trop Sao Paulo. 2015;57(2):153-163. doi: 10.1590/S0036-46652015000200009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sabino EC, Ribeiro AL, Salemi VMC, et al. ; National Heart, Lung, and Blood Institute Retrovirus Epidemiology Donor Study-II (REDS-II), International Component . Ten-year incidence of Chagas cardiomyopathy among asymptomatic Trypanosoma cruzi–seropositive former blood donors. Circulation. 2013;127(10):1105-1115. doi: 10.1161/CIRCULATIONAHA.112.123612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dias JC. The indeterminate form of human chronic Chagas’ disease a clinical epidemiological review. Rev Soc Bras Med Trop. 1989;22(3):147-156. doi: 10.1590/S0037-86821989000300007 [DOI] [PubMed] [Google Scholar]
- 48.Hasslocher-Moreno AM, Xavier SS, Saraiva RM, et al. . Progression rate from the indeterminate form to the cardiac form in patients with chronic Chagas disease: twenty-two–year follow-up in a Brazilian urban cohort. Trop Med Infect Dis. 2020;5(2):76. doi: 10.3390/tropicalmed5020076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nunes MCP, Beaton A, Acquatella H, et al. ; American Heart Association Rheumatic Fever, Endocarditis and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Stroke Council . Chagas cardiomyopathy: an update of current clinical knowledge and management: a scientific statement from the American Heart Association. Circulation. 2018;138(12):e169-e209. doi: 10.1161/CIR.0000000000000599 [DOI] [PubMed] [Google Scholar]
- 50.Benvenuti LA, Roggerio A, Freitas HFG, Mansur AJ, Fiorelli A, Higuchi ML. Chronic American trypanosomiasis: parasite persistence in endomyocardial biopsies is associated with high-grade myocarditis. Ann Trop Med Parasitol. 2008;102(6):481-487. doi: 10.1179/136485908X311740 [DOI] [PubMed] [Google Scholar]
- 51.Bustamante JM, Novarese M, Rivarola HW, et al. . Reinfections and Trypanosoma cruzi strains can determine the prognosis of the chronic chagasic cardiopathy in mice. Parasitol Res. 2007;100(6):1407-1410. doi: 10.1007/s00436-006-0425-3 [DOI] [PubMed] [Google Scholar]
- 52.Bustamante JM, Rivarola HW, Fernandez AR, et al. . Indeterminate Chagas’ disease: Trypanosoma cruzi strain and re-infection are factors involved in the progression of cardiopathy. Clin Sci (Lond). 2003;104(4):415-420. doi: 10.1042/cs1040415 [DOI] [PubMed] [Google Scholar]
- 53.World Health Organization WHO to roll out implementation strategy to eliminate sleeping sickness. News release. November 15, 2013. Accessed December 7, 2019. https://www.who.int/neglected_diseases/HAT_roll_out_strategy_2013/en/
- 54.Olivera MJ, Porras Villamil JF, Toquica Gahona CC, Rodriguez Hernandez JM. Barriers to diagnosis access for Chagas disease in Colombia. J Parasitol Res. 2018;2018:4940796. doi: 10.1155/2018/4940796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sartor P, Colaianni I, Cardinal MV, Bua J, Freilij H, Gurtler RE. Improving access to Chagas disease diagnosis and etiologic treatment in remote rural communities of the Argentine Chaco through strengthened primary health care and broad social participation. PLoS Negl Trop Dis. 2017;11(2):e0005336. doi: 10.1371/journal.pntd.0005336 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Search Strategy, Selection Criteria, and Database Search Strategy
eTable 1. Subgroup Analysis of Patients With the Indeterminate Chronic Form of Chagas Disease
eTable 2. Subgroup Analysis of Patients With the Acute Form of Chagas Disease
eFigure 1. Cumulative Risk of a Cardiac Event in Studies of Patients With the Indeterminate Chronic Form of Chagas Disease
eFigure 2. Cumulative Risk of a Cardiac Event in Studies of Patients With the Acute Form of Chagas Disease
eFigure 3. Subgroup Analysis by Year of Study for Studies of Patients With the Indeterminate Chronic Form of Chagas Disease
eFigure 4. Subgroup Analysis by Study Size for Studies of Patients With the Indeterminate Chronic Form of Chagas Disease
eFigure 5. Subgroup Analysis by Mean Age of Participants for Studies of Patients With the Indeterminate Chronic Form of Chagas Disease
eFigure 6. Subgroup Analysis by Percentage of Men for Studies of Patients With the Indeterminate Chronic Form of Chagas Disease
eFigure 7. Subgroup Analysis by Study’s Country of Origin for Studies of Patients With the Indeterminate Chronic Form of Chagas Disease
eFigure 8. Subgroup Analysis by Use of Antitrypanosomal Treatment Intervention for Studies of Patients With the Indeterminate Chronic Form of Chagas Disease
eFigure 9. Subgroup Analysis by Study Duration (in Years) for Studies of Patients With the Indeterminate Chronic Form of Chagas Disease
eFigure 10. Forest Plot of Cardiomyopathy Risk in Studies of Patients With the Acute Form of Chagas Disease (Including the 6-Month Follow-Up Study)
eFigure 11. Subgroup Analysis by Year of Study for Studies of Patients With the Acute Form of Chagas Disease
eFigure 12. Subgroup Analysis by Study Size for Studies of Patients With the Acute Form of Chagas Disease
eFigure 13. Subgroup Analysis by Study’s Country of Origin for Studies of Patients With the Acute Form of Chagas Disease
eFigure 14. Subgroup Analysis by Route of Transmission for Studies of Patients With the Acute Form of Chagas Disease
eFigure 15. Subgroup Analysis by Percentage of Men for Studies of Patients With the Acute Form of Chagas Disease
eFigure 16. Funnel Plot for Publication Bias
eReferences