Key Points
Question
Is combining immunotherapy with other cancer treatments associated with improved overall survival in patients with brain metastases?
Findings
In this comparative effectiveness study of 3112 adult patients who received definitive surgery of the primary cancer site, those who received any treatment plus immunotherapy had better overall survival than those who received no immunotherapy. Results varied with other combined therapies; immunotherapy plus radiation therapy was associated with improved overall survival compared with radiation therapy alone, but immunotherapy plus chemotherapy was not associated with improved overall survival compared with chemotherapy alone.
Meaning
In this study, immunotherapy plus radiotherapy was associated with improved overall survival compared with radiotherapy alone.
This comparative effectiveness study explores the association of immunotherapy with overall survival in patients with cancer and brain metastases who received definitive surgery of the primary tumor site.
Abstract
Importance
Immunotherapy has shown significant control of intracranial metastases in patients with melanoma. However, the association of immunotherapy combined with other cancer treatments and overall survival (OS) of patients with brain metastases, regardless of primary tumor site, is unknown.
Objective
To explore the association of immunotherapy with OS in patients with cancer and brain metastases who received definitive surgery of the primary site.
Design, Setting, and Participants
This comparative effectiveness study included 3112 adult patients in the National Cancer Database from 2010 to 2016 with non–small cell lung cancer, breast cancer, melanoma, colorectal cancer, or kidney cancer and brain metastases at the time of diagnosis and who received definitive surgery of the primary site. Data analysis was conducted from March to April 2020.
Exposures
Treatment groups were stratified as follows: (1) any treatment with or without immunotherapy, (2) chemotherapy with or without immunotherapy, (3) radiotherapy (RT) with or without immunotherapy, and (4) chemoradiation with or without immunotherapy.
Main Outcomes and Measures
The association of immunotherapy with OS was assessed with Cox proportional hazards regression, adjusted for age at diagnosis, race, sex, place of living, income, education, treatment facility type, primary tumor type, and year of diagnosis.
Results
Of 3112 patients, 1436 (46.14%) were men, 2714 (87.72%) were White individuals, 257 (8.31%) were Black individuals, and 123 (3.98%) belonged to other racial and ethnic groups. The median (range) age at diagnosis was 61 (19-90) years. Overall, 183 (5.88%) received immunotherapy, 318 (10.22%) received chemotherapy alone, 788 (25.32%) received RT alone, and 1393 (44.76%) received chemoradiation alone; 22 (6.47%) received chemotherapy plus immunotherapy, 72 (8.37%) received RT plus immunotherapy, and 76 (5.17%) received chemoradiation plus immunotherapy. In the multivariable analysis, patients who received immunotherapy had significantly improved OS compared with no immunotherapy (hazard ratio, 0.62; 95% CI, 0.51-0.76; P < .001). Treatment with RT plus immunotherapy was associated with significantly improved OS compared with RT alone (hazard ratio, 0.59; 95% CI, 0.42-0.84; P = .003). Chemotherapy plus immunotherapy or chemoradiation plus immunotherapy were not associated with improved OS in the multivariable analysis.
Conclusions and Relevance
In this study, the addition of immunotherapy to RT was associated with improved OS compared with radiotherapy alone in patients with brain metastases who received definitive surgery of the primary tumor site.
Introduction
It is estimated that each year more than 170 000 people are newly diagnosed with brain metastases (BMs) in the United States.1 Brain metastases are the most common intracranial malignant neoplasms in adults and are 10-fold more common than primary intracranial cancer.2,3,4,5 The most common primary tumors associated with BMs are lung cancer (40%-50%), breast cancer (15%-30%), and melanoma (5%-20%), followed by colorectal cancer (3%-8%) and kidney cancer (2%-4%).6 Brain metastases cause significant morbidity and mortality and carry a poor survival prognosis.7 The median survival time is between 4 and 16 months, depending on the primary cancer site.8,9,10
Local therapies, such as whole-brain radiation therapy (RT), stereotactic radiosurgery, and surgical resection, have been the mainstay of treatment in these patients.11,12 These local therapies are associated with neurotoxic effects and represent a significant problem.13
Cytotoxic chemotherapy has shown limited activity in the nervous system due to its inability to cross the blood-brain barrier (BBB). With the progress in the understanding of the pathophysiology of the BBB and the development of new cytotoxic chemotherapies and targeted therapies, a renewed interest has been placed on the use of systemic treatment for BMs.14,15 Currently, there is a compelling rationale that drugs that sufficiently penetrate the BBB can evoke a clinical response in the central nervous system.16
ERBB2 inhibitors were associated with an objective central nervous system response rate of 74% and a median progression-free survival of 7.3 (95% CI, 6.5-8.1) months and a median overall survival (OS) of 10.5 (95% CI, 7.8-13.2) months in patients with breast cancer and BMs.17 Epidermal growth factor receptor inhibitors were associated with 3 months improved median OS in patients with BMs from non–small cell lung cancer (12 months in targeted therapy vs 9 months in chemotherapy).18 Targeted therapies were also associated with improved OS in patients with BMs and kidney cancer or melanoma.19,20 Together, these findings suggest that targeted therapies may be useful in treating intracranial disease.
Immunotherapy has changed the treatment landscape of various malignant neoplasms and may offer an exciting opportunity for the treatment of BMs.15 The brain was long considered as an immune-privileged organ, and it was thought that immunotherapy was ineffective in BMs because it will not cross the BBB or, when it does cross the BBB, its ability to elicit a robust immune response would be limited.14,15 However, preclinical and clinical evidence indicate that monoclonal antibodies can penetrate the BBB both in primary and metastatic brain cancer and thus could be an excellent option for the treatment of brain metastasis.11,21
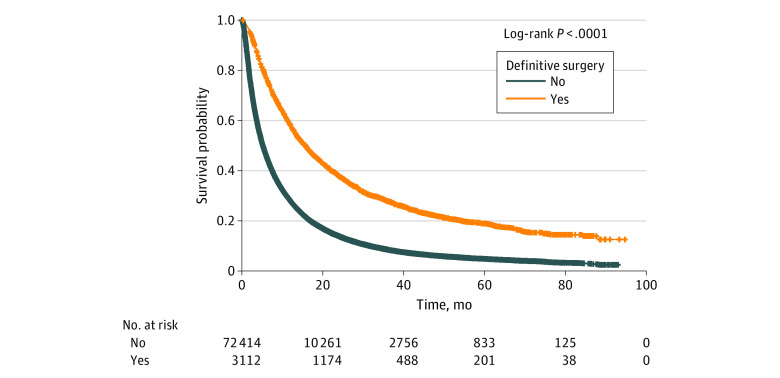
An ongoing phase 2 trial of pembrolizumab among patients with BMs from melanoma or non–small cell lung cancer reported a response rate of 33% (95% CI, 14%-59%) in patients with non–small cell lung cancer and 22% (95% CI, 7%-48%) in patients with melanoma.22 A phase 2 clinical trial of patients with BMs in which participants received a combination of nivolumab and ipilimumab reported 57% intracranial clinical benefit with 56% complete or partial responses.23 Retrospective studies of BMs from melanoma have also reported improved OS in patients who received immunotherapy.24,25,26 However, most clinical trials and retrospective studies only included BMs from melanoma, had a small number of patients, and focused on a single agent, ipilimumab.23,24,25,26,27,28 More extensive studies regarding the potential role of immunotherapy in patients with BMs who receive surgery of the primary tumor are lacking. The surgery distinction was made because the survival of patients with BMs who receive surgery of the primary site is different from those who do not receive surgery (20 vs 9 months) based on our data (Figure 1). Removing the primary tumor will significantly reduce the tumor burden, and immunotherapy may work better in a setting with minimal residual disease, when the bulk of the tumor mass has been optimally reduced. Using the data from the National Cancer Database (NCDB), we explored whether the use of immunotherapy in patients with BMs who received surgery of the primary site is associated with improved OS in patients with non–small cell lung cancer, breast cancer, melanoma, colorectal cancer, and kidney cancer.
Figure 1. Overall Survival With or Without Definitive Surgery of the Primary Tumor Site.
Methods
Data Source
This was a comparative effectiveness study that used data extracted from NCDB. The NCDB is a joint program of the Commission on Cancer of the American College of Surgeons and the American Cancer Society. It captures 70% or more of newly diagnosed malignant neoplasms in the United States annually. The NCDB is a nationwide oncology outcomes database for more than 1500 Commission on Cancer–accredited cancer programs in the United States and Puerto Rico. It is the largest cancer database in the world and now contains approximately 34 million records from hospital registries from all over the United States. Participants in the NCDB database cannot be identified, neither directly nor through identifiers linked to the patients. Therefore, this research is eligible for exemption from IRB approval under 45 CFR 46.101(b)(4). The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Study Population
The study cohort consisted of patients with BMs aged 19 years and older who were diagnosed with the primary cancer of non–small cell lung cancer, melanoma, breast cancer, colorectal cancer, or kidney cancer between 2010 and 2016. These primary cancers account for more than 75% of BM cases. The year 2010 was chosen because this is the first year the NCDB collected information regarding BMs at the time of diagnosis. Patients who did not receive definitive surgery of the primary site and those who were missing radiation therapy, chemotherapy, and immunotherapy were excluded from the study. Patients with unknown or missing information regarding the covariates of interest were not included in the multivariable analysis. This study only includes patients with BMs who had definitive surgery of the primary cancer site. Definitive surgery refers to cancer surgery (removing primary cancer and the regional lymph nodes). Using the site-specific International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) surgery codes, patients with breast cancer with codes 20 to 80, patients with non–small cell lung cancer with codes 30 to 79, patients with melanoma with codes 30 to 60, patients with colorectal cancer with codes 30 to 80, and patients with kidney cancer with codes 30 to 80 were defined as having received definitive surgery and were included in the analysis.
End Points
The primary outcome was OS, which was calculated from the time of diagnosis of BMs to the time of death from any cause. The odds ratios for identifying the factors associated with receiving immunotherapy in the multivariable analysis were also calculated.
Factors and Covariates
The main factors were immunotherapy, immunotherapy plus chemotherapy, immunotherapy plus RT, and immunotherapy plus chemoradiation. Covariates included the patient-level variables of age, sex, and race and the demographic variables of place of residence, income, education (based on the zip code of the patient at the time of diagnosis). The treatment-related variables of insurance, treatment facility type, comorbidity score, and receipt of immunotherapy, chemotherapy, or RT were also included.
Statistical Analysis
The baseline covariates between patients with BMs who received immunotherapy and those who did not were compared. Mean, median, and range were calculated for all continuous variables, while proportions were reported for all categorical variables for those who received immunotherapy and for those who did not. Multiple logistic regression analysis was performed to identify the factors associated with receiving immunotherapy. The odds ratio was reported as the measure of association between the covariate of interest and the outcome of receiving immunotherapy.
The OS rates for those who received immunotherapy and those who did not receive immunotherapy were reported. The OS rates for immunotherapy plus RT vs RT alone, immunotherapy plus chemotherapy vs chemotherapy alone, and immunotherapy plus chemoradiation vs chemoradiation alone were also reported. Kaplan-Meier curves were used to report the median OS, and the log-rank test was used to indicate the significance of the findings. We also reported the Kaplan-Meier curves for the OS of patients who received immunotherapy combined with RT, chemotherapy, or chemoradiation. The Cox proportional hazard regression was used to report the hazard of death. The hazard ratio (HR) and its 95% CI for all the variables of interest were reported. Univariable Cox proportional analysis was performed for all the covariates of interest. Variables with a P < .15 in the univariable analysis were selected for the multivariable analysis. We performed all statistical analyses in SAS version 9.4 (SAS Institute). Statistical significance was set at P < .05, and all tests were 2-tailed.
Results
Patient and Treatment Characteristics
A total of 3112 patients diagnosed between 2010 and 2016 met the inclusion criteria and were identified from the NCDB. Of these, 1436 (46.14%) were men, 2714 (87.72%) were White individuals, 257 (8.31%) were Black individuals, 123 (3.98%) belonged to other racial and ethnic groups, 2959 (97.62%) were living in urban areas, 2924 (95.24%) had health insurance, 1196 (39.93%) were treated at academic centers, and 2254 (72.43%) had a comorbidity score of 0. The median (range) age at diagnosis was 61 (19-90) years. From 3112 patients analyzed, 183 (5.88%) received immunotherapy, 318 (10.22%) received chemotherapy alone, 788 (25.32%) received RT alone, and 1393 (44.76%) received chemoradiation alone. Overall, 22 patients (6.47%) received chemotherapy plus immunotherapy, 72 patients (8.37%) received RT plus immunotherapy, and 76 patients (5.17%) received chemoradiation plus immunotherapy.
Outcomes
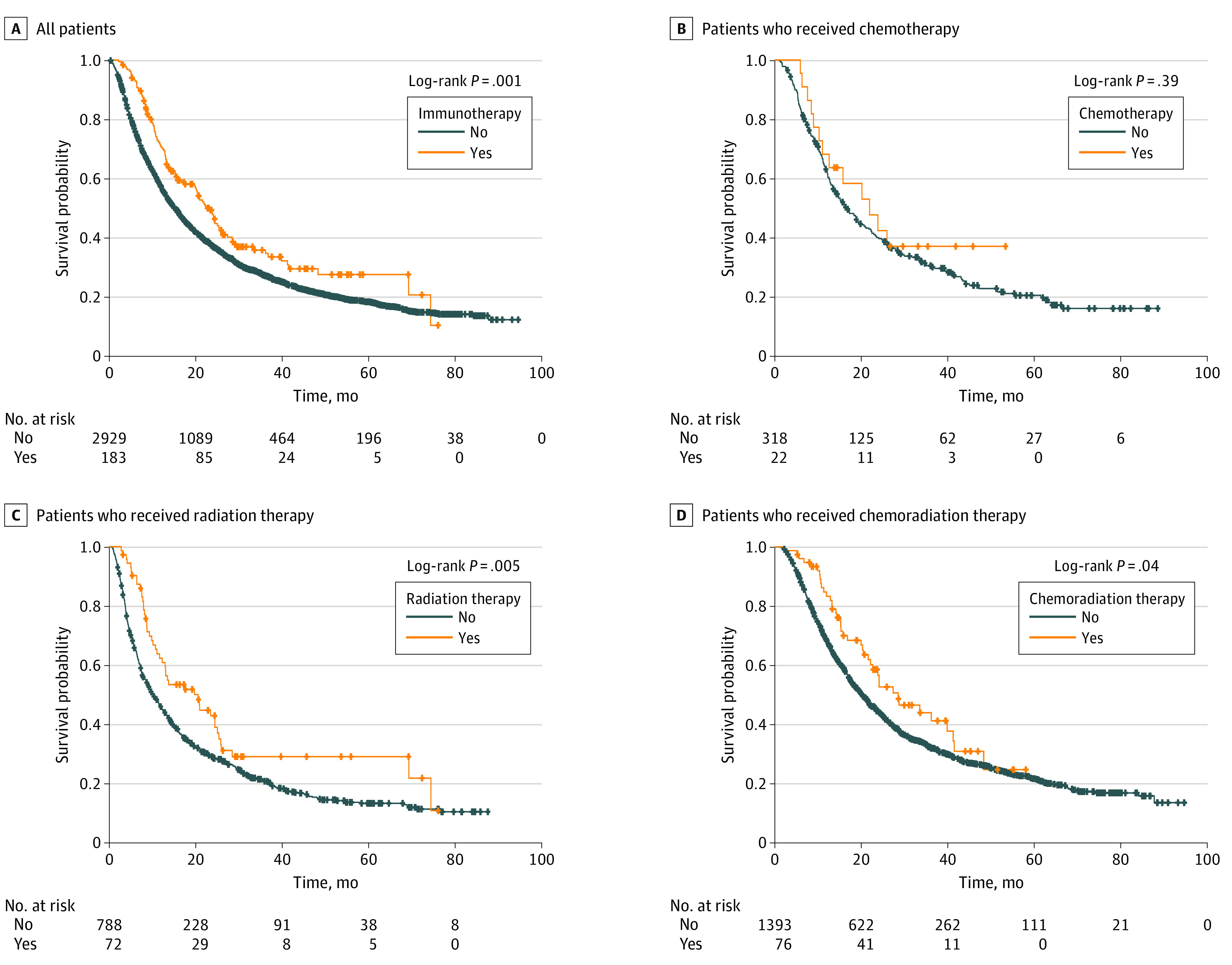
Patients with older age, male sex, comorbidity score of 0, diagnosis in 2014 or after, and primary cancer types of breast cancer, melanoma, and CRC (vs kidney cancer) were positively associated with the use of immunotherapy in the multivariable logistic analysis. The odds ratios of the factors associated with receiving immunotherapy are provided in Table 1. Patients who received immunotherapy had better OS compared with those who did not receive immunotherapy, with an absolute median OS benefit of 7.5 months (22.60 [95% CI, 19.71-25.92] months vs 15.08 [95% CI, 14.13-16.10] months; P < .001) (Figure 2A). Patients who received RT plus immunotherapy had better OS compared with patients who only received RT with an absolute median OS benefit of 10.4 months (20.53 [95% CI, 11.63-25.00] months vs 10.09 [95% CI, 8.77-11.60] months; P = .006) (Figure 2C). Patients who received chemoradiation plus immunotherapy had an overall benefit of 8.3 months (28.52 [95% CI, 21.59-41.23] months vs 20.21 [95% CI, 18.56-21.68] months; P = .04) in median OS compared with chemoradiation alone (Figure 2D). Patients who received chemotherapy plus immunotherapy did not experience survival benefit associated with the addition of immunotherapy (10.25 [95% CI, 21.91 to not reached] vs 16.66 [95% CI, 14.03-19.98]; P = .39) compared with chemotherapy alone (Figure 2B).
Table 1. Multivariable Logistic Regression Analysis of the Factors Associated With the Receipt of Immunotherapy.
| Variable | Patients, No. (%) | OR (95% CI) | P value | ||
|---|---|---|---|---|---|
| Received immunotherapy (n = 183) | No immunotherapy (n = 2929) | Total (N = 3112) | |||
| Age at diagnosis, median (range), y | 58 (26-90) | 61 (19-90) | 61 (19-90) | 0.98 (0.96-0.99) | .009 |
| Sex | |||||
| Men | 96 (52.46) | 1340 (45.75) | 1436 (46.14) | 1 [Reference] | NA |
| Women | 87 (47.54) | 1589 (54.25) | 1676 (53.86) | 0.64 (0.43-0.96) | .03 |
| Racea | |||||
| White | 161 (87.98) | 2553 (87.70) | 2714 (87.72) | NA | NA |
| Black | 13 (7.10) | 244 (8.38) | 257 (8.31) | NA | NA |
| Other | 9 (4.92) | 114 (3.92) | 123 (3.98) | NA | NA |
| Unknown | 0 | 18 | 18 | NA | NA |
| County-level residents without high school diploma, %a | |||||
| ≥13 | 76 (41.53) | 1307 (44.70) | 1383 (44.51) | NA | NA |
| <13 | 107 (58.47) | 1617 (55.30) | 1724 (55.49) | NA | NA |
| Unknown | 0 | 5 | 5 | NA | NA |
| Median county-level income, $a | |||||
| ≥35 000 | 116 (63.39) | 1695 (58.01) | 1811 (58.33) | NA | NA |
| <35 000 | 67 (36.61) | 1227 (41.99) | 1294 (41.67) | NA | NA |
| Unknown | 0 | 7 | 7 | NA | NA |
| Place of residencea | |||||
| Urban | 173 (96.65) | 2786 (97.69) | 2959 (97.62) | NA | NA |
| Rural | 6 (3.35) | 66 (2.31) | 72 (2.38) | NA | NA |
| Unknown | 4 | 77 | 81 | NA | NA |
| Hospital type | |||||
| Academic | 83 (48.82) | 1113 (39.40) | 1196 (39.93) | 1 [Reference] | NA |
| Community | 87 (51.18) | 1712 (60.60) | 1799 (60.07) | 0.72 (0.52-1.01) | .06 |
| Unknown | 13 | 104 | 117 | NA | NA |
| Insurance statusa | |||||
| Insured | 178 (97.27) | 2746 (95.12) | 2924 (95.24) | NA | NA |
| Not insured | 5 (2.73) | 141 (4.88) | 146 (4.76) | NA | NA |
| Unknown | 0 | 42 | 42 | NA | NA |
| Charlson/Deyo score | |||||
| 0 | 152 (83.06) | 2102 (71.77) | 2254 (72.43) | 1 [Reference] | NA |
| ≥1 | 31 (16.94) | 827 (28.23) | 858 (27.57) | 0.65 (0.43-0.99) | .04 |
| Chemotherapya | |||||
| Yes | 98 (53.55) | 1711 (58.42) | 1809 (58.13) | NA | NA |
| No | 85 (45.45) | 1218 (41.52) | 1303 (41.87) | NA | NA |
| Radiation therapy | |||||
| Yes | 148 (80.87) | 2181 (74.46) | 2329 (74.84) | 1 [Reference] | NA |
| No | 35 (19.13) | 748 (25.54) | 783 (25.16) | 0.63 (0.42-0.95) | .03 |
| Cancer type | |||||
| Breast | 43 (23.50) | 560 (19.12) | 603 (19.38) | 2.42 (1.36-4.31) | .003 |
| NSCLC | 24 (13.11) | 1142 (38.99) | 1166 (37.47) | 0.44 (0.26-0.76) | .003 |
| Melanoma | 55 (30.05) | 231 (7.89) | 286 (9.19) | 4.99 (3.10-8.02) | .001 |
| CRC | 24 (13.11) | 326 (11.13) | 350 (11.25) | 1.83 (1.04-3.24) | .04 |
| Kidney | 37 (20.22) | 670 (22.87) | 707 (22.72) | 1 [Reference] | NA |
| Year of diagnosis | |||||
| 2010-2013 | 83 (45.36) | 2036 (69.51) | 2119 (68.09) | 0.32 (0.23-0.44) | <.001 |
| 2014-2016 | 100 (54.64) | 893 (31.49) | 993 (31.91) | 1 [Reference] | NA |
Abbreviations: CRC, colorectal cancer; NA, not applicable; NSCLC, non–small cell lung cancer; OR, odds ratio.
The variables of race, education, income, place of residence, insurance status, and chemotherapy were not included in the multivariable logistic regression analysis above because each of them had a P > .15 in the univariate analysis.
Figure 2. Overall Survival With or Without Immunotherapy.
In the univariable Cox proportional hazard analysis (Table 2), patients who received immunotherapy had improved OS compared with their counterparts (HR, 0.73; 95% CI, 0.60-0.88; P < .001). We also found significantly improved OS among patients who received RT plus immunotherapy vs RT alone (HR, 0.66; 95% CI, 0.49-0.89; P = .006) and chemoradiation plus immunotherapy vs chemoradiation alone (HR, 0.72; 95% CI, 0.53-0.99; P = .04) (Table 3). Younger age, female sex, living in areas with median income of $35 000 or greater, receiving treatment at academic centers, having a comorbidity score of 0, receiving chemotherapy, receiving RT, and having a tumor type of non–small cell lung cancer were also associated with improved OS in the univariable analysis (Table 2).
Table 2. Univariable and Multivariable Cox Proportional Regression Analysis of Factors Associated With Overall Survival.
| Variable | Univariable analysis | Multivariable analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age at diagnosis, continuous | 1.02 (1.02-1.03) | .001 | 1.02 (1.01-1.02) | .001 |
| Sex | ||||
| Men | 1 [Reference] | NA | 1 [Reference] | NA |
| Women | 0.88 (0.81-0.96) | .003 | 0.94 (0.86-1.04) | .24 |
| Race | ||||
| White | 1 [Reference] | NA | 1 [Reference] | NA |
| Black | 1.07 (0.93-1.24) | .36 | 1.09 (0.93-1.27) | .28 |
| Other | 0.75 (0.59-0.94) | .01 | 0.81 (0.64-1.04) | .09 |
| County-level residents without high school diploma, %a | ||||
| ≥13 | 1.06 (0.97-1.15) | .19 | NA | NA |
| <13 | 1 [Reference] | NA | NA | NA |
| Median county-level income, $a | ||||
| ≥35 000 | 1 [Reference] | NA | 1 [Reference] | NA |
| <35 000 | 1.14 (1.05-1.23) | .003 | 1.07 (0.98-1.17) | .12 |
| Place of residencea | ||||
| Urban | 1 [Reference] | NA | NA | NA |
| Rural | 1.10 (0.85-1.43) | .46 | NA | NA |
| Hospital type | ||||
| Academic | 1 [Reference] | NA | 1 [Reference] | NA |
| Community | 1.36 (1.25-1.49) | .001 | 1.27 (1.16-1.38) | .001 |
| Insurance statusa | ||||
| Insured | 1 [Reference] | NA | NA | NA |
| Not insured | 1.08 (0.89-1.32) | .42 | NA | NA |
| Charlson/Deyo score | ||||
| 0 | 1 [Reference] | NA | 1 [Reference] | NA |
| ≥1 | 1.23 (1.12-1.34) | .001 | 1.15 (1.05-1.26) | .004 |
| Year of diagnosisa | ||||
| 2014-2016 | 1 [Reference] | NA | NA | NA |
| 2010-2013 | 1.03 (0.94-1.13) | .54 | NA | NA |
| Type of cancer | ||||
| Kidney | 1 [Reference] | NA | 1 [Reference] | NA |
| Breast | 0.92 (0.81-1.04) | .18 | 0.96 (0.83-1.12) | .60 |
| NSCLC | 0.74 (0.66-0.83) | .001 | 0.80 (0.71-0.90) | .001 |
| CRC | 1.33 (1.14-1.56) | .001 | 1.43 (1.21-1.69) | .001 |
| Melanoma | 1.86 (1.62-2.14) | .001 | 1.97 (1.70-2.29) | .001 |
| Chemotherapy | ||||
| Yes | 0.61 (0.56-0.66) | <.001 | 0.68 (0.62-0.75) | .001 |
| No | 1 [Reference] | NA | 1 [Reference] | NA |
| Radiation therapy | ||||
| Yes | 0.79 (0.72-0.87) | <.001 | 0.94 (0.85-1.04) | .21 |
| No | 1 [Reference] | NA | 1 [Reference] | NA |
| Immunotherapy | ||||
| Yes | 0.73 (0.60-0.88) | <.001 | 0.62 (0.51-0.76) | .001 |
| No | 1 [Reference] | NA | 1 [Reference] | NA |
Abbreviations: CRC, colorectal cancer; HR, hazard ratio; NA, not applicable; NSCLC, non–small cell lung cancer.
These variables were not included in the multivariable analysis because they had P > .15 in the univariable analysis.
Table 3. Univariate and Multivariate Analysis of Combining Immunotherapy With Chemotherapy and Radiation Therapy.
| Group | No. (%) | Univariable analysis | Multivariable analysisa | ||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Chemotherapy treatment group (n = 340) | |||||
| Chemotherapy only | 318 (93.53) | 1 [Reference] | NA | 1 [Reference] | NA |
| Chemotherapy with immunotherapy | 22 (6.47) | 0.79 (0.45-1.37) | .40 | 0.79 (0.44-1.42) | .43 |
| Radiotherapy treatment group (n = 860) | |||||
| Radiotherapy only | 788 (91.63) | 1 [Reference] | NA | 1 [Reference] | NA |
| Radiotherapy with immunotherapy | 72 (8.37) | 0.66 (0.49-0.89) | .006 | 0.59 (0.42-0.84) | .003 |
| Chemoradiation treatment group (n = 1469) | |||||
| Chemoradiation only | 1393 (94.83) | 1 [Reference] | NA | 1 [Reference] | NA |
| Chemoradiation with immunotherapy | 76 (5.17) | 0.72 (0.53-0.99) | .04 | 0.75 (0.54-1.03) | .07 |
Abbreviations: HR, hazard ratio; NA, not applicable.
Three different models were developed for the multivariable analysis because the groups were mutually exclusive. All three models were adjusted for age at diagnosis, race, hospital type, comorbidity score, and tumor type. The chemotherapy plus immunotherapy model also included income, while the chemoradiation plus immunotherapy model included income and year of diagnosis in addition to other factors.
In the multivariable analysis (Table 2) adjusted for age at diagnosis, sex, income, hospital type, comorbidity score, and receipt of chemotherapy or RT, patients who received immunotherapy had significantly improved OS compared with no immunotherapy (HR, 0.62; 95% CI, 0.51-0.76; P < .001). Treatment with RT plus immunotherapy was associated with significantly improved OS compared with RT alone (HR, 0.59; 95% CI, 0.42-0.84; P = .003) in the multivariable analysis. Chemotherapy plus immunotherapy and chemoradiation plus immunotherapy were not associated with improved OS in the multivariable analysis (Table 3). Younger age, academic hospital type, comorbidity score of 0, receiving chemotherapy, and tumor type of non–small cell lung cancer were associated with improved OS in the multivariable analysis.
Discussion
In the current analysis, we found significant OS benefit for patients who received immunotherapy combined with RT compared with RT alone. Younger age, receiving treatment at an academic center, comorbidity score of 0, primary cancer of non–small cell lung cancer, and use of RT were associated with improved OS. Median OS was longer in patients who received RT plus immunotherapy compared with patients who only received RT alone. Most importantly, we found that immunotherapy improved the OS of patients with BMs by 7.5 months regardless of what other treatments they received. Immunotherapy combined with RT improved OS by 10 months compared with those who only received RT.
The median survival time reported in our study is comparable to the median survival time indicated in previous studies.29,30,31 In the multivariable analysis in this study, we found that RT plus immunotherapy was associated with significantly improved OS compared with RT alone (HR, 0.59; 95% CI, 0.42-0.84; P = .003). Previously published studies have also investigated the association of immunotherapy combined with RT and chemotherapy with outcomes.17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37 Most previous studies only included patients with metastatic melanoma who received a single drug (ie, ipilimumab).28,35,36,37 The findings of the current study are consistent with the earlier studies that were focused on patients with melanoma.23,25,26,28,29,30,31,32,35,36,37 However, based on our knowledge, none of the previous studies included patients with non–small cell lung cancer, breast cancer, small cell lung cancer, CRC, or kidney cancer.
What is unique in our study is that we found that immunotherapy was associated with significantly improved survival in patients with BMs who received surgery of the primary site, which has not, to our knowledge, been investigated so far. The improved OS with the addition of immunotherapy to RT may indicate the synergetic or additive effect of immunotherapy with RT. The improved OS in patients who received RT plus immunotherapy may be associated with the abscopal effect of RT. After a tumor is irradiated, injury in the tumor may lead to the release of tumor-associated antigens, which can stimulate a tumor-specific immune response, allowing the immune cells (ie, T-cells) to recognize and attack both the primary tumor and metastatic disease in a sort of autovaccination.38,39,40,41 Immunotherapy may enhance the optimal effect of the abscopal effect by increasing and improving the immune response to tumor-associated antigens, notably when the removal of the primary tumor minimizes the tumor burden. Radiation therapy also causes the release of neoantigens and upregulation of inflammatory cytokines, which promote the presentation of the neoantigens in the tumor microenvironment and thereby increase the immunogenicity of the tumor cells, making them a better target for immunotherapy.42,43,44 For patients with significant extracranial disease, the addition of immunotherapy may improve survival by controlling extracranial disease. However, for those without or with minimal extracranial disease, the association of immunotherapy with survival will be mediated through the control of BMs, which will be influenced by the drug permeability of BBB.
To our knowledge, this study is the first to use an extensive database such as NCDB and to investigate the association of immunotherapy combined with chemotherapy and/or RT with the OS of patients with BMs who received definitive surgery of the primary tumor. The findings of our study, together with the results of the previously published studies of immunotherapy in patients with melanoma, warrant future clinical trials of immunotherapy combined with chemotherapy and/or RT in patients who receive definitive surgery of the primary tumor. Patients with BMs have been historically excluded from clinical trials of immunotherapy due to poor survival and fear of adverse effects. For patients with BMs who do not participate in clinical trials, proper treatment of BM still requires multidisciplinary input regarding appropriate integration from surgery, radiation, and systemic therapies. Furthermore, the quality of life of patients and long-term toxic effects should be carefully weighed when immunotherapy is recommended. Immunotherapy is not a standard-of-care treatment in BMs outside of clinical trials. However, some patients are receiving immunotherapy. These patients may have been taking part in a clinical trial and received immunotherapy as part of a study. Patients who participate in trials are highly selected populations and usually have better prognoses. However, in our study, 83 of 170 patients (48.82%) for whom information regarding treatment facility was available were treated at academic centers, an indication that most patients may have been treated at community centers and may not have been participating in clinical trials. However, we cannot determine whether a patient was participating in a clinical trial by using the treatment facility variable. It is also possible that immunotherapy was recommended for patients who could not tolerate standard-of-care treatments or failed to show any response to standard-of-care treatments. Last but not least, immunotherapy could be recommended for patients who have exhausted many lines of standard-of-care treatments. The strength of this study is the large sample size and the inclusion of patients with various primary tumors.
Limitations
The current study is not without limitations. The limitations are those that are inherent to any secondary analysis of an extensive database such as NCDB and include lack of data regarding the cause of death, type of immunotherapy, lack of information regarding chemotherapy regimens, incomplete data, and ascertainment bias. The immunotherapy group represented only 5.7% of patients who received definitive surgery of the primary tumor site, indicating that this is a highly select group of patients with BMs and many of these patients might have been enrolled in clinical trials. This group may also have characteristics that we were not able to adequately account for from the database, including the decision-making regarding the use of immunotherapy. Not knowing whether patients have received surgery to the brain is another limitation of the current study because the NCDB does not have information on surgery to intracranial lesions for patients with BMs. Another limitation is the lack of information regarding the numbers of intracranial lesions, the extent of the intracranial tumor, and the size of the intracranial tumor. Also, not analyzing whether RT was given intracranially, extracranially, or both and whether it was fully fractionated RT, stereotactic radiosurgery, or stereotactic body RT are other vital limitations and may cause selection bias. Although we found that immunotherapy was associated with improved OS, it is not clear whether improved OS was owing to treating extracranial disease, reducing the incidence of new brain metastasis, or both. Nevertheless, the study analyzed the data from the best available cancer database available in the United States outside the setting of multicenter clinical trials. It investigated the association of immunotherapy with the survival of patients with BMs who received definitive surgery of the primary tumor.
Conclusions
In this study, we found that immunotherapy combined with RT was significantly associated with improved OS. The findings warrant future clinical trials investigating the association of chemotherapy, RT, and chemoradiation combined with immunotherapy with the survival of patients who receive definitive surgery of the primary tumor.
References
- 1.Langer CJ, Mehta MP. Current management of brain metastases, with a focus on systemic options. J Clin Oncol. 2005;23(25):6207-6219. doi: 10.1200/JCO.2005.03.145 [DOI] [PubMed] [Google Scholar]
- 2.Villano JL, Durbin EB, Normandeau C, Thakkar JP, Moirangthem V, Davis FG. Incidence of brain metastasis at initial presentation of lung cancer. Neuro Oncol. 2015;17(1):122-128. doi: 10.1093/neuonc/nou099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feng W, Zhang P, Zheng X, Chen M, Mao WM. Incidence and treatment of brain metastasis in patients with esophageal carcinoma. World J Gastroenterol. 2015;21(19):5805-5812. doi: 10.3748/wjg.v21.i19.5805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis FG, Dolecek TA, McCarthy BJ, Villano JL. Toward determining the lifetime occurrence of metastatic brain tumors estimated from 2007 United States cancer incidence data. Neuro Oncol. 2012;14(9):1171-1177. doi: 10.1093/neuonc/nos152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Curr Oncol Rep. 2012;14(1):48-54. doi: 10.1007/s11912-011-0203-y [DOI] [PubMed] [Google Scholar]
- 6.Achrol AS, Rennert RC, Anders C, et al. . Brain metastases. Nat Rev Dis Primers. 2019;5(1):6. doi: 10.1038/s41572-019-0061-8 [DOI] [PubMed] [Google Scholar]
- 7.Nolan C, Deangelis LM. Overview of metastatic disease of the central nervous system. Handb Clin Neurol. 2018;149:3-23. doi: 10.1016/B978-0-12-811161-1.00001-3 [DOI] [PubMed] [Google Scholar]
- 8.Michl M, Thurmaier J, Schubert-Fritschle G, et al. . Brain metastasis in colorectal cancer patients: survival and analysis of prognostic factors. Clin Colorectal Cancer. 2015;14(4):281-290. doi: 10.1016/j.clcc.2015.05.009 [DOI] [PubMed] [Google Scholar]
- 9.Fabi A, Felici A, Metro G, et al. . Brain metastases from solid tumors: disease outcome according to type of treatment and therapeutic resources of the treating center. J Exp Clin Cancer Res. 2011;30(1):10. doi: 10.1186/1756-9966-30-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esmaeilzadeh M, Majlesara A, Faridar A, et al. . Brain metastasis from gastrointestinal cancers: a systematic review. Int J Clin Pract. 2014;68(7):890-899. doi: 10.1111/ijcp.12395 [DOI] [PubMed] [Google Scholar]
- 11.Cohen JV, Kluger HM. Systemic immunotherapy for the treatment of brain metastases. Front Oncol. 2016;6:49. doi: 10.3389/fonc.2016.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kocher M, Soffietti R, Abacioglu U, et al. . Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29(2):134-141. doi: 10.1200/JCO.2010.30.1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soffietti R, Kocher M, Abacioglu UM, et al. . A European Organisation for Research and Treatment of Cancer phase III trial of adjuvant whole-brain radiotherapy versus observation in patients with one to three brain metastases from solid tumors after surgical resection or radiosurgery: quality-of-life results. J Clin Oncol. 2013;31(1):65-72. doi: 10.1200/JCO.2011.41.0639 [DOI] [PubMed] [Google Scholar]
- 14.Lauko A, Thapa B, Venur VA, Ahluwalia MS. Management of brain metastases in the new era of checkpoint inhibition. Curr Neurol Neurosci Rep. 2018;18(10):70. doi: 10.1007/s11910-018-0877-8 [DOI] [PubMed] [Google Scholar]
- 15.Ahluwalia MS, Vogelbaum MV, Chao ST, Mehta MM. Brain metastasis and treatment. F1000Prime Rep. 2014;6:114. doi: 10.12703/P6-114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Puhalla S, Elmquist W, Freyer D, et al. . Unsanctifying the sanctuary: challenges and opportunities with brain metastases. Neuro Oncol. 2015;17(5):639-651. doi: 10.1093/neuonc/nov023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krop IE, Lin NU, Blackwell K, et al. . Trastuzumab emtansine (T-DM1) versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer and central nervous system metastases: a retrospective, exploratory analysis in EMILIA. Ann Oncol. 2015;26(1):113-119. doi: 10.1093/annonc/mdu486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fan Y, Huang Z, Fang L, et al. . Chemotherapy and EGFR tyrosine kinase inhibitors for treatment of brain metastases from non-small-cell lung cancer: survival analysis in 210 patients. Onco Targets Ther. 2013;6:1789-1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cochran DC, Chan MD, Aklilu M, et al. . The effect of targeted agents on outcomes in patients with brain metastases from renal cell carcinoma treated with Gamma Knife surgery. J Neurosurg. 2012;116(5):978-983. doi: 10.3171/2012.2.JNS111353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knisely JP, Yu JB, Flanigan J, Sznol M, Kluger HM, Chiang VL. Radiosurgery for melanoma brain metastases in the ipilimumab era and the possibility of longer survival. J Neurosurg. 2012;117(2):227-233. doi: 10.3171/2012.5.JNS111929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahluwalia MS, Winkler F. Targeted and immunotherapeutic approaches in brain metastases. Am Soc Clin Oncol Educ Book. 2015;67-74. doi: 10.14694/EdBook_AM.2015.35.67 [DOI] [PubMed] [Google Scholar]
- 22.Goldberg SB, Gettinger SN, Mahajan A, et al. . Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2016;17(7):976-983. doi: 10.1016/S1470-2045(16)30053-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tawbi HA, Forsyth PA, Algazi A, et al. . Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med. 2018;379(8):722-730. doi: 10.1056/NEJMoa1805453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kiess AP, Wolchok JD, Barker CA, et al. . Stereotactic radiosurgery for melanoma brain metastases in patients receiving ipilimumab: safety profile and efficacy of combined treatment. Int J Radiat Oncol Biol Phys. 2015;92(2):368-375. doi: 10.1016/j.ijrobp.2015.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skrepnik T, Sundararajan S, Cui H, Stea B. Improved time to disease progression in the brain in patients with melanoma brain metastases treated with concurrent delivery of radiosurgery and ipilimumab. Oncoimmunology. 2017;6(3):e1283461. doi: 10.1080/2162402X.2017.1283461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qian JM, Yu JB, Kluger HM, Chiang VL. Timing and type of immune checkpoint therapy affect the early radiographic response of melanoma brain metastases to stereotactic radiosurgery. Cancer. 2016;122(19):3051-3058. doi: 10.1002/cncr.30138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Long GV, Atkinson V, Lo S, et al. . Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol. 2018;19(5):672-681. doi: 10.1016/S1470-2045(18)30139-6 [DOI] [PubMed] [Google Scholar]
- 28.Robert C, Thomas L, Bondarenko I, et al. . Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364(26):2517-2526. doi: 10.1056/NEJMoa1104621 [DOI] [PubMed] [Google Scholar]
- 29.Diao K, Bian SX, Routman DM, et al. . Stereotactic radiosurgery and ipilimumab for patients with melanoma brain metastases: clinical outcomes and toxicity. J Neurooncol. 2018;139(2):421-429. doi: 10.1007/s11060-018-2880-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tazi K, Hathaway A, Chiuzan C, Shirai K. Survival of melanoma patients with brain metastases treated with ipilimumab and stereotactic radiosurgery. Cancer Med. 2015;4(1):1-6. doi: 10.1002/cam4.315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silk AW, Bassetti MF, West BT, Tsien CI, Lao CD. Ipilimumab and radiation therapy for melanoma brain metastases. Cancer Med. 2013;2(6):899-906. doi: 10.1002/cam4.140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Di Giacomo AM, Ascierto PA, Pilla L, et al. . Ipilimumab and fotemustine in patients with advanced melanoma (NIBIT-M1): an open-label, single-arm phase 2 trial. Lancet Oncol. 2012;13(9):879-886. doi: 10.1016/S1470-2045(12)70324-8 [DOI] [PubMed] [Google Scholar]
- 33.Weber JS, Amin A, Minor D, Siegel J, Berman D, O’Day SJ. Safety and clinical activity of ipilimumab in melanoma patients with brain metastases: retrospective analysis of data from a phase 2 trial. Melanoma Res. 2011;21(6):530-534. doi: 10.1097/CMR.0b013e32834d3d88 [DOI] [PubMed] [Google Scholar]
- 34.Kiess AP, Wolchok JD, Barker CA, et al. . Stereotactic radiosurgery for melanoma brain metastases in patients receiving ipilimumab: safety profile and efficacy of combined treatment. Int J Radiat Oncol Biol Phys. 2015;92(2):368-375. doi: 10.1016/j.ijrobp.2015.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shoukat S, Marcus DM, Rizzo M, et al. . Outcome with stereotactic radiosurgery (SRS) and ipilimumab (ipi) for malignant melanoma brain metastases (mets). J Clin Oncol. 2014;32(15):9076. doi: 10.1200/jco.2014.32.15_suppl.9076 [DOI] [Google Scholar]
- 36.Acharya S, Mahmood M, Mullen D, et al. . Distant intracranial failure in melanoma brain metastases treated with stereotactic radiosurgery in the era of immunotherapy and targeted agents. Adv Radiat Oncol. 2017;2(4):572-580. doi: 10.1016/j.adro.2017.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murphy B, Walker J, Bassale S, et al. . Concurrent radiosurgery and immune checkpoint inhibition: Improving regional intracranial control for patients with metastatic melanoma. Am J Clin Oncol. 2019;42(3):253-257. doi: 10.1097/COC.0000000000000509 [DOI] [PubMed] [Google Scholar]
- 38.Siva S, MacManus MP, Martin RF, Martin OA. Abscopal effects of radiation therapy: a clinical review for the radiobiologist. Cancer Lett. 2015;356(1):82-90. doi: 10.1016/j.canlet.2013.09.018 [DOI] [PubMed] [Google Scholar]
- 39.Barker HE, Paget JT, Khan AA, Harrington KJ. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer. 2015;15(7):409-425. doi: 10.1038/nrc3958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vatner RE, Cooper BT, Vanpouille-Box C, Demaria S, Formenti SC. Combinations of immunotherapy and radiation in cancer therapy. Front Oncol. 2014;4:325. doi: 10.3389/fonc.2014.00325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mittal D, Gubin MM, Schreiber RD, Smyth MJ. New insights into cancer immunoediting and its three component phases—elimination, equilibrium and escape. Curr Opin Immunol. 2014;27:16-25. doi: 10.1016/j.coi.2014.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haynes NM, van der Most RG, Lake RA, Smyth MJ. Immunogenic anti-cancer chemotherapy as an emerging concept. Curr Opin Immunol. 2008;20(5):545-557. doi: 10.1016/j.coi.2008.05.008 [DOI] [PubMed] [Google Scholar]
- 43.Ma Y, Conforti R, Aymeric L, et al. . How to improve the immunogenicity of chemotherapy and radiotherapy. Cancer Metastasis Rev. 2011;30(1):71-82. doi: 10.1007/s10555-011-9283-2 [DOI] [PubMed] [Google Scholar]
- 44.Germano G, Lamba S, Rospo G, et al. . Inactivation of DNA repair triggers neoantigen generation and impairs tumour growth. Nature. 2017;552(7683):116-120. doi: 10.1038/nature24673 [DOI] [PubMed] [Google Scholar]