Key Points
Question
Does early administration of tranexamic acid to patients with moderate or severe traumatic brain injury improve neurologic outcome at 6 months?
Findings
In this randomized multicenter clinical trial that included 966 participants enrolled in the out-of-hospital setting by paramedics, treatment with tranexamic acid as an out-of-hospital bolus with or without in-hospital infusion, compared with placebo as an out-of-hospital bolus and in-hospital infusion, resulted in a favorable neurologic outcome (defined as Glasgow Outcome Scale-Extended score >4) in 65% vs 62% of patients at 6 months, a difference that was not statistically significant.
Meaning
Among participants suspected of having moderate or severe traumatic brain injury, out-of-hospital administration of tranexamic acid compared with placebo did not significantly improve 6-month neurologic recovery.
Abstract
Importance
Traumatic brain injury (TBI) is the leading cause of death and disability due to trauma. Early administration of tranexamic acid may benefit patients with TBI.
Objective
To determine whether tranexamic acid treatment initiated in the out-of-hospital setting within 2 hours of injury improves neurologic outcome in patients with moderate or severe TBI.
Design, Setting, and Participants
Multicenter, double-blinded, randomized clinical trial at 20 trauma centers and 39 emergency medical services agencies in the US and Canada from May 2015 to November 2017. Eligible participants (N = 1280) included out-of-hospital patients with TBI aged 15 years or older with Glasgow Coma Scale score of 12 or less and systolic blood pressure of 90 mm Hg or higher.
Interventions
Three interventions were evaluated, with treatment initiated within 2 hours of TBI: out-of-hospital tranexamic acid (1 g) bolus and in-hospital tranexamic acid (1 g) 8-hour infusion (bolus maintenance group; n = 312), out-of-hospital tranexamic acid (2 g) bolus and in-hospital placebo 8-hour infusion (bolus only group; n = 345), and out-of-hospital placebo bolus and in-hospital placebo 8-hour infusion (placebo group; n = 309).
Main Outcomes and Measures
The primary outcome was favorable neurologic function at 6 months (Glasgow Outcome Scale-Extended score >4 [moderate disability or good recovery]) in the combined tranexamic acid group vs the placebo group. Asymmetric significance thresholds were set at 0.1 for benefit and 0.025 for harm. There were 18 secondary end points, of which 5 are reported in this article: 28-day mortality, 6-month Disability Rating Scale score (range, 0 [no disability] to 30 [death]), progression of intracranial hemorrhage, incidence of seizures, and incidence of thromboembolic events.
Results
Among 1063 participants, a study drug was not administered to 96 randomized participants and 1 participant was excluded, resulting in 966 participants in the analysis population (mean age, 42 years; 255 [74%] male participants; mean Glasgow Coma Scale score, 8). Of these participants, 819 (84.8%) were available for primary outcome analysis at 6-month follow-up. The primary outcome occurred in 65% of patients in the tranexamic acid groups vs 62% in the placebo group (difference, 3.5%; [90% 1-sided confidence limit for benefit, −0.9%]; P = .16; [97.5% 1-sided confidence limit for harm, 10.2%]; P = .84). There was no statistically significant difference in 28-day mortality between the tranexamic acid groups vs the placebo group (14% vs 17%; difference, −2.9% [95% CI, −7.9% to 2.1%]; P = .26), 6-month Disability Rating Scale score (6.8 vs 7.6; difference, −0.9 [95% CI, −2.5 to 0.7]; P = .29), or progression of intracranial hemorrhage (16% vs 20%; difference, −5.4% [95% CI, −12.8% to 2.1%]; P = .16).
Conclusions and Relevance
Among patients with moderate to severe TBI, out-of-hospital tranexamic acid administration within 2 hours of injury compared with placebo did not significantly improve 6-month neurologic outcome as measured by the Glasgow Outcome Scale-Extended.
Trial Registration
ClinicalTrials.gov Identifier: NCT01990768
This randomized clinical trial compares the effects of an out-of-hospital tranexamic acid bolus vs placebo within 2 hours of traumatic brain injury (TBI) on 6-month functional neurologic outcome (Glasgow Coma Scale-Extended score >4) in patients with moderate or severe TBI.
Introduction
In 2016, an estimated 27 million people worldwide sustained a traumatic brain injury (TBI), an increase of 47% since 1990.1 In 2014, there were an estimated 288 000 TBI-related hospitalizations and more than 56 000 TBI-related deaths in the US alone.2 Effective management for TBI represents a significant unmet need and has the potential to affect morbidity and mortality worldwide. Despite decades of well-designed clinical trials, no drug has been approved by the US Food and Drug Administration to manage acute TBI.3,4,5
Tranexamic acid is a synthetic derivative of the amino acid lysine that acts by competitively inhibiting plasminogen activation and, at higher concentrations, noncompetitively inhibiting plasmin.6 Tranexamic acid binding blocks the interaction of plasminogen with fibrin, preventing breakdown of fibrin clot.6 The clinical use of tranexamic acid to control bleeding was first described in 1966,6 and its use has expanded over the past 50 years to multiple clinical settings.7,8 Because results from the CRASH-2 trial in 2010 demonstrated a survival benefit for patients at risk for traumatic hemorrhage who received tranexamic acid, its use has become common for patients with severe hemorrhagic shock.9 Until publication of the CRASH-3 trial in 2019, which examined the use of tranexamic acid in more than 12 000 patients with TBI, the use of tranexamic acid in patients with TBI has been limited to retrospective analyses and small clinical trials.10,11,12 The current trial was designed to examine whether tranexamic acid administered within 2 hours of injury would result in improved 6-month neurologic outcome in patients with moderate or severe TBI.
Methods
Trial Design and Oversight
The Prehospital TXA for TBI Trial was a randomized, double-blind, 3-group, multicenter phase II trial designed to examine the efficacy and safety of out-of-hospital administration of tranexamic acid compared with placebo in participants with moderate or severe TBI who were not in shock. Outcomes were compared between patients who received tranexamic acid initiated in the out-of-hospital setting within 2 hours of injury and those who received placebo. With the exception of tranexamic acid administration, treatment was not altered during transport or after arrival to the trauma center. Out-of-hospital tranexamic acid administration for TBI was not standard care at any participating site during the trial. The full trial protocol and statistical analysis plan are available in Supplement 1. The trial used the infrastructure of the Resuscitation Outcomes Consortium, a North American clinical trials network dedicated to conducting clinical trials in out-of-hospital cardiac arrest and severe traumatic injury. The study took place in 12 regions, including 20 trauma centers and 39 emergency medical services (EMS) agencies across the US and Canada (eFigure in Supplement 2).
The trial was conducted under US regulations for Exception From Informed Consent Requirements for Emergency Research (21 CFR §50.24) and the Canadian Tri-Council Policy Statement 2 (Ethical Conduct for Research Involving Humans). As required, all individual site ethics review boards and the Human Research Protection Office of the US Department of Defense reviewed and approved the local community consultation process, public disclosure plans, and conduct of the study. Participants or their legally authorized representative were notified of enrollment as soon as feasible and were asked to provide written informed consent for continued participation in the trial. Safety oversight for the trial was performed by a data and safety monitoring board and an independent medical monitor.
Eligible Patients
The target population for the trial was patients aged 15 years or older with moderate or severe blunt or penetrating TBI, a Glasgow Coma Scale (GCS) score of 3 to 12, at least 1 reactive pupil, and systolic blood pressure of at least 90 mm Hg prior to randomization. EMS agencies were provided centralized video and hands-on training to ensure GCS assessment standardization across sites. It was instructed to obtain the GCS score prior to intubation. Patients were eligible only if an intravenous (IV) catheter was in place, the study drug could be administered within 2 hours of injury, and the predefined EMS transport destination was a participating trauma center. Detailed exclusion criteria are provided in the protocol (Supplement 1).
Randomization and Masking
Participants were randomly assigned to study groups in a 1:1:1 ratio using a computer-generated allocation sequence programmed by the data coordinating center. Identical-appearing out-of-hospital study drug kits were packaged in numerical order according to a permuted block design of variable block size (3 and 6) and shipped to EMS agencies for placement on EMS vehicles in random order. Each EMS vehicle or helicopter carried only 1 study kit at a time containing either 2 g of tranexamic acid, 1 g of tranexamic acid, or placebo solution (saline). Any kits that were opened but not used were discarded and a new kit was supplied to each vehicle for the next trip. The order in which kits were used could not be controlled because the location of injury and EMS availability determined which agency and vehicle responded. As a result, the effective randomization was complete rather than a permuted block.
Neither opening the study kit nor administering the study drug revealed the group assignment. Group assignment was blinded to all EMS agencies, pharmacists, coordinators, and providers throughout the study. Emergency unblinding by the coordinating center was performed only when the treating physician determined open-label tranexamic acid was indicated as an adjunct for hemorrhage control.
Study Intervention
Participants were randomly assigned to 1 of 3 treatment groups: 1-g IV tranexamic acid bolus in the out-of-hospital setting followed by a 1-g tranexamic acid IV infusion initiated upon hospital arrival and infused over 8 hours (bolus maintenance group), 2-g IV tranexamic acid bolus in the out-of-hospital setting followed by a placebo infusion (bolus only group), or IV placebo bolus in the out-of-hospital setting followed by an IV placebo infusion (placebo group). The bolus maintenance dose was chosen based on the observed decreased mortality in the CRASH-2 trial using this dose and because it is widely considered standard of care in patients with traumatic hemorrhage. The bolus only dose was chosen as an alternative dosing regimen that could be more feasible in prehospital and military settings. The out-of-hospital bolus was initiated by EMS prior to arrival and completed either out of hospital or in the emergency department. Following completion of the out-of-hospital bolus, the in-hospital infusion was initiated in the emergency department and administered over 8 hours. Infusion stopping rules established to protect patient safety are listed in the protocol (Supplement 1). Adverse events that occurred during the initial 28 days of hospitalization were recorded. A biospecimen and imaging repository was created and maintained at the clinical coordinating center (Supplement 1).
Study Outcomes
The primary outcome was functional neurologic outcome measured 6months after injury using the Glasgow Outcome Scale-Extended (GOSE)13,14,15 and dichotomized into favorable (GOSE score >4 [moderate disability or good recovery]) and poor (GOSE score ≤4 [severe disability, vegetative state, or death]). Prespecified secondary outcomes included 28-day mortality, 6-month Disability Rating Scale (DRS) score (range, 0 [no disability] to 30 [death]),14,15 progression of intracranial hemorrhage (ICH; defined as >33% increase in the combined volume of subdural, epidural, and intraparenchymal hematomas), discharge GOSE score, discharge DRS score, Marshall and Rotterdam scores on initial head computed tomographic imaging, incidence of neurosurgical interventions, hospital-free days, intensive care unit–free days, ventilator-free days, and fibrinolysis at hospital admission. Additional prespecified secondary outcomes included adverse events potentially associated with tranexamic acid administration: incidence of seizures (defined as observed seizure-like activity and administration of antiseizure medication or electroencephalogram confirmation) and incidence of thrombotic events (cerebral ischemia, myocardial infarction, deep vein thrombosis, and pulmonary embolism). Although complete data on all secondary outcomes are reported by treatment group in this study, statistical analyses are only performed on 6-month GOSE score (primary outcome), 28-day mortality, 6-month DRS score, progression of ICH, and the safety-related outcomes. Analyses for other secondary outcomes are planned to be reported separately.
The initial trial design involved a comparison of each of the 2 tranexamic acid dosing regimens separately with placebo, rather than a comparison of the combined tranexamic acid groups with the placebo group. However, the protocol review committee had a concern with study power and the analytic plan was revised by combining the 2 tranexamic acid treatment groups with a comparison of the combined group with the placebo group. The protocol review committee and the data and safety monitoring board approved this change in 2014, and all subsequent protocols have this as the prespecified analytic approach. In addition, the primary outcome measure was the GOSE score 6 months after injury, dichotomized as a favorable neurologic outcome (GOSE score >4) or unfavorable outcome (GOSE score ≤4). This outcome was prespecified in the original protocol and was unchanged in all subsequent versions of the protocol.
At the time of manuscript preparation, we recognized that the original trial registry entry on ClinicalTrials.gov (November 2013) lacked sufficient detail and did not correctly represent the planned 2-group comparison as the primary analysis and did not specify that the GOSE score outcome was to be dichotomized. This analytic plan and this outcome, which were established and prespecified prior to the beginning of trial enrollment, were included in the updated ClinicalTrials.gov entry (July 2018).
Sample Size
The planned sample size of 963 analysis population participants provided 80% power for tests of benefit at a 0.071 absolute higher proportion with favorable outcomes in the combined group of tranexamic acid–treated patients and harm at a 0.095 absolute lower proportion with favorable outcomes in the combined group of tranexamic acid–treated patients. Because no previous studies have evaluated long-term neurologic outcome in patients with TBI who received tranexamic acid, these differences were chosen as part of an overall strategy to use this phase II trial to identify potential treatments for a phase III trial. Because a subsequent phase III trial could test for benefit at conventional levels, power was computed for an asymmetric 2-sided test conducted at the 10% level for benefit and at the 2.5% level for harm under a grouped sequential design that included 1 interim futility analysis. Additional detail of the grouped sequential design and power analysis is available in the protocol (Supplement 1).
Statistical Analysis
In this double-blind study, because caregivers would not be able to discern the contents of study drug containers, our protocol-specified analysis included only participants who received the study drug. This design was chosen to avoid diluting the analysis population with participants who had no potential to benefit from treatment. No follow-up data were collected for participants who received no study drug. The prespecified primary analysis compared the percentage of participants in the analysis population with a favorable neurologic outcome (GOSE score >4) 6 months after injury between the combined tranexamic acid treatment group and the placebo group using logistic regression adjusted for study site after multiply imputing missing outcome data using fully conditional specification.16 The primary analysis pooled both treatment groups because this comparison provided greater power to detect a benefit of tranexamic acid treatment. Because odds ratios derived from logistic regression can provide a misleading representation of risk when the base rate is high, we instead present adjusted differences in the percentages of participants with favorable neurologic outcome using linear regression with robust standard errors; logistic regression results are reported in Supplement 2. Sensitivity analyses were conducted on a GCS-stratified dichotomous GOSE outcome.17 Additional details of the multiple imputation methods are included in eTables 1 to 3 in Supplement 2.
Secondary analyses comparing the combined tranexamic acid treatment group with the placebo group were performed for 28-day mortality, 6-month DRS score, and progression of ICH. Pairwise comparisons between individual treatment groups were also performed for the primary outcome and select secondary outcomes in the full analysis population and in the following 2 exploratory subgroups: participants with unimputed outcomes and participants with ICH on initial computed tomographic imaging. Absolute percent differences or mean differences and 95% CIs are presented for all secondary analyses. Analyses of the full analysis population were adjusted for site only. Subgroup analyses include prespecified adjustment for site, age, sex, penetrating injury, out-of-hospital GCS score, Injury Severity Score (ISS), and Abbreviated Injury Scale (AIS) head score. Imputed outcomes were used for all analyses except for analyses of the progression of ICH and analyses of the unimputed outcomes subgroup.
This National Institutes of Health–sponsored trial required the collection of race and ethnicity data, which were obtained through the medical record of participating sites. Study staff transcribed information from the medical record into fixed categories on the trial case report form.
Statistical analyses were performed using R version 3.6.1 (R Foundation for Statistical Computing) and the R packages mice version 3.6.0, miceadds 3.8-9, mitools 2.4, and sandwich 2.5-1. Because of the potential for type I error due to multiple comparisons, findings for secondary outcomes and secondary analyses of the primary outcome should be interpreted as exploratory.
Results
Study Participants and Enrollment
Between May 2015 and March 2017, a total of 1063 participants were randomized. The study drug was not administered to 96 randomized participants and 1 participant was excluded based on being in police custody prior to enrollment, resulting in 966 participants included in the analysis population (mean age, 42 years; 255 [74%] male participants; mean GCS score, 8) (Figure 1). The study groups were well balanced with respect to demographics and baseline anatomic and physiologic characteristics, with the exception of fewer penetrating injuries in the bolus only group. Injury severity was similar between groups based on the out-of-hospital GCS score, the ISS, and the AIS head score (Table 1). The median estimated time from injury to out-of-hospital study drug administration ranged from 40 to 43 minutes across groups, and the bolus completion percentage ranged from 93% to 95%. The median time from out-of-hospital bolus completion to start of the in-hospital infusion ranged from 86 to 94 minutes, and the in-hospital dose completion percentage ranged from 69% to 77%. (Table 1). Emergency unblinding occurred in 3% of patients, of whom 53% received open-label tranexamic acid.
Figure 1. Flow of Participants in a Study of the Effect of Tranexamic Acid vs Placebo on Neurologic Outcomes in Patients With Traumatic Brain Injury.
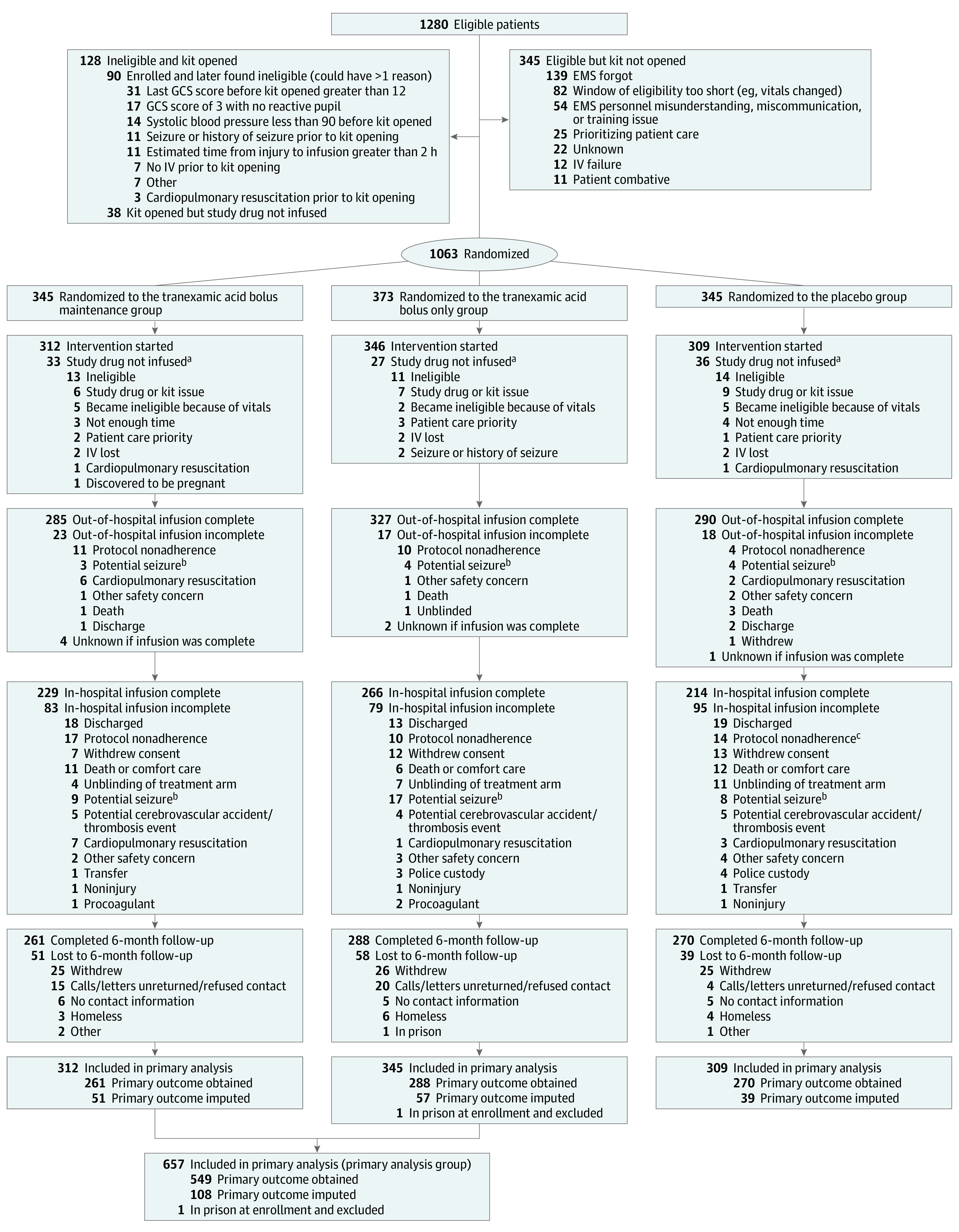
aPatients for whom the blinded study drug was not infused are not included in analyses.
bPatients in this category may have had a seizure prior to study drug administration or seizure-like behavior that was not managed and thus are not necessarily adverse events.
cIncorrect in-hospital assignment was made for 1 patient, who received approximately 125 mg of tranexamic acid prior to infusion being stopped.
Table 1. Baseline Characteristics of Patients in a Study of the Effect of Tranexamic Acid vs Placebo on Neurologic Outcomes in Patients With Traumatic Brain Injury.
| Characteristic | Bolus maintenance (n = 312) | Bolus only (n = 345) | Placebo (n = 309) |
|---|---|---|---|
| Demographics | |||
| Age, median (IQR), y | 39 (26-57) | 40 (26-56) | 36 (25-55) |
| Sex, No. (%) | |||
| Female | 85 (27) | 90 (26) | 76 (25) |
| Male | 227 (73) | 255 (74) | 233 (75) |
| Race, No. (%)a | (n = 273) | (n = 295) | (n = 271) |
| American Indian/Alaska Native | 4 (1) | 4 (1) | 2 (1) |
| Asian | 13 (5) | 10 (3) | 7 (3) |
| Black/African American | 50 (18) | 53 (18) | 46 (17) |
| Native Hawaiian/other Pacific Islander | 1 | 1 | 1 |
| White | 202 (74) | 227 (77) | 213 (79) |
| More than 1 race | 3 (1) | 0 | 2 (1) |
| Ethnicitya | (n = 264) | (n = 294) | (n = 265) |
| Hispanic | 40 (15) | 43 (15) | 40 (15) |
| Injury characteristics | |||
| Injury type, No. (%)b | |||
| Blunt | 302 (97) | 339 (98) | 294 (95) |
| Penetrating (primarily gunshot wounds) | 12 (4) | 5 (1) | 16 (5) |
| Cause of injury | (n = 312) | (n = 340) | (n = 308) |
| Motor vehicle | |||
| Occupant | 103 (33) | 115 (34) | 113 (37) |
| Motorcycle | 32 (10) | 44 (13) | 33 (11) |
| Bicycle/pedestrian | 61 (20) | 62 (18) | 56 (18) |
| Fall at ground level | 44 (14) | 45 (13) | 37 (12) |
| Fall at more than 1 m | 40 (13) | 38 (11) | 32 (10) |
| Assault | 20 (6) | 25 (7) | 24 (8) |
| Suicide attempt | 8 (3) | 5 (1) | 9 (3) |
| Other | 4 (1) | 6 (2) | 4 (1) |
| Out-of-hospital Glasgow Coma Scale score, mean (SD)c | 7.8 (3.3) | 7.8 (3.3) | 7.6 (3.2) |
| Out-of-hospital Glasgow Coma Scale score, No. (%) | |||
| 3-4 | 72 (23) | 81 (23) | 69 (22) |
| 5-6 | 50 (16) | 52 (15) | 62 (20) |
| 7-8 | 47 (15) | 44 (13) | 55 (18) |
| 9-10 | 58 (19) | 77 (22) | 44 (14) |
| 11-12 | 71 (23) | 82 (24) | 71 (23) |
| 13-15 | 14 (4) | 9 (3) | 8 (3) |
| Maximum AIS head score, No. (%)d | (n = 306) | (n = 344) | (n = 301) |
| 0 (none) | 72 (24) | 84 (24) | 65 (22) |
| 1 (minor) | 14 (5) | 19 (6) | 11 (4) |
| 2 (moderate) | 48 (16) | 48 (14) | 46 (15) |
| 3 (serious) | 64 (21) | 66 (19) | 61 (20) |
| 4 (severe) | 56 (18) | 71 (21) | 67 (22) |
| 5 (critical) | 52 (17) | 55 (16) | 50 (17) |
| 6 (unsurvivable) | 0 | 1 | 1 |
| ISS, median (IQR)d | 17 (8-27) | 17 (8-27) | 17 (9-27) |
| Intracranial hemorrhage on initial computed tomographic imaging, No. (%)e | 177 (59) (n = 308) | 197 (58) (n = 339) | 171 (57) (n = 299) |
| Out-of-hospital care | |||
| Advanced airway, No. (%) | 161 (52) | 166 (48) | 168 (54) |
| Emergency medical services time, median (IQR), minf | 49 (36-66) | 47 (33-65) | 48 (35-63) |
| Air transport, No. (%) | 113 (36) | 124 (36) | 104 (34) |
| Out-of-hospital study drug infusion | |||
| Time from injury to start of infusion, median (IQR), ming | 43 (29-62) | 40 (29-65) | 41 (30-58) |
| Entire bolus infused, No. (%) | 285 (93) | 327 (95) | 290 (94) |
| No infusion-related deviations, No. (%)h | 297 (95) | 334 (97) | 304 (98) |
| In-hospital study drug infusion | |||
| Started, No. (%) | 254 (81) | 297 (86) | 244 (79) |
| Time to start of infusion, median (IQR), mini | 88 (60-130) | 94 (65-134) | 86 (60-120) |
| Entire bag infused, No. (%) | 229 (73) | 266 (77) | 214 (69) |
| No infusion-related deviations, No. (%)h | 295 (95) | 335 (97) | 294 (95) |
Abbreviation: IQR, interquartile range.
Race and ethnicity data were obtained through the medical record of participating sites. Study staff at the sites transcribed information from the medical record into fixed categories on the trial case report form. Race and ethnicity are not reported for the Canadian sites.
Four participants with penetrating mechanism also had blunt mechanism (2 in the bolus maintenance group, 1 in the bolus only group, and 1 in the placebo group). Two participants in the bolus only group were found to have no blunt or penetrating injury. All but 3 penetrating injuries were gunshot wounds (2 in the bolus maintenance group and 1 in the bolus only group). Penetrating injury differs between groups (χ2 P value = .03).
The Glasgow Coma Scale (GCS) score is a neurologic scale that ranges from 3 to 15, with lower scores indicating a lower level of consciousness. The overall score is the sum of 3 components. The eye response ranges from 1 (no opening) to 4 (eyes opening spontaneously), the verbal response from 1 (no response) to 5 (oriented), and the motor response from 1 (no motor response) to 6 (obeys commands). GCS scores of 8 or lower generally indicate severe brain injury; 9 to 12, moderate brain injury; and 13 to 15, minor brain injury.
In injury severity scoring, each injury is assigned an Abbreviated Injury Scale (AIS) score that ranges from 1 (minor) to 6 (unsurvivable). Each of 6 regions (head/neck, face, chest, abdomen, extremity, external) is assigned a regional score by taking the highest AIS score in the region. The Injury Severity Score (ISS) is the sum of the squares of each of the 3 highest regional scores. The ISS ranges from 0 to 75, with higher scores indicating greater severity of injury. Patients with injury with an AIS score of 6 in any region are automatically assigned the highest ISS of 75. An ISS greater than 15 is generally considered major trauma. ISS was missing for 18 patients (8 in the bolus maintenance group, 3 in the bolus only group, and 7 in the placebo group).
Participants who died before receiving a head scan and for whom there was no other evidence of intracranial bleeding and patients with “indeterminate” head computed tomographic imaging findings for intracranial bleeding by the central reader are excluded (eTable 12 in Supplement 2). This indicator is not a baseline measure but is included here because it is used for a subgroup definition.
Time from call to dispatch to arrival at the emergency department.
Time of injury was estimated by emergency medical service personnel. If there was no basis to estimate the time, personnel were instructed not to enroll because inclusion criteria included enrolling within 2 hours of injury.
No protocol deviations or violations related to infusion. Eligibility status is not considered a deviation for this measure.
Time from completion of out-of-hospital infusion to start of in-hospital infusion.
Complete data on all prespecified primary and secondary outcomes are presented by treatment group in Table 2. Results from the prespecified primary statistical analysis and exploratory analyses reporting treatment group comparisons for select secondary outcomes and for select subgroups follow.
Table 2. Hospital Course and Outcomesa in a Study of the Effect of Tranexamic Acid vs Placebo on Neurologic Outcomes in Patients With Traumatic Brain Injury.
| Outcome | Treatment group, No. (%) | ||
|---|---|---|---|
| Bolus maintenance (n = 312) | Bolus only (n = 345) | Placebo (n = 309) | |
| Fluids through 24 h | |||
| Median out-of-hospital and in-hospital crystalloid, median (IQR), L | 3.2 (1.9-5.1) | 3.5 (2.3-5.1) | 3.5 (2.2-5.1) |
| Any blood products administered | 65 (21) | 56 (16) | 72 (23) |
| Volume of blood products among patients with some blood products administered, median (IQR), L | 1.3 (0.6-2.2) | 0.8 (0.5-2.1) | 1.4 (0.6-2.4) |
| Any red blood cells administered | 54 (17) | 44 (13) | 62 (20) |
| Red blood cells among patients with some administered, median (IQR), U | 3.0 (2.0-4.5) | 2.0 (0.9-4.0) | 3.0 (1.4-4.8) |
| Outcome from admission blood draw | |||
| Percentage of clot lysed at 30 min following maximum amplitude, %b | (n = 246) | (n = 261) | (n = 240) |
| <0.8 (fibrinolysis shutdown) | 157 (64) | 165 (63) | 148 (62) |
| 0.8-3.0 (normal) | 61 (25) | 65 (25) | 56 (23) |
| >3.0 (hyperfibrinolysis) | 28 (11) | 3 (12) | 36 (15) |
| Outcomes from initial head computed tomographic imaging | |||
| Marshall Classificationc | (n = 290) | (n = 332) | (n = 291) |
| Diffuse injury I | 115 (40) | 134 (40) | 120 (41) |
| Diffuse injury II | 117 (40) | 135 (41) | 106 (36) |
| Diffuse injury III | 12 (4) | 12 (4) | 12 (4) |
| Diffuse injury IV | 4 (1) | 5 (2) | 7 (2) |
| Diffuse injury V/VI | 42 (14) | 46 (14) | 46 (16) |
| Rotterdam Scored | (n = 161) | (n = 187) | (n = 158) |
| 1 | 1 (1) | 5 (3) | 2 (1) |
| 2 | 28 (17) | 36 (19) | 32 (20) |
| 3 | 91 (57) | 98 (52) | 81 (51) |
| 4 | 24 (15) | 28 (15) | 17 (11) |
| 5 | 12 (7) | 17 (9) | 21 (13) |
| 6 | 5 (3) | 3 (2) | 5 (3) |
| Hospital discharge outcomes | (n = 294) | (n = 329) | (n = 292) |
| Glasgow Outcome Scale-Extended score >4e | 101 (34) | 101 (31) | 96 (33) |
| Disability Rating Scalef | (n = 294) | (n = 329) | (n = 291) |
| 0-1 (none to mild disability) | 84 (29) | 89 (27) | 90 (31) |
| 2-6 (partial to moderate) | 92 (31) | 122 (37) | 92 (32) |
| 7-11 (moderately severe) | 37 (13) | 41 (12) | 33 (11) |
| 12-21 (severe to extremely severe) | 23 (8) | 26 (8) | 17 (6) |
| >21 (vegetative to death) | 58 (20) | 51 (16) | 59 (20) |
| Mortality | 53 (18) (n = 294) | 39 (12) (n = 331) | 51 (17) (n = 295) |
| 28-d outcomes | |||
| Neurological procedures, No. (%)g | |||
| Any neurological intervention | 62 (20) | 75 (22) | 54 (17) |
| Craniotomy or craniectomy | 27 (9) | 39 (11) | 27 (9) |
| Intracranial pressure monitoring | 48 (15) | 55 (16) | 48 (16) |
| Adverse events, No. (%)h | |||
| Seizure or seizure-like activity | 5 (2) | 17 (5) | 7 (2) |
| Any thromboembolic event | 13 (4) | 31 (9) | 30 (10) |
| Myocardial infarction | 3 (1) | 2 (1) | 1 (<1) |
| Pulmonary embolism | 3 (1) | 6 (2) | 5 (2) |
| Thrombotic stroke | 3 (1) | 13 (4) | 10 (3) |
| Deep vein thrombosis | 3 (1) | 10 (3) | 9 (3) |
| Other thromboembolic eventi | 1 (<1) | 13 (4) | 9 (3) |
| Hospital-free days, mean (SD)j | 13.6 (10.7) | 14.1 (10.4) | 13.6 (10.7) |
| Intensive care unit–free days, mean (SD)k | 18.1 (10.8) | 19.1 (9.7) | 18.5 (10.6) |
| Ventilator-free days, mean (SD)l | 19.9 (10.8) | 20.9 (9.7) | 20.2 (10.5) |
| Mortality | 53 (19) (n = 285) | 40 (13) (n = 318) | 50 (18) (n = 285) |
| 6-mo outcomesm | |||
| Glasgow Outcome Scale-Extended score >4e | 153 (58) (n = 262) | 178 (62) (n = 289) | 163 (60) (n = 272) |
| Disability Rating Scale scoref | (n = 261) | (n = 287) | (n = 266) |
| 0-1 (none to mild disability) | 123 (47) | 143 (50) | 134 (50) |
| 2-6 (partial to moderate disability) | 64 (25) | 79 (28) | 53 (20) |
| 7-11 (moderately severe disability) | 12 (5) | 10 (3) | 12 (5) |
| 12-21 (severe to extremely severe disability) | 6 (2) | 6 (2) | 11 (4) |
| >21 (vegetative to death) | 56 (21) | 49 (17) | 56 (21) |
| Mortality | 55 (21) (n = 262) | 46 (16) (n = 289) | 54 (20) (n = 272) |
Abbreviation: IQR, interquartile range.
The table includes hospital process measures and outcomes. The sections are organized by chronology from early hospitalization to 6-month follow-up, with some overlap between the sections (eg, some hospital discharges occurred beyond 28 days or even 6 months).
Alterations in fibrinolysis based on fibrinolytic pathway mediators and degree of clot lysis based on kaolin-activated thromboelastography and defined as LY30 (the percent of lysis that occurs 30 minutes after maximum amplitude is achieved). Reasons for missing LY30 are presented in eTable 13 in Supplement 2.
The Marshall classification places patients into 1 of 6 categories of increasing severity based on findings of noncontrast computed tomographic imaging of the brain: I (no visible pathology), II (midline shift 0-5 mm, basal cisterns remain visible, no high or mixed density lesions >25 cm3), III (midline shift 0-5 mm, basal cisterns compressed or completely effaced, no high- or mixed-density lesions >25 cm3), IV (midline shift >5 mm, no high- or mixed-density lesions >25 cm3), V (any lesions evacuated surgically), VI (high- or mixed-density lesion >25 cm3 not surgically removed).
The Rotterdam classification includes 6 categories ranging from 1 (best prognosis) to 6 (worst prognosis). The score is derived from 4 components: basal cisterns (0 indicates normal; 1, compressed; 2, absent), midline shift (0 indicates ≤5 mm and 1 indicates >5 mm), epidural mass lesion (0 indicates present and 1 indicates absent), intraventricular blood or traumatic subarachnoid blood (0 indicates absent and 1 indicates present). The scores for each component are summed and then 1 is added to arrive at the overall score. Only participants with intracranial hemorrhage are included for this outcome. Participants with 1 or more components missing or indeterminant were also excluded.
The Glasgow Outcome Scale-Extended subdivides the categories of severe and moderate disability and good recovery using a scale of 1 to 8, where 1 indicates death; 2, vegetative state; 3, lower severe disability; 4, upper severe disability; 5, lower moderate disability; 6, upper moderate disability; 7, lower good recovery; and 8, upper good recovery. Structured telephone interviews have been developed and validated for the measure and these questions were incorporated into the follow-up survey. The measure was dichotomized into unfavorable (1-4) and favorable (5-8) outcomes for this trial.
The Disability Rating Scale is designed to classify patients based on their degree of function after brain injury, consisting of 8 components: eye opening, communication, motor response, feeding, toileting, grooming, dependence/level of functioning, and psychosocial adaptability/employability. The overall score ranges from 0 (complete recovery) to 30 (death).
Neurosurgical interventions include craniotomy, craniectomy, and placement of a neuromonitoring or drainage device. The follow-up period for interventions continued through hospital discharge or 28 days, whichever occurred first.
Adverse events listed in this section are events listed on the drug insert that are known to be associated with tranexamic acid. The follow-up period for adverse events continued through hospital discharge or 28 days, whichever occurred first. Fifteen patients in the placebo group received open-label tranexamic acid. Among these participants, 1 adverse event (pulmonary embolism) was reported. Each count indicates the number of patients with 1 or more events of the type described in the row label. One patient in the placebo group had 2 instances (or a recurrence) of thrombotic stroke and another patient in the placebo group had 2 instances of deep vein thrombosis.
Other includes cerebral venous sinus thrombosis (7 participants in the bolus only group and 5 in the placebo group), superficial venous thrombosis (2 in the bolus only group and 1 in the placebo group), internal jugular vein thrombus (1 in the bolus only group and 1 in the placebo group), disseminated intravascular coagulation (1 in the bolus maintenance group and 1 in the bolus only group), cerebral vascular emboli (1 in the bolus only group and 1 in the placebo group), left ventricular thrombus (1 in the placebo group), inferior vena cava thrombus (1 in the bolus only group), and “presumed embolic infarcts” (1 in the bolus only group). One patient in the bolus only group had 2 “other” thromboembolic events, thus counts in this footnote do not sum to the count in the table for that group.
Hospital-free days include any day from hospital admission through day 28 that the participant was alive and out of the hospital. Some participants, primarily those who withdrew before discharge, are missing this measure (20 in the bolus maintenance group, 14 in the bolus only group, and 14 in the placebo group).
Intensive care unit–free days include any day from hospital admission through day 28 that the participant is alive and not in the ICU. Participants who died prior to discharge (even if after 28 days) are assigned a value of 0. Some participants, primarily those who withdrew before discharge, are missing this measure (19 in the bolus maintenance group, 14 in the bolus only group, and 14 in the placebo group).
Ventilator-free days include any day from hospital admission through day 28 that the participant is alive and does not require mechanical ventilatory support. Participants who die prior to discharge (even if after 28 days) are assigned a value of 0. Some participants, primarily those who withdrew before discharge, are missing this measure (19 in the bolus maintenance group, 14 in the bolus only group, and 14 in the placebo group).
There were 9 participants for whom the Glasgow Outcome Scale-Extended was taken at 6 months but not the Disability Rating Scale score (1 in the bolus maintenance group, 2 in the bolus only group, and 6 in the placebo group).
Primary Analysis
The primary outcome was obtained in 819 of the 966 participants (85%) treated with the study drug. The percentage of patients who completed follow-up was higher in the placebo group (87%) than in both the bolus maintenance (84%) and bolus only (83%) groups. The primary reasons for failure to follow-up were participant withdrawal from the study and inability to locate the participant 6 months after injury (Figure 1). Participants lost to follow-up were less severely injured and had better outcomes at discharge than other discharged participants (eTable 4 in Supplement 2). The site-adjusted absolute difference in the primary outcome of favorable neurologic outcome (GOSE score >4) between the combined tranexamic acid group and the placebo group was −3.5% (65% vs 62%; [90% 1-sided confidence limit for benefit, −0.9%]; P = .16; [97.5% 1-sided confidence limit for harm, 10.2%]; P = .84) (Table 3). Logistic regression models for the primary outcome yielded similar results (eTable 5 in Supplement 2). Results for the stratified dichotomy of GOSE score based on the qualifying GCS score were similar to those of the GOSE score greater than 4 (eTable 6 in Supplement 2).
Table 3. Adjusted Analyses of Primary and Select Secondary Outcomes in the Tranexamic Acid Groups vs Placebo Group in a Study of the Effect of Tranexamic Acid on Neurologic Outcomes in Patients With Traumatic Brain Injury.
| Outcome | Treatment group, No. (%)a | Adjusted difference (95% CI)b,c | ||
|---|---|---|---|---|
| Combined tranexamic acid group (n = 657) | Placebo (n = 309) | Combined tranexamic acid group vs placebo | P value | |
| Primary outcome | ||||
| 6-mo Glasgow Outcome Scale-Extended score >4d | 425 (65) | 192 (62) | 3.5% (−0.9% to 10.2%)c | .16 (benefit); .84 (harm) |
| Secondary outcomes (exploratory) | ||||
| 28-d mortalityd | 94 (14) | 53 (17) | −2.9% (−7.9% to 2.1%) | .26 |
| 6-mo Disability Rating Scale score, median (IQR)d | 1 (0 to 5) | 1 (0 to 8) | −0.9 (−2.5 to 0.7) | .29 |
| Progression of intracranial hemorrhagee,f | 53 (16) (n = 332) | 30 (20) (n = 148) | −5.4% (−12.8% to 2.1%) | .16 |
Abbreviation: IQR, interquartile range.
See Table 2 for descriptions of the Glasgow Outcome Scale-Extended (GOSE) and Disability Rating Scale (DRS). Imputed outcomes are included for GOSE score, DRS score, and mortality. There were 20 imputed data sets generated, and the mean over both the participants and the 20 data sets is reported. While mean “counts” are rounded to the nearest integer, each participant's mean over 20 imputed data sets may be fractional.
All outcomes are modeled with linear regression with robust standard errors. Adjustments for each model are included in footnotes. eTable 5 in Supplement 2 includes logistic regression results for binary outcomes.
The primary analysis has a 90% (1-sided) confidence limit for benefit and a 97.5% (1-sided) confidence limit for harm.
Model adjusts for regional site only.
To analyze for progression of intracranial hemorrhage (ICH), an initial computed tomographic imaging volume had to be available as well as at least 1 subsequent computed tomographic imaging volume prior to evacuation of a hematoma (eTable 14 in Supplement 2). Progression is defined by a 33% increase or more in combined amount of epidural, subdural, and intraparenchymal hemorrhage volumes on a subsequent computed tomographic image relative to the initial computed tomographic image. The volume difference must be at least 1 mL.
Model adjusts for regional site, age, sex, penetrating vs blunt injury, out-of-hospital Glasgow Coma Scale score, Injury Severity Score, and Abbreviated Injury Scale head score.
Secondary Outcomes
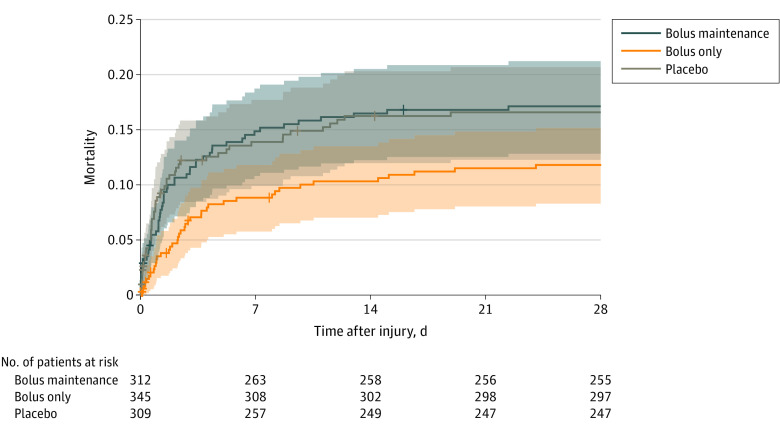
The overall 28-day mortality in this trial was 16%, of which 87% was attributed to neurologic injury (eTable 7 in Supplement 2). The all-cause 28-day mortality was 14% in the combined tranexamic acid group and 17% in the placebo group (adjusted difference, −2.9% [95% CI, −7.9% to 2.1%]; P = .26; Table 3); 28-day mortality was 12% for the bolus only group and 17% for the bolus maintenance group (bolus maintenance vs placebo: adjusted difference, −0.09% [95% CI, −6.1% to 5.9%]; P = .98; bolus only vs placebo: adjusted difference, −5.4% [95% CI, −10.9% to 0.05%]; P = .05; bolus only vs bolus maintenance: adjusted difference, −5.3% [95% CI, −10.8% to 0.1%]; P = .06). Although these mortality differences did not reach statistical significance, they were apparent early (Figure 2) and may have influenced treatment group differences in the rates of adverse events.
Figure 2. Post Hoc Descriptive Analysis of Mortality Through 28 Days in a Study of the Effect of Tranexamic Acid vs Placebo on Neurologic Outcomes in Patients With Traumatic Brain Injury.
Survival data to 28 days was available for 91% of participants in the bolus maintenance group, 92% in the bolus only group, and 92% in the placebo group. Participants who were lost to follow-up after discharge or study withdrawal prior to 28 days and who were notified themselves about their study enrollment rather than family member notification were assumed to survive through 28 days for this plot (n = 52). The remaining participants were censored before 28 days: 5 [2%] in the bolus maintenance group, 9 [3%] in the bolus only group, and 12 [4%] in the placebo group. The shaded areas represent pointwise 95% CIs for each treatment group. The median (interquartile range) observation time for all 3 groups was 28 (28-28) days.
The difference in the DRS score at 6 months was not statistically significant between tranexamic acid–treated and placebo-treated participants (6.8 vs 7.6; adjusted difference, −0.9 [95% CI, −2.5 to 0.7]; P = .29; Table 3). Among participants with multiple head computed tomographic scans, 16% of tranexamic acid–treated participants experienced progression of ICH compared with 20% of placebo-treated participants (adjusted difference, −5.4% [95% CI, −12.8% to 2.1%]; P = .16; Table 3). Pairwise comparisons of the 3 treatment groups for GOSE score greater than 4, DRS score, and progression of ICH revealed no statistically significant differences (Table 3).
Adverse Events
Table 2 and Table 3 list predefined adverse events potentially associated with tranexamic acid administration. Overall, thrombotic events were more frequently observed in the bolus only (9%) and placebo (10%) groups than in the bolus maintenance group (4%). Participants in the bolus only group were more likely to experience seizures (5%) than participants in the bolus maintenance group (2%) or placebo group (2%). In patients without ICH, 6% developed seizures in the bolus only group compared with no patients in the bolus maintenance group and 2% of patients in the placebo group (eTable 8 in Supplement 2). The total numbers of other adverse events were similar between groups (Table 4). Additional details about adverse events are provided in eTable 9 in Supplement 2.
Table 4. Adjusted Analyses of Select Secondary Outcomes and Adverse Events in a Study of the Effect of Tranexamic Acid vs Placebo on Neurologic Outcomes in Patients With Traumatic Brain Injury.
| Outcome | Treatment group, No. (%)a | Adjusted difference (95% CI)b | |||||
|---|---|---|---|---|---|---|---|
| Bolus maintenance | Bolus only | Placebo | Bolus maintenance vs placebo | Bolus only vs placebo | Bolus only vs bolus maintenance | ||
| Secondary outcomes (exploratory) | (n = 312) | (n = 345) | (n = 309) | ||||
| 6-mo Glasgow Outcome Scale-Extended score >4c | 198 (63) | 226 (66) | 192 (62) | 2.0 (−5.8 to 9.8) | 4.8 (−2.9 to 12.5) | 2.8 (−4.6 to 10.2) | |
| 28-d mortalityc | 53 (17) | 41 (12) | 53 (17) | −0.09 (−6.1 to 5.9) | −5.4 (−10.9 to 0.05) | −5.3 (−10.8 to 0.1) | |
| 6-mo Disability Rating Scale score, median (IQR)c | 2 (0 to 7) | 1 (0 to 5) | 1 (0 to 8) | −0.2 (−2.1 to 1.7) | −1.5 (−3.3 to 0.3) | −1.4 (−3.1 to 0.4) | |
| Progression of intracranial hemorrhaged,e | 26 (17) (n = 154) | 27 (15) (n = 178) | 30 (20) (n = 148) | −4.2 (−13.0 to 4.6) | −6.3 (−14.4 to 1.7) | −2.2 (−9.9 to 5.6) | |
| Adverse eventsf | (n = 312) | (n = 345) | (n = 309) | ||||
| Seizure/seizure-like activityc | 5 (2) | 17 (5) | 7 (2) | −0.6 (−2.8 to 1.6) | 2.8 (−0.1 to 5.6) | 3.4 (0.7 to 6.1) | |
| Any thromboembolic eventc | 13 (4) | 31 (9) | 30 (10) | −5.8 (−9.8 to −1.8) | −1.0 (−5.4 to 3.4) | 4.8 (1.1 to 8.5) | |
| Other adverse eventsc,g | 77 (25) | 79 (23) | 76 (25) | −0.4 (−7.1 to 6.2) | −2.3 (−8.8 to 4.3) | −1.8 (−8.3 to 4.6) | |
| Subgroups (exploratory) | |||||||
| Unimputed outcomesh | |||||||
| 6-mo Glasgow Outcome Scale-Extended score >4e | 153 (59) (n = 261) | 178 (62) (n = 288) | 163 (60) (n = 270) | 0.3 (−6.4 to 7.1) | 3.6 (−3.3 to 10.5) | 3.3 (−3.6 to 10.2) | |
| 28-d mortalitye | 53/307 (17) (n = 307) | 40 (12) (n = 336) | 50 (17) (n = 297) | 0.9 (−4.1 to 6.0) | −4.0 (−8.8 to 0.7) | −4.9 (−9.6 to −0.2) | |
| 6-mo Disability Rating Scale score, median (IQR)e | 2 (0 to 8) (n = 261) | 2 (0 to 5) (n = 287) | 1 (0 to 11) (n = 266) | −0.2 (−1.8 to 1.4) | −1.5 (−3.1 to 0.005) | −1.3 (−2.8 to 0.3) | |
| Intracranial hemorrhage on initial computed tomographic imagingi | (n = 177) | (n = 197) | (n = 171) | ||||
| 6-mo Glasgow Outcome Scale-Extended score >4e | 83 (47) | 108 (55) | 86 (50) | −3.9 (−13.0 to 5.2) | 4.8 (−4.4 to 14.0) | 8.7 (−0.3 to 17.8) | |
| 28-d mortalitye | 45 (26) | 35 (18) | 47 (27) | 0.8 (−7.0 to 8.7) | −8.2 (−15.6 to −0.8) | −9.0 (−16.1 to −1.8) | |
| 6-mo Disability Rating Scale score, median (IQR)e | 2 (0 to 30) | 2 (0 to 11) | 4 (0 to 30) | −0.06 (−2.3 to 2.1) | −2.2 (−4.3 to −0.1) | −2.2 (−4.2 to −0.08) | |
See Table 2 for descriptions of the Glasgow Outcome Scale-Extended (GOSE) and Disability Rating Scale (DRS). Imputed outcomes are included for GOSE, DRS, and mortality. There were 20 imputed data sets generated, and the mean over both the participants and the 20 data sets is reported. Although mean “counts” are rounded to the nearest integer, each participant's mean over 20 imputed data sets may be fractional.
All outcomes are modeled with linear regression with robust standard errors. Adjustments for each model are included in footnotes. eTable 5 in Supplement 2 includes logistic regression results for binary outcomes.
Model adjusts for regional site only.
To analyze for progression of intracranial hemorrhage (ICH), an initial computed tomographic imaging volume had to be available as well as at least 1 subsequent computed tomographic imaging volume prior to evacuation of a hematoma (eTable 14 in Supplement 2). Progression is defined by a 33% increase or more in combined amount of epidural, subdural, and intraparenchymal hemorrhage volumes on a subsequent computed tomographic image relative to the initial computed tomographic image. The volume difference must be at least 1 mL.
Model adjusts for regional site, age, sex, penetrating vs blunt injury, out-of-hospital GCS, ISS, and AIS for the head region.
Counts are of participants with 1 or more events of the type listed in the row label.
Includes monitoring for the following events: cardiopulmonary resuscitation, cardiac arrest, cardiac failure, liver failure, acute kidney injury, kidney failure, acute respiratory distress syndrome, cerebral vasospasm, hemorrhagic cerebral vascular accident, central diabetes insipidus, hypernatremia, pseudomembranous colitis, abdominal compartment syndrome, extremity compartment syndrome, fat embolism, posterior ischemic optic neuropathy, anterior ischemic optic neuropathy, pneumonia, sepsis, bloodstream infection, urinary tract infection, meningitis, empyema, cholecystitis, intra-abdominal abscess, pseudomembranous colitis, wound infection, and osteomyelitis.
This section includes participants who completed the 6-month assessment for GOSE and DRS outcomes (ie, outcome was not imputed). For 28-day mortality, it includes participants for whom vital status at 28 days is definitively known and participants who were lost to follow-up prior to 28 days but who were notified of their study participation themselves rather than only family members being notified (n = 52).The latter were assumed to survive through 28 days. Participants are generally notified themselves only if they are sufficiently cognizant to receive and process such information. Among participants with definitive 28-day vital status who were notified themselves of the study only 1 of 362 died before day 28.
Participants with computed tomographic images from which the central reader could not determine whether there was a bleed or not were excluded from both the “Intracranial hemorrhage” and “No intracranial hemorrhage” groups, as were participants who died prior to receiving a computed tomographic image and there was no other evidence to determine whether the participant had an intracranial hemorrhage or not. Among the 26 participants not included in either subgroup, 14 died within 28 days (6 in the bolus maintenance group, 2 in the bolus only group, and 6 in the placebo group).
Exploratory Analyses of Subgroups
Results from between-group comparisons of favorable 6-month neurologic outcome (GOSE score >4), DRS score, and 28-day mortality in the subgroup of participants for whom the outcomes did not need to be imputed were similar to the results of analyses of all patients’ data (Table 4 and eTable 5 in Supplement 2). Among patients who were determined while in the hospital to have had an ICH, 28-day mortality was 18% in the bolus only group, 26% in the bolus maintenance group, and 27% in the placebo group (bolus maintenance vs placebo: adjusted difference, −0.8% [95% CI, −7.0% to 8.7%]; P = .84; bolus only vs placebo: adjusted difference, −8.2% [95% CI, −16.6% to −0.8%]; P = .03; bolus only vs bolus maintenance: adjusted difference, −9.0% [95% CI, −16.1% to −1.8%]; P = .01). Additional subgroup analyses are shown in eTable 10 in Supplement 2.
Resuscitation and Coagulation
Crystalloid volume administered by 24 hours was similar between groups. Participants in the bolus only group received fewer blood transfusions than patients in the other 2 groups. Among those who received a blood transfusion, participants in the bolus only group received less volume (Table 2). Values for all thromboelastography parameters were similar among groups (Table 2 and eTable 11 in Supplement 2).
Discussion
In this phase II trial of patients with moderate or severe traumatic brain injury, there was no statistically significant difference in 6-month neurologic outcome between the combined group of participants with moderate or severe TBI who received either a 1-g out-of-hospital tranexamic acid bolus followed by a 1-g in-hospital tranexamic acid infusion or a 2-g out-of-hospital tranexamic acid bolus followed by an in-hospital placebo infusion compared with participants who received an out-of-hospital placebo bolus followed by an in-hospital placebo infusion.
There are now 2 randomized trials that have examined the use of tranexamic acid in patients with TBI, both of which demonstrated no statistically significant difference in their primary end point comparing participants who received tranexamic acid with those who received placebo.10 This adds to the growing list of neutral trials in individuals with TBI and underscores the importance of developing improved methods for patient selection. In 2019, the CRASH-3 trial collaborators published the results of a pragmatic, placebo-controlled trial that randomized 12 737 adult participants from 29 countries with moderate or severe TBI to receive either a 1-g tranexamic acid bolus (initiated within 3 hours of injury) followed by a 1-g tranexamic acid 8-hour infusion or placebo and found no statistically significant difference in their primary outcome of risk of death from head injury at 28 days (18.5% of patients in the tranexamic acid group vs 19.8% in the placebo group).10 In this trial, similar to the CRASH-3 trial results, all-cause mortality at 28 days was not different between groups (14% for tranexamic acid–treated patients vs 17% for placebo-treated patients). Despite no statistically significant difference in the primary outcome in either trial, there were important differences and findings from both trials that warrant consideration and future investigation. Although this trial did not independently examine patients with less severe injuries, the CRASH-3 trial reported a significantly lower risk of head injury–related death in less severely injured patients (with mild and moderate TBI) who received tranexamic acid compared with placebo (risk ratio, 0.78).10
In contrast to CRASH-3, this trial examined 2 different dosing regimens of tranexamic acid and performed exploratory analyses on the subset of patients with ICH based on the known ability of tranexamic acid to decrease bleeding. Although the CRASH-3 trial investigators did not report the number of participants with ICH and did not independently examine this subset of patients, it is likely that the number of patients enrolled in CRASH-3 with ICH was higher than in this trial because participants were enrolled after imaging was obtained in the hospital setting. Similar to the findings in CRASH-3, most of the mortality difference in this trial was observed by 24 hours after injury. No statistically significant difference in ICH progression was observed between patients who received tranexamic acid and those who received placebo in this trial, suggesting that the antifibrinolytic effect of tranexamic acid on bleeding may be less important than alternative mechanisms in this patient population. Although these findings are only exploratory, future analyses examining different tranexamic acid dosing regimens to determine the true effect of tranexamic acid in both less severely injured patients as well as those with ICH is warranted.
One of the primary concerns surrounding tranexamic acid administration relates to the potential for seizures and thrombotic complications.18,19,20,21 Consistent with multiple trials (including CRASH-3), this trial demonstrated no statistically significant difference in the rate of seizures between participants in the bolus maintenance group (the same tranexamic acid dose used in CRASH-3) and the placebo group, with a seizure rate of approximately 2% across groups in both trials. However, this is the first large-scale trial to administer a 2-g bolus of tranexamic acid, and 5% of participants in that group developed seizures, which is similar to previous studies in different patient populations that demonstrated an association between higher tranexamic acid levels and increased seizures.19,20,21 Although too few patients experienced seizures to draw meaningful conclusions, any potential benefit for tranexamic acid administration in patients with ICH should be balanced by the potential increased seizure risk.
Limitations
This study has several limitations. First, the early mortality difference observed between treatment groups has the potential to lead to survival bias, in which severely injured patients who survived longer in the bolus only group may have lived long enough to experience more complications. Second, determining time of injury prior to hospital arrival is challenging. Although this trial excluded participants without an estimated time of injury, because the actual time of injury is rarely known, the initial 911 call is often used as a surrogate for injury time, which is standard for out-of-hospital trials. Although the time from injury to tranexamic acid administration was well balanced between groups, inaccuracies related to the time from injury to tranexamic acid administration are still likely. Third, enrolling patients in the out-of-hospital setting using the GCS to determine severity of injury has important limitations. Because the GCS has limited ability to discriminate between ICH and other central nervous system depressed states (eg, intoxication, sedation, shock), a fairly low percentage of patients with ICH were enrolled in this trial, which may have diluted treatment differences. Fourth, despite standardized EMS training, 20% of participants enrolled in the trial had a GCS score of 13 or higher on admission, potentially contributing further to an overall low injury severity. Fifth, obtaining 6-month follow-up in participants with TBI enrolled in the out-of-hospital setting, prior to consent, is challenging. In this trial, 15% of participants were lost to follow-up due to study withdrawal or the inability to locate transient participants 6 months after injury. Although considerable prognostic data on participants lost to follow-up were used to impute outcomes, the potential for bias remains. Sixth, participants with both blunt and penetrating injuries were enrolled in this trial to reflect military and civilian populations, potentially introducing heterogeneity into the outcomes. However, because only 3% of participants in this trial experienced penetrating injury, the results may not be generalizable to patients with penetrating TBI. Seventh, several secondary subgroup analyses were based on comparisons of small numbers of participants, potentially leading to a type II error. Eighth, statistical significance was not corrected for multiple testing, potentially leading to type I error; the secondary analyses of the primary outcome as well as the analyses of secondary outcomes should therefore be interpreted with caution.
Conclusions
Among patients with moderate or severe TBI, out-of-hospital tranexamic acid administration within 2 hours of injury did not improve 6-month neurologic outcome as measured by the GOSE.
Trial protocol and statistical analysis plan
eResults
Data sharing statement
References
- 1.Feigin VL, Nichols E, Alam T, et al. ; GBD 2016 Neurology Collaborators . Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(5):459-480. doi: 10.1016/S1474-4422(18)30499-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peterson AB, Xu L, Daugherty J, Breiding MJ. Surveillance Report of Traumatic Brain Injury-related Emergency Department Visits, Hospitalizations, and Deaths—United States, 2014. Centers for Disease Control and Prevention, US Dept of Health and Human Services; 2019. [Google Scholar]
- 3.Alderson P, Roberts I. Corticosteroids for acute traumatic brain injury. Cochrane Database Syst Rev. 2005;(1):CD000196. doi: 10.1002/14651858.CD000196.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewis SR, Evans DJ, Butler AR, Schofield-Robinson OJ, Alderson P. Hypothermia for traumatic brain injury. Cochrane Database Syst Rev. 2017;9(9):CD001048. doi: 10.1002/14651858.CD001048.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma J, Huang S, Qin S, You C, Zeng Y. Progesterone for acute traumatic brain injury. Cochrane Database Syst Rev. 2016;12(12):CD008409. doi: 10.1002/14651858.CD008409.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kobayashi T, Sugiura J. The effect of a new potent antifibrinolytic agent, tranexamic acid. J Jpn Obstet Gynecol Soc. 1966;13(3):158-167. [PubMed] [Google Scholar]
- 7.Gausden EB, Qudsi R, Boone MD, OʼGara B, Ruzbarsky JJ, Lorich DG. Tranexamic acid in orthopaedic trauma surgery: a meta-analysis. J Orthop Trauma. 2017;31(10):513-519. doi: 10.1097/BOT.0000000000000913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pacheco LD, Hankins GDV, Saad AF, Costantine MM, Chiossi G, Saade GR. Tranexamic acid for the management of obstetric hemorrhage. Obstet Gynecol. 2017;130(4):765-769. doi: 10.1097/AOG.0000000000002253 [DOI] [PubMed] [Google Scholar]
- 9.Shakur H, Roberts I, et al. . Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010;376(9734):23-32. doi: 10.1016/S0140-6736(10)60835-5 [DOI] [PubMed] [Google Scholar]
- 10.CRASH-3 trial collaborators Effects of tranexamic acid on death, disability, vascular occlusive events and other morbidities in patients with acute traumatic brain injury (CRASH-3): a randomised, placebo-controlled trial. Lancet. 2019;394(10210):1713-1723. doi: 10.1016/S0140-6736(19)32233-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.CRASH-2 Collaborators Intracranial Bleeding Study C-2 C (Intracranial B. Effect of tranexamic acid in traumatic brain injury: a nested randomised, placebo controlled trial (CRASH-2 Intracranial Bleeding Study). BMJ. 2011;343:d3795. doi: 10.1136/bmj.d3795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weng S, Wang W, Wei Q, Lan H, Su J, Xu Y. Effect of tranexamic acid in patients with traumatic brain injury: a systematic review and meta-analysis. World Neurosurg. 2019;123:128-135. doi: 10.1016/j.wneu.2018.11.214 [DOI] [PubMed] [Google Scholar]
- 13.Clifton GL, Kreutzer JS, Choi SC, et al. . Relationship between Glasgow Outcome Scale and neuropsychological measures after brain injury. Neurosurgery. 1993;33(1):34-38. [DOI] [PubMed] [Google Scholar]
- 14.Shukla D, Devi BI, Agrawal A. Outcome measures for traumatic brain injury. Clin Neurol Neurosurg. 2011;113(6):435-441. doi: 10.1016/j.clineuro.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 15.Narayan RK, Michel ME, Ansell B, et al. . Clinical trials in head injury. J Neurotrauma. 2002;19(5):503-557. doi: 10.1089/089771502753754037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16(3):219-242. doi: 10.1177/0962280206074463 [DOI] [PubMed] [Google Scholar]
- 17.Wright DW, Yeatts SD, Silbergleit R, et al. ; NETT Investigators . Very early administration of progesterone for acute traumatic brain injury. N Engl J Med. 2014;371(26):2457-2466. doi: 10.1056/NEJMoa1404304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boudreau RM, Deshpande KK, Day GM, et al. . Prehospital tranexamic acid administration during aeromedical transport after injury. J Surg Res. 2019;233:132-138. doi: 10.1016/j.jss.2018.07.074 [DOI] [PubMed] [Google Scholar]
- 19.Murkin JM, Falter F, Granton J, Young B, Burt C, Chu M. High-dose tranexamic acid is associated with nonischemic clinical seizures in cardiac surgical patients. Anesth Analg. 2010;110(2):350-353. doi: 10.1213/ANE.0b013e3181c92b23 [DOI] [PubMed] [Google Scholar]
- 20.Keyl C, Uhl R, Beyersdorf F, et al. . High-dose tranexamic acid is related to increased risk of generalized seizures after aortic valve replacement. Eur J Cardiothorac Surg. 2011;39(5):e114-e121. doi: 10.1016/j.ejcts.2010.12.030 [DOI] [PubMed] [Google Scholar]
- 21.Lin Z, Xiaoyi Z. Tranexamic acid-associated seizures: a meta-analysis. Seizure. 2016;36:70-73. doi: 10.1016/j.seizure.2016.02.011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol and statistical analysis plan
eResults
Data sharing statement