Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been discovered as the pathogenic cause of the coronavirus disease 19 (COVID-19). Cellular entry of SARS-CoV-2 are mediated by the spike glycoprotein of virus, and the host specific receptors and proteases. Recently, besides pulmonary complications as the chief symptom, investigations have also revealed that SARS-CoV-2 can trigger neurological manifestations. Herein, to investigate the expression level of receptors and related proteases is important for understanding the neuropathy in COVID-19. We determined the expression levels of receptor ACE2 and CD147, and serine protease TMPRSS2 in human and mouse brain cell lines and mouse different region of brain tissues with qRT-PCR and Western blot. The results showed that the expression pattern of all them was very different to that of lung. ACE2 is lower but CD147 is higher expressed in mostly brain cell lines and mouse brain tissues comparing with lung cell line and tissue, and TMPRSS2 has consistent expression in brain cell lines and mouse lung tissues. It is suggested that SARS-CoV-2 might have a different way of infection to cerebral nervous system. Our finding will offer the clues to predict the possibility of SARS-CoV-2 infection to human brain nervous system and pathogenicity.
Keywords: SARS-CoV-2, Brain nervous system, Receptor and protease, Expression level
1. Introduction
COVID-19 caused by SARS-CoV-2 infection spread around the world and caused a great threat to global public health. The main clinical manifestations are fever, fatigue, dry cough and headache and so on [1]. It is reported that SARS-CoV-2 injures not just human lung but also almost all of organs. The possible mechanism is that virus can infect a variety cells through special receptors and an overactive inflammatory response. It is reported that more than 10% of COVID-19 patients showed neurological symptoms [2]. But whether the brain tissues can be infected directly by SARS-CoV-2 is still unknown.
The presence of receptors is a necessary condition for virus to enter target cells. Although the detail mechanism is not clear, it is reported that angiotensin-converting enzyme 2 (ACE2) and/or CD147 is the important receptor for virus binding to cells, and the transmembrane protease serine 2 (TMPRSS2) is important for virus entry into cells [3,4]. Several studies have clarified that the viral surface spike (S) glycoprotein of SARS-CoV-2 can bind to ACE2 of cell surface to induce membrane fusion, resulting into virus infection [5]. ACE2 is mainly distributed in alveolar epithelial, intestinal epithelial and bronchial epithelial cells, also expressed in vascular endothelial cells, heart and kidney, and in the immune cell spleen, thymus, lymph nodes, bone marrow at lower level [6]. Therefore, the lung and intestine are vulnerable to SARS-CoV-2 and become the main target organs for virus infection. Moreover, the recent experimental and clinical findings suggest that CD147 might also act as a receptor mediating SARS-CoV-2 entry through binding to S protein. CD147 is a type II transmembrane glycoprotein that belongs to the immunoglobulin superfamily and plays a significant role in intercellular recognition in immunology and cellular differentiation [4], and is a widely expressed protein in many cell types including hematopoietic, epithelial, and endothelial cells and so on [7]. After binding to target cells, the S protein is cleaved by the transmembrane protease serine 2 (TMPRSS2), which facilitates virus entry into the cell by inducing the membrane fusion for several coronaviruses, such as SARS-CoV, MERS-CoV, HCoV-229 and SARS-CoV-2 [3]. TMPRSS2 is abundantly expressed in epithelial tissues, including epithelial cells of the respiratory, gastrointestinal, and urogenital tract [8].
Infection by SARS-CoV-2 results in substantial morbidity and mortality. While the lung is the major organ of infection, some studies implied that SARS-CoV-2 might invade the CNS, causing neurological disorders [9]. Neurological symptoms, such as headache, dizziness, and impaired consciousness as well as symptoms involving the cranial nerves (hypogeusia, hyposmia, hypopsia and neuralgia) have been reported in COVID-19 patients [10]. In a case series of 214 patients with COVID-19, 78 (36.4%) patients had neurological manifestations, and severe patients were more likely to have neurological symptoms [11]. Autopsy results of patients with COVID-19 showed that the brain tissue was hyperemic and edematous and some neurons degenerated. Also, SARS-CoV-2 RNA was detected in the CSF specimen of patients [12]. Neurologic injury also has been reported in the infection of other CoVs such as SARS-CoV and MERS-CoV [[13], [14], [15]].
Although tens of thousands of studies about SARS-CoV-2 have been carried out, the understanding of SARS-CoV-2 infection to the brain is still unclear. The S protein on the surface of SARS-CoV-2 binds to specific receptors and induces the membranes fusion of virus and cell with help of various host proteases, completing the first step for virus to invade the host cells. Herein, to investigate the expression levels of receptors and related proteases is important for understanding the pathogens by SARS-CoV-2 infection. This research detected the expressions levels of ACE2, CD147 and serine protease TMPRSS2 in human and mouse brain cell lines, also in mouse different region of brain tissues. The results will give us the clues for the neuroinvasiveness and neuropathy of SARS-CoV-2.
2. Materials and methods
2.1. Cell lines and mouse tissues
One human lung cancer cell line (Calu3) and 3 human brain cell lines (SY5Y, HMC3, U87) and 2 mouse brain cell lines (N2a and BV2) were cultured in DMEM medium (HyClone, Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS, Gibco, Rockville, MD, USA) in a humid atmosphere with 5% CO2 at 37 °C.
The study protocol was approved by the Animal Experimentation Ethics Committee of Jianghan University. Eight-month old C57BL/6 mice (n = 3) were sacrificed and brains were separated, and different regions of brain tissues were separated and collected. All samples were immediately immersed into the PBS after dissection and stored at −80 °C before further experiments.
2.2. Gene expression analysis
Total RNA was extracted from the cells or tissues using TRIzol reagent according to the manufacturer’s protocol (Invitrogen, Carlsbad, CA, USA) and was reverse transcribed using M-MLV Reverse Transcriptase (Invitrogen, Carlsbad, CA, USA). The obtained cDNAs were used as templates for quantitative real-time PCR (qRT-PCR) amplification to determine the mRNA expression levels of ACE2, CD147 and TMPRSS2 with specific primers (Table 1 ) using TB GreenPremix Ex Taq (Takara Bio, Inc., Otsu, Japan). The housekeeping GAPDH gene was used for normalization. The relative expression levels (2−ΔΔCt) of different cell lines and mouse tissues were compared with that of Calu3 cells and mouse lung tissue respectively.
Table 1.
Sequence of primer in qRT-PCR.
| Gene | Sequence of primer (5′ to 3′) |
|---|---|
| ACE2(Human) | F 5′- CGTCTGAATGACAACAGCCTAGA-3′ |
| R 5′-AATGCCAACCACTATCACTCCC-3′ | |
| TMPRSS2(Human) | F 5′-AATCGGTGTGTTCGCCTCTAC-3′ |
| R 5′-CGTAGTTCTCGTTCCAGTCGT-3′ | |
| CD147(Human) | F 5′- CAGAGTGAAGGCTGTGAAGTCG-3′ |
| R 5′- TGCGAGGAACTCACGAAGAAC-3′ | |
| GAPDH(Human) | F 5′-TGACTCTACCCACGGCAAGT-3′ |
| R 5′-TACTCAGCACCAGCATCACC-3′ | |
| ACE2 (Mus) | F 5′- TGAAATAATGGCGACAAGCAC-3′ |
| R 5′- CAGGACCACATACTCTTCATACAAC-3′ | |
| TMPRSS2(Mus) | F 5′- GCACCTCAAAGTCTAAGAAATCG-3′ |
| R 5′- CAGTTGCTGTCCCAGAACCTC-3′ | |
| CD147 (Mus) | F 5′- CCAGGATCAAGGTCGGAAAG-3 |
| R 5′-CAGAACCAATCTGTAATAGGAGGG-3′ | |
| GAPDH (Mus) | F 5′- ATGGGACGATGCTGGTACTGA -3′ |
| R 5′- TGCTGACAACCTTGAGTGAAAT -3′ |
2.3. Western blot analysis
Cells or tissues were homogenized in RIPA buffer (Beyotime Biotechnology, Beijing, China) and the protein concentrations of them were analyzed by the bicinchoninic acid (BCA) method. 30 μg protein of each sample was subjected to 10% SDS-PAGE and transferred to Immobilon-P membrane (Merck Millipore Ltd, Darmstadt, Germany). The membranes were blocked with 5% non-fat milk for 1 h at room temperature, followed by incubation with primary antibodies: anti-ACE2 (#4355 Cell Signaling, 1:1000), anti-CD147 (ab64616 Abcam, 1:1000), anti-TMPRSS2 (14437-1-AP Proteintech, 1:1000) or anti-β-actin antibodies (60008-1-Ig Proteintech, 1:5000) for 1 h and secondary antibodies for 1 h at room temperature. Immune bands were visualized by ChemiDoc™ XRS+ (Bio-Rad Laboratories, Inc., CA, USA) after incubating the membrane with Western Bright Sirius HRP substrate (Milipore, MA, USA). Quantification was performed by using Quantity one software (Bio-Rad Laboratories, Inc., CA, USA).
2.4. Statistical analysis
Each experiment was repeated three times. The expression data were presented as mean ± standard deviation. Mean of the three repeated experiments was compared by one-way analysis of variance with post-hoc Tukey’s test for multiple testing correction. All statistical analyses were performed by the commercially available software (IBM SPSS Statistics 22; SPSS Inc., Chicago, IL). Significance was defined as p < 0.05.
3. Results
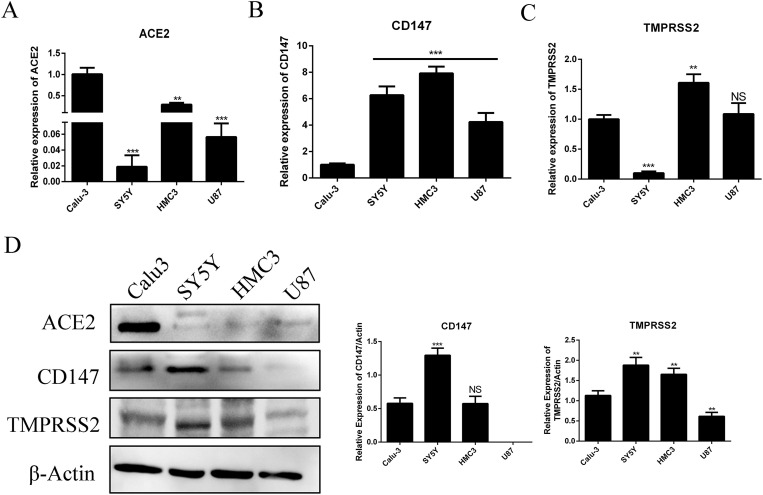
We determined the mRNA and protein levels of ACE2, CD147 and TMPRSS2 in human brain cell lines including neuroblastoma cell SY5Y, microglial cell HMC3 and glioblastoma cell U87. The Calu3 cells, a kind of human lung cell line reported be the target cell of SARS-CoV-2, was used as a control. The mRNA level of ACE2 was significantly weak in brain cell lines comparing with Calu3 cell (Fig. 1 A). Similar to mRNA, the protein of ACE2 was almost not expressed in human brain cell lines, but showed higher expression in Calu3 cell (Fig. 1D). However, there was a higher mRNA level of CD147 in brain cell lines compared with Calu3 cell (Fig. 1B). SY5Y showed significant higher CD147 protein level than that in Calu3, while HMC3 showed similar level (Fig. 1D). Protein CD147 in U87 could hardly be detected although its mRNA showed higher level (Fig. 1B&D). There was no consistent significant differential expression of TMPRSS2 in SY5Y and HMC3 as compared with the Calu3. SY5Y showed significant higher TMPRSS2 protein level but had lower mRNA level. However, there were significant higher level of TMPRSS2 in HMC3 both at mRNA and protein, and lower level of them in U87 (Fig. 1C&D). These results might suggest that SARS-CoV-2 had a different way of infection to brain cells compared with the lung cells.
Fig. 1.
The mRNA and protein levels of ACE2, CD147 and TMPRSS2 in human brain cells. (A–C) Total RNA was extracted from different cells and reverse transcribed, and the mRNA levels of ACE2, CD147 and TMPRSS2 genes was determined by qRT-PCR. Housekeeping gene GAPDH was used for normalization. Relative expression levels of different cells were compared with that of Calu3 cells. (D) Cell lysates were prepared from different cells and analyzed with Western blot using antibodies against ACE2, CD147 or TMPRSS2. β-Actin was used as the loading control. The relative levels of ACE2, CD147 and TMPRSS2 to those of β-actin were estimated by densitometry calculated. ∗∗p < 0.01; ∗∗∗p < 0.001; NS: not significant.
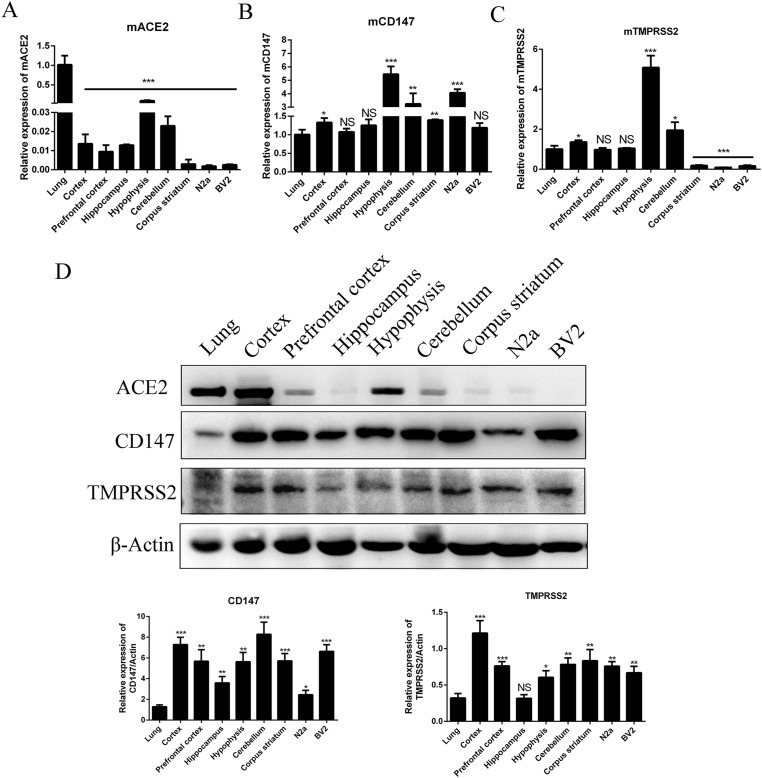
We also determined the mRNA level and protein level of ACE2, CD147 and TMPRSS2 in different region of mouse brain tissues, and mouse brain cell lines neuroblastoma cells N2a and microglial cells BV2. Similar to human brain cell lines, the results showed that the mRNA of ACE2 in almost all regions of mouse brain and cell lines were lower than that in lung tissue except Hypophysis, and both mRNA and protein level of ACE2 in Hypophysis showed higher levels comparing to other region of brain tissues. Cortex showed higher protein level of ACE2 but lower mRNA level (Fig. 2 A&D). Interesting, consistent mRNA of CD147 could be found in all tested tissues and cells, especially in Hypophysis it is more than 5 times of that in lung (Fig. 2B). Moreover, all tested brain tissues and cells showed significant higher CD147 protein levels than that in lung (Fig. 2D). There was high expression of TMPRSS2 mRNA in mouse Cortex, Hypophysis and Cerebellum among the tested tissues, but not in Corpus striatum, N2a and BV2 cells (Fig. 2C). In addition, besides Hippocampus, there were higher expression levels of TMPRSS2 protein among the tested tissues and cells than that in lung tissue (Fig. 2D). Our results indicate that, with reference to the expression of three genes in lung, the Hypophysis, Cortex and Cerebellum could potentially be infected by SARS-CoV-2.
Fig. 2.
The mRNA and protein levels of ACE2, CD147 and TMPRSS2 in different regions of mouse brain tissues and brain cell lines. (A–C) Total RNA was extracted from different regions of mouse brain tissues and cells. The expression of ACE2, CD147 and TMPRSS2 genes was determined by qRT-PCR. Housekeeping gene GAPDH was used for normalization. Relative mRNA levels of different cells were compared with that of mouse lung tissue. (D) Cell lysates were prepared and analyzed by Western blot using antibodies against ACE2, CD147 or TMPRSS2. Beta-actin was used as the loading control. The relative levels of ACE2, CD147 and TMPRSS2 to those of β-actin were estimated by densitometry calculated. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; NS: not significant.
4. Discussion
In this study, we detected the expressions of SARS-CoV-2 receptor ACE2, CD147 and serine protease TMPRSS2 in human and mouse brain cell lines, also in mouse brain tissues. As results shown, mRNA and protein of ACE2 was lower expressed in mostly brain cell lines and tissues comparing with lung cells and tissue. However, another receptor, CD147, had a higher level of expression in brain cells lines and tissues than that in lung cells. Moreover, consistent expression of TMPRSS2 could be found in human brain cell lines. A large-scale immunohistochemical analysis on 45 different human tissues reports that there was not expression of ACE2 in the brain [16]. RNA-seqs detection also indicated that brain showed a consistent lack of ACE2 expression [17,18]. Some RNA-seqs found that expression of TMPRS2 seemed to be higher compared to that of ACE2 in both neuronal and non-neuronal olfactory epithelium (OE) cells [19,20]. However, the detailed expression of these genes in different brain cells and different region of brain tissues has not been reported.
Since cell type and region in brain infected by virus will result in different pathological changes and symptoms, our results might give some information on the neurological symptoms of COVID-19 patients. Compared with ACE2, the cerebral nervous system was more likely to be infected with SARS-CoV2 through CD147 receptor and protease TMPRSS2. What was more, considering the necessity of both CD147 and TMPRSS2 for SARS-CoV-2 infection, neuron and microglia possessed some possibility for SARS-CoV-2 infection, whereas astrocyte would be less likely to be infected by SARS-CoV-2. We also found that mRNA and protein of CD147 and TMPRSS2 showed higher level in Hypophysis, Cortex and Cerebellum of mouse brain. Symptoms related to Hypophysis include frequent headaches, vision changes and impaired pituitary function. Cortex and Cerebellum damage can induce dizziness, ataxia, impaired consciousness and other neurological diseases. Therefore, the susceptibility of these brain regions to SARS-CoV-2 may be one of the reasons for the cognitive and neurological impairment in patients. Moreover, it was reported that S protein of SARS-CoV-2 had lower affinity with CD147 than ACE2 [4], which may explain the low direct infectivity of SARS-CoV-2 to brain. All of these implied that SARS-CoV-2 can infect brain, but the infection level is lower than lung.
In summary, the structural basis of SARS-CoV-2 and several indirect and direct evidence mentioned above have provided proof to support the theory of neurotropic involvement of SARS-CoV-2. Thus, considerably more attention should be paid to the risk of neurological involvement in patients with COVID-19. Therefore, there is a need to investigate which cell and tissue types in the brain accumulate virus particles and whether the virus is transferred between brain cells. Meanwhile, it is recommended that researchers pay more attention to the infection of SARS-CoV-2 in the brain and its impact on brain tissue and function in vivo, so as to provide a theoretical basis for clinical diagnosis and treatment.
Funding
The work was supported by the National Natural Science Foundation of China [grant numbers 31670167]; Wuhan Science and Technology Bureau [grant numbers 2020020601012318] and Jianghan University [grant numbers 1010/08190001 and 2019037]
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We would like to thank Kuan Lin and Xiaokang Gong for technical support.
References
- 1.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in wuhan, China. J. Am. Med. Assoc. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baig A.M., Sanders E.C. Potential neuroinvasive pathways of SARS-CoV-2: deciphering the spectrum of neurological deficit seen in coronavirus disease-2019 (COVID-19) J. Med. Virol. 2020 doi: 10.1002/jmv.26105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffmann M., Kleine-Weber H., Schroeder S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang K., Chen W., Zhou Y.S., et al. SARS-CoV-2 invades host cells via a novel route: CD147-spike protein. bioRxiv. 2020 doi: 10.1101/2020.03.14.988345. 03.14.988345. [DOI] [Google Scholar]
- 5.Shang J., Wan Y., Luo C., et al. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. U. S. A. 2020;117:11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang F., Yang J., Zhang Y., et al. Angiotensin-converting enzyme 2 and angiotensin 1-7: novel therapeutic targets. Nat. Rev. Cardiol. 2014;11:413–426. doi: 10.1038/nrcardio.2014.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nabeshima K., Iwasaki H., Koga K., et al. Emmprin (basigin/CD147): matrix metalloproteinase modulator and multifunctional cell recognition molecule that plays a critical role in cancer progression. Pathol. Int. 2006;56:359–367. doi: 10.1111/j.1440-1827.2006.01972.x. [DOI] [PubMed] [Google Scholar]
- 8.Bugge T.H., Antalis T.M., Wu Q. Type II transmembrane serine proteases. J. Biol. Chem. 2009;284:23177–23181. doi: 10.1074/jbc.R109.021006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou Z., Kang H., Li S., et al. Understanding the neurotropic characteristics of SARS-CoV-2: from neurological manifestations of COVID-19 to potential neurotropic mechanisms. J. Neurol. 2020;267:2179–2184. doi: 10.1007/s00415-020-09929-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yin R., Feng W., Wang T., et al. Concomitant neurological symptoms observed in a patient diagnosed with coronavirus disease 2019. J. Med. Virol. 2020 doi: 10.1002/jmv.25888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mao L., Jin H., Wang M., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in wuhan, China. JAMA Neurol. 2020 doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Health Commission of the People′s Republic of China . 2020. Diagnosis and Treatment of the Novel Coronavirus Pneumonia (Trial Version 7) [Google Scholar]
- 13.Desforges M., Le Coupanec A., Dubeau P., et al. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. 2019;12 doi: 10.3390/v12010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hung E.C., Chim S.S., Chan P.K., et al. Detection of SARS coronavirus RNA in the cerebrospinal fluid of a patient with severe acute respiratory syndrome. Clin. Chem. 2003;49:2108–2109. doi: 10.1373/clinchem.2003.025437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arabi Y.M., Balkhy H.H., Hayden F.G., et al. Middle East respiratory syndrome. N. Engl. J. Med. 2017;376:584–594. doi: 10.1056/NEJMsr1408795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hikmet F., Mear L., Edvinsson A., et al. The protein expression profile of ACE2 in human tissues. Mol. Syst. Biol. 2020;16 doi: 10.15252/msb.20209610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uhlen M., Karlsson M.J., Zhong W., et al. A genome-wide transcriptomic analysis of protein-coding genes in human blood cells. Science. 2019;366 doi: 10.1126/science.aax9198. [DOI] [PubMed] [Google Scholar]
- 18.Uhlen M., Hallstrom B.M., Lindskog C., et al. Transcriptomics resources of human tissues and organs. Mol. Syst. Biol. 2016;12:862. doi: 10.15252/msb.20155865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanageswaran N., Demond M., Nagel M., et al. Deep sequencing of the murine olfactory receptor neuron transcriptome. PLoS One. 2015;10 doi: 10.1371/journal.pone.0113170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saraiva L.R., Ibarra-Soria X., Khan M., et al. Hierarchical deconstruction of mouse olfactory sensory neurons: from whole mucosa to single-cell RNA-seq. Sci. Rep. 2015;5:18178. doi: 10.1038/srep18178. [DOI] [PMC free article] [PubMed] [Google Scholar]