Abstract
Bipolar disorder (BD) is a mood disorder with subtypes characterized by episodes of mania, hypomania, and/or depression. BD is associated with substantial economic burden, and the bipolar I disorder (BD-I) subtype is associated with high medical costs. This review further evaluated the economic burden of BD and BD-I in the United States (US), describing health-care resource utilization (HCRU) and sources of direct medical and indirect costs. Data were obtained from systematic searches of MEDLINE®, EMBASE®, and National Health Service Economic Evaluation Database. Citations were screened to identify primary research studies (published 2008–2018) on the economic burden of BD/BD-I or its treatment in real-world settings. Reported costs were converted to 2018 US dollars. Of identified abstracts (N=4111), 56 studies were included. The estimated total annual national economic burden of BD/BD-I was more than $195 billion, with approximately 25% attributed to direct medical costs. Individuals with BD/BD-I used health-care services more frequently and had higher direct medical costs than matched individuals without the disease. Drivers of higher direct costs included frequent psychiatric interventions, presence of comorbid medical/psychiatric conditions, and both suboptimal medication adherence and clinical management. Indirect costs (eg, unemployment, lost work productivity for patients/caregivers) accounted for 72–80% of the national economic burden of BD/BD-I. Different definitions for study populations and cost categories limited comparisons of economic outcomes. This review builds on existing literature describing the economic burden of BD and confirmed cost drivers of BD/BD-I. Improved clinical management of BD/BD-I and associated comorbidities, together with better medication adherence, may reduce health-care costs and improve patient outcomes.
Keywords: cost of illness, health care costs, indirect costs, mania, mood disorder, resource utilization
Introduction
Bipolar disorder (BD) is a severe and complex mental health disorder composed of different subtypes that present variably. The disorder is characterized by shifts in mood (ie, alternating periods of elation, irritability, and depression), energy, and behavior.1,2 The lifetime prevalence of BD is estimated to be 4.4% in the United States (US), with most cases emerging during adolescence or early adulthood.3 BD is a leading cause of disability among young people,2,4 and is associated with impairments that negatively impact personal, social, and occupational functioning, and reduce quality of life.1,2,5-8
Prior reviews of cost of illness studies have found a substantial economic burden associated with BD, and that cost estimates for the disorder vary considerably across studies. For example, one analysis estimated the per-person total lifetime costs of BD in the US ranged from $11,720 for a single manic episode to $624,785 for a disease course marked by nonresponsive/chronic episodes (1998 US dollars [USD]).9 Sources of direct health-care costs for individuals with BD include medical expenses associated with psychiatric care (both inpatient and outpatient), treatment (pharmacological and non-pharmacological), and emergency room (ER) visits.6,9-11 Persons with BD tend to have higher rates of comorbid medical (eg, metabolic syndrome, hypertension) and psychiatric (eg, substance use disorder, anxiety) conditions, which contribute to higher utilization of general medical services compared to the general population.1,12-15 Fewer studies have examined indirect costs (eg, expenditures associated with reduced work productivity, use of caregivers) for those with BD;6,10,16 yet, their impact is sizable with losses in work productivity previously estimated to represent 20% to 94% of the total societal cost of BD.6
Bipolar I disorder (BD-I) is a subtype of BD in which individuals experience one or more manic episodes, and accounts for approximately one-quarter of all cases of BD.3,17 The disease course for BD-I is typically chronic and is associated with significant functional disability and premature mortality.3,5,18-20 Some evidence suggests that BD-I may also be associated with higher direct medical costs compared to other subtypes of BD; however, the reasons for this are poorly understood.6 Few studies have elucidated the different drivers that may contribute to greater cost burden for those living with BD, in general, and those with BD-I, specifically.
The objective of this systematic review is to provide an updated report of the economic burden of BD in the US, including a broader spectrum of cost and/or health-care resource use (HCRU) estimates compared with previous reviews.6,10 Direct and indirect costs of the disorder are summarized, and drivers of these costs are identified. Where specific data existed for BD-I, these estimates are reported separately from those for BD overall. While BD-I has been associated with higher direct medical costs compared with other BD subtypes,6 this review examines broader cost outcomes and drivers of these costs specifically for patients with BD-I.
Materials and Methods
MEDLINE®, MEDLINE® in-process, EMBASE®, and National Health Service Economic Evaluation Database (NHS EED) databases were searched for primary research studies published between 1 January 2008 and 9 July 2018 on the economic burden of BD and BD-I in the US. Search strategies combined terms related to disease and outcomes and were limited to English-language publications only. The full search strategies and search terms are available in the electronic supplementary materials Tables 1 and 2.
The start year (2008) was selected because it captured a decade of published literature at the time the review was conducted. From 2008 to 2018, several new medications became available for the treatment of BD/BD-I21 and multiple international guidelines, including a major North American clinical guideline, were revised.22–28 In addition, two federal laws were passed in 2008 and 2010 (Mental Health Parity and Addictions Equity Act [MHPAEA] and Affordable Care Act [ACA], respectively) that substantially changed the insurance landscape and availability of mental health benefits in the US.29 Given these collective events, conducting an updated review to understand the contemporary economic burden of BD/BD-I in the US was warranted.
Publications were included if the population of interest was adults with BD (generally or not otherwise specified) or BD-I, and the economic burden of the disorder or its treatment in the US was reported or could be derived. Economic burden was defined broadly; studies that discussed patterns of HCRU without cost estimates and papers that described other economic impacts associated with BD or BD-I (eg, workplace productivity, disability) were included in this review. Inclusion was restricted to studies conducted in a real-world setting (ie, not randomized controlled trials) and studies that included cohorts of at least 100 patients. Studies that focused on bipolar depression only, or on subtypes other than BD-I, were excluded, as were case reports and cost-effectiveness analyses and similar economic evaluations of specific medications. Reviews were not included but their bibliographies were screened for relevant studies.
Citations from all database searches were combined; duplicates and excluded publication types (eg, randomized controlled trials, case reports) were flagged electronically and removed. Titles and abstracts for the remaining articles were screened by one reviewer, with independent review by a second reviewer if inclusion/exclusion was unclear. Inclusion was confirmed by review of full-text publications by one reviewer, with queries resolved by discussion with a second reviewer. Data from included studies were extracted into a structured spreadsheet by two reviewers, and disagreements were resolved by consensus. Data specific to BD-I were extracted separately wherever possible. The extraction spreadsheet was organized to capture discrete categories of economic outcomes to facilitate descriptive summary of the findings for this review. The methodological characteristics of included cost of illness studies were assessed with the checklist utilized by Kleine-Budde et al,10 and these results are included in the electronic supplementary materials Tables 4 and 5 .
Costs were converted to a common year currency (2018 USD) using the Consumer Price Index (CPI) for Medical Care.30 If cost-year was not reported in a study, it was assumed to be the last year of the observation period mentioned in the source publication.
Results
Literature Search Results
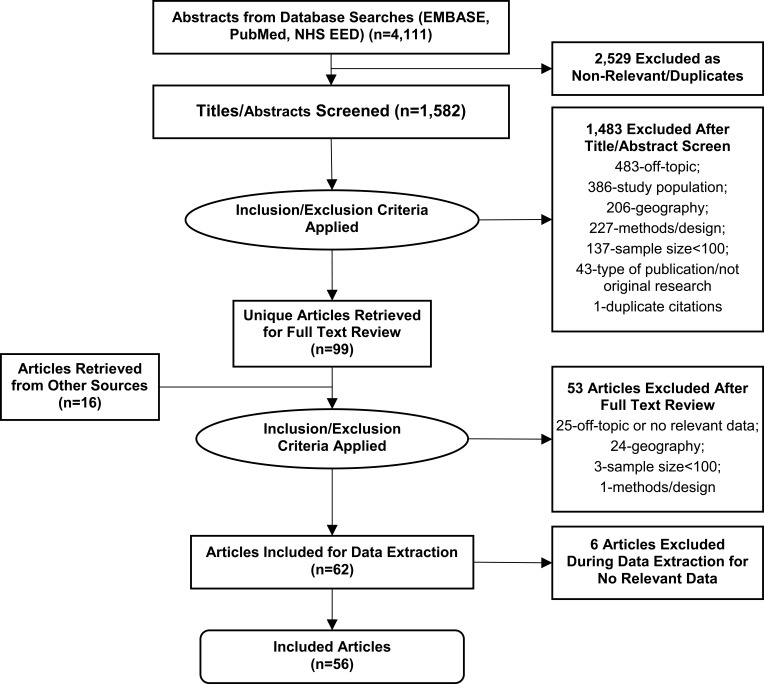
A total of 4111 abstracts were identified. Following screening, 99 articles were selected for full-text review. After inclusion and exclusion criteria were applied, 56 articles were included in the review. Thirteen studies (23.2%) reported data specific to BD-I, whereas the other 43 studies (76.8%) reported data on BD (generally or not otherwise specified). The study selection process is shown in Figure 1.
Figure 1.
PRISMA diagram showing the literature search process.
Notes: The 2529 records excluded prior to title/abstract review were eliminated electronically by identifying duplicate citations (eg, records with duplicate identifiers or citation data fields) and publications indexed for excluded types of publications (eg, randomized controlled trials, case reports). The step “articles retrieved from other sources” refers to papers identified from bibliographic review and other known, relevant research papers.
Abbreviations: BOI, burden of illness; NHS EED, National Health Service Economic Evaluation Database; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Of the 56 included studies, 30 studies (54%) reported cost data. The assessment of methodological characteristics of these studies found that most reported their data sources and analysis perspective; however, only 15 studies (50%) reported the monetary value of all HCRU and 13 studies (23%) provided separate information about the number of services (eg, health-care) and costs for the cost categories described. Inclusion of sensitivity analyses in these cost studies was uncommon. Of the papers not reporting cost data, 4 studies (7%) reported on HCRU, and 22 studies (39%) described other topics associated with economic burden (eg, work productivity, caregiver burden).
The electronic supplementary materials Tables 3–5 provide a list of all studies included in this review and the assessment of methodological characteristics for the cost studies identified.
Total National Economic Burden
Two studies estimated national costs using prevalence data for BD-I and bipolar II disorder (BD-II) in the US population. Cloutier et al estimated the total annual costs of BD-I in the US at $219.1 billion, corresponding to an average of $88,443 per person with BD-I per year. This figure included $50.9 billion in direct health-care costs (ie, medical and pharmacy); $9.7 billion in direct non-healthcare costs (eg, BD-related substance use disorder, criminal justice involvement for those who commit or are victims of crime, prevention/research costs); and $158.5 billion in indirect costs (eg, loss of work productivity or premature mortality). The excess costs of BD-I (the difference between costs incurred by individuals with and without BD-I) were reported to be $129.9 billion annually, an average of $52,413 per person with BD-I per year. Total costs for individuals with BD-I were 2.46 times greater than for controls without BD-I.31
A second study estimated the total annual cost burden of BD-I and BD-II at $194.8 billion, including direct costs of $39.6 billion and indirect costs of $155.2 billion. However, the author explicitly acknowledged that these cost estimates were likely to be substantially underestimated, due to certain assumptions on which the analysis was based (eg, prevalence figures that did not include all subtypes of BD; direct and indirect cost estimates sourced from a 20-year-old cost analysis; the assumption that BD-I and BD-II are equally costly disorders; pharmacy costs that only included lithium).32
Direct Health-Care Costs
Seventeen studies (two for BD-I, 15 for BD) reported on direct all-cause and/or mental health-related costs (Table 1). Some cost estimates for cohorts with BD were higher and spanned a wider range than those reported for patients with BD-I. Several factors may contribute to this variation, including methodological differences between studies, consideration of different cost components (eg, inclusion of emergency room or other costs), and differences in clinical management and available treatments during the periods studied (2004 to 2007 for BD-I and 1998 to 2014 for BD, respectively).
Table 1.
Direct Health-Care Costs (Annualized, per Person, 2018 USD)
| Cohort | Data Source | Study | Study Period | Services Included | All-Cause Costs | Mental Health Costs | ||
|---|---|---|---|---|---|---|---|---|
| Inpatient, Outpatient, Pharmacy | Emergency Room | Other Services† | ||||||
| BD-I | Commercial claims | Bagalman et al, 201133 * | 2004–2007 | ● | $12,913–$19,446 | $4521–$9132 | ||
| Medicaid claims | Durden et al, 201034* | 2004–2006 | ● | ● | $11,239– $15,699 | $4823–$8299 | ||
| BD | Commercial claims | Busch et al, 201344 ** | 1999–2002 | ● | ● | – | $6580 | |
| Centorrino et al, 200912 | 2004–2005 | ● | ● | $19,131 | – | |||
| Guo et al, 200846 | 1998–2002 | ● | ● | ● | $21,707 | $7163 | ||
| Jing et al, 200935 | 2003–2006 | ● | ● | $37,958–$46,791 | $10,545–$21,523 | |||
| Pelletier et al, 201356 | 2007–2008 | ● | $27,665–$29,842 | – | ||||
| Stensland et al, 201048 | 1999–2005 | ● | $14,892–$17,568 | – | ||||
| Williams et al, 201137 | 2004–2007 | ● | $21,694 | – | ||||
| MCO claims | Haskins et al, 201039 | 1999–2005 | ● | – | $6374–$15,189 | |||
| Stensland et al, 201077 | 2002–2003 | ● | $17,201–$37,489 | – | ||||
| Tohen et al, 201736 | 2012–2014 | ● | ● | $11,051–$16,953 | $8540–$12,603 | |||
| Medicaid claims | Qiu et al, 200958 | 2000–2005 | ● | ● | $21,563–$25,328 | $6830–$7179 | ||
| Qiu et al, 201059 | 2000–2005 | ● | ● | $20,500–$24,049 | $6915–$9507 | |||
| Rascati et al, 201157 | 2002–2008 | ● | $25,110 | $15,036 | ||||
| Seabury et al, 201478 | 2001–2008 | ● | $24,209 | – | ||||
| State agencies | Robertson et al, 201579 | 2006–2007 | ● | ● | – | $15,234–$17,753*** | ||
Notes: *Includes deductibles and co-payments; **limited to those with resource use; ***Costs over a 2-year period; – denotes not reported. †Other services include the use of intermediate care or skilled nursing facility, home-based care, ambulance, and laboratory tests.
Abbreviations: BD, bipolar disorder; BD-I, bipolar I disorder; MCO, managed care organization; USD, US dollars.
All-Cause Health-Care Costs
Fourteen studies (two for BD-I, 12 for BD) provided estimates of annual all-cause direct health-care costs (Table 1). Among cohorts with a BD-I diagnosis, annual all-cause direct health-care costs varied from $11,239 to $19,446 per-person-per-year (PPPY).33,34 Estimates of annual all-cause direct costs reported for patients with BD spanned a wider range, from $11,051 to $46,971 PPPY.35,36
A retrospective study of commercial health-care claims reported that PPPY all-cause health-care costs (ie, inpatient, outpatient, prescription medications) for individuals with BD were about four times higher than for matched individuals with no mental health disorders and no psychotropic medication use ($19,131 [BD] vs $4706 [no mental health disorders]).12 A separate study reported that individuals in an employer-based health plan diagnosed with BD had higher all-cause, mean per-member-per-month health-care costs than those with diabetes, depression, asthma, or coronary artery disease. This was largely due to higher costs for medications and psychiatric care (inpatient and outpatient) among those with BD. Only individuals diagnosed with both diabetes and coronary artery disease had higher all-cause health-care costs than those with BD. Sixty-four percent of total costs for the BD group were incurred by a small subgroup (20%) of patients; who were more likely to be female, have frequent hospital stays, and have a higher number of comorbidities.37
In a cohort of community-dwelling dual-eligible Medicare/Medicaid beneficiaries with a mental health disorder in 2005, individuals with a diagnosis of BD had 34% higher medical care costs, and 59% higher prescription drug expenditures than those without a diagnosis of BD. Among members of this group who used Medicaid-paid community-based long-term care services (eg, in-home services), a diagnosis of BD was associated with 5% higher medical care costs, 15% higher long-term care costs, and 55% higher prescription drug expenditures than those without a diagnosis of BD. The authors reported a similar pattern for individuals who resided in Medicaid-paid institutional (eg, nursing home) long-term care facilities, noting the increased medication costs relative to those with other mental health diagnoses were expected due to this population’s greater reliance on pharmacotherapy and having more comorbid conditions.38
Costs Related to Mental Health Care and Psychiatric Hospitalization
Eleven studies (two for BD-I, nine for BD) evaluated mental health-related costs (Table 1). These studies estimated that annual mental health-related costs totaled between $4521 and $9132 PPPY for individuals with BD-I33,34 and between $6374 and $21,523 PPPY for individuals with BD.35,39
Two studies examined the cost of psychiatric hospitalization. The first estimated the cost of a psychiatric hospitalization in patients with BD-I to be $9544.40 This figure is within the range reported by Stensland et al, who reported that the average cost of community hospital-based inpatient psychiatric care for patients with BD was $1159 to $1262 per day, depending on payer, with an average length of stay between 5.5 days (uninsured) and 9.4 days (Medicare).41
Health-Care Resource Utilization
HCRU was reported in four studies (Table 2); however, no study reported data specific to patients with BD-I. A diagnosis of BD was associated with high use of outpatient, inpatient, emergency, pharmaceutical, medical, and mental health services (eg, psychotherapy, BD-related acute care).
Table 2.
Health-Care Utilization (Annualized) in Cohorts with BD
| Study | Study Design [Sample] Evaluation Period |
Brief Methods and Key Findings |
|---|---|---|
| Baldessarini et al, 200843 | Retrospective study [N=7406 (55.4% BD-I)] 1 year |
Described annual mean (per person) medical service use in the 12 months prior to initial psychotropic prescription for BD in a commercially-insured sample.
|
| Busch et al, 201344 | Retrospective study [N=1104 (any BD)] 1 year |
Described annual mean (per person) mental health/SUD service use among BD patients in a commercially-insured sample.
|
| Centorrino et al, 200912 | Retrospective study [N=28,531 (any BD) vs N=85,593 (no mental health disorder)] 1 year |
Compared prevalence of metabolic conditions and health care costs between individuals with BD vs age-/sex-matched individuals with no mental health disorder. The BD cohort had a higher prevalence of metabolic comorbidities, which contributed to significantly greater rates of medical service use (p<0.0001 all comparisons). Annual medical service use (BD vs no mental health disorder):
|
| Lindamer et al, 201242 | Retrospective study [N=1215 (calculated; 12% any BD of N=10,128)] 4 years |
Examined factors associated with high utilization of acute mental health services (defined as ≥3 acute care episodes) from analyses of records from a regional public mental health services database.
|
Abbreviations: BD, bipolar disorder; BD-I, bipolar I disorder; ER, emergency room; OR, odds ratio; SUD, substance use disorder.
One study found that having a BD diagnosis increased the odds of being a “high-use consumer” of health care by 70% relative to a diagnosis of depression (“high-use” was defined as using inpatient, mental health ER services, or crisis residential visits three or more times in a fiscal year).42 Another study found individuals with a diagnosis of BD had greater HCRU compared to age- and gender-matched individuals with no mental health disorders or psychotropic medication use, with the greatest increases reported for acute care services. The percentage of patients in the BD cohort with an inpatient admission was four times higher (22.4% vs 5.0%) and the percentage of BD patients with ER visits was more than twice as high (37.0% vs 14.8%) than the matched cohort. Increased use of outpatient visits (99.9% vs 95.0%) and prescription drugs (97.5% vs 82.0%) was reported for those with BD relative to the matched cohort.12 Two other studies providing descriptive data for annual HCRU among commercially insured patients with BD43,44 and are summarized in Table 2.
Drivers of Direct Health-Care Costs
Eleven studies reported factors that were associated with either increased or decreased direct health-care costs in individuals with BD-I or BD. Factors associated with increased direct health-care costs included having frequent psychiatric interventions (ie, hospitalization, ER visit), the presence of comorbid medical and psychiatric conditions, nonadherence to BD-related medication, approach to pharmacotherapy (eg, use of certain combination treatments), and suboptimal clinical management due to a misdiagnosis of unipolar depression following a BD diagnosis.12,33,34,37,39,45-50
Frequent Psychiatric Intervention
Three studies (two for BD-I, one for BD) examined patients who had “frequent psychiatric intervention” (FPI) over a 12-month period (Year 1), evaluating their health-care costs over the subsequent 12-month period (Year 2), relative to patients without FPI. Two studies defined FPI as ≥2 ER visits or hospitalizations with a principal diagnosis of BD, addition of a new medication to the first observed treatment regimen, or ≥50% increase in BD medication dose, within a 12-month period.33,34 The third study utilized a similar definition for FPI but specified a frequency of ≥4 such events within a 12-month period.39 FPI was common, with a prevalence of 40% to 53% in BD-I cohorts33,34 and 52.5% in a group with BD-I or BD-II.39 Compared to those without FPI, individuals who had FPI incurred greater mental health-related and all-cause medical costs in the year following the FPI (Table 3). They also had a 3.7-fold higher risk of subsequent mental health hospitalization and 3.1-fold higher risk of subsequent ER visits in the year following the FPI.34
Table 3.
Frequent Psychiatric Intervention
| Study | Study Design [Sample] Evaluation Period |
Definition of FPI | Key Findings |
|---|---|---|---|
| Bagalman et al, 201133 | Retrospective study [N=19,191 (BD-I)] 1 year |
Having ≥2 clinically significant events during the 12-month identification period (Year 1) after the first observed diagnosis of BD-I. A clinically significant event included ER visits or hospitalizations with a principal diagnosis of BD (any type), addition of a new medication to the first observed treatment regimen, or ≥50% increase in BD medication dose. |
|
| Durden et al, 201034 | Retrospective study [N=5527 (BD-I)] 1 year |
|
|
| Haskins et al, 201039 | Retrospective study [N=632 (BD-I or BD-II)] 7 years, max |
Having ≥4 clinically significant events requiring intervention in any 12-month period. A clinically significant event was defined as a BD-related ER visit, inpatient psychiatric hospitalization, or a change in psychotropic medication associated with psychiatric symptoms. |
|
Notes: All three studies identified patients who had FPI over a 12-month period (Year 1) and examined health care costs for these patients over the subsequent 12-month period (Year 2) relative to patients without FPI. Costs adjusted to 2018 US dollars.
Abbreviations: BD, bipolar disorder; BD I, bipolar I disorder; BD II, bipolar II disorder; ER, emergency room; FPI, frequent psychiatric interventions; max, maximum; PPPY, per patient per year.
Comorbidities
Seven studies (two for BD-I, five for BD) reported on the economic burden of comorbidities among individuals with the disorder (Table 4). Across studies, cardiometabolic comorbidities (eg, hyperglycemia or diabetes, cardiovascular disease, dyslipidemia, hypertension, and obesity) and psychiatric comorbidities (eg, substance/alcohol abuse, anxiety disorder) were associated with higher medical care costs and/or increased HCRU in patients with the disorder.
Table 4.
Economic Impact of Comorbidities in Persons with BD
| Study | Study Design [Sample] Evaluation Period |
Key Findings |
|---|---|---|
| Bagalman et al, 201133 | Retrospective study [N=19,191 (BD-I)] 1 year |
|
| Centorrino et al, 200912 | Retrospective study [N=28,531 (any BD) vs N=85,593 (no mental health disorder)] 1 year |
|
| Correll et al, 201750 | Retrospective study [N=124,803 (any BD)] 30-days post-index hospitalization |
|
| Durden et al, 201034 | Retrospective study [N=5527 (BD-I)] 1 year |
|
| Guo et al, 200846 | Retrospective study [N=67,862 (any BD)] 5 years, max |
|
| Haskins et al, 201039 | Retrospective study [N=632 (BD-I or BD-II)] 7 years, max |
|
| Williams et al, 201137 | Retrospective study [N=122 (any BD)] 4 years |
|
Note: Costs adjusted to 2018 US dollars.
Abbreviations: BD, bipolar disorder; BD-I, bipolar I disorder; CCI, Charlson Comorbidity Index; ER, emergency room; FPI, frequent psychiatric intervention; HR, hazard ratio; max, maximum; MCO, managed care organization; PPPY, per person per year, RR, rate ratio.
Patients with FPI incurred significantly higher medical care costs and had significantly greater comorbidity burden compared to those without FPI.33,34,39 Comorbidities with significantly higher prevalence rates in patients with FPI included anxiety disorder, substance use disorder, and depressive disorder. Additionally, in the two studies of BD-I populations, patients with FPI had significantly higher comorbidity scores and significantly greater rates of hypertension and dyslipidemia.33,34 In a cost analysis by Durden et al, these three factors were associated with the higher total annual adjusted all-cause medical costs for patients with FPI relative to those without FPI.34
Studies of BD in general also reported that comorbidities contribute significantly to the economic burden of BD. Guo et al estimated that 33% of PPPY direct health-care costs were related to the treatment of BD, while the remaining 67% were attributable to treatment of psychiatric (eg, substance/alcohol use disorders, personality disorder) and medical (eg, obesity, diabetes) comorbidities.46 Similarly, analyses of health-care claims from an employer-based health plan found that health-care costs associated with BD were driven, in part, by patients’ comorbidity burden.37
Another two studies reported associations between cardiometabolic comorbidity burden and increased acute care HCRU and costs for BD patients. In an evaluation of administrative hospital data for 30 days post-discharge, 60.5% of patients with an inpatient diagnosis of BD had at least one cardiometabolic comorbidity, and 33.4% had two or more. Those with one or more cardiometabolic conditions (vs none) had an increased likelihood of hospital readmission in the 30 days post-discharge, higher costs, longer lengths of stay, and higher in-hospital mortality.50 Centorrino et al reported that individuals with BD had significantly more metabolic comorbidities than matched individuals from the general population (prevalence 37% vs 30%, p<0.0001). This was reflected in significantly higher medical service, particularly due to inpatient admissions, ER visits, and prescription costs for these conditions in the BD cohort.12
Adherence to BD-Related Medication
Six studies (one for BD-I, five for BD) reported on economic aspects of adherence to BD-related medications, using various definitions of adherence.40,45,51-54 Suboptimal adherence to BD medication was common. In a claims database study, only 35.3% of individuals with BD were adherent as measured by medication possession ratio (MPR) ≥0.80 over 12 months.53 Individuals with lower antipsychotic adherence, as measured by MPR, had higher direct health-care costs in the form of inpatient and outpatient mental health-related HCRU and expenditures.40,45,51,52 For example, one retrospective study reported that improved adherence to SGA therapy (ie, a 1-unit increase in MPR) was associated with lower quarterly mental health-related medical costs of $192 to $686 per patient.45 Additionally, suboptimal adherence to BD-related medications resulted in higher indirect costs in the form of reduced workplace productivity ($427 - $1156 PPPY)53 and reduced functional status,53,54 compared to those who maintained higher levels of adherence.
Approach to Pharmacotherapy
Seven studies evaluated associations between medication regimens and health-care service use and/or costs among individuals with BD, predominately with antipsychotics.46,47,55-59 One of them, a longitudinal cohort study, found that more than 8% of patients with BD receiving a second-generation antipsychotic (SGA) received combination treatment with more than one SGA concurrently; analyses found no association between disease severity and use of combination SGA treatment. Patients receiving a combination SGA regimen had greater rates of adverse events (eg, dry mouth, tremor, and sedation), nearly two- to three-times greater HCRU for medical and psychiatric services, respectively, and this regimen was associated with slightly worse global functioning relative to those treated with SGA monotherapy.55 A second study of Medicaid claims for patients initiated on SGA therapy found only 58% were prescribed a clinically recommended dose of their index SGA. In this subset, there were no significant differences in annual medical and mental health-related costs across individual SGA treatment groups.57 Other studies evaluated use of SGAs as a class compared to use of traditional mood stabilizers or in combination with traditional mood stabilizers46,59 or examined costs differences for BD patients treated with different SGA agents alone or in combination with a mood stabilizer.47,56,58 In general, studies that reported significant cost differences between treatment groups were driven by the risk or use of hospital services during the study period.46,47,56
BD Patients with Subsequent Diagnoses of Unipolar Depression
Two studies estimated the occurrence of “incongruent diagnoses” among patients previously diagnosed with BD, defined as receipt of a diagnosis of unipolar depression 12 months following an initial BD diagnosis (17.5% to 27.5% of BD patients).48,49 Unipolar depression diagnoses were considered “incongruent” as depressive episodes among BD patients would be expected to be treated as BD. The BD patients who received a subsequent diagnosis of unipolar depression had significantly higher average annual health-care costs (+$2676 PPPY), three times more psychiatric hospitalizations, and twice as many psychiatric ER visits than BD patients without a subsequent unipolar depression diagnosis.48 A chart review for a subset of patients with incongruent diagnoses found different providers were documented for the initial BD diagnosis vs the subsequent unipolar depression diagnosis 76% of the time, suggesting gaps in continuity of care may have contributed to these patterns of “incongruent diagnoses”.49
Direct Non-Health-Care Costs
Criminal Justice System
There were two studies (both in BD-I populations) that reported incremental costs of BD to the criminal justice system, such as costs of incarceration, policing, and legal costs.60,61 In a patient survey, employees with BD-I were more likely to report having been involved in a crime than co-workers without a diagnosis of BD-I.60 The second study examined public expenditures related to criminal justice, medical, mental health, and social welfare services for persons arrested in a large Florida county who had serious mental illnesses (SMIs). This analysis found psychiatric diagnosis influenced total expenditures; individuals with BD-I had the second-highest total quarterly costs ($2525) behind those with a psychotic disorder ($4209).61
Indirect Costs
National Burden
The total annual indirect costs of BD-I in the US was estimated at $158.5 billion, constituting 72.3% of the total economic burden of the disorder.31 About half (50.3%) of indirect costs were related to unemployment; the rest were attributed to caregiving productivity loss (34.1%); all-cause premature mortality among individuals with BD-I (8.6%); productivity loss among individuals with BD-I (6.4%); and direct health-care costs for caregivers (0.6%). The annual all-cause mortality rate for the BD-I population was found to be 3.4- to 11.4-times higher than for the US general population, depending on age group. Suicide was 10.3- to 16.2-times more common than for the general US population, and was responsible for an estimated 19% of the costs (measured as productivity loss) associated with premature deaths among those with BD-I. These findings were similar to another study that estimated total annual indirect costs for BD-I and BD-II of $155.2 billion (79.7% of the total cost burden).32
Workplace Productivity
Seven studies (three for BD-I, four for BD) evaluated effects on workplace productivity or employment, and all found that the disorder had a negative economic impact for employed individuals and their employers (Table 5).
Table 5.
Effects of BD on Workplace Productivity and Employment
| Study | Study Design [Sample] Evaluation Period |
Key Findings |
|---|---|---|
| Bagalman et al, 201053 | Retrospective study [N=1258 (any BD)] 1 year |
|
| Cloutier et al, 201831 | Prevalence-based cost analysis [N=202,019 (BD-I)] 1 year |
|
| McMorris et al, 201060 | Cross-sectional survey [N=219 (BD-I) matched and N=198 (no BD-I)] NA |
|
| O’Donnell et al, 201763 | Longitudinal study [N=273 (63.4% BD-I)] 5 years |
|
| Peters et al, 201664 | Cross-sectional survey [N=909 (BD-I)] NA |
|
| Shippee et al, 201162 | Cross-sectional survey [N=572 (any BD)] 3 years |
|
| Simon et al, 200865 | Structured interviews [N=441 (BD-I or BD-II)] 2 years |
|
Notes: *The EWPS is a 25-item instrument measuring absenteeism and presenteeism. Total EWPS scores range from zero (best) to 100 (worst). Costs adjusted to 2018 US dollars.
Abbreviations: BD, bipolar disorders; BD-I, bipolar I disorder; BD-II, bipolar II disorder; EWPS, Endicott Work Productivity Scale; MDE, major depressive episode; MEPS, Medical Expenditure Panel Survey; MPR, medication possession ratio; NA, not applicable; OR, odds ratio; PPPY, per person per year.
Individuals with BD or BD-I were more likely to be unemployed, miss work, have reduced work hours due to medical or mental health-related reasons, receive disability payments, or have been fired or laid off compared with those with no mood disorders.60,62 Studies of employed persons with BD reported increased indirect costs due to work absence and disability,53 as well as functional deficits that adversely affected work quality, work attendance, and ability to maintain employment.63–65 Moreover, an increased number of lifetime mood episodes was associated with higher likelihood of permanent disability and unemployment.64
Caregivers and Families
The economic burden of BD often extends to families and caregivers of these patients. In an analysis of the national burden of BD-I in the US, it was estimated that caregivers’ productivity loss and direct health-care costs accounted for more than a third of the total annual indirect costs of the disorder. These estimates were based on assumptions that caregivers devoted an average of almost 29 hours per week to caring for an individual with BD-I and that more than half (57.6%) of individuals with BD-I resided with family members.31 A second study reported that total annual health-care costs were 239% higher for families containing a member with BD compared to matched families without a diagnosis of SMI. Specifically, families including a member with BD made more outpatient visits, had more inpatient hospital stays, and filled more prescription medications than the matched families. Notably, most of the total HCRU and costs related to conditions other than BD. The authors suggested that this may be related to the psychological stress of living with and/or caring for a family member with BD. Another possibility for the greater HCRU costs observed is that BD families may have more frequent interactions with the health-care system (on behalf of the member with BD), providing them with additional opportunities to discuss and/or pursue help with their own health concerns compared to families that do not include a member with a SMI.66
Discussion
This literature synthesis presents a comprehensive review of contemporary literature describing the direct and indirect costs associated with BD and BD-I in real-world settings in the US, and the drivers of those costs. It builds on the findings of prior reviews describing the disease burden of BD, which were more focused on methodologic differences among published studies that specifically described cost data related to the burden of BD.6,10 This review included a broader collection of research than prior reviews, such as papers describing resource use or changes in work productivity without associated cost estimates, for additional perspective on the economic burden of BD/BD-I. Because BD encompasses multiple disease subtypes, this review reflected economic drivers that are applicable to both BD as a whole as well as the subset of patients who live with BD-I. While previous research identified differences in direct medical costs between BD-I and other BD subtypes,6 this review allowed for identification of broader cost outcomes and drivers of these costs among patients with BD-I specifically.
National burden estimates for BD and BD-I in this review show the costs associated with this disorder are substantial. Two studies estimated total annual costs of $195 billion (BD-I and BD-II) and $219 billion for BD-I (both 2018 USD), in analyses that assumed a lifetime prevalence of 2.1% (BD-I and BD-II) and 1.0% (BD-I) of the adult population, respectively.31,32 In contrast, the total annual cost of diagnosed diabetes was estimated at $333 billion (2018 USD) for a disease that affects 9.7% of the adult population.67 For both BD and BD-I, the majority of total economic burden (72% to 80%) was attributed to indirect costs, such as losses in work productivity (eg, unemployment, absences associated with morbidity) and caregiving. These population-level findings were aligned with other studies in this review that reported reduced work attendance, functioning, and ability to maintain employment for individuals with BD or BD-I53,60,62,65 as well as increased HCRU and worsening health status for family members of affected individuals.31,66,68 Given the degree to which indirect costs impact the total costs of BD and BD-I, this topic should remain a research priority.
Thirteen studies in this review reported data specific to BD-I populations, summarizing considerable indirect and direct medical costs, with many of the cost drivers reported similar to those in studies of BD as a whole. Functional impairments among individuals with BD-I were associated with increased risk of unemployment or becoming disabled.31,60,64 This risk was higher in persons who experienced recurrent mood episodes.64 High direct medical costs, particularly for acute care, were reported for patients with BD-I specifically.31,33,34,40 Among those with greater HCRU, nonadherence to pharmacotherapy and presence of comorbid conditions (eg, substance use disorder, hypertension) contributed to higher cost burden.33,34,40 These data did not clarify if the BD-I subtype is a more costly form of the disease; thus, additional research into real-world indirect and direct costs associated with the clinical management and treatment of BD-I are needed to help inform key stakeholders and public policy decisions for this population.
Taken together, these observations underscore the need to improve patient outcomes and reduce overall economic burden by implementing strategies of disease and medication management. Treatment guidelines recommend patients receive long-term pharmacotherapy to reduce the recurrence of mood episodes and improve the stability of patients’ psychiatric and general health, their general functioning, and quality of life; however, this is a population in which medication adherence is typically poor.22,28,69 Most currently prescribed mood stabilizing agents have undesirable side effects (eg, changes in cognitive function, tremor) that are poorly tolerated by patients70,71 and also have the potential to induce or exacerbate comorbid conditions that may require intervention.22,28,69 Choice of medication for BD is complex, balancing patient needs, symptoms, and treatment preferences with the risks of available therapies.
Interventions aimed at optimizing care delivery, such as integrated health-care programs combining primary care with specialists (eg, psychiatrists, pharmacists), have promise for improving the clinical management of BD and its comorbidities.72–75 These teams work collaboratively to tailor treatment choices to patients’ psychiatric and medical care needs and to efficiently intervene to address factors that may be barriers to medication adherence.72,75 Collaborative care of this kind has the potential to reduce the need for acute, intensive, or emergency health-care interventions by providing better continuity of care, while simultaneously reducing the indirect costs of BD and improving patients’ lives.75
Our review should be considered in context of its limitations. Only studies that were published between 2008 and 2018 were included. Importantly, many of the studies characterized costs and burden using definitions of BD that predate the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5). Because the DSM-5 criteria broaden the definition of BD,76 cost estimates reported from studies using earlier DSM criteria may not be representative of contemporary clinical experience. Included studies did not shed light on cost differences between persons with BD-I relative to other BD subtypes; however, results from Cloutier et al’s recent analysis of the national burden of BD-I in the US suggest that the cost burden of BD-I is similar to figures reported for BD generally when costs were adjusted to a common year.31 The assessment of methodological characteristics of included cost studies found that most sufficiently reported the components in the quality checklist; however, only 4 studies provided results of sensitivity analyses, which may increase the level of uncertainty around some of these estimates. Categorization of non-medical costs was inconsistent in the literature and limits comparability; for example, costs of criminal justice involvement were categorized differently across studies where it was included in the analysis. In addition, aggregate cost estimates were bundled in ways that made it challenging to reliably separate component costs. Therefore, the CPI for Medical Care as the standard for converting costs to common-year currency (2018) may not accurately reflect BD-related non-health-care costs.
Other limitations inherent to the literature summarized included methodological variability in approaches to costing of data sources, selection of cost items and comorbidities, statistical methods used, and patient selection. Similar to prior reviews,6,10 which discussed the methodological and quality issues in cost studies of BD in greater detail, this review found that greater transparency and specificity in study methods is needed to improve the comparability of results across studies. Many included studies relied on administrative claims data, thus limiting their analyses to those costs for which a payer is responsible. Other relevant costs, such as out-of-pocket payments or expenses carried by other payers (eg, rehabilitation paid from pension funds), were rarely included. Some studies focused on general cost categories (inpatient or outpatient care), while others also included the services of supporting departments such as ER, laboratory, and social work. Some studies matched their samples according to individuals’ age and gender; others used statistical methods to adjust for sociodemographic characteristics. Most studies recruited from special populations (eg, privately insured individuals; recent hospital discharges; employed persons) in which reported costs may not be representative of the general BD population. Some cost studies focused on newly available treatments, which may have resulted in higher reported costs compared to studies that included a broader selection of treatments due to increased pharmacy costs. Additionally, inconsistency in the way that “indirect” costs were defined or apportioned across publications led to heterogeneous definitions of indirect cost categories, resulting in widely differing estimates. This variability in costing methodologies and definitions limited comparisons across and between studies. However, this review summarized a broad range of studies to provide a comprehensive picture of the economic burden of BD and BD-I.
Conclusion
There is clear evidence from the published literature that BD (including BD-I specifically) and its comorbidities exert a large economic burden in the US on patients, caregivers, families, employers, and society. This burden encompasses direct health-care utilization and costs, loss of workplace productivity, caregiving, and other indirect costs. While estimates of indirect costs associated with BD and BD-I are substantial, they are infrequently quantified in the literature and warrant further study. Interventions that target better disease management and medication adherence may reduce the direct and indirect cost burden of BD and BD-I and improve patient outcomes.
Acknowledgments
The authors thank Nancy Neil, PhD (Worldwide Clinical Trials, Inc., USA), who assisted in the planning and conduct of the literature searches and analyses. Medical writing and editorial support for the preparation of the manuscript, under the direction of the authors, was provided by Jo Whelan, MSc (Textpharm Limited, UK) and was funded by Alkermes, Inc.
Funding Statement
Funding for the planning and conduct of the systematic literature review and the preparation of this manuscript was provided by Alkermes, Inc. In May of 2019, data included in this manuscript were presented as a poster at the annual meeting of the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) in New Orleans, Louisiana. The poster’s abstract was published in “Abstracts” in Value in Health, Vol 22: https://doi.org/10.1016/j.jval.2019.04.1065.
Abbreviations
BD, bipolar disorder; BD-I, bipolar I disorder; BD-II, bipolar II disorder; CPI, Consumer Price Index; DSM, Diagnostic and Statistical Manual of Mental Disorders; FPI, frequent psychiatric intervention; HCRU, health-care resource utilization; MPR, medication possession ratio; NHS EED, National Health Service Economic Evaluation Database; PPPY, per person per year; SGA, second-generation antipsychotic; SMI, serious mental illness; US, United States; USD, United States Dollars.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
Leona Bessonova, Michael J. Doane, and Amy K. O’Sullivan are employees of Alkermes, Inc. and may own stock/options in the company. Kristine Ogden is an employee of Worldwide Clinical Trials, Inc., which has received consulting fees from Alkermes, Inc. for conducting this study. Mauricio Tohen was an employee of Lilly (1997 to 2008) and has received honoraria from or consulted for Abbott, AstraZeneca, Alkermes, Allergan, Bristol-Myers Squibb, GlaxoSmithKline, Lilly, Johnson & Johnson, Otsuka, Merck, Gedeon Richter Plc, Sunovion, Forest, Roche, Elan, Lundbeck, Teva, Pamlab, Minerva, Neurocrine, Pfizer, Wyeth and Wiley Publishing. The authors report no other conflicts of interest in this work.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 2.Vieta E, Berk M, Schulze TG, et al. Bipolar disorders. Nat Rev Dis Primers. 2018;4(1):18008. doi: 10.1038/nrdp.2018.8 [DOI] [PubMed] [Google Scholar]
- 3.Merikangas KR, Jin R, He JP, et al. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry. 2011;68(3):241–251. doi: 10.1001/archgenpsychiatry.2011.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vigo D, Thornicroft G, Atun R. Estimating the true global burden of mental illness. Lancet Psychiat. 2016;3(2):171–178. doi: 10.1016/S2215-0366(15)00505-2 [DOI] [PubMed] [Google Scholar]
- 5.Rosa AR, Reinares M, Michalak EE, et al. Functional impairment and disability across mood states in bipolar disorder. Value Health. 2010;13(8):984–988. doi: 10.1111/j.1524-4733.2010.00768.x [DOI] [PubMed] [Google Scholar]
- 6.Jin H, McCrone P. Cost-of-illness studies for bipolar disorder: systematic review of international studies. Pharmacoeconomics. 2015;33(4):341–353. doi: 10.1007/s40273-014-0250-y [DOI] [PubMed] [Google Scholar]
- 7.Moreno C, Hasin DS, Arango C, et al. Depression in bipolar disorder versus major depressive disorder: results from the national epidemiologic survey on alcohol and related conditions. Bipolar Disord. 2012;14(3):271–282. doi: 10.1111/j.1399-5618.2012.01009.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubio JM, Olfson M, Perez-Fuentes G, Garcia-Toro M, Wang S, Blanco C. Effect of first episode axis I disorders on quality of life. J Nerv Ment Dis. 2014;202(4):271–274. doi: 10.1097/NMD.0000000000000117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Begley CE, Annegers JF, Swann AC, et al. The lifetime cost of bipolar disorder in the US: an estimate for new cases in 1998. Pharmacoeconomics. 2001;19(5 Pt 1):483–495. doi: 10.2165/00019053-200119050-00004 [DOI] [PubMed] [Google Scholar]
- 10.Kleine-Budde K, Touil E, Moock J, Bramesfeld A, Kawohl W, Rossler W. Cost of illness for bipolar disorder: a systematic review of the economic burden. Bipolar Disord. 2014;16(4):337–353. doi: 10.1111/bdi.12165 [DOI] [PubMed] [Google Scholar]
- 11.Wyatt RJ, Henter I. An economic evaluation of manic-depressive illness–1991. Soc Psychiatry Psychiatr Epidemiol. 1995;30(5):213–219. doi: 10.1007/BF00789056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centorrino F, Mark TL, Talamo A, Oh K, Chang J. Health and economic burden of metabolic comorbidity among individuals with bipolar disorder. J Clin Psychopharmacol. 2009;29(6):595–600. doi: 10.1097/JCP.0b013e3181bef8a6 [DOI] [PubMed] [Google Scholar]
- 13.Goldstein BI, Liu SM, Zivkovic N, Schaffer A, Chien LC, Blanco C. The burden of obesity among adults with bipolar disorder in the United States. Bipolar Disord. 2011;13(4):387–395. doi: 10.1111/j.1399-5618.2011.00932.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kemp DE, Gao K, Chan PK, Ganocy SJ, Findling RL, Calabrese JR. Medical comorbidity in bipolar disorder: relationship between illnesses of the endocrine/metabolic system and treatment outcome. Bipolar Disord. 2010;12(4):404–413. doi: 10.1111/j.1399-5618.2010.00823.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vancampfort D, Vansteelandt K, Correll CU, et al. Metabolic syndrome and metabolic abnormalities in bipolar disorder: a meta-analysis of prevalence rates and moderators. Am J Psychiatry. 2013;170(3):265–274. doi: 10.1176/appi.ajp.2012.12050620 [DOI] [PubMed] [Google Scholar]
- 16.Fajutrao L, Locklear J, Priaulx J, Heyes A. A systematic review of the evidence of the burden of bipolar disorder in Europe. Clin Pract Epidemiol Ment Health. 2009;5(1):3. doi: 10.1186/1745-0179-5-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blanco C, Compton WM, Saha TD, et al. Epidemiology of DSM-5 bipolar I disorder: results from the national epidemiologic survey on alcohol and related conditions – III. J Psychiatr Res. 2017;84:310–317. doi: 10.1016/j.jpsychires.2016.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Judd LL, Akiskal HS, Schettler PJ, et al. Psychosocial disability in the course of bipolar I and II disorders: a prospective, comparative, longitudinal study. Arch Gen Psychiatry. 2005;62(12):1322–1330. doi: 10.1001/archpsyc.62.12.1322 [DOI] [PubMed] [Google Scholar]
- 19.Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the national comorbidity survey replication. Arch Gen Psychiatry. 2007;64(5):543–552. doi: 10.1001/archpsyc.64.5.543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Westman J, Hallgren J, Wahlbeck K, Erlinge D, Alfredsson L, Osby U. Cardiovascular mortality in bipolar disorder: a population-based cohort study in Sweden. BMJ Open. 2013;3(4):e002373. doi: 10.1136/bmjopen-2012-002373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rhee TG, Olfson M, Nierenberg AA, Wilkinson ST. 20-Year trends in the pharmacologic treatment of bipolar disorder by psychiatrists in outpatient care settings. Am J Psychiatry. 2020;appiajp202019091000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yatham LN, Kennedy SH, Parikh SV, et al. Canadian network for mood and anxiety treatments (CANMAT) and international society for bipolar disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ostacher MJ, Tandon R, Suppes T. Florida best practice psychotherapeutic medication guidelines for adults with bipolar disorder: a novel, practical, patient-centered guide for clinicians. J Clin Psychiatry. 2016;77(7):920–926. doi: 10.4088/JCP.15cs09841 [DOI] [PubMed] [Google Scholar]
- 24.NICE. Bipolar Disorder: Assessment and Management. NICE Clinical Guideline 185. London: National Institute for Health and Care Excellence; 2014. [Google Scholar]
- 25.Malhi GS, Bassett D, Boyce P, et al. Royal Australian and New Zealand college of psychiatrists clinical practice guidelines for mood disorders. Aust N Z J Psychiatry. 2015;49(12):1087–1206. doi: 10.1177/0004867415617657 [DOI] [PubMed] [Google Scholar]
- 26.Grunze H, Vieta E, Goodwin GM, et al. The world federation of societies of biological psychiatry (WFSBP) guidelines for the biological treatment of bipolar disorders: acute and long-term treatment of mixed states in bipolar disorder. World J Biol Psychiatry. 2018;19(1):2–58. doi: 10.1080/15622975.2017.1384850 [DOI] [PubMed] [Google Scholar]
- 27.Goodwin GM, Haddad PM, Ferrier IN, et al. Evidence-based guidelines for treating bipolar disorder: revised third edition recommendations from the British association for psychopharmacology. J Psychopharmacol. 2016;30(6):495–553. doi: 10.1177/0269881116636545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fountoulakis KN, Grunze H, Vieta E, et al. The international college of neuro-psychopharmacology (CINP) treatment guidelines for bipolar disorder in adults (CINP-BD-2017), part 3: the clinical guidelines. Int J Neuropsychopharmacol. 2017;20(2):180–195. doi: 10.1093/ijnp/pyw109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frank RG, Beronio K, Glied SA. Behavioral health parity and the affordable care act. J Soc Work Disabil Rehabil. 2014;13(1–2):31–43. doi: 10.1080/1536710X.2013.870512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.United States Bureau of Labor Statistics. Consumer price index; 2018. Available from: http://www.bls.gov/cpi/. Accessed January18, 2019.
- 31.Cloutier M, Greene M, Guerin A, Touya M, Wu E. The economic burden of bipolar I disorder in the United States in 2015. J Affect Disord. 2018;226:45–51. doi: 10.1016/j.jad.2017.09.011 [DOI] [PubMed] [Google Scholar]
- 32.Dilsaver SC. An estimate of the minimum economic burden of bipolar I and II disorders in the United States: 2009. J Affect Disord. 2011;129(1–3):79–83. doi: 10.1016/j.jad.2010.08.030 [DOI] [PubMed] [Google Scholar]
- 33.Bagalman E, Muser E, Choi JC, et al. Health care resource utilization and costs in a commercially insured population of patients with bipolar disorder type I and frequent psychiatric interventions. Clin Ther. 2011;33(10):1381–1390.e1384. doi: 10.1016/j.clinthera.2011.09.005 [DOI] [PubMed] [Google Scholar]
- 34.Durden E, Bagalman E, Muser E, et al. Characteristics, healthcare utilization and costs of bipolar disorder type I patients with and without frequent psychiatric intervention in a medicaid population. J Med Econ. 2010;13(4):698–704. doi: 10.3111/13696998.2010.531828 [DOI] [PubMed] [Google Scholar]
- 35.Jing Y, Kim E, You M, Pikalov A, Tran QV. Healthcare costs associated with treatment of bipolar disorder using a mood stabilizer plus adjunctive aripiprazole, quetiapine, risperidone, olanzapine or ziprasidone. J Med Econ. 2009;12(2):104–113. doi: 10.3111/13696990903044092 [DOI] [PubMed] [Google Scholar]
- 36.Tohen M, Ng-Mak D, Rajagopalan K, Halpern R, Chuang CC, Loebel A. Patient characteristics associated with use of lurasidone versus other atypical antipsychotics in patients with bipolar disorder: analysis from a claims database in the United States. Prim Care Companion CNS Disord. 2017;19(3). doi: 10.4088/PCC.16m02066 [DOI] [PubMed] [Google Scholar]
- 37.Williams MD, Shah ND, Wagie AE, Wood DL, Frye MA. Direct costs of bipolar disorder versus other chronic conditions: an employer-based health plan analysis. Psychiatr Serv. 2011;62(9):1073–1078. doi: 10.1176/ps.62.9.pss6209_1073 [DOI] [PubMed] [Google Scholar]
- 38.Lum TY, Parashuram S, Shippee TP, et al. Diagnosed prevalence and health care expenditures of mental health disorders among dual eligible older people. Gerontologist. 2013;53(2):334–344. doi: 10.1093/geront/gns163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haskins JT, Macfadden W, Turner N, et al. Clinical characteristics and resource utilization of patients with bipolar disorder who have frequent psychiatric interventions. J Med Econ. 2010;13(3):552–558. doi: 10.3111/13696998.2010.511064 [DOI] [PubMed] [Google Scholar]
- 40.Lang K, Korn J, Muser E, Choi JC, Abouzaid S, Menzin J. Predictors of medication nonadherence and hospitalization in medicaid patients with bipolar I disorder given long-acting or oral antipsychotics. J Med Econ. 2011;14(2):217–226. doi: 10.3111/13696998.2011.562265 [DOI] [PubMed] [Google Scholar]
- 41.Stensland M, Watson PR, Grazier KL. An examination of costs, charges, and payments for inpatient psychiatric treatment in community hospitals. Psychiatr Serv. 2012;63(7):666–671. doi: 10.1176/appi.ps.201100402 [DOI] [PubMed] [Google Scholar]
- 42.Lindamer LA, Liu L, Sommerfeld DH, et al. Predisposing, enabling, and need factors associated with high service use in a public mental health system. Adm Policy Ment Health. 2012;39(3):200–209. doi: 10.1007/s10488-011-0350-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baldessarini R, Henk H, Sklar A, Chang J, Leahy L. Psychotropic medications for patients with bipolar disorder in the United States: polytherapy and adherence. Psychiatr Serv. 2008;59(10):1175–1183. doi: 10.1176/ps.2008.59.10.1175 [DOI] [PubMed] [Google Scholar]
- 44.Busch AB, Yoon F, Barry CL, et al. The effects of mental health parity on spending and utilization for bipolar, major depression, and adjustment disorders. Am J Psychiatry. 2013;170(2):180–187. doi: 10.1176/appi.ajp.2012.12030392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gianfrancesco FD, Sajatovic M, Rajagopalan K, Wang RH. Antipsychotic treatment adherence and associated mental health care use among individuals with bipolar disorder. Clin Ther. 2008;30(7):1358–1374. doi: 10.1016/S0149-2918(08)80062-8 [DOI] [PubMed] [Google Scholar]
- 46.Guo JJ, Keck PE Jr, Li H, Jang R, Kelton CM. Treatment costs and health care utilization for patients with bipolar disorder in a large managed care population. Value Health. 2008;11(3):416–423. doi: 10.1111/j.1524-4733.2007.00287.x [DOI] [PubMed] [Google Scholar]
- 47.Kim E, You M, Pikalov A, Van-Tran Q, Jing Y. One-year risk of psychiatric hospitalization and associated treatment costs in bipolar disorder treated with atypical antipsychotics: a retrospective claims database analysis. BMC Psychiatry. 2011;11(1):6. doi: 10.1186/1471-244X-11-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stensland MD, Schultz JF, Frytak JR. Depression diagnoses following the identification of bipolar disorder: costly incongruent diagnoses. BMC Psychiatry. 2010;10(1):39. doi: 10.1186/1471-244X-10-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stensland MD, Schultz JF, Frytak JR. Diagnosis of unipolar depression following initial identification of bipolar disorder: a common and costly misdiagnosis. J Clin Psychiatry. 2008;69(5):749–758. doi: 10.4088/JCP.v69n0508 [DOI] [PubMed] [Google Scholar]
- 50.Correll CU, Ng-Mak DS, Stafkey-Mailey D, Farrelly E, Rajagopalan K, Loebel A. Cardiometabolic comorbidities, readmission, and costs in schizophrenia and bipolar disorder: a real-world analysis. Ann Gen Psychiatry. 2017;16(1):9. doi: 10.1186/s12991-017-0133-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hassan M, Lage MJ. Risk of rehospitalization among bipolar disorder patients who are nonadherent to antipsychotic therapy after hospital discharge. Am J Health Syst Pharm. 2009;66(4):358–365. doi: 10.2146/ajhp080374 [DOI] [PubMed] [Google Scholar]
- 52.Lage MJ, Hassan MK. The relationship between antipsychotic medication adherence and patient outcomes among individuals diagnosed with bipolar disorder: a retrospective study. Ann Gen Psychiatry. 2009;8(1):7. doi: 10.1186/1744-859X-8-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bagalman E, Yu-Isenberg KS, Durden E, Crivera C, Dirani R, Bunn WB 3rd. Indirect costs associated with nonadherence to treatment for bipolar disorder. J Occup Environ Med. 2010;52(5):478–485. doi: 10.1097/JOM.0b013e3181db811d [DOI] [PubMed] [Google Scholar]
- 54.Perlis RH, Ostacher MJ, Miklowitz DJ, et al. Clinical features associated with poor pharmacologic adherence in bipolar disorder: results from the STEP-BD study. J Clin Psychiatry. 2010;71(3):296–303. doi: 10.4088/JCP.09m05514yel [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brooks JO, Goldberg JF, Ketter TA, et al. Safety and tolerability associated with second-generation antipsychotic polytherapy in bipolar disorder: findings from the systematic treatment enhancement program for bipolar disorder. J Clin Psychiatry. 2011;72(2):240–247. doi: 10.4088/JCP.09m05214yel [DOI] [PubMed] [Google Scholar]
- 56.Pelletier E, Hassan M, Alemayehu B, Smith D, Kim J. Bipolar disorder healthcare costs for quetiapine extended-release versus aripiprazole. Am J Pharm Benefits. 2013;5(3):e73–e79. [Google Scholar]
- 57.Rascati KL, Richards KM, Ott CA, et al. Adherence, persistence of use, and costs associated with second-generation antipsychotics for bipolar disorder. Psychiatr Serv. 2011;62(9):1032–1040. doi: 10.1176/ps.62.9.pss6209_1032 [DOI] [PubMed] [Google Scholar]
- 58.Qiu Y, Christensen DB, Fu AZ, Liu GG. Cost analysis in a medicaid program for patients with bipolar disorder who initiated atypical antipsychotic monotherapy. Curr Med Res Opin. 2009;25(2):351–361. doi: 10.1185/03007990802634077 [DOI] [PubMed] [Google Scholar]
- 59.Qiu Y, Fu AZ, Liu GG, Christensen DB. Healthcare costs of atypical antipsychotic use for patients with bipolar disorder in a medicaid programme. Appl Health Econ Health Policy. 2010;8(3):167–177. doi: 10.2165/11318830-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 60.McMorris BJ, Downs KE, Panish JM, Dirani R. Workplace productivity, employment issues, and resource utilization in patients with bipolar I disorder. J Med Econ. 2010;13(1):23–32. doi: 10.3111/13696990903475833 [DOI] [PubMed] [Google Scholar]
- 61.Petrila J, Andel R, Constantine R, Robst J. Public expenditures related to the criminal justice system and to services for arrestees with a serious mental illness. Psychiatr Serv. 2010;61(5):516–519. doi: 10.1176/ps.2010.61.5.516 [DOI] [PubMed] [Google Scholar]
- 62.Shippee ND, Shah ND, Williams MD, Moriarty JP, Frye MA, Ziegenfuss JY. Differences in demographic composition and in work, social, and functional limitations among the populations with unipolar depression and bipolar disorder: results from a nationally representative sample. Health Qual Life Outcomes. 2011;9(1):90. doi: 10.1186/1477-7525-9-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.O’Donnell LA, Deldin PJ, Grogan-Kaylor A, et al. Depression and executive functioning deficits predict poor occupational functioning in a large longitudinal sample with bipolar disorder. J Affect Disord. 2017;215:135–142. doi: 10.1016/j.jad.2017.03.015 [DOI] [PubMed] [Google Scholar]
- 64.Peters AT, West AE, Eisner L, Baek J, Deckersbach T. The burden of repeated mood episodes in bipolar i disorder: results from the national epidemiological survey on alcohol and related conditions. J Nerv Ment Dis. 2016;204(2):87–94. doi: 10.1097/NMD.0000000000000425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Simon GE, Ludman EJ, Unutzer J, Operskalski BH, Bauer MS. Severity of mood symptoms and work productivity in people treated for bipolar disorder. Bipolar Disord. 2008;10(6):718–725. doi: 10.1111/j.1399-5618.2008.00581.x [DOI] [PubMed] [Google Scholar]
- 66.Chatterton ML, Ke X, Lewis BE, Rajagopalan K, Lazarus A. Impact of bipolar disorder on the family: utilization and cost of health care resources. P T. 2008;33(1):15–34. [PMC free article] [PubMed] [Google Scholar]
- 67.American Diabetes A. Economic costs of diabetes in the U.S. in 2017. Diabetes Care. 2018;41(5):917–928. doi: 10.2337/dci18-0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chessick CA, Perlick DA, Miklowitz DJ, et al. Suicidal ideation and depressive symptoms among bipolar patients as predictors of the health and well-being of caregivers. Bipolar Disord. 2009;11(8):876–884. doi: 10.1111/j.1399-5618.2009.00765.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bobo WV. The diagnosis and management of bipolar I and II disorders: clinical practice update. Mayo Clin Proc. 2017;92(10):1532–1551. doi: 10.1016/j.mayocp.2017.06.022 [DOI] [PubMed] [Google Scholar]
- 70.Baldessarini RJ, Perry R, Pike J. Factors associated with treatment nonadherence among US bipolar disorder patients. Hum Psychopharmacol. 2008;23(2):95–105. doi: 10.1002/hup.908 [DOI] [PubMed] [Google Scholar]
- 71.Mago R, Borra D, Mahajan R. Role of adverse effects in medication nonadherence in bipolar disorder. Harv Rev Psychiatry. 2014;22(6):363–366. doi: 10.1097/HRP.0000000000000017 [DOI] [PubMed] [Google Scholar]
- 72.Kern JS, Cerimele JM. Collaborative care for patients with a bipolar disorder: a primary care FQHC-CMHC partnership. Psychiatr Serv. 2019;70(4):353. doi: 10.1176/appi.ps.201900019 [DOI] [PubMed] [Google Scholar]
- 73.Kilbourne AM, Barbaresso MM, Lai Z, et al. Improving physical health in patients with chronic mental disorders: twelve-month results from a randomized controlled collaborative care trial. J Clin Psychiatry. 2017;78(1):129–137. doi: 10.4088/JCP.15m10301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kilbourne AM, Goodrich DE, O’Donnell AN, Miller CJ. Integrating bipolar disorder management in primary care. Curr Psychiatry Rep. 2012;14(6):687–695. doi: 10.1007/s11920-012-0325-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wright WA, Gorman JM, Odorzynski M, Peterson MJ, Clayton C. Integrated pharmacies at community mental health centers: medication adherence and outcomes. J Manag Care Spec Pharm. 2016;22(11):1330–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Angst J. Bipolar disorders in DSM-5: strengths, problems and perspectives. Int J Bipolar Disord. 2013;1(1):12. doi: 10.1186/2194-7511-1-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stensland MD, Zhu B, Ascher-Svanum H, Ball DE. Costs associated with attempted suicide among individuals with bipolar disorder. J Ment Health Policy Econ. 2010;13(2):87–92. [PubMed] [Google Scholar]
- 78.Seabury SA, Goldman DP, Kalsekar I, Sheehan JJ, Laubmeier K, Lakdawalla DN. Formulary restrictions on atypical antipsychotics: impact on costs for patients with schizophrenia and bipolar disorder in Medicaid. Am J Manag Care. 2014;20(2):e52–60. [PubMed] [Google Scholar]
- 79.Robertson AG, Swanson JW, Lin H, Easter MM, Frisman LK, Swartz MS. Influence of criminal justice involvement and psychiatric diagnoses on treatment costs among adults with serious mental illness. Psychiatr Serv. 2015;66(9):907–909. doi: 10.1176/appi.ps.201500134 [DOI] [PMC free article] [PubMed] [Google Scholar]