Abstract
PURPOSE
Access to a usual source of care is associated with improved health outcomes, but research on how the physician-patient relationship affects a patient’s health, particularly long-term, is limited. The aim of this study was to investigate the longitudinal effect of changes in the physician-patient relationship on functional health.
METHODS
We conducted a prospective cohort study using the Medical Expenditure Panel Survey (MEPS, 2015-2016). The outcome was 1-year change in functional health (12-Item Short-Form Survey). The predictors were quality of physician-patient relationship, and changes in this relationship, operationalized with the MEPS Primary Care (MEPS-PC) Relationship subscale, a composite measure with preliminary evidence of reliability and validity. Confounders included age, sex, race/ethnicity, educational attainment, insurance status, US region, and multimorbidity. We conducted analyses with survey-weighted, covariate-adjusted, predicted marginal means, used to calculate Cohen effect estimates. We tested differences in trajectories with multiple pairwise comparisons with Tukey contrasts.
RESULTS
Improved physician-patient relationships were associated with improved functional health, whereas worsened physician-patient relationships were associated with worsened functional health, with 1-year effect estimates ranging from 0.05 (95% CI, 0-0.10) to 0.08 (95% CI, 0.02-0.13) compared with −0.16 (95% CI, −0.35 to −0.03) to −0.33 (95% CI, −0.47 to −0.02), respectively.
CONCLUSION
The quality of the physician-patient relationship is positively associated with functional health. These findings could inform health care strategies and health policy aimed at improving patient-centered health outcomes.
Key words: United States, primary care, functional health, relationship, impact, Medical Expenditure Panel Survey
INTRODUCTION
Ecologic studies have shown that primary care is associated with better health, improved health care quality, improved access, and lower cost—virtually the definition of value.1–4 Having a usual source of care, defined as access to a regular facility or primary care provider when one is sick or needs medical advice, has been associated with improved health outcomes.5,6 Whereas consistent access to a provider is important, the quality of each clinical encounter is equally important in shaping a patient’s experience and overall health outcomes. Specifically, the quality of the physician-patient relationship warrants closer research. The physician-patient relationship is a valued primary care process on which other primary care processes depend.7–10 A strong physician-patient relationship involves good interpersonal communication, the development of a shared understanding that allows for reliance and trust, and ease of obtaining care, facilitated by the physician serving as a patient advocate.11,12
Despite these promising findings, we currently do not fully understand the underlying processes by which primary care exerts its beneficial effects.13,14 Moreover, whereas the quality of the physician-patient relationship is a known mechanism for improving patient outcomes,15–17 studies have not yet clarified how changes in this fundamental relationship over time affect functional health outcomes.
Primary care research poses challenges, in part due to a shortage of robust measurements capturing unique primary care processes thought to benefit patients.13,15 Efforts to elucidate the protective effect of primary care found that ecologic studies using individual-level data showed mixed results. Robust pragmatic trials aimed at improving functional health outcomes among patients with multimorbidity—a high-risk, high-cost, high-priority primary care population—also showed mixed results.16 Well-designed cohort studies have the potential to provide empirical evidence at a fraction of the cost of randomized trials by tapping readily available, nationally representative data to guide decision making. Whereas reliable physician-patient relationship instruments exist,18–21 until recently no such measure had been specified using the nationally representative Medical Expenditure Panel Survey (MEPS), which provides a rich array of process and outcome data for health services research.22
We recently identified, specified, and validated the primary care composite measure (MEPS-PC)—comprising 3 subscales, each capturing distinct primary care processes—using items from MEPS.23,24 The availability of this measure has made possible the present study and might also inform future research in this critical area of health care inquiry. The aim of this observational study was to investigate the longitudinal effect of changes in the physician-patient relationship on functional health.
METHODS
Data
This observational study relied on secondary data from MEPS. We used the publicly available 2015-2016 Household Component (HC-193) and baseline (2015) Medical Conditions (HC-190) files, both of which can be downloaded at no cost from the Agency for Healthcare Research and Quality’s website.25 The MEPS Household Component collects data from a sample of families and individuals in selected communities across the United States; this sample is drawn from a nationally representative subsample of households that participated in the prior year’s National Health Interview Survey. Data are collected at 5 time points over a 2-year period. This study was guided by the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines and checklist for reports of observational studies26 and was deemed exempt by the Case Western Reserve University Institutional Review Board (IRB-2016-1682).
Study Design
We constructed a prospective observational cohort with 2 years of follow-up comprising adults who were ≥18 years of age at the time of the first MEPS interview, had made ≥1 office-based physician visit in both 2015 and 2016, had not died during follow-up, and whose files contained complete data on the outcome as well as, at minimum, a 50% complete response on items comprising MEPS-PC scores (Figure 1).
Figure 1.
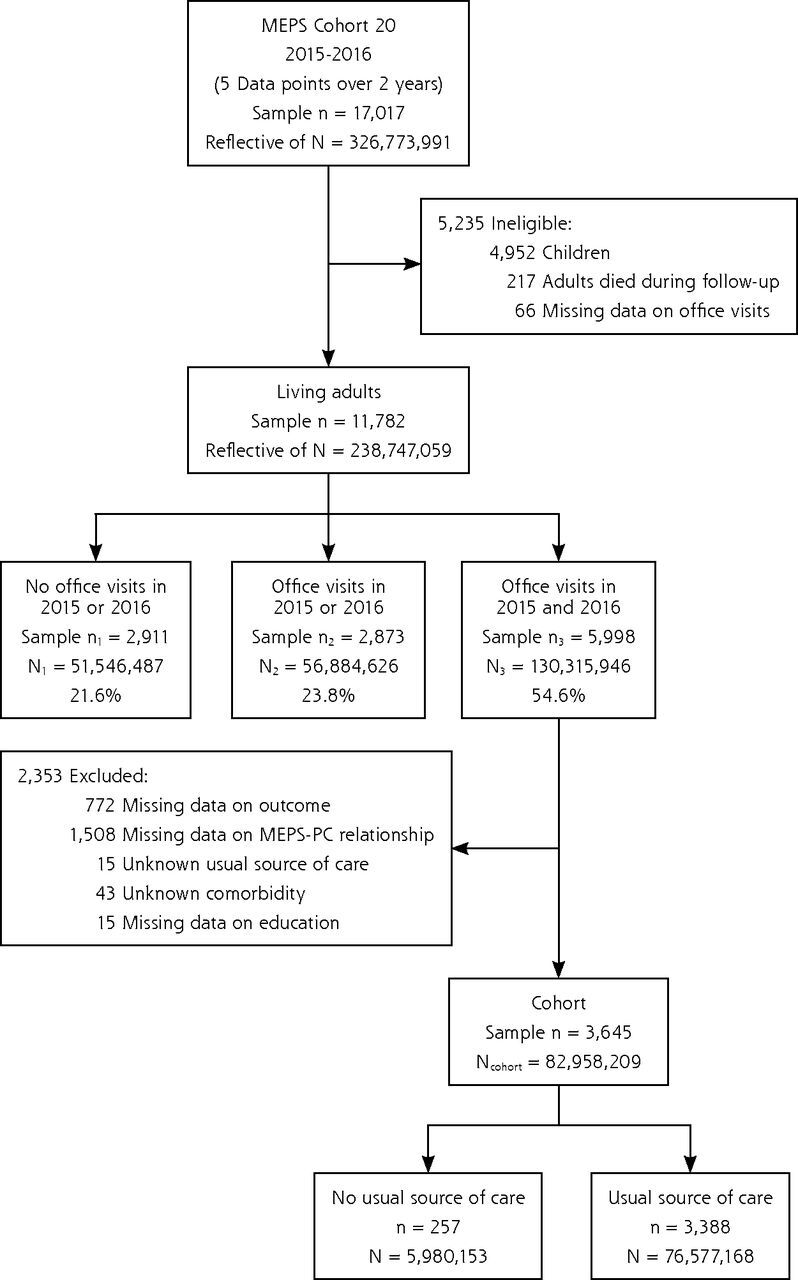
Construction of a prospective cohort study of US adults who had office-based physician visits in 2 consecutive years. Data from MEPS, 2015-2016.
HC = MEPS Household Component; MEPS-PC = Medical Expenditure Panel Survey Primary Care measure.
Note: Source: Agency for Healthcare Research and Quality, Medical Expenditure Panel Survey (MEPS Panel 20 Longitudinal Data File [HC-193, 2015-2016] and Medical Conditions File [HC-190, 2015]).
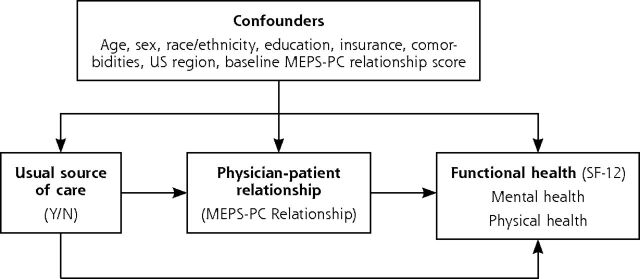
Conceptual Model
This study was guided by a conceptual model in which we hypothesize that the longitudinal trajectory of the physician-patient relationship will affect functional health outcomes. As shown in the directed acyclic graph (Figure 2),27 the quality of the physician-patient relationship lies on the causal pathway between usual source of care and health outcomes.
Figure 2.
Directed acyclic graph of the hypothesized mechanism for how physician-patient relationship advances functional health.
HAVEUS2 = does person have USC provider-R2; MEPS-PC = Medical Expenditure Panel Survey Primary Care measure; N = no; SF-12 = 12-Item Short-Form Survey; Y = yes.
Note: Functional health operationalized with the SF-12 instrument. Physician-patient relationship operationalized with the MEPS-PC Relationship composite subscale. Usual source of care operationalized as having a particular doctor’s office, clinic, health center, or other place usually visited when sick or seeking advice about their health, operationalized with the MEPS HAVEUS2 indicator.
Outcome
The outcome was overall functional health operationalized with the 12-Item Short-Form Survey (SF-12).28 Responses to the SF-12 in MEPS are captured via a mail-in questionnaire, administered twice, approximately 1 year apart.29
Predictor
Physician-patient relationship was operationalized with the MEPS-PC Relationship composite subscale, which has demonstrated preliminary evidence of reliability and validity in published work.23,24 The MEPS-PC comprises 3 composite subscales, identified via a primary care scoping review and specified with variable reduction techniques. Guided by 16 established primary care processes, we found 32 candidate items in MEPS that are valued in primary care. These items are quality indicators verified as important by an advisory group of primary care subject matter experts.
We split our data into a training sample and a testing sample (50-50). Factor analysis of our training data set yielded 3 unique factors involving 24 items. The MEPS-PC Relationship subscale comprises 14 items (Supplemental Appendix 1, https://www.AnnFamMed.org/content/18/5/422/suppl/DC1/) including questions such as, “How often did doctors or other health providers listen carefully to you?” and “How often did doctors or other health providers explain things in a way that was easy to understand?” All 14 questions are scored on a 4-point ordinal scale. The composite measure shows an internal consistency reliability of 0.86, with reproducible results in the testing data (Cronbach α = 0.85, 3 factors with equivalent loadings).24 This continuous measure displays construct validity against the following 3 known measures of primary care: usual source of care, known provider, and family usual source of care.25
To analyze the longitudinal effect of changes in the physician-patient relationship on functional health, we constructed longitudinal trajectories using a 2-step analytic process. First, participants were classified at baseline to 1 of 2 categories, low or high, depending on whether the individual’s baseline physician-patient relationship score was greater than or less than the population median score. Depending on whether the individual’s follow-up score changed ± 0.5 SD compared with their baseline score,30 they were then conditionally assigned to 1 of 3 possible follow-up trajectories (same, worse, better) (Supplemental Figure 1, https://www.AnnFamMed.org/content/18/5/422/suppl/DC1/).
Confounders
Adjustment covariates included age, sex, race/ethnicity, educational attainment, insurance status, multimorbidity, and US region, each represented as categoric variables. The multimorbidity profile was calculated with Clinical Classifications Software (Agency for Healthcare Research and Quality, Healthcare Cost and Utilization Project) using physician-verified diagnoses and procedures readily available in MEPS.
Analytic Approach
Group comparisons were quantified with survey-weighted χ2. To assess demographic and clinical variability within the physician-patient relationship subscale, we used standardized mean difference (SMD), an improvement over P value with large sample sizes.31 To quantify the correlation between the physician-patient relationship at baseline and functional health at follow up, we used the survey-weighted Pearson correlation. To assess the effect of 6 mutually exclusive physician-patient relationship trajectories on functional health, we relied on survey-weighted, covariate-adjusted predicted marginal means. To quantify the absolute size of the difference (controlling for sample size), we converted group differences to effect sizes with the Cohen d statistic. To assess for differences across the 6 trajectories, we used multiple pairwise comparisons with Tukey contrasts. Statistical analyses were conducted with R version 3.5.0 (the R Foundation) and the survey package.
RESULTS
Demographic Characteristics
Descriptively, adults with office-based physician visits in both 2015 and 2016 were on average aged 52.7 years (SD = 17.7 years) and 59.4% female. Compared with adults with no visits in either year, and those with visits in one year but not the other, adults with office-based physician visits in both years differed clinically in age, age group distribution, sex, race/ethnicity, educational attainment, US region, multimorbidity, insurance status, and usual source of care (Table 1).
Table 1.
Demographic and Clinical Characteristics of US Community-Dwelling Adults, Comparing Patients With No Physician Office Visits (2015 or 2016), Patients With Physician Office Visits (2015 or 2016), and Patients With Physician Office Visits (2015 and 2016)
| Baseline Characteristic | MEPS Sample n = 11,782 | Overall, US Adult Population | Patients With No Office Visits (2015 or 2016) | Patients With Office Visits (2015 or 2016) | Patients With Office Visits (2015 and 2016) | P Value |
|---|---|---|---|---|---|---|
|
| ||||||
| Weighted Frequency (in 1,000s) (%) N = 238,747 (100%) | Weighted Frequency (in 1,000s) (%) N1 = 51,546 (21.6%) | Weighted Frequency (in 1,000s) (%) N2 = 56,885 (23.8%) | Weighted Frequency (in 1,000s) (%) N3 = 130,316 (54.6%) | |||
| Age, mean (SD) | 46.0 (17.5) | 46.6 (17.8) | 37.6 (14.2) | 40.8 (15.5) | 52.7 (17.7) | <.001 |
| Age group | <.001 | |||||
| <40 y | 4,707 (40.0) | 93,301 (39.1) | 30,487 (59.1) | 29,532 (51.9) | 33,282 (25.5) | |
| 40-64 y | 5,107 (43.3) | 102,195 (42.8) | 19,112 (37.1) | 22,924 (40.3) | 60,159 (46.2) | |
| ≥65 y | 1,968 (16.7) | 43,251 (18.1) | 1,948 (3.8) | 4,428 (7.8) | 36,874 (28.3) | |
| Sex | <.001 | |||||
| Male | 5,455 (46.3) | 114,958 (48.2) | 32,548 (63.1) | 29,503 (51.9) | 52,908 (40.6) | |
| Female | 6,327 (53.7) | 123,789 (51.8) | 18,998 (36.9) | 27,382 (48.1) | 77,408 (59.4) | |
| Race/Ethnicity | <.001 | |||||
| White, non-Hispanic | 5,037 (42.8) | 152,163 (63.7) | 26,842 (52.1) | 33,429 (58.8) | 91,891 (70.5) | |
| Black, non-Hispanic | 2,113 (17.9) | 27,936 (11.7) | 7,615 (14.8) | 6,994 (12.3) | 13,328 (10.2) | |
| Asian, non-Hispanic | 938 (8.0) | 14,220 (6.0) | 3,480 (6.8) | 4,251 (7.5) | 6,489 (5.0) | |
| Other, non-Hispanic | 312 (2.6) | 6,972 (2.9) | 1,296 (2.5) | 1,397 (2.5) | 4,279 (3.3) | |
| Hispanic | 3,382 (28.7) | 37,456 (15.7) | 12,314 (23.9) | 10,813 (19.0) | 14,328 (11.0) | |
| Educationa | <.001 | |||||
| <High school | 2,331 (19.8) | 30,476 (12.8) | 8,417 (16.3) | 6,978 (12.3) | 15,080 (11.6) | |
| High school graduate | 3,729 (31.6) | 73,042 (30.6) | 18,186 (35.3) | 17,879 (31.4) | 36,977 (28.4) | |
| >High school | 5,611 (47.6) | 133,789 (56.0) | 24,271 (47.1) | 31,643 (55.6) | 77,875 (59.8) | |
| US region | .02 | |||||
| Northeast | 1,971 (16.7) | 43,448 (18.2) | 9,167 (17.8) | 9,717 (17.1) | 24,566 (18.9) | |
| Midwest | 2,242 (19.0) | 50,597 (21.2) | 10,290 (20.0) | 11,451 (20.1) | 28,856 (22.1) | |
| South | 4,410 (37.4) | 88,295 (37.0) | 19,456 (37.7) | 20,431 (35.9) | 48,408 (37.1) | |
| West | 3,159 (26.8) | 56,406 (23.6) | 12,634 (24.5) | 15,286 (26.9) | 28,487 (21.9) | |
| Multimorbiditya | <.001 | |||||
| 0-1 diagnosis | 1,945 (16.5) | 38,428 (16.1) | 12,736 (24.7) | 14,661 (25.8) | 11,032 (8.7) | |
| 2-4 diagnoses | 3,789 (32.2) | 79,785 (33.4) | 12,260 (23.8) | 23,343 (41.0) | 44,183 (33.9) | |
| ≥5 diagnoses | 3,772 (32.0) | 83,426 (34.9) | 2,400 (4.7) | 8,844 (15.5) | 72,183 (55.4) | |
| Insurance | <.001 | |||||
| Private | 6,968 (59.1) | 168,343 (70.5) | 31,780 (61.7) | 42,118 (74) | 94,445 (72.5) | |
| Public | 3,175 (26.9) | 48,635 (20.4) | 7,802 (15.1) | 9,308 (16.4) | 31,525 (24.2) | |
| Uninsured | 1,639 (13.9) | 21,769 (9.1) | 11,964 (23.2) | 5,459 (9.6) | 4,346 (3.3) | |
| Usual source of carea | 8,388 (71.2) | 177,517 (74.4) | 23,090 (44.8) | 39,323 (69.1) | 115,104 (88.3) | <.001 |
| Physician-patient relationship, mean (SD) | 66.6 (14.3) | 67.1 (13.6) | 66.0 (15.8) | 67.4 (14.6) | 67.1 (13.2) | .49 |
HC = MEPS Household Component; MEPS = Medical Expenditure Panel Survey.
Notes: Data are presented as population count (in 1,000s) and percent unless otherwise noted. For MEPS sample, data are shown as count (%), except for continuous measures, which are presented as mean (SD). For physician-patient relationship, there are missing data for n = 5,342. χ2 test performed on all observations with value > 0.
Source: Agency for Healthcare Research and Quality, Medical Expenditure Panel Survey, Longitudinal Panel (HC −193, 2015-2016) and Medical Conditions (HC −190, 2015).
Data missing for education (n = 111), multimorbidity (n = 2,276), and usual source of care (n = 3,394); percentages reflect observed counts and thus do not sum to 100.
Physician-Patient Relationship by Subgroup
Physician-patient relationship scores varied by some, but not all, demographic and clinical characteristics. Adults with multimorbidity, defined as the presence of ≥5 physician-diagnosed conditions, had a significantly lower score than those with 0-1 condition (66.28 [95% CI, 65.61-66.95] vs 71.38 [95% CI, 69.13-73.63]; SMD = 0.26) (Table 2). Uninsured patients had a lower score than those with private insurance (60.59 [95% CI, 57.49-63.69] vs 67.14 [95% CI, 66.49-67.79]; SMD = 0.33). Those with low baseline functional health scored lower than those with high baseline health (63.33 [95% CI, 62.45-64.21] vs 69.97 [95% CI, 69.31-70.64]; SMD = 0.52).
Table 2.
Bivariate Associations Between Baseline Patient Characteristics and Physician-Patient Relationship Among Longitudinal Cohort of US Adults With Physician Office Visits in 2015 and 2016
| Baseline Characteristic | Physician-Patient Relationship Score
|
|
|---|---|---|
| Survey-Weighted Population Mean (95% CI) | SMDa | |
| Age group | 0.14 | |
| <40 y | 65.85 (64.63-67.07) | |
| 40-64 y | 66.31 (65.47-67.15) | |
| ≥65 y | 68.65 (67.77-69.53) | |
| Sex | 0.01 | |
| Male | 66.95 (66.15-67.75) | |
| Female | 67.02 (66.31-67.73) | |
| Race/Ethnicity | 0.17 | |
| White, non-Hispanic | 67.10 (66.43-67.77) | |
| Black, non-Hispanic | 68.39 (67.06-69.72) | |
| Asian, non-Hispanic | 66.62 (64.41-68.83) | |
| Other, non-Hispanic | 62.68 (58.37-65.99) | |
| Hispanic | 66.33 (64.78-67.88) | |
| Education | 0.02 | |
| <High school | 67.08 (65.53-68.63) | |
| High school graduate | 67.24 (66.26-68.22) | |
| >High school | 66.88 (66.17-67.59) | |
| US region | 0.10 | |
| Northeast | 68.06 (66.75-69.37) | |
| Midwest | 67.71 (66.59-68.83) | |
| South | 66.72 (65.86-67.58) | |
| West | 65.85 (64.56-67.14) | |
| Multimorbidity | 0.26 | |
| 0-1 diagnosis | 71.38 (69.13-73.63) | |
| 2-4 diagnoses | 67.65 (66.67-68.63) | |
| ≥5 diagnoses | 66.28 (65.61-66.95) | |
| Insurance | 0.33 | |
| Private | 67.14 (66.49-67.79) | |
| Public | 67.31 (66.29-68.33) | |
| Uninsured | 60.59 (57.49-63.69) | |
| Baseline functional healthb | 0.52 | |
| Low | 63.33 (62.45-64.21) | |
| High | 69.97 (69.31-70.64) | |
HC = MEPS Household Component; SF-12 = 12-Item Short-Form Survey; SMD = standardized mean difference.
Note: Population mean and 95% CI of physician-patient relationship score among adults ≥18 years with office visits in 2015 and 2016, reflective of 83 million patients in 2015.
Source: Agency for Healthcare Research and Quality, Medical Expenditure Panel Survey, Longitudinal Panel (HC −193, 2015-2016) and Medical Conditions (HC −190, 2015).
SMD between the 2 extremes in each category. Difference in means or proportions divided by SD; imbalance defined as absolute value >0.2 (small effect size).
Functional health captured with the SF-12 instrument, cut at the population median.
Physician-Patient Relationship and Functional Health
The survey-weighted correlation between baseline physician-patient relationship (2015) and follow-up functional health (2016) was 0.20 (P <.001) (Supplemental Figure 2 https://www.AnnFamMed.org/content/18/5/422/suppl/DC1/).
Physician-Patient Relationship Trajectories and Functional Health
Physician-patient relationship trajectories were associated with functional health when accounting for possible confounders in both 2015 and 2016. Regardless of patients’ baseline classifications (high, low), improved relationship trajectories for the 2015-2016 interval were associated with improved functional health (effect estimate [EE] = 0.08 [95% CI, 0.02-0.13] and EE = 0.05 [95% CI, 0-0.10], respectively) (Table 3). Flat physician-patient relationship trajectories were associated with worsened functional health (EE = −0.11 [95% CI, −0.21 to 0.02] and EE = −0.10 [95% CI, −0.21 to 0.02], respectively). Worsened physician-patient relationship trajectories were also associated with worsened functional health (EE = −0.33 [95% CI, −0.47 to −0.02] and EE = −0.16 [95% CI, −0.35 to −0.03], respectively). Three of the 15 multiple pairwise comparisons were significantly different (high → same vs high → better; high → worse vs high → better; and low → better vs high → worse, P <.001) (Supplemental Table 1, https://www.AnnFamMed.org/content/18/5/422/suppl/DC1/). No significant difference was found between patients with flat trajectories (better, same, worse), irrespective of baseline score category.
Table 3.
Effect of Physician-Patient Relationship Change Trajectories on Functional Health Using Survey-Weighted, Covariate-Adjusted, Predicted Marginal Means Among a National Representative Cohort of US Adults With Office Visits in 2 Consecutive Years (n = 3,645, Representative of 83 Million US Adults), 2015-2016
| Relationship | MEPS Cohort n | Functional Health (SF-12) | Cohen Effect Estimate | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 2015 Mean (SD) | 2016 Mean (SD) | Change Mean (SD) | 95% CI | Effect Estimate | 95% CI | ||
| High → Better | 1,208 | 97.90 (0.58) | 98.94 (0.60) | 1.04 (0.39) | 0.29 to 1.80 | 0.08 | 0.02 to 0.13 |
| High → Same | 444 | 96.33 (0.83) | 94.82 (0.85) | –1.51 (0.64) | –2.76 to –0.27 | –0.11 | –0.21 to 0.02 |
| High → Worse | 197 | 93.68 (1.58) | 89.62 (1.49) | –4.06 (0.88) | –5.78 to –2.32 | –0.33 | –0.47 to –0.02 |
| Low → Better | 1,397 | 97.89 (0.62) | 98.75 (0.61) | 0.86 (0.46) | –0.04 to 1.76 | 0.05 | 0 to 0.10 |
| Low → Same | 293 | 95.22 (0.99) | 93.90 (1.12) | –1.32 (0.78) | –2.85 to 0.21 | –0.10 | –0.21 to 0.02 |
| Low → Worse | 106 | 93.82 (1.66) | 91.39 (2.03) | –2.43 (1.48) | –5.33 to –0.47 | –0.16 | –0.35 to –0.03 |
HC = MEPS Household Component; MEPS = Medical Expenditure Panel Survey; MEPS-PC = MEPS Primary Care measure; SF-12 = 12-Item Short-Form Survey; US = United States.
Notes: Mean response for each factor, adjusted for all known confounders, including age, sex, race/ethnicity, educational attainment, insurance status, multimorbidity, and US region using survey-weighted predicted marginal means. Relationship is operationalized using the MEPS-PC Relationship composite measure and cut into 6 trajectories based on 2015 score (high or low) and 2016 score (better, same, worse). High Relationship score in 2015 denotes ≥ median Relationship score at baseline (69.23), whereas Low Relationship score denotes < the population median Relationship score at baseline. Better in 2016 reflects a 1-year change in Relationship score ≥ 0.5 SD; Worse reflects a 1-year change greater than −0.5 SD, and change in either direction < 0.5 SD d enotes Same. The SD for Relationship in 2015 was 13.35. Statistical difference between 3 of 15 pairwise comparisons using Tukey pairwise multiple comparison procedures (P = .05, not adjusted for multiple testing [Bonferroni]): High→Same and High→Better, High→Worse and High→Better, Low→Better and Low→Worse. Borderline significance (P < .10) for High→Worse and High→Same, Low→Better and High→Same, Low→Same and Low→Better.
Source: Agency for Healthcare Research and Quality, Medical Expenditure Panel Survey, Longitudinal Panel (HC-193, 2015-2016) and Medical Conditions (HC-190, 2015).
DISCUSSION
Using nationally representative longitudinal data, we found empirical evidence that improved physician-patient relationship trajectories were associated with improved functional health, whereas worsened relationship trajectories were associated with worsened functional health. These findings with individual-level data uphold findings from studies based on nationally representative and convenience samples.32–36
We expanded on prior longitudinal outcomes analyses of individual patients’ experiences with care36 in 2 ways, first by using a measure of physician-patient relationship with preliminary evidence of reliability and validity23,24 and second by modeling additional patient heterogeneity via the use of 6 mutually exclusive time-trend trajectories. These findings provide data-driven evidence of the importance of relationship-centered care as one viable strategy to improve population health outcomes.10,37,38
Relationship-centered care might be especially important among aging adults with a disproportionate burden of chronic diseases, who represent a growing subpopulation in the United States. In the bivariate analysis, we found evidence that adults with ≥5 diagnosed conditions reported physician-patient relationships that were significantly lower in quality than those reported by adults without multimorbidity. This discrepancy might reflect unmet physician-patient relationship needs among adults bearing multimorbidity burdens and therefore signal an opportunity for intervention.39
The present study has several limitations. Despite controlling for known confounders, patients who report high-quality physician-patient relationship scores might differ in ways that are not observable in the data (eg, in health or in attitudes to self/life). Whereas MEPS is a rich data source, measures on personal agency and attitudes are not presently available. It is also possible that the associations observed are due in part to a spurious association as a result of the MEPS self-administered questionnaire format. Ten of 14 (71%) of the items in MEPS-PC originate from a single survey instrument (SF-12). However, our longitudinal approach, coupled with the construction of 6 mutually exclusive trajectories, addresses such possible bias. Some might argue that the 1-year effect estimates are unimportant.40 However, these effect estimates need to be considered within the proper context, especially in light of the single-year follow up. The practical importance of any effect depends both on relative costs and overall benefits.41 From a population health perspective, with 86% of Americans having access to a usual source of care and the majority listing a doctor’s office as the usual place they receive care,42 these small 1-year effects could accumulate to clinically relevant improvements with longer follow up.43 Finally, it is important to note that the MEPS-PC Relationship measure does not quantify patient experiences with a specific physician but rather with the overall physician care team. Many patients, especially those with complex conditions, visit multiple physicians in a given year and undoubtedly encounter a range of physician-patient relationship qualities. At present, MEPS unfortunately does not allow analysis of individual physician encounters.
The present observational study brings forward new questions answerable via the Agency for Healthcare Research and Quality’s publicly available MEPS data. Among the 14 items comprising the MEPS-PC subscale, is any one or combination of physician behaviors more important in improving patients’ self-reported health outcomes? What are the chief drivers that lead to improved physician-patient relationships? For example, are such improvements driven by changes in a patient’s employer-provided health insurance as a consequence of change in employment? Investigators might also wish to assess whether the quality of the physician-patient relationship differs by physician specialty or type of office visit. These important questions and others are ready to be addressed with new data from MEPS released each September.
CONCLUSION
Using nationally representative data, longitudinal analysis suggests that the quality of the physician-patient relationship is positively associated with functional health. These findings might inform health care strategies and health policy aimed at improving patient-centered health outcomes.
Supplementary Material
Acknowledgments
Preliminary results of this work were presented at the 2018 American Public Health Association annual meeting. We thank Dr Mary Assad for editing expertise. We also thank the deputy editor, Dr Diane M. Harper, and the 3 peer reviewers for substantive suggestions that enhanced the content and clarity of this manuscript.
Footnotes
Conflicts of interest: S.M.K. is a site PI on a grant funded by Celgene Corporation. Other authors report none.
To read or post commentaries in response to this article, see it online at https://www.AnnFamMed.org/content/18/5/422.
ORCID ID: https://orcid.org/0000-0002-3673-1599
Disclosure: This analysis was completed while the lead author was a PhD candidate at Case Western Reserve University. He is now a statistician with Substance Abuse and Mental Health Services and the Center for Behavioral Health Statistics and Quality. Dr Stange was supported as a Scholar of The Institute for Integrative Health and as an American Cancer Society Clinical Research Professor. There are no other disclosures of funding or disclosures of potential conflict of interest to report.
Supplementary materials: Available at https://www.AnnFamMed.org/content/18/5/422/suppl/DC1/.
References
- 1.Starfield B, Shi L, Macinko J. Contribution of primary care to health systems and health. Milbank Q. 2005; 83(3): 457–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Macinko J, Starfield B, Shi L. The contribution of primary care systems to health outcomes within Organization for Economic Cooperation and Development (OECD) countries, 1970-1998. Health Serv Res. 2003; 38(3): 831–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Macinko J, Starfield B, Shi L. Quantifying the health benefits of primary care physician supply in the United States. Int J Health Serv. 2007; 37(1): 111–126. [DOI] [PubMed] [Google Scholar]
- 4.Shi L, Starfield B, Politzer R, Regan J. Primary care, self-rated health, and reductions in social disparities in health. Health Serv Res. 2002; 37(3): 529–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim MY, Kim JH, Choi IK, Hwang IH, Kim SY. Effects of having usual source of care on preventive services and chronic disease control: a systematic review. Korean J Fam Med. 2012; 33(6): 336–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sommers BD, Maylone B, Blendon RJ, Orav EJ, Epstein AM. Three-year impacts of the Affordable Care Act: improved medical care and health among low-income adults. Health Aff (Millwood). 2017; 36(6): 1119–1128. [DOI] [PubMed] [Google Scholar]
- 7.Stewart MA. Effective physician-patient communication and health outcomes: a review. CMAJ. 1995; 152(9): 1423–1433. [PMC free article] [PubMed] [Google Scholar]
- 8.Safran DG. Defining the future of primary care: what can we learn from patients? Ann Intern Med. 2003; 138(3): 248–255. [DOI] [PubMed] [Google Scholar]
- 9.Baker R, Mainous AG, III, Gray DP, Love MM. Exploration of the relationship between continuity, trust in regular doctors and patient satisfaction with consultations with family doctors. Scand J Prim Health Care. 2003; 21(1): 27–32. [DOI] [PubMed] [Google Scholar]
- 10.Frankel RM. Relationship-centered care and the patient-physician relationship. J Gen Intern Med. 2004; 19(11): 1163–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McWhinney IR, Freeman T. Textbook of Family Medicine. 3rd ed. Oxford University Press; 2009. [Google Scholar]
- 12.Stange KC. A science of connectedness. Ann Fam Med. 2009; 7(5): 387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Malley AS, Rich EC, Maccarone A, DesRoches CM, Reid RJ. Disentangling the linkage of primary care features to patient outcomes: a review of current literature, data sources, and measurement needs. J Gen Intern Med. 2015; 30 Suppl 3 (Suppl 3): S576–S585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Safran DG, Taira DA, Rogers WH, Kosinski M, Ware JE, Tarlov AR. Linking primary care performance to outcomes of care. J Fam Pract. 1998; 47(3): 213–220. [PubMed] [Google Scholar]
- 15.Stange KC, Etz RS, Gullett H, et al. Metrics for assessing improvements in primary health care. Annu Rev Public Health. 2014; 35: 423–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith SM, Soubhi H, Fortin M, Hudon C, O’Dowd T. Interventions for improving outcomes in patients with multimorbidity in primary care and community settings. Cochrane Database Syst Rev. 2012; 4: CD006560. [DOI] [PubMed] [Google Scholar]
- 17.Shortell SM, Poon BY, Ramsay PP, et al. A multilevel analysis of patient engagement and patient-reported outcomes in primary care practices of accountable care organizations. J Gen Intern Med. 2017; 32(6): 640–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flocke SA. Measuring attributes of primary care: development of a new instrument. J Fam Pract. 1997; 45(1): 64–74. [PubMed] [Google Scholar]
- 19.Safran DG, Kosinski M, Tarlov AR, et al. The Primary Care Assessment Survey: tests of data quality and measurement performance. Med Care. 1998; 36(5): 728–739. [DOI] [PubMed] [Google Scholar]
- 20.Shi L, Starfield B, Xu J. Validating the adult Primary Care Assessment Tool. J Fam Pract. 2001; 50(2): 161–175. [Google Scholar]
- 21.Van der Feltz-Cornelis CM, Van Oppen P, Van Marwijk HW, De Beurs E, Van Dyck R. A patient-doctor relationship questionnaire (PDRQ-9) in primary care: development and psychometric evaluation. Gen Hosp Psychiatry. 2004; 26(2): 115–120. [DOI] [PubMed] [Google Scholar]
- 22.Cohen SB. Design strategies and innovations in the Medical Expenditure Panel Survey. Med Care. 2003; 41(7 Suppl): III5–III12. [DOI] [PubMed] [Google Scholar]
- 23.Olaisen RH, Flocke SA, Smyth KA, Schluchter MD, Koroukian SM, Stange KC. Developing a new measure of primary care using the Medical Expenditure Panel Survey. Med Care. 2019; 57(6): 475–481. [DOI] [PubMed] [Google Scholar]
- 24.Olaisen RH, Flocke SA, Smyth KA, Schluchter MD, Koroukian SM, Stange KC. Validating the new primary care measure in the Medical Expenditure Panel Survey. Med Care. 2020; 58(1): 52–58. [DOI] [PubMed] [Google Scholar]
- 25.Agency for Healthcare Research and Quality . Medical Expenditure Panel Survey (MEPS). https://www.meps.ahrq.gov/mepsweb/. Accessed Nov 22, 2019. [PubMed]
- 26.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007; 147(8): 573–577. [DOI] [PubMed] [Google Scholar]
- 27.Shrier I, Platt RW. Reducing bias through directed acyclic graphs. BMC Med Res Methodol. 2008; 8: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996; 34(3): 220–233. [DOI] [PubMed] [Google Scholar]
- 29.Cheak-Zamora NC, Wyrwich KW, McBride TD. Reliability and validity of the SF-12v2 in the Medical Expenditure Panel Survey. Qual Life Res. 2009; 18(6): 727–735. [DOI] [PubMed] [Google Scholar]
- 30.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003; 41(5): 582–592. [DOI] [PubMed] [Google Scholar]
- 31.Yang DS, Dalton JE. A unified approach to measuring the effect size between two groups using SAS. SAS Global Forum. 2012; 335: 1–6. [Google Scholar]
- 32.Shi L, Green LH, Kazakova S. Primary care experience and racial disparities in self-reported health status. J Am Board Fam Pract. 2004; 17(6): 443–452. [DOI] [PubMed] [Google Scholar]
- 33.Saultz JW, Lochner J. Interpersonal continuity of care and care outcomes: a critical review. Ann Fam Med. 2005; 3(2): 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bazemore A, Petterson S, Peterson LE, Bruno R, Chung Y, Phillips RL., Jr Higher primary care physician continuity is associated with lower costs and hospitalizations. Ann Fam Med. 2018; 16(6): 492–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Etz RS, Zyzanski SJ, Gonzalez MM, Reves SR, O’Neal JP, Stange KC. A new comprehensive measure of high-value aspects of primary care. Ann Fam Med. 2019; 17(3): 221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu X, Buta E, Anhang Price R, Elliott MN, Hays RD, Cleary PD. Methodological considerations when studying the association between patient-reported care experiences and mortality. Health Serv Res. 2015; 50(4): 1146–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beach MC, Inui T, Relationship-Centered Care Research Network . Relationship-centered care. A constructive reframing. J Gen Intern Med. 2006; 21(Suppl 1): S3–S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Epstein RM, Fiscella K, Lesser CS, Stange KC. Why the nation needs a policy push on patient-centered health care. Health Aff (Millwood). 2010; 29(8): 1489–1495. [DOI] [PubMed] [Google Scholar]
- 39.World Health Organization . Preparing a health care workforce for the 21st century: the challenge of chronic conditions. https://apps.who.int/iris/handle/10665/43044/. Published 2005. Accessed Aug 2, 2019.
- 40.Fritz CO, Morris PE, Richler JJ. Effect size estimates: current use, calculations, and interpretation. J Exp Psychol Gen. 2012; 141(1): 2–18. [DOI] [PubMed] [Google Scholar]
- 41.Hojat M, Xu G. A visitor’s guide to effect sizes: statistical significance versus practical (clinical) importance of research findings. Adv Health Sci Educ Theory Pract. 2004; 9(3): 241–249. [DOI] [PubMed] [Google Scholar]
- 42.Ashman JJ, Rui P, Okeyode T, Centers for Disease Control and Prevention. National Center for Health Statistics . Characteristics of office-based physician visits, 2016. NCHS Data Brief No. 331. https://www.cdc.gov/nchs/products/databriefs/db331.htm. Published Jan 2019; Accessed Jul 13, 2020.
- 43.Coe R. It’s the effect size, stupid: what “effect size” is and why it is important. Paper presented at the Annual Conference of the British Educational Research Association, University of Exeter, England, September 12-14, 2002. https://www.leeds.ac.uk/educol/documents/00002182.htm. Accessed Jul 13, 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.