Abstract
Objective:
Elevated intracranial pressure (ICP) and inadequate cerebral perfusion pressure (CPP) may contribute to poor outcomes in hypertensive intraventricular hemorrhage (IVH). We characterized occurrence of elevated ICP and low CPP in obstructive IVH requiring extraventricular drainage (EVD).
Design:
Prospective observational cohort.
Setting:
Intensive care units of 73 academic hospitals.
Patients:
499 patients enrolled in the CLEAR III trial, a multicenter, randomized study to determine if EVD plus intraventricular alteplase improved outcome versus EVD plus saline.
Interventions:
ICP and CPP were recorded every 4 hours, analyzed over a range of thresholds, as single readings or spans (≥2) of readings after adjustment for ICH severity. Impact on 30- and 180-day modified Rankin Scale (mRS) scores was assessed and receiver operating curves analyzed to identify optimal thresholds.
Measurements and Main Results:
Of 21,954 ICP readings, median (IQR) 12 (8–16) mm Hg, 9.7% were >20 mm Hg and 1.8% >30 mm Hg. Proportion of ICP readings from >18 to >30 mm Hg and combined ICP>20+CPP<70 mm Hg were associated with day 30 mortality and partially mitigated by intraventricular alteplase. Proportion of CPP readings from <65 to <90 mm Hg and ICP >20 mm Hg in spans were associated with both 30- and 180-day mortality. Proportion of CPP readings from <65 to <90 mm Hg and combined ICP>20+CPP<60 mm Hg were associated with poor day 30 mRS, while CPP <65 and <75 mm Hg were associated with poor day 180 mRS.
Conclusions:
Elevated ICP and inadequate CPP are not infrequent during EVD drainage for severe IVH, and level and duration both predict higher short- and long-term mortality. Burden of low CPP was also associated with poor short- and long-term outcome and may be more significant than ICP. Adverse consequences of ICP and CPP-time burden should be tested prospectively as potential thresholds for therapeutic intervention.
Keywords: Intracranial pressure, cerebral perfusion pressure, intraventricular hemorrhage, outcomes, thrombolysis, intracerebral hemorrhage
Introduction
Spontaneous intracerebral hemorrhage (ICH) and frequently associated intraventricular hemorrhage (IVH) cause structural changes that can increase intracranial pressure (ICP) and reduce cerebral perfusion pressure (CPP) (1). The impact of intracranial hypertension on mortality and functional outcomes in ICH is controversial because intracranial hypertension generally is not the immediate cause of death (2), may be related to mortality only in comatose patients (3, 4), and has limited relationship with long-term outcome (5). Our prior investigations of ICP recordings in 100 patients with obstructive IVH and small ICH found that ICP >30 mm Hg was an independent predictor of mortality and disability at 30 days, but not at 180 days (5, 6). CPP was not evaluated, and only conventional ICP and CPP thresholds were assessed.
Current management guidelines for ICH contain little evidence regarding indications for invasive ICP monitoring (4,7–9). There is no evidence to guide CPP management, where principles relevant to traumatic brain injury (TBI) are empirically transferred to ICH populations (10). Limited studies of CPP and dynamic autoregulation in ICH (11, 12) exist, but lack objective thresholds for CPP optimization.
This study’s objectives were to determine: 1) the prevalence of high ICP and low CPP in a high severity ICH/IVH population, 2) the temporal profiles of ICP and CPP, 3) the factors associated with high ICP and low CPP, 4) the impact of high ICP and low CPP on clinical outcomes, and 5) the relevance of conventional TBI management thresholds compared to alternative targets for improving outcomes.
We utilized prospectively collected ICP and CPP from the Clot Lysis: Evaluating Accelerated Resolution of Intraventricular Hemorrhage (CLEAR) III trial (13). We hypothesized that both ICP elevation and low CPP are significantly associated with ICH/IVH severity, early mortality, and functional outcomes, and are improved by use of intraventricular thrombolysis (IVT) with alteplase.
Materials and Methods
We performed a prospective observational cohort study using patients enrolled in the CLEAR III trial, a multicenter, randomized, placebo-controlled trial conducted to determine if external ventricular drainage ( EVD) plus intraventricular alteplase improved outcome in patients with spontaneous obstructive IVH by removing IVH and controlling ICP, versus EVD plus saline (13). The CLEAR III trial was performed at 73 sites in Brazil, Canada, Germany, Hungary, Israel, Spain, the UK, and the USA, following local institutional review board and country-specific ethics approval. Written informed consent was obtained from all participants (or legal representatives or surrogates).
Standard of care EVDs were placed into the frontal horn of the lateral ventricle and tunneled under the scalp by neurosurgical staff. Choice of catheter placement ipsilateral or contralateral to dominant lateral ventricular IVH was decided by each site’s neurosurgical team. Use of dual simultaneous EVDs was recommended in cases of large bilateral IVH by the study surgical center.
This study was a pre-specified analysis of ICP and CPP from the CLEAR III trial. Maximum and minimum ICP and CPP were recorded every four hours (q4h) for seven days post randomization. ICP data was also collected retrospectively from EVD placement until randomization. All centers practiced continuous cerebrospinal fluid (CSF) drainage between doses of study agent. Drainage level and EVD management were directed by site physicians. For each recorded ICP and CPP measurement, the EVD was closed for five minutes prior to recording. All EVDs were zeroed at the external auditory meatus. Validation of q4h measurements with hourly measurements to not miss peak values was previously performed (5).
ICP and CPP management was administered per treating physicians and required adherence to Brain Trauma Foundation guidelines (14, 15). Specified interventions included CSF drainage as well as osmotic therapy, hyperventilation, analgesia, sedation, and where indicated to control ICP, induced coma and surgical management. Interpretation of ICP/CPP management guidelines was at the discretion of the treating physicians. We collected use of any ICP therapy and pressors/inotropes, but not adherence data to particular thresholds.
Patient demographics and comorbidities were recorded at enrollment. CT scans were evaluated from admission, randomization (Rnd) (termed “stability” when all bleeding had stabilized), and end of treatment (EOT), 24 hours after last dose of study agent. These were assessed for: ICH and IVH volumes calculated using semi-automated planimetry; modified Graeb score (mGRAEB) (16); and third ventricular obstruction (EOT only), defined as radiographic obstruction of the third ventricle either by IVH or mechanical compression by thalamic hematoma. CT scans were read centrally by trained image readers blinded to treatment and outcomes. Laterality of EVD placement was defined as contralateral or ipsilateral to largest IVH volume on randomization CT. Definition of dual EVDs required ≥2 EVDs in place simultaneously and used for dosing. New ischemic stroke within first 30 days after enrollment was collected as a pre-defined safety event.
Statistical analysis
Maximum ICP (higher reading if ≥1 EVD) and calculated mean CPP (average of maximum and minimum) readings for each time point were utilized for analysis. Wilcoxon rank-sum test or Student’s t-test were used for analyzing continuous variables depending on data distribution. Categorical variables were analyzed using Pearson chi-square tests (Fisher exact test when appropriate). Missing data values were not estimated. Negative ICP measurements (N=112; 0.5% of readings), indicating imprecise levelling of EVD, were changed to zero for analysis.
Several summary variables from the longitudinal ICP and CPP records were derived and compared by treatment group, using generalized linear models. The percentage of ICP readings within each individual subject’s record that were above thresholds of 20, 30, 40, and 50 mm Hg (% ICP readings >threshold) were calculated. A similar procedure calculated the percentage of CPP readings below 50, 60, and 70 mm Hg (% CPP readings <threshold) and percentage of readings combining high ICP and low CPP thresholds. Spans of ICP >20 and CPP <70 mm Hg were defined as ≥2 consecutive q4h ICP or CPP readings per patient exceeding the threshold. The percent of time in these spans was analyzed.
Univariable and multivariable analysis of factors associated with high ICP (>20 mm Hg) and low CPP (<70 mm Hg) and HighICP+lowCPP (ICP>20 + CPP<70 mm Hg vs all other readings) were performed using binomial generalized linear models, with clustering by patient to adjust for within-patient correlations. Risk factors for high ICP were considered for single EVD patients only and for all patients to assess effect of laterality of EVD placement. Covariates for regression models were chosen based on univariable logistic regression with significance of p<0.05. Relationships between ICP/CPP variables and functional outcomes were based on day 30 and 180 modified Rankin Scale (mRS) scores, dichotomized as good (mRS 0–3) and poor outcome (mRS 4–6). All multivariable outcome models were adjusted for Glasgow Coma Scale (GCS), age, stability ICH and IVH volume, thalamic ICH, systolic blood pressure on admission, treatment group, and number of ICP (CPP) measurements taken. These variables were chosen based on their clinical relevance, hypotheses of interest, or previously recognized influences on functional outcome (17, 18).
Mortality and functional outcomes were analyzed with ICP and CPP thresholds to determine whether optimal thresholds could be identified and whether these differed from conventional TBI thresholds. For a given binary outcome, area under the curve (AUC) was calculated following a logistic regression of the percentage of ICP (CPP) measurements for a patient that were greater (less) than a set of defined thresholds. ICP thresholds were: 10, 15, 18, 20, 22, 25, and 30 mm Hg; CPP thresholds were: 90, 85, 80, 75, 70, 65, and 60 mm Hg. The logistic regressions included all covariates used for multivariable outcome models described above. A nonparametric approach (19) was used to compare and test the equality of the AUCs for an outcome across the ICP (CPP) set of thresholds, taking into account correlations between the AUCs since they were based on the same patients.
To evaluate temporal trends of ICP and CPP, a general linear model analysis of daily mean of maximum ICP and daily mean of minimum CPP level was performed to estimate mean ICP/CPP levels and 95% CIs by combinations of day from EVD placement/randomization and day 30 mortality status. This analysis compared the difference in the trajectory slopes for patients alive and dead at 30 days post ICH. To evaluate a dose response relationship between exposure to low CPP and outcomes, contour plots were created based on logistic regression of outcome with time (hours) at each CPP interval (10 mm Hg) clustering by patient and adjusting by number of total CPP readings. Statistical analyses were performed using STATA (STATA Corp., versions 14.0 and 15.1, College Station, TX). Statistical significance for all analyses was determined as p<0.05 (two-tailed).
Results
Analyses were based on 499 patients who contributed ICP and CPP data. The mean (±standard error of the mean) age was 58.5±11.2 years. The median (interquartile range, IQR) for baseline IVH volume was 21.8 mL (12.7–37), and for ICH volume was 7.9 mL (2.5–15). Treatment groups did not differ with respect to age, sex, race/ethnicity, stroke risk factors, or baseline clinical and radiologic parameters (18). The CLEAR III trial was neutral in the primary endpoint of improved functional outcome (mRS 0–3) at 180 days (alteplase group 48% vs saline 45%) (15). There were significantly fewer deaths in alteplase treated patients compared to saline (18.5% vs 29.2%).
Prevalence and Temporal Profile of ICP and CPP Abnormalities
Opening pressure at EVD placement ranged from −3 to 65 mm Hg; median (IQR) was 10 (4,6–13). Opening pressure was >20 mm Hg in 8.4% of patients and was not associated with hemorrhage volumes, but was associated with number of subsequent high ICP readings >20 mm Hg (odds ratio [OR], 1.034; 95% confidence interval [95% CI], 1.028–1.04). The median duration of ICP monitoring was 180 (160–196) hours and was not different between treatment groups (p=0.30). Of 21,954 ICP readings, 9.7% were >20 mm Hg (Table S1, Supplemental Digital Content 1). The percentage of patients with ≥1 ICP reading above threshold was 72.8% and 33.3% for >20 and >30 mm Hg, respectively. The percentage of ICP readings >20 mm Hg was higher in saline vs alteplase treated patients (10.1% vs 9.3%; p=0.046).
Of 18,763 CPP readings, 8.1% were <70 mm Hg. Percentages of patients with ≥1 CPP reading below threshold were 61.9%, 22.7%, and 4.4% for <70, <60, and <50 mm Hg, respectively. The percentage of low CPP readings and number of patients with ≥1 low reading were lower in alteplase vs saline treated subjects (p<0.05 for all three thresholds). ICP elevations with reciprocal decrease in CPP were also generally more frequent in saline vs alteplase treated patients.
Figure 1 shows temporal trends of mean daily ICP and CPP by 30-day mortality status. ICP and CPP trends were not different between survivors and non-survivors. Mean daily CPP was significantly lower in non-survivors over most of the monitoring period, but with values consistently >70 mm Hg.
Figure 1.
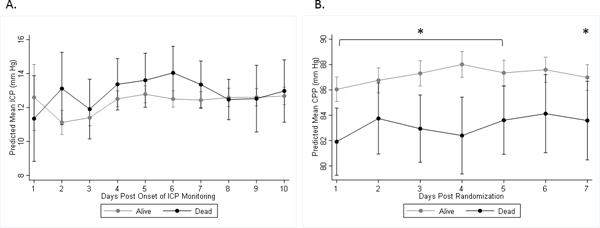
A, Mean of maximum daily ICP readings (with 95% CIs). B, Mean of minimum daily CPP readings (with 95% CIs). Maximum daily ICP was not significantly different between survivors and non-survivors at day 30. Minimum daily CPP was significantly lower in non-survivors versus survivors on days 1–5 and 7 (*:p<0.05). The slopes between groups are not different; however, the slope for ICP in survivors is significantly different from zero (p<0.001).
Treatment intensity was analyzed and one or more ICP therapies were applied to 144 patients (29%). Pressor or inotrope use was administered to 123 (25%) patients. Use of at least one ICP therapy was significantly associated with having any ICP event >20 (single and in spans), >30 and >40 mm Hg, and use of pressors/inotropes was significantly associated with having any CPP event <70 (single and spans) and <60 mm Hg (Table S2, Supplemental Digital Content 2).
Factors Associated with ICP and CPP Abnormalities
Two logistic regression models for high ICP events are reported (Table S3, Supplemental Digital Content 3). In single EVD patients (n=336), ICP >20 mm Hg was associated with younger age, higher GCS, higher ICH and IVH volume, EVD placement ipsilateral to dominant IVH, third ventricular obstruction at EOT, and time from symptom onset to randomization (SO_Rnd) >48 hours. In patients with single or dual EVDs, ICP >20 mm Hg was independently associated with these same factors and with dual EVDs.
CPP <70 mm Hg was associated with lower GCS, higher mGRAEB, lower enrollment DBP, third ventricle obstruction at EOT, SO_Rnd time <48 hrs, saline treatment group, and with new ischemic stroke at day 30 (Table S4, Supplemental Digital Content 4). ICP readings >20 mm Hg associated with CPP <70 mm Hg found the same independent predictors, though younger age was significant, and SO_Rnd time and ischemic stroke were not. New ischemic stroke by day 30 occurred in 23 (4.6%) patients and was detected at median 10 (5–19) days post hemorrhage.
Associations Between ICP and CPP Abnormalities and Functional Outcomes
In the outcome discrimination analysis, the percent of monitoring time at ICP thresholds from >10 to >30 mm Hg, and CPP thresholds from <90 to <60 mm Hg were analyzed. Odds ratios (ORs) for mortality by day 30 were significant for all ICP thresholds at 18 mm Hg and above (Table 1; Figure S1, Supplementary Digital Content 5). No ORs for ICP thresholds and day 180 mortality or poor mRS at either time point were significant. ORs for death at day 30 and 180 were significant for all CPP thresholds except <60 mm Hg at day 180 (Table 2). ORs for poor mRS were significant for CPP thresholds <65 to <90 mm Hg at day 30 and for CPP <75 and <65 mm Hg at day 180. The AUCs were calculated separately for ICP and CPP thresholds (Figure S2, Supplementary Digital Content 6). There were no significant differences between AUC values across ICP thresholds or across CPP thresholds for either mortality or poor functional outcome at either time point. The % of monitoring time in spans of ICP >20 mm Hg was significantly associated with both day 30 and 180 mortality, and % of monitoring time with ICP>20+CPP<70/<60 mm Hg was associated with day 30 mortality and day 30 mRS, respectively. Elevated ICP was not associated with poor functional outcome at either time.
TABLE 1.
Comparison of Odds Ratios and Area Under Receiver Operating Characteristic Curve (AUC) Across Time at ICP Levels for Mortality and Poor mRS (4–6) at Days 30 and 180
| ICP Threshold (mm Hg) | Odds Ratio (95% CI) | p | AUC | Correctly Classified (%) |
|---|---|---|---|---|
| Day 30 Mortality | ||||
| >10 | 1.12 (0.97–1.30) | 0.11 | 0.828 | 88.45 |
| >15 | 1.14 (0.98–1.33) | 0.09 | 0.825 | 88.67 |
| >18 | 1.27 (1.03–1.57) | 0.02 | 0.826 | 89.98 |
| >20 | 1.48 (1.15–1.90) | 0.002 | 0.828 | 90.20 |
| >22 | 1.63 (1.20–2.22) | 0.002 | 0.828 | 90.63 |
| >25 | 2.09 (1.38–3.15) | <0.001 | 0.831 | 90.20 |
| >30 | 2.47 (1.33–4.59) | 0.004 | 0.832 | 89.54 |
| >20 in spans | 1.77 (1.22–2.58) | 0.003 | 0.838 | 88.67 |
| Day 180 Mortality | ||||
| >10 | 1.04 (0.94 – 1.14) | 0.48 | 0.772 | 81.28 |
| >15 | 1.08 (0.96–1.21) | 0.19 | 0.772 | 82.16 |
| >18 | 1.15 (0.98–1.35) | 0.09 | 0.772 | 83.04 |
| >20 | 1.21 (0.99–1.49) | 0.06 | 0.772 | 82.60 |
| >22 | 1.26 (0.98–1.62) | 0.08 | 0.772 | 82.60 |
| >25 | 1.34 (0.95–1.88) | 0.09 | 0.771 | 81.50 |
| >30 | 1.42 (0.84–2.39) | 0.19 | 0.773 | 81.72 |
| >20 in spans | 1.43 (1.03–1.99) | 0.03 | 0.785 | 83.44 |
| Day 30 Poor mRS | ||||
| >10 | 1.06 (0.94–1.20) | 0.34 | 0.895 | 86.75 |
| >15 | 1.07 (0.94–1.22) | 0.33 | 0.895 | 86.75 |
| >18 | 1.11 (0.92–1.35) | 0.27 | 0.895 | 86.98 |
| >20 | 1.17 (0.89–1.53) | 0.27 | 0.895 | 86.75 |
| >22 | 1.12 (0.62–1.30) | 0.55 | 0.894 | 86.98 |
| >25 | 1.10 (0.65–1.88) | 0.71 | 0.893 | 86.98 |
| >30 | 0.85 (0.37–1.96) | 0.71 | 0.895 | 86.75 |
| >20 in spans | 1.55 (0.92–2.62) | 0.10 | 0.899 | 87.42 |
| Day 180 Poor mRS | ||||
| >10 | 0.99 (0.90–1.09) | 0.90 | 0.854 | 77.11 |
| >15 | 1.08 (0.97–1.20) | 0.16 | 0.856 | 78.00 |
| >18 | 1.11 (0.96–1.27) | 0.16 | 0.856 | 78.44 |
| >20 | 1.14 (0.95–1.37) | 0.16 | 0.856 | 78.22 |
| >22 | 1.11 (0.88–1.40) | 0.39 | 0.855 | 77.78 |
| >25 | 1.07 (0.77–1.49) | 0.70 | 0.854 | 77.78 |
| >30 | 1.12 (0.65–1.93) | 0.68 | 0.854 | 77.56 |
| >20 in spans | 1.21 (0.91–1.60) | 0.19 | 0.855 | 78.22 |
CPP = cerebral perfusion pressure, ICP = intracranial pressure. All models adjusted for number of ICP/CPP readings per patient, age, Glasgow Coma Scale, intracerebral hemorrhage (ICH) and intraventricular hemorrhage volume, thalamic ICH location, treatment group, and admission systolic blood pressure.
TABLE 2.
Comparison of Odds Ratios and Area Under Receiver Operating Characteristic Curve (AUC) Across Time at CPP Levels for Mortality and Poor mRS (4–6) at Days 30 and 180
| CPP Threshold (mm Hg) | Odds Ratio (95% CI) | p | AUC | Correctly Classified (%) |
|---|---|---|---|---|
| Day 30 Mortality | ||||
| <90 | 1.18 (1.01–1.37) | 0.04 | 0.835 | 89.96 |
| <85 | 1.17 (1.02–1.34) | 0.02 | 0.838 | 89.52 |
| <80 | 1.17 (1.02–1.34) | 0.03 | 0.836 | 89.30 |
| <75 | 1.22 (1.03–1.44) | 0.02 | 0.836 | 89.30 |
| <70 | 1.36 (1.07–1.74) | 0.01 | 0.838 | 88.43 |
| <65 | 1.77 (1.22–2.57) | 0.003 | 0.839 | 89.52 |
| <60 | 2.82 (1.24–6.42) | 0.01 | 0.841 | 89.30 |
| <70 in spans | 1.58 (0.97–2.59) | 0.07 | 0.841 | 88.86 |
| <70 + ICP >20 | 2.13 (1.46–3.10) | <0.001 | 0.838 | 90.17 |
| Day 180 Mortality | ||||
| <90 | 1.12 (1.01–1.23) | 0.03 | 0.781 | 82.34 |
| <85 | 1.11 (1.01–1.08) | 0.03 | 0.781 | 82.78 |
| <80 | 1.14 (1.03–1.26) | 0.01 | 0.782 | 82.34 |
| <75 | 1.17 (1.04–1.33) | 0.01 | 0.783 | 82.12 |
| <70 | 1.22 (1.01–1.47) | 0.04 | 0.779 | 82.34 |
| <65 | 1.45 (1.05–2.00) | 0.02 | 0.779 | 82.56 |
| <60 | 1.55 (0.82–2.93) | 0.18 | 0.775 | 81.90 |
| <70 in spans | 1.28 (0.88–1.87) | 0.19 | 0.788 | 81.22 |
| <70 + ICP >20 | 1.41 (0.99–2.00) | 0.06 | 0.782 | 82.75 |
| Day 30 Poor mRS | ||||
| <90 | 1.20 (1.08–1.33) | 0.001 | 0.903 | 88.94 |
| <85 | 1.21 (1.09–1.36) | 0.001 | 0.903 | 88.50 |
| <80 | 1.30 (1.13–1.50) | <0.001 | 0.905 | 88.50 |
| <75 | 1.51 (1.20–1.90) | <0.001 | 0.906 | 87.61 |
| <70 | 1.73 (1.18–2.55) | 0.005 | 0.901 | 86.06 |
| <65 | 2.32 (1.08–4.97) | 0.03 | 0.900 | 85.84 |
| <60 | 8.25 (0.74–91.55) | 0.09 | 0.897 | 85.62 |
| <70 in spans | 2.76 (0.75–10.23) | 0.13 | 0.900 | 86.95 |
| <60 + ICP >20 | 13.59 (1.23–150.62) | 0.03 | 0.897 | 87.39 |
| Day 180 Poor mRS | ||||
| <90 | 1.08 (0.98–1.18) | 0.11 | 0.856 | 77.28 |
| <85 | 1.07 (0.98–1.18) | 0.14 | 0.855 | 77.10 |
| <80 | 1.10 (0.98–1.23) | 0.08 | 0.856 | 77.51 |
| <75 | 1.15 (0.99–1.33) | 0.01 | 0.856 | 77.28 |
| <70 | 1.18 (0.95–1.46) | 0.14 | 0.855 | 77.28 |
| <65 | 1.53 (1.02–2.29) | 0.04 | 0.856 | 77.73 |
| <60 | 1.87 (0.82–4.30) | 0.14 | 0.855 | 77.06 |
| <70 in spans | 1.27 (0.77–2.08) | 0.35 | 0.856 | 77.95 |
| <60 + ICP >20 | 1.82 (0.59–5.65) | 0.30 | 0.854 | 76.84 |
CPP = cerebral perfusion pressure, ICP = intracranial pressure. All models adjusted for number of ICP/CPP readings per patient, age, Glasgow Coma Scale, intracerebral hemorrhage (ICH) and intraventricular hemorrhage volume, thalamic ICH location, treatment group, and admission systolic blood pressure.
Figure 2 shows color-coded plots demonstrating the correlation between probability of poor outcome at 180 days and the average duration of CPP events at defined thresholds. This figure illustrates the concept that episodes of lower CPP can only be tolerated for shorter durations before resulting in higher probability of a poor outcome.
Figure 2.
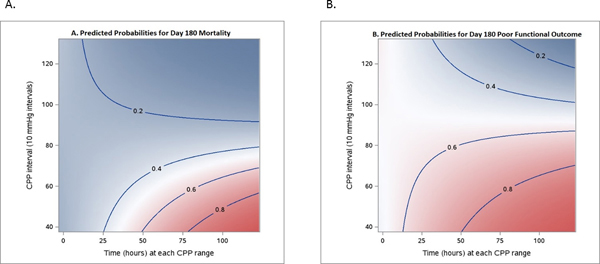
Visualization of correlation between outcomes and average time at observed CPP threshold levels. A, Day 180 mortality (n = 499). B, Day 180 poor mRS (n = 499). Each color-coded point in the graph refers to a probability of the outcome, defined by a certain CPP threshold (Y-axis), and a certain duration (X-axis). Dark pink/blue areas mean that such CPP events, on average, are associated with higher probability of worse outcome (or death)/good outcome (mRS 4–6/0–3).
Discussion
We used an ROC outcome discrimination analysis to compare the association of various thresholds of ICP and CPP with mortality and functional outcomes. Despite relatively infrequent ICP elevation, ICP single events from >18 up to >30 mm Hg predicted higher short-term mortality, and successive events of ICP >20 mm Hg predicted long-term mortality as well. More significant than high ICP, however, is low CPP, which was an independent predictor of both short- and long-term mortality and of short-term poor outcome at all levels tested from <65 to <90 mm Hg, and of long-term poor outcome at <65 and <75 mm Hg. Intraventricular alteplase was associated with lower proportion of both high ICP and low CPP events. This strengthens the results from smaller studies of ICP in severe IVH but raises new questions about optimal thresholds and whether CPP should be prioritized over ICP in this population.
ICP and CPP thresholds
Protocolized interventions for ICP >20–25 mm Hg are a mainstay of neurocritical care, based on observational data from the Traumatic Coma Data Bank confirming that sustained ICP above 20 mm Hg is associated with poor neurological outcome (20). Our finding that early mortality is associated with duration of monitoring time above ICP of 18 mm Hg appears consistent with TBI literature suggesting patients >55 years appear to have lower ICP thresholds (18 vs 22 mm Hg) and higher CPP thresholds for prediction of poor outcome compared to younger patients (21). The importance of CPP management in TBI is controversial; some reports suggest greater contribution to outcomes than intracranial hypertension (22–24), while others report no positive effect on outcomes for CPP >60 mm Hg (25, 26). Of relevance, Young et al reported that aggressive management of CPP can lead to good neurological outcomes in severe TBI despite extremely high ICP (10).
In ICH, infrequent elevations are common. In this study, 73% of IVH subjects had at least one ICP reading >20 mm Hg, and 33% >30 mm Hg, which is consistent with a meta-analysis of 381 ICH patients that reported a pooled prevalence rate for any episode of intracranial hypertension (IHT) (>20 mm Hg) of 67% (95% CI, 51–84%) (27). Moreover, when ICP is elevated, both in comatose patients with supratentorial ICH and aggressively managed patients with obstructive IVH and EVD drainage, ICP >20 or >30 mm Hg was found to be significantly associated with both early mortality and poor outcomes in 2 studies (5, 28), though another study did not find an association between IHT and mRS (29). In this study, we evaluated persistently elevated ICP, suggesting refractoriness; notably, this was the only ICP variable associated with long-term mortality. Unlike ICP, low CPP was also associated with poor functional outcomes, which is consistent with a study of multimodality monitoring in comatose ICH patients that found that CPP <70–80 mm Hg was associated with brain tissue hypoxia and poor outcome (30). Sorrentino et al used pressure reactivity index (PRx) in 459 TBI patients to identify the ICP and CPP thresholds that maximized the statistical difference between death/survival and favorable/unfavorable outcomes. This method identified thresholds of 70 mm Hg for mean CPP and 22 mm Hg for ICP as most discriminative for both survival and favorable outcomes at 6 months (21). We observed no significant difference in AUC for either ICP or CPP thresholds to identify optimal cut points, though odds ratios generally increased at higher ICP and lower CPP thresholds when mortality was the outcome, suggesting a higher mortality risk with increasing duration of monitoring time at the extremes of the thresholds tested. There were fewer CPP readings below 60 mm Hg and very few below 50 mm Hg, which may have reduced statistical power to observe significant risk at these levels. The wide range of CPP, over which significant associations with both mortality and functional outcomes were found, may also be influenced by the autoregulation (AR) status of the patient. Guiza et al (31) found that in TBI patients, the relationship of duration and intensity of CPP below or above a certain threshold with 6-month Glasgow Outcome Score depended on whether AR was active or deficient with a “safe zone” between 60 and 70 mm Hg identified for patients ≤65 years with ICP <25 mm Hg, provided AR was active. The tolerance for low CPP was significantly reduced for patients with deficient AR. In our study, the contour plots suggest that a cumulative time/pressure dose per patient over a wide range of CPP has association with outcomes, which is higher for functional outcome than for mortality. We found a similar effect of ICP burden on day 30 mortality, but not on functional outcomes. Due to the practice of the arterial blood pressure being zeroed at the level of the right atrium instead of the external auditory meatus, overestimation of CPP in this study is a distinct possibility (32), and statements on the association of specific CPP thresholds with outcomes are general and cannot be used to recommend specific thresholds.
Factors Associated with ICP and CPP Abnormalities
We report a significant association between low CPP (<70 mm Hg) and occurrence of new ischemic strokes by day 30, despite low incidence of this finding (<5% of patients). This is significant given that acute ischemic lesions are reported in up to 26.8% of ICH patients, etiology is poorly understood, and these contribute to poor outcomes (33, 34). In ICH, aggressive blood pressure lowering, even within guideline recommendations, has been associated with increased remote cerebral ischemic lesions and acute neurologic deterioration (35). In TBI patients, Robertson et al (26) compared CPP and ICP therapies and reported a decrease in ischemic insults with CPP-targeted therapy (>70 mm Hg vs >50 mm Hg). More recently, studies of continuous monitoring of cerebrovascular pressure reactivity suggest that low CPP (thresholds of 60–70 mm Hg) may result in exhaustion of autoregulatory reserve in TBI patients (36, 37). These data all support a higher CPP treatment threshold in ICH, especially in patients with suspected IHT, and are strengthened by Sorrentino’s evidence that in TBI patients >55 years, the CPP threshold for mortality was 75 mm Hg (21). Nevertheless, policies to therapeutically maintain a high CPP are controversial, and there is no level I evidence confirming that CPP-directed care improves outcomes. Depending on whether cerebral vessels are reactive, increasing CPP may result in hyperemia, increase vasogenic edema, pulmonary complications, and potentially cause secondary increases in ICP (5, 6, 26).
The proportion of ICP events >20 and >30 mm Hg were associated with several treatment-related factors, including placement of the EVD on side of dominant IVH, dual EVDs, persistent obstruction of the third ventricle and SO_Rnd time >48 hrs. With dual EVDs, this association likely indicates more severe IVH for which bilateral EVD placement was recommended. Dual EVD patients had significantly larger mean IVH volume (40.1 vs 22.4 mL). Side of catheter placement is a treatment issue in IVH due to observed greater benefit with IVT being dependent on greater extent of clot removal, which often requires a catheter on dominant side of IVH (18, 38). In this study, we confirm our previous findings that EVD placement ipsilateral to greatest IVH clot burden is associated with higher ICP versus contralateral placement, suggesting an ICP gradient within the ventricular system or perhaps influenced by hematoma location (5, 39). Patients with ipsilateral drains may have been sicker or had local mass effect or a trapped lateral ventricle at time of EVD placement. These explanations argue for a bilateral catheter strategy in more patients than is current practice: an initial EVD to drain CSF and control ICP placed contralateral to dominant IVH and a second ipsilateral treatment catheter to optimize IVH clot removal. The association of high ICP with higher GCS may reflect less sedation, which itself can control ICP in some situations.
Factors independently associated with greater proportion of CPP events <70 mm Hg were related to blood pressure control, ICP elevation, and timing of treatment. The association with earlier time to trial enrollment may reflect more aggressive blood pressure control to meet stable blood pressure requirements.
Temporal Trends of ICP/CPP
Mean daily CPP was significantly lower in non-survivors over most of the monitoring period, but with values consistently >70 mm Hg, supporting the hypothesis that brief episodes of intracranial hypertension or low CPP were sufficient to explain significant differences in mortality and short-term outcomes. Moreover, optimal CPP may be higher than previously considered in patients with obstructive hydrocephalus. Although point estimates are small for odds ratios, they are adjusted for variables known to dominate outcomes after ICH; once ICP and CPP are mitigated, other factors cause mortality, especially in the long-term (18). Further investigation would be required to determine whether goal-directed CPP optimization is beneficial.
Limitations
Use of data from multiple centers may be associated with differential adherence to ICP/CPP management algorithms and time to trigger intervention, especially for patients expected to have poor outcomes. We did not collect site-by-site adherence to ICP/CPP goals or decision-making regarding refractoriness of an individual patient’s ICP/CPP. ICP lowering and inotrope therapies were each utilized in 25 and 29% of patients, respectively, suggesting that ICP lowering therapies may not have been used in all patients with single episodes of elevation, or that CSF drainage alone may have treated elevated ICP in the majority of cases. Although all models were adjusted for known severity variables, the threshold analysis is likely confounded by disease severity in that it does not discriminate between patients with ICP elevation/low CPP who were treated and those who had more severe disease. However, after three distinct analyses of ICP in obstructive IVH with increasing sample size, we show similar proportion of ICP >20 mm Hg across all studies (from 8.5% [n=100] to 14% [n=11]), suggesting results are robust and methodology consistent. Also, do-not-resuscitate orders were an exclusion at trial enrollment and highly discouraged during the active treatment phase. It is not currently possible to collect ICP in the pre-EVD period, but the mechanism of fatal ICP in this population via hydrocephalus is unlikely to present as sudden death due to ICP crisis. Other potential confounders for ICP and CPP estimation include position of head of bed, EVD drainage level, and the short EVD clamping time of 5 minutes, which may not have captured all ICP variation, especially due to pathologic ICP waves, but which was likely sufficient to allow cardiac and respiratory equilibration and capture a stable ICP reading in most patients. Due to therapeutic necessity for continuous CSF drainage in this population, ICP and CPP goals were likely practiced on readings from an open drainage system, which may not always represent closed system measurements. This disconnection governs all usual practice of open EVDs, though trends typically are treated based on closed readings. EVD management was directed by site physicians; it is possible that EVD drainage level was not optimized for ICP/CPP control, leading to bias in measurements across sites. Finally, our findings may not be generalizable to ICH patients monitored with other ICP devices, or without clinical indication for EVD. Nevertheless, this dataset includes the largest cohort of IVH patients studied, well-defined inclusion criteria, single device determination of ICP, and blinded assessment of outcome.
Conclusion
This study supports the concept that unmitigated ICP threshold events perhaps as low as 18 mm Hg are associated with early mortality, and refractory events with more delayed mortality. These are only partially mitigated by current “pragmatic” EVD practices and use of alteplase. On the basis of our findings, the argument could be made that CPP levels and duration of exposure exhibit a dose-response relationship with both mortality and functional outcomes, and that optimal CPP targets for older ICH patients may be higher than in young TBI patients. This is an association study and is hypothesis generating. It cannot imply causality nor any treatment recommendations regarding ICP or CPP control. Many recent studies indicate that ICP/CPP are markers of outcome, but not necessarily “modifiable” therapeutic targets. This study does support merging ICP and CPP management protocols in monitored ICH patients, especially those with obstructive IVH requiring an EVD. Consideration should be given to early timing of EVD placement, and side of EVD placement, both for ICP management and reducing the burden of IVH volume. Alleviating obstruction of the third ventricle may require bilateral EVD placement or other interventions.
Supplementary Material
Supplemental Digital Content 1. Table S1: ICP and CPP Variables by Treatment Group
Supplemental Digital Content 2. Table S2: Association between Use of ICP Therapies and Pressors/Inotropes by ICP/CPP Events
Supplemental Digital Content 3. Table S3: Factors Associated with Elevated Intracranial Pressure (ICP)
Supplemental Digital Content 4. Table S4: Factors Associated with Low Cerebral Perfusion Pressure (CPP) (<70 mmHg) and with High Intracranial Pressure (>20 mmHg) Plus Low CPP (<70 mmHg)
Supplemental Digital Content 5. Figure S1: Comparison of Odds Ratios at ICP Levels Over Monitoring Time (A) and CPP (B) Levels For Day 30 and 180 Mortality and Poor mRS
Supplemental Digital Content 6. Figure S2: Comparison of Receiver Operating Characteristic Curves at ICP Levels (A) and CPP (B) Levels Over Monitoring Time For Day 30 and 180 Mortality and Poor mRS
Acknowledgements
We wish to acknowledge the work of Yi Hao, PhD in the production of Figure 2.
Financial support:
This work was supported in part by the National Institutes of Health grants 5U01NS062851 for Clot Lysis: Evaluating Accelerated Resolution of Intraventricular Hemorrhage (CLEAR III). Genentech assisted by donating the study drug. Drs. Hanley, Awad, and Ziai, Ms. McBee, and Ms. Lane are supported by grant 5U01NS062851. Dr. Hanley is a consultant for BrainScope, Neurotrope, Portola Pharmaceuticals, Op2Lysis, HeadSense, and Medtronic. The remaining authors have disclosed that they do not have any conflicts of interest.
Copyright form disclosure: Dr. Ziai received funding from C. R. Bard, Headsense, and NINDS. Drs. Ziai, Thompason, Mayo, McBee, Ullman, Lane, Awad, and Hanley received support for article research from National Institutes of Health (NIH). Drs. Thompson and Award’s institutions received funding from the NIH. Drs. McBee, Lane, and Hanley’s institutions received funding from NIH/NINDS 5U01NS062851, NIH/NINDS 1U01NS08082, and Genentech (drug donation). Drs. McBee, Ullman, Lane, Awad, and Hanley disclosed off-label product use of alteplase Dr. Ullman’s institution received funding from NIH/NINDS. Dr. Awad received funding form expert reviews and medicolegal opinions. Dr. Hanley received funding from BrainScope, Neurotrope, Portola Pharmaceuticals, Op2Lysis, HeadSense, Medtronic. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Footnotes
No reprints will be ordered
References
- 1.Hemphill JC 3rd, Greenberg SM, Anderson CS, et al. : Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2015; 46:2032–2060 [DOI] [PubMed] [Google Scholar]
- 2.Ropper AH, King RB: Intracranial pressure monitoring in comatose patients with cerebral hemorrhage. Arch Neurol 1984; 41:725–728 [DOI] [PubMed] [Google Scholar]
- 3.Janny P, Papo I, Chazal J, et al. : Intracranial hypertension and prognosis of spontaneous intracerebral haematomas. A correlative study of 60 patients. Acta Neurochir (Wien) 1982; 61:181–186 [DOI] [PubMed] [Google Scholar]
- 4.Fernandes HM, Siddique S, Banister K, et al. : Continuous monitoring of ICP and CPP following ICH and its relationship to clinical, radiological and surgical parameters. Acta Neurochir Suppl 2000; 76:463–466 [DOI] [PubMed] [Google Scholar]
- 5.Ziai WC, Melnychuk E, Thompson CB, et al. : Occurrence and impact of intracranial pressure elevation during treatment of severe intraventricular hemorrhage. Crit Care Med 2012; 40:1601–1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ziai WC, Torbey MT, Naff NJ, et al. : Frequency of sustained intracranial pressure elevation during treatment of severe intraventricular hemorrhage. Cerebrovasc Dis 2009; 27:403–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elliott J, Smith M: The acute management of intracerebral hemorrhage: a clinical review. Anesth Analg 2010; 110:1419–1427 [DOI] [PubMed] [Google Scholar]
- 8.Steiner T, Al-Shahi Salman R, Beer R, et al. : European Stroke Organisation (ESO) guidelines for the management of spontaneous intracerebral hemorrhage. Int J Stroke 2014; 9:840–855 [DOI] [PubMed] [Google Scholar]
- 9.Rodríguez-Yáñez M, Castellanos M, Freijo MM, et al. : Clinical practice guidelines in intracerebral haemorrhage. Neurologia 2013; 28:236–249 [DOI] [PubMed] [Google Scholar]
- 10.Young JS, Blow O, Turrentine F, et al. : Is there an upper limit of intracranial pressure in patients with severe head injury if cerebral perfusion pressure is maintained? Neurosurg Focus 2003; 15:E2. [DOI] [PubMed] [Google Scholar]
- 11.Tian Y, Wang Z, Jia Y, et al. : Intracranial pressure variability predicts short-term outcome after intracerebral hemorrhage: a retrospective study. J Neurol Sci 2013; 330:38–44 [DOI] [PubMed] [Google Scholar]
- 12.Diedler J, Santos E, Poli S, et al. : Optimal cerebral perfusion pressure in patients with intracerebral hemorrhage: an observational case series. Crit Care 2014; 18:R51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ziai WC, Tuhrim S, Lane K, et al. : A multicenter, randomized, double-blinded, placebo-controlled phase III study of Clot Lysis Evaluation of Accelerated Resolution of Intraventricular Hemorrhage (CLEAR III). Int J Stroke 2014; 9:536–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The Brain Trauma Foundation. The American Association of Neurological Surgeons. The Joint Section on Neurotrauma and Critical Care. Intracranial pressure treatment threshold. J Neurotrauma 2000; 17:493–495 [DOI] [PubMed] [Google Scholar]
- 15.The Brain Trauma Foundation. The American Association of Neurological Surgeons. The Joint Section on Neurotrauma and Critical Care. Guidelines for cerebral perfusion pressure. J Neurotrauma 2000; 17:507–511 [DOI] [PubMed] [Google Scholar]
- 16.Morgan TC, Dawson J, Spengler D, et al. : The Modified Graeb Score: an enhanced tool for intraventricular hemorrhage measurement and prediction of functional outcome. Stroke 2013; 44:635–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hemphill JC 3rd, Bonovich DC, Besmertis L, et al. : The ICH score: A simple, reliable grading scale for intracerebral hemorrhage. Stroke 2001; 32:891–897 [DOI] [PubMed] [Google Scholar]
- 18.Hanley DF, Lane K, McBee N, et al. : Thrombolytic removal of intraventricular haemorrhage in treating severe stroke: Results of the CLEAR III trial, a randomized, controlled trial. Lancet 2017; 389:603–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeLong ER, DeLong DM, Clarke-Pearson DL: Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988; 44:837–845 [PubMed] [Google Scholar]
- 20.Marmarou A, Anderson RL, Ward JD, et al. : Impact of ICP instability and hypotension on outcome in patients with severe head trauma. J Neurosurg 1991; 75:S59–S66 [Google Scholar]
- 21.Sorrentino E, Diedler J, Kasprowicz M, et al. : Critical thresholds for cerebrovascular reactivity after traumatic brain injury. Neurocrit Care 2012; 16:258–266 [DOI] [PubMed] [Google Scholar]
- 22.Bullock R, Chesnut RM, Clifton G, et al. : Intracranial pressure treatment threshold. J Neurotrauma 1996; 13:681–683 [DOI] [PubMed] [Google Scholar]
- 23.Chan KH, Dearden NM, Miller JD, et al. : Multimodality monitoring as a guide to treatment of intracranial hypertension after severe brain injury. Neurosurgery 1993; 32:547–553 [DOI] [PubMed] [Google Scholar]
- 24.Rosner MJ: Introduction to cerebral perfusion pressure management. Neurosurg Clin N Am 1995; 6:761–773 [PubMed] [Google Scholar]
- 25.Juul N, Morris GF, Marshall SB, et al. : Intracranial hypertension and cerebral perfusion pressure: influence on neurological deterioration and outcome in severe head injury. The Executive Committee of the International Selfotel Trial. J Neurosurg 2000; 92:1–6 [DOI] [PubMed] [Google Scholar]
- 26.Robertson CS, Valadka AB, Hannay J, et al. : Prevention of secondary ischemic insults after severe head injury. Crit Care Med 1999; 27:2086–2095 [DOI] [PubMed] [Google Scholar]
- 27.Godoy DA, Núñez-Patiño RA, Zorrilla-Vaca A, et al. : Intracranial hypertension after spontaneous intracerebral hemorrhage: a systematic review and meta-analysis of prevalence and mortality rate. Neurocrit Care. Epub 2018 Dec 18. [DOI] [PubMed] [Google Scholar]
- 28.Sykora M, Steinmacher S, Steiner T, et al. : Association of intracranial pressure with outcome in comatose patients with intracerebral hemorrhage. J Neurol Sci 2014; 342:141–145 [DOI] [PubMed] [Google Scholar]
- 29.Kamel H, Hemphill JC: Characteristics and sequelae of intracranial hypertension after intracerebral hemorrhage. Neurocrit Care 2012; 17:172–176 [DOI] [PubMed] [Google Scholar]
- 30.Ko SB, Choi HA, Parikh G, et al. : Multimodality monitoring for cerebral perfusion pressure optimization in comatose patients with intracerebral hemorrhage. Stroke 2011; 42:3087–3092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Güiza F, Meyfroidt G, Piper I, et al. : Cerebral Perfusion Pressure Insults and Associations with Outcome in Adult Traumatic Brain Injury. J Neurotrauma 2017; 34:2425–2431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rao V, Klepstad P, Losvik OK, et al. : Confusion with cerebral perfusion pressure in a literature review of current guidelines and survey of clinical practice. Scand J Trauma Resusc Emerg Med 2013; 21:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rivera-Lara L, Murthy SB, Nekoovaght-Tak S, et al. : Influence of Bleeding Pattern on Ischemic Lesions After Spontaneous Hypertensive Intracerebral Hemorrhage with Intraventricular Hemorrhage. Neurocrit Care 2018; 29:180–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prabhakaran S, Naidech AM: Ischemic brain injury after intracerebral hemorrhage: A critical review. Stroke 2012; 43:2258–2263 [DOI] [PubMed] [Google Scholar]
- 35.Buletko AB, Thacker T, Cho SM, et al. : Cerebral ischemia and deterioration with lower blood pressure target in intracerebral hemorrhage. Neurology 2018; 91:e1058–e1066 [DOI] [PubMed] [Google Scholar]
- 36.Czosnyka M1, Miller C; Participants in the International Multidisciplinary Consensus Conference on Multimodality Monitoring: Monitoring of cerebral autoregulation. Neurocrit Care 2014; 21 Suppl 2:S95–102 [DOI] [PubMed] [Google Scholar]
- 37.Steiner LA, Czosnyka M, Piechnik SK, et al. : Continuous monitoring of cerebrovascular pressure reactivity allows determination of optimal cerebral perfusion pressure in patients with traumatic brain injury. Crit Care Med 2002; 30:733–738 [DOI] [PubMed] [Google Scholar]
- 38.Jaffe J, Melnychuk E, Muschelli J, et al. : Ventricular catheter location and the clearance of intraventricular hemorrhage. Neurosurgery 2012; 70:1258–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chambers IR, Banister K, Mendelow AD: Intracranial pressure within a developing intracerebral haemorrhage. Br J Neurosurg 2001; 15:140–141 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1. Table S1: ICP and CPP Variables by Treatment Group
Supplemental Digital Content 2. Table S2: Association between Use of ICP Therapies and Pressors/Inotropes by ICP/CPP Events
Supplemental Digital Content 3. Table S3: Factors Associated with Elevated Intracranial Pressure (ICP)
Supplemental Digital Content 4. Table S4: Factors Associated with Low Cerebral Perfusion Pressure (CPP) (<70 mmHg) and with High Intracranial Pressure (>20 mmHg) Plus Low CPP (<70 mmHg)
Supplemental Digital Content 5. Figure S1: Comparison of Odds Ratios at ICP Levels Over Monitoring Time (A) and CPP (B) Levels For Day 30 and 180 Mortality and Poor mRS
Supplemental Digital Content 6. Figure S2: Comparison of Receiver Operating Characteristic Curves at ICP Levels (A) and CPP (B) Levels Over Monitoring Time For Day 30 and 180 Mortality and Poor mRS


