Abstract
PURPOSE
We aimed to evaluate the effectiveness and safety of super-selective transarterial chemoembolization (TACE) with doxorubicin-loaded drug-eluting beads (DEB) sized 40–75 μm for hepatocellular carcinoma (HCC) in early and intermediate stages according to Barcelona Clinic Liver Cancer (BCLC) staging system.
METHODS
This single-center retrospective study was conducted with 45 consecutive HCC patients treated by 72 sessions of DEB-TACE during the 2012–2017 period. Thirty-seven patients (82.2%) had single tumor staged BCLC A and B (53.3% and 46.7%, respectively). All procedures were performed by super-selective approach using 1.7–2.0 F microcatheters. Cone beam CT was performed to detect all tumor-feeding arteries and assess the treatment results immediately. Dynamic MRI and laboratory tests were obtained at 1-month follow-up and every 3 months thereafter. Response to treatment according to modified Response Evaluation Criteria in Solid Tumors, demographic and clinical status, laboratory tests, time-to-event durations and rates, complications according to the National Cancer Institute Criteria for Adverse Events were evaluated.
RESULTS
A total of 45 patients (median age, 65.6 years; range, 35–88 years; 33 men, 73.4%) were included. Eight patients (17.7%) underwent liver transplantation after DEB-TACE, and 20 (44.4%) died during the follow-up period. Median follow-up was 22 months (range, 13–31), and 42 (93.3%) patients were followed up for more than 1 year. Overall complete response, partial response, and progressive disease rates were 53.3%, 33.3%, and 13.4% at one year and 22.2%, 26.7%, and 13.3% at three years, respectively. For target lesions, these rates were 60.0%, 26.7%, and 13.3% at one year and 28.9%, 6.7%, and 4.4% at three years, respectively. Median overall survival (OS) duration was 24 months (95% CI, 20.9–31.9 months). At one year and three years, OS rates were 71.0% and 44.4%, respectively. The only statistically significant relationship with OS was presence of chronic liver disease, which worsened the OS rate (P = 0.031). Time-to-progression (TTP) was 23 months (95% CI, 15.1–40.0), and progression-free survival (PFS) was 28 months (95% CI, 6.2–39.8). Post-embolization syndrome occurred in 10 patients (22.2%). Transient grade I/II bilirubin and aminotransferase elevation was observed in 26 (57.7%) and 18 (40%) patients, respectively.
CONCLUSION
Super-selective DEB-TACE with doxorubicin-loaded beads sized 40–75 μm is an effective and safe treatment method with prolonged TTP and PFS in early and intermediate stages of HCC. Presence of chronic liver disease is the only significant factor that worsened OS ratios after DEB-TACE.
Hepatocellular carcinoma (HCC), one of the most common types of cancer in the world, mostly occurs as a result of the structural changes in the liver caused by cirrhosis. Orthotopic liver transplantation and surgical resection are potential curative treatment options in the early stage according to the Barcelona Clinic Liver Cancer (BCLC) staging system (1), but most HCC patients are beyond this stage at the time of diagnosis, and transarterial chemoembolization (TACE) is the standard of care in a palliative manner in the BCLC intermediate stage. Moreover, for patients ineligible for surgical resection or percutaneous ablation, TACE is considered to be a bridging treatment in transition to liver transplantation and a first-line treatment option in the early stage as well. Recently, drug-eluting beads (DEB) loaded with doxorubicin have been introduced and widely used for TACE. A recent meta-analysis showed the superiority of DEB-TACE to conventional TACE performed with lipiodol in terms of effectiveness and safety, but not in terms of tumor response and survival (2). However, randomized controlled trial of DEB-TACE vs. conventional chemoembolization for HCC showed equal effectiveness and safety of both methods, with the only advantage of DEB-TACE being less post-procedural abdominal pain (3). Nevertheless, the size of the beads to be used is still controversial. It has been indicated in animal models that smaller beads permit deeper and more homogeneous penetration into the tumor and allow more permanent drug delivery, which generates distinct tumor necrosis (4). However, serious side effects such as hepatic failure, biliary duct injuries and pulmonary complications following TACE with small-sized beads were reported (5–7). On the other hand, a recent study with a large cohort of patients has suggested that there is no correlation between small beads and increased complication rates (8). In the past decade, a new generation of tightly calibrated DEBs with diameters of 40 μm, 75 μm, and 100 μm have been introduced. The aim of this study was to evaluate the effectiveness and safety of TACE with doxorubicin-loaded small DEBs sized 40–75 μm (TANDEM, Boston Scientific) in early and intermediate-stage HCC.
Methods
Study design and patient selection
All procedures were performed in accordance with the ethical standards of the Institutional Clinical Research Ethical Committee and with the 1964 Helsinki declaration. This study has obtained approval from Institutional Clinical Research Ethical Committee (decision No 75 from March 2, 2018).
This was a single-institution retrospective study including 45 consecutive treatment-naive HCC patients treated by 72 sessions of DEB-TACE between April 2012 and January 2017. Patients were evaluated and discussed at the multidisciplinary tumor board and were scheduled for the treatment with DEB-TACE after a signed informed consent form had been obtained from each patient.
The patients’ inclusion criteria were as follows: HCC diagnosis, histologically proven or in accordance with European Association for the Study of the Liver (EASL) guidelines (9), early or intermediate stage according to BCLC classification. Exclusion criteria were patients with any kind of previous treatment for HCC, Eastern Cognitive Oncology Group (ECOG) performance status score (10) ≥1, decompensated cirrhosis (Child-Pugh score ≥8), refractory ascites, encephalopathy, hepatorenal syndrome, creatinine >2 mg/dL or creatinine clearance <30 mL/min, severe coagulation disorder, portal or hepatic vein invasion, extrahepatic metastases, and very large arteriovenous shunts not suitable for embolization.
DEB-TACE procedure
After common femoral artery puncture under ultrasound guidance and 5 F or 6 F sheath insertion, abdominal aortography, celiac and superior mesenteric artery angiographies were obtained for anatomic evaluation and to determine the treatment strategy. Subsequent to targeted super-selective catheterization, a maximum of 3 vials of microspheres sized 40–75 μm (TANDEM, Boston Scientific) loaded with 50 mg of doxorubicin per one vial were injected according to tumor size and number of feeding arteries. For tumors less than 3 cm, 40 μm sized microspheres were used, while for tumors greater than 3 cm, at first 40 μm and then 75 μm sized microspheres were injected. Saline was not used in the mixture to prevent catheter occlusion due to larger microspheres. Slow injection of the suspension at 1 cc/min was performed in order to saturate the tumor with mixture as much as possible. After achieving intense saturation according to non-contrast cone beam computed tomography (CT) control images, the procedure was finalized. Standard medication protocol for TACE with intravenous injections of 10 mg dexamethasone, 100 mg tramadol, 50 mg ranitidine, 1 g ceftriaxone and 8 mg ondansetron was administered just before and during the procedure. After clinical and liver function tests evaluation, patients were discharged within 24 hours and scheduled for the planned follow-up.
Follow-up and response evaluation
Triphasic CT was the chosen modality for one patient due to claustrophobia, whereas all other patients were evaluated by dynamic contrast-enhanced magnetic resonance imaging (MRI) before and after DEB-TACE at one and three months and then at 3-month intervals. Clinical status and laboratory tests were obtained at the same scheduled intervals. Response assessment of the treated tumors was conducted on Modified Response Evaluation Criteria in Solid Tumors (mRECIST) (11) and, if viable tumor was detected, additional DEB-TACE procedure was made. Complete response (CR), partial response (PR), and progressive disease (PD) rates were calculated in percentages, in accordance with mRECIST criteria. Stable disease (SD) was not found in this data set. Radiological response rates were calculated as overall response, and target-based responses (the local response in the targeted tumor) were calculated separately in order to assess the treatment success specifically. Single reader with 12-year experience in abdominopelvic CT and MRI blinded to the sequence of treatment conducted the radiological response analysis.
Safety analysis
The National Cancer Institute Common Terminology Criteria for Adverse Events (CCTAE) version 5.0 (12) was utilized to assess toxicity; safety was evaluated immediately after the procedure and at every scheduled follow-up. In the first month, patients were followed up in terms of post-embolization syndrome. For this purpose, patients were questioned in concordance with CCTAE chart about abdominal pain, loss of appetite, weakness, and fever. Also, patients were investigated for albumin, total bilirubin, aspartate aminotransferase, alanine aminotransferase values and international standardized ratio within one month after the treatment.
Statistical analysis
Normally distributed continuous variables were presented as mean and standard deviation, while not normally distributed variables were presented as median (min–max). Categorical variables were expressed as numbers and percentages. The descriptive statistics were used for demographic and clinical parameters. Treatment date was chosen for survival analyses and patients with liver transplantation after DEB-TACE were censored. The results were tested with the 95% confidence interval. Time-to-event analyses (overall survival [OS], time-to-progression [TTP], and progression-free survival [PFS]) were performed using Kaplan-Meier method with log-rank test. Accepted P value for statistical significance was <0.05. Statistical analyses were performed with SPSS software version 18.0 (SPSS Inc.).
Results
Table 1 summarizes the patients’ demographic and clinical characteristics. Briefly, almost half of patients (53.3%) were hepatitis B virus infected while alcohol was the minor etiologic factor (4.4%). Thirty-three patients (60.0%) had chronic liver disease according to the clinical decision based on laboratory tests and pre-treatment CT and MRI findings such as parenchyma heterogeneity, irregular contours of the liver, shrinkage of capsule, atrophy of the right lobe, hypertrophy of the left and/or caudate lobe. Thirty-seven patients (82.2%) had single tumor staged BCLC A and B (53.3% and 46.7%, respectively). All patients were in ECOG status 0. Overall, 42 of 45 patients (93.3%) had over one year of follow-up.
Table 1.
Demographic and clinical characteristics of the study population
| Age (years), median (range) | 65.6 (35–88) |
|
| |
| Gender, n (%) | |
| Female | 12 (26.6) |
| Male | 33 (73.4) |
|
| |
| Etiology, n (%) | |
| HBV | 24 (53.3) |
| HCV | 12 (26.7) |
| Alcohol | 2 (4.4) |
| Cryptogenic | 7 (15.6) |
|
| |
| Post DEB-TACE transplantation history | 8 (17.7) |
|
| |
| BCLC stage, n (%) | |
| A | 24 (53.3) |
| B | 21 (46.7) |
|
| |
| α-fetoprotein level (IU/mL), n (%) | |
| <20 | 28 (62.2) |
| 20–400 | 12 (26.7) |
| >400 | 5 (11.1) |
|
| |
| Chronic liver disease, n (%) | |
| No | 22 (40.0) |
| Yes | 33 (60.0) |
|
| |
| Child-Pugh class, n (%) | |
| A | 29 (52.8) |
| B | 26 (47.2) |
|
| |
| Number of tumors, n (%) | |
| Single | 37 (82.2) |
| Multiple | 8 (17.8) |
|
| |
| Size of tumors (cm), n (%) | |
| <3 | 12 (4.6) |
| 3–5 | 26 (57.7) |
| >5 | 17 (37.7) |
HBV, hepatitis B virus; HCV, hepatitis C virus; DEB-TACE, transarterial chemoembolization with doxorubicin-loaded drug-eluting beads; BCLC, Barcelona Clinic Liver Cancer; ECOG, Eastern Cooperative Oncology Group.
A total of 72 sessions (average 1.6 per patient) were performed in order to achieve objective response. Most repeat TACE procedures (77.4%) were performed within the first 3 months depending on the results of control MRI studies, while in others (22.4%), procedures were performed at a later period due to development of local recurrence or new lesions. Twenty patients (44.4%) died during the follow-up period, including two patients (4.4%) who died due to liver transplantation-related complications. Eight patients (17.7%) underwent liver transplantation after DEB-TACE and were therefore censored from time-to-event analyses. The median time from DEB-TACE to liver transplantation was 12 months (range, 4–24 months). Median follow-up of remaining patients was 22 months (range, 1–49 months). Overall CR, PR, and PD rates were 53.3%, 33.3%, and 13.4% at 1 year and 22.2%, 26.7%, and 13.3% at 3 years, respectively. For target lesions, these rates were 60.0%, 26.7% and 13.3% at 1 year and 28.9%, 6.7%, and 4.4% at 3 years, respectively.
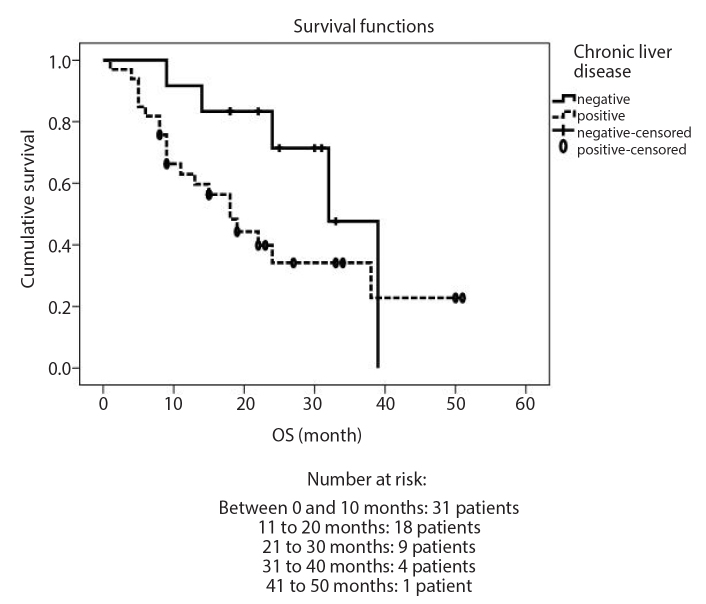
Table 2 summarizes the effects of clinical parameters on overall survival rate, and Figure shows the relationship between presence of chronic liver disease and overall survival after DEB-TACE. Median OS for all patients was 24 months (95% CI, 20.9–31.9 months). OS rates at 1 year and 3 years were 71.0% and 44.4%, respectively. The only statistically significant relationship with OS was presence of chronic liver disease, which worsened the OS rate. Median OS for patients with and without chronic liver disease was 18 months (range, 1–51 months) and 36 months (range, 9–39 months), respectively (P=0.031, hazard ratio 2.172) (Table 2, Fig.). TTP duration was 23 months (95% CI, 15.1–40.0 months) and PFS duration was 28 months (95% CI, 6.2–39.8 months) in this patient population.
Table 2.
Relationship between clinical parameters and overall survival in the study population*
| Parameters | Median (range), months | SE | P |
|---|---|---|---|
| BCLC stage | |||
| A | 24 (4–51) | 4.09 | 0.362 |
| B | 32 (5–39) | 17.24 | |
|
| |||
| Etiology | |||
| HBV | 36 (4–50) | 3.64 | 0.183 |
| HCV | 18 (5–51) | 4.49 | |
| Alcohol and cryptogenic | 14 (1–38) | 4.05 | |
|
| |||
| Chronic liver disease | |||
| Yes | 18 (1–51) | 3.05 | 0.031 |
| No | 36 (9–33) | 3.09 | |
|
| |||
| Tumor size, cm | |||
| <3 | 22 (8–50) | 9.16 | 0.748 |
| 3–5 | 24 (1–51) | 5.36 | |
| >5 | 32 (5–33) | 21.58 | |
|
| |||
| α-fetoprotein value, IU/mL | |||
| <20 | 24 (1–51) | 7.38 | 0.815 |
| 20–400 | 18 (5–38) | 1.92 | |
| >400 | 36 (6–33) | 0.000 | |
|
| |||
| Child-Pugh class | |||
| A | 19 (5–48) | 4.87 | 0.497 |
| B | 15 (1–49) | 287 | |
SE, standard error; BCLC, Barcelona Clinic Liver Cancer; HBV, hepatitis B virus; HCV, hepatitis C virus.
Kaplan-Meier survival analysis with log-rank test.
Figure.
Kaplan-Meier survival analysis with log-rank test of relationship between overall survival and chronic liver disease presence (P = 0.031).
Procedure-related mortality, major adverse events, grade III/IV toxicity or biliary injury were not observed. Also, ascites, liver abscess or non-target liver necrosis were not detected on follow-up images. Nevertheless, post-embolization syndrome (a complex of fever, nausea and/or vomiting, and pain) was encountered in 10 (23%) patients. The syndrome occurred within first 2–3 days after TACE, subsided within 1–2 weeks and was managed by medication in concordance with routine hospital practice. In addition, asymptomatic transient grade I/II (according to CCTAE classification) bilirubin and aminotransferase elevation after DEB-TACE was observed in 26 (57.7%) and 18 (40.0%) patients, respectively. These elevations were not always accompanied by post-embolization syndrome. Manifestations of the syndrome were noted in some patients only after the first treatment and in some patients only after the second one. In these patients, elevated parameters returned spontaneously to pre-intervention levels within one month.
Discussion
In the last few years, it has been indicated that DEB-TACE with smaller particles is more effective than larger ones, by allowing beads to advance more distally into the tumor-feeding arteries and to elute higher doses of the chemotherapeutic agent (13, 14). Distal embolization with smaller beads was reported in an in vivo model, resulting in more necrosis and better local response (15). Thus, theoretically, more distal embolization with smaller beads provides permanent anoxia/ischemia and tumor necrosis with better outcomes. Conversely, incomplete embolization of the tumor, which is more likely to be seen after TACE with larger beads, is a potential stimulator of neoangiogenic and antiapoptotic factors resulting in local recurrence (16).
We intended to assess the overall response and target tumor-based response to DEB-TACE performed with smaller beads, to obtain more accurate and specific data on DEB-TACE outcome. Overall and target lesion-based sum of complete and partial response rates were both 86.7% for the first year, which were superior to recently published studies (17, 18). In addition, at 3 years these rates were 35.7% and 26.7% for target lesion and overall assessment, respectively. This study had longer median follow-up duration (22 months; range, 13–31 months) compared with the previously published studies using DEB-TACE with small particles. Our study exhibited 23 months of median TTP and 28 months of median PFS, which is better than in recently published studies (18, 19). In the present study, median overall survival rate was 24 months (95% CI, 21–32 months), with 1-year and 3-year survival rates of 71% and 44.4%, respectively. All these outcomes revealed better results over a recently published prospective multicenter study (20) and mild superiority over lipiodol chemoembolization according to recently published systematic review data with a median overall survival of 19.4 months, 70.3% 1-year and 40.4% 3-year overall survival rates (21).
The primary concern regarding chemoembolization with small beads in HCC is safety, especially in terms of pulmonary embolization, tumor rupture, and biliary damage, despite the fact that it is more effective than TACE with larger beads (17). In this study, no biliary damage after procedure was recorded, however, 26 patients (57.7%) experienced transient bilirubin elevation within 1 month after procedure, and among them, 5 patients (11.1%) had elevated bilirubin level >2 mg/dL, but within the grade II toxicity criteria according to CCTAE classification. Only one patient (2.22%), who was in Child-Pugh class B7 before DEB-TACE, was diagnosed with liver decompensation after the treatment, and she is still alive 2 years after the procedure. Transient increase of transaminase levels after procedure was detected in 40% (n=18) of the patients during first month after the treatment. These changes were interpreted as grade I/II adverse events. No tumor rupture or clinically significant pulmonary side effect were detected in this study. No non-target embolization or systemic doxorubicin-related toxicity was encountered in this study. However, TACE with larger beads has revealed major complications including mortality, possibly because of non-target embolization which is unlikely with small beads (22, 23). The reason why smaller microspheres are more effective is based on the idea that these microspheres can reach to the more distal part of the arteries feeding the tumor, providing a permanent super-selective occlusion of the tumor-feeding arteries. On the other hand, large microspheres cause earlier stasis as they block the more proximal segments of the tumor-feeding arteries. On the contrary, smaller microspheres do not cause early stasis as they go further distally to the tumor-feeding arteries, and consequently drugs can be delivered directly into the target tumor. Moreover, grade III or IV adverse events were not observed in our treated patients and all patients were discharged within 24 hours after treatment. Ten patients (22.2%) experienced mild symptoms of postembolization syndrome which was easily managed by medication (antiemetic and analgesic drugs such as granisetron 2 mg/day, metoclopramide 10 mg tid, diclofenac 50 mg tid orally). The incidence of postembolization syndrome was lower compared with the other studies using larger beads, and slightly higher compared with a recently published study on DEB-TACE performed with microspheres sized 40 μm (18). In long-term follow-up, no sign of myelosuppression or alopecia was noted.
Consequently, statistically significant relations were found between presence of chronic liver failure and overall survival. No significant correlation was found between overall survival and other investigated factors. However, this could be because of the limited number of enrolled patients.
There are some limitations of the study. First, this was a retrospective study with a relatively small number of patients. Sample size was not calculated due to the retrospective design of this study. In addition, non-comparative design of the study is another significant limitation. In fact, there are few studies assessing safety and efficacy of DEB-TACE with beads smaller than 100 μm, which supports the idea of studying the use of small beads in HCC in terms of safety and efficacy. However, the number of patients in our study was comparable with other studies on this subject and the follow-up period was much longer than in previously published studies. Second, in this study 82% of the patients had only a single session of treatment, which makes it difficult to compare results from studies of patients with different clinical characteristics. Another important limitation is that radiological response analysis was made by single radiologist, though blinded to the sequence of treatment, and intraobserver variability was not evaluated.
In conclusion, TACE with drug-eluting beads sized 40–75 μm and loaded with doxorubicin is an effective and safe treatment method with long durations of TTP and PFS in early and intermediate stages of HCC. Presence of chronic liver disease was the only significant factor to worsen the overall survival ratio after DEB-TACE.
Main points.
DEB-TACE with 40–75 μm beads in hepatocellular carcinoma (HCC) patients resulted in median overall survival rate of 24 months (95% CI, 21–32 months), with 1-year and 3-year survival rates of 71.0% and 44.4% respectively. These rates were better than the survival rates reported in a recently published prospective multicenter study and mildly better than the survival rates after conventional TACE with lipiodol, according to a recently published systematic review.
Among studied demographic and clinical parameters of HCC patients, the only statistically significant relationship was found between the presence of chronic liver failure and overall survival after DEB-TACE with 40–75 μm beads.
DEB-TACE with 40–75 μm beads is an effective and safe treatment method for patients with early and intermediate stage HCC.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Llovet JM, Fuster J, Bruix J. The Barcelona approach: diagnosis, staging, and treatment of hepatocellular carcinoma. Liver Transpl. 2004;10(Suppl 1):S115–120. doi: 10.1002/lt.20034. [DOI] [PubMed] [Google Scholar]
- 2.Zou JH, Zhang L, Ren ZG, Ye SL. Efficacy and safety of cTACE versus DEB-TACE in patients with hepatocellular carcinoma: a meta-analysis. J Dig Dis. 2016;17:510–517. doi: 10.1111/1751-2980.12380. [DOI] [PubMed] [Google Scholar]
- 3.Golfieri R, Giampalma E, Renzulli M, et al. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br J Cancer. 2014;111:255–264. doi: 10.1038/bjc.2014.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dreher MR, Sharma KV, Woods DL, et al. Radiopaque drug-eluting beads for transcatheter embolotherapy: experimental study of drug penetration and coverage in swine. J Vasc Interv Radiol. 2012;23:257–264. doi: 10.1016/j.jvir.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malagari K, Pomoni M, Moschouris H, et al. Chemoembolization of hepatocellular carcinoma with HepaSphere 30–60 μm. Safety and efficacy study. Cardiovasc Interv Radiol. 2014;37:165–175. doi: 10.1007/s00270-013-0777-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown KT. Fatal pulmonary complications after arterial embolization with 40–120-micro m tris-acryl gelatin microspheres. J Vasc Interv Radiol. 2004;15:197–200. doi: 10.1097/01.RVI.0000109400.52762.1F. [DOI] [PubMed] [Google Scholar]
- 7.Ozcinar B, Guven K, Poyanli A, Ozden I. Necrotizing pancreatitis after transcatheter arterial chemoembolization for hepatocellular carcinoma. Diagn Interv Radiol. 2009;15:36–38. [PubMed] [Google Scholar]
- 8.Malagari K, Pomoni M, Spyridopoulos TN, et al. Safety profile of sequential transcatheter chemoembolization with DC Bead: results of 237 hepatocellular carcinoma (HCC) patients. Cardiovasc Interv Radiol. 2011;34:774–785. doi: 10.1007/s00270-010-0044-3. [DOI] [PubMed] [Google Scholar]
- 9.EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. doi: 10.1097/00000421-198212000-00014. [DOI] [PubMed] [Google Scholar]
- 11.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]
- 12. [accessed 17.02.2019]. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50.
- 13.Padia SA, Shivaram G, Bastawrous S, et al. Safety and efficacy of drug-eluting bead chemoembolization for hepatocellular carcinoma: comparison of small-versus medium-size particles. J Vasc Interv Radiol. 2013;24:301–306. doi: 10.1016/j.jvir.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 14.Prajapati HJ, Xing M, Spivey JR, et al. Survival, efficacy, and safety of small versus large doxorubicin drug-eluting beads TACE chemoembolization in patients with unresectable HCC. Am J Roentgenol. 2014;203:W706–714. doi: 10.2214/AJR.13.12308. [DOI] [PubMed] [Google Scholar]
- 15.She HL, Burgmans MC, Coenraadm M, Saraqueta AF. In vivo proof of superselective transarterial chemoembolization with 40-μm drug-eluting beads in a patient with hepatocellular carcinoma. Cardiovasc Interv Radiol. 2016;39:137–140. doi: 10.1007/s00270-015-1154-8. [DOI] [PubMed] [Google Scholar]
- 16.Bonomo G, Pedicini V, Monfardini L, et al. Bland embolization in patients with unresectable hepatocellular carcinoma using precise, tightly size-calibrated, anti-inflammatory microparticles: first clinical experience and one-year follow-up. Cardiovasc Interv Radiol. 2010;33:552–559. doi: 10.1007/s00270-009-9752-y. [DOI] [PubMed] [Google Scholar]
- 17.Spreafico C, Cascella T, Facciorusso A, et al. Transarterial chemoembolization for hepatocellular carcinoma with a new generation of beads: clinical-radiological outcomes and safety profile. Cardiovasc Interv Radiol. 2015;38:129–134. doi: 10.1007/s00270-014-0907-0. [DOI] [PubMed] [Google Scholar]
- 18.Greco G, Cascella T, Facciorusso A, et al. Transarterial chemoembolization using 40 μm drug eluting beads for hepatocellular carcinoma. World J Radiol. 2017;9:245–252. doi: 10.4329/wjr.v9.i5.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aliberti C, Carandina R, Lonardi S, et al. Transarterial chemoembolization with small drug-eluting beads in patients with hepatocellular carcinoma: experience from a cohort of 421 patients at an Italian center. J Vasc Interv Radiol. 2017;28:1495–1502. doi: 10.1016/j.jvir.2017.07.020. [DOI] [PubMed] [Google Scholar]
- 20.Richter G, Radeleff B, Stroszczynski C, et al. Safety and feasibility of chemoembolization with doxorubicin-loaded small calibrated microspheres in patients with hepatocellular carcinoma: results of the MIRACLE I prospective multicenter study. Cardiovasc Interv Radiol. 2018;41:587–593. doi: 10.1007/s00270-017-1839-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lencioni R, de Baere T, Soulen MC, et al. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: a systematic review of efficacy and safety data. Hepatology. 2016;64:106–116. doi: 10.1002/hep.28453. [DOI] [PubMed] [Google Scholar]
- 22.Burrel M, Reig M, Forner A, et al. Survival of patients with hepatocellular carcinoma treated by transarterial chemoembolisation (TACE) using drug eluting beads. Implications for clinical practice and trial design. J Hepatol. 2012;56:1330–1335. doi: 10.1016/j.jhep.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 23.Malagari K, Pomoni M, Moschouris H, et al. Chemoembolization with doxorubicin-eluting beads for unresectable hepatocellular carcinoma: five-year survival analysis. Cardiovasc Interv Radiol. 2012;35:1119–1128. doi: 10.1007/s00270-012-0394-0. [DOI] [PubMed] [Google Scholar]