Abstract
PURPOSE
We aimed to determine the relationship of abnormal labyrinthine signals on heavily T2-weighted three-dimensional fluid-attenuated inversion recovery imaging (HF sequence) with hearing impairment and prognosis in patients with sudden sensorineural hearing loss (SSNHL).
METHODS
Patients with unilateral SSNHL underwent magnetic resonance imaging, including pre-contrast HF sequences and post-contrast HF sequences with a 4-hour scan delay after intravenous gadolinium injection. We measured the signal intensity ratio (SIR) of the vestibule and cochlea relative to the cerebellar medulla on post-contrast HF sequences, and analyzed the relationship of SIR with hearing impairment and prognosis.
RESULTS
Of 61 patients, 23 (37.7%) showed signal abnormalities on post-contrast HF sequences. Initial hearing loss and hearing recovery were worse in the HF+ group than in the HF− group (P < 0.05). Profound hearing loss was more common in the HF+ group (52.2% vs. 23.7%), while moderate hearing loss was more common in the HF− group (18.4% vs. 0.0%; P < 0.05 for both). The rate of partial recovery was higher in the HF− group (42.1%) than in the HF+ group (13.0%; P < 0.05). The SIRs of the vestibule and cochlea were positively correlated with the severity of hearing loss and hearing recovery, with higher SIRs indicating more severe hearing loss and poor recovery.
CONCLUSION
Labyrinthine signal abnormalities were found on post-contrast HF sequences in 37.7% of patients with SSNHL. These abnormalities were found only in patients with severe-to-profound hearing loss. Increased SIR indicated more severe hearing loss and poor prognosis.
In most patients with sudden sensorineural hearing loss (SSNHL), the underlying cause is unknown, and is possibly attributable to viral, vascular, or multiple etiologies (1). Diverse etiological causes can lead to ischemic and hypoxic changes in the cochlear cells, resulting in the breakdown of the blood-labyrinth barrier in the inner ear, which is associated with increased permeability. The increase in permeability leads to changes in the protein composition of the inner ear. These changes are manifested as an increase in signal intensity on three-dimensional fluid-attenuated inversion recovery (3D-FLAIR) sequences (henceforth referred to as FL sequences) (2). A modification of the FL sequence, namely, the heavily T2-weighted FL sequence (henceforth referred to as the HF sequence) with a 4-hour scan delay after intravenous gadolinium injection, can improve the display rate of abnormal signals in the inner ear (3, 4). In SSNHL patients, signal changes on FL sequences are correlated with greater hearing loss and worse prognosis (5, 6). The present study aimed to determine the correlation of signal-intensity changes on HF sequences with the degree of deafness and prognosis in patients with unilateral SSNHL. We evaluated the signal intensity ratio (SIR) of the vestibule and cochlea, as this parameter more objectively reflects the changes in the signal intensity of the inner ear and reduces the influence of human factors on the observer.
Methods
Patient selection
In this prospective study, we enrolled patients who were diagnosed with unilateral SSNHL in our hospital between April 2016 and May 2018. SSNHL was defined as unilateral sensorineural hearing loss of ≥30 dB over three or more contiguous audiometric frequencies that developed over a period of a few hours up to 3 days and had no identifiable cause despite adequate investigation (7). The patients were diagnosed with sudden deafness jointly by an otolaryngologist and the doctor responsible for hyperbaric oxygen therapy, on the basis of factors such as onset time, symptoms, signs, and audiological findings. Patients with middle ear disease, Meniere disease, systemic disease, or definite causes such as inner ear malformation, acoustic neuroma, and autoimmune disease, were excluded from the study. None of the patients had received intratympanic injections for treatment before the imaging. The time interval between SSNHL onset and magnetic resonance imaging (MRI) and the presence of vertigo and tinnitus were recorded. The study protocol was approved by the institutional review board of our hospital (Ethics committee approval number: 2016-科-88).
Audiological evaluation
All patients underwent physical examination, audiological assessments (including tympanic-membrane endoscopy), and speech and pure-tone audiometry. The audiometric assessments were completed immediately after hospitalization. Hearing loss was classified as follows: 26–40 dB, mild; 41–55 dB, moderate; 56–70 dB, moderately severe; 71–90 dB, severe; and >90 dB, profound. Patients were treated with oral prednisone at a dose of 40–60 mg/day, according to the patient’s body weight, for 5 consecutive days. The patients also received drugs to improve blood flow and reduce blood viscosity. Additionally, the patients underwent hyperbaric oxygen treatment. Each course of hyperbaric oxygen treatment lasted 10 days, and each patient received 2–4 such courses, depending on their general condition and response to treatment. Recovery was classified using the Siegel criteria (8): final hearing loss <25 dB, complete recovery; final hearing loss 25–45 dB with >15 dB gain, partial recovery; final hearing loss >45 dB with >15 dB gain, slight improvement; and final hearing loss >75 dB with <15 dB gain, no improvement.
Magnetic resonance imaging
MRI was conducted using a 3.0 T scanner (Magnetom Prisma, Siemens) and a 64-channel array head coil. The following sequences were performed: pre-contrast T1-weighted imaging (repetition time [TR], 700 ms; echo time [TE], 22 ms; thickness, 0.8 mm; and matrix, 256×256), whole-brain T2-weighted imaging (TR, 4000 ms; TE, 99 ms; thickness, 2.0 mm; and matrix, 256×256), MRI water imaging technique 3D-SPACE (TR, 1000 ms; TE, 125 ms; thickness, 0.8 mm; and matrix, 320×320); and pre-contrast 3D-FLAIR sequences (i.e., HF sequences) and post-contrast HF sequences taken 4 h after contrast administration (TR, 9000 ms; TE, 537 ms; inversion time, 2250 ms; thickness, 0.8 mm; matrix, 320×320; variable flip angle echo train; average flip angle, 1100° for turbo spin echo refocusing echo train; echo train length, 96; and scan time, 7 min 41 s). A standard 0.2 mL/kg dose of gadolinium diethylenetriamine pentaacetic acid (Gd-DTPA) was intravenously injected at a rate of 2.5 mL/s for all patients.
Two radiologists who were familiar with inner ear MRI and had more than 10 years of diagnostic experience each assessed the signal abnormalities in each sequence of the inner ear. Any disagreements were resolved through discussion. The vestibule, cochlea, and semicircular canals were examined visually. The procedure for the judgment of abnormalities in the inner ear signal was as follows (9). The signal intensity of the unaffected ears was taken as the reference standard. The normal inner ear has a very low signal on MRI. When the signal of the affected ears was very low or the same as that of the unaffected ears on post-contrast HF sequences images, that is, when there was no abnormal signal in the vestibule, cochlea, and semicircular canals, the ear was said to be HF-signal negative (HF−). The affected ear was said to be HF signal-positive (HF+) when its signal was higher than that of the unaffected ear; in other words, one of the three inner ear structures had an abnormal signal. Regions of interest (ROIs) were drawn according to the method described by Kim et al. (4). For each patient, bilateral ROIs were placed on the vestibule (1 mm2), cochlea (1 mm2), and cerebellar medulla (1 cm2) on post-contrast HF images. Every ROI was examined twice for each patient, and the average values were taken. The signal intensities of the vestibule and cochlea were measured on post-contrast HF sequences images, and ROIs with an area of 1 mm2 were manually placed in the basal turn of the cochlea and the vestibule. The signal intensity of the cerebellar medulla at the same level was also measured within an area of 1 cm2 (Fig. 1). The SIRs of the vestibule and cochlea were calculated as follows: SIR of vestibule or cochlea = vestibular or cochlear signal intensity/cerebellar medulla signal intensity.
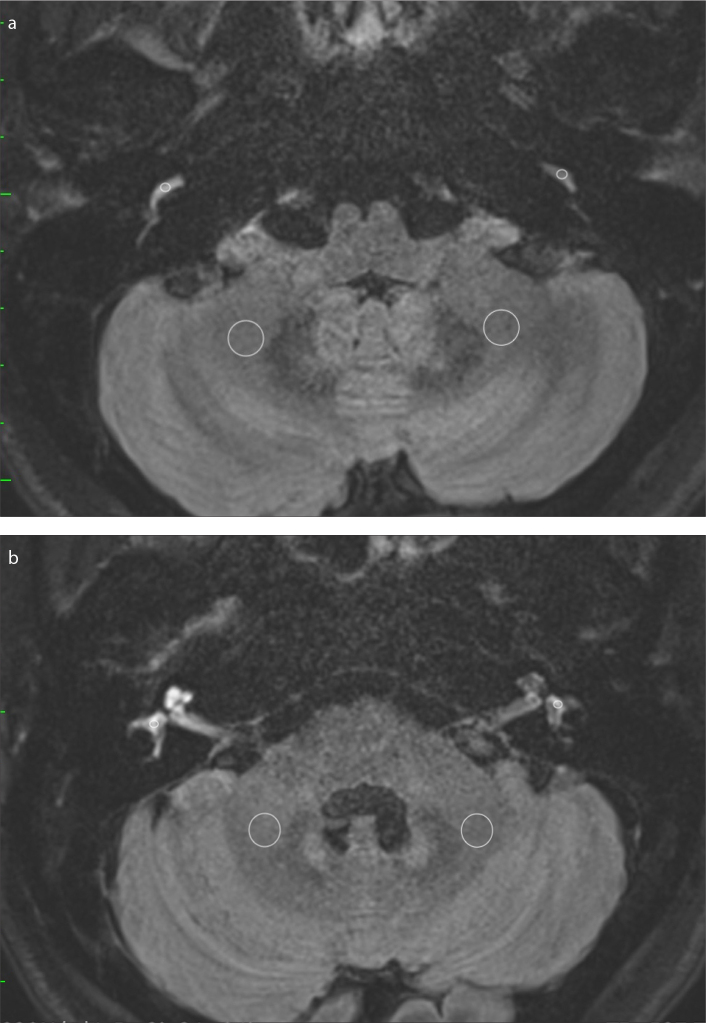
Figure 1. a, b.
Example of the ROIs on post-contrast HF sequences with a 4-hour scan delay after intravenous gadolinium injection. Image (a) shows the ROIs placed bilaterally to measure the signal intensity of the basal turn of the cochlea and the cerebellar medulla. Image (b) shows the ROIs placed bilaterally to measure the signal intensity of the vestibule and cerebellar medulla.
Statistical analysis
Data were analyzed using SPSS for Windows (v22.0; SPSS Inc.). Normal distribution of data was evaluated using the Kolmogorov-Smirnov test. The Fisher exact test or the chi-square test was used to compare categorical variables, namely, prognosis and the severity of the initial hearing loss, between the HF− and HF+ groups. The correlation of the SIR with prognosis and the severity of the initial hearing loss was analyzed using Spearman rank order correlation. A P value of less than 0.05 was used to infer statistical significance.
Results
A total of 61 patients, including 25 men and 36 women, were enrolled in this study. Their mean age was 49.8±12.3 years (range, 19–77 years). The average interval between SSNHL onset and MRI scanning was 16 days (range, 3–60 days). Of the 61 patients with SSNHL, 23 patients (37.7%) showed high signal intensity in the labyrinth of the affected inner ear (HF+ group; Fig. 2). In the remaining patients, the affected ear was HF-signal negative (HF− group). Of the 23 patients in the HF+ group, all 23 (100%) had high signals in the cochlea, 22 (95.7%) had high signals in the vestibule, 11 (47.8%) had high signals in the posterior semicircular canal (PSCC), 5 (21.7%) had high signals in the lateral semicircular canal, and 2 (0.1%) had high signals in the superior semicircular canal. In addition, a total of 8 patients (13.1%) in the overall study cohort showed high signal intensity in the affected inner ear on T1-weighted images (Fig. 3). Of these 8 patients, 5 (62.5%) showed high signal intensity in PSCC on T1-weighted images; the remaining 3 showed high signal intensity in the cochlea (n=1), vestibule (n=1), and both the cochlea and vestibule (n=1). Age, sex, associated symptoms, and the average time between symptom onset and MRI examination did not significantly differ between the two groups (Table 1). None of the patients showed high signal intensity in the unaffected inner ear.
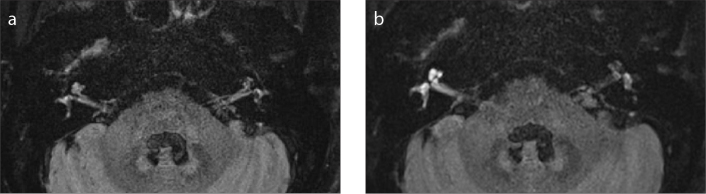
Figure 2. a, b.
Axial MRI with the HF sequence in a 60-year-old female patient with sudden sensorineural hearing loss in the right ear. Pre-contrast image (a) shows that the signals of the right vestibule and cochlea are slightly higher than those of the left vestibule and cochlea. Enhanced imaging with a 4-hour scan delay (b) shows bright signals in the right cochlea and vestibule, which are significantly higher than those in the left cochlea and vestibule.
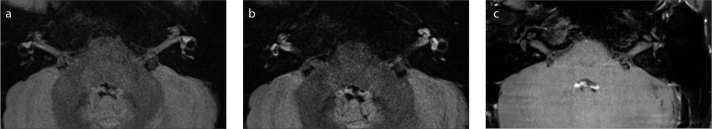
Figure 3. a–c.
Axial MRI with the HF sequence in a 17-year-old female patient with sudden sensorineural hearing loss in the left ear. Pre-contrast image (a) shows that the signals of the left vestibule and cochlea are not significantly higher than those of the right vestibule and cochlea. Enhanced imaging with a 4-hour scan delay (b) shows bright signals in the left cochlea and vestibule that are significantly higher than those in the right cochlea and vestibule. Pre-contrast T1-weighted image (c) shows bilaterally equal signals in the inner ears and punctate high signals (white arrow) in the left vestibule.
Table 1.
Characteristics of patients with SSNHL
| All patients (n=61) | HF+ group (n=23), n (%) | HF− group (n=38), n (%) | P | |
|---|---|---|---|---|
| Mean age (years) | 49.8 | 51.9 | 48.5 | 0.22 |
|
| ||||
| Patients | 61 | 23 (37.7) | 38 (62.3) | 0.43 |
| Male patients | 25 | 11 (44) | 14 (56) | |
| Female patients | 36 | 12 (33.3) | 24 (66.7) | |
|
| ||||
| Affected ear | 0.18 | |||
| Left | 35 | 16 (45.7) | 19 (54.3) | |
| Right | 26 | 7 (26.9) | 19 (73.1) | |
|
| ||||
| Associated symptoms | ||||
| Vertigo | 15 | 6 (40) | 9 (60) | 1.00 |
| Tinnitus | 56 | 21 (37.5) | 35 (62.5) | 1.00 |
|
| ||||
| Onset-to-MRI (days) | 16 | 14 | 18 | 0.34 |
|
| ||||
| T1WI+ | 8 | 8 (100) | 0 (0) | 0.001 |
|
| ||||
| Severity of initial hearing loss | ||||
| Mild | 5 | 0 (0) | 5 (100) | |
| Moderate | 7 | 0 (0) | 7 (100) | |
| Moderately severe | 4 | 0 (0) | 4 (100) | |
| Severe | 24 | 11 (45.8) | 13 (54.2) | |
| Profound | 21 | 12 (57.1) | 9 (42.9) | 0.003 |
|
| ||||
| Improvement by Siegel criteria | ||||
| No improvement | 12 | 7 (58.3) | 5 (41.7) | |
| Slight improvement | 29 | 13 (44.8) | 16 (55.2) | |
| Partial recovery | 19 | 3 (15.8) | 16 (84.2) | 0.050 |
| Complete recovery | 1 | 0 (0) | 1 (100) | |
SSNHL, sudden sensorineural hearing loss; HF+, signal changes on heavily T2-weighted three-dimensional fluid-attenuated inversion recovery sequences with 4-hour scan delay; HF−, no signal changes; MRI, magnetic resonance imaging; T1WI+, signal changes on T1-weighted imaging.
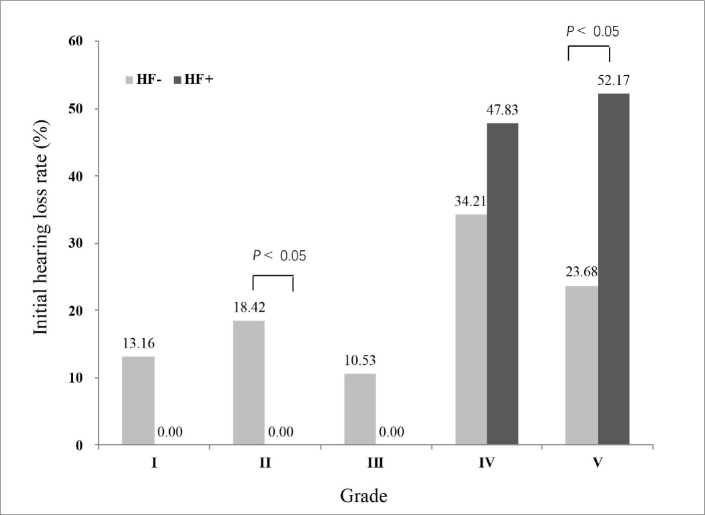
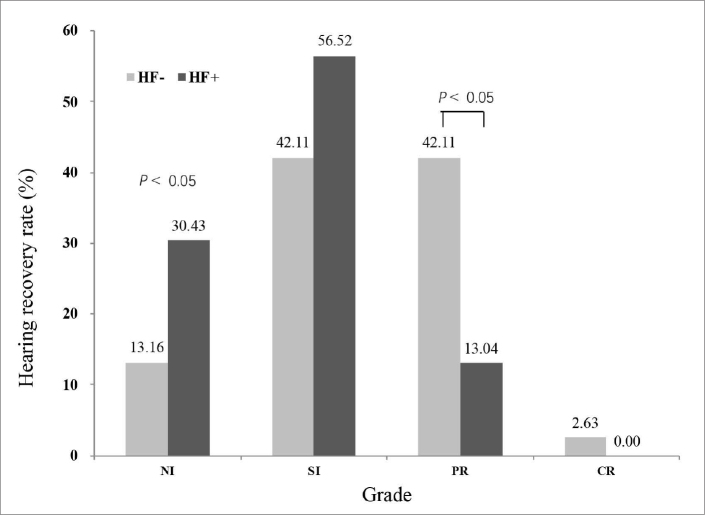
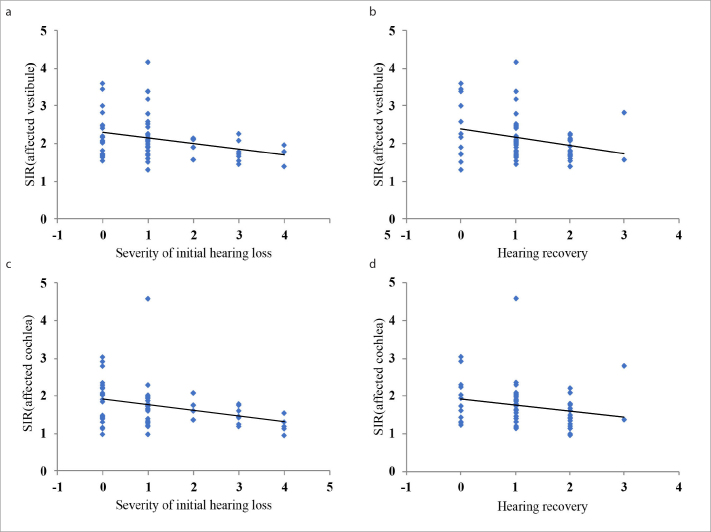
The initial hearing level was significantly worse in the HF+ group than in the HF− group (P < 0.005; Fig. 4). The rate of profound hearing loss was significantly higher in the HF+ group (52.2%) than in the HF− group (23.7%; P < 0.05). Additionally, the rate of moderate hearing loss was significantly lower in the HF+ group (0.0%) than in the HF− group (18.4%; P < 0.05). Recovery of hearing was significantly worse in the HF+ group than in the HF− group (P < 0.05; Fig. 5). The rate of partial recovery was significantly lower in the HF+ group (13.0%) than in the HF− group (42.1%; P < 0.05). The mean SIR value was significantly higher in the vestibule than in the cochlea (P < 0.001) in both the affected and unaffected ear (Table 2). The SIRs of the vestibule and cochlea of the affected ears were positively correlated with the degree of initial hearing loss and the improvement in hearing (P < 0.05), with increased SIR being associated with higher initial hearing loss and poorer improvement (Table 3, Fig. 6).
Figure 4.
Severity of initial hearing loss in the HF+ and HF− groups. I = mild, II = moderate, III = moderately severe, IV = severe, and V = profound hearing loss.
Figure 5.
Hearing recovery in the HF+ group and HF− group. NI, no improvement; SI, slight improvement; PR, partial recovery; CR, complete recovery.
Table 2.
Mean, maximum, minimum SIR values in the cochlea and vestibule of the affected ears and unaffected ears in patients with SSNHL
| Cochlea | Vestibule | P | |
|---|---|---|---|
| Affected | 1.74 (4.58–0.96) | 2.13 (4.18–1.31) | <0.001 |
| Unaffected | 1.41 (2.35–0.76) | 1.76 (3.34–1.20) | <0.001 |
All SIRs were measured using post-contrast HF sequences.
SIR, signal intensity ratio; SSNHL, sudden sensorineural hearing loss; HF, heavily T2-weighted three-dimensional fluid-attenuated inversion recovery.
Table 3.
Correlation of SIR with severity of initial hearing loss and hearing recovery in patients with SSNHL
| Severity of initial hearing loss | Hearing recovery | |||
|---|---|---|---|---|
|
|
|
|||
| r | P | R | P | |
| Affected vestibule | 0.277a | 0.031 | 0.282a | 0.027 |
|
| ||||
| Affected cochlea | 0.339b | 0.007 | 0.272a | 0.034 |
|
| ||||
| Unaffected vestibule | 0.223 | 0.084 | 0.107 | 0.41 |
|
| ||||
| Unaffected cochlea | 0.091 | 0.48 | 0.225 | 0.081 |
All SIRs were measured using post-contrast HF sequences.
SIR, signal intensity ratio; SSNHL, sudden sensorineural hearing loss; r, correlation coefficient; HF, heavily T2-weighted three-dimensional fluid-attenuated inversion recovery.
P < 0.05,
P < 0.01.
Figure 6. a–d.
Correlation of SIR in the vestibule and cochlea on the affected side with severity of initial hearing loss and hearing recovery in patients with sudden sensorineural hearing loss.
Discussion
Previous studies have reported that in patients with sudden deafness, the inner ear labyrinth shows an abnormally high signal intensity on FL sequences (2). This abnormal signal indicates the destruction of the blood-labyrinth barrier and increased permeability in the inner ear. This radiological sign is often present in patients with severe deafness and indicates a poor prognosis (10–13). Studies have found that the HF sequence is more sensitive than the FL sequence for detecting deafness (3, 14). Kim et al. (4) found that enhanced FLAIR imaging of the inner ear with a 4-hour scan delay was more sensitive than plain scanning or scanning with a 10 min delay. Therefore, we employed a scan delay of 4 hours in this study. With these scanning parameters, we found that 23 patients (37.7%) had an abnormally high signal intensity in the inner ear on HF sequences. This rate of HF-positive scans is higher than the rates (11%–35%) reported in previous studies (3, 11, 15). The reason may be that we used a sensitive HF sequence and selected different time points for scanning. In addition, our study included patients who were hospitalized for severe hearing loss requiring hyperbaric oxygen therapy.
Weissman et al. (16) believed that high signal in the inner ear on T1-weighted images was due to an increase in vascular permeability and destruction of the blood-labyrinth barrier, which led to early hemorrhage and high protein concentration. Because hemorrhage may be one of the causes of high signal intensity on TI-weighted images, the careful use of antifibrinogens or anticoagulants is necessary to treat patients with this MRI finding. Inner ear hemorrhage is often accompanied by vestibular dysfunction and severe hearing impairment (17, 18), and it has a worse prognosis than signal changes not associated with inner ear hemorrhage (19, 20). Inner ear hemorrhage can lead to changes in the composition of the endolymphatic fluid in the membranous labyrinth, resulting in irreversible damage to the hair cells in the semicircular canals, utricle, and saccule; moreover, the therapeutic effect of glucocorticoids on such damage is limited (21). Hence, inner ear hemorrhage should be diagnosed and treated as early as possible. In our study, 8 patients (13.1%) had high signal intensity on T1-weighted images as well as post-contrast HF sequences. However, high-signal abnormalities on post-contrast HF sequences are not necessarily associated with similar changes on T1-weighted images. These patients were considered to have idiopathic hearing loss, as they had no history of primary ear diseases, autoimmune diseases, coagulation disorders, or anticoagulant use. All 8 patients also had severe-to-profound hearing loss. In the HF+ group, 30.4% patients (7/23) showed no improvement, while among the patients with signal changes on T1-weighted images, 50% patients (4/8) showed no improvement.
After inner ear hemorrhage, macromolecular substances such as red blood cell debris can settle into the endolymph of the semicircular canals in the membranous labyrinth, causing hearing loss and vertigo (20). The PSCC is the most gravity-dependent canal in both the supine and upright positions. Some studies have reported that the PSCC is the most commonly involved canal in patients with concurrent benign paroxysmal positional vertigo and SSNHL (22, 23). In our study, the high signal intensity observed on T1-weighted images was located in the PSCC in 5 of the 8 patients (62.5%). This suggests that on T1-weighted images, the PSCC is the most common location of abnormally high signal intensity in patients with SSNHL. In our study, abnormal high signals in the inner ear were mainly found in the cochlea (23/23, 100%) and vestibule (22/23, 95.7%). Only 11 patients (47.8%) had abnormal signals in the semicircular canals, with the PSCC (11/23, 47.8%) being the most frequently involved semicircular canal. Compared with abnormal high signal intensity limited to the cochlea or vestibule, extensive abnormal high signal changes in the cochlea, vestibule, and semicircular canals indicate more severe hearing impairment and poor prognosis due to extensive injury to the inner ear (10).
Abnormal high signal intensity in the affected ear on post-contrast HF sequences suggests that the degree of damage to the blood-labyrinth barrier is different from that in the unaffected ear (24). Recent studies have shown that the initial hearing loss was worse in the FL+ group than in the FL− group (15, 24, 25). In our study also, significant differences were present in the initial hearing loss between the HF+ and HF− groups (P < 0.005). Previous studies have reported that among patients with SSNHL, the incidence of abnormal signals on FL sequences is significantly higher in those with severe-to-profound deafness than in those with mild-to-moderate deafness (15, 26). One possible explanation for this is that severe-to-profound deafness is associated with more severe inner ear injury than mild-to-moderate deafness (15, 26). Our results showed that the HF+ group consisted of patients with severe-to-profound hearing loss, exclusively. Moreover, the proportion of patients with profound hearing loss was significantly higher in the HF+ group (52.2%) than in the HF− group (23.7%), while the proportion of patients with moderate hearing loss was significantly lower in the HF+ group (0%) than in the HF− group (18.4%).
The detection of abnormal changes in the MRI signal in the inner ear, which is a tiny anatomical structure, is highly operator dependent, and diagnostic experience is needed for accurate interpretation of these changes. Our measurements showed significant differences in SIR on post-contrast HF sequences between the affected ears and unaffected ears and between the HF+ and HF− groups (P < 0.05). In addition, significant differences in SIR were observed between the cochlea and vestibule in both the affected and unaffected ears (P < 0.001). The mean SIR value was higher in the vestibule than in the cochlea and higher on the affected side than on the unaffected side, a difference that could not be perceived by the naked eye. Only when the signal intensity reaches a certain level can the naked eye sense this change. In contrast, SIR can relatively objectively record the various degrees of signal intensity changes in the affected and unaffected ear without the influence of the observer. Quantitative analysis of this parameter showed that the SIRs of the vestibule and cochlea of the affected ears were positively correlated with the degree of initial hearing loss in all patients with SSNHL, both in the HF− and HF+ groups (P < 0.05), with increased SIR being associated with greater hearing loss. No such correlation was observed for the unaffected ears.
Most studies show that abnormal signals in the inner ear labyrinth on HF sequences are associated with poor prognosis (5, 6, 27). Lee et al. (15) reported that among patients with SSNHL, those with severe or profound hearing loss frequently exhibited abnormalities on post-contrast HF sequences, which were significantly associated with a poor prognosis; in contrast, few abnormal signals were detected in patients with mild-to-moderate hearing loss, and in these patients, no significant correlation was observed between abnormal signals and prognosis. In this study, patient prognosis significantly differed between the HF+ and HF− groups (P < 0.05). Partial recovery was seen in only 13% of patients in the HF+ group and 42.1% of patients in the HF− group. Tagaya et al. (28) found that the ratio of the signal intensities of the inner ear and the cerebellar hemisphere may be a prognostic indicator for evaluating the disruption of the blood-labyrinth barrier in patients with sudden deafness. Increased signal intensity caused by increased permeability suggests an increase in inner ear damage; the repair of damaged hair cells and improvement of hearing takes a long time, and some severe damage may be irreversible. Quantitative analysis of the SIR showed that the SIRs of the vestibule and cochlea were positively correlated with the improvement in hearing in all patients with SSNHL (P < 0.05).
The limitation of this study is that the research population was relatively specific; all participants were serious hospitalized patients. The evaluation of more patients with mild-to-moderate hearing loss is required. Signal characteristics are closely related to time interval between symptom onset and MRI (25). The time from onset to hospitalization widely varied among our cohort, from a few days to several weeks. Although the high signal will exist for a long time, the signal intensity will decrease with time, which will affect the evaluation. Patients with Meniere disease and sudden deafness have similar clinical symptoms, such as hearing loss, dizziness, and tinnitus, and both show increased signals on HF sequences, but the magnitude of this increase is different (29). A single HF sequence cannot be used to make an etiological diagnosis in patients presenting with sudden deafness. In addition, we did not perform long-term follow-up and chronicle the degree of hearing improvement in each patient.
In conclusion, abnormally high signal intensity in the inner ear labyrinth on post-contrast HF sequences was found in 37.7% of patients with SSNHL. This high signal intensity was only found in patients with severe-to-profound initial hearing loss. Increase in the SIR of the affected ears on post-contrast HF sequences was correlated with severe initial hearing loss and poor prognosis.
Main points.
Using heavily T2-weighted 3D-FLAIR (HF sequence), the initial hearing level was significantly worse in the HF+ group than in the HF− group. Recovery of hearing was significantly worse in the HF+ group than in the HF− group.
The signal intensity ratio (SIR) of the vestibule and cochlea of the affected ears were positively correlated with the degree of initial hearing loss and the improvement in hearing, with increased SIR being associated with higher initial hearing loss and poorer improvement.
High signal intensity on T1-weighted images is accompanied by high signal changes on HF sequences. The posterior semicircular canal is the most common location of abnormally high signal intensity in patients with sudden sensorineural hearing loss. This radiological sign indicates more severe hearing impairment and poor prognosis.
This paper builds up on the previous literature and research using the best HF scanning sequence and delay time (4 hours) to improve the image display and lesion detection. In addition, the measurement of SIR is not influenced by the individual factors of the diagnostician. It is more objective than the simple naked eye observation.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Rauch SD. Clinical practice. Idiopathic sudden sensorineural hearing loss. N Engl J Med. 2008;359:833–840. doi: 10.1056/NEJMcp0802129. [DOI] [PubMed] [Google Scholar]
- 2.Sugiura M, Naganawa S, Teranishi M, Nakashima T. Three-dimensional fluid-attenuated inversion recovery magnetic resonance imaging findings in patients with sudden sensorineural hearing loss. Laryngoscope. 2006;116:1451–1454. doi: 10.1097/01.mlg.0000228005.78187.23. [DOI] [PubMed] [Google Scholar]
- 3.Naganawa S, Kawai H, Taoka T, et al. Heavily T(2)-weighted 3D-FLAIR improves the detection of cochlear lymph fluid signal abnormalities in patients with sudden sensorineural hearing loss. Magn Reson Med Sci. 2016;15:203–211. doi: 10.2463/mrms.mp.2015-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim TY, Park DW, Lee YJ, et al. Comparison of inner ear contrast enhancement among patients with unilateral inner ear symptoms in MR images obtained 10 minutes and 4 hours after gadolinium injection. AJNR Am J Neuroradiol. 2015;36:2367–2372. doi: 10.3174/ajnr.A4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshida T, Sugiura M, Naganawa S, Teranishi M, Nakata S, Nakashima T. Three-dimensional fluid-attenuated inversion recovery magnetic resonance imaging findings and prognosis in sudden sensorineural hearing loss. Laryngoscope. 2008;118:1433–1437. doi: 10.1097/MLG.0b013e318172ef85. [DOI] [PubMed] [Google Scholar]
- 6.Lee HY, Jung SY, Park MS, Yeo SG, Lee SY, Lee SK. Feasibility of three-dimensional fluid-attenuated inversion recovery magnetic resonance imaging as a prognostic factor in patients with sudden hearing loss. Eur Arch Otorhinolaryngol. 2012;269:1885–1891. doi: 10.1007/s00405-011-1834-1. [DOI] [PubMed] [Google Scholar]
- 7.Stachler RJ, Chandrasekhar SS, Archer SM, et al. Clinical practice guideline: sudden hearing loss. Otolaryngol Head Neck Surg. 2012;146:S1–35. doi: 10.1177/0194599812436449. [DOI] [PubMed] [Google Scholar]
- 8.Siegel LG. The treatment of idiopathic sudden sensorineural hearing loss. Otolaryngol Clin North Am. 1975;8:467–473. [PubMed] [Google Scholar]
- 9.Zhu H, Ou Y, Fu J, Zhang Y, Xiong H, Xu Y. A comparison of inner ear imaging features at different time points of sudden sensorineural hearing loss with three-dimensional fluid-attenuated inversion recovery magnetic resonance imaging. Eur Arch Otorhinolaryngol. 2015;272:2659–2665. doi: 10.1007/s00405-014-3187-z. [DOI] [PubMed] [Google Scholar]
- 10.Ryu IS, Yoon TH, Ahn JH, et al. Three-dimensional fluid-attenuated inversion recovery magnetic resonance imaging in sudden sensorineural hearing loss: correlations with audiologic and vestibular testing. Otol Neurotol. 2011;32:1205–1209. doi: 10.1097/MAO.0b013e31822e969f. [DOI] [PubMed] [Google Scholar]
- 11.Kuhn M, Heman-Ackah SE, Shaikh JA, Roehm PC. Sudden sensorineural hearing loss: A review of diagnosis, treatment, and prognosis. Trends Amplif. 2011;15:91–105. doi: 10.1177/1084713811408349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao Z, Chi FL. The clinical value of three-dimensional fluid-attenuated inversion recovery magnetic resonance imaging in patients with idiopathic sudden sensorineural hearing loss: a meta-analysis. Otol Neurotol. 2014;35:1730–1735. doi: 10.1097/MAO.0000000000000611. [DOI] [PubMed] [Google Scholar]
- 13.Byun H, Chung JH, Lee SH, et al. The clinical value of 4-hour delayed-enhanced 3D-FLAIR MR images in sudden hearing loss. Clin Otolaryngol. 2019;44:336–342. doi: 10.1111/coa.13305. [DOI] [PubMed] [Google Scholar]
- 14.Shinohara S, Yamamoto E, Saiwai S, et al. Clinical features of sudden hearing loss associated with a high signal in the labyrinth on unenhanced T1-weighted magnetic resonance imaging. Eur Arch Otorhinolaryngol. 2000;257:480–484. doi: 10.1007/s004050000236. [DOI] [PubMed] [Google Scholar]
- 15.Lee JI, Yoon RG, Lee JH, et al. Prognostic value of labyrinthine 3D-FLAIR abnormalities in idiopathic sudden sensorineural hearing loss. AJNR Am J Neuroradiol. 2016;37:2317–2322. doi: 10.3174/ajnr.A4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weissman JL, Curtin HD, Hirsch BE, Hirsch WL., Jr High signal from the otic labyrinth on unenhanced magnetic resonance imaging. AJNR Am J Neuroradiol. 1992;13:1183–1187. [PMC free article] [PubMed] [Google Scholar]
- 17.Wu X, Chen K, Sun L, Yang Z, Zhu Y, Jiang H. Magnetic resonance imaging-detected inner ear hemorrhage as a potential cause of sudden sensorineural hearing loss. Am J Otolaryngol. 2014;35:318–323. doi: 10.1016/j.amjoto.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Cho J, Cheon H, Park JH, et al. Sudden sensorineural hearing loss associated with inner ear lesions detected by magnetic resonance imaging. PLoS One. 2017;12:e0186038. doi: 10.1371/journal.pone.0186038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JW, Park YA, Park SM, et al. Clinical features and prognosis of sudden sensorineural hearing loss secondary to intralabyrinthine hemorrhage. J Audiol Otol. 2016;20:31–35. doi: 10.7874/jao.2016.20.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei F, Wen L, Chen K, et al. Different prognoses in patients with profound sudden sensorineural hearing loss. Acta Otolaryngol. 2019;139:598–603. doi: 10.1080/00016489.2019.1605195. [DOI] [PubMed] [Google Scholar]
- 21.Tsounis M, Psillas G, Tsalighopoulos M, et al. Systemic, intratympanic and combined administration of steroids for sudden hearing loss. A prospective randomized multicenter trial. Eur Arch Otorhinolaryngol. 2018;275:103–110. doi: 10.1007/s00405-017-4803-5. [DOI] [PubMed] [Google Scholar]
- 22.Kim MB, Ban JH. Benign paroxysmal positional vertigo accompanied by sudden sensorineural hearing loss: a comparative study with idiopathic benign paroxysmal positional vertigo. Laryngoscope. 2012;122:2832–2836. doi: 10.1002/lary.23607. [DOI] [PubMed] [Google Scholar]
- 23.Kim CH, Shin JE, Park HJ, Koo JW, Lee JH. Concurrent posterior semicircular canal benign paroxysmal positional vertigo in patients with ipsilateral sudden sensorineural hearing loss: is it caused by otolith particles? Med Hypotheses. 2014;82:424–427. doi: 10.1001/jamaoto.2013.2659. [DOI] [PubMed] [Google Scholar]
- 24.Berrettini S, Seccia V, Fortunato S, et al. Analysis of the 3-dimensional fluid-attenuated inversion-recovery (3D-FLAIR) sequence in idiopathic sudden sensorineural hearing loss. JAMA Otolaryngol Head Neck Surg. 2013;139:456–464. doi: 10.1001/jamaoto.2013.2659. [DOI] [PubMed] [Google Scholar]
- 25.Wang J, Ren T, Sun W, et al. Post-contrast 3D-FLAIR in idiopathic sudden sensorineural hearing loss. Eur Arch Otorhinolaryngol. 2019;276:1291–1299. doi: 10.1007/s00405-019-05285-z. [DOI] [PubMed] [Google Scholar]
- 26.Tanigawa T, Shibata R, Tanaka H, et al. Usefulness of three-dimensional fluid-attenuated inversion recovery magnetic resonance imaging to detect inner-ear abnormalities in patients with sudden sensorineural hearing loss. J Laryngol Otol. 2015;129:11–15. doi: 10.1017/S0022215114003028. [DOI] [PubMed] [Google Scholar]
- 27.Liao WH, Wu HM, Wu HY, et al. Revisiting the relationship of three-dimensional fluid attenuation inversion recovery imaging and hearing outcomes in adults with idiopathic unilateral sudden sensorineural hearing loss. Eur J Radiol. 2016;85:2188–2194. doi: 10.1016/j.ejrad.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Tagaya M, Teranishi M, Naganawa S, et al. 3 Tesla magnetic resonance imaging obtained 4 hours after intravenous gadolinium injection in patients with sudden deafness. Acta Otolaryngol. 2010;130:665–669. doi: 10.3109/00016480903384176. [DOI] [PubMed] [Google Scholar]
- 29.Pakdaman MN, Ishiyama G, Ishiyama A, et al. Blood-labyrinth barrier permeability in Meniere disease and idiopathic sudden sensorineural hearing loss: findings on delayed postcontrast 3D-FLAIR MRI. AJNR Am J Neuroradiol. 2016;37:1903–1908. doi: 10.3174/ajnr.A4822. [DOI] [PMC free article] [PubMed] [Google Scholar]