Abstract
PURPOSE
The purpose of this study was to determine the safety and efficacy of liver-directed therapies in patients with unresectable metastatic leiomyosarcoma to the liver. Liver-directed therapies included in this study were transarterial chemoembolization with doxorubicin eluting beads (DEB-TACE), yttrium-90 (Y90) radioembolization, and percutaneous microwave ablation.
METHODS
This is a single institution retrospective study of unresectable metastatic leiomyosarcoma to the liver treated with DEB-TACE, radioembolization, or microwave ablation. DEB-TACE was performed using 70–150 or 100–300 μ doxorubicin-loaded drug-eluting LC beads. Radioembolization was performed using Y90 glass microspheres. Electronic medical records were retrospectively reviewed to evaluate clinical and biochemical toxicities, tumor response on imaging, overall survival (OS), and liver progression-free survival (PFS).
RESULTS
A total of 24 patients with metastatic leiomyosarcoma to the liver who underwent liver-directed treatment were identified (8 males, 16 females; average age, 62.8±11.4 years). Of these patients, 13 underwent DEB-TACE, 6 underwent Y90, and 5 underwent ablation. Three patients received a combination of treatments: one received Y90 followed by DEB-TACE, one received ablation followed by DEB-TACE, and one received ablation followed by Y90. Of the 24 patients, 19 received prior chemotherapy. At 3-month follow-up, grade 1 or 2 lab toxicities were found in 20 patients; 3 patients had grade 3 toxicities. A grade 3 clinical toxicity was reported in one patient. MELD score was 7.5±1.89 at baseline and 8.8±4.2 at 3 months. Median OS was 59 months (95% CI, 39.8–78.2) from diagnosis, 27 months (95% CI, 22.9–31.0) from development of liver metastasis, and 9 months (95% CI, 0–21.4) from first liver-directed treatment. Median liver PFS was 9 months (95% CI, 1.4–16.6).
CONCLUSION
Treatment with liver-directed therapies for patients with unresectable metastatic leiomyosarcoma to the liver is safe and can improve overall survival, with OS after liver-directed therapy being similar to patients who underwent surgical resection.
Leiomyosarcomas are malignant tumors originating from the smooth muscle with an incidence of 1 per 100 000. Surgical resection of the primary tumor is common practice, but recurrence rate is high (1, 2). Of the patients, 10–30% will eventually develop distant metastasis, with the most common sites being the lungs and liver (1–4). Liver metastasis is often the limiting factor for survival with reported survival no more than 15 months (5–7). Factors associated with the development of metastases include high grade histology, large size, deep location and recurrent disease (1, 4, 8). Hepatic metastasectomy can improve survival in sarcoma patients, but many patients are not surgical candidates (9–11). Treatment for these patients is limited and traditionally includes chemotherapy (10). Doxorubicin is the first-line chemotherapy for patients with unresectable metastatic leiomyosarcoma. However, chemotherapy response is low with reported median overall survival (OS) as high as 21.9 month and progression-free survival (PFS) is 6.9 months (12, 13).
Liver-directed therapies are promising alternatives to systemic chemotherapy in patients with liver-dominant metastatic disease (7). The safety and efficacy of liver-directed treatments in primary and metastatic hepatic malignancies are well documented (7, 14–20). Transarterial chemoembolization (TACE), radioembolization with Yttrium-90 (Y90) loaded particles, and heat-based ablations have been proven to improve survival of patients with primary liver malignancies like hepatocellular carcinoma and cholangiocarcinoma (14). Studies have also reported improved survival and decreased toxicity in patients with metastatic liver disease (7). However, beyond case reports, there are few studies that have examined the survival benefit of liver-directed therapies in treating leiomyosarcoma liver metastasis. The purpose of this study is to report on our experience with liver-directed treatments (TACE, Y90 radioembolization, and percutaneous microwave ablation) on patients with metastatic leiomyosarcoma to the liver.
Methods
Patients
This study was approved by the Institutional Review Board, (IRB #Pro00022620). Medical records of 24 patients (8 males, 16 females; mean age, 62.8±11.4 years) with liver dominant metastatic leiomyosarcoma who received liver-directed treatment from June 2011 to August 2018 at Moffitt Cancer Center were retrospectively reviewed and analyzed to evaluate median OS, liver PFS, radiographic response and clinical and biochemical toxicities. The liver-directed treatments included: TACE utilizing doxorubicin-loaded drug-eluting LC beads (DEB-TACE) (BTG International), radioembolization utilizing Y90 labeled glass microspheres (TheraSphere, BTG International), and microwave ablation utilizing Accu2i or Solero (Angiodynamics) microwave ablation probe. Of the 24 patients, 13 underwent DEB-TACE, 6 underwent Y90, and 5 underwent ablation. Three patients received two different liver-directed treatments; one received radioembolization followed by DEB-TACE, one received ablation followed by DEB-TACE, and one received ablation followed by radioembolization. Nineteen out of the 24 patients received prior chemotherapy. Reasons for not receiving prior chemotherapy included multiple comorbidities, small burden of extra-hepatic disease, and patient refusal. Two patients had prior hepatic metastasectomy. Patient demographics are presented in Table 1.
Table 1.
Patient demographics
| n (%) | |
|---|---|
| Age | |
| <60 years | 10 (41.7) |
| >60 years | 14 (58.3) |
|
| |
| Sex | |
| Female | 16 (66.7) |
| Male | 8 (33.3) |
|
| |
| First intervention | |
| DEB-TACE | 13 (54.2) |
| Y90 | 6 (25.0) |
| Ablation | 5 (20.8) |
|
| |
| Extrahepatic disease | |
| Yes | 15 (62.5) |
| No | 9 (37.5) |
|
| |
| Survival status | |
| Dead | 17 (70.8) |
| Alive | 7 (29.2) |
|
| |
| Prior chemotherapy | |
| Yes | 19 (79.2) |
| No | 5 (20.8) |
|
| |
| ECOG score | |
| 0 | 16 (66.7) |
| 1 | 8 (33.3) |
|
| |
| Lobar disease | |
| Bilobar | 20 (83.3) |
| Unilobar | 4 (16.7) |
|
| |
| Hepatic lesions | |
| Multiple | 20 (83.3) |
| Solitary | 4 (16.7) |
|
| |
| Primary disease site | |
| Retroperitoneum | 8 (33.3) |
| Pelvis | 5 (20.8) |
| Extremities | 5 (20.8) |
| Mesentery | 2 (8.3) |
| IVC | 2 (8.3) |
| Head/Neck | 1 (4.2) |
| Unknown | 1 (4.2) |
DEB-TACE, doxorubicin eluting bead transarterial chemoembolization; Y90, yttrium 90; ECOG, Eastern Cooperative Oncology Group; IVC, inferior vena cava.
Criteria for receiving liver-directed treatment included liver-dominant metastases, Eastern Cooperative Oncology Group (ECOG) performance status of ≤2, total serum bilirubin ≤2 mg/dL, serum creatinine ≤2 mg/dL, international normalized ratio (INR) correctable to ≤1.5, and platelet count correctable to ≥ 50 000/μL. Patients underwent microwave ablation if they had 3 or fewer liver metastasis and each was less than 3 cm in size in an ablatable location. All other patients underwent embolization, either DEB-TACE or Y90 radioembolization. The preferred embolization method was DEB-TACE. Patients received radioembolization if radioembolization was the patient’s preference. Patients who had received previous liver-directed therapy or multiple lines of chemotherapy before the liver-directed treatment were not excluded from the study. Patients underwent evaluation of baseline and follow-up liver function parameters: albumin, bilirubin, INR, alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase, as well as MELD score. In addition, patients underwent contrast-enhanced cross-sectional imaging to evaluate radiographic response. Response evaluation criteria in solid tumors (RECIST) and modified RECIST (mRECIST) criteria were used to evaluate radiographic response (21).
DEB-TACE
Embolizations were performed as selectively as possible according to the Society of Interventional Radiology quality improvement guidelines (22). When patients had multiple metastases in a single hepatic lobe, DEB-TACE was performed via the proximal right or left hepatic artery. When patients had bilobar metastases, treatment of left and right lobes was done separately, approximately 5–7 weeks apart. DEB-TACE was performed using 70–150 μm or 100–300 μm DEBs (LC Beads, BTG International) loaded with 50 mg doxorubicin per 1 mL DEB. Maximum of 2 mL DEB loaded with 100 mg doxorubicin was used per embolization session.
Y90 radioembolization
Radioembolization was performed utilizing Y90 labeled glass microspheres (TheraSphere; BTG International). When patients had bilobar metastases, treatment of the left and right lobes were done separately, approximately 5–7 weeks apart.
Ablation
All percutaneous microwave ablation procedures were performed under general anesthesia using 14-gauge saline-cooled antenna (Acculis or Solero; AngioDynamics). Ablation zones extended beyond the tumor margin by at least 5 mm.
Clinical outcome measures
Overall survival was calculated from the date of leiomyosarcoma diagnosis, from the date of liver metastasis, and from the date of first liver-directed treatment to the date of death or last encounter. Clinical and laboratory toxicities were assessed at 3-month follow-up visit after first liver-directed treatment. Clinical toxicity was based on subjective reporting by the patient. Subjective reporting included: pain, fatigue, gastrointestinal symptoms (anorexia, nausea, vomiting), or other. Laboratory toxicities were defined according to the Common Terminology Criteria for Adverse Events scoring system (version 5.0). Tumor response on computed tomography (CT) was evaluated 3 months after the liver-directed treatment utilizing RECIST and mRECIST (21).
Statistical analysis
Survival data were assessed by the Kaplan-Meier method with the last date of contact or death used for censoring. Clinico-pathological and treatment-related variables were analyzed for an association with OS from the first liver-directed treatment by univariate Cox regression models. Statistical analysis was performed with SPSS version 22 (IBM Corp.). Data are presented as mean ± standard deviation.
Results
The median follow-up period from the liver-directed treatment was 9.4 months (range, 2.5–44.9 months). At the time of the data analysis, 7 out of 24 patients (29.2%) were still alive. Median OS from leiomyosarcoma diagnosis was 59 months (95% CI, 39.8–78.2 months) (Fig. 1). Median OS from liver metastasis diagnosis was 27 months (95% CI, 22.9–31.0 months) (Fig. 2). Median OS from first liver-directed treatment was 9 months (95% CI, 0–21.4 months) (Fig. 3). Median liver PFS was 9 months (95% CI, 1.4–16.6 months) (Fig. 4).
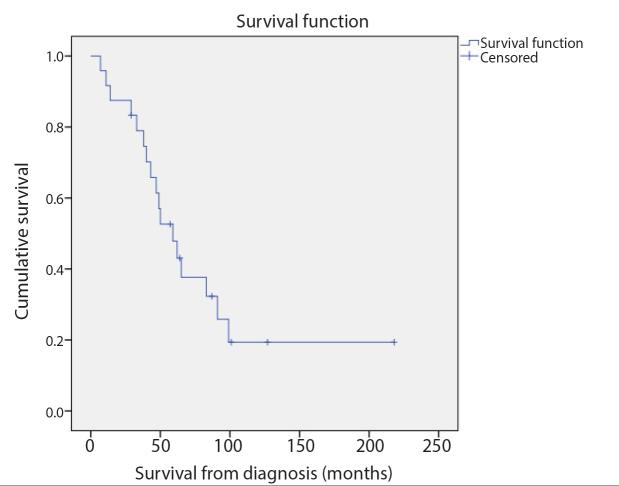
Figure 1.
Kaplan-Meier survival analysis revealed that the median OS from leiomyosarcoma diagnosis was 59 months (95% CI, 39.8–78.2 months).
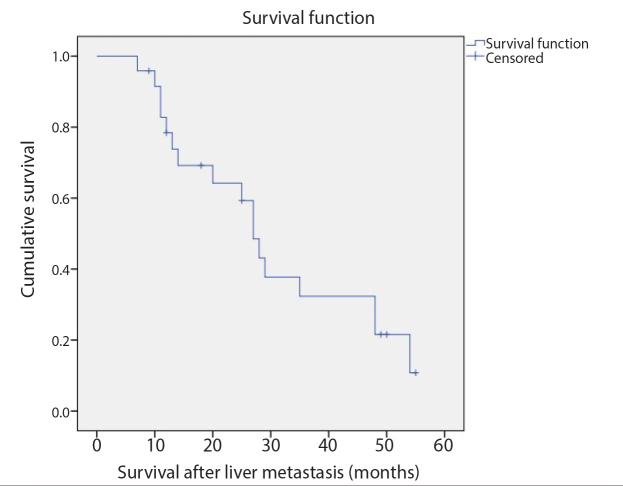
Figure 2.
Kaplan-Meier survival analysis revealed that the median OS from diagnosis of liver metastasis was 27 months (95% CI, 22.9–31.0 months).
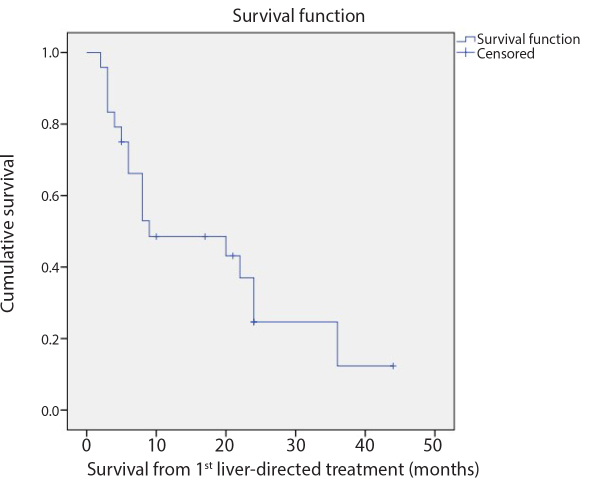
Figure 3.
The Kaplan-Meier survival analysis revealed that the median OS from the first liver-directed treatment was 9 months (95% CI, 0–21.4 months).
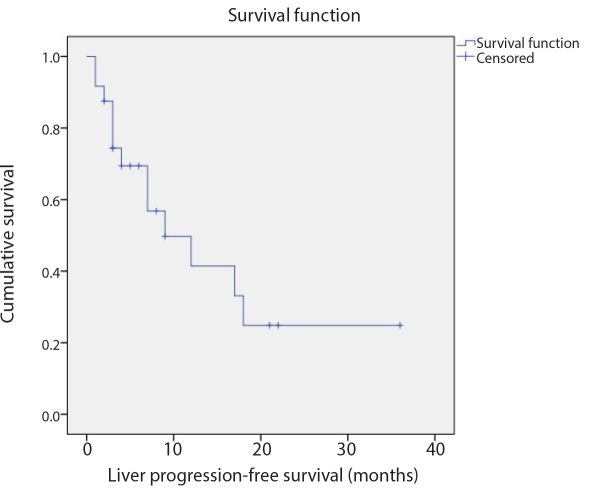
Figure 4.
The Kaplan-Meier method revealed that the median liver progression-free survival from the first liver-directed treatment was 9 months (95% CI, 1.4–16.6 months).
Patients without extrahepatic disease (n=9) had longer median OS compared with patients with extrahepatic disease (n=15) at the time of the first liver-directed treatment (22 months vs. 8 months, P = 0.763). Patients with stable or decreased MELD score at 3 months after the liver-directed treatment (n=16) had longer OS compared with patients with increased MELD score (n=8) (20 months vs. 4 months, P = 0.142). Patients who received liver-directed treatment starting ≤4 months (n=11) from liver metastasis diagnosis had a greater median survival compared with those who received liver-directed treatment >4 months (n=13) after liver metastasis diagnosis (24 months vs. 6 months, P = 0.102). Although the survival difference between the above groups are considerable, the small sample size did not allow for statistically significant results (Table 2). Among the other variables that were compared, the median OS from diagnosis and from first treatment of patients who were responders by mRECIST criteria vs non-responders was not statistically significant (Table 2). A cox-regression hazard analysis to investigate a correlation between OS and lab values at baseline and at 3 month follow-up after first liver-directed treatment was preformed and the results were not statically significant.
Table 2.
Effects of categorical variables on median overall survival
| Median OS (months) | 95% CI | P | |
|---|---|---|---|
| Extrahepatic disease | |||
| No | 22 | 4.7–39.3 | 0.763 |
| Yes | 8 | 5.2–10.8 | |
|
| |||
| MELD score | |||
| Decreased or stable | 20 | 9.3–30.8 | 0.143 |
| Increased | 4 | 0–21.4 | |
|
| |||
| Time form liver metastasis to treatment | |||
| ≤4 months | 24 | 7.5–40.5 | 0.102 |
| >4 months | 6 | 2.5–9.5 | |
|
| |||
| Imaging response by mRECIST criteria | |||
| Responders | 9 | 0.0–28.1 | 0.753 |
| Non-responders | 9 | 0.0–19.7 | |
OS, overall survival; CI, confidence interval; MELD, model for end-stage liver disease; mRECIST, modified response evaluation criteria in solid tumors.
Among 23 out of 24 patients, grade 1 or 2 clinical toxicities were reported during the first 3 months after liver-directed treatment. Most patients endorsed some degree of nausea and right upper quadrant or epigastric pain as well as fatigue. There were two reports of grade 2 acute cholecystitis and one grade 3 postembolization syndrome resulting in 6-day hospitalization following DEB-TACE. At 3-month follow-up, 21 out of 24 patients were found to have grade 1 or 2 lab toxicities; 3 patients had grade 3 toxicities due to either elevated bilirubin, ALT, or alkaline phosphatase. Average MELD score at baseline was not significantly different from MELD score at 3-month follow-up (7.5±1.9 vs. 8.9±4.2, P = 0.062).
Abdominal CT was performed in all patients prior to liver-directed treatment and at 3-month follow-up. Out of 24 patients, 20 were found to have bilobar disease and 15 were found to have extrahepatic metastasis at the time of the treatment. At 3 months, 9 patients were found to have progressive disease (PD), 5 had stable disease (SD), 5 had partial response (PR), and 5 had complete response (CR) by mRECIST criteria. Therefore, objective response rate (CR+PR) was 41.7% (10/24) and disease control rate was 62.5% (15/24) by mRECIST criteria. By RECIST criteria, 13 patients had PD, 10 had SD, and one had CR (Table 3). Among those patients with PD, 4 had extrahepatic progression.
Table 3.
Imaging response
| Total n (%) |
DEB-TACE n (%) |
Y90 n (%) |
Ablation n (%) |
|
|---|---|---|---|---|
| RECIST | ||||
| PD | 13 (54.2) | 7 (30.4) | 2 (33.3) | 4 (80.0) |
| SD | 10 (41.7) | 6 (26.1) | 4 (66.7) | 0 (0) |
| PR | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| CR | 1 (4.2) | 0 (0) | 0 (0) | 1 (20.0) |
|
| ||||
| mRECIST | ||||
| PD | 9 (37.5) | 3 (13.0) | 3 (50.0) | 3 (60.0) |
| SD | 5 (20.8) | 3 (13.0) | 2 (33.3) | 0 (0) |
| PR | 5 (20.8) | 4 (17.4) | 1 (16.7) | 0 (0) |
| CR | 5 (20.8) | 3 (13.0) | 0 (0) | 2 (40.0) |
DEB-TACE, doxorubicin eluting bead transarterial chemoembolization; Y90, yttrium-90; RECIST, response evaluation criteria in solid tumors; PD, progressive disease; SD, stable disease; PR, partial response; CR, complete response; mRECIST, modified response evaluation criteria in solid tumors.
Discussion
Soft tissue sarcomas are rare tumors accounting for less than 1% of all malignancies, and leiomyosarcomas account for 10%–24% of soft tissue sarcomas (23). Metastases arise via hematogenous spread to the lungs, liver, bone, and other soft tissues (23). As liver metastases are often the limiting factor for survival, optimal treatment strategies are currently under investigation.
Most patients with metastatic sarcoma to the liver are treated with chemotherapy. However, response to chemotherapy is poor. For patients who are surgical candidates, resection of liver metastases can prolong survival (9–11). In a study of 126 patients who underwent liver resection for metastatic sarcomas, Goumard et al. (9) reported a median OS of 58.0 months and recurrence-free survival (RFS) of 12.5 months. Another study reported 7.9 months RFS with metastatic leiomyosarcoma who underwent hepatic metastasectomy (11). However, most of the patients who present with liver metastasis are not surgical candidates. In a series of 331 patients with sarcoma liver metastasis, only 17% (56 patients) underwent resection. In addition, the recurrence rate after sarcoma metastasectomy is high, DeMatteo et al. (10) reported an 84% recurrence rate, with the liver as the most common site of tumor recurrence.
For patients who fail to respond to chemotherapy and are not surgical candidates, the treatment options are limited. Liver-directed therapies are emerging new technologies to treat these patients. Percutaneous ablation was our primary liver-directed therapy choice, since ablation can provide the best local tumor control eliminating the entire metastasis. In our study only 5 out of 24 patients received ablation since patients needed to have limited disease (1–3 lesions) and lesions <3 cm to qualify for ablation. The primary embolization method was DEB-TACE and radioembolization was used if that was the patient’s preference (patients with low pain tolerance and/or the patient did not want to stay in the hospital for overnight observation). In our study, liver-directed treatments with DEB-TACE, Y90 radioembolization, or ablation, resulted in an OS of 59 months from leiomyosarcoma diagnosis, 27 months from hepatic metastasis diagnosis, and 9 months from liver-directed treatment. Our results are similar to previous reports; however, due to the rarity of leiomyosarcoma and soft tissue sarcomas as a whole, most previous studies included heterogeneous groups of soft tissue sarcomas. In a study of 28 patients who underwent embolization for sarcoma liver metastases, 12 of which underwent bland embolization, 6 radioembolization, 8 TACE, and 2 who underwent a combination of liver-directed treatment, Pierce et al. (24) reported a median OS of 26.7 months and PFS of 14.2 months from treatment. Out of these 28 patients, 11 had leiomyosarcoma (24). Maluccio et al. (25) reported promising response of sarcoma hepatic metastases to bland embolization with a median OS from treatment of 18 months in a study of 24 patients with metastatic sarcoma, 7 of which were leiomyosarcoma. In a larger study including 30 patients with sarcoma hepatic metastases treated with TACE, Chapiro et al. (7) reported a median OS from first treatment of 21.2 months and a median PFS of 6.3 months. Out of the 30 patients, 25 had leiomyosarcoma (7). In regards to Y90 radioembolization, two smaller studies have reported OS ranging from 26–30 months from treatment; both studies consisted of mixed hepatic metastatic soft tissue sarcoma patients, with leiomyosarcoma being the predominant subtype (26, 27). As in our study, ablation is used in patients with limited hepatic disease burden. In a study of 25 patients with sarcoma liver metastases treated with ablation, including 5 patients with hepatic leiomyosarcoma metastasis, local ablation treatment was limited to patients with ≤5 local hepatic lesions and size ≤3.5 cm. In this study, median OS was 19 months (95% CI, 12–26 months) from treatment and the median PFS at the ablation site was 9 months (95% CI, 8–10 months) (28). While our reported OS from treatment is lower than other reports, our study is unique in that our patient population consists entirely of leiomyosarcoma patients whereas all other previously presented studies include sarcoma patients of various histological subtypes (23–28). It is worth to mention that in our study, the OS from the liver-directed treatment was 22 months in patients without extrahepatic disease at the time of treatment and it was 24 months in patients who underwent liver-directed therapy within 4 months after diagnosis of hepatic metastasis.
In the current study, objective tumor response (CR+PR) by mRECIST was 42% overall, and 54%, 17%, and 40% for TACE, radioembolization, and ablation, respectively. Chapiro et al. (7) reported an objective tumor response rate of 48% for TACE using mRECIST criteria. Miller et al. (26) reported a 36% radiographic response to Y90 radioembolization therapy in patients with sarcoma hepatic metastasis. Pierce et al. (24) reported radiographic response of 38.9% at 3 months after transarterial embolization treatment with TACE, Y90, or bland beads.
Our study reports a favorable median OS compared with doxorubicin (9 vs. 12.8 months) and liver-directed therapies are better tolerated (12). In our study, most patients endorsed grade 1 nausea and/or right upper quadrant or epigastric pain. Only one patient was reported to have a grade 3 postembolization syndrome resulting in 6-day hospitalization after DEB-TACE. The use of doxorubicin for chemotherapy is associated with grade 3 and 4 toxic effects, with the most common being leukopenia (18%), neutropenia (37%), febrile neutropenia (13%), anemia (5%), and thrombocytopenia (<1%) (12). The lower rate of toxicity associated with liver-directed therapies in our study is consistent with other reports. Chapiro et al. (7) reported right upper quadrant pain as the most frequently reported adverse event after DEB-TACE. The most common reported clinical toxicity in the study of Pierce et al. (24) was postembolization syndrome reported in 3 patients and defined as fever, nausea and vomiting, and pain within 72 hours. They also reported one patient who developed a hepatic abscess 3 months after radioembolization which resolved with percutaneous drainage and antibiotics (24). Development of liver abscess was also reported in two patients in the Maluccio et al. (25) study which required treatment with either drainage or resection. Few studies report lab toxicities. We followed albumin, INR, ALT, AST, alkaline phosphatase, and bilirubin and reported mostly grade 1 or 2 lab toxicities and 3 patients with grade 3 toxicities due to either elevated bilirubin, ALT, or alkaline phosphatase. We also calculated MELD score before and after treatment and found no significant difference between the two. Transue et al. (27) also followed lab toxicity, and similar to our study, reported a single grade 3 bilirubin toxicity that occurred at 3 months in a study of 18 patients with metastatic soft tissue sarcomas to the liver treated with Y90.
The main deficiencies of this study are its retrospective nature and the low number of subjects which did not allow for significant subgroup analysis. Although we report large differences in survival among various subgroups, due to the small size of our study group, we were unable to identify significant predictors of OS. In addition, this was a single institution study. As such, criteria for treatment may vary between our institution and many others. Our decision to treat was based on discussions at a multidisciplinary tumor board; therefore, selection bias may have altered the results. In our study, patients were not selected for or against treatment based on presence or absence of extrahepatic metastasis or primary tumor control.
In conclusion, our findings are consistent with previous studies showing that liver-directed treatments (TACE, Y-90 radioembolization, ablation) are effective and safe treatment options for unresectable hepatic leiomyosarcoma metastasis. Our study may indicate that patients who have extrahepatic disease and patients who undergo liver-directed therapy within 4 months after diagnosis of hepatic metastasis benefit the most from the treatment. To our knowledge, this is the largest single-institution study consisting exclusively of patients with leiomyosarcoma metastasis to the liver.
Main points.
Liver-directed treatments (DEB-TACE, Y90 radioembolization, ablation) are effective and safe treatment options for unresectable hepatic leiomyosarcoma metastasis.
In our study, mean overall survival was 59 months (95% CI, 39.8–78.2) from diagnosis, 27 months (95% CI, 22.9–31.0) from development of liver metastasis, and 9 months (95% CI, 0–21.4) from first liver-directed treatment. Median liver progression-free survival was 9 months (95% CI, 1.4–16.6).
Our study may indicate that patients without extrahepatic disease and patients who undergo liver-directed therapy within 4 months after diagnosis of liver metastasis benefit the most from the treatment.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Gladdy RA, Qin L-X, Moraco N, Agaram NP, Brennan MF, Singer S. Predictors of survival and recurrence in primary leiomyosarcoma. Ann Surg Oncol. 2013;20:1851–1857. doi: 10.1245/s10434-013-2876-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alldinger I, Yang Q, Pilarsky C, Saeger HD, Knoefel WT, Peiper M. Retroperitoneal soft tissue sarcomas: prognosis and treatment of primary and recurrent disease in 117 patients. Anticancer Res. 2006;26:1577–1581. [PubMed] [Google Scholar]
- 3.Mendenhall WM, Zlotecki RA, Hochwald SN, Hemming AW, Grobmyer SR, Cance WG. Retroperitoneal soft tissue sarcoma. Cancer. 2005;104:669–675. doi: 10.1002/cncr.21264. [DOI] [PubMed] [Google Scholar]
- 4.Tan MC, Brennan MF, Kuk D, et al. Histology-based classification predicts pattern of recurrence and improves risk stratification in primary retroperitoneal sarcoma. Ann Surg. 2016;263:593–600. doi: 10.1097/SLA.0000000000001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaques DP, Coit DG, Hajdu SI, Brennan MF. Management of primary and recurrent soft-tissue sarcoma of the retroperitoneum. Ann Surg. 1990;212:51–59. doi: 10.1097/00000658-199007000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bramwell VH, Anderson D, Charette ML Sarcoma Disease Site Group. Doxorubicin-based chemotherapy for the palliative treatment of adult patients with locally advanced or metastatic soft tissue sarcoma. Cochrane Database Syst Rev. 2003;2003:CD003293. doi: 10.1002/14651858.CD003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chapiro J, Duran R, Lin M, et al. Transarterial chemoembolization in soft tissue sarcoma metastases to the liver - the use of imaging biomarkers as predictors of patient survival. Eur J Radiol. 2015;84:424–430. doi: 10.1016/j.ejrad.2014.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Svarvar C, Böhling T, Berlin O, et al. Scandinavian Sarcoma Group. Leiomyosarcoma I 225 patients from the Scandinavian Sarcoma Group. Cancer. 2007;109:282–291. doi: 10.1002/cncr.22395. [DOI] [PubMed] [Google Scholar]
- 9.Goumard C, Marcal LP, Wang WL, et al. Long-term survival according to histology and radiologic response to preoperative chemotherapy in 126 patients undergoing resection of non-GIST sarcoma liver metastases. Ann Surg Oncol. 2018;25:107–116. doi: 10.1245/s10434-017-6144-4. [DOI] [PubMed] [Google Scholar]
- 10.DeMatteo RP, Shah A, Fong Y, et al. Results of hepatic resection for sarcoma metastatic to liver. Ann Surg. 2001;231:540–548. doi: 10.1097/00000658-200110000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brudvik KW, Patel SH, Roland CL, et al. Survival after resection of gastrointestinal stromal tumor and sarcoma liver metastatsis in 146 patients. J Gastrointest Surg. 2015;19:1476–1483. doi: 10.1007/s11605-015-2845-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Judson I, Verweij J, Gelderblom H, et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for first-line treatment of advanced or metastatic soft-tissue sarcoma: a randomized controlled phase 3 trial. Lancet Oncol. 2014;15:415–423. doi: 10.1016/S1470-2045(14)70063-4. [DOI] [PubMed] [Google Scholar]
- 13.Tap W, Wagner AJ, Papai Z, Ganjoo N, Ganjoo KN, Yen C. ANNOUNCE: A randomized, placebo (PBO)-controlled, double-blind, phase (Ph) III trial of doxorubicin (dox) + olaratumab versus dox + PBO in patients (pts) with advanced soft tissue sarcomas (STS) J Clin Oncol. 2019;37(suppl) doi: 10.1200/JCO.2019.37.18_suppl.LBA3. abstr LBA3. [DOI] [Google Scholar]
- 14.Gangi A, Shah J, Hatfield N, et al. Intrahepatic cholangiocarcinoma treated with transarterial Yttrium-90 glass microsphere radioembolization: results of a single institution retrospective study. J Vasc Interv Radiol. 2018;29:1101–1108. doi: 10.1016/j.jvir.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Llovet JM, Real MI, Montaña X, et al. Barcelona Liver Cancer Group. Arterial embolization or chemoembolization versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 16.Tellez C, Benson AB, 3rd, Lyster MT, et al. Phase II trial of chemoembolization for the treatment of metastatic colorectal carcinoma to the liver and review of the literature. Cancer. 1998;82:1250–1259. doi: 10.1002/(sici)1097-0142(19980401)82:7<1250::aid-cncr7>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 17.Kennedy AS, Coldwell D, Nutting C, et al. Resin 90Y-microsphere brachytherapy for unresectable colorectal liver metastases: modern USA experience. Int J Radiat Oncol Biol Phys. 2006;65:412–425. doi: 10.1016/j.ijrobp.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 18.Sato KT, Lewandowski RJ, Mulcahy MF, et al. Unresectable chemorefractory liver metastases: radioembolization with 90Y microspheres—safety, efficacy, and survival. Radiology. 2008;247:507–515. doi: 10.1148/radiol.2472062029. [DOI] [PubMed] [Google Scholar]
- 19.Cianni R, Pelle G, Notarianni E, et al. Radioembolization with (90) Y-labelled resin microsphere in the treatment of liver metastasis from breath cancer. Eur Radiol. 2013;23:182–189. doi: 10.1007/s00330-012-2556-5. [DOI] [PubMed] [Google Scholar]
- 20.Martin RC, Scoggins CR, McMasters KM. Safety and efficacy of microwave ablation of hepatic tumors: a prospective review of a 5-year experience. Ann Surg Oncol. 2010;17:171. doi: 10.1245/s10434-009-0686-z. [DOI] [PubMed] [Google Scholar]
- 21.Nishino M, Jagannathan JP, Ramaiya NH, Van den Abbeele AD. Revised RECIST guideline version 1.1: What oncologists want to know and what radiologists need to know. AJR Am J Roentgenol. 2010;195:281–289. doi: 10.2214/AJR.09.4110. [DOI] [PubMed] [Google Scholar]
- 22.Brown DB. Quality improvement guidelines for transhepatic arterial chemoembolization, embolization, and chemotherapeutic infusion for hepatic malignancy. J Vasc Interv Radiol. 2012;23:287–294. doi: 10.1016/j.jvir.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 23.D’Angelo SP, Tap WD, Schwartz GK, Carvajal RD. Sarcoma immunotherapy: past approaches and future directions. Sarcoma. 2014;2014 doi: 10.1155/2014/391967. 391967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pierce DB, Johnson GE, Monroe E, et al. Safety and efficacy outcomes of embolization in hepatic sarcomas. AJR Am J Roentgenol. 2018;210:175–182. doi: 10.2214/AJR.16.17573. [DOI] [PubMed] [Google Scholar]
- 25.Maluccio MA, Covey AM, Schubert J, et al. Treatment of metastatic sarcoma to the liver with bland embolization. Cancer. 2006;107:1617–1623. doi: 10.1002/cncr.22191. [DOI] [PubMed] [Google Scholar]
- 26.Miller MD, Sze DY, Padia SA, et al. Response and overall survival for yttrium-90 radioembolization of hepatic sarcoma: a multicenter retrospective study. J Vasc Interv Radiol. 2018;29:867–873. doi: 10.1016/j.jvir.2018.01.775. [DOI] [PubMed] [Google Scholar]
- 27.Transue DL, Hackworth J, Johnson MS, et al. Multi-institutional experience treating metastatic soft tissue sarcomas using yttrium-90 microspheres. J Vasc Interv Radiol. 2015;26:S133. doi: 10.1016/j.jvir.2014.12.356. [DOI] [Google Scholar]
- 28.Jones RL, McCall J, Adam A, O’Donnell D, Ashley S, Al-Muderis O, et al. Radiofrequency ablation is a feasible therapeutic option in the multi modality management of sarcoma. Eur J Surg Oncol. 2010;36:477–482. doi: 10.1016/j.ejso.2009.12.005. [DOI] [PubMed] [Google Scholar]