Abstract
PURPOSE
We aimed to evaluate which morphologic features on magnetic resonance imaging (MRI) could predict the progression of pancreatic cystic lesions (PCLs) that are suitable for follow-up.
METHODS
A total of 2176 MRI findings of PCLs were retrospectively reviewed between January 2009 and December 2016. The study population was composed of 223 patients. Clinical data and morphologic features of PCLs were recorded. We divided the individuals into two sub-groups according to the final features on MRI. Univariable and multivariable regression analyses were performed to identify independent risk factors for progression of PCLs.
RESULTS
A total of 84 PCLs (37.7%) progressed during follow-up, while 139 PCLs (62.3%) were stable. Age (odds ratio [OR], 1.042; P = 0.017), number of lesions (OR, 0.491; P = 0.048), communication to pancreatic duct (PD) (OR, 2.425; P = 0.007) and presence of septa (OR, 6.105; P < 0.001) were significant independent factors for progression of PCLs. Among 84 lesions that progressed, 23 lesions (27.4%) increased to ≥ 30 mm in diameter or showed worrisome imaging features at the end of follow-up that needed clinical intervention. The initial size and communication to PD were independent factors for progression of PCLs necessitating clinical intervention (P < 0.001 and P = 0.011, respectively).
CONCLUSION
Age, number of the lesions, communication to PD and presence of septa were independent risk factors for the progression of PCLs, and the initial size and communication to PD could potentially predict PCLs needing clinical interventions.
More and more pancreatic cystic lesions (PCLs) are incidentally detected on abdominal imaging with computed tomography (CT) and magnetic resonance imaging (MRI). The prevalence of PCLs detected by MRI is reported as 2.2%–44.7% (1–5). A recent study showed that MRI combined with magnetic resonance cholangiopancreatography (MRCP) is superior to endoscopic ultrasound in classifying malignant or benign intraductal papillary mucinous neoplasm (IPMN) and mucinous cystic neoplasm (MCN) (6). MRI is preferred for the detection and follow-up of PCLs, since it has high contrast resolution, no ionizing radiation, and with the use of MRCP, shows the internal morphology such as septa, mural nodules, and dilatation of pancreatic duct (PD) better than CT (7–10).
PCLs consist of various pathologic types and some have the potential for malignant transformation. However, it is difficult to identify malignant PCLs only by imaging. Due to the low rate of malignant transformation from pancreatic cysts to invasive ducal adenocarcinoma of the pancreas (33.2 per 100 000) (11) and the relatively higher mortality rate of resection (12), most guidelines recommended that incidental PCLs without worrisome imaging features and symptoms could be followed up. Guidelines also expressed that management of follow-up should be stratified based on imaging features and size (7, 8). The latest American Gastroenterological Association (AGA) Institute guideline noted that cyst size is the most convenient surrogate we have (8). Hoffman et al. (13) showed that the risk of malignancy in cysts ≥30 mm was four times higher that of smaller cysts (RR 4.32, 95%CI 1.55–12.07). Other studies reported that an increase in size of between 2 and 5 mm/year is related to an increased risk of malignancy (14, 15). Before embarking on management of PCLs, clinicians need to consider the rate of malignant transformation, cost of follow-up, patient’s approximate life expectancy and comorbidity.
Guidelines indicate that the management should be timely adjusted by the final size and imaging features (7, 8), but which factors may influence the progression of PCLs during follow-up is not widely investigated. This study is particularly interesting since it could help to identify which cysts may show high-grade dysplasia or early malignancy, which could be timely intervened and get a better prognosis.
The aim of this study was to evaluate the MRI-detected morphologic features which can predict the progression of pancreatic cystic lesions that are suitable for follow-up.
Methods
The Ethics Committee at Zhongshan Hospital of Fudan University approved this retrospective research and waived the informed patient consent. The decision number was B2014 – 019.
Study subjects
We retrospectively reviewed consecutive diagnostic reports and selected patients with incidental PCLs detected by contrast-enhanced abdominal MRI from January 2009 to December 2016. Four abdominal radiologists with 6 to 7 years of experience reviewed the diagnostic reports in chronological order.
The images were re-evaluated by two radiologists together. The inclusion criteria for the study included: (a) PCL diagnosis by MRI; (b) availability of consecutive contrast-enhanced abdominal MRI with follow-up of more than 2 years; (c) magnetic resonance cholangiopancreatography (MRCP) included in the conventional MRI protocol. Exclusion criteria consisted of: (a) patients with a known or suspected history of pancreatic disease such as pancreatic solid tumors, acute/chronic pancreatitis, Von Hippel-Lindau disease; (b) the PCLs showing classic imaging features of serous cystadenoma, such as a microcystic appearance with a central scar; (c) initial size of PCLs ≥ 30 mm or other worrisome imaging features such as the presence of a mural nodule or solid component either within the cyst or in the pancreatic parenchyma, dilatation of the main pancreatic of >5 mm, a focal dilatation of the PD concerning for main duct IPMN or an obstructing lesion, and change in main duct caliber with upstream atrophy (8); (d) follow-up time less than 2 years after initial diagnosis or followed by other imaging methods. The flowchart for the enrollment of this study is shown in Fig. 1.
Figure 1.
Flow diagram shows inclusion and exclusion criteria for the study.
MRI protocol
Abdominal MRI examinations were performed on either 1.5 T MRI scanners (Avanto or Aera, Siemens Medical) or 3.0 T MRI scanners (Magnetom Verio, Siemens Medical; Signa HDx, GE Healthcare). The conventional abdominal sequences were as follows: axial T2-weighted fat-suppressed fast/turbo-spin-echo (FSE/TSE) sequence; In- and opposed-phase axial T1-weighted imaging sequence; diffusion-weighted imaging (DWI) (b=0, 500 s/mm2) sequence with a free-breathing single-shot echo-planar technique, with corresponding ADC maps automatically generated; coronal oblique thick-slab single-section T2-weighted MRCP using fast or turbo SE technique with breath-hold; coronal thin-slab multi-section T2-weighted MRCP using single-shot fast SE sequence or the half-Fourier rapid acquisition with relaxation enhancement sequence with breath-hold. Arterial, portal venous and delayed phases were obtained at 20–30 s, 70–80 s, and 180 s after a 0.2 mL/kg bolus injection of gadopentetate dimeglumine (Magnevist, Bayer Healthcare) at a rate of 2 mL/s. MRI sequences are summarized in Table 1.
Table 1.
MRI acquisition parameters
| Sequence | Area 1.5 T | Avanto 1.5 T | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| TSE T2WI | DWI | 3D GRE T1WI | T1WI | TSE T2WI | DWI | 3D GRE T1WI | T1WI | |
| Repetition time (ms) | 3500 | 3200 | 4.38 | 230 | 3300 | 2400~2600 | 5.04 | 112 |
|
| ||||||||
| Echo time (ms) | 84 | 56 | 1.93 | 2.47 | 70 | 66 | 2.31 | 4.76 |
|
| ||||||||
| Matrix size | 194×256 | 84×128 | 216×288 | 180×256 | 207×384 | 112×128 | 250×512 | 144×256 |
|
| ||||||||
| Field of view (mm2) | 360×360 | 380~400×300 ~ 324 | 380~400×300 ~ 324 | 330×330~ 380×380 | 330×330~ 380×380 | 330×330~ 380×380 | 330×330~ 380×380 | 330×330~ 380×380 |
|
| ||||||||
| Slice thickness (mm) | 8 | 5.5 | 5 | 7 | 7 | 7 | 4–5 | 7 |
|
| ||||||||
| Sequence | Verio 3.0 T | Signa HDX 3.0 T | ||||||
|
|
|
|||||||
| TSE T2WI | DWI | 3D GRE T1WI | T1WI | TSE T2WI | DWI | 3D GRE T1WI | T1WI | |
|
| ||||||||
| Repetition time (ms) | 3000 | 3400 | 4.1 | 140 | 4500 | 2300 | 2.9 | 285 |
|
| ||||||||
| Echo time (ms) | 83 | 70 | 1.4 | 2.5 | 88 | 52 | 1.3 | 2.1 |
|
| ||||||||
| Matrix size | 165×320 | 128 ×80 | 200×352 | 168×320~ 180×320 | 224×320 | 96×130 | 200×352 | 192×256 |
|
| ||||||||
| Field of view (mm2) | 285×214~ 285×380 | 285×214~ 285×380 | 285×214~ 285×380 | 285×214 285×380 | 300×400 | 280×400 | 420×420 | 400×400 |
|
| ||||||||
| Slice thickness (mm) | 5.5 | 6 | 3 | 5 | 5 | 5 | 3 | 5 |
MRI, magnetic resonance imaging; TSE, turbo spin-echo, T2WI, T2-weighted imaging; DWI, diffusion-weighted imaging; 3D, three-dimensional; GRE, gradient echo; T1WI, T1-weighted imaging.
Data collection
The following series of data were collected: clinical features (age, gender, the history of malignant tumor, follow-up time), imaging morphologic features of PCLs (initial and final sizes, locations, numbers, presence of septa, presence of communication with or dilatation of PD, shape of lesion) and pathologic results. The maximum diameter of the largest lesion was recorded for multiple lesions in transverse or coronary position. Some laboratory result such as tumor markers during follow-up was not collected because the data of some out-patients were not properly documented or examined.
Cyst progression was defined as increasing ≥2 mm/year in diameter or showing high-risk characteristics such as the presence of a mural nodule or solid component either within the cyst or in the pancreatic parenchyma, dilatation of the main pancreatic of >5 mm, a focal dilatation of the PD concerning for main duct IPMN or an obstructing lesion, cysts measuring ≥30 mm in diameter and change in main duct caliber with upstream atrophy (8). The rounded lesion was defined as a ball-shaped lesion with a smooth nonlobulated surface. If the lesion was ≥30 mm in diameter or showed high-risk characteristics, clinical intervention such as endoscopic ultrasound-guided fine needle aspiration biopsy was planned or referral to a multidisciplinary group for further evaluation was considered (8).
Statistical analysis
We divided the progression group into two subgroups to evaluate which factors influence the cysts increasing in size ≥30 mm or show high-risk characteristics. The Kolmogorov-Smirnov test was used to determine whether the variables were normally distributed. Descriptive statistics of the data are presented as n (%) for categorical variables, median (min–max) for non-normalized continuous variables, and mean ± standard deviation (SD) for normally distributed continuous variables. Independent t test was used to compare two groups for normally distributed variables and Pearson chi-square test was used to compare categorical variables. Univariable and multivariable logistic regression analyses using the method of Backward: Wald were performed to identify independent risk factors associated with progression of PCLs. Factors with a P value ≤ 0.10 at univariate analyses were entered into the multivariate model. Odds ratios and 95% confidence intervals (CIs) were calculated. A two-sided P value < 0.05 was considered indicative of a significant difference. All statistical analyses were conducted in SPSS (version 24.0, IBM Corp.).
Results
A total of 223 patients were enrolled, including 101 men (45.3%). Median age of patients at initial diagnosis was 62 years (25–85 years). Median follow-up duration was 43 months (range, 24–151 months). Among 223 patients, 158 (70.8%) had a single lesion. Communication to the PD and the presence of septa were respectively reported in 103 (46.2%) and 86 (38.6%). The initial size was 12 mm (3–29 mm) and the final size was 12 mm (0–40 mm). The largest PCLs were located throughout the pancreas: uncinate (7.6%, 17/223), head (24.7%, 55/223), neck (16.1%, 36/223), body (31.4%, 70/223), and tail (20.2%, 45/223). Totally 70 patients had the history of malignant tumors, which included: hepatic malignant tumor (n=63), gastrointestinal cancer (n=6), and thyroid cancer (n=1). Baseline characteristics of the patients and imaging features are summarized in Table 2.
Table 2.
Characteristics of patients and PCLs detected by MRI from 2009 to 2016
| Characteristics | |
|---|---|
| Age (years), median (range) | 62 (25–85) |
|
|
|
| Sex (M/F), n (%) | 101 (45.3)/122 (54.7) |
|
|
|
| Single/Multiple, n (%) | 158 (70.8)/65 (29.2) |
|
|
|
| Communication to PD, n (%) | 103 (46.2) |
|
|
|
| Presence of septa, n (%) | 86 (38.6) |
|
|
|
| Location, n (%) | |
| Uncinate | 17 (7.6) |
| Head | 55 (24.7) |
| Neck | 36 (16.1) |
| Body | 70 (31.4) |
| Tail | 45 (20.2) |
|
|
|
| Follow-up period (months), median (range) | 43 (24–151) |
|
|
|
| Presence of history of malignant tumors (Yes/No), n (%) | 70 (31.4)/153 (68.6) |
|
|
|
| Initial size (mm), median (range) | 12 (3–29) |
|
|
|
| Final size (mm), median (range) | 12 (0–40) |
PCL, pancreatic cystic lesion; MRI, magnetic resonance imaging; SD, standard deviation; M/F, male/female; PD, pancreatic duct.
Progression was determined in 84 patients (37.7%), while 139 patients (62.3%) were progression-free during follow-up. Patients in the progression group were older than the patients in the stable group (63.55±9.18 years vs. 60.53±10.17 years, P = 0.029). The initial cyst size of the progression group was 14.83±6.68 mm compared with 11.52±6.15 mm of the stable group (P < 0.001). Single lesion was found in 52 patients (61.9%) in the progression group, whereas in 106 patients (76.2%) in the stable group (P = 0.023). Rounded lesion was present in 101 patients (72.7%) in the stable group compared with 41 patients (48.8%) in the progression group (P < 0.001). Communication to the PD and the presence of septa also showed a significant correlation with progression (P < 0.001).
On univariable analysis, age (OR = 1.033; 95% CI, 1.003–1.064; P = 0.029), initial size (OR = 1.082; 95% CI, 1.036–1.130; P < 0.001), morphology (OR = 0.359; 95% CI, 0.203–0.633; P < 0.001), number (OR = 0.506; 95% CI, 0.281–0.911; P = 0.023), communication to PD (OR = 3.915; 95% CI, 2.206–6.949; P < 0.001) and the presence of septa (OR = 6.019; 95% CI, 3.317–10.922; P < 0.001) were significant independent risk factors for lesion progression, whereas sex and history of malignant tumors had no statistical significance (P = 0.170 and P = 0.111, respectively). Age (OR = 1.042; 95% CI, 1.007–1.078; P = 0.017), number (OR = 0.491; 95% CI, 0.243–0.992; P = 0.048), communication to PD (OR = 2.425; 95% CI, 1.269–4.634; P = 0.007) and the presence of septa (OR = 6.105; 95% CI, 3.131–11.903; P <0.001) remained significant after multivariable analysis. Univariable and multivariable analyses for risk factors associated with PCLs are presented in Tables 3 and 4.
Table 3.
Comparison between subgroups of pancreatic cystic lesions
| Risk factors | Progression (n=84) | Stable (n=139) | P | <30 mm and without high-risk characteristics (n=61) | ≥30 mm or with high-risk characteristics (n=23) | P |
|---|---|---|---|---|---|---|
| Age (y), mean±SD | 63.55±9.18 | 60.53±10.17 | 0.027 | 62.59±8.44 | 66.09±10.69 | 0.120 |
|
| ||||||
| Sex, n (%) | 0.169 | 0.705 | ||||
| Male | 43 (51.2) | 58 (41.7) | 32 (52.5) | 11 (47.8) | ||
| Female | 41 (48.8) | 81 (58.3) | 29 (47.5) | 12 (52.2) | ||
|
| ||||||
| Initial size (mm), mean±SD | 14.83±6.68 | 11.52±6.15 | <0.001 | 12.46±5.28 | 21.13±5.93 | <0.001 |
|
| ||||||
| Morphology, n (%) | <0.001 | 0.039 | ||||
| Rounded | 41 (48.8) | 101 (72.7) | 34 (55.7) | 7 (30.4) | ||
| Not rounded | 43 (51.2) | 38 (27.3) | 27 (44.3) | 16 (69.6) | ||
|
| ||||||
| Single/Multiple, n (%) | 52 (61.9)/32 (38.1) | 106 (76.3)/33 (23.7) | 0.022 | 35 (57.4)/26 (42.6) | 17 (73.9)/6 (26.1) | 0.164 |
|
| ||||||
| Communication to PD, n (%) | <0.001 | 0.015 | ||||
| Present | 56 (66.7) | 47 (33.8) | 36 (59.0) | 20 (87.0) | ||
| Absent | 28 (33.3) | 92 (66.2) | 25 (41.0) | 3 (13.0) | ||
|
| ||||||
| Septa, n (%) | <0.001 | 0.031 | ||||
| Present | 54 (64.3) | 32 (23.0) | 35 (57.4) | 19 (82.6) | ||
| Absent | 30 (35.7) | 107 (77.0) | 26 (42.6) | 4 (17.4) | ||
|
| ||||||
| History of malignant tumors, n (%) | 0.110 | 0.120 | ||||
| Yes | 21 (25.0) | 49 (35.2) | 18 (29.5) | 3 (13.0) | ||
| No | 63 (75.0) | 90 (64.8) | 43 (70.5) | 20 (87.0) | ||
SD, standard deviation; PD, pancreatic duct.
Table 4.
Univariate and multivariate analyses of risk factors for progression of pancreatic cystic lesions
| Risk factors | Progression vs. stable | <30 mm and without high-risk characteristics vs. ≥30 mm or with high-risk characteristics | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||
|
|
|
|
|
|||||
| OR | P | OR | P | OR | P | OR | P | |
| Age | 1.033 (1.003, 1.064) | 0.029 | 1.042 (1.007, 1.078) | 0.017 | 1.045 (0.988, 1.106) | 0.122 | ||
|
| ||||||||
| Sex | ||||||||
| Male | 1.465 (0.850, 2.525) | 0.170 | 0.831 (0.318, 2.170) | 0.705 | ||||
| Female | 1 | 1 | ||||||
|
| ||||||||
| Initial size | 1.082 (1.036, 1.130) | <0.001 | 1.028 (0.969, 1.089) | 0.362 | 1.313 (1.160, 1.486) | <0.001 | 1.346 (1.117, 1.540) | <0.001 |
|
| ||||||||
| Morphology | ||||||||
| Rounded | 0.359 (0.203, 0.633) | <0.001 | 0.991 (0.444, 2.210) | 0.982 | 0.347 (0.125, 0.965) | 0.043 | 1.182 (0.265, 5.273) | 0.826 |
| Not rounded | 1 | 1 | 1 | 1 | ||||
|
| ||||||||
| Number | ||||||||
| Single | 0.506 (0.281, 0.911) | 0.023 | 0.491 (0.243, 0.992) | 0.048 | 2.105 (0.729, 6.075) | 0.169 | ||
| Multiple | 1 | 1 | 1 | |||||
|
| ||||||||
| Communication to PD | ||||||||
| Present | 3.915 (2.206, 6.949) | <0.001 | 2.425 (1.269, 4.634) | 0.007 | 4.630 (1.241, 17.268) | 0.023 | 8.112 (1.604, 41.020) | 0.011 |
| Absent | 1 | 1 | 1 | 1 | ||||
|
| ||||||||
| Septa | ||||||||
| Present | 6.019 (3.317, 10.922) | <0.001 | 6.105 (3.131, 11.903) | <0.001 | 3.529 (1.072, 11.618) | 0.038 | 1.452 (0.329, 6.418) | 0.632 |
| Absent | 1 | 1 | 1 | |||||
|
| ||||||||
| History of malignant tumors | ||||||||
| Yes | 0.612 (0.335, 1.120) | 0.111 | 0.358 (0.095, 1.358) | 0.131 | ||||
| No | 1 | 1 | ||||||
Data in parentheses are 95% confidence intervals.
PD, pancreatic duct; OR, odd ratio.
Of 84 enlarged PCLs, 23 (27.4%) increased to ≥30 mm in size or showed worrisome imaging features. By using univariable analysis, we found that initial size (OR = 1.313; 95% CI, 1.160–1.486; P < 0.001), morphology (OR = 0.347; 95% CI, 0.125–0.965; P = 0.043) and communication to PD (OR = 4.630; 95% CI, 1.241–17.268; P =0.023) were significantly related to a size increase ≥30 mm or worrisome imaging features. The initial size (OR = 1.346; 95% CI, 1.117–1.540; P < 0.001) and communication to PD (OR = 8.112; 95% CI, 1.604–41.020; P =0.011) showed significant correlation with progression after multivariable analysis. The results are summarized in Tables 3 and 4. Exemplary PCLs followed up by MRI are presented in Figs. 2–4.
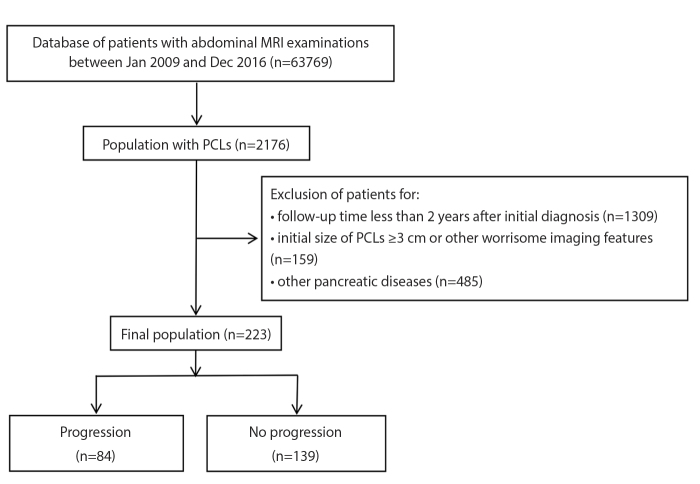
Figure 2. a–d.
Axial T2-weighted image (a) of a patient with a small rounded cyst (arrow) in the head of the pancreas. No septa or communication to pancreatic duct was found. There was no size increase or dilatation of pancreatic duct after 47 months of follow-up (b). Axial T2-weighted image (c) of a patient with a small rounded cyst (arrow) in the body of the pancreas. No septa or communication to pancreatic duct was found. No size increase or dilatation of pancreatic duct was found after 38 months of follow-up (d).
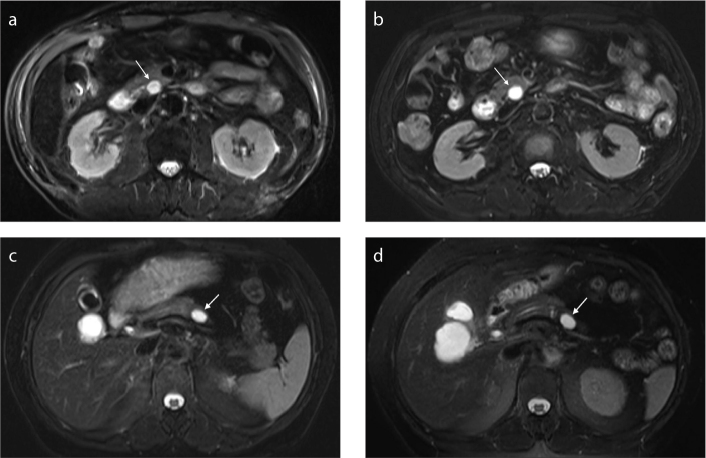
Figure 3. a–d.
Portal venous phase image (a) shows a cyst (arrow) with well-defined homogeneous signal intensity in the head of pancreas. Axial T2-weighted image (b) shows the largest cyst with septum, closely related to pancreatic duct. Portal venous phase image (c) shows that the cyst has enlarged after a 51-month follow-up period. Axial T2-weighted image (d) shows the largest cyst with septum within the lesion.
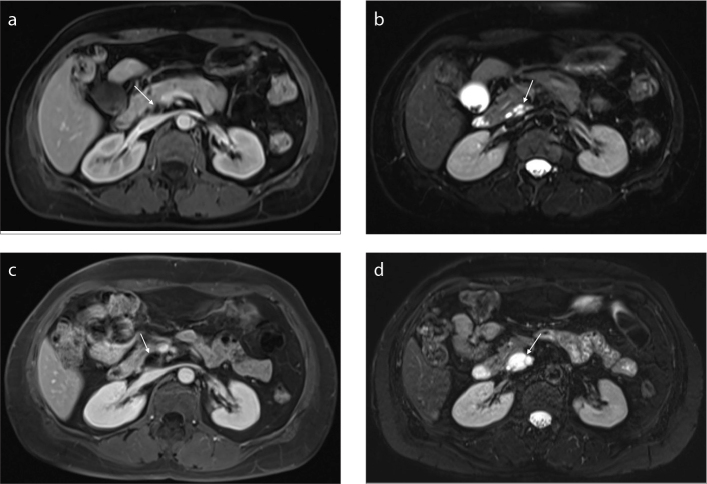
Figure 4. a–d.
A patient with a cyst (arrow) in the body of the pancreas. Axial T2-weighted image (a) shows a lobulated cyst with septum, communicating to pancreatic duct (a). Portal venous phase image (b) shows the mildly enhancing septa within the lesion. Axial T2-weighted image (c) shows that the cyst has enlarged after a 38-month follow-up period. Portal venous phase (d) shows mildly enhancing septa and cyst wall. This patient underwent surgical resection after 39-month follow-up and the pathologic diagnosis was branch-duct IPMN with low-grade dysplasia.
Among the 23 patients who met the criteria for clinical intervention, 5 (21.7%) underwent surgical resection after discussion by a multidisciplinary team. At pathology, 2 patients (40%) had MCNs with high-grade dysplasia, 2 (40%) had branch-duct IPMNs with low-grade dysplasia and 1 (20%) had serous cystic neoplasm. An 81-year-old patient with malignant MCN confirmed by biopsy chose to undergo selective radiotherapy. The other 17 patients (73.9%) who were not fit for surgery, selected follow-up by MRI.
Discussion
Identifying risk factors related to progressive PCLs might be helpful to determine different follow-up strategies, further exanimations (e.g., fine needle biopsy) or surgical resection. The present study demonstrated that age, number of lesions, communication to PD and the presence of septa were related to progression of PCLs, and the initial size and communication to PD were associated with lesion increase to ≥30 mm in diameter or presence of high-risk characteristics necessitating clinical intervention or change of follow-up strategy.
Within our cohort, 84 patients (37.7%) had an increase in PCL size, compared with 139 patients (62.3%) with stable lesion. This rate is similar to the findings of Arlix et al. (16) who showed 18 patients (38.3%) had an increase in cyst size of BD-IPMNs (16). However, our progression rate is less than 47.1% reported by Del Chiaro et al. (17), because they used 1 mm/year instead of 2 mm/year to define the standard of progression (17). The definition of progression (≥ 2 mm increase in diameter per year or development of high-risk characteristics) is based on clinical relevance and several researches applied this cutoff in large multicenter trials (14, 18, 19).
In the present study, age was identified to be different between progression and stable groups. Several researchers showed that the incidence of PCLs increased with increasing age, and the cumulative prevalence is as high as 40% in patients older than 70 years (4). The reason may be that part of those patients had PCLs several years before the initial diagnosis and the PCLs could increase in size as time goes on. In addition, presence of multiple lesions is also a risk factor for progression. Raman et al. (20) reported that the presence of multiple cysts should raise suspicion and these cysts have to be followed very closely due to the relatively high risk of high-grade dysplasia or invasive carcinoma. Furthermore, Matthaei et al. (21) demonstrated that the majority of multifocal IPMNs arise independently and exhibit a gastric-foveolar subtype, with low to intermediate dysplasia.
It is well recognized that the presence of PCLs with a solid component is a strong indicator of malignancy (21, 22). However, septa can appear in both mucinous cystic neoplasm, which could be malignant or potentially malignant, and a benign serous cystadenoma, which does not always mean malignancy (23, 24). Sahani et al. (25) reported that pancreatic cysts ≤3 cm without septa or solid components were almost always benign, but 20% of PCLs with septa were associated with a borderline or in situ malignancy. Our study found that the presence of septa was an independent risk factor for progression, further supporting that septa within PCLs should raise concern for malignancy.
Interestingly, we found that communication to PD was an independent risk factor not only for progressive PCLs but also for progression to ≥3 cm or having high-risk characteristics. Communication with the PD is the characteristic of IPMN which is a mucin-producing cyst with malignant potential. Previous study showed that the median annual growth rate of branch-duct of IPMNs was 0.8 mm and high-risk characteristics were shown in 15.3% of patients during surveillance (26). Furthermore, initial size of the cyst was also a factor affecting whether the PCLs need clinical interventions in our study. This was on keeping with the previous studies (26, 27). Han et al. (26) demonstrated that the growth rates and the incidence of new development of high-risk characteristics varied with different initial size of PCLs.
Our study presents several limitations. First, we selected patients by reviewing the diagnostic reports, which could produce selection bias; however, the imaging findings of morphologic features were reviewed carefully. Second, we did not collect the laboratory results such as CEA, CA19-9, because the data of out-patients could not be obtained completely. Third, due to the difference in magnetic field strength (1.5 T vs. 3.0 T), image resolution could vary, but most patients had undergone multiple examinations during follow-up. Furthermore, we unfortunately did not obtain the 1-, 3- or 5-year cumulative risk of malignancy of PCLs because the number of malignant cases confirmed by pathology was too few.
In conclusion, this study indicated that 62.3% of incidental PCLs followed more than 2 years were stable. Age, number of lesions, presence of septa, and communication to PD were significant risk factors for progression of PCLs. The initial size and communication to PD were associated with the lesion increasing to ≥30 mm in diameter or developing worrisome imaging features. PCLs with the above features have a higher risk of progression. Clinicians need to pay more attention to those patients and adjust the management timely to achieve a better prognosis.
Main points.
Most PCLs remained stable during the follow-up, as detected by MRI.
Age, number of lesions, presence of septa, and communication to pancreatic duct (PD) were the risk factors for progression of PCLs.
The initial size and communication to PD were associated with PCL increasing to ≥30 mm or developing worrisome imaging features.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Chang YR, Park JK, Jang JY, Kwon W, Yoon JH, Kim SW. Incidental pancreatic cystic neoplasms in an asymptomatic healthy population of 21,745 individuals: Large-scale, single-center cohort study. Medicine (Baltimore) 2016;95:e5535. doi: 10.1182/blood-2010-10-299529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang XM, Mitchell DG, Dohke M, Holland GA, Parker L. Pancreatic cysts: depiction on single-shot fast spin-echo MR images. Radiology. 2002;223:547–553. doi: 10.1016/S1470-2045(14)70442-5. [DOI] [PubMed] [Google Scholar]
- 3.de Jong K, Nio CY, Hermans JJ, et al. High prevalence of pancreatic cysts detected by screening magnetic resonance imaging examinations. Clin Gastroenterol Hepatol. 2010;8:806–811. doi: 10.14694/EDBK_159009. [DOI] [PubMed] [Google Scholar]
- 4.Lee KS, Sekhar A, Rofsky NM, Pedrosa I. Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am J Gastroenterol. 2010;105:2079–2084. doi: 10.2214/AJR.11.8010. [DOI] [PubMed] [Google Scholar]
- 5.Girometti R, Intini S, Brondani G, et al. Incidental pancreatic cysts on 3D turbo spin echo magnetic resonance cholangiopancreatography: prevalence and relation with clinical and imaging features. Abdom Imaging. 2011;36:196–205. doi: 10.1002/cncr.26467. [DOI] [PubMed] [Google Scholar]
- 6.Hwang J, Kim YK, Min JH, Jeong WK, Hong SS, Kim HJ. Comparison between MRI with MR cholangiopancreatography and endoscopic ultrasonography for differentiating malignant from benign mucinous neoplasms of the pancreas. Eur Radiol. 2018;28:179–187. doi: 10.1002/ajh.25117. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka M, Fernandez-Del Castillo C, Kamisawa T, et al. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology. 2017;17:738–753. doi: 10.1002/jmri.25769. [DOI] [PubMed] [Google Scholar]
- 8.Elta GH, Enestvedt BK, Sauer BG, Lennon AM. ACG clinical guideline: diagnosis and management of pancreatic cysts. Am J Gastroenterol. 2018;113:464–479. doi: 10.1371/journal.pone.0180562. [DOI] [PubMed] [Google Scholar]
- 9.European Study Group on Cystic Tumours of the Pancreas. European evidence-based guidelines on pancreatic cystic neoplasms. Gut. 2018;67:789–804. doi: 10.1016/j.ejrad.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sainani NI, Saokar A, Deshpande V, Fernandez-del Castillo C, Hahn P, Sahani DV. Comparative performance of MDCT and MRI with MR cholangiopancreatography in characterizing small pancreatic cysts. AJR Am J Roentgenol. 2009;193:722–731. doi: 10.1586/17474086.2014.873347. [DOI] [PubMed] [Google Scholar]
- 11.Gardner TB, Glass LM, Smith KD, et al. Pancreatic cyst prevalence and the risk of mucin-producing adenocarcinoma in US adults. Am J Gastroenterol. 2013;108:1546–1550. doi: 10.1155/2013/298675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scheiman JM, Hwang JH, Moayyedi P. American gastroenterological association technical review on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015;148:824–848. doi: 10.1002/jmri.25496. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman RL, Gates JL, Kochman ML, et al. Analysis of cyst size and tumor markers in the management of pancreatic cysts: support for the original Sendai criteria. J Am Coll Surg. 2015;220:1087–1095. doi: 10.1007/s00247-017-3856-3. [DOI] [PubMed] [Google Scholar]
- 14.Kwong WT, Lawson RD, Hunt G, et al. Rapid growth rates of suspected pancreatic cyst branch duct intraductal papillary mucinous neoplasms predict malignancy. Dig Dis Sci. 2015;60:2800–2806. doi: 10.1148/radiology.153.1.6089263. [DOI] [PubMed] [Google Scholar]
- 15.Kang MJ, Jang JY, Kim SJ, et al. Cyst growth rate predicts malignancy in patients with branch duct intraductal papillary mucinous neoplasms. Clin Gastroenterol Hepatol. 2011;9:87–93. doi: 10.5152/dir.2018.17320. [DOI] [PubMed] [Google Scholar]
- 16.Arlix A, Bournet B, Otal P, et al. Long-term clinical and imaging follow-up of nonoperated branch duct form of intraductal papillary mucinous neoplasms of the pancreas. Pancreas. 2012;41:295–301. doi: 10.3389/fendo.2018.00141. [DOI] [PubMed] [Google Scholar]
- 17.Del Chiaro M, Ateeb Z, Hansson MR, et al. Survival analysis and risk for progression of intraductal papillary mucinous neoplasia of the pancreas (IPMN) under surveillance: a single-institution experience. Ann Surg Oncol. 2017;24:1120–1126. doi: 10.1148/radiology.215.3.r00jn42910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nougaret S, Reinhold C, Chong J, et al. Incidental pancreatic cysts: natural history and diagnostic accuracy of a limited serial pancreatic cyst MRI protocol. Eur Radiol. 2014;24:1020–1029. doi: 10.1002/mrm.24775. [DOI] [PubMed] [Google Scholar]
- 19.Kayal M, Luk L, Hecht EM, et al. Long-term surveillance and timeline of progression of presumed low-risk intraductal papillary mucinous neoplasms. AJR Am J Roentgenol. 2017;209:320–326. doi: 10.1002/mrm.21561. [DOI] [PubMed] [Google Scholar]
- 20.Raman SP, Kawamoto S, Blackford A, et al. Histopathologic findings of multifocal pancreatic intraductal papillary mucinous neoplasms on CT. AJR Am J Roentgenol. 2013;200:563–569. doi: 10.14218/JCTH.2018.00021. [DOI] [PubMed] [Google Scholar]
- 21.Matthaei H, Norris AL, Tsiatis AC, et al. Clinicopathological characteristics and molecular analyses of multifocal intraductal papillary mucinous neoplasms of the pancreas. Ann Surg. 2012;255:326–333. doi: 10.1002/jcsm.12343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allen PJ, Jaques DP, D’Angelica M, Bowne WB, Conlon KC, Brennan MF. Cystic lesions of the pancreas: selection criteria for operative and nonoperative management in 209 patients. J Gastrointest Surg. 2003;7:970–977. doi: 10.18383/j.tom.2017.00011. [DOI] [PubMed] [Google Scholar]
- 23.Hammond N, Miller FH, Sica GT, Gore RM. Imaging of cystic diseases of the pancreas. Radiol Clin North Am. 2002;40:1243–1262. doi: 10.1002/ajh.24402. [DOI] [PubMed] [Google Scholar]
- 24.Sarr MG, Murr M, Smyrk TC, et al. Primary cystic neoplasms of the pancreas. Neoplastic disorders of emerging importance-current state-of-the-art and unanswered questions. J Gastrointest Surg. 2003;7:417–428. doi: 10.1038/oby.2009.352. [DOI] [PubMed] [Google Scholar]
- 25.Sahani DV, Saokar A, Hahn PF, Brugge WR, Fernandez-Del Castillo C. Pancreatic cysts 3 cm or smaller: how aggressive should treatment be? Radiology. 2006;238:912–919. doi: 10.1016/j.mri.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 26.Han Y, Lee H, Kang JS, et al. Progression of pancreatic branch duct intraductal papillary mucinous neoplasm associates with cyst size. Gastroenterology. 2018;154:576–584. doi: 10.2214/AJR.10.4384. [DOI] [PubMed] [Google Scholar]
- 27.Yoen H, Kim JH, Lee DH, Ahn SJ, Yoon JH, Han JK. Fate of small pancreatic cysts (<3 cm) after long-term follow-up: analysis of significant radiologic characteristics and proposal of follow-up strategies. Eur Radiol. 2017;27:2591–2599. doi: 10.1002/nbm.3420. [DOI] [PubMed] [Google Scholar]