Abstract
The critical role of the innate immune system has been confirmed in driving local and systemic inflammation and the cytokine release storm in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This dysregulated immune response is focused on interferon (IFN) and complement activation, which are crucial for the development of metabolic inflammation, local lung tissue damage, and systemic multi-organ failure. IFNs control viral infections by inducing expression of IFN-stimulated genes (ISGs) that restrict distinct steps of viral replication. Therefore, in this review article, we propose the mechanism of SARS-CoV-2-associated acute respiratory disease syndrome, and assess treatment options by considering IFNs and by targeting IFN-antagonist SARS-CoV-2 virulent gene products. Furthermore, we elaborate on the mechanism of the amplified complement-mediated inflammatory cytokine storm, and propose an antiviral and immunotherapeutic strategy against coronavirus disease 2019 (COVID-19).
Keywords: SARS-CoV-2, innate immune system, cytokines, complement, IFNs, virulent
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is an enveloped novel virus with a single-strand RNA (+SSRNA) genome in the subfamily Coronaviridae.1,2 The virus is a club-shaped spherical virion with spike projections from the virus membrane.1,3,4 The organization and expression of the genome are similar for all coronaviruses, in which the 16 non-structural proteins (Nsp1–Nsp16), encoded by open reading frame (ORF)1a/b at its 5ʹ-terminal, are followed by the structural protein spike (S), envelope (E), membrane (M), and nucleocapsid (N), which are encoded by another ORF at its 3ʹ-terminal.5 Clinically, patients with SARS-CoV-2 present with fever, cough, shortness of breath, and acute respiratory distress syndrome (ARDS).6–8 However, some patients have been diagnosed positive by real-time reverse-transcriptase polymerase chain reaction (RT-PCR) or nucleic acid test results without relevant clinical symptoms or who are only minimally symptomatic.9 Current research evidence shows that asymptomatic individuals have the same infectivity as those with symptomatic infections and can spread the virus efficiently.10 In turn, the emergence of these silent transmissions of SARS-CoV-2 has caused difficulties in controlling the COVID-19 pandemic.11 Both innate and adaptive immune responses are activated by SARS-CoV-2 infection to overcome the problem.12 However, uncontrolled inflammatory innate immune responses may lead to harmful tissue damage. Commonly, the symptoms resulted from the consequent infiltration of inflammatory cells and the release of proinflammatory cytokines.12 These include interleukin (IL)-1β, IL-6, and IL-8, which are responsible for fever and fibrosis and/or plural effusion of the lung, which provoke severe acute respiratory syndrome.13,14 In addition, CXCL9 and CXCL16, chemoattractants of T cells and/or natural killer (NK) cells, CCL8 and CCL2, recruiting monocytes/macrophages, CXCL8, and other classic neutrophil chemoattractants are profoundly elevated. Therefore, we suggest that the presence of these cells may be a primary driver of the signature pathology observed in coronavirus disease 2019 (COVID-19) patients. This review describes the interaction between SARS-CoV-2 and innate immunity, and discusses the escaping mechanism against interferons (IFNs), predominantly Nsps, as well as the pathology of the disease, related to exuberant activation of the complement system. Therefore, a detailed understanding of the interactions of the virus with innate immunity and the immunopathology behind COVID-19 could lead to new approaches aimed at improving immunotherapy for SARS-CoV-2.
Innate Immune Response to SARS-CoV-2s,
For effective recognition of viral infection and successive activation of an antiviral state, innate immune responses are vital and essential host defense mechanisms.15 The innate immune response is the first-line inborn host defense mechanism against non-specific pathogens. This innate immunity to viruses involves complex interactions between soluble factors and cells. These cells understand and sense the foreign microbes through the presence of pattern recognition receptors (PRRs), commonly named toll-like receptors (TLRs). TLRs, a family of sensor proteins, assist the immune system in discriminating between “self” and “non-self”.16 The TLR detects a specific and conserved microbial domain called the pathogen-associated molecular pattern (PAMP), which acts as a ligand with elements such as lipids, lipoproteins, proteins, RNAs, and DNA of the microbe’s structure.17,18 TLR family members TLR-3, TLR-7, TLR-8, and TLR-9 are involved exclusively in the membrane’s intracellular compartments, such as endosomes, in the recognition of viral nucleotides. The TLR signaling cascade is mediated by the TIR (toll/IL-1 receptor) domain, which contains an adaptor molecule, such as myeloid differentiation primary response gene 88 (MyD88), TIR-domain containing adaptor protein (TIRAP), or TIR-domain-containing adapter (TRIF).18–20 The TIR complex plays a role in the inflammatory immune response via the production of proinflammatory cytokines, type I IFN, and up-regulation of costimulatory molecules.21,22 In a small COVID-19 patient cohort, levels of IFN-α and IFN-stimulated gene (ISG) were associated with viral load as well as disease severity.23 Taking this a step further, these studies indicate that severe infections lead to high IFN signatures but fail to clear and bring down the viral load. In contrast, owing to their virulence gene product, the expression and IFN-α/β response are decreased (Figure 1). In the same way as SARS-CoV,20 SRAS-CoV-2 uses its spike glycoprotein to bind with the angiotensin-converting enzyme-2 (ACE2) receptor to gain entry to cells,24 suggesting a similar cellular tropism and route of entry. This receptor is expressed not only in cardiopulmonary tissues, particularly in type I alveolar cells, but also in some hematopoietic cells, including monocytes, dendritic cells, and macrophages.19 Prior to the translocation of IFN regulator factors (IRF-7) and nuclear factor-κB (NF-κB) from the cytoplasm to the nucleus, the single-stranded viral RNA should be recognized by TLR-7 with the following recruited signal transducing adaptor proteins, MyD88, playing a key role. Hence, it promotes the expression of type I IFN (primarily IFN-α and IFN-βs), chemokines, and inflammatory cytokines (Figure 2).25 The major antiviral chemical mediators in the innate immune response are IFNs, which act to limit the spread of the virus and enhance macrophage phagocytosis of the pathogen, as well as NK cell restriction of infected target cells.
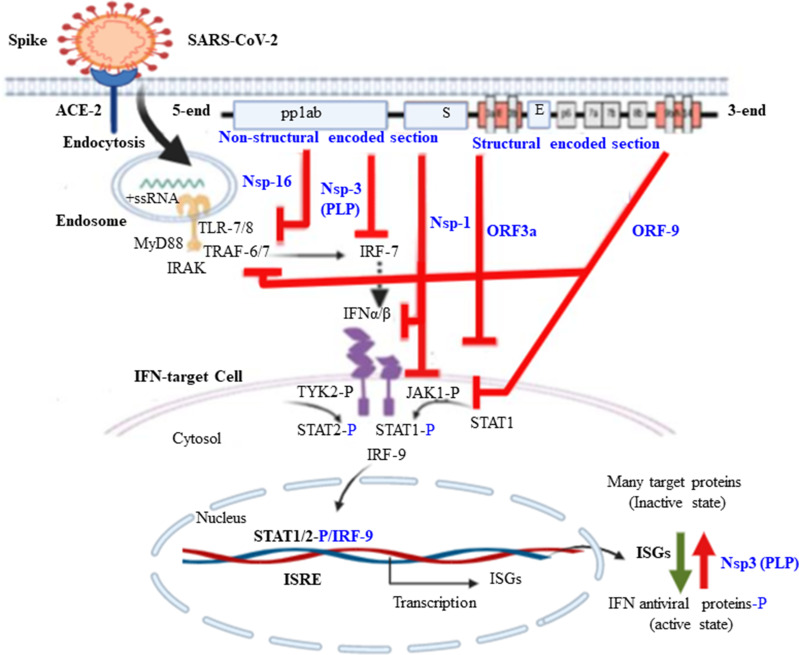
Figure 4.
Proposed mechanism of SARS-CoV-2 induced inhibition of IFN production and it signaling during action on target cells. Some SARS-CoV-2 genes act as virulence factor that antagonize innate immune response by inhibiting IFN gene expressions such as Nsp16, Nsp3, and ORF9. Other gene components of the SARS virus act as inhibitors of INF signaling on different target cells mainly ORF9, ORF3a and Nsp1.
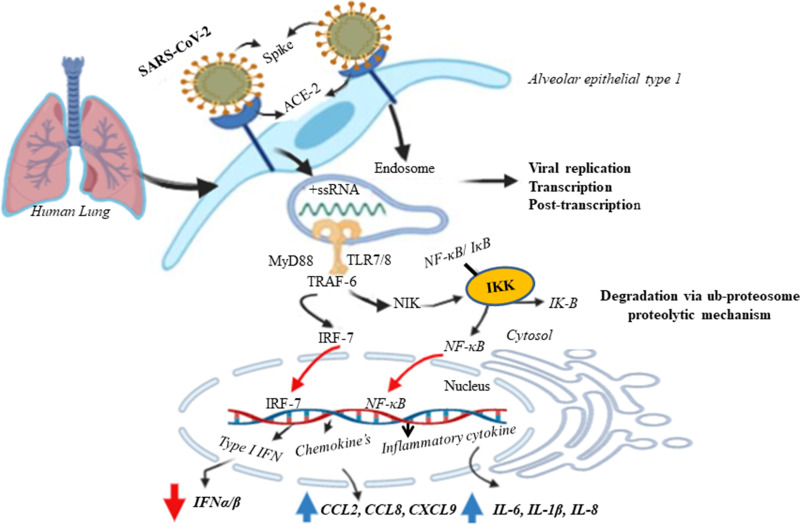
Figure 1.
Possible mechanism of SARS-CoV-2 mediated innate immune response at the lung alveolar cell. The SARS-CoV -2 infects alveolar epithelial cells (mainly Alveolar epithelial type 1) through ACE2 receptor. Recognition of ssRNA by TLR-7 in the endosomal membrane recruits MyD88 to the receptor, which induces proinflammatory cytokines and type I IFNs through MyD88-TRAF6-NIK-IKK-NF-Kb pathway and MyD88-TRAF-6-IRF-7 respectively. The destruction of epithelial cells and the increase of cell permeability lead to the release of virus. Phosphorylated IRF-7 and translocate to the nucleus results expression of type I IFN genes and release IFNα/β. IkappaB kinase (IKKs) directly phosphorylate the inhibitory IκB family members, which normally sequester NF-κB as inactive form (NF-κB/ IκB) in the cytosol. However, phosphorylation of IκBs leads to their polyubiquitination and degradation via ubiquitin proteasome proteolytic mechanism. The activation of the NF-κB pathway results in the induction of aberrant inflammatory cytokines and chemokine’s secretion by alveolar cells including IL-6, IL-1β, IL-8, CCL2, CCL8 and CXCL9.
Interferon
Interferons are a large family of genetically and functionally related proteins. These molecules were discovered in the 1950s by Isaacs and Lindemann. They are recognized as essential first-line biological protein defense against viral infection in mammals.26,27 The term “IFN” was coined to describe a special substance, possibly produced by cells, which is centrally important in the innate immune response to the virus through “interfering” with virus replication, with antiviral activity, antiproliferative activity, stimulation of cytotoxic T cells and NK cells, and immune modulation.28,29 IFNs are divided into three types, named type I, type II, and type III.20 Type I IFNs are the largest IFN family, including IFN-α (13 subtypes) encoded at chromosome 9, IFN-β encoded at chromosome 12, IFN-ε, IFN-κ, and IFN-ω.26,30,31 IFN-δ, IFN-τ, and IFN-ζ (or limitin) have also been identified as type I IFNs in swine, ruminants, and mice, respectively.32 Furthermore, four IFN-like cytokines have been reported: limitin (found only in mice), IL-28A, IL-28B, and IL-29, found in humans and other mammals. Almost all cell types are capable of producing IFN-α/β; however, plasmacytoid dendritic cells (pDC) are specialist type I IFN-producing cells, particularly producing IFN-α, β, and ω during the course of an infection.
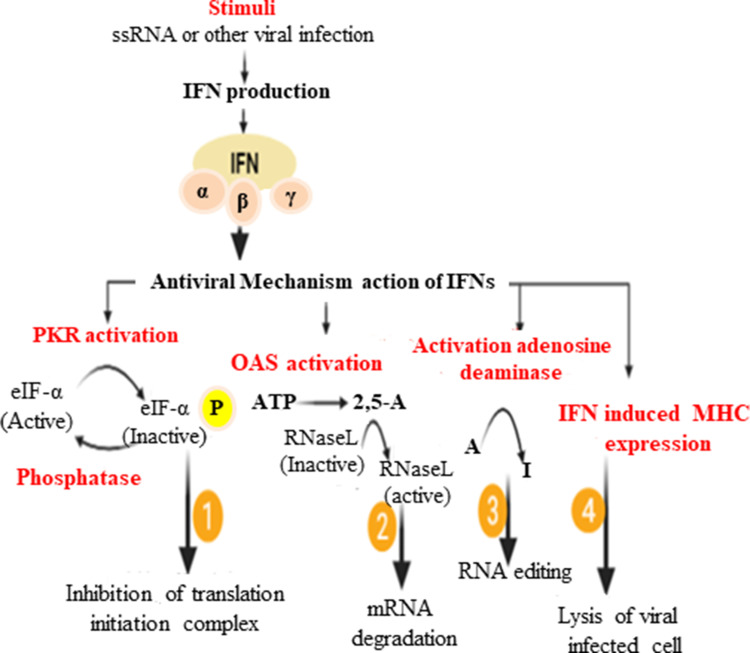
The activation of the IFN system is the most important defense for containing the initial stages of a viral infection. IFN is mostly produced when TLRs on the cell surface (TLR-1, TLR-2, TLR-4, TLR-5, TLR-6, and TLR-10) are induced by lipopolysaccharides of Gram-negative bacteria, and virion proteins.33 On the other hand, some TLRs are anchored to the endosomal membrane (TLR-3, TLR-7, TLR-8, and TLR-9), which is stimulated by PAMPs such as dsRNA, ssRNA virus, and viral dsDNA. Upon the attachment or binding of viral RNAs to TLR-7/8, it can induce genes encoding for inflammatory cytokines and type I IFNs via NF-κB and IRF-7 (Figure 3).16,33,34 In addition, cytoplasmic viral RNA genomes or viral replicative intermediates can be recognized by the family of RNA helicases, such as retinoic acid-induced gene-I (RIG-I) and melanoma differentiation-associated gene-5 (MDA-5), which ultimately can control genes encoded for IFN production.35–37 In the foundation of the innate immune response system, type I IFNs are first line of defense against viral infection. Immediately after the body encounters a viral infection, host cell PRRs, such as TLRs, RLR, Mda5, protein kinase R (PKR), and 2′-5′oligoadenylate synthetase (OAS), serve in discriminating self from non-self ligands at a cellular level, which stimulates the cells to produce and secrete IFN. The IFN signaling cascades are initiated upon its binding with its receptor, IFRAR1/2, leading to cytoplasmic kinase activation that allows the recruitment of JAK-1 and TYK, which phosphorylate STAT1 and STAT2, respectively. Both transcription factors then form heterodimers with IRF-9, and translocate to act on the IFN-stimulated response element (ISRE) and promote the binding of RNA polymerase II to expressISG.20 After translation followed by posttranslational modification, the product of ISG brings an antiviral effect and immunomodulation, and by inhibiting replication it can limit both the spread and the load of the virus (Figure 3).
Figure 2.
Schematic representation of how interferons induce antiviral state in host cells.
Interferon as a Treatment Option for COVID-19
IFNs are rapidly secreted and induce a wide range of effects that not only act upon innate immune cells but also modulate the adaptive immune system. Type I IFNs-α/β are broad-spectrum antivirals, both exhibiting direct inhibitory effects on viral replication and supporting an immune response to clear the viral infection.38 A study conducted in China showed that treatment with IFN-α2b (manufactured using recombinant DNA technology) with or without Arbidol® (an inhibitor of Nsp12 cofactors called Nsp7/8) significantly reduced the duration of detectable virus in the upper respiratory tract. At the same time, IFN-α2b reduced the duration of elevated blood levels of the inflammatory markers IL-6 and C-reactive protein. Surprisingly, from the onset of symptoms, the mean time to viral clearance was 27.9, 21.1, and 20.3 days for patients treated with Arbidol (umifenovir) alone, IFN-α2b alone, and a combination of Arbidol and IFN-α2b, respectively.39 Therefore, these research findings illustrate that IFN treatment accelerated viral clearance by approximately 7 days.39,40 Moreover, for better clinical outcomes of patients with cytokine storm, the IL-6 receptor inhibitor tocilizumab (branded as Actemra), a recombinant human IL-6 monoclonal antibody, is useful as a therapeutic adjunct.41,42
Complement Activation in SARS-CoV-2 Infection
The complement system is a key player in innate immunity and consists of both soluble factors and cell surface receptors that interact to sense and respond against invading pathogens.43 It can also act as a bridge between innate and adaptive immunity to enhance humoral immunity and T-cell function.44 Complements recognize non-self-structures which present on the surface of pathogens, and initiate activation of zymogens via proteolytic cascades, resulting in inflammation, opsonization, and/or cell lysis.45 However, the defensive function of the complement system can exacerbate immune, inflammatory, and degenerative responses in various pathological conditions. In general, in response to foreign pathogen, there are three major complement activation pathways. The first one is the classical pathway, characterized by C1q-mediated antigen–antibody complexes. The second is the lectin pathway, initiated by mannose-binding lectin (MBL), which recognizes the microbial surface glycan domain, called mannose. Mannose-associated serine protease (MASP) cleaves complement (C)4 and 2. C4 and C2 cleavage products form the classical and lectin pathway C3 convertase (C4bC2a), which cleaves C3 into C3b and C3a. A second molecule of C3b can associate with C4bC2a to form C5 convertase (C4bC2aC3b) of the classical and lectin pathways.45 The third is the alternative pathway, mediated by spontaneous cleavage of C3 binding with the pathogen’s cell surface components.44–46 While complements play a central role in innate immunity and provide abridge with the adaptive immune response against pathogens, their overactivation may cause excessive alveolar air sac infiltration byneutrophils, and monocytes secondary to excessive production of C3a and C5a (Figure 4). Subsequently, this leads to collateral lung injury and exacerbating factors for SARS-CoV-associated ARDS. Since SARS-CoV-2 is a new virus, the body does not have memory antibodies or specific IgG to detect the spike glycoprotein through activation of adaptive immunity in the early phase of COVID-19 infection.47,48 Since SARS-CoV-2 is a respiratory disease, the primary antibody secreted might be IgA, which is not reliable for activating the classical complement pathway, unless based on an aggregated antibody. Instead, the virus may form IgA complexes or SARS-CoV–S-N-glycan (N-330) MBL binding to activate the lectin complement cascade defense against the invading virus in mucosal immunity.49
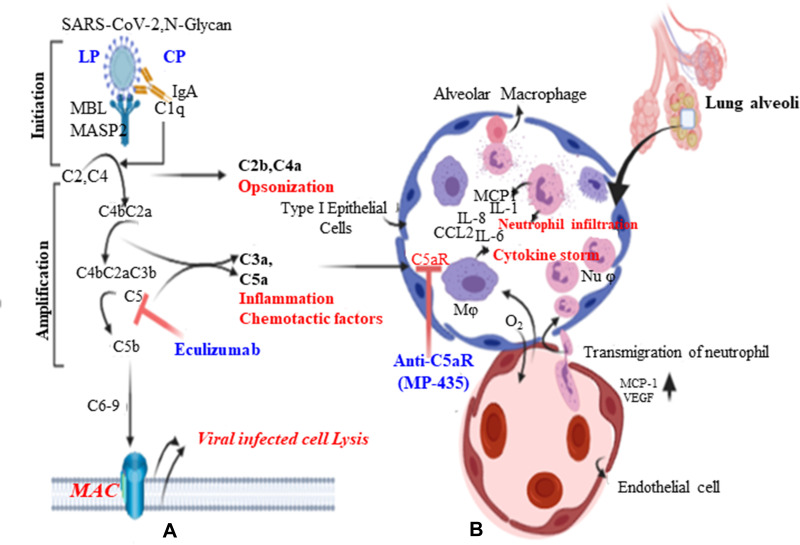
Figure 3.
Complement pathways and pathology of ARDS. A:lectin(LP) and classical(CP) pathways use C3 as common intermidate. Exuberant activation of complement pathways results over production of chemo attractants, C3a and C5a (anaphylatoxins) and promote inflammation. Ecluizumab humanized monoclonal antibody targat aganist C5 preventing breakdown into C5a (anaphylatoxin action of complement component) and C5b, which is an integral component of the membrane attack complex(MAC). B:Overproduction of C3a and C5a which acts on type I epithelium of the air sack (pulmonary edema and fibrosis or hyaline membrane) as well as the endothelial wall of the vasculature (increased vascular permeability), results recruitment of inflammatory cells mainly monocytes and nutrophil, andrelease of many inflammatory cytokines known as inflammatory cytokine storm.
Overriding activation of complement pathways leads to the formation of bioactive molecules, mainly C3a and C5a. C5a is the most potent chemoattractant involved in the migration and recruitment of inflammatory cells in the activation of phagocytic cells and the release of granular enzymes and free radicals.50 Since SARS-CoV-2 has a similar identity to SARS-CoV, C3a products of the complement cascade exacerbate SARS-CoV-associated ARDS.51 A study showed that C3 knockout mice infected with SARS-CoV had less respiratory dysfunction, decreased infiltration of neutrophils and monocytes, and low levels of cytokines and chemokines in both lungs as well as sera. However, they showed similar viral load in the lung alveoli.51 SARS-CoV-2-mediated C5a triggers inflammatory cell activation and cytokine release. This results in epithelial cell degradation and increased vascular permeability. Together, large numbers of inflammatory infiltrates, fluid, and blood cells shift to the alveoli and lung pleura, resulting in dyspnea and respiratory insufficiency. Many scholars have shown that C5a signaling contributes greatly to inflammation and lung damage, and is targeted at treating highly pathogenic virus infections. Moreover, recently C5aR targeting has shown more promising beneficial effects than C5 in small animal models of ARDS by reducing proinflammatory cytokines.52–54 In addition, different research output elaborates that C5 is a bottleneck for both the release of C5a as an inflammatory mediator and the membrane attack complex (MAC) (C5b–C9) as a defense against viral infection. Thus, anti-C5a and anti-C5aR only block the effects of C5a without affecting the formation of the MAC, thus protecting against not only viral infection but also superimposed bacterial infections of the lung.55,56 Eculizumab is an anti-C5 antibody; however, C5aR antagonists such as MP-435 target the action of C5a alone and do not inhibit MAC formation Figure 4).57 Therefore, receptor antagonists (anti-C5aR) may lessen a patient’s susceptibility to bacterial infection, so caution is needed when anti-C5 monoclonal antibodies are used.
SARS-CoV-2 Subversion of Innate Immunity
Viruses have evolved a number of strategies to evade the immune response.58,59 Even though SARS-CoV-2 and other related viruses, such as SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV), are sensitive to the IFN-α/β immune response system, this novel coronavirus is still highly pathogenic. Recent and previous research experience on SARS-CoV showed that the genome contains virulence factors that target core molecules to evade the host response. One of the major mechanisms used by SARS-CoV-2 to escape from host innate immunity is encoded COVID-19 proteins inhibiting IFN production and signaling cascade. This allows the virus to replicate, transcribe, and be transmitted. The detailed understanding of the mechanism of hindrance of IFN production and actions, promoting COVID-19 replication and proliferation, is essential in planning therapeutic options and controls against viral infection.
Nsp16 encodes 2′-O-methyltransferase, which can methylate the RNA cap at 2ʹ-O-positions.60 The first strategies inhibit type I IFN production by viral genes encoding for Nsp16 or S-adenosyl-L-methionine (Ado Met)-dependent 2ʹ-O-methylase. This involves posttranscriptional modification of the ssRNA at the 5ʹ-terminal and changing 5′-triphosphate groups into methylated 5ʹ-cap-1 formation, and mimicking the host cell’s mRNA. Therefore, the viral RNA is capable of mimicking the host RNA and is not detected by MDA-5 and TLR-7/8, allowing the virus to escape innate immune recognition.61 Yet, any non-self-recognizable RNA (having 5′-triphosphate groups) in the cytoplasm can be specifically recognized by RIG-I or TLR-7/8 or Mda5, which then amplify type I IFN production.62,63 In contrast, eukaryotic mRNA, which is not recognized by retinoic acid-inducible protein I (RIG-I) or MDA-5, usually has a 5′-cap structure methylated at the N7 position of the capping guanosine residue (cap 0), the resulting ribose-2′-O position (cap 1), and sometimes at adjoining residues (cap 2) during posttranscriptional modification.64 Also, Nsp10 works with Nsp16 to bind to SAM and m7GpppA-RNA as substrated for its 2ʹ-O-MTase activity.64,65 Thus, eukaryotic mRNA 5′-cap structures are known to increase mRNA stability, protecting against 5′-exonuclease. This facilitates the binding of translational initiation factor proteins with both 40s and 60s ribosomes, and enhances translational efficacy.66
Nsp3 (1922 aa) is the largest multidomain protein among all the Nsps of β-CoV. It is cleaved off from ORF1a/ORF1ab by the papain-like protease domain or PL2pro domain that is within Nsp3 itself.67,68 It is a central player during the formation of viral replication associated with other Nsps, especially Nsp4 and Nsp6.69 SARS-CoV-2 Nsp3 contains 16 domains, namely ubiquitin-like domain 1 (Ubl1), hypervariable region (HVR) or acidic domain, macrodomain I/II/III (MacI/II/III), domain preceding Ubl2 and PL2pro (DPUP), ubiquitin-like domain 2 (Ubl2), papain-like protease 2 domain (PL2pro), nucleic acid binding domain (NAB), beta coronavirus-specific marker domain (βSM), transmembrane domain 1 (TM1), Nsp3 ecto-domain (3Ecto), transmembrane domain 2 (TM2), amphipathic helix region (AH1), and domains specific to Nidoviriales and Coronaviridae (Y1 and CoV-Y).68 After Nsp16, Nsp3 acts as the second most important virulent gene product, mainly by inhibiting RIG-I (cytoplasmic cascade) and TLR-3/7 (endosomal)-dependent IFN-α/β production, probably by interaction of the papain-like protease (PLP) domain with protein stimulators of IFN gene (STING). PLP of MERS-CoV down-regulates the gene encoding for IFN-β via its deubiquitinating activity.70 Moreover, even though the mechanism is not yet clear, different scholars have indicated that the PLP of SARS-CoV inhibits the host antiviral innate immune response by inhibiting phosphorylation, dimerization, and nuclear translocation of IRF-7 or -3, likely by forming a complex with IRF-3.71,72
Nsp1 is found in the closest portion of the 5ʹ end encoded by ORF1a/b. This Nsp is considered as the third virulent factor, by inhibiting IFN-β mRNA from being translated into the protein product and by promoting degradation of overexpressed host endogenous mRNA. Its mechanism is to bind with 40s ribosomal subunits and cause conformational change (loss of function), and prevent 80s formation, resulting in translational inhibition. Furthermore, the Nsp1–40s ribosomal complex induces the modification of 5ʹ-capped mRNA and renders the template RNA translationally incompetent.64,73,74 Nsp1 also inhibits the action of IFN-α/β on its signaling cascade intermediates, mainly on the JAK-STAT pathway via blocking its phosphorylation (Figure 1).72
The SARS-CoV-1 or -2 genome consists of nine unique ORFs. Of these, ORF3a is the largest and encodes a protein of 274 amino acids.75 The 3a protein is part of the virus particle and is expressed abundantly in infected cells.76 The major virulence of this sectioned viral protein is achieved by down-regulation of the expression of the type I IFN receptor (IFNAR), leading to a blockade on type I IFN signaling.72,77 IFNs are known to be crucial regulators of the development of Treg cells. In the regulatory immune system of patients with COVID-19, Treg cell counts have been shown to correlate inversely with disease severity.78
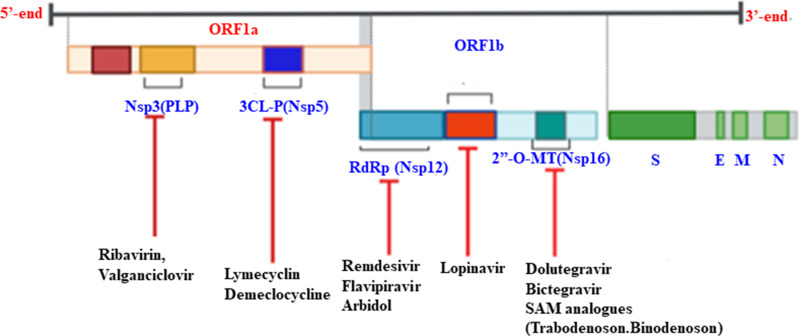
Non-Structural Protein as a Potential Therapeutic Target for SARS-CoV-2
The genomes of replicases are involved during viral RNA gene expression. They are also considered as virulent gene products of the viruses to enable escape from the host immune response.70,79 The multidomain Nsp3 (PLP), Nsp5 (3CLpro), Nsp12 (the main RNA-dependent RNA polymerase), Nsp16 (2ʹ-O-methyltransferase), and Nsp13 (ATP-dependent RNA helicase) are the most important popular drug targets because of their vital biological function (Figure 5).70
Figure 5.
Therapeutic targets of non-structural proteins of SARS-CoV-2.
Since COVID-19 does not have a confirmed drug option, many broad-spectrum antibiotics and antiviral drugs are under clinical trial to reduce and treat the virus.80–86 Drugs that have shown high binding affinity to Nsp3 include ribavirin, valganciclovir, thymidine, and levodropropizine. In addition, FDA-approved drugs (remdesvir, saquinavir, and darunavir) and two small molecules (flavone and coumarin derivatives) are potential inhibitors of 3CLpro.87 Moreover, antibacterial drugs (lymecyclin, demeclocycline, and chlorhexidine) also target3CLpro.79
COVID-19 uses unique 2ʹ-O-methyltransferase (2ʹ-O-MTase) as capping machinery of its RNA at the 5ʹ-end. As a result of this, the viral genome mimics the host mRNA and escapes from innate immune sensing factors, such as RIG-I and MDA-5, and type I IFN antiviral activities.61,88,89 A known HIV inhibitor drug, dolutegravir (DG) showed an estimated binding free energy of 9.4 kcal/mol, and inhibits 2ʹ-O-MTase, while bictegravir conferred an estimated binding free energy (DG) of 8.4 kcal/mol against 2ʹ-O-MTase. Moreover, natural ligand (SAM) analogues, namely trabodenoson, binodenoson, and regadenoson, may be potent inhibitors of the substrate-binding pockets of Nsp16.86,89
Nsp1 inhibits host gene expression via targeting the 40s ribosomal subunit, which induces host mRNA degradation and IFN-dependent signaling. Therefore, Nsp1 is a promising drug target, and its inhibition would make SARS-CoV more susceptible to the host’s antiviral defenses. This implies that the innate immune activities via type I IFN would become free of being inhibited by Nsp1 and work actively against the virus.90,91
The other protein that is most conserved in SARS-CoV-2 is RNA-dependent RNA polymerase (Nsp12), which is particularly involved in low-fidelity replication and transcription at the time of viral priming.92,93 In addition, Nsp7 and Nsp8 complexes act as cosubstrates to promote the binding of Nsp12 to RNA and boost enzyme activity at the time of transcription.79,92 The other potential alternative COVID-19 broad-spectrum antiviral drug therapy, Arbidol (an indole derivative), also targets Nsp7/8 complex and blocks viral replication by inhibiting SARS-CoV-2 fusion to the host cell.79,80
Remdesvir (RDV) is a nucleoside analogue and shows the most promising broad-spectrum antiviral activity in SARS-CoV-2.41 This drug was originally developed by Gilead Sciences (USA) for treating Ebola virus disease. Subsequent studies showed that RDV is a monophosphoramidate prodrug that has metabolized into C-adenosine nucleoside analogue by targeting RNA-dependent RNA polymerase (RdRp) as a chain terminator.80,85,94–96 Similarly, favipiravir is a guanine analogue, also used as an inhibitor of RNA-dependent RNA polymerase, but it has been less approved than remdesvir (Table 1).79,97
Table 1.
Summary of Some Non-Structural Protein Targeting Drugs of COVID-19 Registered Under Clinicaltrials.gov
| Drug(s) | Class | Site of Study | Clinical Trial Status | Available Clinical Trial Outcomes |
|---|---|---|---|---|
| Remdesvir (GS-5734) | Ebola virus (EBOV) | USA, Birmingham | Completed (Phase III) | Time to recovery by day |
| Favipiravir | Antiviral for flu and Ebola | USA, Arizona | Recruiting (Phase IV) | Time to clinical improvement until 14 days later |
| Lopinavir and ritonavir | Antiretroviral (PI) | Canada (Nova Scotia Health Authority) | Recruiting | No clear benefit* |
| Ribavirin | Antiviral and anti-HCV | USA, Colorado | Recruiting | NA |
| Trabodenoson | SAM ligand analogue | USA | Recruiting | NA |
Note: *WHO discontinued this clinical trial.
Abbreviations: PI, protease inhibitor; HCV, hepatitis C virus; NA, not available.
The other multifunctional non-structural protein is Nsp13 (helicase), for unwinding of dsDNA as well as RNA from 5ʹ–3ʹ polarity, coupled with NTP (ATP) as an energy source.98,99 Because this SARS-CoV helicase enzyme is absolutely necessary for subsequent viral replication and proliferation, it is thought to be an attractive target of anti-SARS-CoV-2 drugs. Lopinavir (known as a protease inhibitor of HIV infection) possibly targets Nsp3b/3c and helicase, with ritonavir being used to increase the half-life of lopinavir via inhibition of cytochrome 450 (Table 1).94,97 Collectively, targeting the Nsp of the novel coronavirus helps to boost the innate immune recognition ability as well as the activity of the type I IFN antiviral signaling cascade.
Conclusions and Future Directions
In innate immunity, IFNs are key players against viral infection; however, SARS-CoV-2 infecting cells must overcome the IFN-mediated antiviral response to replicate and propagate to new host cells. Because of different evasion mechanisms of the SARS-CoV-2 genome, containing virulent accessory as well as non-structural gene products, such as Nsp16, Nsp3, Nsp1 ORF3a, and ORF9, designing drugs that inhibit these gene products is vital for boosting the action of IFNs against COVID-19. Dysregulated complement systems are the most likely cause of the pathogenesis of ARDS or cytokine storms in COVID-19. We have much research evidence to show that anti-C5a and/or C5aR antibody can provide promising therapeutic options by reducing histopathological injury of lung alveoli and decreasing inflammatory cytokines. Furthermore, we suggest IFN-α2b and Arbidol as potential therapeutic options to reduce the viral load as well as its pathological sequelae. SARS-CoV-2 induces an inflammatory cytokine storm linked with an exuberant elevation of C5a and downregulation of IFNα/β secretion, and its action is associated with the disease severity. Therefore, it is reasonable to hypothesize that blocking of C5aR, IL-6, and virulent Nsp of SARS-CoV-2 might be an effective therapeutic avenue for COVID-19-mediated ARDS and its complications.
Acknowledgments
We would like to forward our gratitude to the authors of the articles that we used to generate this review report.
Funding Statement
For this review, we did not receive any grants from any funding agencies.
Abbreviations
C, complement; Nsp, non-structural protein; ORF, open reading frame; IL-6, interleukin-6; MERS, Middle East respiratory syndrome; SARS, severe acute respiratory syndrome; COVID-19, coronavirus disease 2019.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no any competing interest.
References
- 1.Su S, Wong G, Shi W, et al. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24(6):490–502. doi: 10.1016/j.tim.2016.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonilla-Aldana DK, Holguin-Rivera Y, Cortes-Bonilla I, et al. Coronavirus infections reported by ProMED, February 2000–January 2020. Travel Med Infect Dis. 2020;35:101575. doi: 10.1016/j.tmaid.2020.101575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiss SR, Navas-Martin S. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol Mol Biol Rev. 2005;69(4):635–664. doi: 10.1128/MMBR.69.4.635-664.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Veit S, Jany S, Fux R, Sutter G, Volz A. CD8+ T cells responding to the middle east respiratory syndrome coronavirus nucleocapsid protein delivered by vaccinia virus MVA in mice. Viruses. 2018;10(12):718. doi: 10.3390/v10120718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu J, Zhao S, Teng T, et al. Systematic comparison of two animal-to-human transmitted human coronaviruses: SARS-CoV-2 and SARS-CoV. Viruses. 2020;12(2):244. doi: 10.3390/v12020244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan JF-W, Yuan S, Kok K-H, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akalu Y, Ayelign B, Molla MD. Knowledge, attitude and practice towards COVID-19 among chronic disease patients at Addis Zemen Hospital, Northwest Ethiopia. Infect Drug Resist. 2020;13:1949. doi: 10.2147/IDR.S258736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long Q-X, Tang X-J, Shi Q-L, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;1–5. [DOI] [PubMed] [Google Scholar]
- 10.Gao Z, Xu Y, Sun C, et al. A systematic review of asymptomatic infections with COVID-19. J Microbiol Immunol Infect. 2020. doi: 10.1016/j.jmii.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo Y, Trevathan E, Qian Z, et al. Asymptomatic SARS-CoV-2 infection in household contacts of a healthcare provider, Wuhan, China. Emerg Infect Dis. 2020;26(8):1930–1933. doi: 10.3201/eid2608.201016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frieman M, Heise M, Baric R. SARS coronavirus and innate immunity. Virus Res. 2008;133(1):101–112. doi: 10.1016/j.virusres.2007.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Satija N, Lal SK. The molecular biology of SARS coronavirus. Ann N Y Acad Sci. 2007;1102(1):26–38. doi: 10.1196/annals.1408.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muller MP, McGeer A Severe acute respiratory syndrome (SARS) coronavirus. Paper presented at: Seminars in respiratory and critical care medicine; 2007. [DOI] [PubMed] [Google Scholar]
- 15.Wang L, Wang K, Zou Z-Q. Crosstalk between innate and adaptive immunity in hepatitis B virus infection. World J Hepatol. 2015;7(30):2980. doi: 10.4254/wjh.v7.i30.2980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Totura AL, Whitmore A, Agnihothram S, et al. Toll-like receptor 3 signaling via TRIF contributes to a protective innate immune response to severe acute respiratory syndrome coronavirus infection. MBio. 2015;6(3):e00638–00615. doi: 10.1128/mBio.00638-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Le Goffic R, Balloy V, Lagranderie M, et al. Detrimental contribution of the toll-like receptor (TLR)3 to influenza A virus–induced acute pneumonia. PLoS Pathog. 2006;2(6):e53. doi: 10.1371/journal.ppat.0020053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takeda K, Akira S TLR signaling pathways. Paper presented at: Seminars in immunology; 2004. [DOI] [PubMed] [Google Scholar]
- 19.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21(1):335–376. doi: 10.1146/annurev.immunol.21.120601.141126 [DOI] [PubMed] [Google Scholar]
- 20.Totura AL, Baric RS. SARS coronavirus pathogenesis: host innate immune responses and viral antagonism of interferon. Curr Opin Virol. 2012;2(3):264–275. doi: 10.1016/j.coviro.2012.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2(8):675–680. doi: 10.1038/90609 [DOI] [PubMed] [Google Scholar]
- 22.Hoebe K, Beutler B. LPS, dsRNA and the interferon bridge to adaptive immune responses: trif, Tram, and other TIR adaptor proteins. J Endotoxin Res. 2004;10(2):130–136. doi: 10.1177/09680519040100021001 [DOI] [PubMed] [Google Scholar]
- 23.Wei L, Ming S, Zou B, et al. Viral invasion and type i interferon response characterize the immunophenotypes during COVID-19 infection. SSRN 3555695; 2020.
- 24.Chen J. Pathogenicity and transmissibility of 2019-nCoV—a quick overview and comparison with other emerging viruses. Microbes Infect. 2020;22(2):69–71. doi: 10.1016/j.micinf.2020.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma DY, Suthar MS. Mechanisms of innate immune evasion in re-emerging RNA viruses. Curr Opin Virol. 2015;12:26–37. doi: 10.1016/j.coviro.2015.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sen GC. Viruses and interferons. Annu Rev Microbiol. 2001;55(1):255–281. doi: 10.1146/annurev.micro.55.1.255 [DOI] [PubMed] [Google Scholar]
- 27.Isaacs A, Lindenmann J. Virus interference. I. The interferon. Proc R Soc Lond B Biol Sci. 1957;147(927):258–267. [DOI] [PubMed] [Google Scholar]
- 28.Crommelin DJ, Sindelar R, Meibohm B. Pharmaceutical Biotechnology. Springer-Verlag: New York; 2016 [Google Scholar]
- 29.De Weerd NA, Samarajiwa SA, Hertzog PJ. Type I interferon receptors: biochemistry and biological functions. J Biol Chem. 2007;282(28):20053–20057. doi: 10.1074/jbc.R700006200 [DOI] [PubMed] [Google Scholar]
- 30.Pestka S. The interferons: 50 years after their discovery, there is much more to learn. J Biol Chem. 2007;282(28):20047–20051. doi: 10.1074/jbc.R700004200 [DOI] [PubMed] [Google Scholar]
- 31.Pestka S, Krause CD, Walter MR. Interferons, interferon‐like cytokines, and their receptors. Immunol Rev. 2004;202(1):8–32. doi: 10.1111/j.0105-2896.2004.00204.x [DOI] [PubMed] [Google Scholar]
- 32.Fensterl V, Sen GC. Interferons and viral infections. Biofactors. 2009;35(1):14–20. doi: 10.1002/biof.6 [DOI] [PubMed] [Google Scholar]
- 33.Wu J, Chen ZJ. Innate immune sensing and signaling of cytosolic nucleic acids. Annu Rev Immunol. 2014;32:461–488. doi: 10.1146/annurev-immunol-032713-120156 [DOI] [PubMed] [Google Scholar]
- 34.Nelemans T, Kikkert M. Viral innate immune evasion and the pathogenesis of emerging RNA virus infections. Viruses. 2019;11(10):961. doi: 10.3390/v11100961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoneyama M, Fujita T. RNA recognition and signal transduction by RIG‐I‐like receptors. Immunol Rev. 2009;227(1):54–65. [DOI] [PubMed] [Google Scholar]
- 36.Kell AM, Gale M Jr. RIG-I in RNA virus recognition. Virology. 2015;479:110–121. doi: 10.1016/j.virol.2015.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loo Y-M, Fornek J, Crochet N, et al. Distinct RIG-I and MDA5 signaling by RNA viruses in innate immunity. J Virol. 2008;82(1):335–345. doi: 10.1128/JVI.01080-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang BX, Fish EN Global virus outbreaks: interferons as 1st responders. Paper presented at: Seminars in immunology; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou Q, Chen V, Shannon CP, et al. Interferon-α2b Treatment for COVID-19. Front Immunol. 2020;11:1061. doi: 10.3389/fimmu.2020.01061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lian N, Xie H, Lin S, Huang J, Zhao J, Lin Q. Umifenovir treatment is not associated with improved outcomes in patients with coronavirus disease 2019: a retrospective study. Clin Microbiol Infect. 2020;26(7):917–921. doi: 10.1016/j.cmi.2020.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang W, Zhao Y, Zhang F, et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): the experience of clinical immunologists from China. Clin Immunol. 2020;214:108393. doi: 10.1016/j.clim.2020.108393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu R, Wang L, Kuo H-CD, et al. An update on current therapeutic drugs treating COVID-19. Curr Pharmacol Rep. 2020:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma Y, Liu Y, Zhang Z, Yang G-Y. Significance of complement system in ischemic stroke: a comprehensive review. Aging Dis. 2019;10(2):429. doi: 10.14336/AD.2019.0119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Avirutnan P, Mehlhop E, Diamond MS. Complement and its role in protection and pathogenesis of flavivirus infections. Vaccine. 2008;26:I100–I107. doi: 10.1016/j.vaccine.2008.11.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dunkelberger JR, Song W-C. Complement and its role in innate and adaptive immune responses. Cell Res. 2010;20(1):34. doi: 10.1038/cr.2009.139 [DOI] [PubMed] [Google Scholar]
- 46.Jack DL, Klein NJ, Turner MW. Mannose‐binding lectin: targeting the microbial world for complement attack and opsonophagocytosis. Immunol Rev. 2001;180(1):86–99. doi: 10.1034/j.1600-065X.2001.1800108.x [DOI] [PubMed] [Google Scholar]
- 47.Okba NM, Muller MA, Li W, et al. SARS-CoV-2 specific antibody responses in COVID-19 patients. medRxiv. 2020. [Google Scholar]
- 48.Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin Infect Dis. 2020. doi: 10.1093/cid/ciaa344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou Y, Lu K, Pfefferle S, et al. A single asparagine-linked glycosylation site of the severe acute respiratory syndrome coronavirus spike glycoprotein facilitates inhibition by mannose-binding lectin through multiple mechanisms. J Virol. 2010;84(17):8753–8764. doi: 10.1128/JVI.00554-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guo R-F, Ward PA. Role of C5a in inflammatory responses. Annu Rev Immunol. 2005;23:821–852. doi: 10.1146/annurev.immunol.23.021704.115835 [DOI] [PubMed] [Google Scholar]
- 51.Gralinski LE, Sheahan TP, Morrison TE, et al. Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis. MBio. 2018;9(5):e01753–01718. doi: 10.1128/mBio.01753-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bosmann M, Ward PA. Protein-based therapies for acute lung injury: targeting neutrophil extracellular traps. Expert Opin Ther Targets. 2014;18(6):703–714. doi: 10.1517/14728222.2014.902938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bhatia M, Moochhala S. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J Pathol. 2004;202(2):145–156. doi: 10.1002/path.1491 [DOI] [PubMed] [Google Scholar]
- 54.Wang R, Xiao H, Guo R, Li Y, Shen B. The role of C5a in acute lung injury induced by highly pathogenic viral infections. Emerg Microbes Infect. 2015;4(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Campbell CM, Kahwash R. Will Complement Inhibition Be the New Target in Treating COVID-19–Related Systemic Thrombosis? Circulation. 2020;141(22):1739––1741. [DOI] [PubMed] [Google Scholar]
- 56.Wang R, Xiao H, Guo R, Li Y, Shen B. The role of C5a in acute lung injury induced by highly pathogenic viral infections. Emerging microbes & infections. 2015;4(1):1––7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Horiuchi T, Tsukamoto H. Complement-targeted therapy: development of C5-and C5a-targeted inhibition. Inflammation and Regeneration. 2016;36(1):1––5.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ploegh HL. Viral strategies of immune evasion. Science. 1998;280(5361):248–253. doi: 10.1126/science.280.5361.248 [DOI] [PubMed] [Google Scholar]
- 59.García-Sastre A. Ten strategies of interferon evasion by viruses. Cell Host Microbe. 2017;22(2):176–184. doi: 10.1016/j.chom.2017.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Decroly E, Imbert I, Coutard B, et al. Coronavirus nonstructural protein 16 is a cap-0 binding enzyme possessing (nucleoside-2′ O)-methyltransferase activity. J Virol. 2008;82(16):8071–8084. doi: 10.1128/JVI.00407-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Züst R, Cervantes-Barragan L, Habjan M, et al. Ribose 2′-O-methylation provides a molecular signature for the distinction of self and non-self mRNA dependent on the RNA sensor Mda5. Nat Immunol. 2011;12(2):137. doi: 10.1038/ni.1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pichlmair A, Schulz O, Tan CP, et al. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5ʹ-phosphates. Science. 2006;314(5801):997–1001. doi: 10.1126/science.1132998 [DOI] [PubMed] [Google Scholar]
- 63.Hornung V, Ellegast J, Kim S, et al. 5ʹ-Triphosphate RNA is the ligand for RIG-I. Science. 2006;314(5801):994–997. doi: 10.1126/science.1132505 [DOI] [PubMed] [Google Scholar]
- 64.Decroly E, Ferron F, Lescar J, Canard B. Conventional and unconventional mechanisms for capping viral mRNA. Nat Rev Microbiol. 2012;10(1):51–65. doi: 10.1038/nrmicro2675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen Y, Su C, Ke M, et al. Biochemical and structural insights into the mechanisms of SARS coronavirus RNA ribose 2′-O-methylation by nsp16/nsp10 protein complex. PLoS Pathog. 2011;7(10):e1002294. doi: 10.1371/journal.ppat.1002294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fabrega C, Hausmann S, Shen V, Shuman S, Lima CD. Structure and mechanism of mRNA cap (guanine-N7) methyltransferase. Mol Cell. 2004;13(1):77–89. doi: 10.1016/S1097-2765(03)00522-7 [DOI] [PubMed] [Google Scholar]
- 67.Ziebuhr J, Thiel V, Gorbalenya AE. The autocatalytic release of a putative RNA virus transcription factor from its polyprotein precursor involves two paralogous papain-like proteases that cleave the same peptide bond. J Biol Chem. 2001;276(35):33220–33232. doi: 10.1074/jbc.M104097200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lei J, Kusov Y, Hilgenfeld R. Nsp3 of coronaviruses: structures and functions of a large multi-domain protein. Antiviral Res. 2018;149:58–74. doi: 10.1016/j.antiviral.2017.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Angelini MM, Akhlaghpour M, Neuman BW, Buchmeier MJ. Severe acute respiratory syndrome coronavirus nonstructural proteins 3, 4, and 6 induce double-membrane vesicles. MBio. 2013;4(4):e00524–00513. doi: 10.1128/mBio.00524-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Báez-Santos YM, John SES, Mesecar AD. The SARS-coronavirus papain-like protease: structure, function and inhibition by designed antiviral compounds. Antiviral Res. 2015;115:21–38. doi: 10.1016/j.antiviral.2014.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sun L, Xing Y, Chen X, et al. Coronavirus papain-like proteases negatively regulate antiviral innate immune response through disruption of STING-mediated signaling. PLoS One. 2012;7(2):e30802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.DeDiego ML, Nieto-Torres JL, Jimenez-Guardeño JM, et al. Coronavirus virulence genes with main focus on SARS-CoV envelope gene. Virus Res. 2014;194:124–137. doi: 10.1016/j.virusres.2014.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kamitani W, Huang C, Narayanan K, Lokugamage KG, Makino S. A two-pronged strategy to suppress host protein synthesis by SARS coronavirus Nsp1 protein. Nat Struct Mol Biol. 2009;16(11):1134. doi: 10.1038/nsmb.1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Narayanan K, Huang C, Lokugamage K, et al. Severe acute respiratory syndrome coronavirus nsp1 suppresses host gene expression, including that of type I interferon, in infected cells. J Virol. 2008;82(9):4471–4479. doi: 10.1128/JVI.02472-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Law PT, Wong C-H, Au TC, et al. The 3a protein of severe acute respiratory syndrome-associated coronavirus induces apoptosis in Vero E6 cells. J Gen Virol. 2005;86(7):1921–1930. doi: 10.1099/vir.0.80813-0 [DOI] [PubMed] [Google Scholar]
- 76.Padhan K, Minakshi R, Towheed MAB, Jameel S. Severe acute respiratory syndrome coronavirus 3a protein activates the mitochondrial death pathway through p38 MAP kinase activation. J Gen Virol. 2008;89(8):1960–1969. doi: 10.1099/vir.0.83665-0 [DOI] [PubMed] [Google Scholar]
- 77.Minakshi R, Padhan K, Rani M, Khan N, Ahmad F, Jameel S. The SARS Coronavirus 3a protein causes endoplasmic reticulum stress and induces ligand-independent downregulation of the type 1 interferon receptor. PLoS One. 2009;4(12):e8342. doi: 10.1371/journal.pone.0008342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wu C, Liu Y, Yang Y, et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm Sin B. 2020;10(5):766–788. doi: 10.1016/j.apsb.2020.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu C, Zhou Q, Li Y, et al. Research and Development on Therapeutic Agents and Vaccines for COVID-19 and Related Human Coronavirus Diseases. ACS Publications; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shereen MA, Khan S, Kazmi A, Bashir N, Siddique R. COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J Adv Res. 2020;24:91–98. doi: 10.1016/j.jare.2020.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Richardson P, Griffin I, Tucker C, et al. Correspondence Baricitinib as potential. Lancet. 2020;6736:2019–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sheahan TP, Sims AC, Leist SR, et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun. 2020;11(1):1–14. doi: 10.1038/s41467-019-13940-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brown AJ, Won JJ, Graham RL, et al. Broad spectrum antiviral remdesivir inhibits human endemic and zoonotic deltacoronaviruses with a highly divergent RNA dependent RNA polymerase. Antiviral Res. 2019;169:104541. doi: 10.1016/j.antiviral.2019.104541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Khan RJ, Jha RK, Amera G, et al. Targeting SARS-Cov-2: A systematic drug repurposing approach to identify promising inhibitors against 3C-like Proteinase and 2ʹ-O-RiboseMethyltransferase. J Biomol Struct Dyn. 2020:1–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Khan SA, Zia K, Ashraf S, Uddin R, Ul-Haq Z. Identification of chymotrypsin-like protease inhibitors of SARS-CoV-2 via integrated computational approach. J Biomol Struct Dyn. 2020:1–13. [DOI] [PubMed] [Google Scholar]
- 88.Encinar JA, Menendez JA. Potential drugs targeting early innate immune evasion of SARS-Coronavirus 2 via 2ʹ-O-Methylation of viral RNA. Viruses. 2020;12(5):525. doi: 10.3390/v12050525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jiang Y, Liu L, Manning M, Bonahoom M, Lotvola A, Yang Z-Q. Repurposing therapeutics to identify novel inhibitors targeting 2ʹ-O-ribose methyltransferase nsp16 of SARS-CoV-2. 2020. [DOI] [PMC free article] [PubMed]
- 90.Jauregui AR, Savalia D, Lowry VK, Farrell CM, Wathelet MG. Identification of residues of SARS-CoV nsp1 that differentially affect inhibition of gene expression and antiviral signaling. PLoS One. 2013;8(4):e62416. doi: 10.1371/journal.pone.0062416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gordon DE, Jang GM, Bouhaddou M, et al. A SARS-CoV-2-human protein-protein interaction map reveals drug targets and potential drug-repurposing. BioRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Imbert I, Guillemot JC, Bourhis JM, et al. A second, non‐canonical RNA‐dependent RNA polymerase in SARS Coronavirus. EMBO J. 2006;25(20):4933–4942. doi: 10.1038/sj.emboj.7601368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jang KJ, Lee N-R, Yeo W-S, Jeong Y-J, Kim D-E. Isolation of inhibitory RNA aptamers against severe acute respiratory syndrome (SARS) coronavirus NTPase/Helicase. Biochem Biophys Res Commun. 2008;366(3):738–744. doi: 10.1016/j.bbrc.2007.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tu Y-F, Chien C-S, Yarmishyn AA, et al. A review of SARS-CoV-2 and the ongoing clinical trials. Int J Mol Sci. 2020;21(7):2657. doi: 10.3390/ijms21072657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cao Y-C, Deng Q-X, Dai S-X. Remdesivir for severe acute respiratory syndrome coronavirus 2 causing COVID-19: an evaluation of the evidence. Travel Med Infect Dis. 2020;35:101647. doi: 10.1016/j.tmaid.2020.101647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Agostini ML, Andres EL, Sims AC, et al. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. MBio. 2018;9(2):e00221–00218. doi: 10.1128/mBio.00221-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dhama K, Sharun K, Tiwari R, et al. COVID-19, an emerging coronavirus infection: advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics. Hum Vaccin Immunother. 2020;1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cho J-B, Lee J-M, Ahn H-C, Jeong Y-J. Identification of a novel small molecule inhibitor against SARS coronavirus helicase. J Microbiol Biotechnol. 2015;25(12):2007–2010. doi: 10.4014/jmb.1507.07078 [DOI] [PubMed] [Google Scholar]
- 99.Millet JK, Whittaker GR. Host cell proteases: critical determinants of coronavirus tropism and pathogenesis. Virus Res. 2015;202:120–134. doi: 10.1016/j.virusres.2014.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]