Abstract
Objectives
Cerebral infections related to the presence of an intraparenchymal intracranial pressure transducer (ICPT) are rare. We assessed the incidence of ICPT-related infections and colonization using culture, molecular biology, and electron microscopy.
Methods
All consecutive patients in a neurosurgical intensive care unit who had an ICPT inserted between March 2017 and February 2018 were prospectively included. Presence of colonization on the ICPTs was assessed after removal using culture, scanning electron microscopy (SEM), and next-generation sequencing (NGS).
Results
Fifty-three ICPTs (53 patients), indwelling for a median of 4 (range 3–7) days, were studied. Median patient follow-up was 3 months. SEM, microbial culture, and NGS were performed for 91%, 79%, and 72% of ICPTs, respectively; 28 ICPTs (53%) were assessed using all three techniques. No patient developed ICPT-related infection. Microbial cultures were positive for two of the ICPTs (5%); colonization was identified on all ICPTs using NGS and SEM. Mature biofilm was observed on 35/48 (73%) of ICPTs. A median of 10 (8–12) operational taxonomic units were identified for each ICPT, most being of environmental origin. There was no association between biofilm maturity and antimicrobial treatment or duration of ICPT insertion. Antimicrobial treatment was associated with decreased alpha and beta-diversity (p = 0.01).
Conclusions
We observed no ICPT-related cerebral infections although colonization was identified on all ICPTs using NGS and SEM. Mature biofilm was the main bacterial lifestyle on the ICPTs.
Electronic supplementary material
The online version of this article (10.1007/s12028-020-01096-x) contains supplementary material, which is available to authorized users.
Keywords: Biofilm, Colonization, Device-related infection, Intracranial pressure monitoring, DNA sequencing
Introduction
The use of continuous intracranial pressure (ICP) monitoring has been associated with improved outcomes in traumatic brain injury [1]. Intraventricular pressure measurement using a fluid-filled catheter is considered the gold standard for ICP monitoring [2], but despite the lack of specific guidelines, intraparenchymal fiber-optic devices are now widely used because of the low frequency of complications [3–5]. ICP transducers (ICPTs) cross the various brain-protecting barriers (the scalp and the skull, the meninges, and the blood brain barrier) and may therefore theoretically expose the brain to bacterial contamination and subsequent infection. For example, use of external ventricular drains (EVD) is associated with infections in 10% of cases [6] and it has been previously demonstrated that bacteria move along EVDs, from the skin to the brain, mainly as biofilms [6, 7].
Surprisingly, however, intraparenchymal fiber-optic ICPTs are rarely responsible for cerebral infections [5]. In the largest published series, none of 1000 consecutive patients experienced clinically significant intraparenchymal ICPT-related infection [8]. Some authors have reported infectious complications limited to superficial skin (0–2.4%) or bone, but no parenchymal infections or meningitis [3, 4, 8, 9]. Among 101 patients with an ICPT in situ, Martínez-Mañas et al. [4] reported meningitis in 2.9%, but the infections were considered to be related to an EVD and not the ICPT. Recent studies reported that 8.5–17% of ICPT tips were culture positive, mainly with Gram-positive bacteria [4, 8–10], but there were no associated cases of parenchymal infection. However, none of these large studies performed electron microscopy or next-generation sequencing (NGS) to address the infectious risk. Given the numerous similarities between ICPTs and EVDs, in terms of positioning, the differences in terms of frequency of microbial colonization and related infections remain unexplained.
The objective of this study was therefore to assess the bacterial burden on ICPTs [with biofilm formation studied using scanning electron microscopy (SEM) and NGS] and to determine how it may correlate with patient characteristics, ICPT colonization, and related infections.
Methods
Study Setting and Population
This prospective study was conducted between March 2017 and February 2018 in the neurosurgical intensive care unit (ICU) of a tertiary teaching hospital. All consecutive patients with an ICPT (Sophysa, Orsay, France) inserted were included, unless the ICPT had been inserted for management of a central nervous system infection or prior to ICU admission. ICPTs not removed in aseptic conditions were not included. The Institutional Ethical Committee approved the study (Claude Galien). Informed consent was collected from each patient or his/her next of kin.
Definitions
Culture-based colonization was defined by a positive bacterial culture (see below) of the ICPT tip without clinical infection. Any degree of positive culture was considered sufficient to define colonization because no cutoff threshold has been proposed in the literature [4, 8–10].
Infectious meningitis was defined by the presence of a cerebrospinal fluid (CSF) sample with altered laboratory variables (pleocytosis, elevated protein level, and decreased glucose level) and a positive microbiological culture [11, 12].
ICPT-related infection was defined by the presence of meningitis or brain abscess with a CSF or abscess culture positive for the same microbial strain (same species and same antibiotic susceptibility) as that identified in the culture of the ICPT tip.
Patient Management
Patients who are admitted to our center with traumatic brain injury (TBI) all receive initial resuscitation followed by a whole-body computed tomography (CT) scan. Patients with severe TBI (Glasgow Coma Scale score ≤ 8) undergo ICP monitoring per current guidelines [1] or, in case of emergency, extracranial surgery. During the study period, only ICPTs (Sophysa, Orsay, France) were used for ICP monitoring. The ICPT was inserted (on the healthy side, preferably on the right) under general anesthesia in the operating room (OR) or at the bedside in the ICU according to national guidelines. The patient’s hair was clipped, and the skin scrubbed gently using a 10% povidone-iodine solution diluted 50:50 with water. The area was then dried with a sterile towel and washed a second time with the 10% povidone-iodine solution. After inserting the ICPT and the bolt, a new round of alcohol-based hand rubbing and sterile glove exchange, a sterile gauze was wrapped at the base of the bolt (see Supplemental Fig. S1), and another one was used to wind the ICPT cable. A tightly occlusive sterile dressing was used to maintain a closed system. ICPT insertion sites were cleaned (povidone-iodine) at the bedside, and the dressing changed every 48 h, unless soiled. No antibiotic prophylaxis was administered for ICPT placement, but in some patients prophylaxis was indicated for surgery.
When ICP monitoring was no longer clinically indicated, the ICPT was removed under aseptic conditions, and three 0.5-cm portions were cut from the ICPT tip (Fig. 1). The first section was sent to the clinical microbiology laboratory for culture; the second was immediately fixed in a mixture of 4% paraformaldehyde–2.5% glutaraldehyde–0.1% picric acid in 0.1 M phosphate buffer (pH 7.0) for 1 h at room temperature and then kept at 4 °C until SEM examination was performed; the third was stored at − 80 °C for later analysis using NGS.
Fig. 1.
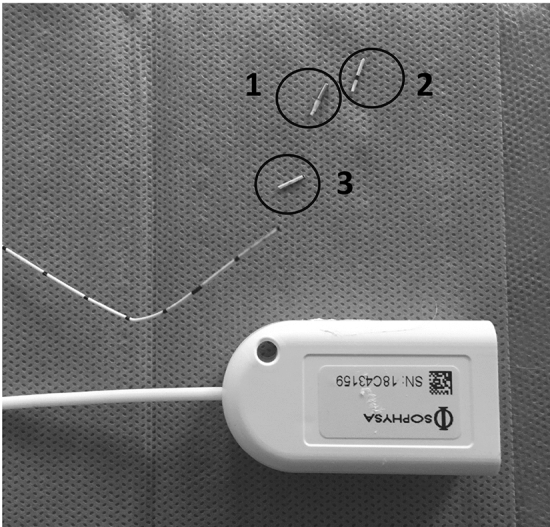
Intraparenchymal intracranial pressure transducer (ICPT) processing. After removal, the intracerebral portion of the ICPT was immediately cut into three sections of 0.5 cm (labeled 1–3). Section 1 was used for bacterial culture; section 2 was assessed by scanning electron microscopy; and section 3 was assessed using next-generation sequencing
CT scans were performed routinely at admission and whenever clinically indicated. CT scans were also performed after the removal of the ICPT, at ICU discharge, at discharge from the neurosurgery unit, and at 3- and 6-month follow-up appointments to rule out subsequent infection.
Standard Bacteriological Procedure
After receipt at the Clinical Microbiology Laboratory, the first ICPT tip section was vortexed for 10 s in 1 mL of sterile saline (bioMérieux, Marcy l’Etoile, France). One hundred microliters of the obtained dilution was plated on blood and on Polivitex agar plates using the automatic WASP system (Wasp, Beckman Coulter, Villepinte, France) and incubated at 35 °C for 48 h in an aerobic atmosphere and in 5% CO2 for 5 days in a chocolate broth, respectively. When cultures were positive, identification was performed using the Andromas MALDI-TOF system (Andromas, Beckman Coulter, Villepinte, France). Antibiotic susceptibility testing was performed using the WalkAway microdilution automatic system (Beckman Coulter, Villepinte, France) for species belonging to the Staphylococcus genus. The agar diffusion method was used for all other species, and results were interpreted according to CA-SFM–EUCAST 2017 recommendations (www.sfm-microbiologie.org).
Scanning Electron Microscopy Examination
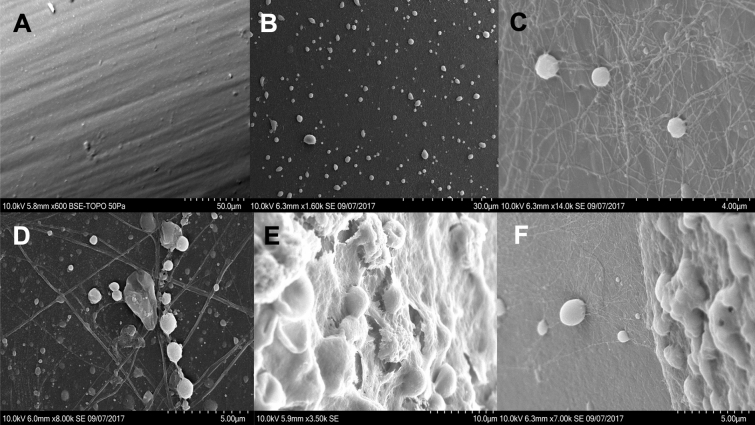
Samples were washed for 30 min in 1 mL of Sörensen’s phosphate buffer, then rinsed with distilled water, dehydrated with increasing ethanol concentrations, and dried with liquid CO2 (without critical-point drying). After one night at room temperature, samples were coated with gold in a Jeol FJC-1200 sputter coater and examined under a Hitachi SU3500 SEM. An examination was also performed using a new ICPT tip as a control (Fig. 2a). SEM pictures were categorized by two physicians experienced in SEM into:
Isolated bacteria (Fig. 2b): bacteria attached to the ICPT without matrix;
Adhesion phase (Fig. 2c, d): bacteria attached to the ICPT in one or more layers, with little extracellular matrix;
Mature biofilm (Fig. 2e): bacteria encapsulated in a thick extracellular matrix.
When several phenotypes were observed on a single SEM picture, for example isolated bacteria and mature biofilm (Fig. 2f), the category representing the most advanced bacterial lifestyle was selected.
Fig. 2.
Categorization of the bacterial lifestyle observed on intraparenchymal intracranial pressure transducers (ICPTs) using scanning electron microscopy. a Control ICPT. b Isolated bacteria. c, d Adhesion bacteria with some extracellular matrix. e Mature biofilm: bacteria are encapsulated in a thick matrix. f Presence of isolated bacteria (on the left) and mature biofilm (on the right) on the same SEM picture
Uncertainty regarding classification of the images was resolved by discussion with a third expert.
Next-Generation Sequencing
Microbial genomic material from the third section of each catheter, from a new ICPT (control), and from a negative control of milliQ water was extracted according to a pre-extraction protocol based on bead-beating disruption of the biofilm and bacteria in a lysis buffer. The pre-extract material was then extracted using the DSP DNA Midi Kit with QIAsymphony instrument (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Finally, the extracts were library-prepared using the “16S Metagenomic Sequencing Library Preparation” protocol (https://support.illumina.com/documents/documentation/chemistry_documentation/16s/16s-metagenomic-library-prep-guide-15044223-b.pdf) (Illumina, San Diego, California, USA). The universal primers used to amplify the bacterial 16S rRNA gene (loop V3–V4) were 341F and 785R (see Supplemental data and Table S1).
Microbiome composition was analyzed with respect to alpha- and beta-diversity and differential abundance of operational taxonomic units (OTUs) across samples. Alpha-diversity was assessed using quantitative and qualitative approaches by Chao1 (richness) and Simpson (evenness) indexes, respectively, as previously described [13]. We also used the PielouE index to measure evenness. Relative frequency was defined at the phylum or genus/OTU levels as the frequency of each clone relative to the number of total clones. This relative frequency was studied for each ICPT but also for the total clone library from all removed ICPTs.
Beta-diversity was studied using Jaccard dissimilarity and weighted UniFrac distance [14].
Samples of used reagents, materials, blanks (water used as the template in the procedure), and a control ICPT were taken as procedure controls and also submitted to 16S rRNA sequencing to determine potential sources of bacterial contamination.
Statistical Analysis
Qualitative data are expressed as incidence and percentage, and quantitative data as median and interquartile range. Categorical data were compared using a two-way Fisher exact test or a chi-square test with a Pearson correction-based on the number of groups. Quantitative data were compared using a Mann–Whitney–Wilcoxon U test or Kruskall–Wallis test as a function of the number of groups. A PERMANOVA test was used to compare beta-diversity between groups. A p value ≤ 0.05 was considered significant. Statistical analyses for diversity were performed using JMP® 14.1.0 and Qiime® (v2018.4.0) software. The differential OTU abundance was assessed using phyloseq (v1.26.1) and DESeq2 (v1.22.2) packages in R software (v3.5.0) to fit generalized linear models of abundance based on the negative binomial distribution. Candidate OTUs with an adjusted p value < 0.01 were validated as being differentially abundant between groups if the OTU was not driven by a single sample or an alone sample with a raw read count > 300 000.
Results
During the study period, 69 ICPTs were inserted in 67 adult patients (2 patients had 2 consecutive ICPTs). Of these 69 ICPTs, 14 were excluded because they were not removed under aseptic conditions, and 2 were not sent to the laboratory when removed in the OR. Fifty-three ICPTs in 53 patients were therefore included in the study (Table 1). The median distance from the skin insertion site to the parenchymal end of the ICPT was 4 (3.4–4.5) cm.
Table 1.
Baseline characteristics of the 53 patients and intraparenchymal intracranial pressure transducers (ICPTs) prospectively included
| Patients | n = 53 |
|---|---|
| Sex (male) | 40 (75) |
| Age (years) | 43 (31–55) |
| Cause of admission | |
| Polytrauma | 36 (68) |
| Isolated TBI | 13 (25) |
| Intraparenchymal hemorrhage | 4 (8) |
| GCS score at admission | 7 (4–8) |
| SAPS 2 | 45 (37–54) |
| Breaking of the BBB | 28 (53) |
| Neurosurgical intervention within the 24 h of admission | 16 (30) |
| Treatment of increased ICP | |
| EVD | 6 (11) |
| Hypothermia, neuromuscular blocking agent | 19 (36) |
| Phenobarbital | 10 (19) |
| Motive for antimicrobial treatment | |
| Ventilator-associated pneumonia | 11 (21) |
| Bacteremia | 1 (2) |
| Ventriculostomy-related infection | 0 |
| ICPT-related infection | 0 |
| Surgical Site Infection | 2 (4) |
| ICU stay (days) | 14 (7–40) |
| In-ICU Mortality | 23 (43) |
| ICP transducers | n = 53 |
|---|---|
| Location of placement | |
| Operating room | 16 (30) |
| ICU | 37 (70) |
| Antimicrobial prophylaxis at ICPT insertion | 17 (32) |
| Duration of placement (days) | 4 (3–7) |
| Cause of removal | |
| End of monitoring | 43 (81) |
| Death | 10 (19) |
| Microbiological culture | 42 (79) |
| SEM performed | 48 (91) |
| NGS performed | 38 (72) |
| ICPT assessed with the three techniques | 28 (53) |
ICPT-related infection was defined as a meningitis or a brain abscess. Antimicrobial treatment means treatment during ICP monitoring. Breaking of the BBB means skull depression fracture, skull base fracture, or brain surgery
Data are shown as the median (25th–75th percentile) or number (%), unless otherwise indicated
BBB blood brain barrier, EVD external ventricular drain, GCS Glasgow Coma Scale, ICP intracranial pressure, ICPT intracranial pressure transducer, ICU intensive care unit, NGS next-generation sequencing, SAPS Simplified Acute Physiology Score, SEM scanning electron microscopy, TBI traumatic brain injury
Forty of the patients were men (75%) and 36 (68%) were admitted for polytrauma. The median Glasgow Coma Scale score was 7 (4–8). The ICPTs were mainly inserted in the ICU, and patients were monitored for 4 (3–7) days. The median ICU length of stay was 14 (7–40) days and in-ICU mortality was 43% (23/53). Median follow-up was 3 months. We observed no bleeding, even minor, around the probes.
During ICP monitoring, 12 patients (23%) received antimicrobial treatment for extraneurological infection (Table 1); amoxicillin–clavulanic acid and cefotaxime were the most commonly used empirical antimicrobial therapies, in 33% (4/12) and 58% (7/12) of cases, respectively. Cefepime was used in one case. After microbial identification, amoxicillin–clavulanic acid and cefotaxime were used in 58% (7/12) and 42% (5/12) of cases, respectively. The median time to start of antimicrobial treatment was 4.5 (3.3–6.5) days. Blood cultures were performed in 78% of our patients (52/67).
ICPT-Related Infection
No patient developed ICPT-related infection or cutaneous infection at the site of bolt insertion. Among the 16 patients who had an emergency neurosurgical intervention, two developed a surgical site infection: one a bone flap infection and the other a superficial surgical site infection.
Colonization
Microbial culture, SEM, and NGS were performed for 42 (79%), 48 (91%), and 38 (72%) of ICPTs, respectively, and 28 ICPTs (53%) were assessed using all three techniques.
Bacterial Culture
Among the 42 ICPTs cultured, only 2 (5%) showed colonization, both with S. epidermidis and one with Bacillus spp. These 2 ICPTs were not studied using NGS, but mature biofilm was observed on both of them with SEM (see Supplemental Fig. S2). These 2 ICPTs were from patients who did not receive antimicrobial treatment.
Scanning Electron Microscopy
All SEM examinations showed the presence of bacteria (48/48, 100%). Isolated bacteria were observed on 33 of the 48 (70%) ICPTs, adhesion phase bacteria on 37/48 (78%), and mature biofilm on 35/48 (73%). A single (2%) ICPT showed only isolated bacteria. Thirteen (25%) ICPTs showed isolated and adhesion phase bacteria without mature biofilm (see Supplemental Fig. S3). There was no significant association between the presence of mature biofilm and receipt of intravenous antimicrobial treatment or duration of ICP monitoring (Table 2).
Table 2.
Clinical factors associated with the presence of mature biofilm on intraparenchymal intracranial pressure transducers (ICPTs) using scanning electron microscopy (SEM)
| Mature biofilm | OR (IC95%) | p values | ||
|---|---|---|---|---|
| No ( n = 13) | Yes ( n = 35) | |||
| Cause of admission | ||||
| Polytrauma | 8 (61) | 24 (68) | – | 0.87 |
| Isolated TBI | 4 (31) | 8 (23) | ||
| Intraparenchymal hemorrhage | 1 (8) | 3 (9) | ||
| Location of placement | ||||
| Operating room | 3 (23) | 13 (37) | 0.51 (0.12–2.19) | 0.5 |
| ICU | 10 (77) | 22 (63) | ||
| Breaking of the BBB | 8 (62) | 18 (51) | 0.66 (0.18–2.43) | 0.53 |
| Concomitant EVD | 1 (8) | 3 (9) | 1.12 (0.11–11.89) | 1 |
| Hypothermia and neuromuscular blocking agent | 3 (23) | 15 (43) | 2.5 (0.58–10.7) | 0.32 |
| Phenobarbital | 1 (8) | 7 (20) | 3 (0.33–27.12) | 0.42 |
| Antimicrobial treatment | 3 (23) | 6 (17) | 0.69 (0.14–3.29) | 0.69 |
| Monitoring duration | ||||
| ≤ 5 days | 9 (69) | 22 (65) | 1.23 (0.31–4.84) | 1 |
| > 5 days | 4 (31) | 12 (35) | ||
Data presented are limited to the 48 ICPTs assessed by SEM
Breaking of the BBB means skull depression fracture, skull base fracture, or brain surgery
Data are shown as the median (25th–75th percentile) or number (%), unless otherwise indicated
BBB blood brain barrier, EVD external ventricular drain, ICP intracranial pressure, ICU intensive care unit, TBI traumatic brain injury
Next-Generation Sequencing
The median number of raw reads per sample was 37,933 (25,134–85,695). After trimming and host contamination removal, the median number of reads per sample was 10,604 (4,378–36,861). The rate of OTUs identified was 99.87% (98.25–100%). 16S rRNA-based molecular analysis detected bacterial DNA on all ICPTs. The main phylum was Proteobacteria, identified on all ICPTs, representing 80% of all reads, followed by Actinobacteria found on 79% (30/38) of ICPTs (see Supplemental Fig. S4). Firmicutes were present on 23/38 samples (61%), and Bacteroidetes on 6/38 samples (16%).
For the 38 ICPTs studied by NGS, we found a median of 10 [8–12] OTUs (Fig. 3). Across the 38 ICPTs, 91 OTUs were identified, but only 11 of them had a relative frequency greater than 1% (Fig. 4). Many of these species can be considered to be of environmental and skin origin, including Delftia spp. and Herbaspirillum spp. Stenotrophomonas spp. and Serratia spp. were identified on 38 (100%) and 22 (58%) of ICPTs, respectively. Commensal bacteria such as Corynebacterium spp. and Staphylococcus spp. were each identified on 3 (8%) ICPTs.
Fig. 3.
Heatmap of genus/OTU and their relative abundance in clone libraries from each of 38 intraparenchymal intracranial pressure transducers (ICPTs). The genus subset was assessed according to Callahan et al. [33], and normalization was conducted by sample (grayscale: The OTU count was centered to the mean and the scale to unit variance). Numbers 1–38 represent the individual ICPTs
Fig. 4.
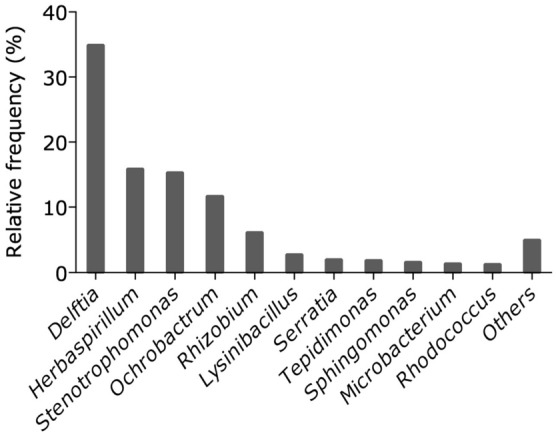
Relative frequencies of each genus/OTU in the entire cohort of removed ICPTs. Genus/OTUs are ranked according to the abundance of their clone library relative to the number of total clones. Subsets were created according to Callahan et al. [33], and the category “Others” represents genus clusters with a relative abundance ≤ 1%
Microbiome Diversity
Alpha-Diversity
The number of OTUs representing more than 90% of the sequences found by sample was 6 (5.25–8). No clinical or therapeutic factor or the presence of mature biofilm on SEM was associated with a decrease in the number of OTUs representing more than 90% of the sequences or with an alteration in the richness (Chao1) of α-diversity (Table 3). ICPT placement in the OR, antimicrobial treatment during monitoring, and surgical antimicrobial prophylaxis were associated with reduced evenness [p = 0.01, p = 0.01, and p = 0.006, respectively (PielouE)].
Table 3.
Comparison of the α-diversity found on intraparenchymal intracranial pressure transducer (ICPT), according to different variables
| ICPTs | Richness | Evenness | |||||
|---|---|---|---|---|---|---|---|
| n = 38 | Chao 1 | p | Simpson | p | PielouE | p | |
| Location of placement | |||||||
| Operating room | 14 | 14.5 (14–19.25) | 0.36 | 0.82 (0.77–0.83) | 0.04 | 0.78 (0.73–0.79) | 0.01 |
| ICU | 24 | 17.5 (15.5–19) | 0.86 (0.83–0.89) | 0.84 (0.79–0.85) | |||
| Antimicrobial prophylaxis | |||||||
| Yes | 17 | 16 ()14–20) | 0.8 | 0.83 (0.78–0.85) | 0.05 | 0.78 (0.75–0.80) | 0.006 |
| No | 21 | 17 (14–19)) | 0.87 (0.82–0.89) | 0.84 (0.80–0.86) | |||
| Breaking of the BBB | |||||||
| Yes | 23 | 16 (14–18.5) | 0.31 | 0.83 (0.80–0.87) | 0.23 | 0.8 (0.75–0.84) | 0.28 |
| No | 15 | 18 (16–19) | 0.87 (0.83–0.88) | 0.84 (0.77–0.85) | |||
| Treatment of increased ICP | |||||||
| EVD | |||||||
| Yes | 4 | 17 (13.5–20) | 0.96 | 0.82 (0.79–0.84) | 0.23 | 0.75 (0.74–0.78) | 0.32 |
| No | 34 | 16.5 (14–18.75) | 0.85 (0.81–0.89) | 0.81 (0.77–0.85) | |||
| Hypothermia | |||||||
| Yes | 12 | 16 (13.5–19.25) | 0.52 | 0.83 (0.78–0.87) | 0.29 | 0.77 (0.74–0.86) | 0.59 |
| No | 26 | 17 (14–18.75) | 0.85 (0.82–0.89) | 0.81 (0.78–0.84) | |||
| Curarization | |||||||
| Yes | 13 | 16 (14–19) | 0.64 | 0.83 (0.78–0.87) | 0.35 | 0.78 (0.74–0.86) | 0.84 |
| No | 25 | 17 (14–19) | 0.85 (0.82–0.89) | 0.81 (0.77–0.84) | |||
| Phenobarbital | |||||||
| Yes | 8 | 16 (14–19.25) | 0.93 | 0.85 (0.80–0.87) | 0.52 | 0.78 (0.73–0.86) | 0.86 |
| No | 30 | 17 (14–18.75) | 0.85 (0.81–0.89) | 0.80 (0.77–0.84) | |||
| Antimicrobial treatment | |||||||
| Yes | 7 | 16 (14.5–19.5) | 0.85 | 0.81 (0.67–0.83) | 0.02 | 0.73 (0.62–0.77) | 0.01 |
| No | 31 | 17 (14.5–18.5) | 0.86 (0.82–0.89) | 0.82 (0.78–0.85) | |||
| Monitoring duration | |||||||
| ≤ 5 days | 25 | 17 (14–19) | 0.64 | 0.86 (0.82–0.89) | 0.26 | 0.82 (0.77–0.85) | 0.11 |
| > 5 days | 13 | 16 (15–19) | 0.83 (0.78–0.88) | 0.78 (0.73–0.84) | |||
| Mature biofilm (SEM) | |||||||
| Yes | 26 | 16.5 (14–19) | 0.96 | 0.83 (0.81–0.87) | 0.19 | 0.80 (0.75–0.84) | 0.19 |
| No | 12 | 16.5 (14.75–18.25) | 0.87 (0.82–0.89) | 0.84 (0.79–0.86) | |||
Antimicrobial prophylaxis means that ICPT was placed under surgical antimicrobial prophylaxis. Breaking of the BBB means skull depression fracture, skull base fracture, or brain surgery
BBB blood brain barrier, EVD external ventricular drain, ICPT intraparenchymal intracranial pressure transducer, ICU intensive care unit, OTU operational taxonomic units, TBI traumatic brain injury
Data are shown as the median (25th–75th percentile) or number (%), unless otherwise indicated
Significant p-values are shown in bold
Beta-Diversity
ICPT placement in the OR and antimicrobial prophylaxis decreased the beta-diversity (weighted UniFrac: p = 0.02 and p = 0.03, respectively) (Table 4).
Table 4.
Comparison of the beta-diversity found on intraparenchymal intracranial pressure transducer (ICPT), according to different variables
| Metric | Group | p values |
|---|---|---|
| Jaccard | Antimicrobial prophylaxis | 0.82 |
| ICU versus OR | 0.71 | |
| Breaking of the BBB | 0.52 | |
| Curarization | 0.48 | |
| Hypothermia | 0.28 | |
| Phenobarbital | 0.03 | |
| Antimicrobial treatment | 0.09 | |
| Monitoring duration | 0.08 | |
| Biofilm presence | 0.98 | |
| Weighted UniFrac | Antimicrobial prophylaxis | 0.03 |
| ICU versus OR | 0.02 | |
| Breaking of the BBB | 0.88 | |
| Curarization | 0.76 | |
| Hypothermia | 0.76 | |
| Phenobarbital | 0.63 | |
| Antimicrobial treatment | 0.14 | |
| Monitoring duration | 0.83 | |
| Biofilm presence | 0.34 |
ICU versus OR means ICPT placement location in the intensive care unit versus in the operating room. Antimicrobial prophylaxis means that ICPT was placed under surgical antimicrobial prophylaxis. Breaking of the BBB means skull depression fracture, skull base fracture, or brain surgery
Significant p-values are shown in bold
Differential Abundance (See Supplemental Table S2)
Antimicrobial treatment was associated with a decrease in Serratia and Curvibacter genera. Some genera were differentially abundant depending on where the ICPT had been inserted: Microbacterium and Curvibacter were more abundant in ICPTs inserted in the ICU, whereas Staphylococcus was more abundant in ICPTs inserted in the operating room. The information provided by the sequencing of 16S rDNA was not discriminative enough to differentiate all staphylococcal species.
Discussion
In this prospective study including 53 ICPTs, SEM and NGS always identified the presence of bacteria, whereas bacterial culture was only positive for 5% of transducer tips, suggesting that ICPT colonization may be largely underestimated using standard techniques.
In large clinical studies, intraparenchymal ICP monitors have occasionally been associated with clinically relevant infections [8–10, 15]. However, analysis of published data may be hindered by the concomitant insertion of other intracranial devices, such as EVDs, which are more frequently associated with infectious complications [4, 16–21]. When restricted to studies that included only intraparenchymal transducers, reported infections were rare and mainly superficial [3, 9]. Indeed, the largest series, including more than 1000 patients, reported no infections [4, 8].
Several reasons may explain this low risk of infection. First, because known risk factors for ventriculostomy-related infection are CSF leak, elevated ICP, and duration of ICP monitoring [4, 6, 17, 22, 23], one can hypothesize that the shorter duration of ICPT placement (< 10 days), as compared to EVD (≥ 10 days), may play a key role in this observation [3, 6, 9, 10, 12, 24, 25]. Second, the bolt, present for ICPTs but not EVDs, may also act as a physical barrier, limiting CSF leaks or delaying bacterial progression from the skin to the ICPT tip. Third, ICPTs have a thinner diameter (1 mm) than EVDs (3 mm).
When focusing on the frequency of tip colonization, one can also observe that ICPTs are less frequently colonized (8.5 to 17%) than EVDs (10–36%) [4, 6, 8–10, 26, 27]. Using SEM, one study reported the presence of biofilm on 75% of the analyzed EVDs, whereas colonization and ventriculostomy-related infection diagnosed by culture occurred in only 38% and 19% of cases, respectively [7]. Using both SEM and NGS, we identified bacteria on all studied ICPTs, although only 5% of ICPTs were culture-positive. The main bacterial lifestyle was mature biofilm, seen on 73% of ICPTs; NGS confirmed this high frequency of bacterial colonization. However, we did not observe any cerebral infections, despite a follow-up period of several months. The absence of ICPT-related infection, despite almost universal ICPT colonization (as shown with SEM or NGS), may suggest a role of bacterial virulence traits [28]. Indeed, most of the culture-positive ICPTs reported in the literature were colonized by S. epidermidis [4, 9, 10]. However, more virulent bacteria, such as Escherichia coli, Enterobacter spp., Klebsiella spp., Serratia spp. and Pseudomonas spp., have also been recovered from ICPT cultures, without clinically relevant infection [4, 8, 9]. In our study, NGS identified mostly environmental or skin bacteria [29], suggesting possible ICPT colonization during insertion or handling.
Finally, a seductive hypothesis to explain the low risk of infection despite almost universal bacterial colonization is that the mature biofilm we observed may act as a protective microbiota on the surface of the ICPT [6, 30, 31]. Studying another type of indwelling device (totally implanted venous access port, TIVAP) using NGS, Stressmann et al. [30] identified two distinct patterns of biofilm composition, depending on the status of the patient at TIVAP removal (clinical suspicion of infection [“symptomatic”] or not [“asymptomatic”]). Among samples from asymptomatic patients, they identified Herbaspirillum, Paracoccus and Stenotrophomonas maltophilia more abundantly than in symptomatic patients. Interestingly, we also identified Stenotrophomonas and Herbaspirillum on all ICPTs. Furthermore, Stressmann et al. reported that the mean species richness (represented by the number of OTUs/sample) in the overall sample set was significantly higher (p = 0.0002) in asymptomatic (5.4 OTUs/sample) than in symptomatic (2.5 OTUs/sample) samples. Strikingly, we noted that the main factor associated with a decrease in bacterial diversity was antimicrobial treatment.
Our study has some limitations. First, positive results achieved using NGS may have been the result of contamination during removal or contamination of commonly used DNA extraction kits or PCR reagents [32]. To reduce this risk, ICPTs were excluded if there was any suspicion of contamination during removal. The SEM findings also suggest that the bacteria were actually on the surface of the catheter (and not the result of contamination) as the process from bacterial adhesion to biofilm formation takes time. As we performed fast-acting fixation immediately after ICPT removal, the observation of isolated bacteria using SEM could correspond to contamination during ICPT removal. However, “isolated bacteria” alone were observed for just a single (2%) ICPT. Second, the small number of ICPTs included reduced our statistical power to identify factors associated with the formation of mature biofilm. Third, meningitis may have been under-recognized. Head trauma patients with elevated ICP are usually sedated with temperature control, which may interfere with clinical examination. In addition, because of the increased intracranial pressure, lumbar puncture may not be feasible. However, CSF was sampled daily for microbiological analysis in patients with an EVD in situ and when clinically indicated by lumbar puncture, when possible. Fourth, the study was performed in a single center, thereby limiting its generalizability, especially as the method for ICP probe insertion may differ from center to center. Fifth, we limited our analysis to the bacterial kingdom, without taking into account the possible role of fungi or viruses. Sixth, in the absence of infection and because of universal colonization, no multivariate analysis could be performed. Finally, a quantitative analysis (such as quantitative PCR) may have improved our understanding of colonization dynamics and its microbial composition.
Conclusion
In conclusion, we observed no ICPT-related cerebral infections, although colonization was always observed using NGS and SEM, mostly with bacteria of environmental origin. Mature biofilm was the main lifestyle for bacteria on ICPTs.
Supporting Information
Data from NGS are available under the number BioProject: PRJNA560770.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Karen Pickett for editorial assistance.
Author Contributions
RM, FC, SS drafted study conception and design. VD, RM, VM, NK, SP, CD, P-L Woerther, BA, MA acquisition of data. GG, CR, RM, DL, NK, BN, DL analysis and interpretation of data. RM, DL, FC drafting of manuscript. RM, DL, GD critical revision.
Source of Support
Support was provided solely from institutional and/or departmental sources.
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical Approval/Informed Consent
The Institutional Ethical Committee approved the study (Claude Galien). Informed consent was collected from each patient or his/her next of kin.
Footnotes
The original article was updated to correct the co-author name Suhan Senova in author list. The details mentioned here are correct.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
10/15/2020
A Correction to this paper has been published: 10.1007/s12028-020-01130-y
References
- 1.Carney N, Totten AM, O’Reilly C, Ullman JS, Hawryluk GWJ, Bell MJ, et al. Guidelines for the management of severe traumatic brain injury. Fourth Ed Neurosurg. 2017;80:6–15. doi: 10.1227/NEU.0000000000001432. [DOI] [PubMed] [Google Scholar]
- 2.Lang J-M, Beck J, Zimmermann M, Seifert V, Raabe A. Clinical evaluation of intraparenchymal Spiegelberg pressure sensor. Neurosurgery. 2003;52:1455–1459. doi: 10.1227/01.NEU.0000065136.70455.6F. [DOI] [PubMed] [Google Scholar]
- 3.Shapiro S, Bowman R, Callahan J, Wolfla C. The fiberoptic intraparenchymal cerebral pressure monitor in 244 patients. Surg Neurol. 1996;45:278–282. doi: 10.1016/0090-3019(95)00359-2. [DOI] [PubMed] [Google Scholar]
- 4.Martínez-Mañas RM, Santamarta D, de Campos JM, Ferrer E. Camino intracranial pressure monitor: prospective study of accuracy and complications. J Neurol Neurosurg Psychiatry. 2000;69:82–86. doi: 10.1136/jnnp.69.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morton R, Lucas TH, Ko A, Browd SR, Ellenbogen RG, Chesnut RM. Intracerebral abscess associated with the Camino intracranial pressure monitor: case report and review of the literature. Neurosurgery. 2012;71:E193–198. doi: 10.1227/NEU.0b013e318232e250. [DOI] [PubMed] [Google Scholar]
- 6.Mounier R, Lobo D, Cook F, Martin M, Attias A, Aït-Mamar B, et al. From the skin to the brain: pathophysiology of colonization and infection of external ventricular drain, a prospective observational study. PLoS ONE. 2015;10:e0142320. doi: 10.1371/journal.pone.0142320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramírez P, Gordón M, Soriano A, Gil-Perotin S, Marti V, Gonzalez-Barbera EM, et al. Assessment of the in vivo formation of biofilm on external ventricular drainages. Eur J Clin Microbiol Infect Dis. 2013;32:1437–1443. doi: 10.1007/s10096-013-1895-8. [DOI] [PubMed] [Google Scholar]
- 8.Gelabert-González M, Ginesta-Galan V, Sernamito-García R, Allut AG, Bandin-Diéguez J, Rumbo RM. The Camino intracranial pressure device in clinical practice. Assessment in a 1000 cases. Acta Neurochir (Wien) 2006;148:435–441. doi: 10.1007/s00701-005-0683-3. [DOI] [PubMed] [Google Scholar]
- 9.Poca M-A, Sahuquillo J, Arribas M, Báguena M, Amorós S, Rubio E. Fiberoptic intraparenchymal brain pressure monitoring with the Camino V420 monitor: reflections on our experience in 163 severely head-injured patients. J Neurotrauma. 2002;19:439–448. doi: 10.1089/08977150252932398. [DOI] [PubMed] [Google Scholar]
- 10.Jensen RL, Hahn YS, Ciro E. Risk factors of intracranial pressure monitoring in children with fiberoptic devices: a critical review. Surg Neurol. 1997;47:16–22. doi: 10.1016/S0090-3019(96)00276-5. [DOI] [PubMed] [Google Scholar]
- 11.Lozier AP, Sciacca RR, Romagnoli MF, Connolly ES., Jr Ventriculostomy-related infections: a critical review of the literature. Neurosurgery. 2002;51:170–181. doi: 10.1097/00006123-200207000-00024. [DOI] [PubMed] [Google Scholar]
- 12.Mounier R, Lobo D, Cook F, Fratani A, Attias A, Martin M, et al. Clinical, biological, and microbiological pattern associated with ventriculostomy-related infection: a retrospective longitudinal study. Acta Neurochir (Wien) 2015;157:2209–2217. doi: 10.1007/s00701-015-2574-6. [DOI] [PubMed] [Google Scholar]
- 13.Magurran AE. Biological diversity. Curr Biol. 2005;15:R116–118. doi: 10.1016/j.cub.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Lozupone CA, Hamady M, Kelley ST, Knight R. Quantitative and qualitative beta diversity measures lead to different insights into factors that structure microbial communities. Appl Environ Microbiol. 2007;73:1576–1585. doi: 10.1128/AEM.01996-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Artru F, Terrier A, Gibert I, Messaoudi K, Charlot M, Naous H, et al. Monitoring of intracranial pressure with intraparenchymal fiberoptic transducer. Technical aspects and clinical reliability. Ann Fr Anesth Reanim. 1992;11:424–429. doi: 10.1016/S0750-7658(05)80342-9. [DOI] [PubMed] [Google Scholar]
- 16.Bekar A, Doğan S, Abaş F, Caner B, Korfali G, Kocaeli H, et al. Risk factors and complications of intracranial pressure monitoring with a fiberoptic device. J Clin Neurosci. 2009;16:236–240. doi: 10.1016/j.jocn.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 17.Rebuck JA, Murry KR, Rhoney DH, Michael DB, Coplin WM. Infection related to intracranial pressure monitors in adults: analysis of risk factors and antibiotic prophylaxis. J Neurol Neurosurg Psychiatry. 2000;69:381–384. doi: 10.1136/jnnp.69.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winfield JA, Rosenthal P, Kanter RK, Casella G. Duration of intracranial pressure monitoring does not predict daily risk of infectious complications. Neurosurgery. 1993;33:424–430. doi: 10.1227/00006123-199309000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Stoikes NF, Magnotti LJ, Hodges TM, Weinberg JA, Schroeppel TJ, Savage SA, et al. Impact of intracranial pressure monitor prophylaxis on central nervous system infections and bacterial multi-drug resistance. Surg Infect (Larchmt) 2008;9:503–508. doi: 10.1089/sur.2007.032. [DOI] [PubMed] [Google Scholar]
- 20.Clark WC, Muhlbauer MS, Lowrey R, Hartman M, Ray MW, Watridge CB. Complications of intracranial pressure monitoring in trauma patients. Neurosurgery. 1989;25:20–24. doi: 10.1227/00006123-198907000-00004. [DOI] [PubMed] [Google Scholar]
- 21.May AK, Fleming SB, Carpenter RO, Diaz JJ, Guillamondegui OD, Deppen SA, et al. Influence of broad-spectrum antibiotic prophylaxis on intracranial pressure monitor infections and subsequent infectious complications in head-injured patients. Surg Infect (Larchmt) 2006;7:409–417. doi: 10.1089/sur.2006.7.409. [DOI] [PubMed] [Google Scholar]
- 22.Mayhall CG, Archer NH, Lamb VA, Spadora AC, Baggett JW, Ward JD, et al. Ventriculostomy-related infections. A prospective epidemiologic study. N Engl J Med. 1984;310:553–559. doi: 10.1056/NEJM198403013100903. [DOI] [PubMed] [Google Scholar]
- 23.Schultz M, Moore K, Foote AW. Bacterial ventriculitis and duration of ventriculostomy catheter insertion. J Neurosci Nurs. 1993;25:158–164. doi: 10.1097/01376517-199306000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Gupta DK, Bisht A, Batra P, Mathur P, Mahapatra AK. A cost effectiveness based safety and efficacy study of resterilized intra-parenchymal catheter based intracranial pressure monitoring in developing world. Asian J Neurosurg. 2016;11:416–420. doi: 10.4103/1793-5482.165785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mounier R, Birnbaum R, Cook F, Jost P-H, Martin M, Aït-Mamar B, et al. Natural history of ventriculostomy-related infection under appropriate treatment and risk factors of poor outcome: a retrospective study. J Neurosurg. 2018;2018:1–10. doi: 10.3171/2018.6.JNS18853. [DOI] [PubMed] [Google Scholar]
- 26.Hetem DJ, Woerdeman PA, Bonten MJM, Ekkelenkamp MB. Relationship between bacterial colonization of external cerebrospinal fluid drains and secondary meningitis: a retrospective analysis of an 8-year period. J Neurosurg. 2010;113:1309–1313. doi: 10.3171/2010.6.JNS10258. [DOI] [PubMed] [Google Scholar]
- 27.Aucoin PJ, Kotilainen HR, Gantz NM, Davidson R, Kellogg P, Stone B. Intracranial pressure monitors. Epidemiologic study of risk factors and infections. Am J Med. 1986;80:369–376. doi: 10.1016/0002-9343(86)90708-4. [DOI] [PubMed] [Google Scholar]
- 28.Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med. 2004;350:1422–1429. doi: 10.1056/NEJMra035415. [DOI] [PubMed] [Google Scholar]
- 29.Grice EA, Kong HH, Renaud G, Young AC, NISC Comparative Sequencing Program. Bouffard GG, et al. A diversity profile of the human skin microbiota. Genome Res. 2008;18:1043–1050. doi: 10.1101/gr.075549.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stressmann FA, Couve-Deacon E, Chainier D, Chauhan A, Wessel A, Durand-Fontanier S, et al. Comparative analysis of bacterial community composition and structure in clinically symptomatic and asymptomatic central venous catheters. mSphere. 2017;2:e00146–e217. doi: 10.1128/mSphere.00146-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grice EA, Segre JA. The skin microbiome. Nat Rev Microbiol. 2011;9:244–253. doi: 10.1038/nrmicro2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glassing A, Dowd SE, Galandiuk S, Davis B, Chiodini RJ. Inherent bacterial DNA contamination of extraction and sequencing reagents may affect interpretation of microbiota in low bacterial biomass samples. Gut Pathog. 2016;8:24. doi: 10.1186/s13099-016-0103-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Callahan BJ, Sankaran K, Fukuyama JA, McMurdie PJ, Holmes SP. Bioconductor workflow for microbiome data analysis: from raw reads to community analyses. F1000Res. 2016;5:1492. doi: 10.12688/f1000research.8986.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.