ABSTRACT
Funding of research by industry in general can lead to sponsorship bias. The aim of the current study was to conduct an initial exploration of the impact of sponsorship bias in observational alcohol research by focusing on a broad spectrum of health outcomes. The purpose was to determine whether the outcome depended on funding source. We focused on moderate alcohol consumption and used meta-analyses that are the basis of several international alcohol guidelines. These meta-analyses included observational studies that investigated the association of alcohol consumption with 14 different health outcomes, including all-cause mortality, several cardiovascular diseases and cancers, dementia, and type 2 diabetes. Subgroup analyses and metaregressions were conducted to investigate the association between moderate alcohol consumption and the risk of different health outcomes, comparing findings of studies funded by the alcohol industry, ones not funded by the alcohol industry, and studies with an unknown funding source. A total of 386 observational studies were included. Twenty-one studies (5.4%) were funded by the alcohol industry, 309 studies (80.1%) were not funded by the alcohol industry, and for the remaining 56 studies (14.5%) the funding source was unknown. Subgroup analyses and metaregressions did not show an effect of funding source on the association between moderate alcohol intake and different health outcomes. In conclusion, only a small proportion of observational studies in meta-analyses, referred to by several international alcohol guidelines, are funded by the alcohol industry. Based on this selection of observational studies the association between moderate alcohol consumption and different health outcomes does not seem to be related to funding source.
Keywords: alcohol industry funding, sponsorship bias, all-cause mortality, cardiovascular disease, cancer, type 2 diabetes
Introduction
Industry funding of health research has been a topic of discussion for many years (1–6). Because of the potential sponsorship bias, it is argued that industry funding in scientific research is undesirable (5). More specifically, concerns have been raised about the integrity of research funded by the pharmaceutical and food industry (1–3, 5–7). With respect to the pharmaceutical industry, Lundh and colleagues (1) demonstrated that empirical studies funded by the pharmaceutical industry reported results favorable to the sponsors’ products more often than non–industry-sponsored studies (RR: 1.27; 95% CI: 1.17, 1.37). Similar results are found for food industry funding in nutrition research. Biased results and conclusions have been found in industry-funded studies for sugar sweetened beverages (SSBs), the fat substitute olestra, and nonalcoholic drinks (8–10). For example, industry-sponsored systematic reviews on SSBs and weight gain have a greater probability of reporting no positive association between weight gain and SSBs than systematic reviews with no financial conflict of interest with the food industry (RR: 5.0; 95% CI: 1.3, 19.3) (8). In addition, a study that investigated the association between authors’ published position on the fat substitute olestra and financial relations with this sector, discovered that 80% of the authors in favor of olestra have ≥1 financial relation with this sector, in contrast to only 11% of the critical and 21% of the neutral authors (9). Food industry–funded intervention studies on nonalcoholic drinks (e.g., milk, soft drinks, and juices) have not reported any unfavorable conclusions compared with 37% of non–industry-funded intervention studies reporting unfavorable results (10). Such bias in health research can have major consequences for public health, because these studies are essential to the development of new medication and treatments, or are the basis for the formulation of dietary guidelines and design of public health interventions.
It has also been suggested that the involvement of the alcohol industry in scientific research could affect the objectivity of independent scientists and the integrity of science (4, 11). So far, only 1 study has examined this. McCambridge and Hartwell (12) investigated whether findings of alcohol's protective effects on cardiovascular disease (CVD) were biased by industry funding. This study gave no specific grounds for concern that alcohol industry funding has biased what is known about the protective effects on CVD, apart from stroke. Studies giving some concern about receipt of alcohol industry funding found a protective effect of alcohol consumption against stroke (RR: 0.88; 95% CI: 0.81, 0.94), whereas studies without such concerns found no effect (RR: 1.07; 95% CI: 0.97, 1.17). However, it is important to stress the limited sample size. For this analysis, there were only 8 studies with “some concern” of receiving alcohol industry funding compared with 10 studies with “no concern.” The authors indicate that the findings are preliminary and stress the importance of more sophisticated research on the issue (12). The aim of the current study was to conduct an initial exploration of the impact of sponsorship bias in observational alcohol research by focusing on a broad spectrum of health outcomes. Studies focusing on the effect of moderate alcohol consumption on CVD, stroke, coronary heart disease (CHD), different types of cancer, type 2 diabetes, dementia, and all-cause mortality were included. These health outcomes were selected because they are often associated with moderate alcohol consumption and also referred to by international nutrition guidelines. The purpose was to determine whether study outcome was associated with the funding source.
Methods
Selection of meta-analyses
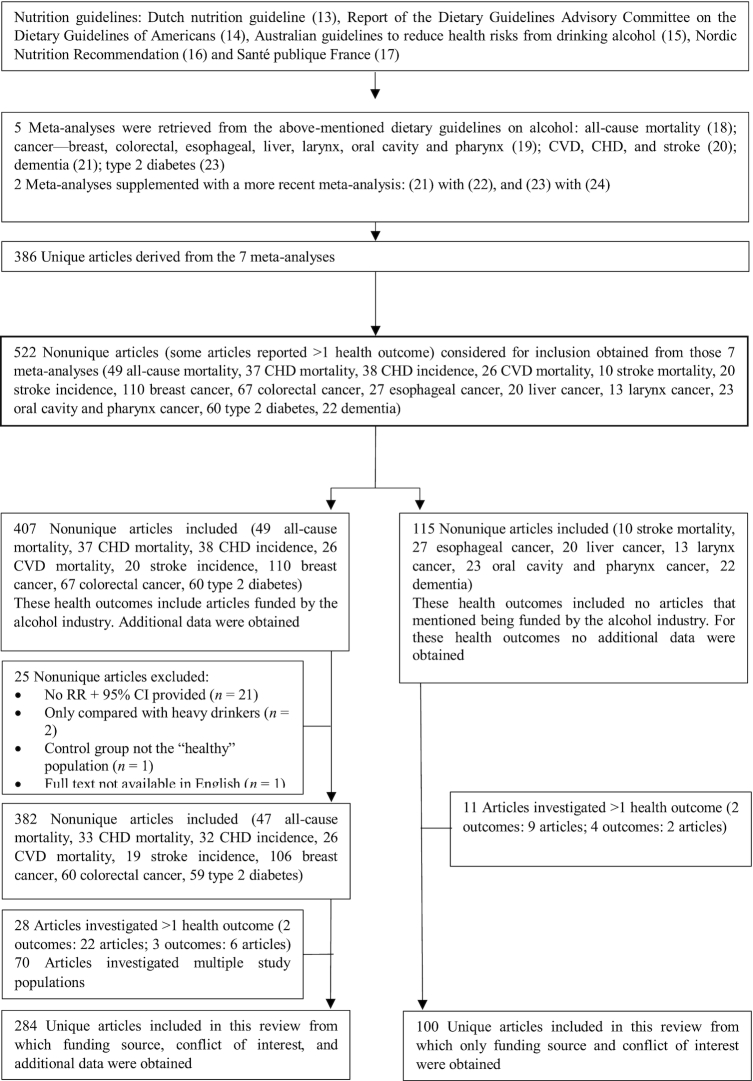
The study was based on meta-analyses investigating the effect of alcohol consumption on several health outcomes that are referred to by several international guidelines on alcohol, including the Dutch nutrition guidelines on alcohol (13), the Report of the Dietary Guidelines Advisory Committee on the Dietary Guidelines of Americans (14), Australian guidelines to reduce health risks from drinking alcohol (15), Nordic Nutrition Recommendation (16), and Santé publique France (17). An additional manual search was performed to find recently published meta-analyses that were not yet included in the guidelines. When a more recent meta-analysis for a specific health outcome was found, the initial and recently published meta-analyses were compared with respect to the included individual studies. In case of limited overlap in included studies, both the initial and more recent meta-analysis were taken into account. When the more recently published meta-analysis included the same studies as the initial meta-analysis, plus additional recent studies, only the recently published meta-analysis was included. In this case, the recent meta-analysis was seen as an “update” of the initial meta-analysis. A flow diagram of the study selection and identification process is provided in Figure 1.
FIGURE 1.
Flow diagram of the study selection and identification process.
Health outcomes
The meta-analyses retrieved from the guidelines focused on several health outcomes, including all-cause mortality (18); cancer (breast, colorectal, esophageal squamous cell carcinoma, liver, larynx, oral cavity, and pharynx) (19); CVD, CHD, and stroke (20); dementia (21, 22); and type 2 diabetes (23, 24). The inclusion and exclusion criteria of these meta-analyses are given in Supplemental Table 1. The outcome variables for CVD, CHD, and stroke were divided into 2 categories, incidence and mortality. The measures presented in the original articles were used; these can be HR, HR ratio (HRR), incidence rate ratio (IRR), OR, or RR. The majority of the measures were RRs, and other effect estimates used in the original publications were interpreted to reflect RRs (25, 26).
The included meta-analyses were published between 2006 and 2017, and the publication year of the original research articles derived from the meta-analyses ranged between 1961 and 2016.
Data collection
All individual studies within the meta-analyses were examined. From each study, the funding source and information on conflict of interest were obtained from the original article. When for a certain health outcome none of the original studies were funded by the alcohol industry, no further data were obtained from these studies and this health outcome was excluded from further analysis. For the health outcomes where ≥1 study was funded by the alcohol industry, the following data were collected: first author, year of publication, study design, gender and number of study subjects, the most adjusted outcome measures for moderate alcohol consumption, and corresponding 95% CI. The current study focused on moderate alcohol consumption, defined as 10, 20, or 15 g alcohol/d for, respectively, women, men, and both sexes combined. The outcome measures obtained from each study were based on this definition of moderate alcohol consumption. Because some studies expressed the alcohol consumption in different units, milliliters and ounces were converted to grams of alcohol by the following conversion factors: 1 mL = 0.789 g and 1 oz = 28.350 g. When alcohol consumption was expressed in drinks, units, or servings, 1 of each of these expressions was considered equivalent to 10 g of alcohol. Besides different alcohol intake levels, in some of the articles the data were stratified for other characteristics of the study population. In case of additional stratification of disease (e.g., colon and rectal cancer in case of colorectal cancer), either the most prevalent disease outcome, the disease outcome most similar to other end points, or the least stratified option (e.g., colorectal cancer) was included in the analysis. In case of separate effect estimates for the time point at which alcohol intake was determined (e.g., baseline, updated/recent, or lifetime alcohol intake), lifetime alcohol intake was obtained as first choice. If not available, updated/recent alcohol intake was obtained. For stratification based on other factors, for each individual study the effect estimate for the largest subgroup was obtained. For stratification of gender, the effect estimates for both men and women were obtained. See Supplemental Tables 2–23 for all the collected data.
Studies were excluded from further analysis when: 1) no effect estimate and corresponding 95% CI could be derived from the publication; 2) the risk of alcohol consumption for a certain health outcome was only given for heavy drinkers; 3) the control group was not a representation of a “healthy” population; and 4) the full text was not available in English (Figure 1). With respect to source of funding, when this information could not be derived from the study, the corresponding author was approached by e-mail if correspondence details were available or could be tracked.
Data processing
For each individual study within every meta-analysis, categories were defined for funding source and the presence of conflict of interest. The categorization was double-checked by 1 of the other authors. Any disagreement was resolved by discussion. Based on funding source, each study was categorized in 1 of the following categories: “alcohol industry,” “not alcohol industry,” and “unknown.” “Not alcohol industry” was further specified in “other industry,” “government,” “nongovernmental organization (NGO),” and “both government and NGO.” Studies sponsored by both the alcohol industry and another source were labeled as “alcohol industry.” Studies funded by both another industry and the government or an NGO were labeled as “other industry.” When no information on funding source was provided in the study and no response by e-mail was given, the study was categorized as “unknown.” Regarding conflict of interest, each study was categorized into “conflict,” “no conflict,” or “unknown.” When a study declared a conflict of interest, meaning that 1 of the authors was affiliated or had received funding in the past from the alcohol industry, the study was labeled as “conflict.” When the study declared having no conflict of interest, the study was categorized as “no conflict.” In case no information on conflict of interest was provided, it was categorized as “unknown.”
Statistical analysis
The complete statistical analysis was conducted by using R 3.6.2 (27). Effect estimates and/or corresponding 95%CIs could not be retrieved directly from 36 studies. For 15 of these studies, these measures could be calculated indirectly using available data in the publication using the epitools package (28). See footnotes in Supplemental Tables 16–23 for the concerned studies. The dmetar (29), meta (30), and metafor (31) packages were used for the calculation of the standard error (SE) and for the different analyses. To calculate the pooled RR, a random-effects model was used for every health outcome per study population (men, women, both sexes) (30), for which sub datasets were created accordingly. In these random-effects models the Sidik and Jonkman estimator was used as τ2 (32). The level of statistical significance was set at a 2-sided P value < 0.05. The Q and I2 statistics were estimated to indicate any substantial between-study heterogeneity and its significance. In the subgroup analyses, the between-subgroup heterogeneity (Q) was estimated (29). For the metaregression a mixed-effects model was applied with funding source as factor added to the model. From the model the indicators of the unaccounted variability (I2) and the accounted variability (explained by the model, R2) were estimated. Additionally, a test for moderators was conducted, by this means the QM statistic (with corresponding P value, level of statistical significance set a P < 0.05), which explains the accounted variability of the factor(s) in the model, was estimated. So, the QM statistic indicates to what extent the factor Funding Source explains the accounted variability (30, 31). The devtools (33) and tidyverse (34) packages were used as supplementary packages.
Finally, 2 sensitivity analyses were conducted. Decisions for the sensitivity analyses were based on an influence analysis. This influence analysis was done using the meta (30) package. The primary sensitivity analysis omitted outliers with significant influence; significance was based on the formulas DFFITS >3 ×√(p/k-p) and hat >3 ×p/k (35). The secondary sensitivity analysis excluded both significant and debatable outliers; debatable outliers are regarded as influential based on the context of the sub dataset (35).
Results
This investigation included 5 meta-analyses obtained from international guidelines on alcohol, supplemented with 2 more recent meta-analyses. In total, 14 different health outcomes were assessed. The 7 meta-analyses included 386 unique articles, from which 44 articles reported on >1 health outcome, resulting in 452 nonunique articles (Figure 1). Funding of the alcohol industry was declared in 21 articles (5.4%), 309 articles (80.1%) were not funded by the alcohol industry, and for 56 articles (14.5%) the funding source was neither mentioned in the article nor provided via personal correspondence with the authors (Table 1). Regarding conflict of interest, 71 studies (18.4%) provided information on conflict of interest and 315 did not (81.6%). From the 71 studies, 2 studies reported a conflict of interest with the alcohol industry and the other 69 declared having no conflict of interest. Recently published studies, that is, <10 y ago, declared funding source and conflict of interest more often compared with articles published >10 y ago (Supplemental Table 24).
TABLE 1.
Funding source per health outcome1
| Health outcome | Alcohol industry, n (%) | Non–alcohol industry, n (%) | Unknown, n (%) | All, n |
|---|---|---|---|---|
| All studies2 | 21 (5.4) | 309 (80.1) | 56 (14.5) | 386 |
| All-cause mortality | 3 (8.8) | 26 (76.5) | 5 (14.7) | 34 |
| CVD mortality | 4 (19.0) | 14 (66.7) | 3 (14.3) | 21 |
| CHD mortality | 3 (9.4) | 24 (75.0) | 5 (15.6) | 32 |
| Incident CHD | 2 (7.1) | 23 (82.1) | 3 (10.7) | 28 |
| Stroke mortality | 0 | 7 (70.0) | 3 (30.0) | 10 |
| Incident stroke | 1 (5.9) | 13 (76.5) | 3 (17.6) | 17 |
| Breast cancer | 7 (6.4) | 91 (82.7) | 12 (10.9) | 110 |
| Colorectal cancer | 4 (7.4) | 39 (72.2) | 11 (20.4) | 54 |
| Esophageal cancer | 0 | 21 (77.8) | 6 (22.2) | 27 |
| Liver cancer | 0 | 18 (90.0) | 2 (10.0) | 20 |
| Larynx cancer | 0 | 11 (84.6) | 2 (15.4) | 13 |
| Oral cavity and pharynx cancer | 0 | 17 (73.9) | 6 (26.1) | 23 |
| Dementia | 0 | 20 (90.9) | 2 (9.1) | 22 |
| Type 2 diabetes | 1 (2.4) | 35 (85.4) | 5 (12.2) | 41 |
CHD, coronary heart disease; CVD, cardiovascular disease.
2The sum of health outcomes does not add up to “all studies,” because multiple studies have reported >1 health outcome.
Health outcomes
For several health outcomes, none of the studies declared funding by the alcohol industry. This was the case for 6 of 14 health outcomes (stroke mortality, esophageal cancer, liver cancer, larynx cancer, oral cavity and pharynx cancer, and dementia) (Table 1 and Supplemental Tables 2–15). For the other 8 health outcomes, ≥1 study was funded by the alcohol industry and further data were collected for 407 nonunique articles (Figure 1). A total of 25 nonunique articles were excluded because they did not meet the inclusion criteria. Twenty-eight articles reported on >1 health outcome. Another 70 articles reported on multiple study populations. Accordingly, 284 unique articles were included.
Pooling of the effect estimates
A pooled effect estimate for every sub dataset was estimated to obtain an indication of the overall association between moderate alcohol intake and the health outcome. A moderate-to-large between-study heterogeneity (25% ≤ I2 ≥ 100%) was found in 13 of the 22 sub datasets. In 12 of these sub datasets the Q statistic was significant. In general, a protective association between moderate alcohol intake and the different health outcomes was observed from the effect estimate pooling. Only for “breast cancer” was the association harmful (RR = 1.05) and statistically significant. More detailed results can be found in Supplemental Table 25.
Subgroup analyses
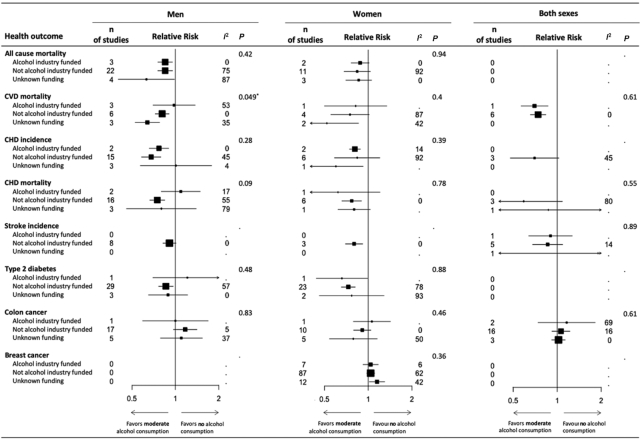
A subgroup analysis was conducted to investigate the effect of funding source on the association between moderate alcohol intake and the health outcome for every sub dataset. Two of the 22 sub datasets did not contain enough studies to be included in this step of the analysis. Only the sub dataset “CVD mortality men” showed a significant Q statistic (P = 0.049). This indicates that results of the subgroups according to funding source were significantly different. The corresponding RR values were 0.98 (P = 0.22), 0.81 (P = 0.0002), and 0.64 (P < 0.0001) for “alcohol industry funded,” “not alcohol industry funded,” and “unknown funding,” respectively. In the other 19 sub datasets funding source most likely did not have an effect on the variability. See Figure 2 for a summary overview of the subgroup analyses. More detailed results can be found in Supplemental Table 26 and Supplemental Figures 1–19.
FIGURE 2.
Summary overview of the subgroup analyses on the influence of funding source on the RR of different health outcomes due to moderate alcohol consumption. Data are presented as the average RR with 95% CI according to the random effects model. P values refer to the difference in RR among funding sources per health outcome. I2 is presented in percentages and indicates the level of between-study heterogeneity. *Statistically significant, P < 0.05. CHD, coronary heart disease; CVD, cardiovascular disease.
Metaregression
A metaregression was conducted to investigate which level(s) (“alcohol industry funded,” “not alcohol industry funded,” and “unknown funding”) of the factor funding source had an effect on the between-study variability within the sub datasets. Five of the 22 sub datasets either did not contain enough studies, or did not have enough studies per level to be included in this step of the analysis. Funding source is tested as a factor in all mixed-effects models. Only the model of sub dataset “CVD mortality men” suggested substantial accounted variability (R2 = 42.75%). In this sub dataset the level “unknown funding” was the only significant factor level.
These findings suggest that the factor funding, and especially the level “alcohol industry funded” and “not alcohol industry funded,” does not explain the accounted variance of the corresponding models in each sub dataset. For more detailed results see Supplemental Table 27.
Sensitivity analysis
In the primary sensitivity analysis 4 influential outliers (all not alcohol industry–funded studies) were removed from the analysis. For the secondary analysis, an additional 43 studies detected as debatable outliers were excluded (2 studies funded by the alcohol industry, 35 studies not funded by the alcohol industry, and 6 studies with unknown funding). Neither sensitivity analysis showed different results compared with the initial analysis (data not shown), indicating that deviating studies did not change outcomes and conclusions of this study.
Discussion
To the best of our knowledge, the current study is the first to examine the association between funding source and study outcomes in observational studies focusing on moderate alcohol consumption and various health outcomes. In total 386 articles were investigated, of which 21 studies (5.4%) were funded by the alcohol industry, 309 studies (80.1%) were not funded by the alcohol industry, and for the remaining 56 studies (14.5%) the funding source was unknown. Based on this selection of observational studies the association between moderate alcohol consumption and different health outcomes does not seem to be related to funding source.
The findings of the present study are not in line with similar studies investigating funding bias in pharmaceutical and nutrition research. As stated earlier, potential funding bias has been identified for studies of SSBs, nonalcoholic drinks, and the fat substitute olestra. In the current study, no differences were observed between observational studies funded by the alcohol industry and ones not funded by the alcohol industry. This could be due to the low number of observations for alcohol industry–funded studies. Furthermore, the present study only assessed the effect of funding with regard to moderate alcohol consumption (10–20 g/d). It remains unknown to what extend alcohol industry funding affects observational research in terms of heavy drinking.
Interestingly, the alcohol industry has funded research not only on health outcomes positively associated with moderate alcohol consumption (such as CHD and type 2 diabetes), but also on health outcomes negatively associated with alcohol consumption, including breast and colorectal cancer. This at least suggests that there is no bias in the selection of research topics for studies funded by the alcohol industry. For the 6 health outcomes for which none of the studies declared alcohol industry funding, it could be that the alcohol industry intentionally avoids funding these areas of research because of a well-established harmful impact. However, this does not seem to be the case; moderate alcohol consumption compared with alcohol abstinence is associated with a reduced risk of stroke mortality (20) and dementia (21, 22), no difference in risk for liver cancer and larynx cancer, and an increased risk for esophageal squamous cell carcinoma and oral cavity and pharynx cancer (19).
Of 386 studies investigated, funding source could be determined for 330 studies (85.5%). This percentage is high compared with other investigations focusing on sponsorship bias in nutrition research. For example, funding source could be derived in 54% and 76% of included studies when investigating the role of industry funding in studies of nonalcoholic drinks (10) and infant formula (36), respectively.
In contrast to the funding source, the majority of the studies (n = 315; 81.6%) did not disclose information on conflict of interest. The disclosure of conflict of interest has become more common in the last 10 y. Because the majority of the selected studies, 291 of 386 (75%), were published before 2007, this might explain the low percentage of conflict of interest disclosures. Among the studies that did disclose information on conflict of interest, only 2 of 71 declared a conflict of interest with the alcohol industry. Due to the lack of available data from the articles on potential conflicts of interest and the low number of conflicts of interest with the alcohol industry, this factor is not further explored in the current study.
The findings from our analysis should be considered in light of its limitations. First, our method to select individual studies did not meet the guidelines for meta-analyses, which include a rigorous process of literature search (37) and an updated search within 12 mo of publication (38). Instead we focused on meta-analyses referred to by several international guidelines, with an additional manual search to find more recent meta-analyses. Only the observational studies included in these meta-analyses were used for our research. This might have led to bias and therefore the conclusion can only be made for those studies included in these specific meta-analyses. Second, information on research funding was obtained by extracting funding information from the article or by e-mail correspondence. Current or past relationship of any (co-)author or institute with the alcohol industry cannot be ruled out. Third, this study only focused on data presented in the articles. The formulation of the results and conclusions in text could deviate between studies funded and not funded by the alcohol industry. Fourth, it could also be possible that observational studies funded by the alcohol industry reporting unfavorable results are less often submitted for publication and hence produce publication bias. Furthermore, there could be additional original research articles published that were not included in the meta-analyses because they did not fit the specific criteria of the meta-analysis, or because for a specific health outcome insufficient data were available for a meta-analysis to estimate the summary effect. Moreover, a quality assessment of the individual studies used in this analysis was not performed. However, scientific experts who are responsible for formulating national dietary guidelines selected these meta-analyses based on the quality of the research. Finally, the current research only investigated the effect of alcohol industry funding on outcomes of observational studies. Future research should also focus on the impact of alcohol industry funding in intervention studies.
In conclusion, only a small proportion of observational studies in meta-analyses, referred to by several international alcohol guidelines, are funded by the alcohol industry. Based on this selection of observational studies the association between moderate alcohol consumption and different health outcomes does not seem to be related to funding source.
Supplementary Material
ACKNOWLEDGEMENTS
The author's responsibilities were as follows—AS, MLJ: contributed to the conception and design of the research; MV, APMvS, TvW: collected and analyzed the data, interpreted the results, and drafted the manuscript; EJMF: advised on the statistical analysis; RMD, RJB, IdK: advised on the (revised) content of the manuscript; and all authors: read and approved the final manuscript.
Notes
The authors reported no funding received for this study.
Author disclosures: MV, APMvS, TvW, MLJ, RMD, RJB, IdK, and AS were employed by the Dutch Beer Institute during the study and writing of the manuscript. This Institute is funded by Dutch Brewers, which is the trade organization of the 14 largest beer brewers in the Netherlands. EJMF reports no conflicts of interest.
Supplemental Figures 1–19 and Supplemental Tables 1–27 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: CHD, coronary heart disease; CVD, cardiovascular disease; HRR, hazard rate ratio; IRR, incidence rate ratio; NGO, nongovernmental organization; SSB, sugar-sweetened beverage.
References
- 1. Lundh A, Lexchin J, Mintzes B, Schroll JB, Bero L. Industry sponsorship and research outcome. Cochrane Database Syst Rev. 2017;2:MR000033 doi:10.1002/14651858.MR000033.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tierney WM, Meslin EM, Kroenke K. Industry support of medical research: important opportunity or treacherous pitfall?. J Gen Intern Med. 2016;31(2):228–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mandrioli D, Kearns CE, Bero LA. Relationship between research outcomes and risk of bias, study sponsorship, and author financial conflicts of interest in reviews of the effects of artificially sweetened beverages on weight outcomes: a systematic review of reviews. PLoS One. 2016;11(9):e0162198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stenius K, Babor TF. The alcohol industry and public interest science. Addiction. 2010;105(2):191–8. [DOI] [PubMed] [Google Scholar]
- 5. Aveyard P, Yach D, Gilmore AB, Capewell S. Should we welcome food industry funding of public health research?. BMJ. 2016;353:i2161. [DOI] [PubMed] [Google Scholar]
- 6. Nestle M, Barnes DE, Cho MK, Relman AS, Moses H III, Martin JB, Angell M, Wazana A, Blumenthal D, Campbell EG et al.. Food company sponsorship of nutrition research and professional activities: a conflict of interest?. Public Health Nutrition. 2001;4(5):1015–22. [DOI] [PubMed] [Google Scholar]
- 7. Bekelman JE, Li Y, Gross CP. Scope and impact of financial conflicts of interest in biomedical research: a systematic review. J Am Med Assoc. 2003;289(4):454–65. [DOI] [PubMed] [Google Scholar]
- 8. Bes-rastrollo M, Schulze MB, Ruiz-Canela M, Martinez-Gonzalez MA. Financial conflicts of interest and reporting bias regarding the association between sugar-sweetened beverages and weight gain: a systematic review of systematic reviews. PLoS Med. 2013;10(12):e1001578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Levine J, Gussow JD, Hastings D, Eccher A. Authors’ financial relationships with the food and beverage industry and their published positions on the fat substitute olestra. Am J Public Health. 2003;93(4):664–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lesser LI, Ebbeling CB, Goozner M, Wypij D, Ludwig DS. Relationship between funding source and conclusion among nutrition-related scientific articles. PLoS Med. 2007;4(1):e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Babor TF. Alcohol research and the alcoholic beverage industry: issues, concerns and conflicts of interest. Addiction. 2009;104(Suppl 1):34–47. [DOI] [PubMed] [Google Scholar]
- 12. McCambridge J, Hartwell G. Has industry funding biased studies of the protective effects of alcohol on cardiovascular disease? A preliminary investigation of prospective cohort studies. Drug Alcohol Rev. 2015;34(1):58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Health Council of the Netherlands. Alcohol – background document for the Dutch Dietary Guidelines 2015. The Hague: Health Council of the Netherlands;2015. Publication no. A15/05. [Google Scholar]
- 14. USDA. Report of the Dietary Guidelines Advisory Committee Dietary Guidelines for Americans, 2010. Nutr Rev. 2010;53(12):376–9. [DOI] [PubMed] [Google Scholar]
- 15. The Health Council of Australia. Australian guidelines to reduce health risks from drinking alcohol. National Health and Medical Research Council (HHMRC); 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nordic Co-operation. Nordic nutrition recommendations 2012: integrating nutrition and physical activity. 5th ed. Copenhagen: Nordic Council of Ministers; 2014. [Google Scholar]
- 17. Santé publique France. Avis d'experts relatif à l’évolution du discours public en matière de consommation d'alcool en France. Saint-Maurice: Santé publique France; 2017. [Google Scholar]
- 18. Di Castelnuovo A, Costanzo S, Bagnardi V, Donati MB, Iacoviello L, de Gaetano G. Alcohol dosing and total mortality in men and women. Arch Intern Med. 2006;166(22):2437–45. [DOI] [PubMed] [Google Scholar]
- 19. Bagnardi V, Rota M, Botteri E, Tramacere I, Islami F, Fedirko V, Scotti L, Jenab M, Turati F, Pasquali E et al.. Light alcohol drinking and cancer: a meta-analysis. Ann Oncol. 2013;24(2):301–8. [DOI] [PubMed] [Google Scholar]
- 20. Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ. 2011;342:d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Anstey KJ, Mack HA, Cherbuin N. Alcohol consumption as a risk factor for dementia and cognitive decline: meta-analysis of prospective studies. Am J Geriatr Psychiatry. 2009;17(7):542–55. [DOI] [PubMed] [Google Scholar]
- 22. Xu W, Wang H, Wan Y, Tan C, Li J, Tan L, Yu JT. Alcohol consumption and dementia risk: a dose–response meta-analysis of prospective studies. Eur J Epidemiol. 2017;32(1):31–42. [DOI] [PubMed] [Google Scholar]
- 23. Baliunas DO, Taylor BJ, Irving H, Roerecke M, Patra J, Mohapatra S, Rehm J. Alcohol as a risk factor for type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2009;32(11):2123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Knott C, Bell S, Britton A. Alcohol consumption and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis of more than 1.9 million individuals from 38 observational studies. Diabetes Care. 2015;38(9):1804–12. [DOI] [PubMed] [Google Scholar]
- 25. Greenland S. Quantitative methods in the review of epidemiologic literature 1. Epidemiol Rev. 1987;9(1):1–30. [DOI] [PubMed] [Google Scholar]
- 26. A'Court C, Stevens R, Heneghan C. Against all odds? Improving the understanding of risk reporting. Br J Gen Pract. 2012;62(596):220–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing; Vienna (Austria): 2019. https://www.R-project.org/. [Google Scholar]
- 28. Tomas J. Aragon. epitools: Epidemiology Tools. R package version 0.5-10.1. 2020. https://CRAN.R-project.org/package=epitools. [Google Scholar]
- 29. Harrer M, Cuijpers P, Furukawa T, Ebert DD. dmetar: Companion R Package For The Guide 'Doing Meta-Analysis in R'. R package version 0.0.9000. 2019. http://dmetar.protectlab.org. [Google Scholar]
- 30. Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R : a practical tutorial, Evidence-Based Mental Health 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Statistical Software. 2010;36(3):1–48.. https://www.jstatsoft.org/v36/i03/. [Google Scholar]
- 32. Sidik K, Jonkman JN. A comparison of heterogeneity variance estimators in combining results of studies. Stat Med2007;26(9);1964–81. [DOI] [PubMed] [Google Scholar]
- 33. Wickham H, Hester J, Chang W. devtools: Tools to Make Developing R Packages Easier. R package version 2.2.2. 2020.https://CRAN.R-project.org/package=devtools. [Google Scholar]
- 34. Wickham H, Averick M, Bryan J, Chang W, McGowan LDA, François R, Grolemund G, Hayes A, Henry L, Hester J et al.. Welcome to the tidyverse. J Open Source Software. 2019;4(43):1686 10.21105/joss.01686. [DOI] [Google Scholar]
- 35. Viechtbauer W, Cheung MW-L. Outlier and influence diagnostics for meta-analysis. Res Synth Methods. 2010;1(2):112–25. [DOI] [PubMed] [Google Scholar]
- 36. Mugambi MN, Musekiwa A, Lombard M, Young T, Blaauw R. Association between funding source, methodological quality and research outcomes in randomized controlled trials of synbiotics, probiotics and prebiotics added to infant formula: a systematic review. BMC Med Res Methodol. 2013;13(1):137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cooper C, Booth A, Varley-Campbell J, Britten N, Garside R. Defining the process to literature searching in systematic reviews: a literature review of guidance and supporting studies. BMC Med Res Methodol. 2018;18(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cochrane. Cochrane Editorial and Publishing Policy Resource. Reporting search dates in Cochrane reviews [Internet]. [cited 2020 Mar 26]. Available from: https://documentation.cochrane.org/display/EPPR/Reporting+search+dates+in+Cochrane+Reviews [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.