ABSTRACT
Despite almost 25 y of fish oil supplementation (FS) research in athletes and widespread use by the athletic community, no systematic reviews of FS in athletes have been conducted. The objectives of this systematic review are to: 1) provide a summary of the effect of FS on the athlete's physiology, health, and performance; 2) report on the quality of the evidence; 3) document any side effects as reported in the athlete research; 4) discuss any risks associated with FS use; and 5) provide guidance for FS use and highlight gaps for future research. Electronic databases (PubMed, Embase, Web of Science, Google Scholar) were searched up until April 2019. Only randomized placebo-controlled trials (RCTs) in athletes, assessing the effect of FS on a health, physiological/biochemical, or performance variable were included. Of the 137 papers identified through searches, 32 met inclusion criteria for final analysis. Athletes varied in classification from recreational to elite, and from Olympic to professional sports. Mean age for participants was 24.9 ± 4.5 y, with 70% of RCTs in males. We report consistent effects for FS on reaction time, mood, cardiovascular dynamics in cyclists, skeletal muscle recovery, the proinflammatory cytokine TNF-α, and postexercise NO responses. No clear effects on endurance performance, lung function, muscle force, or training adaptation were evident. Methodological quality, applying the Physiotherapy Evidence Database (PEDro) scale, ranged from 6 to a maximum of 11, with only 4 RCTs reporting effect sizes. Few negative outcomes were reported. We report various effects for FS on the athlete's physiology; the most consistent findings were on the central nervous system, cardiovascular system, proinflammatory cytokines, and skeletal muscle. We provide recommendations for future research and discuss the potential risks with FS use.
Keywords: performance, injury, inflammation, mood state, recovery, DHA, EPA, exercise
Introduction
The effects of fish oil supplementation (FS) on athlete health and performance have been researched for the past ∼25 y. The early research focused on the potential of FS to modify the inflammatory response to exercise. However, subsequent studies have investigated the effects of FS on metabolism, the immune response, and the respiratory and cardiovascular, musculoskeletal, or central and peripheral nervous systems, with most recent research exploring the effect of FS on neuronal injury.
DHA (22:6n–3) and EPA (20:5n–3) are the long-chain polyunsaturated n–3 (ω-3) fatty acids present in FS. They are natural constituents of seafood including algae, crustaceans, and fish, and to a much a lesser extent in dairy and meat (the diet of the animal influencing the n–3 fatty acid content). In addition to both dietary intake (e.g., eating fish) and FS changing n–3 fatty acid status, endurance training is known to alter skeletal muscle membrane composition, leading to changes in the muscle phospholipids including increasing DHA content and decreasing the n–6:n–3 fatty acid ratio (1, 2). By virtue of training, athletes can acquire a “superior” n–3 fatty acid status compared with the nonathlete and therefore have less need to use supplements. For example, endurance training alters muscle DHA content significantly (1). Therefore, dietary requirements for the specific long-chain n–3 fatty acids can differ in athletes, both collectively in terms of recovery and performance effects, or individually for the respective fatty acids. EPA and DHA have been reported to have differential effects on the antioxidant systems and anabolic-catabolic pathways in skeletal muscle (3, 4). Moreover, EPA has inhibitory effects on cyclooxygenase-2 expression, and metabolism of arachidonic acid (an n–6 fatty acid) (5). Furthermore, EPA is the precursor for 3-series prostanoids and 5-series leukotrienes (eicosanoids), whereas DHA is the precursor for protectins and maresins, with both fatty acids producing the anti-inflammatory and proresolving, aptly named, resolvins (5).
Case studies have demonstrated that elite athletes use FS (6, 7). A review focusing on nutritional recovery strategies in team sports athletes concluded there was emerging evidence for n–3 fatty acid supplementation (the studies referenced used FS) to support recovery in season (8). However, the American College of Sports Medicine position statement on nutrition and athletic performance (9), although comprehensive, provides no statement or guidance on the use of FS (9). According to the International Olympic Committee's recent consensus statement on dietary supplements, there is “limited support” for FS in modifying inflammation and reducing upper respiratory tract infections, and although low risk, it is “unclear if FS should be pursued by athletes” (10). A lack of consensus for FS in athletes is evident within the literature.
FS use constitutes a billion-dollar industry, in which growth has been exponential, with the fish oil market expected to reach USD 5 billion by 2025 if trends continue (11). Concerns have been raised, however, over the quality of some fish oil products (12, 13). Furthermore, FS products are marketed by sports nutrition manufacturers specifically for the athlete, varying in formulation, and it is well recognized that nutritional supplements are not without risk for the athlete (10). To our knowledge no systematic review of FS in athletes has been conducted to date, despite numerous publications, including many narrative reviews. A systematic review of the evidence is warranted. In this review we will focus specifically on FS (EPA and DHA) in relation to the athlete. The aims of this systematic review are to: 1) provide a summary of the effect of FS on the health and performance of athletes; 2) report on the quality of the evidence; 3) document any side effects as reported in the athlete research; 4) discuss any risks associated with FS; and 5) provide guidance for FS use and highlight gaps for future research.
Methods
Search strategy
The Preferred Reporting Items for Systematic Reviews and Meta-analysis protocols (PRISMA) checklist was followed. Figure 1 summarizes the study selection process. Electronic searches were performed up until April 2019 with no date restrictions in PubMed, Embase, Web of Science, and Google Scholar using the search terms “athlete” and “omega-3,” and “athlete” and “fish oil” with the terms being included in the study title, abstract, and/or keywords. To ensure the search strategy captured all relevant studies, searches within the electronic databases were conducted under each of the following specific terms—cognition, performance, injury, recovery, adaptation, skeletal muscle, muscle soreness, endurance, strength—and the following methods were applied to the searches: in PubMed, Medical Subject Headings were used and combined, whereas in Embase, Emtree was used with the expansion of the subject search term with the subject added to query builder. Reference lists of individual study publications and reviews were consulted for additional studies. The publication abstracts and titles were screened individually to ensure removal of studies not meeting the inclusion/exclusion criteria. If there was any doubt over article inclusion or exclusion, the article was obtained in full for clarification. The remaining publications were obtained in full, with each author independently reviewing the screened articles according to the inclusion and exclusion criteria.
FIGURE 1.
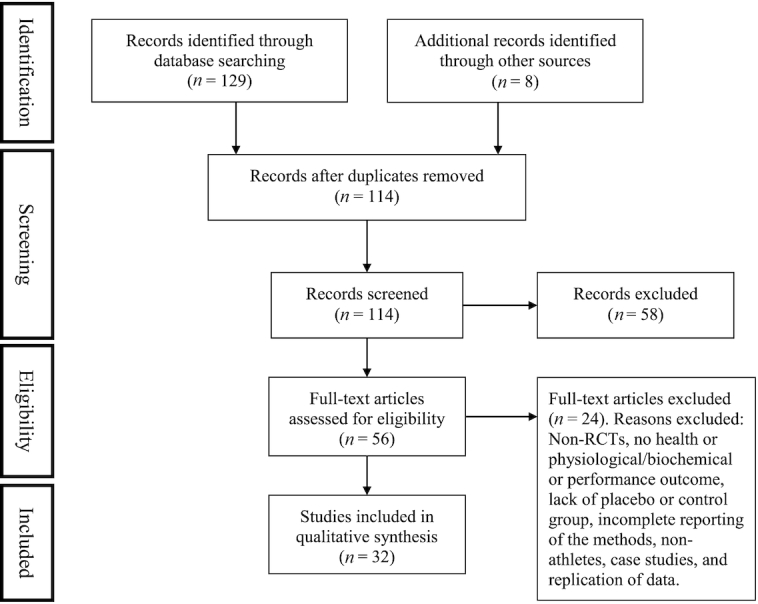
Flow diagram of study selection criteria. RCT, randomized placebo-controlled trial.
Eligibility criteria for studies
Only publications written in the English language were included. Due to the plethora of research papers on FS, we focused on studies in which the research participants were characterized as male or female athletes, whether recreational, well-trained, or elite athletes. We chose not to include research on healthy physically active individuals and extrapolate such findings to athletes, 1) due to the known effects of training on increasing muscle phospholipid n–3 fatty acid content (1); 2) to summarize the evidence for practitioners in sport settings; and 3) to identify gaps in the literature as they pertain to the athlete. Only randomized placebo-controlled trials (RCTs), assessing the effect of FS on health, and/or physiological/biochemical or performance variables were included. For example, studies that did not include a matched control or placebo group were excluded (6, 7, 14–16), as were nonrandomized trials (17). Publications that failed to report fully the methodology and statistical approach were excluded (18, 19), as were studies where the participants were not classified as athletes (i.e., recreationally or physically active, or resistance trained were excluded) (20–30). Figure 1 summarizes the studies excluded. To ensure we captured a broad spectrum of FS studies, no restrictions were imposed on sport, sex of participants, fish oil dose, duration of FS, whether supplements were EPA or DHA only, or lack of reporting of dietary or blood n–3 fatty acid status, or whether FS was combined with other ingredients (e.g., antioxidants). The latter point is relevant because these are the products that make it to market and are used by athletes. We chose to extract and tabulate separately both the statistically significant and nonsignificant findings, given the wide range of outcomes and variables assessed (Tables 1–4).
TABLE 1.
Summary of studies of fish oil supplementation (FS) in athletes relating to oxidative stress1
| Author | Sport | Athlete status | Sex and sample size | Biomarkers affected by FS | Dosing period, wk | Active treatment, mg/d | ω-3 biomarker response to FS | No effect for FS2 |
|---|---|---|---|---|---|---|---|---|
| Żebroswka et al. (32) | Cycling | Competitive | Male participants n = 13 | ↑NO at rest and postexercise | 3 | 1320 EPA880 DHA | Not measured | Endothelium independent vasodilatation, peak power, TGs, LDL and HDL cholesterol, total cholesterol, HR, SBP, DBP, glycerol, TAS, PWV |
| Filaire et al. (35) | Judo | Elite | Male participants n = 20 | ↓TGs, ↑MDA at rest only, ↑MDA, Rmax, CDmax post-ex, ↑NO post-ex | 6 | 600 EPA400 DHA | Not measured | GPx |
| Filaire et al. (36) | Judo | Elite | Male participants n = 28 | ↑MDA, Rmax, CDmax at rest, and ↓post-ex | 6 | 600 EPA400 DHAVitamin C, Vitamin E,β-carotene | Not measured | |
| Filaire et al. (36) | Judo | Elite | Male participants n = 28 | ↑MDA, Rmax, CDmax at rest and post-ex, ↑NO post-ex | 6 | 600 EPA400 DHA | Not measured | |
| McAnulty et al. (37) | Cycling | Competitive | Male and female participants n = 48 | ↑F2-isoprostane post-ex | 6 | 2000 EPA400 DHA | ↑Plasma DHA (55.8%), EPA (61.5%) | |
| McAnulty et al. (37) | Cycling | Competitive | Male and female participants n = 48 | Prevented F2-isoprostane ↑post-ex | 6 | 2000 EPA400 DHAVitamins, Minerals,Antioxidants | ↑Plasma DHA (95.3%), EPA (77.8%) | |
| McAnulty et al. (38) | Cycling | Competitive | Male and female participants n = 39 | Prevented F2-isoprostane ↑post-ex | 2 | 220 EPA180 DHAVitamin C, EGCG,Quercetin, B vitamins | Not measured | ORAC, ferric-reducing ability of plasma |
| Martorell et al. (39) | Soccer | Semiprofessional | Male participants n = 15 | ↑RBC SOD and GRd activity, ↓GPx | 5 d/wk for 8 wk | 1140 DHACHO, Proteins,Almonds | ↑RBC DHA (26.4%) | RBC MDA, carbonyl index, nitrotyrosine, CAT |
| Ghiasvand et al. (19) | Basketball | Well-trained | Male participants n = 34 | Resting: ↑MDA | 6 | 2000 EPA | Not measured | No change TNF-α |
CAT, catalase; CDmax, maximum amount of conjugated dienes; CHO, carbohydrate; DBP, diastolic blood pressure; EGCG, epigallocatechin 3-gallate; FS, fish oil supplementation; GPx, glutathione peroxidase; GRd, glutathione reductase; HR, heart rate; MDA, malondialdehyde; ORAC, oxygen radical absorption capacity; post-ex, postexercise; PWV, pulse wave velocity; Rmax, maximum rate of oxidation during the propagating chain reaction; SBP, systolic blood pressure; SOD, superoxide dismutase; TAS, total antioxidant status; TG, triglyceride.
All nonsignificant findings for the effects of FS.
TABLE 4.
Summary of studies of fish oil supplementation (FS) in athletes relating to cardiovascular performance, cognition and mood, and respiratory function1
| Author | Sport | Athlete status | Sex and sample size | Mood and cognition | Immune and inflammation | Cardiovascular and respiratory | Skeletal/muscle | Biomarkers | Physical performance | Dosingperiod, wk | Active treatment, mg/d | ω-3 biomarker response to FS | No effect for FS2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Raastad et al. (54) | Soccer | Professional-elite | Male participants n = 28 | ↓TGs | 10 | 1600 EPA1004 DHA | ↑Plasma EPA (175%), DHA (40%) | V̇O2max, running speed at anaerobic threshold, run time to exhaustion | |||||
| Buckley et al. (55) | Aussie Rules | Professional-elite | Male participants n = 25 | ↓DBP, ↓sub-max exercise HR | ↓TGs | 5 | 360 EPA1560 DHA | ↑RBC EPA (116%) DHA (100%), total n–3 (74%) | Run time to exhaustion, V̇O2/running economy | ||||
| Hingley et al. (57) | Runners and cyclists | Well-trained, recreational | Male participants n = 26 | ↓Sub-max V̇O2 during 5-min cycling time trial | 8 | 140 EPA 560 DHA | ↑OM3I: 4.5% to 6% | Endurance performance (time-trial), Wingate, MVC | |||||
| Peoples et al. (58) | Cyclists | Competitive | Male participants n = 16 | ↓Exercise HR, ↑cycling economy (↓sub-max V̇O2) | 8 | 800 EPA2400 DHA | ↑RBC DHA (41.4%), total n–3 (24.3%) | ||||||
| Żebroswka et al. (32) | Cyclists | Competitive | Male participants n = 13 | ↑V̇O2max (+5%), FMD (%) | ↑NO at rest, postexercise, ↑glucose and FFA at rest | 3 | 1320 EPA880 DHA | Not measured | Endothelium independent vasodilatation, peak power, TGs, LDL and HDL cholesterol, total cholesterol, HR, SBP, DBP, glycerol, TAS, BM, PWV | ||||
| Fontani et al. (33) | Athletics | Recreational | Male and female participants n = 33 | ↑Vigor, attention, ↓reaction time, anger, anxiety, depression | ↓Homocysteine | 5 | 1600 EPA800 DHA | ↓AA/EPA | |||||
| Fontani et al. (59) | Karate | Competitive | Male and female participants n = 18 | ↑POMS, ↓reaction time | 3 | 4800 EPA2400 DHAPolicosanol | Not measured | ||||||
| Guzmán et al. (60) | Soccer | Professional-elite | Female participants n = 34 | ↓Complex reaction time | 4 | 3500 DHA | Not measured | ||||||
| Black et al. (51) | Rugby | Professional-elite | Male participants n = 20 | ↓Fatigue, ↑sleep quality | ↓Muscle soreness | ↑Mean CMJpeak force | 5 | 1102 EPA1102 DHAWhey PRO,CHO, Vitamin D | ↑Plasma ω-3 fatty acids (240%) | ||||
| Mickleborough et al. (34) | Endurance sports | Competitive | Male and female participants n = 20 | ↓Urinary LTE4 and ↓9α,11β-PGF2; ↓plasma LTB4, TNF-α, IL-1β | Improved lung function (FEV1) | 3 | 3200 EPA2200 DHA | ↑Neutrophil EPA (1206%), but no change DHA | |||||
| Tartibian et al. (61) | Wrestling | Recreational | Male participants n = 40 | ↑FEV1, ↑FVC, ↑VC, ↑MVV, ↑FEF25–75, ↑FIV1 | 12 | 180 EPA120 DHA | Not measured | FEV1% and FIV1% | |||||
| Gravina et al. (53) | Soccer | Competitive | Male and female participants n = 26 | ↑Anaerobicendurance | 4 | 4900 EPA1400 DHA | ↑Blood ω-3 fatty acids (100%) | Respiratory function (FEV1, FVC), MVC, 20-m sprints, vertical jump height, yo-yo level 1 test |
AA, arachidonic acid; BM, body mass; CHO, carbohydrate; CMJ, counter movement jump; DBP, diastolic blood pressure; FEF, forced expiratory flow from 25% to 75%; FEV1, forced expiratory volume in 1 s; FFA, free fatty acid; FIV1, forced inspiratory volume in 1 s; FMD, flow mediated dilation; FS, fish oil supplementation; FVC, forced vital capacity; HR, heart rate; LT, leukotriene; MVC, maximal voluntary contraction; MVV, maximal voluntary ventilation; OM3I, ω-3 index; POMS, profile of mood states; PRO, protein; PWV, pulse wave velocity; SBP, systolic blood pressure; TAS, total antioxidant status; TG, triglyceride; VC, vital capacity; V̇O2, oxygen uptake; V̇O2max, maximal oxygen uptake; 9α,11β-PGF2, prostaglandin metabolite.
All nonsignificant findings for the effects of FS.
Assessment of study quality and risk of bias
Studies were rated using the Physiotherapy Evidence Database (PEDro) scale (https://www.pedro.org.au/english/downloads/pedro-scale/), an 11-point validated scale for the assessment of RCTs. Any differences of opinion regarding the rating of study quality were resolved between authors.
Data synthesis and extraction
A meta-analysis was not conducted due to the heterogeneity across studies and the variety of outcomes reported. Data extraction captured information relating to: 1) study design (e.g., randomized, placebo-controlled trial); 2) sample characteristics (e.g., age, sample size, sex, sport, athlete status); 3) health and performance variables (e.g., mood and cognition, cardiovascular and respiratory, skeletal muscle, immune and inflammation, biomarkers, physical performance); 4) fish oil dose and dosing period; 5) additions to the fish oil (e.g., vitamin D, vitamin E, etc.); 6) measurement of n–3 fatty acid biomarkers (e.g., plasma EPA and DHA); and 7) nonsignificant effects and findings.
Results
Study selection and sports
We identified a total of 137 papers using our search strategy, of which 32 RCTs (level of evidence 1B) met the inclusion criteria (Figure 1). The mean sample size of included studies was 27 participants (range: 15–81 participants).
The 32 RCTs were grouped into the following areas (some studies represented twice): oxidative stress only (n = 5); immunity, inflammation, and oxidative stress (n = 10); muscle recovery (n = 5); team sports and training adaptation (n = 5); cardiovascular physiology (n = 5); cognition and mood state (n = 4); respiratory health (n = 2); and injury related (n = 2) (Tables 1–4). Only significant findings are reported in the results and discussed. However, Tables 1–4 provide a summary of both the significant and nonsignificant findings.
Subject characteristics
The majority of investigations (n = 22) studied men only. Nine RCTs included participants of both sexes, and one RCT included women only. The mean age for participants in the FS and placebo groups across the RCTs was 24.9 ± 4.5 y; 1 RCT failed to report the age of the participants (31). Athletes varied in classification, from recreational (e.g., noncompetitive) to elite (referring to a professional or national-level competitive standard) and from a range of summer Olympic sports (e.g., judo, swimming, cycling, marathon) and professional sports (i.e., American football, soccer, rugby, Australian Rules football, and basketball) (Tables 1 –4).
Methodological quality and risk of bias
The methodological quality of the RCTs, applying the PEDro scale (maximum score obtainable 11), ranged from a score of 6 (2 studies) to a maximum of 11 (1 study), with an average score of 9. Of the 32 RCTs included, 25 (75%) were double-blind in design, 7 (20%) were single-blind, and 3 incorporated a crossover design with a wash-out period ranging from 2 wk to 35 d (32–34). A major methodological flaw affecting 28 of the 32 RCTs (88%), was that the size of the treatment effect (i.e., effect sizes) was not reported. Sponsorship and research funding by the fish oil industry was clearly reported in 7 RCTs.
Seventeen studies (53%) measured various biomarkers of n–3 fatty acid status to confirm compliance with the FS. Only 1 RCT ensured subjects were matched at baseline for n–3 fatty acid status, thus reducing the potential confounding effect of different baseline status on outcome (53).
Main findings
The findings were analyzed and grouped in relation to the main outcomes assessed. Inflammation was the most frequently studied variable in athletes (19, 40–48), with doses of EPA ranging from 300 to 2400 mg/d, and of DHA from 400 to 1500 mg/d. For the majority of pro- and anti-inflammatory mediators measured, the effects were inconsistent: for example, despite the variation in the timing of blood sampling and source of the cytokines, IL-6 (measured at rest) showed a reduction in 4 RCTs (19, 45, 46, 48) and an increase in 1 RCT (41), with no effect at rest or postexercise in 3 RCTs (43, 45, 46). However, there was evidence in 4 of the 5 RCTs for an effect of FS on attenuating the production of TNF-α by peripheral blood mononuclear cells (PBMCs) in ex vivo culture (41, 42, 45, 46). Only 1 RCT reported on upper respiratory tract illness (URTI), via an illness log and questionnaire, in which a moderate dose of fish oil reduced the total number of symptom days, but not the number of URTI episodes, symptom severity score, or URTI duration; however, the FS included vitamin D, whey protein, and 100 kcal more energy than the control group condition (44).
Muscle recovery and team sport training adaptations were examined across 7 RCTs (Table 3). Four RCTs reported positive effects on measures of recovery (e.g., muscle soreness, counter movement jump, creatine kinase activity) (49–52), a single RCT noted a positive effect on anaerobic endurance in soccer players but not on a battery of other physiological measures (53), whereas 2 RCTs reported no effect on physiological adaptations and performance (54, 55). Overall effects were consistent for muscle recovery, but inconsistent for training adaptation and performance outcomes in team sport athletes. The RCT with the largest change in plasma n–3 fatty acids (240%) with FS did observe effects on subjective and objective measures of recovery in professional rugby players (51). The 2 RCTs using the same FS in a recovery product formulation (i.e., administered posttraining with whey protein), reported positive effects on muscle recovery (51, 52).
TABLE 3.
Summary of studies of fish oil supplementation (FS) in athletes relating to recovery and injury1
| Author | Sport | Athlete status | Sex and sample size | Mood and cognition | Cardiovascular and respiratory | Skeletal/muscle | Biomarkers | Physical performance | Dosing period, wk | Active treatment, mg/d | ω-3 biomarker response to FS | No effect for FS2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Jakeman et al. (49) | Athletes | Recreational | Male participants n = 27 | >Recovery of CMJ | <1 | 7500 EPA500 DHA | Not measuredNo. Please this as the omega, as opposed to n-3, this is correct as per Professor Calder final edits | Muscle soreness, CK, IL-6 | ||||
| Lewis et al. (50) | Summer Olympic sports | Competitive | Male participants n = 31 | ↓Fatigue (% drop Wingate power), ↑VL EMG | 3 | 375 EPA510 DHAVitamin D | Plasma EPA↑ (65.8%), DHA no change | MVC, squat jump, CMJ, back squat, push-ups, endurance performance (time trial) | ||||
| Black et al. (51) | Rugby | Professional-elite | Male participants n = 20 | ↓Fatigue, ↑sleep quality | ↓Muscle soreness | ↑Mean CMJ peak force | 5 | 1102 EPA1102 DHAWhey PRO,CHO, Vitamin D | Plasma ω-3 fatty acids (240%)↑ | |||
| Philpott et al. (52) | Soccer | Competitive | Male participants n = 30 | Muscle soreness↓ | ↓CK conc. | 6 | 1102 EPA1102 DHAWhey PRO,CHO, Vitamin D | Blood ω-3 fatty acids↑ (58%) | CRP, MVC, soccer passing test, yo-yo level 2 test | |||
| Gravina et al. (53) | Soccer | Competitive | Male and female participants n = 26 | ↑Anaerobic endurance (enhanced training effect) | 4 | 4900 EPA1400 DHA | Blood ω-3 fatty acids↑ (100%) | Respiratory function (FEV1, FVC), MVC, 20-m sprints, vertical jump height, yo-yo level 1 test | ||||
| Raastad et al. (54) | Soccer | Professional-elite | Male participants n = 28 | ↓TGs | 10 | 1600 EPA1004 DHA | Plasma EPA (175%), DHA (40%) ↑ | V̇O2max, running speed at anaerobic threshold, run time to exhaustion | ||||
| Buckley et al. (55) | Aussie Rules | Professional-elite | Male participants n = 25 | ↓DBP, ↓sub-max exercise HR | ↓TGs | 5 | 360 EPA1560 DHA | RBC EPA (116%) DHA (100%), ↑total n–3 (74%) | Run time to exhaustion, V̇O2/running economy | |||
| Oliver et al. (31) | American football | Competitive | Male participants n = 81 | ↓NFL | 27 | 2000–6000 DHA | ↑Plasma DHA (98–99.9%), dose dependent | |||||
| Mavrogenis et al. (56) | All sports | Recreational | Male participants n = 31 | ↓Pain | ↑Activity | 32 d | 3000 EPA2112 DHAVitamins and minerals,GLA | Not measured |
CHO, carbohydrate; CK, creatine kinase; CMJ, counter movement jump; CRP, C-reactive protein; DBP, diastolic blood pressure; EMG, electromyography recording from vastus lateralis; FEV1, forced expiratory volume in 1 s; FS, fish oil supplementation; FVC, forced vital capacity; GLA, γ-linolenic acid; HR, heart rate; MVC, maximal voluntary contraction; NFL, neurofilament light; PRO, protein; TG, triglyceride; VL, vastus lateralis; V̇O2max, maximal oxygen uptake.
All nonsignificant findings for the effects of FS.
Positive effects of EPA and DHA at various doses were observed on cardiovascular and oxygen kinetics in all studies of cyclists [cycling efficiency, maximal oxygen uptake (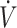 O2max)] (32, 57, 58) but there were no improvements in endurance performance [e.g., time trial (57)]; see Table 4. An effect on endurance was not observed in the 2 RCTs of individuals involved in team sports (54, 55), or in athletes from 4 summer Olympic sports (50).
O2max)] (32, 57, 58) but there were no improvements in endurance performance [e.g., time trial (57)]; see Table 4. An effect on endurance was not observed in the 2 RCTs of individuals involved in team sports (54, 55), or in athletes from 4 summer Olympic sports (50).
Three RCTs with various doses of EPA and DHA showed that FS increased biomarkers of lipid peroxidation (i.e., malondialdehyde, F2-isoprostanes) at rest (19, 35, 36), and 4 RCTs reported this postexercise (35–38) (Table 1). Two RCTs showed the postexercise effect for FS on increasing F2-isoprostanes was prevented with the addition of various antioxidants (36, 37). Antioxidant enzyme activity (i.e., superoxide dismutase, glutathione peroxidase, glutathione reductase) was increased with FS in 3 RCTs (19, 39, 47), and decreased in 2 RCTs (45, 46). Finally, 3 RCTs showed an effect on increasing NO postexercise (32, 35, 36) (Table 1).
Of the studies assessing cognitive variables, a positive effect on reaction time and mood state was seen across all RCTs where measured (n = 4), regardless of sport and ability, that is, professional rugby (51), soccer (60), athletics (33), and karate (59) (Table 4).
Few studies have examined injury risk. A positive effect for DHA was observed on biomarkers of neuronal injury (31), and a single RCT incorporating high doses of both EPA and DHA showed an effect on tendinopathy pain and subsequent activity level (56) (Table 3). Three RCTs explored the effect of FS on respiratory function (Table 4), 1 of which examined the effect of FS on exercise-induced bronchoconstriction (EIB) (34). The RCT reporting positive findings in EIB was of the highest methodological quality score (11 out of 11). In healthy athletes, a positive effect for FS on lung function was identified in 1 RCT in wrestlers (61), but this was not corroborated when examined in soccer players (53).
Adverse effects and product analysis
Of the included RCTs, only 1 reported on adverse effects with FS (DHA use only), citing poor palatability, gastrointestinal distress, and nausea in a small number of participants (∼10%) (31). Three RCTs reported the laboratory analysis of the FS and thus verification of the product contents (55, 31, 58), with analysis independent of the manufacturer in just 2 studies (55, 31). No RCTs reported on FS analysis confirming the absence of traces of banned substances in the batches studied, which is relevant for the elite athlete participants.
Discussion
Summary of key findings
We present to our knowledge, the first systematic review of FS in athletes. We report evidence from a number of RCTs for an impact of FS upon the athlete's physiology. The most consistently reported findings to date relate to skeletal muscle recovery, postexercise NO response, biomarkers of lipid peroxidation, TNF-α production by immune cells, and cardiovascular dynamics in cyclists. Inflammation was the most studied variable in athletes, with FS modifying various inflammatory markers at rest and postexercise (via immune cell activation), with a consistent effect on attenuating the production of TNF-α by PBMCs. No effects on endurance performance, muscle force, or training adaptation were evident across sports. Few negative outcomes were reported. Where relevant, we will contextualize findings from studies in athletes with the inclusion of research published in nonathletes and conclude with recommendations for future research and guidance for practitioners in sport.
Cardiovascular and performance effects in endurance athletes
DHA is preferentially increased in skeletal muscle phospholipids in response to training (2), whereas both EPA and DHA are incorporated into mitochondrial phospholipids with FS (62). We found evidence for a consistent effect of FS on advantageously modifying cardiovascular physiology and whole-body oxygen consumption in athletes. A mechanism for this effect could be related to the capacity for DHA and EPA to moderate sympathetic activation of blood vessels and blood flow, via the expression and activation of endothelial NO synthase and production of NO (63). Indeed, 3 RCTs reported an effect for FS on increasing NO. Nevertheless, effects for FS on aerobic performance were not evident. Furthermore, the positive findings extended only to controlled laboratory studies in cyclists. Indeed, FS consistently showed no effect on tests of endurance undertaken in competitive and professional team sport athletes. It is possible that greater intra- and intersubject variability for performance tests could have confounded the findings. Finally, a recently published review article on the effects of FS on performance (encompassing both sedentary and athletic populations) was in agreement with our findings; an effect for FS in endurance-based athletes on oxygen efficiency, but no clear effects on performance (64).
Muscle recovery and adaptation
The research into muscle recovery in athletes has been focused on a greater dose of EPA compared with DHA administered, with 1 study showing that EPA, but not DHA, stimulates muscle protein synthesis in the presence of leucine and inhibits muscle protein breakdown in muscle cell culture (4). We identified 7 RCTs investigating muscle recovery and training adaptation, in which differences in FS formulations and types of sport participation could account for divergent findings. For example, the positive effects on muscle soreness (i.e., perceived), damage (i.e., creatine kinase activity), and recovery (i.e., counter movement jump peak force) were evident in professional rugby and competitive soccer players when administered over several weeks, in which the FS also included vitamin D and whey protein (51, 52). This was notwithstanding the fact that the control groups in both studies received matched timed protein and carbohydrate intakes. In addition, the 3-wk FS study in Olympic athletes showing an effect on fatigue (i.e., percentage drop in Wingate power), also included vitamin D (50). It is conceivable that the strongest effects for FS on accelerating muscle recovery can be most evident when administered alongside other nutrients known to impact on skeletal muscle remodeling (i.e., vitamin D, whey protein) as part of a recovery drink. The strength of the evidence for FS on enhancing adaptations to training in team sport athletes is very weak, with 3 RCTs reporting no effect on performance (52, 54, 55), and just 1 observing an effect for FS on increasing anaerobic capacity over 4 wk of training (53). An effect of FS on muscle force (maximal voluntary contraction) was absent across all RCTs. Three separate RCTs of recreational, competitive, and professional athletes reported an effect for FS on neuromuscular performance measures, for example, muscle activation, Wingate power, counter movement jumps (49–51). Further research is needed to clarify the effect of FS on the neuromuscular system, and whether EPA is more effective for enhancing muscle recovery in vivo, given the effects on muscle recovery that were seen using a 1:1 ratio of EPA and DHA.
Respiratory function
EIB causes narrowing of the airways leading to decrements in pulmonary function and is well known to adversely affect elite athlete performance. Inflammation is part of the pathophysiology of EIB, and as such FS has been tested as a nonpharmacological therapeutic treatment option. FS reduced the postexercise decline in forced expiratory volume, systemic inflammation, and bronchodilator use in elite athletes (34). However, the evidence for an effect of FS on modifying lung function in healthy athletes without EIB is weak, with only 1 RCT showing benefit after 12 wk of FS (61). In short, FS can reduce airway inflammation in elite athletes with EIB and can be considered as an adjunctive nonpharmacological low-risk treatment option; clearly, further research is needed to better understand the effects of FS on respiratory function. However, the absence of EIB is likely to limit the potential application of FS on respiratory function.
Inflammation and immunity
For the healthy athlete, the evidence summarized in Table 2 suggests that FS can modify the PBMC inflammatory response, when assessed through changes in cytokines, for example, TNF-α (40–42, 45, 46). However, we acknowledge the conflicting evidence with regard to immunomodulation in athletes; with differences in study design (e.g., pre- compared with postexercise sampling), training and immune function status, n–3 fatty acid dose, measures of immunity, and the underlying fatty acid status affecting the consistency of study outcomes (19, 40–43, 45–47, 65). In contrast to our findings, a systematic review published in 2012 reported that moderate n–3 fatty acid consumption (900–2000 mg/d) does not lead to changes in inflammatory biomarkers in healthy nonathletes (66), suggesting a differential effect between trained and untrained individuals. The biological material sampled and thus the source of the cytokines [e.g., plasma compared with in vitro (ex vivo) assessments of immune cell function], the timing (at rest compared with postexercise), and dose of n–3 fatty acids are critical factors in determining consistency of study outcomes with regard to inflammation. Finally, complicating the findings, using modest doses (400 mg/d EPA and DHA) combined with polyphenols such as green tea and quercetin and administered during an intensified period of training, Nieman et al. (48) showed an anti-inflammatory effect (i.e., a reduction in high sensitivity C-reactive protein and IL-6 compared with placebo) both at rest and postexercise (48). Because antioxidant supplements can modify adaptive responses to exercise (67), caution should be applied to their use.
TABLE 2.
Summary of studies of fish oil supplementation (FS) in athletes relating to immunity and inflammation1
| Author | Sport | Athlete status | Sex and sample size | Biomarkers affected by FS | Dosing period, wk | Active treatment, mg/d | ω-3 biomarker response to FS | No effect for FS2 |
|---|---|---|---|---|---|---|---|---|
| Andrade et al. (40) | Swimmers | Elite | Male participantsn = 20 | Resting: ↑PBMC, ↓plasma PGE2, ↓IFN-γ | 6 | 950 EPA500 DHA | ↑Plasma total ω-3 fatty acids (45.4%), EPA (196%), DHA (96%) | PBMC TNF-α, IL-2, IL-4, cortisol, insulin |
| Delfan et al. (41) | Paddlers | Elite | Male participants n = 22 | Resting PBMCs: ↓TNF-α, ↓IL-1β, ↑IL-6, ↑IL-10, ↓IFN-γ | 4 | 2400 EPA1200 DHA | Not measured | IL-4, Th1/Th2 ratio |
| Santos et al. (42) | Marathon | Competitive | Male participants n = 21 | Resting: ↑lymphocyte proliferation; ↓IL-2, ↓TNF-α, ↓IL-10Postrace: ↑lymphocyte proliferation | 8.5 | 300 EPA1500 DHA | Not measured | Lymphocyte death. Postrace lymphocyte: IL-2, IL-4, TNF-α, IL-10 |
| Nieman et al. (43) | Cyclists | Competitive | Male and female participants n = 23 | 6 | 2000 EPA400 DHA | ↑Plasma EPA (311%), DHA (40%) | Total blood leukocytes, CK, CRP, ratio sIgA:protein, myeloperoxidase, IL-6, IL-8, IL-1Ra, endurance performance | |
| Da Boit et al. (44) | Athletic, nonspecific | Recreational | Male and female participants n = 30 | ↓Total number of symptom days | 16 | 550 EPA550 DHAWhey protein,Vitamin D | Not measured | URTI incidence, severity and duration of URTI, visits to medical doctor, sIgA conc. and secretion rate |
| Capó et al. (45, 46) | Soccer | Semiprofessional | Male participants n = 15 | ↓Post-ex MIP1-α. Post-ex LPS-stimulated PBMCs: ↓TNF-α, ↓IL-6, ↓TLR-4 protein concentrations↑PBMCs UCP-3, ↓GPx | 5 d/wk for 8 wk | 1140 DHACHO, Proteins,Almonds | ↑RBC DHA (36%) | Oxidative damage in PBMCs, ROS production by PBMCs. Resting and postexercise plasma: IL-2, IL-4, IL-6, IL-8, IL-10, VEGF, IFN-γ, TNF-α, IL-1α, IL-1β, TNF-β, EGF, IL-5, IL-15, MCP-1, GMCSF |
| Capó et al. (47) | Soccer | Semiprofessional | Male participants n = 15 | Resting: neutrophil gene expression (IL-8, NFκB); ↑neutrophil total MPO, ↑neutrophil total CAT activity | 5 d/wk for 8 wk | 1140 DHACHO, Proteins,Almonds | ↑RBC DHA (36%) | Neutrophils: nitrate, nitrite; NO; MPO, TNF-α, gene expression (COX2, TNF-α, MPO) |
| Nieman et al. (48) | Cyclists | Competitive | Male and female participants n = 39 | Resting: serum ↓CRP, plasma IL-6, total leukocytes, ↓granulocyte oxidative burst | 24 d | 400 EPA400 DHAEGCG, Quercetin | Not measured | Plasma IL-10, IL-1Ra, TNF-α, CK, MCP, MPO, sIgA protein, HSP-70 |
| Ghiasvand et al. (19) | Basketball | Well-trained | Male participants n = 34 | Resting: ↓IL-6, ↑GPx | 6 | 2000 EPAVitamin E +/− | Not measured | No change GPx in EPA-only group |
CAT, catalase; CHO, carbohydrate; CK, creatine kinase; COX, cycloxygenase; CRP, C-reactive protein; EGCG, epigallocatechin 3-gallate; EGF, epidermal growth factor; FS, fish oil supplementation; GMCSF, granulocyte macrophage colony-stimulating factor; GPx, glutathione peroxidase; HSP, heat-shock protein; MCP, monocyte chemotactic protein; MIP, macrophage inflammatory protein; MPO, myeloperoxidase; PBMC, peripheral blood mononuclear cell; ROS, reactive oxygen species; sIgA, salivary immunoglobulin A; Th1/2; T helper cell 1/2; TLR, toll-like receptor; UCP, mitochondrial uncoupling protein; URTI, upper respiratory tract illness; VEGF, vascular endothelial growth factor.
All nonsignificant findings for the effects of FS.
We did not find any convincing evidence that FS provided protection from illness. Of the 10 RCTs exploring the effects of FS on immune and inflammatory variables in athletes, only 1 RCT included data on infectious symptoms and illness (44). FS in combination with 10 μg vitamin D-3 and 8 g whey protein reduced the number of illness symptom days. Further research is needed to corroborate these findings.
Cognition
We observed consistent findings for an effect of FS on aspects of cognition and mood state in athletes, namely reaction time, using combinations of EPA and DHA (51, 33,59,60). Improvements in reaction times related to working memory and episodic memory using DHA (68), and complex reaction times with EPA (69) have also been reported in healthy male and female nonathletes. Furthermore, higher RBC DHA concentrations are associated with improved working memory performance and are predictive of baseline performance (70). Thus FS, with an emphasis on DHA, could be anticipated to improve mood states and cognitive performance in athletes; mental tasks requiring more complex cortical processing are likely to benefit the most, as could athletes with poor n–3 fatty acid status prior to FS (e.g., low RBC DHA, bottom quartile ω-3 fatty acid index). Mechanisms by which FS might modify brain function and therefore mood (e.g., vigor and fatigue), include the increased incorporation of DHA into neuronal membranes leading to alterations in membrane fluidity and speed of signal transduction and neurotransmission (71), and via neuroprotective and antidepressant-like effects in the face of a physiological stress as observed with FS in rodents (72). Decreased n–3 fatty acid status has been reported in chronic fatigue patients compared with healthy controls (73).
Injury
Tendinopathy
FS as a means of treating tendinopathy in athletes in conjunction with pharmacological therapy has been previously proposed (74). We identified 1 positive clinical trial in athletes, which reported a reduction in pain with FS compared with the placebo (99% compared with 31%; P < 0.001), and greater increases in voluntary sporting activity (56). Two FS trials in nonathletes have been published and are discussed (75): in contrast to the findings of Mavrogenis et al. (56) in athletes, Røe et al. (75) using the same FS supplement found no effect for the FS compared with placebo when administered to patients (n = 55) with unilateral epicondylitis (i.e., tennis elbow) over several months. Neither study assessed the participants’ underlying n–3 fatty acid status. In a recent multicenter clinical trial (n = 73), Sanford et al. (76), recruiting patients with unilateral shoulder pain, found no significant difference between FS (EPA 1530 mg/d plus DHA 1035 mg/d) compared with placebo in the primary outcome measure (the Oxford shoulder score). However, the FS group experienced a more rapid improvement from baseline to 3 mo, and a modest effect (P < 0.05) on shoulder pain and disability, flexion, and abduction at 3, but not 2, 6, or 12 mo, and strength at 12 mo. More patients in the placebo group were using analgesic medication (P = 0.02). Moreover, a significant relation between increasing plasma EPA and DHA and reduction in pain was reported. An effect of DHA and EPA on tendinopathy might result from alterations in the formation of specialized proresolving mediators (SPMs) leading to alterations in pain and inflammatory signaling. For example, in arthritis patients taking FS, plasma SPMs were negatively correlated with erythrocyte sedimentation rate, and synovial fluid resolvin E2 was negatively associated with pain score (77). Thus, at present the evidence for an effect of FS is inconclusive; however, in lieu of the above and the reported minimal side effects, FS could provide a nonpharmacological, low-risk means of supporting tendinopathy rehabilitation in athletes, exerting a modest effect dependent on dose and n–3 fatty acid status.
Concussion and head trauma
We identified only 1 publication in American football (31), a sport well recognized for having a high incidence of concussions (78). The RCT was designed to test the effects of DHA (3 different DHA dosing strategies) on neuronal injury across a playing season in National Collegiate Athletic Association Division 1 American football players (n = 81). The authors demonstrated that FS (DHA only) attenuated the rise in neurofilament light (a biochemical marker of axonal injury that is observed to rise across the playing season in American football players). Caution should be exercised with regard to dosing strategies for DHA, given that the lowest dose used (2000 mg/d) was the most effective. Further work in other sports that present with a high incidence of concussion is clearly warranted.
Surgery and rehabilitation
FS is sometimes prescribed to elite athletes following surgery (7) and, despite the emerging role of resolvins, protectins, and maresins in inflammatory control and resolution, no RCTs have examined the effects of FS on wound healing in athletes postsurgery. Of relevance, FS (1660 mg/d EPA and 1100 mg/d DHA) administered to healthy young subjects failed to enhance wound closure at any of the time points examined after initiation of the wound compared with placebo (79). Research in healthy animals corroborates these findings, with FS and linseed (a source of plant n–3 fatty acids but not EPA and DHA) delaying the percentage wound closure and re-epithelialization compared with controls at 14 d postwound (80). In contrast, obese diseased and therefore “inflamed” animals, display enhanced wound healing with an n–3 fatty acid–rich “high-fat” diet when compared with controls, and compared with diets rich in n–6 fatty acids and saturated fat (81). Given the lack of supportive scientific evidence in athletes or indeed healthy young participants, caution is advised over the use of FS in otherwise healthy athletes recovering from acute surgery or an acute traumatic injury (with the exception of neuronal injury and the use of DHA and the correction of any underlying n–3 fatty acid deficiency). The decision whether to use FS for rehabilitation purposes is further complicated by the findings of recent experimental research using a nonsurgical unilateral limb immobilization approach (no actual injury or wound and therefore no activation of an immune inflammatory response). FS prevented muscle disuse atrophy in nonathletes, preserving both muscle mass and mitochondrial respiration (82, 83).
Bone health
Bone stress injuries are common injuries sustained by athletes (84), and interventions that can mitigate the risk are warranted. There have been no RCTs in athletes that examined the effects of FS on bone metabolism, or bone mineral density. However, the National Aeronautics and Space Administration agency conducted a series of research studies from cell culture to human interventions in association with bed rest and space flight, to assess the potential for FS to ameliorate bone loss, with an effect for n–3 fatty acids (85). Those astronauts who ate more n–3 fatty acid–rich fish experienced less decline in bone mineral density as a result of short-duration space flight. Moreover, a higher intake of n–3 fatty acids (i.e., fish intake) was associated with reduced bone resorption (measured via N-telopeptide) with bed rest in nonathletes (85). In conclusion, a higher fish intake and better n–3 fatty acid status can serve to protect against bone loss. Speculatively this might also allow attainment of a higher bone mineral density for the athlete. However, research in athletes would clearly be needed to support this hypothesis.
Adverse effects of FS
Only 1 RCT reported adverse effects of FS, in which the athletes’ complaints were mild and affected a small proportion (∼10%). Thus, given the wide range of FS used, doses administered, and time frames, FS appears to be safe in athletes. The most commonly stated concerns with regard to the use of chronic high doses of FS are: 1) increased oxidative stress, driven by oxidation of the polyunsaturated fats in FS; 2) a hypocoagulant effect; 3) exposure to heavy metals and toxins concentrated in the oils; and 4) mild gastrointestinal side effects such as nausea. Each area will be briefly discussed below. Table 1 provides a summary of the RCTs measuring the effect of FS on oxidative stress in athletes, with or without added nutrients. Based on these studies in athletes, FS use increases lipid peroxidation at rest and postexercise compared with a placebo or control (35, 36); however, the increase in oxidative stress postexercise is prevented when FS is consumed with antioxidant nutrients, for example, polyphenols and/or vitamins (36–38). In addition, 3 RCTs reported an effect for FS on increasing NO postexercise (32, 35, 36). It should be noted that well-designed human studies examining the effect of FS on intracellular antioxidant enzymes and systems, have found that ∼3 wk of FS increases the expression and activity of antioxidant enzymes (86). Others have reported differential effects for the fatty acids, EPA and DHA, on muscle antioxidant enzymes (3).
Anecdotally, athletes sometimes consume supplements in excess of recommendations, in accordance with the “more is better” mantra. Excessive FS doses over a prolonged period of time can pose an increased risk of bleeding. For example, an amateur athlete consuming 20 g/d of n–3 fats from supplements presented with a duodenal ulcer and bleeding (87). A recent systematic review including 52 publications incorporating data on both healthy and surgical patients, concluded that FS reduces platelet aggregation in healthy subjects; however, in RCTs there is no increased risk of bleeding during or after surgery with FS (88). The European Food Safety Authority (89) scientific opinion states, “Long-term supplemental intakes of EPA and DHA combined up to about 5g day−1 do not appear to increase the risk of spontaneous bleeding episodes or bleeding complications, or affect glucose homeostasis, immune function or lipid peroxidation, provided the oxidative stability of the n–3 long chain polyunsaturated fatty acids is guaranteed.” Athletes should be educated to avoid excessively high doses of FS. Overall, we are not aware of any RCTs in which FS exerted a negative outcome on performance or recovery. Furthermore, mild gastrointestinal side effects (belching, nausea, fishy taste) in the studies reviewed were rare occurrences.
Quality of FS products
Only 2 studies sought independent laboratory analysis of the FS used, which is somewhat concerning given that 9 studies were conducted in elite athletes, and a further 2 in semiprofessional athletes. A number of studies from different countries have raised concerns over both the quantity and the quality of the fatty acids contained within various commercial products (12, 13, 90). For example, in the United States, Kleiner et al. (12) found that >70% of the supplements analyzed (47 FS products were selected) did not contain the amounts of EPA and DHA stated on the product label. Moreover, a study in New Zealand identified just 3 of 32 supplements contained quantities of EPA and DHA that were ≥100% of the label content. Two-thirds contained <69% of label-claimed content (13). Of greater concern is the finding that 83% of supplements exceeded concentrations of peroxide markers, with 50% exceeding recommended total oxidation values calculated (13). Only 3 supplements met international recommendations. Such results are not without controversy, however, with the analytical methods in the latter study (13) receiving criticism from industry scientists (91). In fact, in an industry-sponsored study, all 10 fish oil products met international guidelines (91). In summary, care needs to be taken when recommending FS products for athletes, and ideally the products should be analyzed not only from an antidoping perspective, but also for the presence and concentration of heavy metals, dioxins, and polychlorinated biphenyls.
Study Limitations and Bias
Only randomized placebo-controlled trials were included in the review to minimize selection bias associated with the nonrandomization of participants. However, our initial search strategy captured 6 nonrandomized and/or non–placebo-controlled studies in athletes (14, 15, 17, 53, 65, 92), which could offer further insight into this field albeit without the same scientific rigor. We included both single (20%) and double-blind (80%) studies, however, and thus there is a risk of bias due to the lack of blinding of researchers in the single-blind studies. Removing the 7 single-blind studies (32, 36, 40, 42, 44, 54, 59) does not change the conclusions, but it weakens the strength of the findings for inflammation because 2 single-blind studies reported on TNF-α (40, 42), and 2 on postexercise NO (32, 36).
With regard to the specific FS characteristics, some included additional nutrients, as stated in Tables 1 –4. Such studies were not excluded from the review. In fact, by including studies with additional nutrients (e.g., antioxidants), an effect for antioxidants on attenuating lipid peroxidation was observed. Furthermore, FS sold into the marketplace contains various antioxidants (i.e., α-tocopherol or carotenoids) in order to reduce oxidation of the fatty acids, ensuring greater product stability, and to prolong the product's shelf life. The inclusion of such studies gives the review ecological validity.
Conclusion
We provide a summary on FS research in athletes (Tables 1 –4), which demonstrates broadly positive effects and serves as a resource for practitioners. Indeed, FS exerts positive effects on cognition, cardiovascular dynamics in cyclists, and muscle recovery. FS also attenuates proinflammatory cell responses and can increase lipid peroxidation and postexercise NO. An effect for FS on endurance exercise performance was absent across all studies. We are not aware of any RCTs that have demonstrated a negative effect of FS on performance, and the reported side effects with FS use are mild. Many of the RCTs that report positive effects have used doses of FS that are achievable through the consumption of oily fish. It is recommended that future research on FS and n–3 fatty acid–rich diets should measure biomarkers of n–3 fatty acid status, to allow the proper investigation and understanding of the impact of n–3 fatty acid status and the dose–response on outcomes. Furthermore, FS research is needed in athletes to further understand the impact on neuromuscular performance, bone metabolism, rehabilitation from injury (e.g., surgical compared with nonsurgical outcomes including bone stress), EIB, risk of illness, and risk of sudden cardiac death in athletes (93) with high compared with low n–3 fatty acid status. Finally, future FS studies should include effect sizes and have the supplement analyzed for contaminants and the supplement contents verified independently from the manufacturer.
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—NAL, CRP: designed the study; NAL, CRP, DD: conducted the systematic review; NAL, CRP, DD, LMC, PCC: prepared the manuscript; and all authors: read and approved the final manuscript.
Notes
The authors reported no funding received for this study.
Author disclosures: The authors report no conflicts of interest.
Abbreviations used: EIB, exercise-induced bronchoconstriction; FS, fish oil supplementation; PBMC, peripheral blood mononuclear cell; PEDro, Physiotherapy Evidence Database; RCT, randomized placebo-controlled trial; SPM, specialized proresolving mediator; URTI, upper respiratory tract illness.
References
- 1. Helge JW, Wu BJ, Willer M, Daugaard JR, Storlien LH, Kiens B. Training affects muscle phospholipid fatty acid composition in humans. J Appl Physiol. 2001;90:670–7. [DOI] [PubMed] [Google Scholar]
- 2. Andersson A, Sjödin A, Hedman A, Olsson R, Vessby B. Fatty acid profile of skeletal muscle phospholipids in trained and untrained young men. Am J Physiol Endocrinol Metab. 2000;279:E744–51. [DOI] [PubMed] [Google Scholar]
- 3. da Silva EP, Nachbar RT, Levada-Pires AC, Hirabara SM, Lambertucci RH. Omega-3 fatty acids differentially modulate enzymatic anti-oxidant systems in skeletal muscle cells. Cell Stress Chaperones. 2016;21:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kamolrat T, Gray SR. The effect of eicosapentaenoic and docosahexaenoic acid on protein synthesis and breakdown in murine C2C12 myotubes. Biochem Biophys Res Commun. 2013;432:593–8. [DOI] [PubMed] [Google Scholar]
- 5. Calder PC. Omega-3 fatty acids and inflammatory processes: from molecules to man. Biochm Soc Trans. 2017;45:1105–15. [DOI] [PubMed] [Google Scholar]
- 6. Rosimus C. Case study: the effect of nutritional intervention on body composition and physical performance of a female squash player. Int J Sport Nutr Exerc Metab. 2018;28:279–83. [DOI] [PubMed] [Google Scholar]
- 7. Milsom J, Barreira P, Burgess DJ, Iqbal Z, Morton JP. Case study: muscle atrophy and hypertrophy in a premier league soccer player during rehabilitation from ACL injury. Int J Sport Nutr Exerc Metab. 2014;24:543–52. [DOI] [PubMed] [Google Scholar]
- 8. Heaton LE, Davis JK, Rawson ES, Nuccio RP, Witard OC, Stein KW, Baar K, Carter JM, Baker LB. Selected in-season nutritional strategies to enhance recovery for team sport athletes: a practical overview. Sports Med. 2017;47:2201–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thomas DT, Erdman KA, Burke LM. American College of Sports Medicine joint position statement. Nutrition and athletic performance. Med Sci Sports Exerc. 2016;48:543–68. [DOI] [PubMed] [Google Scholar]
- 10. Maughan RJ, Burke LM, Dvorak J, Larson-Meyer DE, Peeling P, Phillips SM, Rawson ES, Walsh NP, Garthe I, Geyer H et al.. IOC consensus statement: dietary supplements and the high-performance athlete. Int J Sport Nutr Exerc Metab. 2018;28:104–25. [DOI] [PubMed] [Google Scholar]
- 11. Finco AM, de Oliveira Finco AM, Mamani LDG, de Carvalho JV, de Melo Pereira GV, Thomaz-Soccol V, Soccol CR. Technological trends and market perspectives for production of microbial oils rich in omega-3. Crit Rev Biotechnol. 2017;37:656–71. [DOI] [PubMed] [Google Scholar]
- 12. Kleiner AC, Cladis DP, Santerre CR. A comparison of actual versus stated label amounts of EPA and DHA in commercial omega-3 dietary supplements in the United States. J Sci Food Agric. 2014;95:1260–7. [DOI] [PubMed] [Google Scholar]
- 13. Albert BB, Derraik JGB, Cameron-Smith D, Hofman PL, Tumanov S, Villas-Boas SG, Garg ML, Cutfield WS. Fish oil supplements in New Zealand are highly oxidised and do not meet label content of n-3 PUFA. Sci Rep. 2015;5:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Malaguti M, Baldini M, Angeloni C, Biagi P, Hrelia S. High-protein-PUFA supplementation, red blood cell membranes, and plasma antioxidant activity in volleyball athletes. Int J Sport Nutr Exerc Metab. 2008;18:301–12. [DOI] [PubMed] [Google Scholar]
- 15. Marques CG, Santos VC, Levada-Pires AC, Jacintho TM, Gorjão R, Pithon-Curi TC, Cury-Boaventura MF. Effects of DHA-rich fish oil supplementation on the lipid profile, markers of muscle damage, and neutrophil function in wheelchair basketball athletes before and after acute exercise. Appl Physiol Nutr Metab. 2015;40:596–604. [DOI] [PubMed] [Google Scholar]
- 16. Drobnic F, Rueda F, Pons V, Banquells M, Cordobilla B, Domingo JC. Erythrocyte omega-3 fatty acid content in elite athletes in response to omega-3 supplementation: a dose-response pilot study. J Lipids. 2017;2017:1472719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Price OJ, Hull JH, Howatson G, Robson-Ansley P, Ansley L. Vitamin D and omega-3 polyunsaturated fatty acid supplementation in athletes with exercise-induced bronchoconstriction: a pilot study. Expert Rev Respir Med. 2015;9:369–78. [DOI] [PubMed] [Google Scholar]
- 18. Ghiasvand R, Djalali M, Djazayery S, Keshavarz S, Hosseini M, Askari G, Jani N, Fardad N, Fatehi F. Effect of eicosapentaenoic acid (EPA) and vitamin E on the blood levels of inflammatory markers, antioxidant enzymes, and lipid peroxidation in Iranian basketball players. Iran J Public Health. 2010; 39:15–21. [PMC free article] [PubMed] [Google Scholar]
- 19. Ghiasvand R, Djalali M, Djazayery SA, Keshavarz SA, Hosseini M. Effects of eicosapentaenoic acid and vitamin E on the plasma levels of antioxidant vitamins and inflammatory markers, and on erythrocyte antioxidant enzyme activities, in male basketball players. Acta Medica Iranica. 2009;47(4):269–74. [Google Scholar]
- 20. Bloomer RJ, Larson DE, Fisher-Wellman KH, Galpin AJ, Schilling BK. Effect of eicosapentaenoic and docosahexaenoic acid on resting and exercise-induced inflammatory and oxidative stress biomarkers: a randomized, placebo controlled, cross-over study. Lipids Health Dis. 2009;8:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Corder KE, Newsham KR, McDaniel JL, Ezekiel UR, Weiss EP. Effects of short-term docosahexaenoic acid supplementation on markers of inflammation after eccentric strength exercise in women. J Sports Sci Med. 2016;15:176–83. [PMC free article] [PubMed] [Google Scholar]
- 22. Gerling CJ, Whitfield J, Mukai K, Spriet LL. Variable effects of 12 weeks of omega-3 supplementation on resting skeletal muscle metabolism. Appl Physiol Nutr Metab. 2014;39:1083–91. [DOI] [PubMed] [Google Scholar]
- 23. Lewis EJH, Stucky F, Radonic PW, Metherel AH, Wolever TMS, Wells GD. Neuromuscular adaptations to sprint interval training and the effect of mammalian omega-3 fatty acid supplementation. Eur J Appl Physiol. 2017;117:469–82. [DOI] [PubMed] [Google Scholar]
- 24. Lembke P, Capodice J, Hebert K, Swenson T. Influence of omega-3 (n3) index on performance and wellbeing in young adults after heavy eccentric exercise. J Sports Sci Med. 2014;13:151–6. [PMC free article] [PubMed] [Google Scholar]
- 25. McGlory C, Wardle SL, Macnaughton LS, Witard OC, Scott F, Dick J, Bell JG, Phillips SM, Galloway SDR, Hamilton DL et al.. Fish oil supplementation suppresses resistance exercise and feeding-induced increases in anabolic signaling without affecting myofibrillar protein synthesis in young men. Physiol Rep. 2016;4:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huffman DM, Altena TS, Mawhinney TP, Thomas TR. Effect of n-3 fatty acids on free tryptophan and exercise fatigue. Eur J Appl Physiol. 2004;92:584–91. [DOI] [PubMed] [Google Scholar]
- 27. Tsuchiya Y, Yanagimoto K, Nakazato K, Hayamizu K, Ochi E. Eicosapentaenoic and docosahexaenoic acids-rich fish oil supplementation attenuates strength loss and limited joint range of motion after eccentric contractions: a randomized, double-blind, placebo-controlled, parallel-group trial. Eur J Appl Physiol. 2016;116:1179–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tinsley GM, Gann JJ, Huber SR, Andre TL, La Bounty PM, Bowden RG, Gordon PM, Grandjean PW. Effects of fish oil supplementation on postresistance exercise muscle soreness. J Dietary Suppl. 2017;14:89–100. [DOI] [PubMed] [Google Scholar]
- 29. Gray P, Gabriel B, Thies F, Gray SR. Fish oil supplementation augments post-exercise immune function in young males. Brain, Behav Immun. 2012;26:1265–72. [DOI] [PubMed] [Google Scholar]
- 30. Gray P, Chappell A, Jenkinson AM, Thies F, Gray SR. Fish oil supplementation reduces markers of oxidative stress but not muscle soreness after eccentric exercise. Int J Sport Nutr Exerc Metab. 2014;24:206–14. [DOI] [PubMed] [Google Scholar]
- 31. Oliver JM, Jones MT, Kirk KM, Gable DA, Repshas JT, Johnson TA, Andereasson U, Norgren N, Blennow K, Zetterberg H. Effect of docosahexaenoic acid on a biomarker of head trauma in American football. Med Sci Sports Exer. 2016;48:974–82. [DOI] [PubMed] [Google Scholar]
- 32. Żebrowska A, Mizia-Stec K, Mizia M, Gąsior Z, Poprzecki S. Omega-3 fatty acids supplementation improves endothelial function and maximal oxygen uptake in endurance-trained athletes. Eur J Sport Sci. 2015;15:305–14. [DOI] [PubMed] [Google Scholar]
- 33. Fontani G, Corradeschi F, Felici A, Alfatti F, Migliorini S, Lodi L. Cognitive and physiological effects of omega-3 polyunsaturated fatty acid supplementation in healthy subjects. Eur J Clin Invest. 2005;35:691–9. [DOI] [PubMed] [Google Scholar]
- 34. Mickleborough TD, Murray RL, Ionescu AA, Lindley MR. Fish oil supplementation reduces severity of exercise-induced bronchoconstriction in elite athletes. Am J Respir Crit Care Med. 2003;168:1181–9. [DOI] [PubMed] [Google Scholar]
- 35. Filaire E, Massart A, Portier H, Rouveix M, Rosado F, Bage AS, Gobert M, Durand D. Effect of 6 weeks of n-3 fatty-acid supplementation on oxidative stress in judo athletes. Int J Sport Nutr Exerc Metab. 2010;20:496–506. [DOI] [PubMed] [Google Scholar]
- 36. Filaire E, Massart A, Rouveix M, Portier H, Rosado F, Durand D. Effects of 6 weeks of n-3 fatty acids and antioxidant mixture on lipid peroxidation at rest and postexercise. Eur J Appl Physiol. 2011;111:1829–39. [DOI] [PubMed] [Google Scholar]
- 37. McAnulty SR, Nieman DC, Fox-Rabinovich M, Duran V, McAnulty LS, Henson DA, Jin F, Landrum MJ. Effect of n-3 fatty acids and antioxidants on oxidative stress after exercise. Med Sci Sports Exerc. 2010;42:1704–11. [DOI] [PubMed] [Google Scholar]
- 38. McAnulty SR, Nieman DC, McAnulty LS, Lynch WS, Jin F, Henson DA. Effect of mixed flavonoids, n-3 fatty acids, and vitamin C on oxidative stress and antioxidant capacity before and after intense cycling. Int J Sport Nutr Exerc Metab. 2011;21:328–37. [DOI] [PubMed] [Google Scholar]
- 39. Martorell M, Capó X, Bibiloni MM, Sureda A, Mestre-Alfaro A, Batle JM, Llompart I, Tur JA, Pons A. Docosahexaenoic acid supplementation promotes erythrocyte antioxidant defense and reduces protein nitrosative damage in male athletes. Lipids. 2015;50:131–48. [DOI] [PubMed] [Google Scholar]
- 40. Andrade PMM, Ribeiro BG, Bozza MT, Costa Rosa LFB, do Carmo MGT. Effects of the fish-oil supplementation on the immune and inflammatory responses in elite swimmers. Prostaglandins Leukot Essent Fatty Acids. 2007;77:139–45. [DOI] [PubMed] [Google Scholar]
- 41. Delfan M, Ebrahim K, Baesi F, Mirakhori Z, Ghalamfarsa G, Bakhshaei P, Saboor-Yaraghi AA, Razavi A, Setayesh M, Yousefi M et al.. The immunomodulatory effects of fish-oil supplementation in elite paddlers: a pilot randomized double blind placebo-controlled trial. Prostaglandins Leukot Essent Fatty Acids. 2015;99:35–40. [DOI] [PubMed] [Google Scholar]
- 42. Santos VC, Santos VC, Levada-Pires AC, Levada-Pires AC, Alves SR, Alves SR, Pithon-Curi TC, Pithon-Curi TC, Curi R, Cury-Boaventura MF et al.. Effects of DHA-rich fish oil supplementation on lymphocyte function before and after a marathon race. Int J Sport Nutr Exerc Metab. 2013;23:161–9. [DOI] [PubMed] [Google Scholar]
- 43. Nieman DC, Henson DA, McAnulty SR, Jin F, Maxwell KR. n-3 polyunsaturated fatty acids do not alter immune and inflammation measures in endurance athletes. Int J Sport Nutr Exerc Metab. 2009;19:536–46. [DOI] [PubMed] [Google Scholar]
- 44. Da Boit M, Gabriel B, Gray P, Gray S. The effect of fish oil, vitamin D and protein on URTI incidence in young active people. Int J Sports Med. 2015;36:426–30. [DOI] [PubMed] [Google Scholar]
- 45. Capó X, Martorell M, Sureda A, Llompart I, Tur JA, Pons A. Diet supplementation with DHA-enriched food in football players during training season enhances the mitochondrial antioxidant capabilities in blood mononuclear cells. Eur J Nutr. 2014;54:35–49. [DOI] [PubMed] [Google Scholar]
- 46. Capó X, Martorell M, Llompart I, Sureda A, Tur JA, Pons A. Docosahexaenoic acid diet supplementation attenuates the peripheral mononuclear cell inflammatory response to exercise following LPS activation. Cytokine. 2014;69:155–64. [DOI] [PubMed] [Google Scholar]
- 47. Capó X, Martorell M, Sureda A, Tur JA, Pons A. Effects of docosahexaenoic supplementation and in vitro vitamin C on the oxidative and inflammatory neutrophil response to activation. Oxid Med Cell Longev[Internet] 2015. doi:10.1155/2015/187849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nieman DC, Henson DA, Maxwell KR, Williams AS, McAnulty SR, Jin F, Shanely RA, Lines TC. Effects of quercetin and EGCG on mitochondrial biogenesis and immunity. Med Sci Sports Exerc. 2009;41:1467–75. [DOI] [PubMed] [Google Scholar]
- 49. Jakeman JR, Lambrick DM, Wooley B, Babraj JA, Faulkner JA. Effect of an acute dose of omega-3 fish oil following exercise-induced muscle damage. Eur J Appl Physiol. 2017;3:1–8. [DOI] [PubMed] [Google Scholar]
- 50. Lewis EJH, Radonic PW, Wolever TMS, Wells GD. 21 days of mammalian omega-3 fatty acid supplementation improves aspects of neuromuscular function and performance in male athletes compared to olive oil placebo. J Int Soc Sports Nutr. 2015;12:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Black KE, Witard OC, Baker D, Healey P, Lewis V, Tavares F, Christensen S, Pease T, Smith B. Adding omega-3 fatty acids to a protein-based supplement during pre-season training results in reduced muscle soreness and the better maintenance of explosive power in professional Rugby Union players. Eur J Sport Sci. 2018;18:1357–67. [DOI] [PubMed] [Google Scholar]
- 52. Philpott JD, Donnelly C, Walshe IH, MacKinley EE, Dick J, Galloway SDR, Tipton KD, Witard OC. Adding fish oil to whey protein, leucine, and carbohydrate over a six-week supplementation period attenuates muscle soreness following eccentric exercise in competitive soccer players. Int J Sport Nutr Exerc Metab. 2018;28:26–36. [DOI] [PubMed] [Google Scholar]
- 53. Gravina L, Brown FF, Alexander L, Dick J, Bell G, Witard OC, Galloway SDR. n-3 Fatty acid supplementation during 4 weeks of training leads to improved anaerobic endurance capacity, but not maximal strength, speed, or power in soccer players. Int J Sport Nutr Exerc Metab. 2017;27:305–13. [DOI] [PubMed] [Google Scholar]
- 54. Raastad T, Høstmark AT, Strømme SB. Omega-3 fatty acid supplementation does not improve maximal aerobic power, anaerobic threshold and running performance in well-trained soccer players. Scand J Med Sci Sports. 1997;7:25–31. [DOI] [PubMed] [Google Scholar]
- 55. Buckley JD, Burgess S, Murphy KJ, Howe PRC. DHA-rich fish oil lowers heart rate during submaximal exercise in elite Australian Rules footballers. J Sci Med Sport. 2009;12:503–7. [DOI] [PubMed] [Google Scholar]
- 56. Mavrogenis S, Johannessen E, Jensen P, Sindberg C. The effect of essential fatty acids and antioxidants combined with physiotherapy treatment in recreational athletes with chronic tendon disorders. Phys Ther Sport. 2004;5:194–9. [Google Scholar]
- 57. Hingley L, Macartney MJ, Brown MA, McLennan PL, Peoples GE. DHA-rich fish oil increases the omega-3 index and lowers the oxygen cost of physiologically stressful cycling in trained individuals. Int J Sport Nutr Exerc Metab. 2017;27:335–43. [DOI] [PubMed] [Google Scholar]
- 58. Peoples GE, McLennan PL, Howe PRC, Groeller H. Fish oil reduces heart rate and oxygen consumption during exercise. J Cardiovasc Pharmacol. 2008;52:540–7. [DOI] [PubMed] [Google Scholar]
- 59. Fontani G, Lodi L, Migliorini S, Corradeschi F. Effect of omega-3 and policosanol supplementation on attention and reactivity in athletes. J Am Coll Nutr. 2009;28(Suppl):473S–81S. [DOI] [PubMed] [Google Scholar]
- 60. Guzmán JF, Esteve H, Pablos C, Pablos A, Blasco C, Villegas JA. DHA-rich fish oil improves complex reaction time in female elite soccer players. J Sports Sci Med. 2011;10:301–5. [PMC free article] [PubMed] [Google Scholar]
- 61. Tartibian B, Maleki BH, Abbasi A. The effects of omega-3 supplementation on pulmonary function of young wrestlers during intensive training. J Sci Med Sport. 2010;13:281–6. [DOI] [PubMed] [Google Scholar]
- 62. Herbst EAF, Paglialunga S, Gerling C, Whitfield J, Mukai K, Chabowski A, Heigenhauser GJF, Spriet LL, Holloway GP. Omega-3 supplementation alters mitochondrial membrane composition and respiration kinetics in human skeletal muscle. J Physiol. 2014;592:1341–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Walser B, Stebbins CL. Omega-3 fatty acid supplementation enhances stroke volume and cardiac output during dynamic exercise. Eur J Appl Physiol. 2008;104:455–61. [DOI] [PubMed] [Google Scholar]
- 64. Philpott JD, Witard OC, Galloway SD. Applications of omega-3 polyunsaturated fatty acid supplementation for sport performance. Res Sports Med. 2019;27(2):219–37. [DOI] [PubMed] [Google Scholar]
- 65. Toft AD, Thorn M, Ostrowski K, Asp S, Møller K, Iversen S, Hermann C, Sondergaard SR, Pedersen BK. N-3 polyunsaturated fatty acids do not affect cytokine response to strenuous exercise. J Appl Physiol. 2000;89:2401–6. [DOI] [PubMed] [Google Scholar]
- 66. Rangel-Huerta OD, Aguilera CM, Mesa MD, Gil A. Omega-3 long-chain polyunsaturated fatty acids supplementation on inflammatory biomakers: a systematic review of randomised clinical trials. Br J Nutr. 2012;107(Suppl 2):S159–70. [DOI] [PubMed] [Google Scholar]
- 67. Merry TL, Ristow M. Mitohormesis in exercise training. Free Radic Biol Med. 2016;98:123–30. [DOI] [PubMed] [Google Scholar]
- 68. Stonehouse W, Conlon CA, Podd J, Hill SR, Minihane AM, Haskell C, Kennedy D. DHA supplementation improved both memory and reaction time in healthy young adults: a randomized controlled trial. Am J Clin Nutr. 2013;97:1134–43. [DOI] [PubMed] [Google Scholar]
- 69. Bauer I, Crewther DP, Pipingas A, Rowsell R, Cockerell R, Crewther SG. Omega-3 fatty acids modify human cortical visual processing—a double-blind, crossover study. PLoS One. 2011;6:e28214–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Narendran R, Frankle WG, Mason NS, Muldoon MF, Moghaddam B. Improved working memory but no effect on striatal vesicular monoamine transporter type 2 after omega-3 polyunsaturated fatty acid supplementation. PLoS One. 2012;7:e46832–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Horrocks LA, Farooqui AA. Docosahexaenoic acid in the diet: its importance in maintenance and restoration of neural membrane function. Prostaglandins Leukot Essent Fatty Acids. 2004;70:361–72. [DOI] [PubMed] [Google Scholar]
- 72. Dang R, Zhou X, Tang M, Xu P, Gong X, Liu Y, Jiao H, Jiang P. Fish oil supplementation attenuates neuroinflammation and alleviates depressive-like behavior in rats submitted to repeated lipopolysaccharide. Eur J Nutr. 2018;57:893–906. [DOI] [PubMed] [Google Scholar]
- 73. Maes M, Mihaylova I, Leunis J-C. In chronic fatigue syndrome, the decreased levels of omega-3 poly-unsaturated fatty acids are related to lowered serum zinc and defects in T cell activation. Neuro Endocrinol Lett. 2005;26:745–51. [PubMed] [Google Scholar]
- 74. Fallon K, Purdam C, Cook J, Lovell G. A “polypill” for acute tendon pain in athletes with tendinopathy?. J Sci Med Sport. 2008;11:235–8. [DOI] [PubMed] [Google Scholar]
- 75. Røe C, Ødegaard TT, Hilde F, Maehlum S, Halvorsen T. No effect of supplement of essential fatty acids on lateral epicondylitis. Tidsskr Nor Laegeforen. 2005;125:2615–8. [PubMed] [Google Scholar]
- 76. Sandford FM, Sanders TA, Wilson H, Lewis JS. A randomised controlled trial of long-chain omega-3 polyunsaturated fatty acids in the management of rotator cuff related shoulder pain. BMJ Open Sport Exerc Med. 2018;4:e000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Barden AE, Moghaddami M, Mas E, Phillips M, Cleland LG, Mori TA. Specialised pro-resolving mediators of inflammation in inflammatory arthritis. Prostaglandins Leukot Essent Fatty Acids. 2016;107:24–9. [DOI] [PubMed] [Google Scholar]
- 78. Larrabee GJ, Rohling ML, Binder LM. Age of first exposure to football and later-life cognitive impairment in former NFL players. Neurology. 2015;85:1007–8. [PubMed] [Google Scholar]
- 79. McDaniel JC, Belury M, Ahijevych K, Blakely W. Omega-3 fatty acids effect on wound healing. Wound Repair Regen. 2008;16:337–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Otranto M, Do Nascimento AP, Monte-Alto-Costa A. Effects of supplementation with different edible oils on cutaneous wound healing. Wound Repair Regen. 2010;18:629–36. [DOI] [PubMed] [Google Scholar]
- 81. Wu C-L, Jain D, McNeill JN, Little D, Anderson JA, Huebner JL, Kraus VB, Rodriguiz RM, Wetsel WC, Guilak F. Dietary fatty acid content regulates wound repair and the pathogenesis of osteoarthritis following joint injury. Ann Rheum Dis. 2015;74:2076–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. McGlory C, Gorissen SHM, Kamal M, Bahniwal R, Hector AJ, Baker SK, Chabowski A, Phillips SM. Omega-3 fatty acid supplementation attenuates skeletal muscle disuse atrophy during two weeks of unilateral leg immobilization in healthy young women. FASEB J. 2019;33:4586–97. [DOI] [PubMed] [Google Scholar]
- 83. Miotto PM, McGlory C, Bahniwal R, Kamal M, Phillips SM, Holloway GP. Supplementation with dietary ω-3 mitigates immobilization-induced reductions in skeletal muscle mitochondrial respiration in young women. FASEB J. 2019;33:8232–40. [DOI] [PubMed] [Google Scholar]
- 84. Tenforde AS, Kraus E, Fredericson M. Bone stress injuries in runners. Phys Med Rehabil Clin N Am. 2016;27:139–49. [DOI] [PubMed] [Google Scholar]
- 85. Zwart SR, Pierson D, Mehta S, Gonda S, Smith SM. Capacity of omega-3 fatty acids or eicosapentaenoic acid to counteract weightlessness-induced bone loss by inhibiting NF-κB activation: from cells to bed rest to astronauts. J Bone Miner Res. 2010;25:1049–57. [DOI] [PubMed] [Google Scholar]
- 86. Anderson EJ, Thayne KA, Harris M, Shaikh SR, Darden TM, Lark DS, Williams JM, Chitwood WR, Kypson AP, Rodriguez E. Do fish oil omega-3 fatty acids enhance antioxidant capacity and mitochondrial fatty acid oxidation in human atrial myocardium via PPARγ activation?. Antioxid Redox Signal. 2014;21:1156–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Detopoulou P, Papamikos V. Gastrointestinal bleeding after high intake of omega-3 fatty acids, cortisone and antibiotic therapy: a case study. Int J Sport Nutr Exerc Metab. 2014;24:253–7. [DOI] [PubMed] [Google Scholar]
- 88. Begtrup KM, Krag AE, Hvas A-M. No impact of fish oil supplements on bleeding risk: a systematic review. Dan Med J. 2017;64(5):A5366. [PubMed] [Google Scholar]
- 89. EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific opinion on the tolerable upper intake level of eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA) and docosapentaenoic acid (DPA). EFSA J. 2012;10:827–48. [Google Scholar]
- 90. Fernandes AR, Rose M, White S, Mortimer DN, Gem M. Dioxins and polychlorinated biphenyls (PCBs) in fish oil dietary supplements: occurrence and human exposure in the UK. Food Addit Contam. 2006;23:939–47. [DOI] [PubMed] [Google Scholar]
- 91. Nichols P, Dogan L, Sinclair A. Australian and New Zealand fish oil products in 2016 meet label omega-3 claims and are not oxidized. Nutrients. 2016;8:703–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Oostenbrug GS, Mensink RP, Hardeman MR, De Vries T, Brouns F, Hornstra G. Exercise performance, red blood cell deformability, and lipid peroxidation: effects of fish oil and vitamin E. J Appl Physiol. 1997;83:746–52. [DOI] [PubMed] [Google Scholar]
- 93. Von Scacky C, Kemper M, Haslbauer R, Halle M. Low omega-3 index in 106 German elite winter endurance athletes: a pilot study. Int J Sport Nutr Exerc Metab. 2014;24:559–64. [DOI] [PubMed] [Google Scholar]


