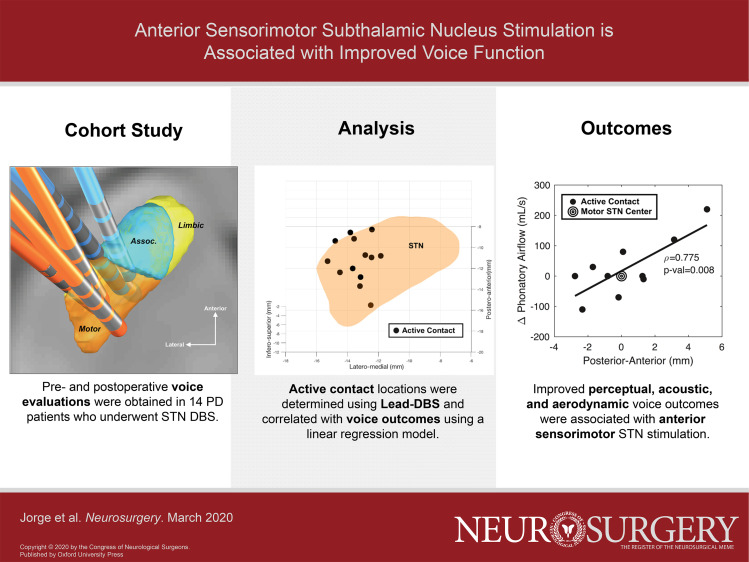
Abstract
Background
Despite the impact of Parkinson disease (PD) on speech communication, there is no consensus regarding the effect of lead location on voice-related outcomes in subthalamic nucleus (STN) deep brain stimulation (DBS).
Objective
To determine the relationship of stimulation location to changes in cepstral analyses of voice following STN DBS.
Methods
Speech pathology evaluations were obtained from 14 PD subjects, before and after STN DBS, including audio-perceptual voice ratings (overall severity, loudness, hoarseness changes), measured indices of dysphonia (cepstral peak prominence and cepstral spectral index of dysphonia), and phonatory aerodynamics. The contact locations used for active stimulation at the time of postoperative voice evaluations were determined and assessed in relation to voice outcomes.
Results
Voice outcomes remained relatively unchanged on average. Stimulation locations in the anterior portion of the sensorimotor region of the left STN, however, were associated with improvements in voice severity scores, cepstral spectral index of dysphonia, shortness of breath, and phonatory airflow during connected speech. Posterior locations were associated with worsening of these outcomes. Variation in the medial-lateral or dorsal-ventral position on the left, and in any direction on the right, did not correlate with any voice outcome.
Conclusion
Active contact placement within the anterior sensorimotor STN was associated with improved perceptual and acoustic-aerodynamic voice-related outcomes. These findings suggest an STN topography for improving airflow for speech, in turn improving how PD patients’ voices sound.
Keywords: Cepstral, Deep brain stimulation, Parkinson disease, Phonatory airflow, Voice, Subthalamic nucleus
Graphical Abstract
Graphical Abstract.
ABBREVIATIONS
- ADSV
Analysis of Dysphonia in Speech and Voice
- CI
confidence interval
- CAPE-V
Consensus Auditory Perceptual Evaluation-V
- CPP
cepstral peak prominence
- CSID
cepstral spectral index of dysphonia
- CT
computed tomography
- DBS
deep brain stimulation
- IQR
interquartile range
- LEDD
levodopa equivalent daily dose
- MRI
magnetic resonance imaging
- PD
Parkinson disease
- SLP
speech-language pathologist
- SOB
shortness of breath
- SPL
sound pressure level
- STN
subthalamic nucleus
- UPDRS
Unified Parkinson's Disease Rating Scale
- VAS
visual analog scale
Speech communication often becomes a struggle for patients with Parkinson disease (PD). Advanced stages of PD often are marked by hypophonia, a breathy and harsh voice quality, and unintelligible speech, ultimately resulting in worsening or total lack of ability for verbal communication.1 In fact, approximately 90% of patients with PD have been shown to develop a specific motor speech disorder, hypokinetic dysarthria, which presents as various deficits in fluency, phonation, articulation, and prosody.2,3 There is no consensus, however, for how stimulation location within target structures affects voice-related outcomes in deep brain stimulation (DBS).
Previous studies have reported variable relationships between stimulation location and voice and speech-related function, including worsening postoperative dysarthria, and decreases in both syllable repetition rates and verbal fluency.4-11 Some investigators have proposed that there is a necessary trade-off between motor, speech and neuropsychological outcomes, with respect to lead location in the subthalamic nucleus (STN).10,12 An alternate view is that an improved understanding of functional subterritories may allow for differential modulation of separate behaviors that are mediated in part by the STN. In this light, understanding the millimetric location effects of STN stimulation is important when targeting voice dysfunction during preoperative planning,4 especially given that voice and speech deficits significantly impact patients’ self-identity and social functioning.1,3,13 Thus, we investigated whether the position of active stimulation within the STN was associated with perceptual and acoustic-aerodynamic measures of voice function.
METHODS
Participants and Surgery
For approximately the past 3 yr, all movement disorders patients in our DBS program have been encouraged to undergo voice evaluation preoperatively and at 6 mo postoperatively. Under an Institutional Review Board-approved protocol and patient consent, a retrospective review was performed on all DBS subjects who underwent STN DBS between September 2015 and September 2018 (n = 54), to identify those having STN implantation and both pre- and postoperative voice evaluations. The STN was targeted using standard clinical techniques for STN DBS in either awake, microelectrode-guided surgery (n = 12),14 or asleep, interventional magnetic resonance imaging (MRI)-guided surgery (n = 2).15,16 All patients underwent bilateral simultaneous implantation, with the left side always being the first side implanted. Preoperative Unified Parkinson's Disease Rating Scale (UPDRS) III scores, including speech subscores, were obtained by certified movement disorders specialists, and levodopa equivalent daily doses (LEDD) were calculated.17
Voice Pathology Evaluations
A fellowship trained laryngologist and voice specialized speech-language pathologist (SLP) evaluated vocal function in patients with PD before and after DBS surgery. Audio-perceptual voice evaluation using an ordinal scale of 0 (normal), 1 (slight degree of abnormality), 2 (medium degree of abnormality), 3 (high degree of abnormality) was used for the following parameters: roughness, breathiness, strain, pitch low and pitch high, loudness (soft and loud), and hoarseness. Subjective ratings of vocal quality severity were completed using standard procedures in SLP, which do not include clear cutoff values.18 Overall severity of voice pathology was measured via a visual analog scale (VAS).19 The VAS ranges from 0 to 100, and lower scores are correlated with normal voice.20 Additionally, a patient assessment of vocal effort and shortness of breath (SOB) during speech was made using a VAS.
Acoustic and aerodynamic voice measures were also obtained pre- and postoperatively by SLPs as part of routine clinical care. Patients produced a sustained/a/and read the sentence “We were away a year ago” from the Consensus Auditory Perceptual Evaluation-V (CAPE-V) protocol, with recordings completed via Analysis of Dysphonia in Speech and Voice (ADSV: KayPENTAX, Montvale, New Jersey). Connected speech samples were screened for extraneous background noise and disfluencies in reading; only samples with subjectively quiet background noise were included for analysis. Acoustic parameters of voice included the following: cepstral peak prominence (CPP), CPP fundamental frequency F0, and cepstral spectral index of dysphonia (CSID) (sustained vowel/a/and connected speech “we were away a year ago”). CPP is the Fourier transform of the voice spectrum measuring dysphonia severity, where changes of ∼2 dB are considered to be significant and increased values indicate improvement. CPP F0 is the mean fundamental frequency, which typically falls between 60 and 200 Hz. CSID is a multifactorial estimate of dysphonia severity, where a change of 20 units is considered significant and decreased values indicate improvement in dysphonia.21-23
Phonatory aerodynamics in connected speech24 was elicited using the Rainbow Passage.25 Aerodynamic measures were collected using the Phonatory Aerodynamic System 6600 (PAS 6600, KayPENTAX) in accordance with the previously described protocol by Gartner-Schmidt et al.26 The following aerodynamic measures were taken from the reading passage: number of breaths taken, duration of reading passage, mean dB sound pressure level (SPL), mean fundamental frequency, and mean phonatory airflow (total volume of airflow during voicing divided by time, a surrogate for respiratory drive).
Active Contact Localization
Contact locations were determined using the open-source Lead-DBS platform.27 Raw pre and postoperative DICOM images were coregistered using hybrid SPM and ANTs algorithms and then normalized to the ICMB 152 Nonlinear 2009b template. Leads were first localized automatically using the TRAC/CORE algorithm for postoperative MRI (n = 7) or PACER if postoperative computed tomography (CT) was available (n = 7) and then visually adjusted as needed.27 Coordinates in MNI space were recorded, and the origin was translated to the centroid of the sensory-motor STN as defined by the DISTAL Atlas.28 The stimulation parameters (ie, voltage, current, frequency, pulse width, and active contacts) recorded during the movement disorders clinic visit that immediately preceded the postoperative voice evaluation were obtained by the chart review. The active contact location was translated to MNI coordinate space; for monopolar settings, the centroid of the active contact lead was used, and for bipolar settings the centroid between the 2 active contact leads was used.
Statistical Analysis
Nonparametric Mann-Whitney tests were used to compare pre- and postoperative LEDD, UPDRS scores, auditory-perceptual, acoustic, and aerodynamic changes in voice, with P-values <.05 considered significant and with all results corrected for multiple comparisons using the false discovery rate method. To explore the relationship between active contact and voice outcomes, we fitted a linear regression model and calculated Pearson's correlation coefficients with their corresponding 95% CI. Computational and statistical analyses were performed in MATLAB (Mathworks, Natick, Massachusetts) and R (R Foundation, Vienna, Austria).
RESULTS
DBS Patient Characteristics
A total of 14 patients (13 male, 1 female; mean age = 72 ± 5 yr) were identified who underwent STN DBS and received both pre- and postoperative voice evaluations, all of whom underwent simultaneous bilateral implanted lead implantation (Medtronic, model 3389: n = 11, St. Jude, model 6172: n = 2 and Boston Scientific, Vercise: n = 1). All patients reported dysphonia symptoms consistent with the diagnosis of hypokinetic dysarthria (n = 14), with some patients displaying concurrent voice diagnoses such as primary muscle tension dysphonia (n = 2) and vocal fold atrophy (n = 7). Postoperative UPDRS scores were available for only 5 patients, and only from the on-medication state; the mean values are reported for these patients, along with mean pre- and postoperative LEDD for all patients, in Table 1. As expected, LEDD was significantly reduced by STN DBS (P < .05).
TABLE 1.
Clinical Characteristics
| Preoperative values | Postoperative values | |||||
|---|---|---|---|---|---|---|
| n | Mean (SD) | Range | n | Mean (SD) | Range | |
| LEDD (mg)* | 14 | 1045 (646) | 300-2946 | 14 | 549 (389) | 0-1630 |
| UPDRS III | ||||||
| Off meds | 13 | 38 (12) | 19-62 | n/a | n/a | n/a |
| Speech | 13 | 1.1 (0.6) | 0-2 | n/a | n/a | n/a |
| On meds | 13 | 21 (8.5) | 11-39 | 5 | 17 (9.6) | 8-33 |
| Speech | 13 | 1 (0.7) | 0-2 | 5 | 0.5 (0.6) | 0-1 |
| Stimulation | ||||||
| L Frequency | – | – | – | 14 | 152 (17) | 130-174 |
| L Voltage | – | – | – | 14 | 2.82 (0.64) | 1.5-3.6 |
| L Pulse width | – | – | – | 14 | 58.5 (5.5) | 40-60 |
| R Frequency | – | – | – | 14 | 151 (16) | 125-180 |
| R Voltage | – | – | – | 14 | 2.33 (0.71) | 1.6-3.8 |
| R Pulse Width | – | – | – | 14 | 65.4 (11.2) | 60-90 |
*P < .05 and n/a = not available.
Voice Outcomes
In the overall cohort, perceived overall severity of voice quality improved by a median of −6/100 (interquartile range [IQR] −16.5 to 20) at the time of postoperative evaluation (median follow-up = 7 mo, range 6-35 mo). Eight patients improved by a median of −12/100 (IQR −20 to −6) points, while 5 patients worsened by a median of 18/100 (14 to 28) points and 1 patient's voice quality was unchanged. There were no significant differences, however, between mean pre- and postoperative voice severity scores (average preoperative severity = 31.0 ± 16.6 points, average postoperative severity = 30.8 ± 16.9 points, P = .95), when stimulation location was not considered.
Audio-perceptual voice evaluations of roughness, breathiness, strain, low pitch, high pitch, loudness (soft and loud), and hoarseness are shown in Table 2. Low pitch was the only perceptual outcome that worsened significantly after surgery. Acoustic measures of voice and phonatory aerodynamics in connected speech are also shown in Table 2. There were no significant differences between pre- and postoperative measures, when stimulation location was not considered.
TABLE 2.
Change in voice measures across the cohort
| Preoperative Mean (SD) | Postoperative Mean (SD) | P | |
|---|---|---|---|
| Perceptual measures | |||
| Roughness | 0.6 (0.5) | 0.9 (0.5) | .179 |
| Breathiness | 0.4 (0.6) | 0.4 (0.5) | .822 |
| Strain | 0.1 (0.3) | 0.1 (0.4) | .577 |
| Low pitch | 0.1 (0.3) | 0.4 (0.5) | .035 |
| High pitch | 0.5 (0.8) | 0.1 (0.3) | .067 |
| Loudness | 0.8 (0.7) | 0.6 (0.5) | .452 |
| Hoarseness | 0.2 (0.6) | 0.6 (0.8) | .112 |
| Acoustic measures | |||
| CPP vowel (dB) | 10.6 (2.2) | 9.8 (2.5) | .308 |
| CSID vowel | 25.6 (29.6) | 25.8 (14.7) | .332 |
| CPP speech (dB) | 7.7 (1.3) | 7.3 (1.3) | .510 |
| CPP F0 (dB) | 137.1 (21.5) | 139.4 (18.7) | .752 |
| CSID speech | 8.3 (13.1) | 3.0 (17.8) | .174 |
| Passage reading | |||
| Quantity of breaths | 6.6 (3.1) | 6.2 (2.5) | .859 |
| Duration (seconds) | 25.8 (7.0) | 26.5 (8.3) | .990 |
| Mean SPL (dB) | 72.5 (5.6) | 68.8 (4.9) | .139 |
| Mean pitch (Hz) | 134.2 (23.7) | 138.2 (20.7) | .681 |
| Mean pitch range (Hz) | 157.3 (89.2) | 114.6 (66.9) | .213 |
| Mean phonatory airflow | 155.0 (72.6) | 171.8 (79.1) | .283 |
| (mL/s) |
CPP = cepstral peak prominence, CSID = cepstral spectral index of dysphonia, F0 = fundamental frequency, SPL = sound pressure level.
Stimulation Location
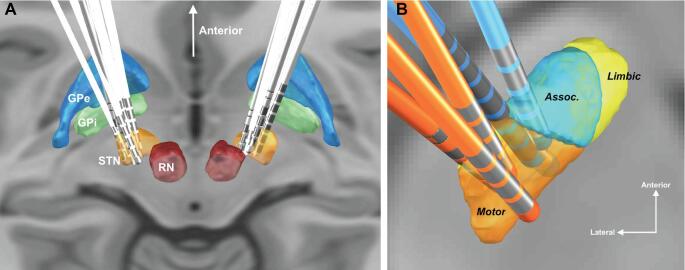
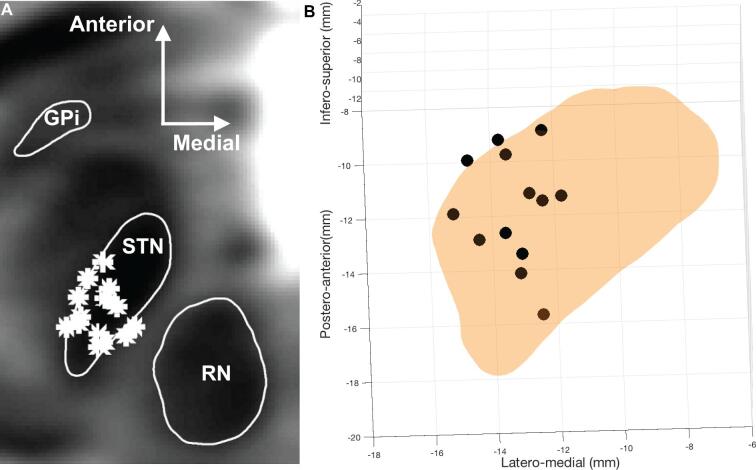
All 14 lead pairs were localized successfully using Lead-DBS (Figure 1A).27 To facilitate visualization, the 3 most anterior and 3 most posterior contacts are shown in Figure 1B, along with the 3 functional STN subdivisions, as defined by the DISTAL Atlas28: limbic, associative, and motor. All left-sided active contact locations projected onto an axial plane at −4 mm in the anterior/posterior commissure coordinate system are shown in Figure 2A and active contact locations with respect to the STN are show in Figure 2B. Note that for one patient, the left lead was turned off due to a lack of right-side symptomology and therefore is not plotted in Figure 2B.
FIGURE 1.
Representation of lead locations in MNI space. A, 3D reconstruction of all leads in common atlas space. B, Visualization of selected leads in the left subthalamic nucleus (blue: 3 most anterior; orange: 3 most posterior).
FIGURE 2.
Representation of stimulation locations in MNI space. A, T2-weigthed axial reconstruction with active contacts (*) plotted on the left subthalamic nucleus. B, Left subthalamic nucleus active contacts in 3-dimensional group space (black circles).
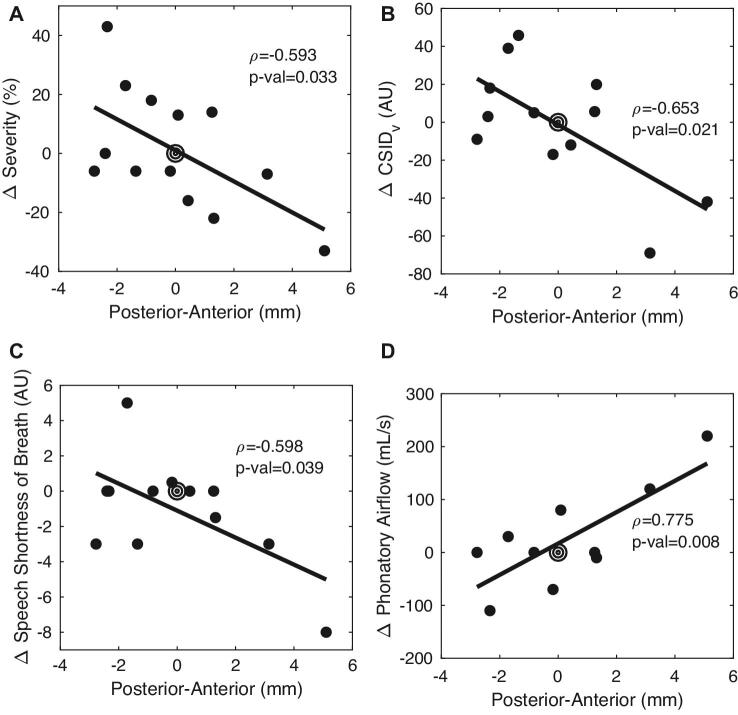
When a linear regression model was used to evaluate the relationship between active contact location and voice metrics, we observed several location-dependent changes between pre- and postoperative values. Change in severity (ie, the difference between pre and postoperative scores, in a 100-point scale) was correlated with the anterior-posterior coordinate of the subthalamic left active contact (rho = −0.592, CI = −0.862 to −0.062, P = .032, Figure 3A). This location-outcome correlation implied that the anterior-posterior contact location accounted for approximately a 40% change in voice severity. CSID was also significantly associated with anterior-posterior stimulation location (rho = −0.598, CI = −0.872 to −0.0371, P = .039, Figure 3B). In addition, speech-associated change in SOB on a 10-point scale was correlated with the anterior-posterior stimulation location (Figure 3C, rho = −0.598, CI = −0.872 to −0.0371, P = .039), as was change in phonatory airflow (Figure 3D, rho = 0.775, CI = 0.284 to 0.944, P = .008). Neither CSID in speech nor any other acoustic measure of voice or other perceptual outcome showed a location-dependent change. No other phonatory aerodynamic changes were significant. No voice outcome was independently associated with stimulation voltage, frequency or pulse width.
FIGURE 3.
A-D, Stimulation location-dependent effects on voice. Target symbol at 0 mm represents the motor subthalamic nucleus centroid. AU: arbitrary units, CSID: cepstral spectral index of dysphonia.
Evaluating contact positions in group space can be challenging, given the variation of individual patient STN anatomy. To understand whether contacts that appeared to border the lateral STN in group space might contribute to worsening voice function due to stimulation of internal capsule fibers, we examined whether position of the active contact was associated with a lower threshold for capsular side effects during initial programming. There was no association between lead positions in the medial-lateral plane with thresholds for a capsular side effect during initial programming, suggesting that capsular effects were not responsible for worsening voice function. Finally, we calculated the Euclidean distance (ie, absolute distance) between the center of the left motor STN and each of the left active contact locations and repeated it for the right side. We then compared the variance of the absolute distances between the right side and the left side (analysis of variance, F(1,24) = 1.51, P = .23). We found that there is no significant difference between the variance of left and right contact locations.
DISCUSSION
For the past several years, we have included formal preoperative and postoperative voice evaluations as standard components of our movement disorders DBS program. Unlike neuropsychological testing, we do not require voice evaluation prior to surgery; similar to neuropsychological testing, however, patients who do well postoperatively often fail to return for postoperative evaluation. Nonetheless, complete speech pathology data from 14 patients were available for this study. Changes in both perceptual and acoustic-aerodynamic measures of voice were correlated with the site of stimulation, as mapped into common STN space using a state-of-the-art atlas that defines the STN on a multimodal high-resolution brain template. We found that stimulation locations in the anterior portion of the sensorimotor region of the left, but not right, STN were associated with improvements in voice severity scores, cepstral spectral index of dysphonia scores, SOB, and phonatory airflow during connected speech.
Of note, patients did not experience a significant change in several subjective and objective voice measures following STN DBS, on average, when stimulation location was not taken into consideration. For example, the median improvement in voice severity ratings was −7 points on a scale of 100 points, a change regarded as not clinically significant. These findings match well with reported trends,29,30 including a recent study reporting no significant changes in multiple perceptual speech scores and acoustic analyses.29 Similarly, stimulation location within the STN vs above the STN previously was not shown to account for changes in SPL acoustic measures.9 Thus, incorporating the active contact location (derived from Lead-DBS, a sophisticated platform for representing DBS contacts in high-resolution group space) into a linear regression model allowed us to elucidate a topography for voice outcomes that would otherwise have gone unobserved.
Perceptual Measures of Voice
When accounting for the location of the active contact, we discovered a significant linear relationship between voice outcomes and left anterior-posterior position within the left STN. Voice severity improved with anterior subthalamic locations and worsened with posterior subthalamic locations. Although voice severity scores measure vocal quality and not articulation, our data generally support previous findings related to articulation outcomes (eg, intelligibility), where worsening speech intelligibility was reported in 2 patients with more posterior STN electrodes (10 patient cohort). In that study, however, other locations were neither definitively localized nor associated with speech intelligibility improvement.7 A similar study reported intelligibility worsening of about 31% with more posterior STN locations; however, electrode locations included contacts slightly outside the STN.31 In our case, we observed a significant clinical improvement in voice severity of about 40% when comparing the most anterior to most posterior stimulation locations. Importantly, no previous studies of DBS effects on voice, to our knowledge, have employed normalization of active contact locations in 3-dimensional space.
Cepstral Voice Measures
We also discovered a relationship between cepstral measures of voice function and anterior-posterior position of the active contact location within the left STN. Cepstral analyses involve the power spectrum of the logarithm of the power spectrum of the acoustic signal and are used to determine the fundamental frequency of human speech. Acoustic analyses such as CPP and CSID are both indices intended to capture dysphonia severity.22,32 The CPP and CSID values reported from our cohort are well within previously reported ranges for PD patients,33 but no previous studies have evaluated the effects of DBS on cepstral voice measures. After STN DBS, we found that worsening CSID scores were correlated with posterior stimulation locations. In contrast, CPP was not correlated to stimulation location, possibly due to the fact that it is a simple frequency measure,34 whereas CSID, a multiparameter model, is able to capture more complex relationships. For instance, a recent study in PD patients reported no significant change in CPP with medication ON vs OFF.35 We note that frequency-based voice measures, such as CSID and CPP, are voice-disorder dependent, and thus not all dysphonia measures may be relevant for each patient.32
Aerodynamic Voice Measures
In addition, we found a linear relationship between improvement in SOB during speech and anterior-posterior left STN contact location. Mean phonatory airflow and STN contact location followed a similar trend, and improvements in both outcomes were observed with more anterior STN locations. It is well known that patients with PD are hypophonic, have a decreased respiratory drive, and show a decrease expelled lung volume per syllable when compared to controls.36 Moreover, studies have reported that STN DBS can affect respiratory drive.37 Therefore, it is meaningful that an improvement in both sound and phonatory airflow corresponds to the same electrode location. The fact that both phonatory airflow measures and CSID scores increased with more anterior locations indicates that improved airflow for speech may have allowed the improvement in how patients sound (CSID). In fact, both vocal severity and CSID are related to regular vocal fold vibration, which is fueled by adequate respiratory drive. It is possible that improvements in respiratory drive are mediated by a more anterior electrode location, leading to improved sound quality in those patients. These results need to be verified in a larger number of subjects, but offer a clue into factors affecting voice after DBS.
Functional Anatomy
Many studies have demonstrated changes in speech/voice behavior because of DBS; however, the underlying mechanism is poorly understood. Multiple theories have been postulated in regard to voice changes after subthalamic stimulation, including spread of stimulation to surrounding structures, a functionally relevant somatotopy within the STN, or simply altered skeletal motor function in line with overall motor responses.9 Spread of the electrical field to adjacent structures, such as corticobulbar or internal capsule fibers, has been suggested to result in speech impairments,38,39 by affecting laryngeal muscle, lip, and tongue circuits via stimulation that is too lateral to the STN target.40-42 Tsuboi et al studied electrode location and voice outcomes but found conflicting results with lateral STN implants (with this location correlating with both worsening and improvement in voice outcomes depending on patient characteristics) which they explained as spread of electrical field to surrounding internal capsule or corticobulbar structures.40 These previous results, however, do not explain the within STN location dependence of our findings.
Other studies have suggested an STN somatotopy where speech-related musculature is represented toward the middle of the STN in PD patients.43 In this case, our anterior electrodes may be stimulating regions controlling truncal speech musculature in a manner that improves voice function, consistent with the idea that improvements in respiratory drive contribute to the presented results. Moreover, posterior locations have been implicated in hypophonic speech,44 which aligns with our results of worsened voice outcomes with posterior STN stimulation locations. Interestingly, none of the outcomes were associated with stimulation location within the right STN, in agreement with previous STN data,31,45 demonstrating left language dominance at the level of basal ganglia participation in speech motor control.
Limitations
Perceptual evaluations were prone to intersubject variability, and subjective observations were taken by different raters. Acoustic measures of voice and phonatory aerodynamics in connected speech were measured once before and once after surgery, but these measures can vary depending on multiple factors. Finally, the software used to normalized patient's anatomy and localize leads is sensitive to patient imaging variations and has a reported mean Euclidean distance when comparing the same contacts using MRI and CT imaging in the order of 0.66 ± 0.43 mm.27
CONCLUSION
This study is the first to include cepstral analyses and state-of-the-art atlas registration to study voice outcomes following DBS. These data suggest that perceptual, cepstral, and aerodynamic improvements in voice function co-localize to stimulation locations within the anterior sensorimotor region of the left STN. The laterality specificity of these effects is intriguing and warrants further investigation.
Disclosures
Funding was from the National Institutes of Health U01NS098969. The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Notes
An abstract of this data was accepted for poster presentation at the International Movement Disorders Society Meeting, September 22-26, 2019 in Nice, France
Contributor Information
Ahmed Jorge, Department of Neurological Surgery, University of Pittsburgh, Pittsburgh, Pennsylvania.
Christina Dastolfo-Hromack, Department of Neurological Surgery, University of Pittsburgh, Pittsburgh, Pennsylvania; Department of Otolaryngology, University of Pittsburgh, Pittsburgh, Pennsylvania.
Witold J Lipski, Department of Neurological Surgery, University of Pittsburgh, Pittsburgh, Pennsylvania.
Ian H Kratter, Department of Neurological Surgery, University of Pittsburgh, Pittsburgh, Pennsylvania; Department of Psychiatry, University of Pittsburgh, Pittsburgh, Pennsylvania.
Libby J Smith, Department of Otolaryngology, University of Pittsburgh, Pittsburgh, Pennsylvania.
Jackie L Gartner-Schmidt, Department of Otolaryngology, University of Pittsburgh, Pittsburgh, Pennsylvania.
R Mark Richardson, Department of Neurosurgery, Massachusetts General Hospital, Boston, Massachusetts; Harvard Medical School, Boston, Massachusetts.
REFERENCES
- 1. Brabenec L, Mekyska J, Galaz Z, Rektorova I. Speech disorders in Parkinson's disease: early diagnostics and effects of medication and brain stimulation. J Neural Transm. 2017;124(3):303-334. [DOI] [PubMed] [Google Scholar]
- 2. Gates S, Iansek R, Marigliani C, Ho AK, Bradshaw JL. Speech impairment in a large sample of patients with Parkinson's disease. Behav Neurol. 2014;11(3):131-137. [PubMed] [Google Scholar]
- 3. Aldridge D, Theodoros D, Angwin A, Vogel AP. Speech outcomes in Parkinson's disease after subthalamic nucleus deep brain stimulation: a systematic review. Park Relat Disord. 2016;33:3-11. [DOI] [PubMed] [Google Scholar]
- 4. Matsumoto JY, Fossett T, Kim M et al. . Precise stimulation location optimizes speech outcomes in essential tremor. Park Relat Disord. 2016;32:60-65. [DOI] [PubMed] [Google Scholar]
- 5. Imai R, Kamada T, Araki N et al. . Carbon ion radiation therapy for unresectable sacral chordoma: an analysis of 188 cases. Int J Radiat Oncol Biol Phys. 2016;95(1):322-327. [DOI] [PubMed] [Google Scholar]
- 6. Smith KM, O’Connor M, Papavassiliou E, Tarsy D, Shih LC. Phonemic verbal fluency decline after subthalamic nucleus deep brain stimulation does not depend on number of microelectrode recordings or lead tip placement. Park Relat Disord. 2014;20(4):400-404. [DOI] [PubMed] [Google Scholar]
- 7. Åström M, Tripoliti E, Hariz MI et al. . Patient-specific model-based investigation of speech intelligibility and movement during deep brain stimulation. Stereotact Funct Neurosurg. 2010;88(4):224-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fenoy AJ, McHenry MA, Schiess MC. Speech changes induced by deep brain stimulation of the subthalamic nucleus in Parkinson disease: involvement of the dentatorubrothalamic tract. J Neurosurg. 2016;126(6):2017-2027. [DOI] [PubMed] [Google Scholar]
- 9. Tripoliti E, Tisch S, Hariz MI et al. . Effects of contact location and voltage amplitude on speech and movement in bilateral subthalamic nucleus deep brain stimulation. Mov Disord. 2008;23(16):2377-2383. [DOI] [PubMed] [Google Scholar]
- 10. Floden D, Matias C, Wathen C, Ozinga G, Hogue O, Machado A. Contact location and neuropsychological outcomes in subthalamic deep brain stimulation. Neurosurgery. 2017;83(4):666-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ehlen F, Schoenecker T, Kühn AA, Klostermann F. Differential effects of deep brain stimulation on verbal fluency. Brain Lang. 2014;134:23-33. [DOI] [PubMed] [Google Scholar]
- 12. Tsai S-T, Lin S-H, Lin S-Z, Chen J-Y, Lee C-W, Chen S-Y. Neuropsychological effects after chronic subthalamic stimulation and the topography of the nucleus in Parkinson's disease. Neurosurgery. 2007;61(5):950-955. [DOI] [PubMed] [Google Scholar]
- 13. Whitehead B. The psychosocial impact of communication changes in people with Parkinson's disease. Br J Neurosci Nurs. 2010;6(1):30-36. [Google Scholar]
- 14. Starr PA, Theodosopoulos P V., Turner R et al. . Surgery of the subthalamic nucleus: use of movement-related neuronal activity for surgical navigation. Neurosurgery. 2003;53(5):1146-1149. [DOI] [PubMed] [Google Scholar]
- 15. Lee PS, Richardson RM. Interventional MRI–guided deep brain stimulation lead implantation. Neurosurg Clin N Am. 2017;28(4):535-544. [DOI] [PubMed] [Google Scholar]
- 16. Lee PS, Weiner GM, Corson D et al. . Outcomes of interventional-MRI versus microelectrode recording-guided subthalamic deep brain stimulation. Front Neurol. 2018;9:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rick C, Clarke CE, Tomlinson CL, Patel S, Gray R, Stowe R. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord. 2010;25(15):2649-2653. [DOI] [PubMed] [Google Scholar]
- 18. Kempster GB, Gerratt BR, Verdolini Abbott K, Barkmeier-Kraemer J, Hillman RE. Consensus auditory-perceptual evaluation of voice: development of a standardized clinical protocol. Am J Speech Lang Pathol. 2009;18(2):124-132. [DOI] [PubMed] [Google Scholar]
- 19. Coleman TC, Childes JM, Karnell MP, Melton SD, Hoffman HT, Dailey SA. Reliability of clinician-based (GRBAS and CAPE-V) and patient-based (V-RQOL and IPVI) documentation of voice disorders. J Voice. 2006;21(5):576-590. [DOI] [PubMed] [Google Scholar]
- 20. Borgt MJ, Dejonckere PH, Martens JP, Gillis M, Peleman M, Moerman MBJ. Perceptual evaluation of substitution voices: development and evaluation of the (I)INFVo rating scale. Eur Arch Otorhinolaryngol. 2005;263(2):183-187. [DOI] [PubMed] [Google Scholar]
- 21. Heman-Ackah Y, Heuer R, Deirdre M et al. . Cepstral peak prominence: a more reliable measure of dysphonia. Ann Otol Rhinol Laryngol. 2003;112:324-333. [DOI] [PubMed] [Google Scholar]
- 22. Peterson EA, Merrill RM, Banks R, Roy N, Tanner K, Awan SN. Toward validation of the cepstral spectral index of dysphonia (CSID) as an objective treatment outcomes measure. J Voice. 2013;27(4):401-410. [DOI] [PubMed] [Google Scholar]
- 23. Shim H-J, Jung H, Koul R, Ko D-H. Spectral and cepstral based acoustic features of voices with muscle tension dysphonia. Clin Arch Commun Disord. 2016;1(1):42-47. [Google Scholar]
- 24. Dastolfo C, Gartner-Schmidt J, Yu L, Carnes O, Gillespie AI. Aerodynamic outcomes of four common voice disorders: moving toward disorder-specific assessment. J Voice. 2016;30(3):301-307. [DOI] [PubMed] [Google Scholar]
- 25. Baken RJ, Orlikoff RF.. Clinical Measurement of Speech and Voice. 2nd ed San Diego: Singular Thomson Learning; 2000. [Google Scholar]
- 26. Gartner-Schmidt JL, Hirai R, Dastolfo C, Rosen CA, Yu L, Gillespie AI. Phonatory aerodynamics in connected speech. Laryngoscope. 2015;125(12):2764-2771. [DOI] [PubMed] [Google Scholar]
- 27. Horn A, Kühn AA. Lead-DBS: A toolbox for deep brain stimulation electrode localizations and visualizations. Neuroimage. 2015;107:127-135. [DOI] [PubMed] [Google Scholar]
- 28. Ewert S, Plettig P, Li N et al. . Toward defining deep brain stimulation targets in MNI space: a subcortical atlas based on multimodal MRI, histology and structural connectivity. Neuroimage. 2018;170:271-282. [DOI] [PubMed] [Google Scholar]
- 29. Schnitzler A, Skodda S, Grönheit W, Schlegel U, Wojtecki L, Südmeyer M. Effect of subthalamic stimulation on voice and speech in Parkinson's disease: for the better or worse? Front Neurol. 2014;4:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Skodda S. Effect of deep brain stimulation on speech performance in Parkinson's disease. Parkinsons Dis. 2012;2012:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tripoliti E, Zrinzo L, Martinez-Torres I et al. . Effects of subthalamic stimulation on speech of consecutive patients with Parkinson disease. Neurology. 2011;76(1):80-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gillespie AI, Dastolfo C, Magid N, Gartner-Schmidt J. Acoustic analysis of four common voice diagnoses: moving toward disorder-specific assessment. J Voice. 2014;28(5):582-588. [DOI] [PubMed] [Google Scholar]
- 33. Byeon H, Cho S.. Cepstral analysis of connected speech of hypokinetic dysarthria and normal speakers. Healthc Nurs. 2016;132:212-215. [Google Scholar]
- 34. Awan SN, Roy N, Dromey C. Estimating dysphonia severity in continuous speech: application of a multi-parameter spectralcepstral model estimating dysphonia severity in continuous speech. Clin Linguist Phonetics. 2009;23(11):825-841. [DOI] [PubMed] [Google Scholar]
- 35. Adams S, Gilmore G, Cushnie-Sparrow D, Abeyesekera A, Pieterman M, Jog M. Voice quality severity and responsiveness to levodopa in Parkinson's disease. J Commun Disord. 2018;76:1-10. [DOI] [PubMed] [Google Scholar]
- 36. Hammer MJ. Aerodynamic assessment of phonatory onset in Parkinson's disease: evidence of decreased scaling of laryngeal and respiratory control. J Parkinsons Dis. 2013;3(2):173-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hammer MJ, Barlow SM, Lyons KE, Pahwa R. Subthalamic nucleus deep brain stimulation changes speech respiratory and laryngeal control in Parkinson's disease. J Neurol. 2010;257(10):1692-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Krack P, Gentil M, Pinto S et al. . Changes induced by levodopa and subthalamic nucleus stimulation on parkinsonian speech. Mov Disord. 2005;20(11):1507-1515. [DOI] [PubMed] [Google Scholar]
- 39. Klostermann F, Ehlen F, Vesper J et al. . Effects of subthalamic deep brain stimulation on dysarthrophonia in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2008;79(5):522-529. [DOI] [PubMed] [Google Scholar]
- 40. Tsuboi T, Watanabe H, Tanaka Y et al. . Distinct phenotypes of speech and voice disorders in Parkinson's disease after subthalamic nucleus deep brain stimulation. J Neurol Neurosurg Psychiatry. 2015;86(8):856-864. [DOI] [PubMed] [Google Scholar]
- 41. Pinto S, Ozsancak C, Tripoliti E, Thobois S, Limousin-Dowsey P, Auzou P. Treatments for dysarthria in Parkinson's disease. Lancet Neurol. 2004;3(9):547-556. [DOI] [PubMed] [Google Scholar]
- 42. Limousin P, Tripoliti E, Foltynie T et al. . Pyramidal tract activation due to subthalamic deep brain stimulation in Parkinson's disease. Mov Disord. 2017;32(8):1174-1182. [DOI] [PubMed] [Google Scholar]
- 43. Rodriguez-Oroz MC. The subthalamic nucleus in Parkinson's disease: somatotopic organization and physiological characteristics. Brain. 2002;124(9):1777-1790. [DOI] [PubMed] [Google Scholar]
- 44. Plaha P, Ben-Shlomo Y, Patel NK, Gill SS. Stimulation of the caudal zona incerta is superior to stimulation of the subthalamic nucleus in improving contralateral parkinsonism. Brain. 2006;129(7):1732-1747. [DOI] [PubMed] [Google Scholar]
- 45. Hariz MI, Foltynie T, Aviles-Olmos I et al. . Predictive factors of speech intelligibility following subthalamic nucleus stimulation in consecutive patients with Parkinson's disease. Mov Disord. 2014;29(4):532-538. [DOI] [PMC free article] [PubMed] [Google Scholar]