ABSTRACT
Upon dietary exposure, the endogenous metabolism responds to the diet-derived nutrients and bioactive compounds, such as phytochemicals. However, the responses vary remarkably due to the interplay with other dietary components, lifestyle exposures, and intrinsic factors, which lead to differences in endogenous regulatory metabolism. These physiological processes are evidenced as a signature profile composed of various metabolites constituting metabolic phenotypes, or metabotypes. The metabolic profiling of biological samples following dietary intake hence would provide information about diet—that is, as the intake biomarkers and the ongoing physiological reactions triggered by this intake—thereby enable evaluation of the metabolic basis required to distinguish the different metabotypes. The capacity of nontargeted metabolomics to also encompass the unprecedented metabolite species has enabled the profiling of multiple metabolites and the corresponding metabotypes with a single analysis, decoding the complex interplay between diet, other relevant factors, and health. In this systematic review, we screened 345 articles published in English in January 2007–July 2018, which applied the metabolomics approach to profile the changes of endogenous metabolites in the blood related to dietary interventions, either derived by metabolism of gut microbiota or the human host. We excluded all the compounds that were directly derived from diet, and also the dietary interventions focusing on supplementation with individual compounds. After the removal of less relevant studies and assessment of eligibility, 49 articles were included in this review. First, we mention the contribution of individual factors, either modifiable or nonmodifiable factors, in shaping metabolic profile. Then, how different aspects of the diet would affect the metabolic profiles are disentangled. Next, the classes of endogenous metabolites altered following included dietary interventions are listed. We also discuss the current challenges in the field, along with future research opportunities.
Keywords: metabotype, endogenous metabolite, blood, dietary interventions, nontargeted metabolomics, metabolic profiling
Introduction
Recent progress in the metabolomics field has enabled the characterization of an enormous range of chemical compounds, basically as many metabolites as amenable for the chosen analytical technique. The numbers typically exceed several hundred. The application of nontargeted metabolite profiling in a biological sample, such as blood, urine, feces, and saliva, could assist in exploration and monitoring of alteration in human metabolism (1–4). In the field of nutrition and health specifically, metabolic profiling may provide information about diet—that is, as food intake biomarkers, as well as the ongoing physiological responses triggered by the diet. Investigations of dietary responses, however, remain challenging due to the complex interplay between any bioactive compound with other nutritional components, and an immense variation between individuals, as previously reported (5, 6).
Factors contributing to individual variations include the balance between lifestyle and environmental exposures, which could be affected by personal knowledge and choices. This balance includes diet, physical activity, and psychological stress that could interact with genetic factors via epigenetic modifications and the gut microbiome. As a system, the human body hence would respond to these triggers by tightly regulated endogenous metabolisms. Within certain genetics-bound limits, modulation of the endogenous metabolisms would elevate or diminish the concentrations of endogenous metabolites as the byproducts of metabolic processes, mediators of exposures, or even phenotype modulators (7). The interaction of these factors would then shape metabolic phenotypes (metabotypes) (8). Or, individual metabotypes would represent the net results of all intrinsic and extrinsic factors.
In this context, metabolomics is an essential platform to identify metabotypes by performing large-scale metabolic profiling following dietary interventions. Besides giving an overview of the physiological changes in the body during an intervention, metabolic profiling also enables identification of metabotypes, detangles the interaction of dietary and individual factors in shaping metabotypes, and shows how different metabotypes would respond differently towards the given intervention. Combined with a comprehensive mapping of the affected metabolites in their respected metabolic pathways, this technique may improve understanding to decode the encrypted mechanisms underlying different dietary responses, which may elucidate the variation in dietary responses and enable the future realization of personalized nutrition.
Scope of the Current Review
In this systematic review, we focus on applications of a metabolomics approach in the past decade to profile the changes of endogenous metabolites with prior dietary interventions, either derived by metabolisms of gut microbiota or the human host. We included only human studies utilizing metabolomics approaches to assess the effects of diet on metabolic profiles. In the first part, we briefly discuss the contribution of factors other than diet, both modifiable and nonmodifiable factors, that would shape metabolic profiles, which, in turn, would also affect endogenous metabolism and dietary responses. Next, we discuss how different aspects of diet would affect the metabolic profile. Various aspects that we address include how nutritional components, habitual patterns, or processing methods may alter endogenous metabolites. The scope of metabolites is restricted to ones that are endogenously produced in the human body, not principally derived or modified from the diet. We also give examples of how different baseline profiles may affect dietary responses.
In the second part, we discuss how different compound classes belonging to particular metabolic pathways changed following dietary interventions. Included in this section, we also summarize how microbial metabolites reflecting the gut microbial community are affected by diet. To keep the focus on the effect of dietary intervention on metabolic profiles, we will not discuss the impact of diet on the composition or population of gut microbiota themselves, but how their metabolites change following dietary interventions. Finally, we address some challenges and gaps in the field that offer some insight into future research opportunities directed to the more precise approach of health optimization.
Methods and Results
The literature search was performed in 3 different search engines (PubMed, Scopus, Web of Science). To restrict the search results, we used the search terms (biomarker* OR marker* OR metabolite* OR biokinetics OR biotransformation OR conversion OR metabolome*) AND (trial OR experiment OR study OR intervention) AND (human* OR men OR women OR volunteer* OR subject* OR participant* OR individual*) AND (urine OR plasma OR serum OR blood) AND (intake OR meal OR diet OR ingestion OR consumption OR eating OR drink* OR food) AND (endogenous OR intrinsic OR phenotype* OR metabotype* OR gut bacteria* OR gut microb*) AND (NMR OR LC-MS OR LC/MS OR chromatography OR spectrometry OR lipidomic*) and articles published in English. We included research articles published within January 2007–July 2018. Both targeted and nontargeted metabolomics were allowed for inclusion. Because the plasma sample was reported to better reflect individual lipid and energy metabolism due to environmental factors and risk of metabolic diseases (9), the included research articles and the discussion thereafter will focus on metabolites discovered from blood samples. We restricted the results to only dietary interventions that investigated whole diets or dietary components/factors, but not supplementation with individual compounds. We also excluded the findings that solely focused on the biotransformation of phytochemicals. Other reasons for exclusion included irrelevant content with the purpose of the review paper. The search found 345 records after the removal of duplicate items. Forty-three records were obtained from other sources. After the removal of less relevant studies, 229 full-text articles were assessed for eligibility. Eventually, 49 studies were included in this review (Figure 1). The complete list of affected endogenous metabolites following dietary interventions can be found in Supplemental Table 1.
FIGURE 1.
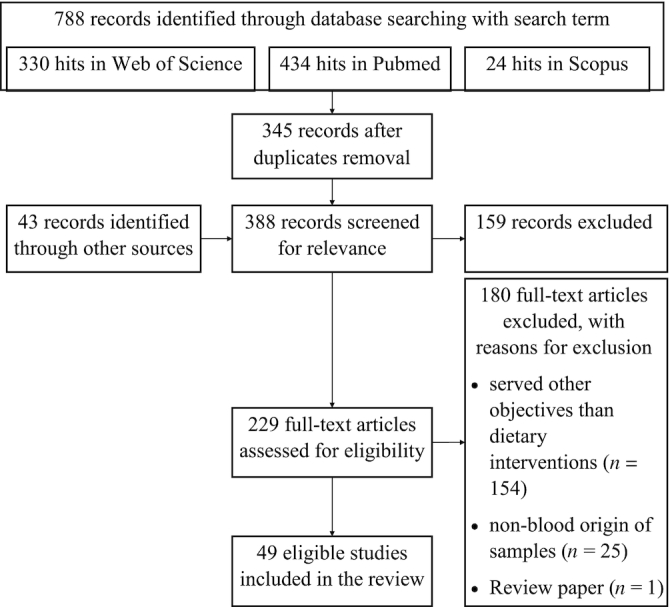
The workflow of the literature search.
Contribution of Individual Factors to Metabotypes
Every individual is different, including in their dietary responses. This biological variation often exists in a wide range of spectra due to the contribution of factors other than diet in shaping metabolic profile, as they play an essential role in diet-related responses and endogenous metabolisms.
We cannot deny the influence of the fixed genetic factors in metabotypes, both at baseline as well as in response to a dietary intervention (10, 11) or postprandial challenge (12). Nevertheless, lifestyle or environmental exposures may modulate the gene expression via, for example, epigenetics modification. Sex is another important factor to consider, as males and females differ in many aspects that would affect baseline phenotypes and overall metabolisms (11–14), such as steroid hormones, body composition, fat distribution, etc.
The partly modifiable factors include the individual's characteristics shaped by the interaction between both individual-specific factors and environmental exposure over time. Such factors could be reflected in the metabolic profile at baseline and also would determine the future response to diets, creating a bidirectional relation between the factors and the phenotypes. The first example of this group is the baseline metabolic phenotype (e.g., obesity) (Table 1). Another important example is the gut microbiota, which has been shown to be individual-specific, diet-dependent, but resilient (4, 15, 16). The gut microbiota has been proposed to affect the bioavailability, bioactivity, and physiological effects of diet-derived bioactive compounds (17–22) and dietary fiber (23), as well as reflect higher inflammation and the risk of metabolic diseases due to adoption of a Western diet (23, 24).
TABLE 1.
Differences in blood metabolites in obese and lean individuals at baseline and after the intervention1
| Phenotypes | |||
|---|---|---|---|
| Intervention and metabolites | Obese | Lean | Reference |
| Baseline | (14) | ||
| Carnitines | ↑ | ||
| Fatty acids | ↑ | ||
| TCA cycle intermediates | ↑ | ||
| Amino acids (BCAAs, acidic amino acids, Pro) | ↑ | ||
| Carboxylic acids | ↑ | ||
| Uric acids | ↑ | ||
| Low-carbohydrate (20 g/d) diet | (14) | ||
| Oleamide | ↓ | ↓↓ | |
| Ribose | ↓ | ↓↓ | |
| 4-Hydroxy-3-methoxymandelate | ↓ | ↓↓ | |
| Mannose | ↑↑ | ↑ | |
| Nicotinamide | ↑↑ | ↑ | |
| Fat-rich overload | (33) | ||
| Acylcarnitines (11:1 and 12:1) | ↑ | ↓ | |
| Glycerol | ↓↓ | ↓ | |
| Triglycerides | ∼ | ↑ | |
| Fish oil | (34) | ||
| PUFAs | ↑ | ↑↑ | |
| 9,12,13-Trihydroxyoctadecenoic acid | ↑ | ↓ | |
| LA- and AA-derived oxylipins | ↓ | ||
AA, arachidonic acid; BCAA, branched-chain amino acid; LA, linoleic acid; Pro, Proline; TCA, tricarboxylic acid; ↑↑, higher increment than ↑; ↓↓, more pronounced reduction than ↓; ~, remained similar after the intervention.
The modifiable factors, such as physical activity and psychological stress, also have a capability to enhance certain metabolic pathways and thereby modulate metabolic profiles. Exercise has been shown to increase metabolic stress and activate energy-generating pathways (25, 26). Stress, on the other hand, may activate the sympathetic-adrenal-medullary axis that triggers acute responses and the hypothalamic-pituitary-adrenal axis for more chronic ones (27, 28).
Effect of Dietary Aspects on Metabotypes
In this section, we discuss how different aspects of interventions shaped the metabolic phenotype, ranging from individual dietary components to a dietary pattern as a whole entity, from energy deficit to surplus, as well as the effect of various preparation methods. Generally, changes in energy intake, either a chronic calorie restriction or an acute postprandial challenge, offer the most widespread changes to the metabolic profile. It is noteworthy that baseline metabolic phenotypes deserve some attention because they could affect dietary responses, with more pronounced metabolic changes in individuals with less favorable phenotypes. Adoption of a particular dietary pattern, meanwhile, allows the interaction of each dietary component, which most likely also provides a synergistic or an antagonistic effect.
Macronutrients
Based on the macromolecular composition, we separately discuss the dietary aspect of fat, protein, and carbohydrate. To keep the focus of this paper, we will not discuss fortifications of vitamins (29, 30), minerals (31), or other food supplements. The complete list of endogenous metabolites (altogether, 545 metabolites) altered following these dietary interventions can be found in Supplemental Table 1.
Fat
Fat covers a wide range of compounds, depending on the forms, sources, and fatty acid composition. With this complexity, dietary fat intake potentially affects a long sequence of events, from its absorption, transport, oxidation, synthesis, and storage. Additionally, it may also impact the metabolic rate and satiety. The physiological responses following different fat-rich interventions hence differ considerably between meals and fat sources (32).
The first important aspect of dietary fat is the fatty acid composition. The dietary fatty acids, especially the essential ones, will be incorporated into various lipid pools. For example, consumption of fish as a rich source of omega-3 fatty acids (35) has increased blood EPA (20:5) and DHA (22:6), either in the free form, bound to phospholipids (36) or cholesteryl esters (11), or modified to oxylipins (11, 37), which may suggest the potential effect of fish oil on inflammatory mediators. Other susceptible downstream products are ethanolamides, as a diet rich in oleic acid (18:1) increased oleoylethanolamide (OEA), whereas a diet high in α-linolenic acid (18:3) increased α-linolenoyl-ethanolamide (ALEA) (38). Both OEA and ALEA play a different role in the regulation of lipid metabolism and satiety (39).
Besides the direct derivatives, consumption of a particular fat source would also alter the composition of other lipid molecules. Besides increasing PUFA-containing lipid pools, fish intake increased acylcarnitines that contained short- and saturated medium-chain fatty acids (MCFAs), which may imply alteration of lipid transport and oxidation (40). Also, it increased plasmalogens that contain odd-chain fatty acids (OCFAs) and decreased those containing MCFAs (40). Another example came from a breakfast trial with either soy- or dairy-rich products (32). A dairy-rich breakfast increased phosphatidylcholine (PC) containing OCFAs and SCFAs, ceramides, sphingomyelin, and the plasmalogens alkyl-PC [PC(O)] and alkenylphosphatidylethanolamine [PE(P)], while a soy-rich breakfast induced the opposite effects (32), highlighting the complexity of lipid metabolism in response to a fat-containing diet.
Another critical factor in a fat-containing diet is its proportion in the total daily intake. In an 8-wk overfeeding trial, the lipid-enriched diet blunted the systemic regulation of the fed-or-fasted state, as it increased insulin, insulin resistance, leptin, and free fatty acids (33). Additionally, a fat-rich diet may affect homocysteine and lipid metabolisms, lipid transport, and suppress creatinine, bilirubin, arachidonic acid (AA), (acetyl)carnitine, glycine betaine, as well as most lysoPCs except for lysoPC (14:0) and lysoPE (16:0) (33). These facts may relate to the role of fatty acids or their derivatives, for example, as ligands for nuclear receptors for transcription regulation. Therefore, a comprehensive investigation on the biological function of dietary fat should also consider the proportion, composition, and availability of fat in different molecular forms. Furthermore, the mapping of the findings in the human metabolic map is encouraged to depict the whole picture on the contribution of dietary fat to metabotypes and modulation of dietary responses and metabolic health.
Protein
Unlike fat that is easily stored, released, and fluctuates in the body, protein metabolism is tightly associated with energy balance. Excessive protein intake would be excreted as urea after protein turnover, whereas an energy-deficient diet would induce the catabolism of dietary protein as an energy source. Hence, dietary protein would not necessarily increase the pool of circulating amino acids in the blood, depending on the energy balance.
Protein intake has been shown to affect some metabolic pathways, including the metabolism of amino acids and their derivatives, such as β-alanine, norleucine, 2-,4-hydroxyproline, 5-methylcysteine (41), citrulline, and kynurenine (42, 43). Kynurenine is a tryptophan metabolite that can pass the blood–brain barrier and was previously associated with the immune response mediated by IFN-γ (44), neurotoxicity, and several neural impairments (45). Specifically, gluten increased dopamine-3-O-sulfate (43), the endogenous neurotransmitter that is involved in various functions of the nervous system.
Interestingly, different protein sources also interfere with other pathways that affect disaccharides and lipid pools. For example, beef intake increased isomaltose and lactulose (41), whereas casein increased SCFAs and short-chain keto acids, propionylcarnitine, and reduced hydroxydecanoic, lauric, and myristic acids (43). These examples emphasize a tight connection between the metabolism of energy sources (carbohydrates and lipids) with amino acids, as in the widely known transaminase reaction between pyruvate and glutamate yielding alanine and α-ketoglutarate (46). This link between amino acids and energy metabolism hence reminds one how investigations of the metabolic impact of protein intake should also consider the surrounding physiological functions, including, but not limited to, immune response, nervous system, as well as metabolisms of carbohydrates and lipids.
Carbohydrates
The included studies did not investigate much about different types of carbohydrates, although the wide range of structures carry various physiological impacts. Carbohydrates, especially the complex ones, constitute the dietary fiber that provides energy and carbon for gut microbial fermentation and also contributes to our daily energy requirement (16). The complex carbohydrate from a plant-rich diet often carries over phenolic compounds having a potential role in the metabolic system. The metabolic effect of complex carbohydrates, for example, derived from whole-grain products, can be observed in several compound classes other than carbohydrates, which is discussed in the section on dietary patterns. No literature was found in the included references on simple sugar, nonstarch polysaccharides, or refined starch, except for the glucose in the oral-glucose-tolerance test (OGTT), which will be discussed as a postprandial challenge. As an energy source, the contribution of carbohydrates to the total daily energy intake with protein and fat is discussed in the section about energy intake.
Energy intake
Postprandial challenges
When any or a mixture of these macronutrients is given in a large amount, it provides an additional burden for metabolic capacity. Aligned with the concept of health as the capacity to flexibly respond to challenges (47) (i.e., allostasis), nutrient overload can be perceived as an additional challenge that perturbs the homeostatic balance. The application of extensive metabolic phenotyping after a challenge hence is useful to measure phenotypic flexibility—that is, how the body responds to restore the balance. The simpler the challenge is, the less necessary actions are to restore the homeostatic balance. By mapping the altered metabolites, this technique hence enables detection of the perturbed metabolic pathways, elucidation of physiological response to the challenge, and assessment of health as the capacity to restore the homeostatic balance.
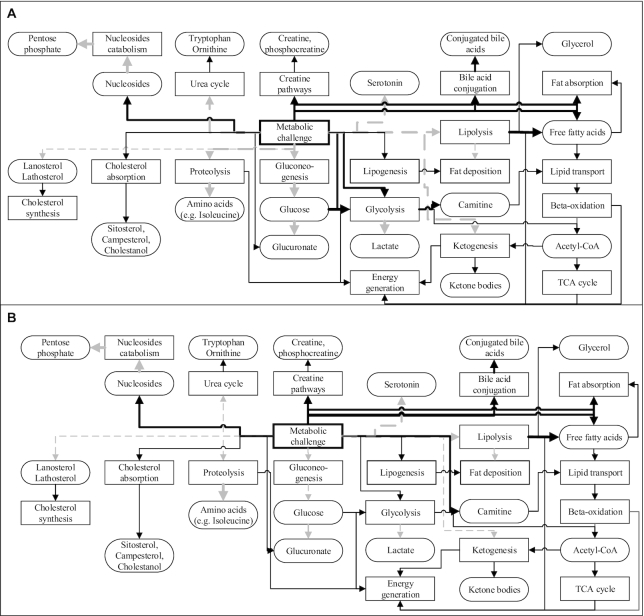
One of the most widely used challenge tests is the OGTT, which was explicitly meant to challenge glycemic response (48) and thereby was more sensitive than fasting blood glucose to detect type 2 diabetes (T2D). Besides widely known pathways related to insulin action, such as elevated glycolysis and inhibited lipolysis, ketogenesis, and proteolysis, the OGTT also seems to increase concentrations of free carnitine, (phospho)creatine, and decreased dicarboxylic MCFAs, serotonin metabolites, B vitamins, glucuronate, nucleosides, and their catabolism products, ribose/ribulose phosphate (12). In response to an OGTT, the blunted effect of insulin on ketogenesis, proteolysis, and glycolysis in insulin-resistant individuals was shown by differences of β-hydroxybutyrate, isoleucine, and lactate (Figure 2). Also, they had a smaller reduction in tryptophan and ornithine, showing alteration of the urea cycle (12), which suggested a broad metabolic shift due to a meal challenge.
FIGURE 2.
The potentially altered metabolic pathways and respective metabolites by energy-based metabolic challenge both in normal (A) and insulin-resistant (B) individuals, as suggested by the current literature. Solid black lines/arrows indicate stimulation; dashed gray lines/arrows indicate inhibition. Thick lines indicate stronger stimulation or inhibition.
Compared with an OGTT, a challenge with higher complexity (e.g., high-fat or mixed-meal challenge) would give a more severe perturbation to the metabolic capacity and trigger more complex responses to restore the homeostatic balance. A high-fat challenge seems to increase fat and cholesterol absorption, as proven by higher circulating concentrations of free fatty acids, β-sitosterol, campesterol, and cholestanol (48). Also, it inhibited the endogenous cholesterol synthesis, demonstrated by reduced concentrations of its precursors (lanosterol and lathosterol) (48).
Over the longer term, however, an energy surplus seems to have an opposite effect compared with an acute metabolic challenge. Only 1 included study (33) discussed the effect of a prolonged energy surplus with reduction in PUFAs (48), betaines, and metabolites in the urea cycle (12), as opposed to the acute energy surplus. It is unclear if these discrepancies were due to the duration of the intervention (acute or chronic) or the prandial state (fed or fasted) during the sampling.
In conclusion, challenges to metabolic capacity by energy overload raised circulating concentrations of free fatty acids, indicators of cholesterol absorption, glycolysis, conjugation of bile acids, and lysoPC or lysoPE containing MCFAs. In contrast, indicators of cholesterol synthesis, tricarboxylic acid (TCA) cycle, ketogenesis, proteolysis, along with B vitamins and nucleosides as cofactors of many metabolic enzymes, decreased similarly to PUFA-containing (lyso)PC (12, 33, 48). This involvement of seemingly irrelevant pathways such as B vitamins reveals the knowledge gap in the metabolic design of the human body, particularly in response to the amount of nutrient intake. This gap hence highlights the importance of metabolic profiling to fully phenotype and follow the physiological mechanisms over time, and in assessing the contribution of intrinsic factors to individual variations.
Energy restriction
As metabolic diseases have been generally linked to excessive consumption, energy restriction seems promising to reverse the unfavorable phenotypes. In some interventions, energy restriction successfully improved phenotypic markers, such as fasting glucose (49), body weight, and waist circumference, and reduced total fat, diastolic blood pressure, transaminase, circulating and stored fat, and total, HDL, and LDL cholesterol (50). Metabotyping during energy restriction hence may provide insight into the mechanisms underlying the reversal processes.
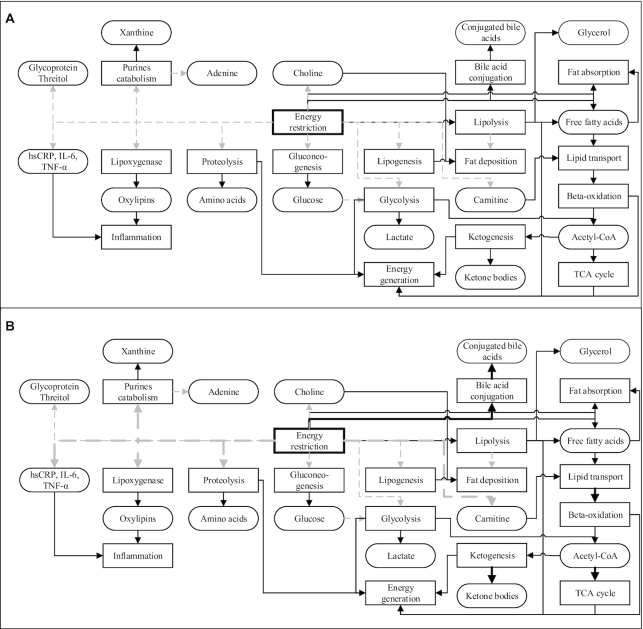
An interesting observation is that the energy restriction did not have the opposite effect of a postprandial challenge. The activation and inhibition of different metabolic pathways due to energy restriction in normal individuals are depicted in Figure 3A. The general mechanisms involve the reduction in blood glucose and elevated concentrations of free fatty acids (51) and ketone bodies, such as acetoacetate and 3-hydroxybutyrate (49), indicating inhibition of gluconeogenesis and energy generation via lipolysis and β-oxidation instead of glycolysis and the TCA cycle. The increased β-oxidation or lowered endogenous fatty acid production due to energy restriction reduced circulating and stored fat, as predicted by palmitoleic acid (C16:1) (50).
FIGURE 3.
The potentially altered metabolic pathways and respective metabolites by energy restriction both in normal individuals (A) and those with metabolic syndrome (B), as suggested by the current literature. Solid black lines/arrows indicate stimulation; dashed gray lines/arrows indicate inhibition. Thick lines indicate stronger stimulation or inhibition. hsCRP, high-sensitivity C-reactive protein; TCA cycle, tricarboxylic acid/Krebs cycle.
In addition to known effects in energy-generating pathways, some studies focusing on energy restriction observed lowered concentrations of tyrosine, lactate, and glycoprotein (49), in addition to lower circulating concentrations of choline and carnitine, as they were associated with visceral adiposity (52). As obesity is associated with low-grade chronic inflammation (34), energy restriction has been shown to lower the inflammatory markers high-sensitivity C-reactive protein (hsCRP), IL-6, TNF-α (50), and oxylipins (34), although their concentrations were still affected by the fatty acid content in the diet (11, 37). Additionally, energy restriction increased adenine and urea but lowered xanthine and ornithine (14, 42), revealing the possibility of perturbed purine catabolism and a higher ornithine turnover in the urea cycle. These observations could also indicate that energy restriction may ameliorate the obesity-related impaired metabolisms of fatty acids and amino acids, and reversibly affect the inflammation and oxidation processes (14).
Since obese individuals seemed to have lower metabolic flexibility than their lean counterparts (Table 1), especially in the adaptation to β-oxidation and inflammation (18), they seemed to improve both processes more substantially following a weight-loss intervention compared with their lean counterparts (16) (Figure 3B). Also, energy restriction reduced the profile gap between obese and lean individuals (e.g., in the TCA cycle intermediates), besides increased ketone bodies and conjugated bile acids and decreased amino acids, threitol, glycerol, xanthine, and PUFAs (14). Following a weight-loss program, some metabolites exclusively changed in participants with less favorable phenotypes at baseline, such as reduced acylcarnitines, glycine, serine, dimethylarginine, kynurenine, and increased lysoPC (6). Some metabolites related to energy metabolisms also predicted a more significant weight loss in some participants (responders) than in their counterparts (nonresponders) (6, 49) (Table 2). The improvement in these metabolic processes, however, did not directly correspond to a more significant weight loss in obese individuals (6), which implies that weight loss may occur more slowly than the improvement in physiological functions or involve functions other than the known improved processes. Most of these studies, however, were conducted in the short term (maximum: 8 wks), which suggests the need for a prolonged intervention to investigate if the metabolic shift remains.
TABLE 2.
Differences in blood metabolites between responders and nonresponders at baseline and in response to weight-loss interventions1
| Intervention and metabolites | Phenotypes | ||
|---|---|---|---|
| Nonresponders | Responders | Reference | |
| Baseline | (49) | ||
| Ketone bodies | ↑ | ↑↑ | |
| Lactate | ↑ | ↑↑ | |
| Serum lipids | ↑↑ | ↑ | |
| Mixed-meal challenge | (6) | ||
| Glucose (AUC) | ↑ | ↑↑ | |
| Insulin (AUC) | ↑ | ↑↑ | |
| Free fatty acids | ↑ | ↑↑ | |
| 3-Hydroxybutyrate | ↑ | ↑↑ | |
| Ratio of medium- to long-chain acylcarnitines | ↑ | ↑↑ | |
Responders were defined as individuals who had greater weight loss than the nonresponders. AUC: area under the curve. ↑↑, higher increment than ↑.
Other dietary components
When one particular element is investigated, the replacement diet is usually maintained as isocaloric. However, this replacement may alter the overall composition of the habitual diet, total energy intake, or even introduce an additional component. As an example, replacing meat protein by a plant-based protein would likely provide additional fiber and plant phytochemicals to the diet. Therefore, one should keep in mind that metabolic profiling would detect the contribution of not only the modified factors but also other factors during the intervention, highlighting the importance of measuring the background diet before and throughout the intervention period.
Among the included articles, the investigated dietary components are, for example, food products rich in polyphenols, including soy, cranberry, bilberry, dark chocolate, and tea (10, 18, 19, 21, 25, 26, 53, 54). The consumption of these products seems to promote energy generation by glycolysis, TCA cycle, lipid transport, and lipolysis, which may provide a mechanistic explanation behind the health claim of polyphenols to combat metabolic disruption. Interestingly, the polyphenol intake also seems to increase circulating concentrations of nucleosides and microbial metabolites, in addition to reducing amino acids and their derivatives (10, 18, 19, 21, 25, 26, 53, 54). Considering the role of these metabolites in normal homeostasis, the biological implication of how dietary polyphenols affect, for example, nucleosides, amino acids, and their derivatives, remains to be elucidated.
Dietary patterns
Regardless of how beneficial a single component can be, eventually we do not eat only one type of food throughout the day. Therefore, how each dietary component comprises the daily intake, and as a habitual pattern, is one of the most important contributors to the metabotype. Within a dietary pattern, a dietary component interacts with the food matrix, other diet components, the gut microbial community shaped by the habitual dietary patterns, or other individual factors. Therefore, the dietary responses reflected in the metabolic profiles have a higher complexity because they are not dependent solely on a single component but reflect the net responses of all the interactions. Since most metabolomics studies on dietary patterns reported the changes of metabolites that are directly derived from the diet, which are not the focus of this current article, the numbers of studies included in this section are limited. Here, we discuss both inclusion of dietary components with expected beneficial properties, such as dietary patterns rich in phytosterol, and the combination of various elements such as the Mediterranean or Nordic diet.
One important component of both Mediterranean and Nordic diets is whole-grain cereals. Whole grains could affect the abundances of some methyl donors, microbial metabolites, and some metabolites involved in lipid metabolism (55–57). In addition, a whole-grain–rich diet increased circulating lysine, arginine, tyrosine, PC (16:0/16:1), propionylcarnitine, cortisol, creatinine, ornithine, N-acetylspermidine (57), dimethylsulfone, and hypoxanthine (55, 56). In 2 studies with a high-fiber content from rye and oats (40, 58), 1 study proposed the microbial metabolites 2,6-dihydroxybenzoic acid and 2-aminophenol sulfate as intake biomarkers of fiber (58), but it was not confirmed in the other study with an optimized Nordic diet (40). This observation hence shows the potential of whole grains to directly affect methyl and lipid metabolism as well as gut microbiota due to their betaine and fiber contents, along with the indirect effect on the metabolism of amino acids and nitrogen bases, as well as the gut–brain axis via cortisol.
The consumption of a phytosterol-rich diet, including whole-grain cereals, vegetable oils, sesame seeds, and almonds, has been shown to modulate, for example, cholesterol metabolism by lowering its absorption and promoting its excretion via feces (59). Regardless of the high phytosterol consumption in the intervention group compared with the control, it is unclear if the observed effect was solely due to the phytosterol or other dietary components in the diet.
Unfortunately, the studies on the Mediterranean diet that were found after the literature search mostly investigated urine metabolome or biochemical conversion of the phytochemicals in the human body, which were not covered in this review. The only included study observed how the consumption of mixed nuts reduced decanedioic acid (C10 dicarboxylic) and increased dodecanedioic acid (C12 dicarboxylic) and urolithin A glucuronide, a microbial metabolite that was associated with lower abdominal adiposity and better glycemic control (20).
Food-processing methods
Last, food-preparation processes may also affect the chemical composition of the food by introducing new compounds or converting one compound to another. In an intervention study with herring, the baked herring raised plasma concentrations of aspartic acid, creatinine, leucine, and norleucine, whereas pickled herring elevated plasma concentrations of benzoic acid, hippuric acid, isomaltose, and N-acetylmannosamine (41). Fermentation is also included in this section, as the microbial activity can introduce microbial-derived metabolites that may not be present in nonfermented food. Pimentel and colleagues (60) reported smaller-size metabolites in the serum metabolome after the consumption of yogurt than after milk, suggesting microbial catabolic activity during the fermentation. It is noteworthy that the differences in metabolic profile were not only restricted to the metabolite content of the dairy products but also due to the alteration of endogenous metabolic pathways (60), drawing more attention to food processing in the impact assessment of any dietary intervention.
Dietary Responses Reflected by Compound Classes or Respective Metabolic Pathways
Considering the complexity of human metabolic pathways, we classify dietary responses based on the compound classes or the respective metabolic pathways. Also, how various dietary interventions may affect microbial metabolites will be exemplified. A summary of the dietary responses following the given intervention for each compound class is provided in Table 3, and a detailed list of reported studies and compounds is presented in Supplemental Table 1.
TABLE 3.
Summary of dietary responses towards a given intervention for each class of compound or respective metabolic pathways1
| Compound classes/pathways | Side chains | Soy | Dairy | Meat Protein | High-fat diet | Energy overload | Energy restriction | Whole-grain–rich diet | Polyphenol-rich diet2 | Fish (oil)–rich diet | Health-optimized diet3 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (Lyso)PE/PC4 | ETE (20:3), AA (20:4) | — | — | — | — | — | — | — | — | — | ↓ (38) |
| (Lyso)PE/PC4 | EPA (20:5), DHA (22:6) | — | — | — | ↓ (39) | — | — | — | — | ↑ (11, 56, 57, 59) | ↑ (38) |
| (Lyso)PC | MCFAs | ↓ (30, 65) | ↑ (30) | — | ↑ (39) | — | — | — | — | — | ↓ (38) |
| (Lyso)PC | OCFAs | ↓ (30, 65) | — | — | — | — | — | — | — | — | ↑ (38) |
| FFAs | SCFAs | — | — | — | — | ↓ (12) | — | — | ↑ (53, 65) | — | — |
| FFAs | MCFAs | — | ↑ (35) | — | — | ↑ (47) | ↓ (49) | — | — | — | — |
| FFAs | Furan fatty acids | — | — | — | — | — | — | — | — | ↑ (11, 56, 57, 59) | ↑ (38) |
| FFAs | LC-SFAs | — | — | — | — | ↑ (47) | ↓ (14, 49) | — | — | — | — |
| FFAs | MUFAs | — | — | — | — | ↑ (47) | ↓ (14, 49, 64) | — | ↓ (34) | — | — |
| FFAs | PUFAs | — | — | — | ↓ (39) | ↑ (47) | ↓ (14, 49, 64) | — | — | ↑ (11, 35, 42, 56, 57, 59) | ↑ (38) |
| Oxylipins | LA (18:2), ALA (18:3), DGLA (20:3), AA derivatives | — | — | — | — | — | ↓ (52) | — | — | ↓ (11, 35) | — |
| Oxylipins | EPA, DHA derivatives | — | — | — | — | — | ↓ (52) | — | — | ↑ (11, 35) | — |
| Acylcarnitines | SCFAs | ↓ (65) | ↑ (42) | — | ↓ (39) | — | ↓ (6) | ↑ (57) | ↑ (21) | — | ↑ (38) |
| Acylcarnitines | MCFAs | ↓ (65) | — | — | — | ↑ (6) | ↓ (6) | — | ↓ (21) | — | ↑ (38) |
| Acylcarnitines | LCFAs | ↓ (65) | — | — | — | — | ↓ (6) | — | ↑ (34) | — | ↓ (38) |
| Lipolysis | Ketone bodies | ↑ (33) | — | — | — | ↓ (12) | ↑ (14, 48) | ↑ (56) | ↑ (33, 53) | — | — |
| Sterols | Cholesterol absorption | — | — | — | — | ↑ (47) | — | — | — | — | ↑ (59) |
| Sterols | Cholesterol synthesis | — | — | — | — | ↓ (47) | — | — | — | — | — |
| Cholesterol esters | n–3 PUFAs | — | — | — | — | — | — | — | — | ↑ (11) | — |
| Cholesterol esters | n–6 PUFAs | — | — | — | — | — | — | — | — | ↓ (11) | — |
| Cholesterol esters | Other cholesterol esters | — | — | — | — | — | — | — | ↑ (34) | — | — |
| Bile acids | Cholate and deoxycholate | — | — | — | — | ↓ (12) | — | — | — | — | — |
| Bile acids | Other bile acids | — | ↑ (60) | — | — | ↑ (12) | ↑ (14) | — | — | — | — |
| Methyl donors | Trimethylamine | — | — | — | — | ↑ (12) | ↓ (48, 51) | — | — | — | — |
| Methyl donors | Other methylamines | — | — | — | — | ↓ (12) | ↓ (51) | — | ↑ (10) | — | — |
| Methyl donors | Betaines | ↑ (65) | — | — | ↓ (39) | ↑ (12) | — | ↑ (55,57) | — | — | — |
| Methyl donors | Dimethylglycine | — | — | — | — | ↓ (12) | — | ↑ (55, 56) | — | — | — |
| Homocysteine metabolism | Homocysteine, (di)methylarginine | — | — | — | — | ↓ (12) | ↓ (6) | ↓ (56) | — | — | — |
| Sugars | Glucose | — | — | — | ↑ (66) | ↑ (12) | ↓ (48, 50) | — | ↓ (53) | — | — |
| Sugars | Other hexoses | — | — | — | — | — | ↑ (14) | — | — | ↑ (40) | — |
| Sugars | Pentoses | — | — | — | — | ↓ (12) | ↑ (14) | — | ↑ (33) | — | — |
| Sugars | Sugar alcohols | — | — | — | — | ↓ (12) | ↓ (14) | — | ↓ (34) | — | — |
| Glycolysis | — | — | — | — | ↑ (12) | ↑ (14) | — | ↑ (34, 53) | — | — | |
| TCA cycle | — | — | — | — | ↓ (12) | ↓ (14) | — | ↑ (18, 34) | — | — | |
| Amino acids | Essential | ↓ (65) | ↑ (42, 60) | — | — | ↓ (12) | ↓ (14) | ↑ (57) | ↓ (21, 34) | — | — |
| Amino acids | Nonessential | ↓ (65) | ↑ (42, 60) | — | — | ↓ (12) | ↓ (6, 14, 48) | ↑ (57) | ↓ (10, 34) | — | — |
| Amino acids | BCAAs | ↓ (65) | ↑ (42) | ↑ (40) | — | ↓ (12) | ↓ (6, 14, 49) | ↓ (10) | ↓ (34) | ↓ (42) | — |
| Amino acids | Nonproteogenic | — | ↑ (42, 60) | ↑ (40) | — | ↓ (12) | ↑ (14) | — | ↓ (34) | ↑ (42) | — |
| Amino acid derivatives | Serotonins | — | — | — | — | ↓ (12) | ↓ (14) | — | — | — | — |
| Amino acid derivatives | Other tryptophan metabolites | — | — | — | — | ↓ (12) | ↓ (6, 41) | — | ↑ (18) | ↑ (42) | — |
| Amino acid derivatives | Tyrosine metabolites | — | — | — | — | — | ↑ (14) | — | ↑ (10, 34) | — | — |
| Amino acid derivatives | Others | ↑ (65) | ↑ (42) | ↑ (40, 42) | — | ↓ (12) | ↓ (14) | ↑ (57) | ↓ (18, 34) | — | — |
| Urea cycle | — | — | ↑ (40) | ↓ (39) | ↑ (12) | ↑ (14, 41) | ↑ (57) | ↑ (34) | ↑ (42) | — | |
| Nucleosides | Pyrimidines | — | — | — | — | ↓ (12) | — | — | ↑ (34) | — | — |
| Nucleosides | Purines | — | — | — | — | ↓ (12) | ↑ (14) | — | — | — | — |
| Nucleosides | Purine catabolites | ↓ (65) | — | — | — | ↓ (12) | ↓ (14) | ↑ (57) | ↑ (33) | — | — |
| B vitamins | — | — | — | — | ↓ (12) | ↓ (14) | — | — | — | — | |
| Microbial metabolites | Hippuric acid and derivatives | — | — | — | — | ↑ (12) | — | ↑ (57) | ↑ (18, 19, 33, 34, 54, 57) | — | ↑ (57) |
| Microbial metabolites | Indoles | — | ↑ (60) | — | — | ↓ (12) | — | — | ↓ (34) | — | ↑ (38) |
| Microbial metabolites | Other microbial metabolites | — | — | — | — | — | ↑ (14) | ↑ (56) | ↑ (18) | — | — |
| Catechols | — | — | — | — | — | — | ↑ (57) | ↑ (18, 33, 34) | — | ↑ (38, 57) |
Numbers in the parentheses indicate references. AA, arachidonic acid; ALA, ɑ-linolenic acid; BCAA, branched-chain amino acid; DGLA, di-homo-γ-linolenic acid; ETE, eicosatrienoic acid; LA, linoleic acid; LC-SFA, long-chain saturated fatty acid; MCFA, medium-chain fatty acid; OCFA, odd-chain fatty acid; PC, phosphatidylcholine; PE, phosphatidylethanolamine; TCA cycle, tricarboxylic acid cycle; ↑, higher levels; ↓, lower levels.
Polyphenol-rich diet included black tea (53), green tea (25, 26, 53), black soybean (67), dark chocolate (10, 21), blueberry (25), cranberry (18, 54), and bilberry (19).
The health-optimized diet consisted of dietary pattern rich in phytosterols (59), fatty fish, whole grains, bilberries (57), and prebiotic, fiber, low-glycemic-impact meal, and food with blood-lipid–lowering properties (40).
Phospholipids: PC or PE, including plasmalogens.
Lipophilic molecules
The lipophilic molecules in the blood vary greatly based on the molecular structures or biological functions, amounting to up to thousands of molecules with varying acyl chains, location of double bonds, head group, etc. With the emerging lipidomics platforms, we have only started to understand how diet affects this lipid composition and further indicate dietary responses. Due to the immense variation in lipophilic molecules, we focus our discussion on several reported classes in the included studies, as follows: phospholipids, free fatty acids and their oxidation products (oxylipins), steroids, and carnitines.
Phospholipids
Phospholipids are a versatile class of metabolites as they consist of a large group of molecules varying according to their polar head group and fatty acid composition at the sn-1 and sn-2 position. Based on the head group, phospholipids consist of 6 major classes: PC, PE, phosphatidylinositol (PI), phosphatidylserine (PS), phosphatidic acid (PA), and phosphatidylglycerol (PG) (61). The differences in analytical platforms, with their unique technical advantages and resolution, may allow the identification of certain classes or acyl side chains in one chosen platform while restricting the characterization of others (62). Also, the inconsistent nomenclature of phospholipids sets particular challenges in concluding the behavior of fatty acids and the phospholipid carriers. Thus, we only highlight some aspects that deserve further attention.
Among many critical biological roles including cell and organelle membrane components, phospholipids also act as a versatile reservoir for fatty acids, which can be readily converted to other molecules with their specific functions, for example, prostaglandins, leukotrienes, thromboxanes, or platelet-activating factor (62). Compared with the classes, the acyl side chains of phospholipids seem more flexible as they quickly adapt to various exposures, including diet. The classical example is how the consumption of fish rich in omega-3 fatty acid increased EPA- and DHA-containing phospholipids and decreased a phospholipid containing AA (20:4) (36).
Besides reflecting the consumption of a specific fatty acid, phospholipids with various acyl side chains have also been associated with risk factors for cardiovascular diseases (CVD) (12). Phospholipids and plasmalogens with eicosatetraenoic acid (20:3) and AA side chains had a positive association, whereas those with EPA (20:5) and DHA (22:6) side chains had a negative association with blood cholesterol and other cardiovascular risk predictors, such as BMI and insulin (12). PCs with 2 acyl chains reflected a higher risk of T2D, whereas PCs with 1 acyl chain (lysoPCs) or those with OCFAs were more prevalent in those who remained free of T2D (63). As phospholipids were associated not only with cardiometabolic risk factors but also with diet (63), they may hold a potential role in mediating or moderating the effect of diet on cardiometabolic health.
Among phospholipids, plasmalogens have received less attention. In these phospholipids, the polar heads are bound to acyl side chains with ether bonds, instead of more commonly found ester bonds. Plasmalogens were shown to protect against lipid peroxidation in vitro (64). Several members of PC(O) and PE(P) have been reported to increase following the consumption of a dairy-rich breakfast and decrease after a soy-rich breakfast and dark chocolate (21, 32), although the composition of the acyl chains was not defined in the latter study (21). Moreover, it is unclear if the changes in the abundance of plasmalogens were related to the fatty acyl chains or the plasmalogen class, in general, or if there was a direct link between the intervention and the suggested protective effect. Therefore, more studies on plasmalogens are encouraged to comprehend their biological roles in the human body.
Free fatty acids and oxylipins
MCFAs seem to be quite responsive to various dietary exposures, in addition to a diet that contains a specific source of fatty acids, such as canola, flax, or fish oil (37, 38). For example, substituting cooking oil rich in linoleic acid (18:2) by one rich in oleic acid (18:1) decreased the concentration of palmitic acid (16:0), which may indicate activated biosynthesis of unsaturated fatty acids (38). Total energy intake would also affect the concentrations of MCFAs (14). Both myristic (14:0) and palmitic acids increased following additional fat intake (48) but decreased after energy restriction (48, 50, 68).
Another important category to discuss is OCFAs. OCFAs have been previously associated with a lower risk of T2D (69, 70). Besides being recognized as intake biomarkers of dairy products (71), OCFAs could also increase after the consumption of nuts, seeds, fruits, and vegetables (58). The endogenous production of OCFAs has also been proposed, as propionyl-CoA competed with acetyl-CoA for de novo lipogenesis after intake of inulin and its microbial degradation product, propionate, increasing the concentrations of pentadecanoic acid (15:0) and heptadecanoic acid (17:0) (72). The OCFAs were not distinctively reported as aliphatic or branched-chain fatty acids, which may include the possibility of those OCFA as branched-chain fatty acids. A recent study showed an endogenous elongation of branched-chain fatty acids following the conversion of branched-chain amino acids (BCAAs) in vitro and in vivo (65), which may represent a new link between OCFAs with BCAAs and lipid metabolism. Further studies to investigate the causal relation between diet, OCFA abundance, lipid metabolism, and metabolic health are required.
Steroids
This compound class includes sterols, bile acids, and cholesterol esters. Despite the lack of results within the included studies for this compound class, cholesterol esters seem to be affected mainly by fatty acid content in the diet (11). An acute challenge of glucose or fat triggered cholesterol absorption and inhibited the synthesis of cholesterol and primary bile acids (12, 48). The same effect was observed in the fasted state following an optimized dietary pattern rich in phytosterol (59), which emphasizes the consideration of sampling time in results interpretation.
Lipid transport and lipolysis
The dietary response of carnitine, the transport molecule of fatty acids into mitochondria for β-oxidation, is not that conclusive due to the wide variation between studies (12, 33, 52). The bound forms (acylcarnitines) increased during the fed state and decreased during the fasted state (6). Energy restriction seems to lower acylcarnitines and elevate ketone bodies (6, 14, 49), showing stimulation of lipolysis and ketogenesis as in the fasted state. The fasting-like profile could also be observed after the consumption of black soybean peptide (25, 67), whereas other dietary patterns with expected health benefits did not always give consistent results (14, 21, 33, 40, 67).
Based on the acyl side chains, the behavior of acylcarnitines after dietary challenges has been inconsistent. The optimized Nordic diet seemed to uniformly increase fasting medium-chain acylcarnitines [carnitine (10:2), (13:0), (13:1), (14:2)] that were inversely associated with traditional risk factors of CVD, whereas acylcarnitine with longer chains, such as palmitate (16:0) and stearate (18:0) tended to decrease (40). A polyphenol-rich diet seemed to stimulate lipolysis, as indicated by lowered acylcarnitines containing MCFAs, but at the same time, also increased acylcarnitines with short- and long-chain fatty acids (21, 25, 26, 53).
Sugars, glycolysis, and TCA cycle
Blood sugar is one of the most widely applied measurements to indicate glycemic function with well-established relations with relevant pathways such as glycolysis and gluconeogenesis. Interestingly, elevated blood glucose or any other hexose did not always trigger glycolysis and the TCA cycle. As an example, glucose and glycolysis-associated metabolites increased following an OGTT, but TCA intermediates decreased (12). As expected, fasting or energy restriction lowered glucose but increased other hexoses and pentoses, which triggered glycolysis and reduced TCA intermediates (14, 49, 51). A polyphenol-rich diet, conversely, seems to stimulate both glycolysis and the TCA cycle despite the lower glucose concentration (18, 26, 53). The biological explanation behind these observations remains to be elucidated.
Blood sugars are also affected by other dietary factors, such as increased rhamnose after herring intake as well as isomaltose and lactulose after beef intake (41). Blood glucose decreased following vitamin D supplementation, but only in the responsive group with lower 25-hydroxyvitamin D and higher adiponectin and resistin at baseline (29). These findings highlight, once again, the importance of metabolic profiling to give a complete picture of physiological responses to dietary intervention.
Methyl donors
The metabolites included in this class are choline, betaines, other trimethylamines, and dimethylglycine. Choline is an essential nutrient whose amount is dependent on the dietary source (73), while the metabolism of trimethylamines involves the contribution of gut microbiota (74, 75). Glycine betaine, however, can be supplied by food or derived from choline, as a choline-rich diet can increase glycine betaine independently of gut microbiota (73). Whole-grain products, especially rye, are rich in betaines. Expectedly, dietary patterns containing whole-grain products elevated N,N-dimethylglycine and fasting plasma concentrations of betaines, such as glycine betaine, pipecolic acid betaine, and γ-butyrobetaine (55–57). The diminished homocysteine concentration (55–57) in parallel may suggest how increased betaines promote methyl transfer by betaine-homocysteine S-methyltransferase to homocysteine, yielding methionine and N,N-dimethylglycine, which may imply the potential of whole-grain products to lower CVD risk by restoring homocysteine metabolism. Unfortunately, it is still unclear whether this effect relies on dietary betaines, the involvement of gut microbiota, endogenous modulation of methyl metabolism, or the interaction of those 3. Besides whole-grain products, another dietary item that has been shown to increase betaines is black soybean peptide (67).
Besides dietary sources, metabolic challenges seem to affect the circulating concentrations of methyl donors. An OGTT triggered choline and glycine betaine but suppressed trimethylamine (12). Lower choline and glycine betaine were observed after energy restriction (49, 52) and a lipid-rich overfeeding (33), respectively. The fasted state, meanwhile, seems to restrain these methyl donors, regardless of the intervention. These observations hence may imply how the dynamic between fed–fasted states is as crucial as energy-deficit surplus in the modulation of methyl donors and respective methyl-dependent pathways.
It is noteworthy that not all betaines may be beneficial or promote a favorable impact. The higher concentration of arsenobetaine after cod intake was most likely due to the fish's adaptation to arsenic contamination in the water (43).
Amino acids and their derivatives
As mentioned earlier, the tight regulation of protein metabolism ensures almost balanced fluxes of nitrogen in the body. Different sources of dietary protein may still, nevertheless, increase a specific pool of amino acids in the circulation. For example, the concentrations of some amino acids seem to increase by dairy or meat intake (41, 43, 60) but diminish by soy protein intake (67), and other nonprotein-based interventions, including metabolic challenge (12), energy restriction (6, 14, 49, 50), or consumption of food containing bioactive compounds, such as green tea or dark chocolate (10, 21, 26). Exceptions included a whole-grain–rich Nordic diet that increased both essential and nonessential amino acid pools (57) but reduced BCAAs (55). BCAA concentrations have been reported as a risk predictor of T2D (70), although some studies also reported a neutral or favorable association with improvement in insulin sensitivity (76). The gut microbiota has been proposed to modulate the insulin sensitivity by enhancing the microbial BCAA biosynthesis and restricting BCAA transport, which increased circulating BCAAs in insulin-resistant individuals (77).
One of the ways to maintain the balance of amino acids in the body is by tight regulation of (muscle) protein decomposition to urea and creatinine (78). The intake of animal protein (herring, beef, and fish) also similarly increased the pool of creatine and creatinine (41, 43, 79) as the breakdown products of muscle protein. Interestingly, the same effect was demonstrated by other foods with expected health outcomes, such as whole grain or green tea (26, 57), which may suggest enhanced protein turnover despite the limited evidence on the biological implication.
The nonproteogenic amino acids are those that do not constitute structural proteins, including citrulline, β-alanine, norleucine, 2-aminobutyrate, theanine, 4-acetamido-2-aminobutanoic acid, and taurine (Supplemental Table 1). Various dietary interventions have affected the members of this group differently. Their concentrations seem to decrease after an OGTT (12) and increase by the intake of animal-based protein (41, 43, 60) or a very-low-carbohydrate diet (14). Furthermore, the same food can both increase or decrease metabolites in this group—for example, yogurt consumption led to increased citrulline but decreased taurine (60). Nonetheless, the balance of these nonproteogenic amino acids may provide valuable information about how a dietary intervention may activate or suppress particular pathways, independently of the protein content. For example, the elevated γ-glutamylated amino acids after intake of casein and whey (43) may imply a higher activity of γ-glutamyl transferase after consumption of dairy protein.
Microbial metabolites
Due to their abundant genetic and metabolic attributes, gut microbial species partake in many biochemical conversions. Microbial metabolites do not seem to have a similar behavior following dietary interventions, which makes sense due to their great diversity (Table 3). Moreover, not all microbial metabolites can be found in the circulation because some microbial metabolites are used within the digestive system or carried into feces. Nevertheless, almost all types of dietary intervention could affect microbial metabolites. Some examples presented here include secondary bile acids, SCFAs, and indoles (80).
Secondary bile acids are conversion products of primary bile acids by the gut microbiota. Included in this group are deoxycholic and lithocholic acid, as well as their conjugates of taurine and glycine. They have been reported to increase following various interventions, including OGTT, yogurt, acidified milk, and wheat flour fortified with vitamin B and minerals, with the exception of lower deoxycholate concentrations after OGTT (12, 31).
Meanwhile, free SCFAs vary across molecules due to differences in utilization in the body. Butyrate is usually undetectable in the blood circulation due to its rapid usage (81). Hence, the undetectable concentration in circulation does not necessarily indicate an absence of SCFA fermentation in the gut. The other SCFAs, however, can be found in the circulation and seem to be affected by various interventions. Propionate decreased after an OGTT (12) but increased after the consumption of cranberry juice (18) and green tea (53). Acetate, conversely, decreased following consumption of dark chocolate (10) and black tea (53). When SCFA is bound to carnitine, a broad range of diets affected its abundance, ranging from a soy-, dairy-, polyphenol-, or fat-rich diet, to energy restriction, to a Nordic diet (6, 21, 31, 33, 40, 43, 57).
Indoles are microbial metabolites of tryptophan, despite their possible availability in dietary sources (82), especially in fermented food products. Molecules with this structure include tryptamine, indoxyl sulfate, and other indole-containing metabolites, such as indolepropionic acid. Indolepropionic acid has been shown to increase following an optimized Nordic diet and acidified milk, but decrease after an OGTT (12, 40, 60). Similarly, the circulating concentrations of hippuric acid and its hydroxylated forms have increased due to a healthy lifestyle, such as exercise and consumption of berries in the Nordic diet, B-carotene, or polyphenol-rich food (25, 30, 57, 79). Overall, these examples suggest how various dietary interventions can modulate the gut microbial community and the metabolites.
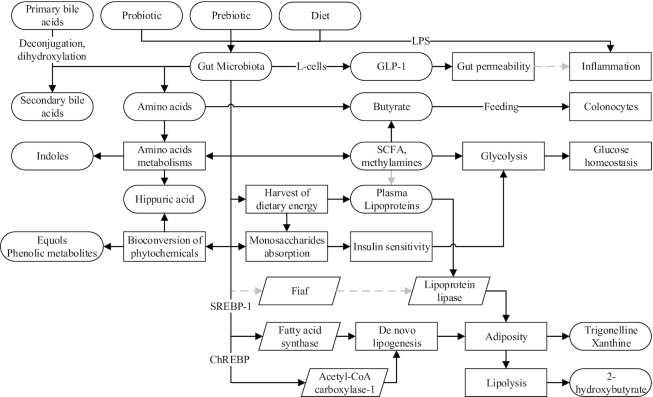
Although causality cannot be directly inferred, several microbial metabolites have been associated with host phenotypes, including the differences in dietary responses and susceptibility of metabolic diseases. Both indolepropionic and hippuric acids have been associated with better insulin secretion and a lower risk of T2D (55, 83). Baseline hippurate significantly predicted the postprandial response for 2 h after an OGTT (12). It is yet unclear if the risk reduction was attributable to the reduction of BCAAs, hippuric acid and other microbial metabolites (56, 57), or other microbial activity. Along with hippuric acid, a lower concentration of other microbial metabolites, such as trigonelline and xanthine, and a higher concentration of 2-hydroxy-isobutyrate have been observed in obese persons as compared with their lean counterparts (84). These discrepancies in metabolite concentrations were restored after bariatric surgery–induced weight loss (52). Fecal acetate and propionate have also been shown to predict the extent of weight loss (49), showing the possible role of the gut microbiota in the modulation of net energy intake, glycemic regulation, and risk of metabolic diseases. The roles of the gut microbiota in various metabolic pathways shared with the host as well as the intermediary metabolites are depicted in Figure 4.
FIGURE 4.
How the gut microbiota, modified by diet or other factors, may potentially modulate host metabolic pathways and blood metabolites, as suggested by the current literature. Solid black lines/arrows indicate stimulation; dashed gray lines/arrows indicate inhibition. ChREBP, carbohydrate-response element-binding protein; Fiaf, fasting-induced adipose factor; GLP-1, glucagon-like peptide-1; SREBP-1, sterol regulatory element-binding protein-1.
Opportunities and Challenges
Due to the increased sensitivity and accuracy of technologies used to profile metabolites in the circulation, we have more possibilities than ever to maximize our understanding related to diet and its effect on health. This capacity enables a meticulous assessment of the physiological impact of food, not only as an individual component but also in interaction with other parts of the habitual dietary pattern. However, the assessment should also take into account each of the multistep processes involved in food processing and metabolism. These processes begin with how the food is prepared, sensory stimulation during serving, followed by mechanical and enzymatic digestion in the mouth as a trigger for further metabolic processes, which are largely neglected at this time. The digestion and absorption of nutrients, along with other diet-derived compounds in the intestine, involve the contribution of other metabolic organs. In the colon, the role of the gut microbiota with its abundant metabolic properties deserves more attention. Understanding the metabolic properties of the gut microbiota, however, is challenging due to the shared metabolic functions between various microbial compositions (4) and their potential roles as the actor, mediator, and object in the interplay of dietary components, food matrix, and endogenous metabolism, along with other intrinsic factors. Therefore, analyzing the circulating metabolites could be more practical than determining the composition of bacterial diversity in understanding the metabolic roles of the gut microbiota. Additionally, the secondary metabolism in the liver and cross-talk between the metabolic organs affected by individual factors may also be reflected in the circulation, although the local utilization of some metabolites may restrict the detection of such metabolites in the blood (85). Taken together, each of the multistep processes within the framework of absorption, digestion, metabolism, and excretion of the food is important to fully comprehend how endogenous metabolites in the circulation may reflect the physiological effects of a diet.
To analyze the metabolic profiles of endogenous metabolites, researchers all over the globe use different platforms with their technical advantages. The methodological advantages of each analytical platform should bridge the gap that cannot be filled by a single tool. Therefore, conformity to standard guidelines in metabolomics workflow and reporting (86) is a prerequisite to ensure the accurate dissemination of information and reproducibility of results.
To obtain a complete overview of the biological mechanisms, however, conformity to standard metabolomics guidelines is not enough. To date, the application of metabolomics approaches in nutrition studies mainly aims to obtain a metabolic signature of a specific condition, to underline altered metabolic pathways by a particular condition, or to understand the biotransformation of bioactive metabolites. After the discovery stage, the metabolites of interest could be mapped to respective metabolic pathways using available tools (e.g., Virtual Metabolic Human Map) (87). Further efforts would then be required to merge the biochemical conversion pathways of the diet-derived metabolites, the metabolic capacity of the gut microbiota, as well as the interaction with other individual factors, for example, via lifestyle-driven epigenetics modification or gene expression modulation, to understand the biological mechanisms. To confirm the findings and further elucidate the underlying mechanism, collaboration with other research groups focusing on basic research is therefore essential. The integration of multi-omics platforms would then provide better insight and reveal the mechanisms linking diet and health optimization or disease prevention.
Conclusions
We have summarized the findings in the past decade on diet-induced alterations in blood endogenous metabolic profiles, which shape distinct metabotypes. Different methodological approaches were compiled, not compared, regardless of their technical advantages, demonstrating both the complementary capacity and challenges in merging information to generate hypotheses about diet-modifiable metabolic pathways. Despite variations in the analytical platform, terminology, or the resolution of the detection, lipids still appear as one of the most versatile endogenous metabolites affected by various dietary interventions. The metabolites’ interconnectivity shows how one intervention could affect endogenous metabolites from other seemingly less relevant pathways, as shown by how various protein interventions affected lipid metabolisms, or how energy overload or restriction could affect not only energy generation but also metabolism of methyl donors and lipid-mediated inflammation. We also have highlighted the vital contribution of the gut microbiota in this interaction, as evidenced by the enormous variation in microbial metabolites affected by dietary interventions, as well as their associations with the host phenotypes. Therefore, a thorough investigation of endogenous metabolites with their relevant biochemical conversions within the body seems necessary to obtain a comprehensive understanding of how these metabolites could mediate the role of diet in disease prevention or health maintenance. The combination of these results with other omics-based methodologies and with a strong understanding of basic physiology is a prerequisite to translate the findings and to elucidate the underlying mechanisms between nutrition and health. With this foundation, the common goal of a personalized approach for health and well-being seems feasible in the near future.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—SN and KH: designed the research; SN: performed the systematic search and wrote the manuscript; KH and MK: critically reviewed the manuscript and had primary responsibilities for final content: and all authors: read and approved the final manuscript.
Notes
SN received funding from the Juho Vainio Foundation, Finnish Cultural Foundation (grant numbers 65171618 and 00180775), and a salary from Kuopio University Hospital (project number VTR 510RA17). KH received funding from the Academy of Finland (grant numbers 277986, 283454, and 305396).
Author disclosures: KH is affiliated with Afekta Technologies, Ltd, which provides metabolomics analysis services. The other authors report no conflicts of interest. The funders had no contribution in study design, data collection, data analysis, preparation of the manuscript, or decision to publish.
Supplemental Table 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances.
Abbreviations used: AA, arachidonic acid; ALEA, α-linolenoyl-ethanolamide; BCAA, branched-chain amino acid; CVD, cardiovascular disease; MCFA, medium-chain fatty acid; OCFA, odd-chain fatty acid; OEA, oleoylethanolamide; OGTT, oral-glucose-tolerance test; PC, phosphatidylcholine; PE, phosphatidylethanolamine; TCA, tricarboxylic acid; T2D, type 2 diabetes.
References
- 1. Holmes E, Wilson ID, Nicholson JK. Metabolic phenotyping in health and disease. Cell. 2008;134(5):714–17. [DOI] [PubMed] [Google Scholar]
- 2. Suarez M, Caimari A, del Bas JM, Arola L. Metabolomics: an emerging tool to evaluate the impact of nutritional and physiological challenges. Trends Anal Chem. 2017;96:79–88. [Google Scholar]
- 3. Zheng H, Yde CC, Clausen MR, Kristensen M, Lorenzen J, Astrup A, Bertram HC. Metabolomics investigation to shed light on cheese as a possible piece in the French paradox puzzle. J Agric Food Chem. 2015;63(10):2830–9. [DOI] [PubMed] [Google Scholar]
- 4. Costea PI, Hildebrand F, Arumugam M, Bäckhed F, Blaser MJ, Bushman FD, De Vos WM, Ehrlich SD, Fraser CM, Hattori M. Enterotypes in the landscape of gut microbial community composition. Nat Microbiol. 2018;3(1):8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zeevi D, Korem T, Zmora N, Israeli D, Rothschild D, Weinberger A, Ben-Yacov O, Lador D, Avnit-Sagi T, Lotan-Pompan M. Personalized nutrition by prediction of glycemic responses. Cell. 2015;163(5):1079–94. [DOI] [PubMed] [Google Scholar]
- 6. Fiamoncini J, Rundle M, Gibbons H, Thomas EL, Geillinger-Kastle K, Bunzel D, Trezzi JP, Kiselova-Kaneva Y, Wopereis S, Wahrheit J et al.. Plasma metabolome analysis identifies distinct human metabotypes in the postprandial state with different susceptibility to weight loss-mediated metabolic improvements. FASEB J. 2018;32(10):5447–58. [DOI] [PubMed] [Google Scholar]
- 7. Guijas C, Montenegro-Burke JR, Warth B, Spilker ME, Siuzdak G. Metabolomics activity screening for identifying metabolites that modulate phenotype. Nat Biotechnol. 2018;36(4):316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu X, Locasale JW. Metabolomics: a primer. Trends Biochem Sci. 2017;42(4):274–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dumas ME, Barton RH, Toye A, Cloarec O, Blancher C, Rothwell A, Fearnside J, Tatoud R, Blanc V, Lindon JC et al.. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proc Natl Acad Sci. 2006;103(33):12511–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Martin F-PJ, Rezzi S, Peré-Trepat E, Kamlage B, Collino S, Leibold E, Kastler JR, Rein D, Fay LB, Kochhar S. Metabolic effects of dark chocolate consumption on energy, gut microbiota, and stress-related metabolism in free-living subjects. J Proteome Res. 2009;8(12):5568–79. [DOI] [PubMed] [Google Scholar]
- 11. Nording ML, Yang J, Georgi K, Karbowski CH, German JB, Weiss RH, Hogg RJ, Trygg J, Hammock BD, Zivkovic AM. Individual variation in lipidomic profiles of healthy subjects in response to omega-3 fatty acids. PLoS One. 2013;8(10):e76575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ho JE, Larson MG, Vasan RS, Ghorbani A, Cheng S, Rhee EP, Florez JC, Clish CB, Gerszten RE, Wang TJ. Metabolite profiles during oral glucose challenge. Diabetes. 2013;62(8):2689–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Armah CN, Traka MH, Dainty JR, Defernez M, Janssens A, Leung W, Doleman JF, Potter JF, Mithen RF. A diet rich in high-glucoraphanin broccoli interacts with genotype to reduce discordance in plasma metabolite profiles by modulating mitochondrial function. Am J Clin Nutr. 2013;98(3):712–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gu Y, Zhao A, Huang F, Zhang Y, Liu J, Wang C, Jia W, Xie G, Jia W. Very low carbohydrate diet significantly alters the serum metabolic profiles in obese subjects. J Proteome Res. 2013;12(12):5801–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444(7122):1022. [DOI] [PubMed] [Google Scholar]
- 16. Marchesi JR, Adams DH, Fava F, Hermes GD, Hirschfield GM, Hold G, Quraishi MN, Kinross J, Smidt H, Tuohy KM. The gut microbiota and host health: a new clinical frontier. Gut. 2016;65(2):330–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bolca S, Van de Wiele T, Possemiers S. Gut metabotypes govern health effects of dietary polyphenols. Curr Opin Biotechnol. 2013;24(2):220–5. [DOI] [PubMed] [Google Scholar]
- 18. Liu H, Garrett TJ, Su Z, Khoo C, Gu L. UHPLC-Q-Orbitrap-HRMS-based global metabolomics reveal metabolome modifications in plasma of young women after cranberry juice consumption. J Nutr Biochem. 2017;45:67–76. [DOI] [PubMed] [Google Scholar]
- 19. de Mello VD, A Lankinen M, Lindström J, Puupponen‐Pimiä R, E Laaksonen D, Pihlajamäki J, Lehtonen M, Uusitupa M, Tuomilehto J, Kolehmainen M et al.. Fasting serum hippuric acid is elevated after bilberry (Vaccinium myrtillus) consumption and associates with improvement of fasting glucose levels and insulin secretion in persons at high risk of developing type 2 diabetes. Mol Nutr Food Res. 2017;61(9):1700019. [DOI] [PubMed] [Google Scholar]
- 20. Mora‐Cubillos X, Tulipani S, Garcia‐Aloy M, Bulló M, Tinahones FJ, Andres‐Lacueva C. Plasma metabolomic biomarkers of mixed nuts exposure inversely correlate with severity of metabolic syndrome. Mol Nutr Food Res. 2015;59(12):2480–90. [DOI] [PubMed] [Google Scholar]
- 21. Martin F-PJ, Montoliu I, Nagy KL, Moco S, Collino S, Guy P, Redeuil K, Scherer M, Rezzi S, Kochhar S. Specific dietary preferences are linked to differing gut microbial metabolic activity in response to dark chocolate intake. J Proteome Res. 2012;11(12):6252–63. [DOI] [PubMed] [Google Scholar]
- 22. Smit S, Szymańska E, Kunz I, Roldan VG, Tilborg MWEM, Weber P, Prudence K, van der Kloet FM, van Duynhoven JPM, Smilde AK et al.. Nutrikinetic modeling reveals order of genistein phase II metabolites appearance in human plasma. Mol Nutr Food Res. 2014;58(11):2111–21. [DOI] [PubMed] [Google Scholar]
- 23. Shanahan F, van Sinderen D, O'Toole PW, Stanton C. Feeding the microbiota: transducer of nutrient signals for the host. Gut. 2017;66(9):1709–17. [DOI] [PubMed] [Google Scholar]
- 24. Zinöcker M, Lindseth I. The Western diet—microbiome-host interaction and its role in metabolic disease. Nutrients. 2018;10(3):365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nieman DC, Gillitt ND, Knab AM, Shanely RA, Pappan KL, Jin F, Lila MA. Influence of a polyphenol-enriched protein powder on exercise-induced inflammation and oxidative stress in athletes: a randomized trial using a metabolomics approach. PLoS One. 2013;8(8):e72215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jacobs DM, Hodgson AB, Randell RK, Mahabir-Jagessar-T K, Garczarek U, Jeukendrup AE, Mela DJ, Lotito S. Metabolic response to decaffeinated green tea extract during rest and moderate-intensity exercise. J Agric Food Chem. 2014;62(40):9936–43. [DOI] [PubMed] [Google Scholar]
- 27. Piazza JR, Almeida DM, Dmitrieva NO, Klein LC. Frontiers in the use of biomarkers of health in research on stress and aging. J Gerontol B Psychol Sci Soc Sci. 2010;65B(5):513–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Read S, Grundy E. Allostatic load—a challenge to measure multisystem physiological dysregulation. NCRM Working Paper 04/12, Pathways Node at NCRM United Kingdom: National Centre for Research Methods; 2012. [Google Scholar]
- 29. O'Sullivan A, Gibney MJ, Connor AO, Mion B, Kaluskar S, Cashman KD, Flynn A, Shanahan F, Brennan L. Biochemical and metabolomic phenotyping in the identification of a vitamin D responsive metabotype for markers of the metabolic syndrome. Mol Nutr Food Res. 2011;55(5):679–90. [DOI] [PubMed] [Google Scholar]
- 30. Mondul AM, Sampson JN, Moore SC, Weinstein SJ, Evans AM, Karoly ED, Virtamo J, Albanes D. Metabolomic profile of response to supplementation with beta-carotene in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study. Am J Clin Nutr. 2013;98(2):488–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jiang Z, Liang Q, Wang Y, Zheng X, Pei L, Zhang T, Wang Y, Luo G. Metabonomic study on women of reproductive age treated with nutritional intervention: screening potential biomarkers related to neural tube defects occurrence. Biomed Chromatogr. 2011;25(7):767–74. [DOI] [PubMed] [Google Scholar]
- 32. Meikle PJ, Barlow CK, Mellett NA, Mundra PA, Bonham MP, Larsen A, Cameron-Smith D, Sinclair A, Nestel PJ, Wong G. Postprandial plasma phospholipids in men are influenced by the source of dietary fat. J Nutr. 2015;145(9):2012–18. [DOI] [PubMed] [Google Scholar]
- 33. Morio B, Comte B, Martin J-F, Chanseaume E, Alligier M, Junot C, Lyan B, Boirie Y, Vidal H, Laville M. Metabolomics reveals differential metabolic adjustments of normal and overweight subjects during overfeeding. Metabolomics. 2015;11(4):920–38. [Google Scholar]
- 34. Möller K, Ostermann AI, Rund K, Thoms S, Blume C, Stahl F, Hahn A, Schebb NH, Schuchardt JP. Influence of weight reduction on blood levels of C-reactive protein, tumor necrosis factor-α, interleukin-6, and oxylipins in obese subjects. Prostaglandins Leukotrienes Essent Fatty Acids. 2016;106:39–49. [DOI] [PubMed] [Google Scholar]
- 35. Chung H, Nettleton JA, Lemaitre RN, Barr RG, Tsai MY, Tracy RP, Siscovick DS. Frequency and type of seafood consumed influence plasma (n-3) fatty acid concentrations. J Nutr. 2008;138(12):2422–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Guertin KA, Moore SC, Sampson JN, Huang WY, Xiao Q, Stolzenberg-Solomon RZ, Sinha R, Cross AJ. Metabolomics in nutritional epidemiology: identifying metabolites associated with diet and quantifying their potential to uncover diet-disease relations in populations. Am J Clin Nutr. 2014;100(1):208–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schuchardt JP, Schmidt S, Kressel G, Willenberg I, Hammock BD, Hahn A, Schebb NH. Modulation of blood oxylipin levels by long-chain omega-3 fatty acid supplementation in hyper-and normolipidemic men. Prostaglandins Leukotrienes Essent Fatty Acids. 2014;90(2):27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jones PJ, Lin L, Gillingham LG, Yang H, Omar JM. Modulation of plasma N-acylethanolamine levels and physiological parameters by dietary fatty acid composition in humans. J Lipid Res. 2014;55(12):2655–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Verme JL, Gaetani S, Fu J, Oveisi F, Burton K, Piomelli D. Regulation of food intake by oleoylethanolamide.Cell Mol Life Sci. 2005;62(6):708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tovar J, de Mello VD, Nilsson A, Johansson M, Paananen J, Lehtonen M, Hanhineva K, Björck I. Reduction in cardiometabolic risk factors by a multifunctional diet is mediated via several branches of metabolism as evidenced by nontargeted metabolite profiling approach. Mol Nutr Food Res. 2017;61(2):1600552. [DOI] [PubMed] [Google Scholar]
- 41. Ross AB, Svelander C, Undeland I, Pinto R, Sandberg AS. Herring and beef meals lead to differences in plasma 2-aminoadipic acid, beta-alanine, 4-hydroxyproline, cetoleic acid, and docosahexaenoic acid concentrations in overweight men. J Nutr. 2015;145(11):2456–63. [DOI] [PubMed] [Google Scholar]
- 42. Poesen R, Mutsaers HAM, Windey K, van den Broek PH, Verweij V, Augustijns P, Kuypers D, Jansen J, Evenepoel P, Verbeke K. The influence of dietary protein intake on mammalian tryptophan and phenolic metabolites. PLoS One. 2015;10(10):e0140820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stanstrup J, Schou SS, Holmer-Jensen J, Hermansen K, Dragsted LO. Whey protein delays gastric emptying and suppresses plasma fatty acids and their metabolites compared to casein, gluten, and fish protein. J Proteome Res. 2014;13(5):2396–408. [DOI] [PubMed] [Google Scholar]
- 44. Karlsson T, Strand E, Dierkes J, Drevon CA, Øyen J, Midttun Ø, Ueland PM, Gudbrandsen OA, Pedersen ER, Nygård O. Associations between intake of fish and n-3 long-chain polyunsaturated fatty acids and plasma metabolites related to the kynurenine pathway in patients with coronary artery disease. Eur J Nutr. 2017;56:261–72. [DOI] [PubMed] [Google Scholar]
- 45. Owe‐Young R, Webster NL, Mukhtar M, Pomerantz RJ, Smythe G, Walker D, Armati PJ, Crowe SM, Brew BJ. Kynurenine pathway metabolism in human blood-brain-barrier cells: implications for immune tolerance & neurotoxicity. J Neurochem. 2008;105(4):1346–57. [DOI] [PubMed] [Google Scholar]
- 46. Gray LR, Tompkins SC, Taylor EB. Regulation of pyruvate metabolism and human disease. Cell Mol Life Sci. 2014;71(14):2577–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. van Ommen B, van den Broek T, de Hoogh I, van Erk M, van Someren E, Rouhani-Rankouhi T, Anthony JC, Hogenelst K, Pasman W, Boorsma A. Systems biology of personalized nutrition. Nutr Rev. 2017;75(8):579–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Irvin MR, Zhi D, Aslibekyan S, Claas SA, Absher DM, Ordovas JM, Tiwari HK, Watkins S, Arnett DK. Genomics of post-prandial lipidomic phenotypes in the Genetics of Lipid lowering Drugs and Diet Network (GOLDN) study. PLoS One. 2014;9(6):e99509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schmedes MS, Yde CC, Svensson U, Håkansson J, Baby S, Bertram HC. Impact of a 6-week very low-calorie diet and weight reduction on the serum and fecal metabolome of overweight subjects. Eur Food Res Technol. 2015;240(3):583–94. [Google Scholar]
- 50. Perez-Cornago A, Brennan L, Ibero-Baraibar I, Hermsdorff HHM, O'Gorman A, Zulet MA, Martínez JA. Metabolomics identifies changes in fatty acid and amino acid profiles in serum of overweight older adults following a weight loss intervention. J Physiol Biochem. 2014;70(2):593–602. [DOI] [PubMed] [Google Scholar]
- 51. Hammer S, van der Meer RW, Lamb HJ, Schär M, de Roos A, Smit JWA, Romijn JA. Progressive caloric restriction induces dose-dependent changes in myocardial triglyceride content and diastolic function in healthy men. J Clin Endocrinol Metab. 2008;93(2):497–503. [DOI] [PubMed] [Google Scholar]
- 52. Trøseid M, Hov JR, Nestvold TK, Thoresen H, Berge RK, Svardal A, Lappegård KT. Major increase in microbiota-dependent proatherogenic metabolite TMAO one year after bariatric surgery. Metab Syndr Relat Disord. 2016;14(4):197–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Van Dorsten FA, Daykin CA, Mulder TPJ, Van Duynhoven JPM. Metabonomics approach to determine metabolic differences between green tea and black tea consumption. J Agric Food Chem. 2006;54(18):6929–38. [DOI] [PubMed] [Google Scholar]
- 54. Feliciano RP, Mills CE, Istas G, Heiss C, Rodriguez-Mateos A. Absorption, metabolism and excretion of cranberry (poly)phenols in humans: a dose response study and assessment of inter-individual variability. Nutrients. 2017;9(3):268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Moazzami AA, Bondia-Pons I, Hanhineva K, Juntunen K, Antl N, Poutanen K, Mykkänen H. Metabolomics reveals the metabolic shifts following an intervention with rye bread in postmenopausal women—a randomized control trial. Nutr J. 2012;11(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Moazzami AA, Zhang JX, Kamal-Eldin A, Aman P, Hallmans G, Johansson JE, Andersson SO. Nuclear magnetic resonance-based metabolomics enable detection of the effects of a whole grain rye and rye bran diet on the metabolic profile of plasma in prostate cancer patients. J Nutr. 2011;141(12):2126–32. [DOI] [PubMed] [Google Scholar]
- 57. Hanhineva K, Lankinen MA, Pedret A, Schwab U, Kolehmainen M, Paananen J, de Mello V, Sola R, Lehtonen M, Poutanen K et al.. Nontargeted metabolite profiling discriminates diet-specific biomarkers for consumption of whole grains, fatty fish, and bilberries in a randomized controlled trial. J Nutr. 2015;145(1):7–17. [DOI] [PubMed] [Google Scholar]
- 58. Johansson-Persson A, Barri T, Ulmius M, Önning G, Dragsted LO. LC-QTOF/MS metabolomic profiles in human plasma after a 5-week high dietary fiber intake. Anal Bioanal Chem. 2013;405(14):4799–809. [DOI] [PubMed] [Google Scholar]
- 59. Lin X, Racette SB, Lefevre M, Spearie CA, Most M, Ma L, Ostlund RE Jr.. The effects of phytosterols present in natural food matrices on cholesterol metabolism and LDL-cholesterol: a controlled feeding trial. Eur J Clin Nutr. 2010;64(12):1481–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pimentel G, Burton KJ, von Ah U, Butikofer U, Pralong FP, Vionnet N, Portmann R, Vergeres G. Metabolic footprinting of fermented milk consumption in serum of healthy men. J Nutr. 2018;148(6):851–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Postle AD. Phospholipid lipidomics in health and disease. Eur J Lipid Sci Technol. 2009;111(1):2–13. [Google Scholar]
- 62. Peterson BL, Cummings BS. A review of chromatographic methods for the assessment of phospholipids in biological samples. Biomed Chromatogr. 2006;20(3):227–43. [DOI] [PubMed] [Google Scholar]
- 63. Noerman S, Kärkkäinen O, Mattsson A, Paananen J, Lehtonen M, Nurmi T, Tuomainen T-P, Voutilainen S, Hanhineva K, Virtanen JK. Metabolic profiling of high egg consumption and the associated lower risk of type 2 diabetes in middle-aged Finnish men. Mol Nutr Food Res. 2019;63(5):1800605. [DOI] [PubMed] [Google Scholar]
- 64. Sindelar PJ, Guan Z, Dallner G, Ernster L. The protective role of plasmalogens in iron-induced lipid peroxidation. Free Radic Biol Med. 1999;26(3):318–24. [DOI] [PubMed] [Google Scholar]
- 65. Wallace M, Green CR, Roberts LS, Lee YM, McCarville JL, Sanchez-Gurmaches J, Meurs N, Gengatharan JM, Hover JD, Phillips SA. Enzyme promiscuity drives branched-chain fatty acid synthesis in adipose tissues. Nat Chem Biol. 2018;14(11):1021–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bakker LE, van Schinkel LD, Guigas B, Streefland TC, Jonker JT, van Klinken JB, van der Zon GC, Lamb HJ, Smit JW, Pijl H et al.. A 5-day high-fat, high-calorie diet impairs insulin sensitivity in healthy, young South Asian men but not in Caucasian men. Diabetes. 2014;63(1):248–58. [DOI] [PubMed] [Google Scholar]
- 67. Kim MJ, Yang HJ, Kim JH, Ahn C-W, Lee JH, Kim KS, Kwon DY. Obesity-related metabolomic analysis of human subjects in black soybean peptide intervention study by ultraperformance liquid chromatography and quadrupole-time-of-flight mass spectrometry. J Obesity. 2013;2013:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kunesova M, Hlavaty P, Tvrzicka E, Stankova B, Kalouskova P, Viguerie N, Larsen TM, van Baak MA, Jebb SA, Martinez JA et al.. Fatty acid composition of adipose tissue triglycerides after weight loss and weight maintenance: the DIOGENES study. Physiol Res. 2012;61(6):597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Forouhi NG, Koulman A, Sharp SJ, Imamura F, Kröger J, Schulze MB, Crowe FL, Huerta JM, Guevara M, Beulens JWJ et al.. Differences in the prospective association between individual plasma phospholipid saturated fatty acids and incident type 2 diabetes: the EPIC-InterAct case-cohort study. Lancet Diabetes Endocrinol. 2014;2(10):810–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. de Mello VD, Paananen J, Lindstrom J, Lankinen MA, Shi L, Kuusisto J, Pihlajamaki J, Auriola S, Lehtonen M, Rolandsson O et al.. Indolepropionic acid and novel lipid metabolites are associated with a lower risk of type 2 diabetes in the Finnish Diabetes Prevention Study. Sci Rep. 2017;7:46337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Riserus U, Marklund M. Milk fat biomarkers and cardiometabolic disease. Curr Opin Lipidol. 2017;28(1):46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Weitkunat K, Schumann S, Nickel D, Hornemann S, Petzke KJ, Schulze MB, Pfeiffer AFH, Klaus S. Odd-chain fatty acids as a biomarker for dietary fiber intake: a novel pathway for endogenous production from propionate. Am J Clin Nutr. 2017;105(6):1544–51. [DOI] [PubMed] [Google Scholar]
- 73. Tang WHW, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368(17):1575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Brial F, Le Lay A, Dumas M-E, Gauguier D. Implication of gut microbiota metabolites in cardiovascular and metabolic diseases. Cell Mol Life Sci. 2018;75(21):3977–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Koistinen VM, Kärkkäinen O, Borewicz K, Zarei I, Jokkala J, Micard V, Rosa-Sibakov N, Auriola S, Aura A-M, Smidt H et al.. Contribution of gut microbiota to metabolism of dietary glycine betaine in mice and in vitro colonic fermentation. Microbiome. 2019;7:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Shah SH, Crosslin DR, Haynes CS, Nelson S, Turer CB, Stevens RD, Muehlbauer MJ, Wenner BR, Bain JR, Laferrère B et al.. Branched-chain amino acid levels are associated with improvement in insulin resistance with weight loss. Diabetologia. 2012;55(2):321–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Pedersen HK, Gudmundsdottir V, Nielsen HB, Hyotylainen T, Nielsen T, Jensen BA, Forslund K, Hildebrand F, Prifti E, Falony G et al.. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature. 2016;535(7612):376. [DOI] [PubMed] [Google Scholar]
- 78. Dragsted LO. Biomarkers of meat intake and the application of nutrigenomics. Meat Sci. 2010;84(2):301–7. [DOI] [PubMed] [Google Scholar]
- 79. Feliciano RP, Mecha E, Bronze MR, Rodriguez-Mateos A. Development and validation of a high-throughput micro solid-phase extraction method coupled with ultra-high-performance liquid chromatography-quadrupole time-of-flight mass spectrometry for rapid identification and quantification of phenolic metabolites in human plasma and urine. J Chromatogr A. 2016;1464:21–31. [DOI] [PubMed] [Google Scholar]
- 80. Donia MS, Fischbach MA. Small molecules from the human microbiota. Science. 2015;349(6246):1254766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Pryde SE, Duncan SH, Hold GL, Stewart CS, Flint HJ. The microbiology of butyrate formation in the human colon. FEMS Microbiol Lett. 2002;217(2):133–9. [DOI] [PubMed] [Google Scholar]
- 82. Wu Y, Li RW, Huang H, Fletcher A, Yu L, Pham Q, Yu L, He Q, Wang TT. Inhibition of tumor growth by dietary indole-3-carbinol in a prostate cancer xenograft model may be associated with disrupted gut microbial interactions. Nutrients. 2019;11(2):467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Martin FP, Dumas ME, Wang Y, Legido-Quigley C, Yap IK, Tang H, Zirah S, Murphy GM, Cloarec O, Lindon JC et al.. A top-down systems biology view of microbiome-mammalian metabolic interactions in a mouse model. Mol Syst Biol. 2007;3:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Calvani R, Miccheli A, Capuani G, Miccheli AT, Puccetti C, Delfini M, Iaconelli A, Nanni G, Mingrone G. Gut microbiome-derived metabolites characterize a peculiar obese urinary metabotype. Int J Obes. 2010;34(6):1095–8. [DOI] [PubMed] [Google Scholar]
- 85. Zierer J, Jackson MA, Kastenmüller G, Mangino M, Long T, Telenti A, Mohney RP, Small KS, Bell JT, Steves CJ. The fecal metabolome as a functional readout of the gut microbiome. Nat Genet. 2018;50(6):790–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Sumner LW, Amberg A, Barrett D, Beale MH, Beger R, Daykin CA, Fan TWM, Fiehn O, Goodacre R, Griffin JL. Proposed minimum reporting standards for chemical analysis. Metabolomics. 2007;3(3):211–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Brunk E, Sahoo S, Zielinski DC, Altunkaya A, Dräger A, Mih N, Gatto F, Nilsson A, Preciat Gonzalez GA, Aurich MK et al.. Recon3D enables a three-dimensional view of gene variation in human metabolism. Nat Biotechnol. 2018;36:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.