Abstract
BACKGROUND
Skull base osteoradionecrosis (ORN) is a challenging treatment-related complication sometimes seen in patients with cancer. Although ORN management strategies for other anatomic sites have been reported, there is a paucity of data guiding the management of skull base ORN.
OBJECTIVE
To report a single-center tertiary care series of skull base ORN and to better understand the factors affecting ORN recurrence after surgical management.
METHODS
We conducted a retrospective cohort study of patients with skull base ORN treated at our center between 2003 and 2017. Univariate and multivariate binary logistic regressions were performed to identify predictors of recurrence.
RESULTS
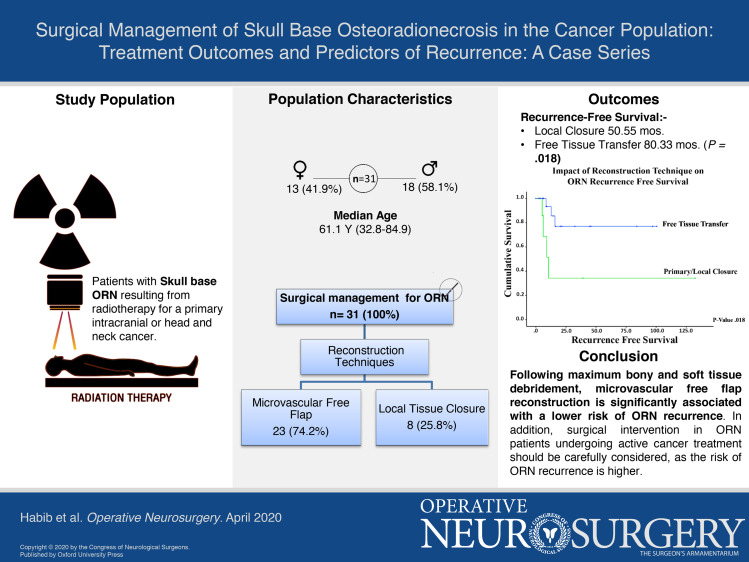
A total of 31 patients were included in this study. The median age at ORN diagnosis was 61.1 yr (range, 32.8-84.9 yr). Of these 31 patients, 15 (48.4%) patients were initially treated medically. All 31 patients underwent surgery. Three (14.3%) of 21 patients treated with a free flap and 4 (50.0%) of 8 patients who underwent primary closure experienced recurrence. Cox regression analysis revealed that reconstruction with local tissue closure (P = .044) and ongoing treatment for active primary cancer (P = .022) were significant predictors of recurrence. The median overall survival from index surgery for ORN treatment was 83.9 mo. At 12-mo follow-up, 78.5% of patients were alive.
CONCLUSION
In this study, we assess the outcomes of our treatment approach, surgical debridement with vascularized reconstruction, on recurrence-free survival in patients with skull base ORN. Further studies with larger cohorts are needed to assess current treatment paradigms.
Keywords: Osteoradionecrosis, Skull base, Cancer, Surgery, Management
Graphical Abstract
Graphical Abstract.
ABBREVIATIONS
- CT
computed tomography
- CI
confidence interval
- HBO
hyperbaric oxygen
- HR
hazard ratio
- MRI
magnetic resonance imaging
- ORN
osteoradionecrosis
- OD
overall survival
- RFS
recurrence-free survival
Osteoradionecrosis (ORN) is a treatment-related complication primarily reported in patients within the head and neck cancer population defined as necrosis and exposure of bone as a complication of radiation therapy that fails to heal over a period of 3 mo.1,2 The hypothesized pathogenesis implicates radiation-induced damage to vascular endothelial cells in bone, which hampers osteoblast activity and increases bone matrix absorption.3-7 It is additionally known that social habits, like alcohol abuse and smoking, are significantly associated with ORN.5,8 In patients with ORN at nonskull base sites, conservative management, including hyperbaric oxygen (HBO) therapy, antibiotics for superimposed infection, and medical therapy with pentoxifylline and tocopherol, are usually the first lines of treatment and lead to complete resolution of ORN within 1 yr in 8% to 33% of patients.9,10 If medical therapy fails in these patients, surgery has been shown to be effective.11-16
The majority of the published studies on ORN pertain to mandibular ORN; there are few studies in the literature regarding the management of skull base ORN, which has thus far only been described in case reports and small descriptive series.17,18 Due to advances in radiation therapy and systemic therapy regimens, a significant number of patients with malignancies involving the skull base undergo multimodal therapy.19-22 Additionally, with incremental improvements in cancer survival rates, an increasing number of patients are undergoing salvage treatments, which often consist of re-irradiation, possibly with concurrent chemotherapy. As a result, cancer survivorship is an increasing focus of care along with the management of treatment-related sequelae – including ORN.
Due to the involvement of multiple anatomic compartments and the exposure of critical neurovascular structures, skull base ORN is particularly challenging to manage. Tissue necrosis and infection in this region can affect critical neurovascular structures and lead to internal carotid artery rupture (Figure 1) or recalcitrant cerebrospinal fluid leaks.23,24 Given the unique anatomic challenges in the skull base, there is a need to better understand the factors affecting ORN outcomes after surgical management. Accordingly, the goal of this study was to present and analyze our approach to surgical treatment and reconstruction of skull base ORN and to identify possible predictors of ORN recurrence after surgical management. To our knowledge, this is the largest series discussing skull base ORN.
Figure 1.
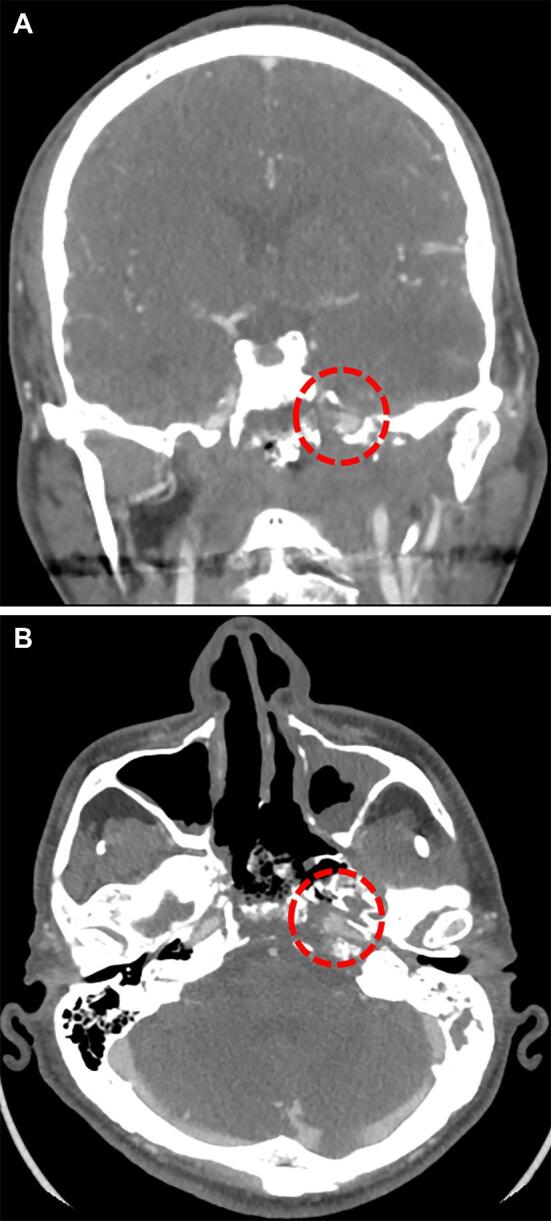
CT-angiogram demonstrating findings of radiation-induced injury to the internal carotid artery. This is a patient with a history of nasopharyngeal carcinoma treated with chemoradiation at initial diagnosis and then recurrent, who subsequently presented with massive epistaxis. CT-angiography at the time demonstrated a pseudoaneurysm involving the horizontal petrous segment of the left internal carotid artery along with significant treatment-related changes in the adjacent bony skull base A and B. After passing a balloon test occlusion, the carotid artery was sacrificed.
METHODS
Study Design
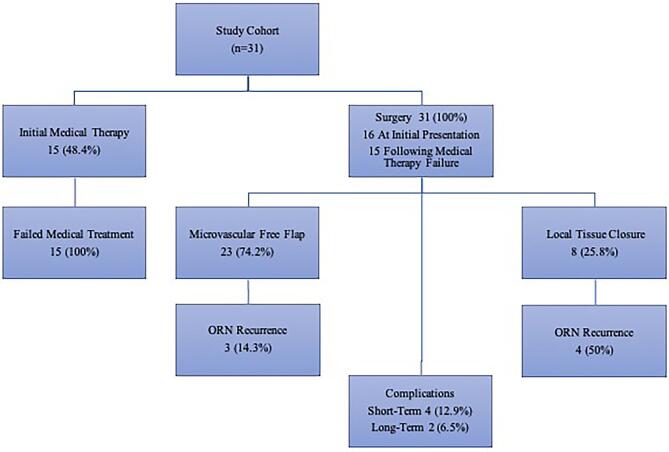
A retrospective review of all patients treated surgically for ORN at our institution between 2003 and 2017 for skull base ORN was performed under an Institutional Review Board-approved protocol in compliance with institutional regulations with regard to the study of human subjects (Figure 2). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study, formal consent was not required. Diagnosis and anatomic involvement were assessed clinically and on magnetic resonance imaging (MRI) and computed tomography (CT). All surgical procedures were performed to achieve a wide resection and debridement of the invaded bony skull base and soft tissue compartments, followed by reconstruction tailored to the individual patient and site of involvement. The extent of bony resection required for each patient was based on preoperative imaging in addition to intraoperative decision-making after the overlying involved soft tissue had been debrided. In general, free tissue transfer was the preferred method of reconstruction unless the area of debridement needed was thought to be limited and could be reconstructed with local/regional flaps (ie, scalp advancement and rotational temporalis flap).
Figure 2.
Patient flowchart.
A retrospective review of patients’ clinical charts was performed. Demographics and clinical variables, including age and sex; medical comorbidities, including any history of atherosclerotic disease and history of smoking; and information about the primary cancer's histopathological diagnosis and treatment (surgical resection, chemotherapy, and radiation) were also collected. For each patient, the anatomic location of the ORN and the medical treatment, surgical treatment, and reconstruction type were recorded. The primary outcome of the study was recurrence-free survival (RFS), defined as the time from the initial surgical treatment for ORN to the time of ORN recurrence. ORN recurrence was defined as the need for reoperation following failure of a prior surgical treatment.
Statistical Analysis
Categorial variables were assessed with frequencies and percentages, while continuous variables were summarized with means and ranges. Descriptive statistics were calculated for all variables. Kaplan-Meier estimates of RFS curves were compared using the log-rank test. Univariate predictors of risk of overall mortality and ORN recurrence were assessed using the Cox proportional hazards model with 95% (CIs). A P value < .05 was considered significant for all analyses. Analyses were performed using the statistical software SPSS V.24 (IBM, Armonk, New York).
RESULTS
Patient Demographics and Prior Cancer Treatments
A total of 31 patients were included in this study; patient demographic and clinical information is summarized in Table 1. The median age at the time of ORN diagnosis was 61.1 yr (range, 32.8-84.9 yr). The median time from radiation for the primary cancer to ORN occurrence was 52.0 mo (range, 1.1-305.8 mo). The most common primary cancer diagnoses were squamous cell carcinoma (n = 7, 22.6%) and esthesioneuroblastoma (n = 5, 16.1%), followed by basal cell carcinoma, atypical meningioma, astrocytoma, and World Health Organization Grade I meningioma (each n = 2, 6.5%). All patients had previously received tumor-directed radiation therapy (mean dose, 60 Gy) and had undergone surgical resection for their primary tumor; additional tumor-directed treatments included adjuvant chemotherapy (n = 18, 58.1%), concurrent, adjuvant chemoradiation therapy (n = 9, 29%), and neoadjuvant chemotherapy (n = 7, 22.6%). The median overall survival (OS) from the index surgery for ORN was 83.9 mo.
TABLE 1.
Demographic and Previous Cancer History
| N (%) | |
|---|---|
| Number of patients | 31 |
| Median age, years (range)* | 61.1 (32.8-84.9) |
| Sex | |
| Male | 18 (58.1) |
| Female | 13 (41.9) |
| Primary cancer | |
| Squamous cell carcinoma | 7 (22.6) |
| Esthesioneuroblastoma | 5 (16.1) |
| Astrocytoma | 2 (6.5) |
| Atypical meningioma | 2 (6.5) |
| Basal cell carcinoma | 2 (6.5) |
| Meningioma | 2 (6.5) |
| Lymphoma | 1 (3.2) |
| Chondrosarcoma | 1 (3.2) |
| Ewing sarcoma | 1 (3.2) |
| Fibroxanthoma | 1 (3.2) |
| Hemangiopericytoma | 1 (3.2) |
| Melanoma | 1 (3.2) |
| Oligoastrocyroma | 1 (3.2) |
| Oligodendroglioma | 1 (3.2) |
| Osteosarcoma | 1 (3.2) |
| Pleomorphic adenoma | 1 (3.2) |
| Seminoma | 1 (3.2) |
| Treatments for primary cancer | |
| Adjuvant chemotherapy | 18 (58.1) |
| Surgery | 31 (100) |
| Adjuvant radiation | 31 (100) |
| Median radiation dose, Gy (range) | 60 (30-70) |
| Radiation type | |
| IMRT | 9 (29.03) |
| SRS | 2 (6.5) |
| Proton beam | 2 (6.5) |
| Electron beam | 1 (3.2) |
| Unknown | 17 (54.8) |
IMRT, intensity-modulated radiation therapy; SRS, stereotactic radiosurgery.
Presentation and Treatment Strategies
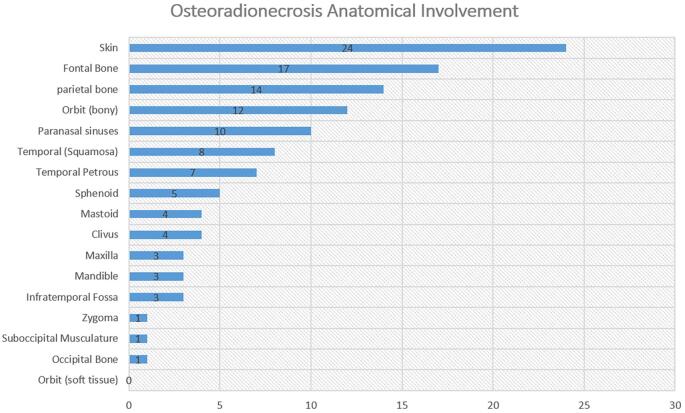
At the time of presentation, 4 patients (12.9%) were undergoing treatment for active cancer. The anatomic extent of skull base ORN involvement is summarized in Figure 3. We assessed skull base involvement by the Irish et al25 classification. Zone I is defined as the central skull base extending laterally to the mid-pupillary line of the orbit. Zone II is defined as extending from the mid-pupillary line to the external auditory meatus while Zone III extends from the external auditory meatus to the occipital condyle. Zone I was most commonly affected (n = 17), followed by zone II (n = 14) and zone III (7).25 ORN involved the skin in the majority of cases (n = 24; 77.4%). Treatments for ORN are summarized in Table 2. In this cohort, 15 (48.4%) were initially managed medically at our institution. Medical treatment strategies included the use of antibiotics, HBO, and/or dressing changes. The surgical approaches employed were essentially multicompartmental skull base approaches. The diseased bony skull base was resected and any adjacent soft tissue compartments debrided of necrotic tissue (Figure 4). Reconstruction was performed by plastic surgeons in all cases. Reconstruction techniques included microvascular free flaps (n = 23, 74.2%) or local closure with local tissue advancement or regional flaps (n = 8, 25.8%). Free flaps were designed to provide coverage for the bony defect while also providing a skin paddle for reconstruction of any involved overlying epithelial (ie, scalp) or mucosal surfaces (ie, within the nasopharynx). The types of free flaps employed are shown in Table 2; the recipient vessels included superficial temporal (12 patients), facial (9 patients), and transverse cervical (2 patients). The following rotations flaps were employed in the remaining patients: temporalis flaps (2 patients), pericranial flap (1 patient), and galeal flap (1 patient). In the remaining patients, after bony debridement, primary closure was performed via local scalp advancement (4 patients). Tissue specimens were sent for microbiological analysis, and 19 (61.3%) patients were tested positive for microorganisms. The most commonly found bacterial species was Staphylococcus aureus, and methicillin-sensitive Staphylococcus aureus was present in 2 patients. B-hemolytic streptococci, Staphylococcus epidermidis, and Pseudomonas aeruginosa were also found. A prolonged antibiotic course was administered to all patients with positive microbiology culture results.
FIGURE 3.
Anatomic extent of skull base ORN.
TABLE 2.
ORN Characteristics and Treatment Strategies
| N (%) | |
|---|---|
| Number of patients | 31 |
| Median time to ORN following radiation treatment, months (range) | 52.0 (1.1-305.8) |
| Initial medical treatment for ORN | 15 (48.4) |
| Surgery for ORN | 31 (100) |
| Reconstruction techniques | |
| Local tissue closure | 8 (25.8) |
| Microvascular free flap | 23 (74.2) |
| Anterolateral thigh | 13 |
| Latissmus dorsi | 6 |
| Serratus anterior | 2 |
| Rectus abdominus | 1 |
| Radial forearm | 1 |
| Median follow-up time for ORN, months (range) | 16.3 (0-132.7) |
ORN, osteoradionecrosis.
FIGURE 4.
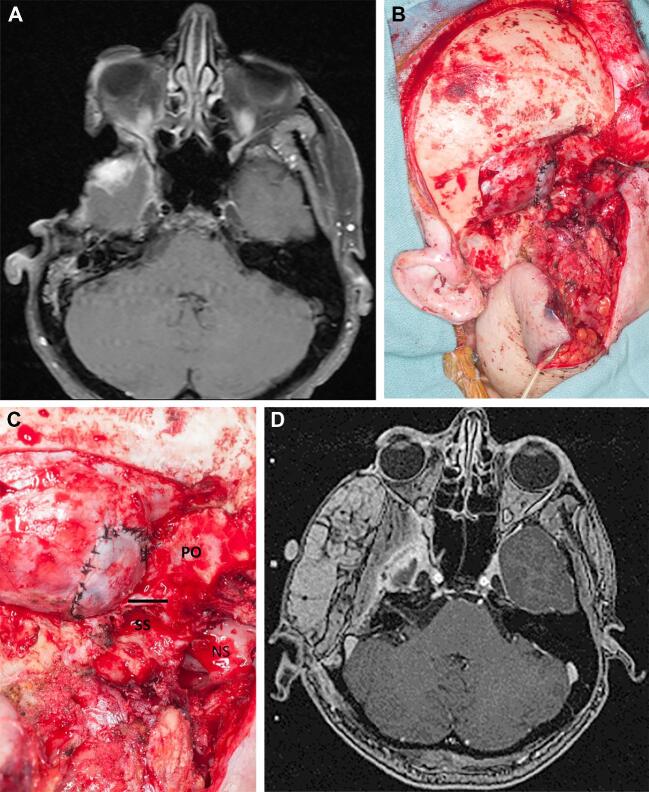
Representative case showing ORN of the anterolateral skull base. The image shows an example of a patient with a previous parotid gland malignancy. The patient had undergone a mandibulectomy/infratemporal fossa resection and adjuvant chemoradiation therapy and presented 3 yr post-treatment with extensive ORN involving the middle cranial fossa, temporal bone, remnant infratemporal fossa, and overlying skin. Preoperative MRI A demonstrates the extent of exposure of the skull base and the soft tissue defect with temporal lobe necrosis but shows no evidence of tumor recurrence. Intraoperative images B and C show the extent of the debridement of the middle fossa, dura, and infratemporal fossa. Postoperative MRI D demonstrates soft tissue reconstruction with an anterolateral thigh flap. Abbreviations: NS, nasal septum viewed through the posterior aspect of the maxillary sinus (solid line); PO, posterior orbital apex; SS, sphenoid sinus; and V2, skeletonized at foramen rotundum.
Treatment Outcomes and Predictors of ORN Recurrence
The median follow-up duration for the study cohort was 16.3 mo and the mean ORN RFS was 89.3 mo. The median OS was 83.9 mo where at last follow-up 18 patients (58.1%) were alive. Short-term complications within 30 d occurred in 4 (12.9%) patients, including 3 with facial cellulitis and 1 with poly-microbial skin inflammation. Long-term complications occurring greater than 30 d postoperatively were reported in 2 (6.5%) patients, including cerebrospinal fluid leakage (n = 1) and frontal sinus fistula and mucocele (n = 1). Of the 21 patients who underwent free flap reconstruction, only 3 patients (14.3%) experienced recurrence. Of the 8 patients who underwent local closure, 4 patients (50.0%) experienced recurrence. All 7 patients that experienced recurrence were subsequently treated via reoperation and reconstruction involving microvascular free flaps (4 patients including 3 patients who also underwent cranioplasty with polyether ether ketone implants), split-thickness skin grafts (2 patients), or primary closure (1 patient). None of these patients experienced recurrence after their repeat treatment for ORN.
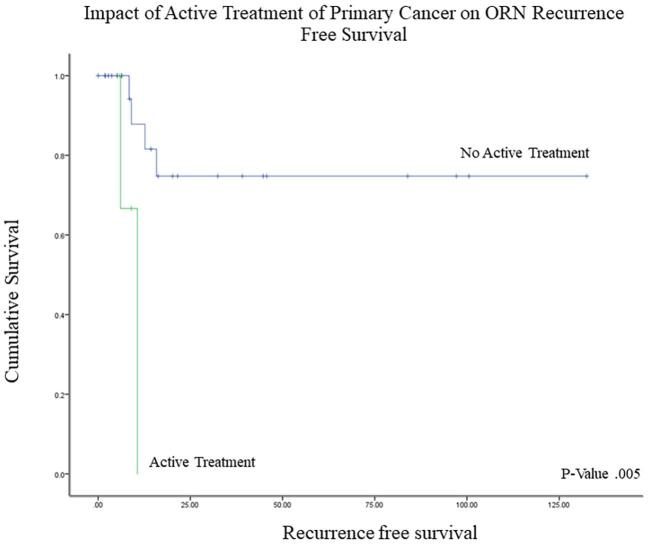
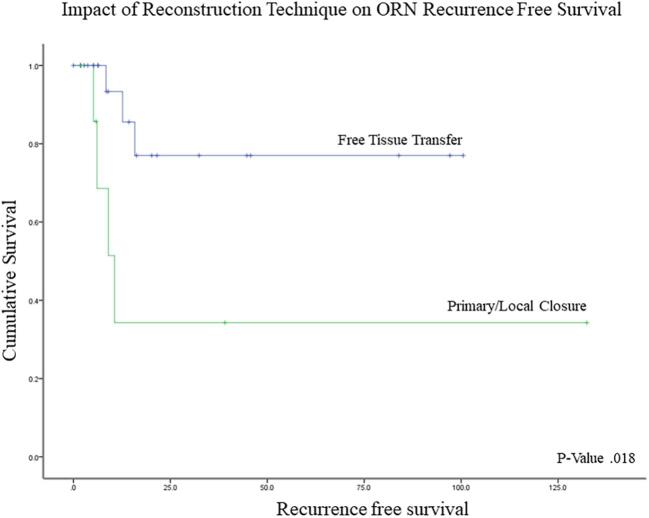
The Kaplan-Meier and Cox regression analyses for predictors of ORN recurrence are summarized in Table 3. Patients undergoing concurrent treatment of ORN and an active malignancy had significantly shorter recurrence-free intervals (9.1 vs 102.0 mo; P = .005) and were at higher risk for recurrence (hazard ratio (HR) = 10.3; 95% CI, 1.4-75.6; P = .022) (Figure 5). In assessing the impact of reconstruction, local closure (rotational flap or primary closure), as compared with free tissue transfer, was significantly linked with poorer outcomes (50.6 mo vs 80.3 mo, P = .018) and a 5-fold increase in the risk of ORN recurrence (95% CI, 1.1-23.3; P = .033) (Figure 6). Primary and regional flaps were assessed as a single category due to the relatively small numbers of each that precluded analysis in addition to the hypothesis that both vascular supply of both types of tissue closure are likely to have been affected by previous cancer-directed local therapies. When rotational flaps (4 patients) were assessed separately, a trend toward a higher risk of ORN recurrence on Cox regression analysis was noted (HR 1.7, P = .196). To assess for confounding factors that could have influenced why a local closure was performed, log-rank analysis demonstrated no survival difference between those undergoing local closure vs free tissue transfer (P > .05). A 2-tailed student's t-test was also performed to assess for active cancer as a confounding factor – no correlation between cancer status and type of reconstruction was noted (P > .05). Of the 9 patients with active cancer at the time of ORN diagnosis, 5 underwent free tissue transfer and 4 underwent local closure. On further analysis between the 2 groups, no significant differences in any of the factors evaluated was noted (P < .05). Statistically nonsignificant risk factors for shorter RFS included smoking (44.0 vs 105 mo), chronic obstructive pulmonary disease (18.7 vs 89.0 mo), and cardiac disease (76.8 vs 86.9 mo).
TABLE 3.
Predictors of ORN Recurrence-Free Survival
| Log-rank analysis of RFS | Cox regression analysis of RFS | |||
|---|---|---|---|---|
| Variable | Median RFS (months) | P value | HR (95% CI) | P value |
| Patient characteristics | ||||
| Age | ||||
| Below 65 | 69.3 | .052 | .16 (.02-1.32) | .088 |
| 65 or above | 90.0 | |||
| Sex | ||||
| Male | 25.9 | .073 | .25 (.047-1.29) | .097 |
| Female | 110.7 | |||
| Type I/II diabetes | ||||
| Yes | N/A | .46 | .044 (0-13 976.2) | .63 |
| No | N/A | |||
| History of smoking | ||||
| Yes | 44.02 | .19 | 2.65 (.588-11.945) | .21 |
| No | 105.59 | |||
| History of COPD | ||||
| Yes | 18.74 | .72 | .68 (.081-5.63) | .72 |
| No | 89.04 | |||
| History of cardiac disease | ||||
| Yes | 76.8 | .54 | .52 (.062-4.39) | .55 |
| No | 86.9 | |||
| Primary cancer history | ||||
| Neoadjuvant chemotherapy | ||||
| Yes | N/A | .086 | .028 (0-27.4) | .31 |
| No | N/A | |||
| Concurrent chemoradiation | ||||
| Yes | 59.13 | .895 | .895 (.17-4.63) | .895 |
| No | 88.29 | |||
| Adjuvant chemotherapy | ||||
| Yes | 83.62 | .51 | 1.73 (.34-8.93) | .51 |
| No | 74.93 | |||
| Previous radiation dose | ||||
| 60 Gy or below | N/A | .20 | .031 (0-179.25) | .43 |
| Above 60 Gy | N/A | |||
| Previous IMRT | ||||
| Yes | 67.65 | .94 | .94 (.18-4.84) | .94 |
| No | 88.41 | |||
| Previous SRS | ||||
| Yes | N/A | .63 | .044 (0-9 329 347.2 | .75 |
| No | N/A | |||
| Previous proton beam therapy | ||||
| Yes | N/A | .33 | .040 (0-879.85) | .53 |
| No | N/A | |||
| Ongoing treatment for primary cancer at ORN diagnosis | ||||
| Yes | 9.1 | .005 * | 10.32 (1.41-75.57) | .022 * |
| No | 101.95 | |||
| Treatments for ORN | ||||
| Medical treatment before surgery | ||||
| Yes | N/A | .76 | .79 (.17-3.62) | .76 |
| No | N/A | |||
| Antibiotric course after ORN surgery | ||||
| Yes | 81.72 | .34 | 2.67 (.32-22.28) | .36 |
| No | 38.37 | |||
| Reconstruction type | ||||
| Local closure | 50.55 | .018 * | 5.15 (1.14-23.3) | .033 * |
| Free tissue transfer | 80.33 | |||
*P values < .05 were considered statistically significant.
COPD, chronic obstructive pulmonary disease; HR, hazard ratio; IMRT, intensity-modulated radiation therapy; ORN, osteoradionecrosis; RFS, recurrence-free survival; SRS, stereotactic radiosurgery. N/A – median values not reached.
FIGURE 5.
Kaplan-Meier curve demonstrating impact of active treatment for primary cancer on ORN recurrence-free survival.
FIGURE 6.
Kaplan-Meier curve demonstrating impact of reconstruction technique on ORN recurrence-free survival.
DISCUSSION
As cancer survival improves with advancements in therapy, survivorship and management of treatment-related complications are becoming increasingly important in cancer care. ORN of the skull base poses substantial quality-of-life issues and can be challenging to manage because of numerous anatomic factors. The current published literature has insufficient data guiding the proper management of skull base ORN. Accordingly, in this manuscript, we demonstrated the role of surgery along with the impact of vascularized reconstruction and active systemic disease on ORN recurrence.
Presentation and Medical Management of ORN
The presentation of skull base ORN in our series appears to be similar in nature to that of ORN of the head and neck; both types of ORN present with exposed bone and devitalized overlying soft tissue.26-31 Another finding at presentation in our cohort was concomitant infection with either soft tissue cellulitis, osteomyelitis, or sinusitis. Typically, ORN is diagnosed by clinical examination of the exposed areas, which can be difficult in cases involving the skull base. Thus, imaging, including MRI and CT, is important in assessing the extent of bony skull base involvement. MRI, for example, can demonstrate irregular or decreased marrow signals and post-gadolinium enhancements.12,32,33
Currently, there is no universally accepted medical approach to ORN. This is likely reflective of the shift in the hypothesized pathophysiology that has occurred over time. In 1983, Marx et al proposed ORN was a result of tissue hypoxia and hypovascularity resulting from cellular events triggered by radiation therapy ultimately causing tissue breakdown and a chronic nonhealing wound. This hypoxia theory has been the primary motivation to pursue HBO as a therapy. While there are concerns that HBO could stimulate growth in cancer cells based on conflicting early studies, the current body of literature indicates that HBO does not promote cancer development as tested in Vivo models.34,35 In ORN, the rationale is that HBO could increase oxygenation in hypoxic tissue while also promoting angiogenesis and having bactericidal and bacteriostatic effects.1 Data from Wong et al16 showed that conservative treatment with antibiotics, HBO, and local wound care resulted in improvement in 25% to 44% of patients. Ultimately, the efficacy of HBO in ORN management is contentious; furthermore, several published case series concluded negative results.36-38 Due to the poor quality of currently available data, the Dana‐Farber/Brigham and Women's Cancer Center Multidisciplinary Guideline39 recommends against the routine of HBO as monotherapy for ORN treatment, especially in patients who received radiation therapy for head and neck cancers.
More recently, an alternative etiology for ORN has been proposed, suggesting that radiation-induced dysregulation of fibroblast activity in the bone results in unhealthy fibrotic tissue.40 This fibroatrophic mechanism is based on endothelial dysfunction, microvascular thrombosis, and bone necrosis. This has paved the way for the use of antioxidant and antifibrotic therapies, such as pentoxifylline, alpha-tocopherol, and clondronate, in the treatment of ORN.41 In a study of 54 mandibular ORN patients, the use of all 3 agents, along with prednisone and ciprofloxacin, resulted in complete recovery in all cases after a median treatment length of 9 mo.9 Based on all currently available data for head and neck ORN, a suggested approach for early and limited ORN could rely on the use of antibiotics along with the above medical therapies and nonsurgical wound care.
Surgical and Reconstruction Strategies for ORN
Surgical management of ORN is reserved for patients in whom conservative medical treatment has failed or for those who present with extensive advanced lesions. While extensive skull base debridement with the need for soft tissue reconstruction could be considered a large undertaking, the observed median OS of 83.9 mo in our study indicates that intervention is justified given the long-term negative impact of an extensive non-healing wound. This is bolstered by the increasing number of salvage therapy options for patients dealing with the progression of their primary malignancy. The head and neck literature describes surgical options for ORN based on the severity of the disease.14,42, Anatomically limited cases can be managed with sequestrectomy and primary closure.14,43 Advanced cases with extensive osteomyelitis and larger skin defects require extensive bone resection followed by advanced reconstruction.12,14,23,42-44 In our surgical practice, we have employed the strategy of extensive bony skull base resection with debridement of any infected soft tissue, followed by coverage of the dura and reconstruction with vascularized soft tissue.
Multicompartmental skull base resections – even for ORN – can unify multiple intracranial and subcranial compartments, including the upper aerodigestive tract and paranasal sinuses, while leaving previously irradiated vascular structures exposed. Adequate reconstruction must separate the subcranial and intracranial cavities, provide vascularized coverage of any exposed bone and vessels, and produce an optimal cosmetic outcome. Our data demonstrate that reconstruction with free vascular flaps is significantly associated with longer RFS and better healing and tissue viability. The inferiority of regional flaps (ie, temporalis and pericranial flaps) may be due to the disruption of their vascular supply by previous local treatments (ie, surgery and/or radiation therapy), as well as their limited size and reach. Although a wide spectrum of reconstruction techniques, including skin/bone grafts, rotational and regional flaps, and vascularized flaps, have been described in the literature, several prior studies have found vascularized flaps to be the most effective option.13,14,42,45 A consideration for why patients with local closure had poorer outcomes is that intervention may have been performed too early in this cohort before their ORN had fully peaked. Hence, the true extent of diseased tissue may have been underestimated – influencing the reconstruction strategy. Also, while we combined regional flaps with other local closure methods for analysis given the relatively small numbers, there may be a subset of patients where there still might be viable regional flaps whose vascularity has not been compromised.
In 2011, Hanasono et al46 published an updated approach to skull base reconstruction in their report of a 250-case series. In comparing the results of 39 local or regional pedicled flap reconstructions with those from 211 free flap reconstructions, Hanasono et al demonstrated a significant improvement in wound-healing outcomes with the use of free tissue transfer. Not only do free flaps bring in well-vascularized, nonirradiated tissue to facilitate healing, but they also permit more complete resection of ORN lesions because larger flaps than those available locally or regionally can be utilized. This publication also summarizes our algorithm for approaching reconstruction of skull base defects. Generally, the skull base location and size of the defect along with the number of skin paddles needed to reconstruct any cutaneous or mucosal defects dictates the type of free tissue transferred employed. Typically, the anterolateral thigh flap is the most versatile and has been our preferred method of reconstruction due to the reduced donor site morbidity, the ability to harvest additional bulk, and multiple skin paddles on separate perforators. This flap also permits the harvesting of nerve grafts and fascia if needed while also allowing 2 teams to work together while performing the resection and graft harvest simultaneously. Although microvascular free flaps are beyond the scope of this study, these flaps may be associated with improved functional and aesthetic outcomes and, thus, a greatly improved quality of life for patients because they allow the transfer of various tissue types, including skin, fat, bone, and even functional muscle. The value of upfront free tissue transfer for head and neck ORN has been demonstrated in several other small series.47-49
The potential morbidity associated with free tissue transfer must be considered. With the use of intraoperative vascular dyes (ie, indocyanine green), decisions regarding the reliability of regional flaps versus free tissue transfers can be made case by case. The importance of adequate wound healing is likely the explanation for why ongoing treatment for active cancer was found in our study to be linked with higher ORN recurrence rates. Particularly in patients who have previously undergone intensive treatments for their primary malignancies, the salvage systemic therapies used in skull base cancers can be associated with higher rates of toxicity, including immune suppression and impaired wound healing.
Study Limitations
Although this study is one of the largest skull base ORN cohorts, its relatively small sample size limited its statistical power. The study was also limited by a patient cohort heterogeneous in terms of primary cancer pathology and ORN anatomical involvement. Because many of the patients received radiation therapy at other institutions, radiation dose and modality information were missing for several patients. Finally, the findings of this study do not provide insight regarding the best initial medical management strategies and the identification of which patients should initially be treated primarily with noninvasive strategies vs surgical intervention.
CONCLUSION
There are limited data regarding the surgical management of skull base ORN. In the cohort presented, we employed the strategy of maximum bony and soft tissue debridement with soft tissue reconstruction. We found that microvascular free flap reconstruction was significantly associated with a lower risk of ORN recurrence; it is feasible that in select cases, rotational flaps can also be effective when well vascularized tissue is available. Thus, the reconstruction strategy following surgical treatment of skull base ORN should be thoughtfully selected. In addition, surgical intervention in patients with ORN who are undergoing active cancer treatment should be carefully considered, as the risk of ORN recurrence is higher for these patients. This study sheds light on the management of skull base ORN and opens the discussion regarding the optimal surgical management of this understudied treatment-related complication.
Disclosures
This study was supported by the NIH/NCI under award number P30CA016672 and used the Clinical Trials Support Resource. The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Contributor Information
Ahmed Habib, Department of Neurosurgery, The University of Texas MD Anderson Cancer Center, Houston, Texas.
Matthew M Hanasono, Department of Plastic and Reconstructive Surgery, The University of Texas MD Anderson Cancer Center, Houston, Texas.
Franco DeMonte, Department of Neurosurgery, The University of Texas MD Anderson Cancer Center, Houston, Texas.
Ali Haider, Department of Neurosurgery, The University of Texas MD Anderson Cancer Center, Houston, Texas.
Jonathan D Breshears, Department of Neurosurgery, The University of Texas MD Anderson Cancer Center, Houston, Texas.
Marc-Elie Nader, Department of Head and Neck Surgery, The University of Texas MD Anderson Cancer Center, Houston, Texas.
Paul W Gidley, Department of Head and Neck Surgery, The University of Texas MD Anderson Cancer Center, Houston, Texas.
Shirley Y Su, Department of Head and Neck Surgery, The University of Texas MD Anderson Cancer Center, Houston, Texas.
Ehab Y Hanna, Department of Head and Neck Surgery, The University of Texas MD Anderson Cancer Center, Houston, Texas.
Shaan M Raza, Department of Neurosurgery, The University of Texas MD Anderson Cancer Center, Houston, Texas.
REFERENCES
- 1. Teng MS, Futran ND. Osteoradionecrosis of the mandible. Curr Opin Otolaryngol Head Neck Surg. 2005;13(4):217-221. [DOI] [PubMed] [Google Scholar]
- 2. Marx RE, Johnson RP. Studies in the radiobiology of osteoradionecrosis and their clinical significance. Oral Surg Oral Med Oral Pathol. 1987;64(4):379-390. [DOI] [PubMed] [Google Scholar]
- 3. Hoff AO, Toth B, Hu M, Hortobagyi GN, Gagel RF. Epidemiology and risk factors for osteonecrosis of the jaw in cancer patients. Ann N Y Acad Sci. 2011;1218(1):47-54. [DOI] [PubMed] [Google Scholar]
- 4. Glanzmann C, Gratz KW. Radionecrosis of the mandibula: a retrospective analysis of the incidence and risk factors. Radiother Oncol. 1995;36(2):94-100. [DOI] [PubMed] [Google Scholar]
- 5. Goldwaser BR, Chuang SK, Kaban LB, August M. Risk factor assessment for the development of osteoradionecrosis. J Oral Maxillofac Surg. 2007;65(11):2311-2316. [DOI] [PubMed] [Google Scholar]
- 6. Nabil S, Samman N. Risk factors for osteoradionecrosis after head and neck radiation: a systematic review. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;113(1):54-69. [DOI] [PubMed] [Google Scholar]
- 7. Zhuang Q, Zhang Z, Fu H, He J, He Y. Does radiation-induced fibrosis have an important role in pathophysiology of the osteoradionecrosis of jaw? Med Hypotheses. 2011;77(1):63-65. [DOI] [PubMed] [Google Scholar]
- 8. Madrid C, Abarca M, Bouferrache K. Osteoradionecrosis: an update. Oral Oncol. 2010;46(6):471-474. [DOI] [PubMed] [Google Scholar]
- 9. Delanian S, Chatel C, Porcher R, Depondt J, Lefaix JL. Complete restoration of refractory mandibular osteoradionecrosis by prolonged treatment with a pentoxifylline-tocopherol-clodronate combination (PENTOCLO): a phase II trial. Int J Radiat Oncol Biol Phys. 2011;80(3):832-839. [DOI] [PubMed] [Google Scholar]
- 10. Delanian S, Depondt J, Lefaix JL. Major healing of refractory mandible osteoradionecrosis after treatment combining pentoxifylline and tocopherol: a phase II trial. Head Neck. 2005;27(2):114-123. [DOI] [PubMed] [Google Scholar]
- 11. Jacobson AS, Buchbinder D, Hu K, Urken ML. Paradigm shifts in the management of osteoradionecrosis of the mandible. Oral Oncol. 2010;46(11):795-801. [DOI] [PubMed] [Google Scholar]
- 12. Chronopoulos A, Zarra T, Ehrenfeld M, Otto S. Osteoradionecrosis of the jaws: definition, epidemiology, staging and clinical and radiological findings. A concise review. Int Dent J. 2018;68(1):22-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hirsch DL, Bell RB, Dierks EJ, Potter JK, Potter BE. Analysis of microvascular free flaps for reconstruction of advanced mandibular osteoradionecrosis: a retrospective cohort study. J Oral Maxillofac Surg. 2008;66(12):2545-2556. [DOI] [PubMed] [Google Scholar]
- 14. Shaha AR, Cordeiro PG, Hidalgo DA et al. Resection and immediate microvascular reconstruction in the management of osteoradionecrosis of the mandible. Head Neck. 1997;19(5):406-411. [DOI] [PubMed] [Google Scholar]
- 15. Morrish RB Jr., Chan E, Silverman S Jr., Meyer J, Fu KK, Greenspan D. Osteonecrosis in patients irradiated for head and neck carcinoma. Cancer. 1981;47(8):1980-1983. [DOI] [PubMed] [Google Scholar]
- 16. Wong JK, Wood RE, McLean M. Conservative management of osteoradionecrosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84(1):16-21. [DOI] [PubMed] [Google Scholar]
- 17. Marwan H, Green JM 3rd, Tursun R, Marx RE. Recurrent malignancy in osteoradionecrosis specimen. J Oral Maxillofac Surg. 2016;74(11):2312-2316. [DOI] [PubMed] [Google Scholar]
- 18. Hao SP, Chen HC, Wei FC, Chen CY, Yeh AR, Su JL. Systematic management of osteoradionecrosis in the head and neck. Laryngoscope. 1999;109(8):1324-1327; discussion 1327-1328. [DOI] [PubMed] [Google Scholar]
- 19. Trotti A, Bellm LA, Epstein JB et al. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiother Oncol. 2003;66(3):253-262. [DOI] [PubMed] [Google Scholar]
- 20. Ma J, Mai HQ, Hong MH et al. Results of a prospective randomized trial comparing neoadjuvant chemotherapy plus radiotherapy with radiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma. J Clin Oncol. 2001;19(5):1350-1357. [DOI] [PubMed] [Google Scholar]
- 21. Lin S, Lu JJ, Han L, Chen Q, Pan J. Sequential chemotherapy and intensity-modulated radiation therapy in the management of locoregionally advanced nasopharyngeal carcinoma: experience of 370 consecutive cases. BMC Cancer. 2010;10(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen Y, Liu MZ, Liang SB et al. Preliminary results of a prospective randomized trial comparing concurrent chemoradiotherapy plus adjuvant chemotherapy with radiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma in endemic regions of China. Int J Radiat Oncol Biol Phys. 2008;71(5):1356-1364. [DOI] [PubMed] [Google Scholar]
- 23. Chang KP, Tsang NM, Chen CY, Su JL, Hao SP. Endoscopic management of skull base osteoradionecrosis. Laryngoscope. 2000;110(7):1162-1165. [DOI] [PubMed] [Google Scholar]
- 24. Huang XM, Zheng YQ, Zhang XM et al. Diagnosis and management of skull base osteoradionecrosis after radiotherapy for nasopharyngeal carcinoma. Laryngoscope. 2006;116(9):1626-1631. [DOI] [PubMed] [Google Scholar]
- 25. Irish JC, Gullane PJ, Gentili F et al. Tumors of the skull base: outcome and survival analysis of 77 cases. Head Neck. 1994;16(1):3-10. [DOI] [PubMed] [Google Scholar]
- 26. Beumer J, Harrison R, Sanders B, Kurrasch M. Osteoradionecrosis: predisposing factors and outcomes of therapy. Head Neck. 1984;6(4):819-827. [DOI] [PubMed] [Google Scholar]
- 27. Epstein JB, Rea G, Wong FL, Spinelli J, Stevenson-Moore P. Osteonecrosis: study of the relationship of dental extractions in patients receiving radiotherapy. Head Neck. 1987;10(1):48-54. [DOI] [PubMed] [Google Scholar]
- 28. Harris M. The conservative management of osteoradionecrosis of the mandible with ultrasound therapy. Br J Oral Maxillofac Surg. 1992;30(5):313-318. [DOI] [PubMed] [Google Scholar]
- 29. Store G, Eribe ER, Olsen I. DNA-DNA hybridization demonstrates multiple bacteria in osteoradionecrosis. Int J Oral Maxillofac Surg. 2005;34(2):193-196. [DOI] [PubMed] [Google Scholar]
- 30. Marx RE. Osteoradionecrosis: a new concept of its pathophysiology. J Oral Maxillofac Surg. 1983;41(5):283-288. [DOI] [PubMed] [Google Scholar]
- 31. Rivero JA, Shamji O, Kolokythas A. Osteoradionecrosis: a review of pathophysiology, prevention and pharmacologic management using pentoxifylline, alpha-tocopherol, and clodronate. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;124(5):464-471. [DOI] [PubMed] [Google Scholar]
- 32. Jereczek-Fossa BA, Orecchia R. Radiotherapy-induced mandibular bone complications. Cancer Treat Rev. 2002;28(1):65-74. [DOI] [PubMed] [Google Scholar]
- 33. Poort LJ, Postma AA, Stadler AAR, Bockmann RA, Hoebers FJ, Kessler P. Radiological changes with magnetic resonance imaging and computed tomography after irradiating minipig mandibles: the role of T2-SPIR mixed signal intensities in the detection of osteoradionecrosis. J Craniomaxillofac Surg. 2017;45(5):607-613. [DOI] [PubMed] [Google Scholar]
- 34. Moen I, Stuhr LE. Hyperbaric oxygen therapy and cancer–a review. Target Oncol. 2012;7(4):233-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stepien K, Ostrowski RP, Matyja E. Hyperbaric oxygen as an adjunctive therapy in treatment of malignancies, including brain tumours. Med Oncol. 2016;33(9):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Marx RE, Ehler WJ, Tayapongsak P, Pierce LW. Relationship of oxygen dose to angiogenesis induction in irradiated tissue. Am J Surg. 1990;160(5):519-524. [DOI] [PubMed] [Google Scholar]
- 37. Annane D, Depondt J, Aubert P et al. Hyperbaric oxygen therapy for radionecrosis of the jaw: a randomized, placebo-controlled, double-blind trial from the ORN96 study group. J Clin Oncol. 2004;22(24):4893-4900. [DOI] [PubMed] [Google Scholar]
- 38. Myers RA, Marx RE. Use of hyperbaric oxygen in postradiation head and neck surgery. NCI Monogr. 1990(9):151-157. [PubMed] [Google Scholar]
- 39. Sultan A, Hanna GJ, Margalit DN et al. The use of hyperbaric oxygen for the prevention and management of osteoradionecrosis of the jaw: a dana-farber/brigham and women's cancer center multidisciplinary guideline. Oncologist. 2017;22(3):343-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Delanian S, Lefaix JL. The radiation-induced fibroatrophic process: therapeutic perspective via the antioxidant pathway. Radiother Oncol. 2004;73(2):119-131. [DOI] [PubMed] [Google Scholar]
- 41. Robard L, Louis MY, Blanchard D, Babin E, Delanian S. Medical treatment of osteoradionecrosis of the mandible by PENTOCLO: preliminary results. Eur Ann Otorhinolaryngol Head Neck Dis. 2014;131(6):333-338. [DOI] [PubMed] [Google Scholar]
- 42. Ang E, Black C, Irish J et al. Reconstructive options in the treatment of osteoradionecrosis of the craniomaxillofacial skeleton. Br J Plast Surg. 2003;56(2):92-99. [DOI] [PubMed] [Google Scholar]
- 43. Curi MM, Oliveira dos Santos M, Feher O, Faria JC, Rodrigues ML, Kowalski LP. Management of extensive osteoradionecrosis of the mandible with radical resection and immediate microvascular reconstruction. J Oral Maxillofac Surg. 2007;65(3):434-438. [DOI] [PubMed] [Google Scholar]
- 44. Rommel N, Kesting MR, Rohleder NH, Wolff KD, Weitz J. Surgical management of severe osteoradionecrosis of the mandibular bone by using double free flap reconstruction. J Craniomaxillofac Surg. 2018;46(1):148-154. [DOI] [PubMed] [Google Scholar]
- 45. Pitak-Arnnop P, Sader R, Dhanuthai K et al. Management of osteoradionecrosis of the jaws: an analysis of evidence. Eur J Surg Oncol. 2008;34(10):1123-1134. [DOI] [PubMed] [Google Scholar]
- 46. Hanasono MM, Silva A, Skoracki RJ et al. Skull base reconstruction. Plast Reconstr Surg. 2011;128(3):675-686. [DOI] [PubMed] [Google Scholar]
- 47. Mericli AF, Schaverien MV, Hanasono MM et al. Using a second free fibula osteocutaneous flap after repeated mandibulectomy is associated with a low complication rate and acceptable functional outcomes. Plast Reconstr Surg. 2017;140(2):381-389. [DOI] [PubMed] [Google Scholar]
- 48. Shan XF, Li RH, Lu XG, Cai ZG, Zhang J, Zhang JG. Fibular free flap reconstruction for the management of advanced bilateral mandibular osteoradionecrosis. J Craniofac Surg. 2015;26(2):e172-e175. [DOI] [PubMed] [Google Scholar]
- 49. Lee M, Chin RY, Eslick GD, Sritharan N, Paramaesvaran S. Outcomes of microvascular free flap reconstruction for mandibular osteoradionecrosis: a systematic review. J Craniomaxillofac Surg. 2015;43(10):2026-2033. [DOI] [PubMed] [Google Scholar]