ABSTRACT
As the science surrounding population sodium reduction evolves, monitoring and evaluating new studies on intake and health can help increase our understanding of the associated benefits and risks. Here we describe a systematic review of recent studies on sodium intake and health, examine the risk of bias (ROB) of selected studies, and provide direction for future research. Seven online databases were searched monthly from January 2015 to December 2019. We selected human studies that met specified population, intervention, comparison, outcome, time, setting/study design (PICOTS) criteria and abstracted attributes related to the study population, design, intervention, exposure, and outcomes, and evaluated ROB for the subset of studies on sodium intake and cardiovascular disease risks or indicators. Of 41,601 abstracts reviewed, 231 studies were identified that met the PICOTS criteria and ROB was assessed for 54 studies. One hundred and fifty-seven (68%) studies were observational and 161 (70%) focused on the general population. Five types of sodium interventions and a variety of urinary and dietary measurement methods were used to establish and quantify sodium intake. Five observational studies used multiple 24-h urine collections to assess sodium intake. Evidence mainly focused on cardiovascular-related indicators (48%) but encompassed an assortment of outcomes. Studies varied in ROB domains and 87% of studies evaluated were missing information on ≥1 domains. Two or more studies on each of 12 outcomes (e.g., cognition) not previously included in systematic reviews and 9 new studies at low ROB suggest the need for ongoing or updated systematic reviews of evidence on sodium intake and health. Summarizing evidence from assessments on sodium and health outcomes was limited by the various methods used to measure sodium intake and outcomes, as well as lack of details related to study design and conduct. In line with research recommendations identified by the National Academies of Science, future research is needed to identify and standardize methods for measuring sodium intake.
Keywords: dietary sodium, health indicators, reduction, risk of bias, cardiovascular health
Here we describe a systematic review of recent studies on sodium intake and health, examine the risk of bias of selected studies, and provide direction for future research.
Introduction
Based on the large body of evidence demonstrating the adverse health effects of excess sodium intake, numerous public health organizations and authoritative scientific bodies recommend dietary sodium reduction (1–6). In 2013, the Institute of Medicine (IOM) convened an expert panel “to examine the designs, methodologies, and conclusions of emerging” scientific evidence on sodium and health outcomes (2). Although the committee concluded the available evidence indicated a positive relation between higher sodium intake and risk of cardiovascular disease (CVD) outcomes (including stroke, CVD mortality, and all-cause mortality), consistent with efforts to reduce population sodium intake, they found limited evidence that suggested decreasing sodium intake could possibly reduce risk of gastric cancer and no consistent evidence on other health outcomes. Further, they also identified several areas for future research based on a number of methodological and data gaps (2). Research recommendations applicable to this review included standardizing methodological approaches to measuring sodium intake, using sodium levels for analyses (i.e., 1500–2300 mg) corresponding with current guidelines, using appropriate methods to account for potential confounding, and a need for randomized controlled trial (RCT) research (2). As the science on sodium reduction evolves, monitoring and evaluating newly published studies on intake and health can increase our understanding of the reported health benefits and risks and drive directions for future research.
Since the 2013 IOM report, there have been several meta-analyses and reports reviewing evidence related to sodium and health [including the 2019 National Academies of Science, Engineering, and Medicine (NASEM) report updating DRIs for sodium and potassium] (3, 7–9); however, reviews focused on specific outcomes and conclusions can become outdated as new evidence emerges. The 2019 DRI for sodium included Adequate Intake (AI) levels at 1500 mg/d and Chronic Disease Risk Reduction (CDRR) levels (i.e., individuals should lower their intake if it is above this level to reduce chronic disease risk) at 2300 mg/d for individuals aged ≥14 y. Lower AI and CDRR levels were set for children aged ≤13 y (3). To our knowledge, there is only 1 ongoing systematic review (i.e., The Science of Salt) (10) of studies related to sodium intake and health outcomes that is published and regularly updated (11). Although the aim of our ongoing systematic review of the literature is similar in relation to health outcomes from The Science of Salt, the scope and methods differ. In brief, the Science of Salt review uses key criteria to select studies that are relevant to clinical and public health (i.e., 24-h urine collections for prospective studies, studies conducted in non-ill populations, intervention periods > 4 wk) (10), whereas our search is broader and includes additional databases and study designs, and is not limited by duration of study or intervention nor the levels of actual sodium intake achieved. The objectives of this ongoing review are to 1) describe the characteristics of recent studies examining the effects and associations of sodium intake on health risks or indicators; 2) evaluate the strengths and biases of the study design and methods of prospective cohort studies and intervention trials examining cardiovascular disease (CVD) risks or indicators; and 3) provide direction for future research. For this report, we evaluated the current literature with respect to the research recommendations outlined in the 2013 IOM report to determine if emerging evidence since that time addressed the aforementioned selected methodological and data gaps, including those not meeting criteria in other systematic reviews (2). In this article, we report the results for the period of January 2015 through December 2019.
Methodology
This systematic literature review was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (12) (Supplemental Table 1).
Eligibility criteria
Articles reporting results from studies with an objective to examine the effect or association of dietary sodium intake with ≥1 health indicator were included if they met the population, intervention, comparison, outcome, time, setting/study design (PICOTS) criteria (Supplemental Table 2). Briefly, we included studies focused on the general healthy population and populations with specific chronic diseases (Supplemental Table 2). We excluded trials if the independent effect of sodium in the intervention could not be determined and studies that did not quantify sodium intake exposure. We did not exclude any health risks or indicators. We included intervention trials regardless of randomization, observational studies, and systematic reviews/meta-analyses (Supplemental Table 2). Secondary analyses of participants from the same study were treated as independent studies if the authors focused on mutually exclusive health indicators. Systematic reviews/meta-analyses were treated independently as long as the objectives, outcomes, or methods differed.
Search strategy
Relevant abstracts were identified on a monthly basis through an electronic search of 7 online databases (Supplemental Table 3). Our search was restricted to humans.
Study selection
Each month, titles and abstracts of potential articles were manually screened by a single reviewer for studies examining ≥1 health indicator in relation to dietary sodium intake. Full-text articles were ordered for selected abstracts. Two researchers independently reviewed the full-text articles against our PICOTS criteria (Supplemental Table 2). Disagreements in assessments were resolved by discussion or a third reviewer. Articles published in a language other than English were reviewed with assistance from a native speaker.
Data extraction
Information from an included article was transcribed by 1 author into tables specific to the study design. For all studies, we abstracted the number of participants, percentage male, mean age, country, study name (if applicable), participant selection criteria, duration of trial/follow-up, health indicators, and methods used to quantify dietary sodium intake. The WHO's regions were reported, if studies were conducted in ≥5 countries (Supplemental Tables 4–6) (13). Standard conversions were used to report all sodium intake in milligrams per day (14). Health risks and indicators were categorized similarly to groupings of intermediate markers for health outcomes and clinical health outcomes described previously (2).
We evaluated studies with respect to the following methodological and data gaps adapted from the 2013 IOM report (2). Did studies (or systematic reviews) 1) focus specifically on African Americans, adults aged 51–70 y, ≥70 y, or other higher-risk subgroups (particularly through RCTs); 2) include recommended methods to measure sodium intake (e.g., use of multiple 24-h urine collections in observational studies); or 3) evaluate dietary sodium intake consistent with DRI levels (e.g., 1500–2300 mg/d)? Further, we specifically identified whether published RCTs evaluated the effects of a range of sodium levels 1) on risk of CVD events, stroke, and mortality (particularly among patients in controlled environments such as chronic care facilities); and 2) among chronic heart failure (CHF) patients receiving therapeutic treatments typically used in the United States. Lastly, did observational studies examine associations between sodium intake and cancer (particularly, gastric cancer) in the US population (2)?
Sodium intake measures were classified according to collection approach and method: dietary (i.e., FFQs, diet recalls, or food diaries) and urinary [i.e., partial (spot or <24-h urine) or 24-h urine]. Each assessment method (e.g., single 24-h urine) comes with particular strengths, limitations, and applications (3, 15). For example, sodium intake and excretion vary from day to day, thus accurate estimation of long-term dietary sodium intake in observational studies requires >1 nonconsecutive 24-h dietary recall or urine collection to account for random measurement error (3, 16, 17). A 24-h urine collection, when complete, is considered an unbiased indicator of short-term sodium intake and is not subject to systematic error (representing ∼90% of sodium consumed from all sources over the last few days), thus it can be used for characterizing differences in group mean intake in intervention studies (18). Estimation of sodium intake based on dietary methods may be subject to errors in self-report or nutrient databases, whereas spot urine sodium concentration may be subject to errors due to diurnal variation or in other variables used in equations to predict sodium intake (15, 18). Thus, we reported the approach, assessment method, number of collections, time period/duration for the collection, and, if applicable, succession of collections (i.e., on consecutive or nonconsecutive days) (19).
Risk of bias assessment
The totality of evidence from well-designed trials and cohort studies forms the basis for conclusions about causal relations between particular exposures and health indicators (20). Thus, we assessed the risk of bias (ROB) within trials and cohort studies. Owing to the range of health indicators included in the review and the need to develop ROB criteria specific to the outcome of interest, we limited the ROB assessment to all-cause mortality, CVD events (e.g., mortality or hospitalization), subclinical CVD indicators [e.g., pulse wave velocity (PWV)], and blood pressure (BP) (2, 20). ROB criteria used for assessment of trials were adapted from the Cochrane ROB tool (RCTs), Risk of Bias in Non-Randomised Studies (ROBINS-I) tool (nonrandomized trials and cohort studies), and Cobb's criteria (cohort studies) (21, 22). The formation of the ROB assessment tools was guided by study design, focused on studies’ internal validity, and required both methodological and subject matter expertise to address challenges inherent in the design, conduct, and analyses of included studies. The ROB abstraction and instruction forms (specific to the study's design and health outcome) went through testing by multiple reviewers and several iterations before the completion of the final tools used for the ROB assessment presented in this review (Supplemental Tables 7–9). Adherence to the intervention (defined as a ratio of the measured difference in sodium intake to the expected difference between intervention groups of 90%–110%), a measure that assessed the extent to which participants in each of the intervention groups followed the treatment regimen, diet, or counseling prescribed by the researchers, was also examined in trials to determine the uptake and impact of the intervention. Two researchers independently assessed each study included in the ROB review and any discrepancies were resolved through discussion or by a third reviewer.
Owing to the nature of the review (current, rather than complete, assessment of the literature) and variation in health indicators (e.g., PWV and QT-interval dispersion are both measures of cardiac function) and analyses (e.g., marginal models compared with ANOVA), we did not perform a meta-analysis. However, results of included studies on sodium intake and mortality, CVD, and BP were summarized qualitatively.
Results
Study selection and characteristics
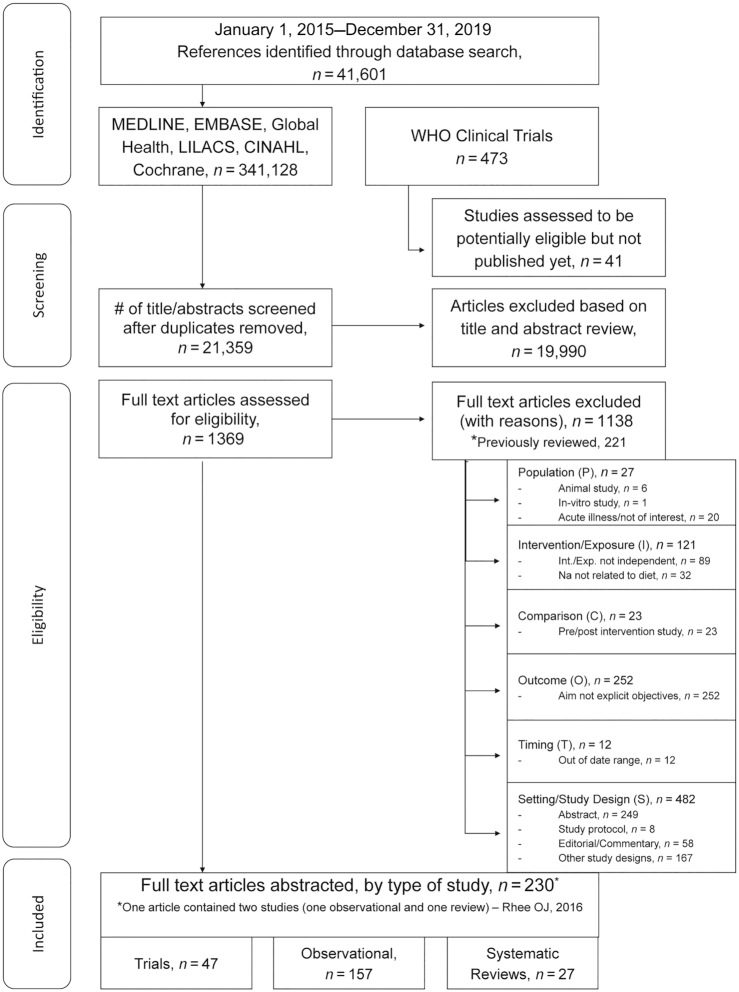
The search identified 41,601 potential articles published between 1 January, 2015 and 31 December, 2019 for inclusion in the current report. Overall, 1369 articles were eligible for full-text review and 230 articles, comprising 231 studies, were included (23–251) (Figure 1). One article included both a meta-analysis and a cross-sectional study (182) (Figure 1). Forty-seven intervention trials (34 RCTs and 13 non-RCTs) (23–69), 157 observational studies (52 cohort, 4 case-control, and 101 cross-sectional) (70–226), and 27 systematic reviews/meta-analyses (26 meta-analyses and 1 systematic review) (227–251) were included (Figure 1, Supplemental Tables 4–6).
FIGURE 1.
Flow diagram depicting the screening and selection of studies.
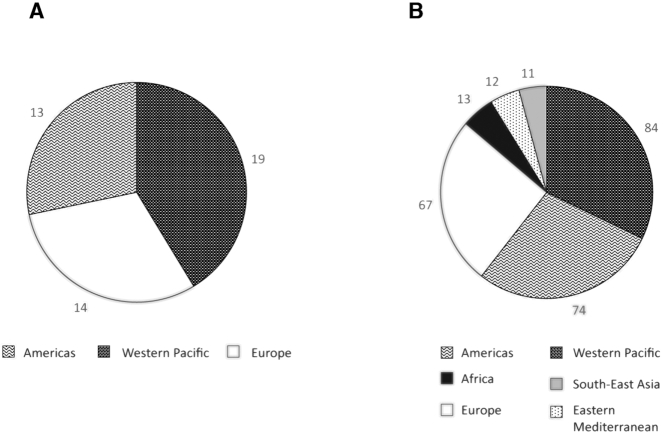
Most studies were conducted in developed countries and several cohort studies included participants from multiple countries. For example, the Prospective Urban Rural Epidemiological (PURE) cohort recruited participants from 21 countries (136 , 152). Trials were conducted in 3 of the 6 WHO regions (Western Pacific, Europe, Americas), whereas observational studies were conducted in all of the WHO regions, with the most studies also coming from the Western Pacific, Americas, and Europe (Figure 2A, B). With the exception of 6 meta-analyses that did not specify the locations of their included studies, all meta-analyses included participants from ≥4 countries (Supplemental Table 6).
FIGURE 2.
Distribution of study location sites by WHO region among trials (A) and observational studies (B), 2015–2019. The number of countries is not equal to the number of studies, because 1 study could enroll participants from multiple countries [e.g., the Prospective Urban Rural Epidemiological (PURE) study was conducted in 21 countries (136 , 152)].
Population characteristics
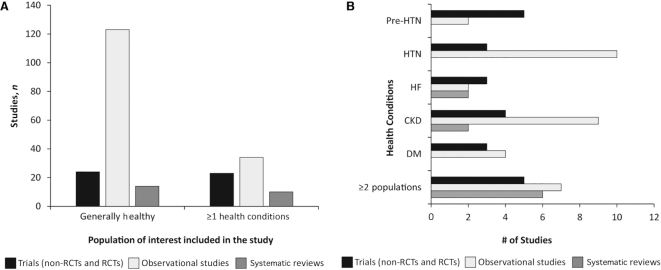
Among the studies evaluated, 161 enrolled generally healthy participants and 67 targeted and specifically enrolled participants with ≥1 of the selected chronic conditions [e.g., 15 studies specifically enrolled persons with chronic kidney disease (CKD)]. Eighteen studies recruited participants that fit in ≥2 groupings of interest owing to the analytic design of the study [n = 6, 4 case-control (80, 145, 150, 200) and 2 stratified analyses (34, 232)], health-specific inclusion criteria (n = 5) (28, 32, 44, 53, 186), or the inclusion of multiple cohorts/studies (n = 7) (152, 154, 233–235, 247) (Figure 3A, B, Supplemental Tables 4–6). Three systematic reviews did not report participants’ health selection criteria (228, 236, 239). Roughly 49% (n = 23) of all RCTs included in this review were conducted among persons with ≥1 specific chronic disease conditions, as opposed to 22% (n = 34) of included observational studies (Figure 3A). At least 2 studies (trials and observational studies combined) were conducted among participants with each of the conditions of interest (Figure 3B). Three parallel RCTs (31, 33, 42) and 1 cohort study (186) specifically recruited patients with heart failure (HF) (Figure 3B, Supplemental Table 4). Of these, 2 RCTs evaluated subclinical CVD indicators (31, 42), 1 RCT evaluated serum sodium (33), and 1 cohort evaluated CVD events (186) (Supplemental Table 4) and their results are discussed below in the ROB assessment.
FIGURE 3.
Distribution of the population health status (i.e., generally healthy populations compared with populations with health conditions of interest) of included studies by study design (A) and distribution of specific health conditions of interest among studies of populations with ≥1 health conditions (n = 64) (B). Healthy population refers to recruiting participants from the general population which can include healthy participants with health conditions (i.e., DM, HTN, HF, and/or pre-HTN). CKD, chronic kidney disease; DM, diabetes mellitus; HF, heart failure; HTN, hypertension; pre-HTN, prehypertension; RCT, randomized controlled trial.
The majority of trials and observational studies were conducted among persons aged 18–79 y of both sexes; however, there were a few exceptions where the study focused on participants of a specific sex, age group, or higher-risk population as defined by the IOM (Supplemental Tables 4–6). Two trials (47, 55) and 1 observational study (191) enrolled only male participants, whereas 1 trial (57) and 9 observational studies (76, 81, 93, 109, 126, 134, 171, 172, 175) enrolled only female participants (Supplemental Tables 4, 5). One trial (37, 38) (Supplemental Table 4) and 19 observational studies (72, 76, 77, 85, 90, 92, 101, 110, 132, 133, 150, 159, 169, 176, 190, 196, 203, 214, 226) enrolled children or adolescents (Supplemental Table 5). Four trials (25, 29, 42, 43) and 9 observational studies (74, 81, 109, 138, 140, 154, 175, 184, 194) specifically enrolled adults aged 50–80 y. While no trials specifically enrolled adults aged ≥70 y, 4 observational studies (117, 123, 131, 165) focused on this population. One RCT recruited only untreated, African-American hypertensives to examine the effects of dietary sodium reduction on changes in metabolomics profiling in this population (30). Further, 7 trials included African-American participants (24, 27, 29, 32, 44, 53, 58), although none had objectives to examine the effects of sodium on health indicators among this group separately, whereas 13 observational studies had objectives specific to examining the association between sodium and health indicators among African Americans (76, 94, 123, 139, 147, 178, 188, 197, 209, 226), American Indians (108), or Mexican Americans (97, 158).
Sodium intake exposure
RCTs
Among the 47 trials included in this review, researchers administered 5 types of sodium interventions: feeding trials of different levels of sodium in foods (n = 23), dietary trials of 1 level of sodium in food plus sodium supplements and/or placebos (n = 5), dietary counseling trials (n = 12), a trial using warning stickers on high-sodium foods (n = 1), and trials using a combination of ≥2 intervention types (n = 6) (Table 1). Of the 15 parallel trials, 10 (67%) were dietary counseling trials, whereas of the 32 crossover trials, 20 (63%) were feeding trials. The assigned dietary interventions took place in a variety of locations: 17 at a study center, 10 in the participant's home, 2 in a hospital, and 18 in >1 location.
TABLE 1.
Characteristics of dietary sodium interventions among 46 published trials with health indicators, January 2015–December 20191
| Duration, wk3 | Intended sodium intakes, mg/d | Actual sodium intakes, mg/d | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Type2 | Place | LS | HS | Diff | Sodium measure | LS | HS | Diff | Adherence4 | |
| Parallel RCT | |||||||||||
| Fabricio et al. (33) | Diet + supp | Hospital | 1 | 1200 | 2800 | 1600 | Multiple, 24-h recalls | 998 | 2467 | 1469 | 103% |
| Hummel et al. (42)5 | Diet | Home | 4 | 1500 | 2000 | 500 | Single, 24-h urine | NR | NR | NR | Cannot be calculated |
| Kang et al. (45) | Diet | Home | 8 | 2000 | 5000 | 3000 | Single, 24-h urine | 2848 | 3517 | 669 | 22% |
| Serizawa et al. (57)6 | Diet | Hospital | 2.3 | 2400 | 4800 | 2400 | Multiple, spot urine samples | NR | NR | NR | NR |
| Chen L et al. (29) | Edu | Study center | 156 | <1800 | NA | NA | Multiple, non consecutive, 24-h urine collections | 2371 | 3314 | 943 | Cannot be calculated |
| Colin-Ramirez et al. (31) | Edu | Home | 24 | 1500 | 2300 | 800 | Multiple, 3-d diet recalls | 1398 | 1461 | 63 | 8% |
| Gant et al. (35) | Edu | Home | 6 | 1200 | 4800 | 3600 | Single, 24-h urine | 2047 | 4600 | 2553 | 71% |
| He FJ et al. (37)7 | Edu | School + home | 14 | — | — | Reduce intake by 20% | Multiple, consecutive, 24-h urine collections | 2574 (C); 4056 (A) | 3120 (C); 4719 (A) | 741 (C); 1131 (A) | Reduced by 27% (C) and 25% (A) |
| He FJ et al. (38)7 | Edu | School + home | 14 | — | — | Reduce intake by 20% | Multiple, consecutive, 24-h urine collections | 2574 (C); 4056 (A) | 3120 (C); 4719 (A) | 741 (C); 1131 (A) | Reduced by 27% (C) and 25% (A) |
| Keyzer et al. (46) | Edu | Study center + home | 8 | 1150 | 4600 | 3450 | Multiple, nonconsecutive, 24-h urine collections | 2484 | 4002 | 1518 | 44% |
| Meuleman et al. (49) | Edu | Study center + home | 12 | NR | NR | NR | Multiple, nonconsecutive, 24-h urine collections | 3181 | 4069 | 888 | Cannot be calculated |
| Nakano et al. (51) | Edu | Study center | 12 | <2340 | NA | NA | Single, 24-h urine | 2652 | 3354 | 702 | Cannot be calculated |
| Parvanova et al. (52) | Edu | Study center + home | 8 | 2400 | 4800 | 2400 | Multiple, nonconsecutive, 24-h urine collections | 3831 | 4523 | 692 | 29% |
| Takada et al. (59) | Edu | Study center + home | 4 | 3432 | 3822 | 390 | Multiple, consecutive, overnight urine collections | 3354 | 3498 | 144 | 37% |
| Pinjuh Markota et al. (48) | Warning stickers | Home | 8 | 4025 | 4600 | 575 | Single, 24-h urine | 4057 | 4600 | 543 | 94% |
| Median value of parallel trials | 8 | 1900 | 4600 | 2340 | 2848; 2652 (with C) | 4002; 3517 (with C) | 888; 741 (with C) | ||||
| Crossover RCT | |||||||||||
| Babcock et al. (23) | Diet | Home | 1 | 460 | 6900 | 6440 | Single, 24-h urine | 483 | 4545 | 4062 | 63% |
| Babcock et al. (24) | Diet | Home | 1.4 | 1000 | 2300 | 1300 | Single, 24-h urine | 846 | 1950 | 1104 | 85% |
| Brian et al. (27) | Diet | Home | 1 | 520 | 7119 | 6599 | Single, 24-h urine | 661 | 5405 | 4744 | 72% |
| Derkach et al. (32)8 | Diet | Study center + home | 4 | 1150 | 3450 | 2300 | Single, 24-h urine | NR | NR | NR | Cannot be calculated |
| Muth et al. (50)9 | Diet | Home | 1 | 460 | 6900 | 6440 | Single, 24-h urine | ∼900 | ∼5750 | ∼4850 | 75% |
| Juraschek et al. (44)8 | Diet | Study center + home | 4 | 1150 | 3450 | 2300 | Single, 24-h urine | NR | NR | NR | Cannot be calculated |
| Peng et al. (53)8 | Diet | Study center + home | 4 | 1150 | 3450 | 2300 | Single, 24-h urine | NR | NR | NR | Cannot be calculated |
| Rorije et al. (55) | Diet | Study center + home | 2 | <1200 | >4800 | ≥3600 | Multiple, nonconsecutive, 24-h urine collections | 920 | 6072 | 5152 | 143% |
| Foo et al. (34) | Diet + supp | Study center | 0.86 | 920 | 4140 | 3220 | Single, 24-h urine | 1804 | 5653 | 3849 | 119% |
| Gijsbers et al. (36) | Diet + supp | Study center + home | 4 | 2000 | 5000 | 3000 | Single, 24-h urine | 2417 | 4667 | 2250 | 75% |
| Riphagen et al. (54) | Diet + supp | Study center + home | 4 | 2400 | 5400 | 3000 | Single, 24-h urine | 2346 | 4623 | 2277 | 76% |
| Baqar et al. (25) | Comb (edu + supp) | Study center | 3 | NR | NR | NR | Single, 24-h dietary recall | 3541 | 4715 | 1174 | Cannot be calculated |
| Cashman et al. (28) | Comb (edu + bread) | Study center + home | 5 | NR | NR | NR | Single, 24-h urine | 1784 | 2438 | 654 | Cannot be calculated |
| Jablonski et al. (43) | Comb (supp + edu) | Study center + home | 5 | 1150 | 3450 | 2300 | Multiple, nonconsecutive, 24-h urine collections | 1610 | 3519 | 1909 | 83% |
| Suckling et al. (58) | Comb (supp + edu) | Study center + home | 6 | 2070 | 4140 | 2070 | Multiple, consecutive, 24-h urine collections | 2682 | 3797 | 1115 | 54% |
| Chen L et al. (30) | Comb (supp + edu) | Study center + home | 6 | <2000 | NA | NA | Multiple, consecutive, 24-h urine collections | 2650 | 3751 | 1101 | Cannot be calculated |
| Todd et al. (60) | Comb (diet—tomato juice + supp) | Home | 4 | 1380 | 5750 | 4370 | Multiple, nonconsecutive, spot urine samples | NR | NR | NR | Cannot be calculated |
| Saran et al. (56) | Edu | Study center | 4 | <2000 | NA | NA | Single, 24-h urine | 2419 | 3928 | 1509 | Cannot be calculated |
| Toering et al. (61) | Edu | Study center + home | 1 | 1150 | 4600 | 3450 | Single, 24-h urine | 920 | 4842 | 3922 | 114% |
| Crossover non-RCT | |||||||||||
| Baric et al. (26) | Diet + supp | Study center + home | 1 | 1400 | 5880 | 4480 | Single, 24-h urine | 2461 | 5750 | 3289 | 73% |
| He M et al. (39)9 | Diet | Study center | 1 | 1170 | 7020 | 5850 | Single, 24-h urine | ∼1150 | ∼6325 | ∼5175 | 88% |
| Hu J-W et al. (40) | Diet | Study center | 1 | 1170 | 7020 | 5850 | Single, 24-h urine | 1819 | 6483 | 4664 | 80% |
| Hu J-W et al. (41) | Diet | Study center | 1 | 1170 | 7020 | 5850 | Single, 24-h urine | 1868 | 3855 | 1987 | 34% |
| Liu F-Q et al. (47) | Diet | Study center | 1 | 1170 | 7020 | 5850 | Single, 24-h urine | 2291 | 5624 | 3333 | 57% |
| Wan et al. (62) | Diet | Study center | 1 | 1170 | 7020 | 5850 | Multiple, consecutive, overnight urine collections | NR | NR | NR | Cannot be calculated |
| Wang Y et al. (64) | Diet | Study center | 1 | 1170 | 7020 | 5850 | Single, 24-h urine | 2335 | 5808 | 3473 | 59% |
| Wang Y et al. (65) | Diet | Study center | 1 | 1170 | 7020 | 5850 | Single, 24-h urine | 2332 | 5824 | 3491 | 60% |
| Wang Y-Y et al. (67) | Diet | Study center | 1 | 1170 | 7020 | 5850 | Single, 24-h urine | 2328 | 5789 | 3462 | 59% |
| Wang K et al. (63) | Diet | Study center | 1 | 1170 | 7020 | 5850 | Single, 24-h urine | 1819 | 6484 | 4665 | 80% |
| Wang Y et al. (66) | Diet | Study center | 1 | 1170 | 7020 | 5850 | Single, 24-h urine | 2098 | 6134 | 4036 | 69% |
| Zhang et al. (69) | Diet | Study center | 1 | 1170 | 7020 | 5850 | Single, 24-h urine | 2272 | 6164 | 3892 | 67% |
| Zhang et al. (68)8 | Diet | Study center | 1 | 1170 | 7020 | 5850 | Single, 24-h urine | ∼2300 | ∼5750 | ∼3450 | 59% |
| Median values of crossover trials | 1 | 1170 | 6900 | 5850 | 1983 | 5515 | 3456 | ||||
| Median values of all trials | 4 | 1170 | 5000 | 3450 | 2310; 2334 (with C) | 4645; 4612 (with C) | 2119; 1948 (with C) | ||||
| Range of all trials | 0.86–156 | 460–4025 | 2000–7119 | 390–6599 | 483–4057 | 1461–6484 | 63–5175 | 8%–143% | |||
A, adults; C, children; Comb, combination; Diff, difference; Edu, education; HS, high sodium; LS, low sodium; NA, not applicable; NR, not reported; RCT, randomized controlled trial; Supp, supplements.
Diet was defined as studies that prepared food on-site and provided all foods and beverages to participants (i.e., feeding trials). Diet + Supp intervention was where participants were prescribed an LS “baseline” diet and then were allocated to receive either placebo tablets or salt supplements. Edu was where participants were either counseled on diet, provided personalized meal plans, or provided dietary materials such as menus. A Comb intervention was defined as the use of ≥1 intervention type (e.g., dietary education/counseling + supplements).
Duration refers to the period of time for each intervention (e.g., participants received tablets or placebo for 6 wk, crossing over to take the opposite tablet for a further 6 wk) (58). Six crossover RCTs used a washout period between intended sodium levels of 4-wk (24, 34), 3-wk (25) and 2-wk (56, 60) duration. A standard dietary run-in period was used in 12 of the 19 crossover RCTs (range: 7–14 d) (23, 27, 30, 32, 36, 43, 44, 50, 53, 54, 58, 60).
Adherence was calculated by taking the ratio of the measured difference in sodium intake to the expected difference between intervention groups. Inadequate adherence was defined as <90% or >110%.
Hummel et al. (42) reported changes in urinary sodium excretion over the duration of the intervention in a supplementary figure; however, they did not provide numbers. The figure indicates that the LS and HS groups were similar in “actual sodium levels.”
Secondary analysis of a 2004 metabolic study (253). No data on actual measured sodium levels were reported.
Baseline sodium intake was 3822 mg/d in the control group and 4212 mg/d in the intervention group (mean: 4017 mg/d). Actual difference estimates were adjusted for age, sex, BMI, stratification variables at randomization (school location/class size), and indoor and outdoor temperature.
Secondary analyses of the Dietary Approaches to Stop Hypertension-Sodium trial using subsets of participants from the original trial. Actual low and high sodium intake levels were not reported in these subpopulations.
Data on the actual measured sodium levels were presented graphically in the publication. Reported levels are estimated from the graph.
The median duration of the dietary interventions included in this review was 4 wk (range: 5 d–6 mo) (Table 1); however, intervention duration varied by trial design. Parallel trials had a median duration of 8 wk (range: 1–156 wk), whereas crossover trials had a median duration of 1 wk (range: 5 d–6 wk). A standard dietary run-in period was used in 12 of the 19 randomized crossover trials (range: 7–14 d) (23, 27, 30, 32, 36, 43, 44, 50, 53, 54, 58, 60). One randomized crossover trial required a 6-wk washout period before trial entry for participants prescribed antihypertensive agents capable of affecting the renin-angiotensin-aldosterone system (RAAS) (25). Of the 32 crossover trials included, 5 had a defined washout period of ≥2 wk (25, 56, 60) or ≥4 wk (24, 34), within the design of the dietary intervention. Participants could resume their usual diets during 5-d breaks in 3 ancillary reports of the Dietary Approaches to Stop Hypertension (DASH)-Sodium trial (32, 44, 53), because the investigators reported that the intervention period at each level of sodium intake was long enough to minimize the potential for carryover effects, i.e., 4 and 8 wk, respectively (252). Two studies reported that intervention periods were not separated by a washout (36, 55) and, of these, only 1 had an intervention period lasting ≥4 wk (36). One randomized crossover trial, that had an intervention period ≥4 wk with no washout, tested for and found no significant carryover or residual effects for each outcome (28).
Among the included trials, the intended level of sodium intake in the low-sodium (LS) group ranged from 460 (23) to 4025 mg/d (48) (median: 1170 mg/d) and in the high-sodium group from 2000 (42) to 7119 mg/d (27) (median: 3450 mg/d). With the exception of 7 trials included in this review that did not report actual sodium values (32, 42, 44, 53, 57, 60, 62) and 4 trials that used a single dietary recall (25), multiple dietary recalls (31, 33), or overnight urine samples (59), investigators quantified the actual difference in sodium between intake groups using the mean (average) of one or more 24-h urine collections per participant (n = 36) (Table 1). The range of the mean actual difference in sodium consumed between intake groups was 63 (31) to 5175 mg/d (39) (median: 2310 mg/d) for trials with only adult participants (median: 2334 mg/d including 1 cohort of children). This varied by trial design where the median actual difference in sodium consumed between groups was 888 mg/d in parallel trials (adults only, n = 15) compared with 3456 mg/d in crossover trials (n = 32). Of the 33 trials that reported intended and actual sodium measures, only 3 trial populations (33, 37, 38, 48) adhered to the intervention. Fewer than half (48%, n = 40) of the included trials reporting actual intake examined dietary sodium intake levels in the LS arm ≤2300 mg/d. The actual mean 24-h urinary sodium excretion at the end of the intervention in the LS arm was <1500 mg/d in 9 trials (23, 24, 27, 31, 33, 39, 50, 55, 61), within 1500–2300 mg/d in 10 trials (28, 34, 35, 40, 41, 43, 47, 63, 66, 69), and ≥2300 mg/d in the remaining 21 trials. The actual mean sodium 24-h urinary sodium excretion value at the end of the trial could not be determined from the figure presented in 1 trial (42) and was not reported in 2 trials (60, 62), 3 ancillary studies of the DASH-Sodium trial (32, 44, 53), or 1 ancillary study of a metabolic balance trial (57). Except for 1 trial (57), the target intake levels in the LS arms of these trials were ≤1500 mg/d.
Observational studies
Methods to assess sodium intake exposure among observational studies varied. In the majority of studies, researchers estimated sodium intake exposure using a variety of urinary biomarkers (n = 111, 70%) (Table 2); the remainder were dietary methods, i.e., FFQs (n = 27, 17%) or 24-h dietary recall or food diaries (n = 20, 13%).
TABLE 2.
The distribution of exposure assessment methods used to quantify dietary sodium intake among observational studies by study design1
| Study design | Prospective cohorts | Cross-sectional studies | Case-control studies | All observational studies |
|---|---|---|---|---|
| Urinary measures | ||||
| Multiple 24-h urine collections2 | 93 | 7 | 0 | 16 |
| Single 24-h urine | 7 | 39 | 1 | 47 |
| Multiple spot urine samples4 | 3 | 3 | 0 | 6 |
| Single spot urine | 135 | 28 | 1 | 42 |
| Dietary measures | ||||
| Multiple FFQs | 63 | 0 | 0 | 6 |
| Single FFQ | 12 | 7 | 2 | 21 |
| Multiple, multiple-day diet recalls/records/diaries | 1 | 0 | 0 | 1 |
| Single, multiple-day diet recalls/records/diaries6 | 1 | 1 | 0 | 2 |
| Multiple 24-h diet recalls/records/diaries | 0 | 5 | 0 | 5 |
| Single 24-h recall/record/diary | 1 | 11 | 0 | 12 |
| Totals | 53 | 101 | 4 | 1583 |
n = 157. WLVS, Women's Lifestyle Validation Study.
All multiple 24-h urine collections were collected on nonconsecutive days with the exception of 3 observational studies [1 prospective (127) and 2 cross-sectional (102, 208)].
One prospective cohort study conducted by Cortese et al. (93) used both a dietary and a urinary measurement to estimate dietary sodium intake and is counted in both categories. Sodium excretion was measured using multiple 24-h urine samples from women in the WLVS to correct the sodium intake estimated by FFQ in the study for measurement error. The correction equation was based on a linear regression with energy-adjusted sodium intake assessed by FFQ in the WLVS as exposure and urinary sodium as outcome: [corrected sodium intake = 1455.83 + (0.767* uncorrected FFQ sodium intake)] (93). This study was counted under both categories of multiple nonconsecutive 24-h urine collections and multiple FFQs.
Multiple spot urine samples were collected nonconsecutively in 4 studies (93, 143, 174, 185), whereas the sodium intake was averaged from early-morning urine samples collected on 3 consecutive days in 1 study (162, 163).
Takase et al. (201) instructed participants to “collect overnight urine in a paper cup and to bring in a sample of the urine in a plastic tube.” It is unclear if the overnight collection was timed or if just a spot sample from the overnight urine was used.
All of the studies assessed sodium using a multiple, consecutive-day diet recall (e.g., one 3-d recall from foods eaten on Monday, Tuesday, and Wednesday).
In most observational studies (n = 122), researchers estimated sodium intake exposure at a single time point and mostly based on short-term indicators, i.e., urinary biomarkers (spot or 24-h urine) (n = 89) or 24-h dietary recall/diary collected on a single day (n = 12). For FFQs measured at a single time point (n = 21), the duration of exposure for the majority of studies was 1 y, with the exception of 4 studies with a duration < 1 y (72, 109, 150, 166) and 1 study whose duration of exposure could not be determined (196) (Supplemental Table 10). Researchers in the remaining studies (n = 36) estimated sodium intake exposure at ≥1 time point, the majority of which had a cohort design (n = 19). In 5 cohort studies (91, 93, 110, 133, 156), sodium intake exposure was estimated using ≥3 nonconsecutive 24-h urine collections with a duration of exposure ranging from 1 (93) to 3 y (111, 133, 156) (median: 3 y). In 2 cohort studies sodium intake exposure was estimated using >3 spot urine samples collected on ≥3 d over a duration of 2 seasons (143) or 5 y (185). For the remainder of the cohort studies which estimated sodium intake exposure at ≥1 time point (n = 10), the duration between exposure measures ranged from 3 mo (174) to 8 y (76). Researchers in most studies (n = 15) estimated sodium intake using the mean of multiple measures, except in 4 studies where researchers estimated the temporal change in sodium intake (146, 154, 158, 163).
Researchers categorized sodium intake in the majority of observational studies (n = 104, 66%) (Supplemental Table 10). Of these, in 23 studies, mean sodium intake in the LS group was <1500 mg/d (77, 83, 87, 101, 114, 122, 123, 134, 144, 151, 173, 176–179, 189, 192, 194, 197, 206, 207, 222, 224); in 30, 1500–2300 mg/d (86, 91, 92, 95, 98, 109, 111, 124, 128–131, 149, 155, 161, 165, 172, 183, 184, 187, 190, 198, 199, 204, 205, 208, 210, 213, 217, 221); and in 51, ≥2300 mg/d (71, 74, 76, 78, 81, 82, 84, 85, 90, 93, 96, 99, 100, 102, 105, 106, 113, 115, 116, 118, 127, 136–138, 142, 143, 148, 152, 153, 156, 160, 162, 164, 167, 174, 180, 182, 185, 186, 191, 195, 196, 201–203, 211, 212, 214, 218, 223, 226).
Health indicators and outcomes
Health indicators and outcomes varied widely (Table 3). More than 1 health indicator or outcome was examined in 53 studies (23%) included in this review (Supplemental Tables 4–6). The most frequently studied health indicators over this time period were BP (n = 875), followed by renal function/CKD indicators (n = 45), subclinical CVD indicators (n = 30), and clinical CVD indicators (n = 24) (Table 3). In 2 observational studies, a cross-sectional study in Korea (195) and a cohort study in Japan (206), investigators evaluated the association between sodium intake and gastric cancer (Supplemental Table 5). Other indicators evaluated included body fatness or weight (24 studies), insulin resistance/glucose tolerance (12 studies), RAAS (12 studies), bone measures (8 studies), blood lipids (7 studies), and metabolic syndrome or rheumatoid arthritis (4 studies each). In addition, 45 focused on indicators that did not fit in prespecified categories (e.g., indicators related to cognition or gastric function). The health indicators/outcomes varied by study design. Whereas BP, followed by renal function/CKD, were the most frequently reported indicators for trials, observational studies, and meta-analyses, clinical CVD outcomes and mortality were frequently reported as outcomes in observational studies, but not in trials, during this time period (Table 3).
TABLE 3.
Categorization and definition of health indicators assessed by study design1
| Trials | Observational | Systematic review/meta-analyses | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Health outcome | Indicators assessed | Total | RCT | Non-RCT | Total | Case control | Cross-sectional | Prospective | Total outcomes2 | |
| All-cause mortality and CVD indicators | ||||||||||
| All-cause mortality | Death from causes other than cardiovascular-related problems | 0 | 0 | 0 | 12 | 0 | 0 | 12 | 3 | 15 |
| Clinical CVD indicators | Fatal or nonfatal cardiovascular events (e.g., stroke, CHD, CVD, MI, TIA, HF, arrhythmia, pulmonary edema, aneurysm, atrial fibrillation, angina pectoris) | 0 | 0 | 0 | 19 | 0 | 1 | 18 | 5 | 24 |
| Subclinical CVD indicators | Cardiovascular functional measures (e.g., ejection fraction, heart rate, atrial filling fraction, pulse wave velocity, β-type natriuretic peptide, augmentation index); cardiovascular structural measures (e.g., LV mass index, LA diameter) | 12 | 10 | 2 | 13 | 0 | 10 | 3 | 5 | 30 |
| Blood pressure | SBP; DBP; hypertension; blood pressure variability (ARV index) | 17 | 17 | 0 | 59 | 1 | 45 | 13 | 11 | 87 |
| Other indicators | ||||||||||
| Renal function/CKD | CKD (incidence, prevalence, progression); markers of renal function (e.g., urinary albumin:creatinine ratio, eGFR); fluid measures (e.g., overload, volume); albuminuria | 13 | 10 | 3 | 26 | 0 | 10 | 16 | 6 | 45 |
| Gastric cancer | Gastric cancer | 0 | 0 | 0 | 2 | 0 | 1 | 1 | 0 | 2 |
| Indicators of body fatness | BMI; adiposity; total body percentage fat; waist-to-hip ratio; waist circumference; predictive body fatness | 5 | 4 | 1 | 16 | 0 | 15 | 1 | 3 | 24 |
| Blood lipids | Total cholesterol; HDL cholesterol; LDL cholesterol; triglycerides | 1 | 1 | 0 | 4 | 0 | 4 | 0 | 2 | 7 |
| Indicators of IR/glucose tolerance | Insulin resistance; fasting glucose; metabolic clearance rate of glucose | 2 | 1 | 1 | 8 | 0 | 8 | 0 | 2 | 12 |
| Bone measures | Bone mineral density; osteoporosis; bone turnover markers (e.g., CTX-I, OC, ALP) | 1 | 1 | 0 | 6 | 0 | 5 | 1 | 1 | 8 |
| RAAS | Renin; angiotensin; aldosterone | 7 | 7 | 0 | 1 | 0 | 1 | 0 | 4 | 12 |
| Metabolic syndrome | Metabolic syndrome | 0 | 0 | 0 | 4 | 0 | 4 | 0 | 0 | 4 |
| Rheumatoid arthritis | Rheumatoid arthritis | 0 | 0 | 0 | 4 | 3 | 1 | 0 | 0 | 4 |
| Other | Cognition (e.g., headaches, function, decline, mental distress, lightheadedness); gastric dysfunction (e.g., Crohn disease, ulcerative colitis, Helicobacter pylori); cataracts; age-related body composition (sarcopenia, frailty); NAFLD; LTL; metabolite profile; QoL measurement; hormones (e.g., serum dopamine, leptin, adiponectin, gastrin, cortisol, XO, corin, cardiotrophin 1, ghrelin); oxidative stress damage; inflammation (e.g., hsCRP, GlycA, IL-6, TNF-α, pentraxin-3); endothelial dysfunction (e.g., microparticles, plasma PAI-1); plasma OPG; iodine; lower urinary tract symptoms; multiple sclerosis; fibroblast growth factor 23; hyponatremia; minerals; hearing loss | 20 | 13 | 7 | 27 | 2 | 18 | 7 | 1 | 48 |
ALP, alkaline phosphatase; ARV, average real variability; CAD, coronary artery disease; CKD, chronic kidney disease; CTX-I, C-telopeptides of type 1 collagen; CVD, cardiovascular disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; GlycA, glycoprotein acetylation; HF, heart failure; hsCRP, high-sensitivity C-reactive protein; IR, insulin resistance; LA, left atrium; LTL, leukocyte telomere length; LV, left ventricular; MI, myocardial infarction; NAFLD, nonalcoholic fatty liver disease; OC, osteocalcin; OPG, osteoprotegerin; PAI-1, plasminogen activator inhibitor-1; QoL, quality of life; RAAS, renin-angiotensin-aldosterone system; RCT, randomized controlled trial; SBP, systolic blood pressure; TIA, transient ischemic attack; XO, xanthine oxidase.
Counts are of the number of health indicator categories by study type. One study may assess multiple health indicator categories, which will be counted separately (e.g., an RCT trial examined subclinical CVD indicators and BP—1 count would be put in both categories under RCT). Therefore, the total outcomes will not equal the number of studies included. One study may have assessed >1 indicator per category (i.e., clinical CVD indicators assessed were stroke and CHD); however, we will only count 1 for assessing the category of clinical CVD indicators.
ROB assessment
In total, 54 studies [22 RCT (23–25, 28, 31, 33, 34, 36, 37, 42, 46, 48–52, 54–56, 58, 59), 2 non-RCT (39, 64), and 30 cohort studies (76, 91, 96, 108, 111, 122, 123, 128, 133, 136, 138, 143, 152–154, 156, 158, 163, 164, 174, 175, 185, 186, 189, 190, 192, 201, 205, 216, 224)] evaluated sodium intake in relation to the health indicators/outcomes selected for the ROB assessment in this review (Table 4). Although not included in the ROB assessment, results of 18 meta-analyses (228, 231–239, 241–243, 249–251) were summarized for each of the selected outcomes.
TABLE 4.
Risk of bias assessment of prospective studies examining dietary sodium and its association with all-cause mortality1
| Reference | Association | Control of key confounding | Selection unrelated to sodium/outcome | Coinciding follow-up/baseline exam | Departures from intended exposure | Potential for systematic error | Potential for random error | Adequate follow-up |
|---|---|---|---|---|---|---|---|---|
| Cook et al. (91) | + | L | L | L | U | L | L | L |
| Lamelas et al. (136) | + | H | M | U | U | H | H | U |
| Doukky et al. (96) | 0 | H | H | U | U | H | L | U |
| He J et al. (111) | 0 | L | H | L | U | L | L | U |
| Kalogeropoulos et al. (123) | 0 | H | H | H | U | H | L | U |
| Liu H et al. (143) | 0 | L | L | H | U | H | H | L |
| Merino et al. (154) | 0 | L | U | L | U | H | H | L |
| Singer et al. (192) | 0 | H | H | H | H | H | H | U |
| Welsh et al. (216) | 0 | L | L | L | U | H | H | U |
| Lelli et al. (138) | − | L | M | H | U | L | H | U |
| Saulnier et al. (189) | − | H | H | U | U | H | H | U |
| Mente et al. (152) | U-shaped | H | H | U | U | H | H | U |
| Total high rankings | 7 | 6 | 3 | 1 | 8 | 7 | 0 |
n = 12. H, high; L, low; M, moderate; U, unclear.
All-cause mortality
Twelve cohort studies and 3 meta-analyses evaluated the association of sodium intake with all-cause mortality during this time period. Not accounting for ROB, in 2 cohort studies (91, 136) higher sodium intake was associated with higher mortality (positive association). In 7 cohort studies (96, 111, 123, 143, 154, 192, 216) and 3 meta-analyses (241, 243, 251), no significant association was observed between sodium intake and mortality (null association). In 2 cohort studies, higher sodium was associated with lower mortality [inverse association (138, 178)]. In 1 cohort study, mortality was associated with both low (<4000 mg/d) and high (>7000 mg/d) estimated sodium intake (i.e., a U-shaped association) (152) (Table 4).
The most common biases were systematic or random measurement error in sodium assessment (8 and 7 studies, respectively), potential for confounding (7 studies), and selection bias (6 studies). One study was judged to be at low ROB, except for an unclear ROB due to potential departure from the intended exposure (91). However, all evaluated cohort studies on sodium intake and mortality were judged to be unclear or high ROB on this criterion and this bias most likely attenuated results, e.g., owing to nonadherence to the intervention. Despite potential attenuation, in this low-ROB study, higher sodium intake (estimated using ≥3 nonconsecutive, high-quality, 24-h urinary excretions) was positively associated with higher mortality in a linear dose–response relation (91).
In 2 meta-analyses of observational studies, a null association was observed between sodium intake and mortality and high levels of heterogeneity were found between studies included in both reviews (243, 251). One systematic review of RCTs in adults with HF (n = 4 studies) lacked enough information for a meta-analysis to evaluate the effects of reduced dietary sodium intake on mortality (241).
Clinical CVD measures
Despite the fact that no RCTs evaluated CVD events during this time, 17 cohort studies and 5 meta-analyses evaluated the association of sodium intake with CVD events (Table 5). Not accounting for ROB, the reported association with sodium intake was positive for 8 cohort studies (136, 143, 153, 154, 156, 174, 186, 224) and 2 meta-analyses (243, 250); null for 6 cohort studies (96, 123, 138, 175, 192, 216) and 1 meta-analysis (241); inverse for 2 cohort studies (128, 189) and 1 meta-analysis (251); and U-shaped for 1 cohort study (152). For HF events, the reported association with sodium intake was positive for 2 studies (156, 175); null for 2 (123, 216); and inverse for 1 (96). For stroke events, the association with sodium intake was positive for 2 cohort studies (143, 156) and 2 meta-analyses (236, 251); inverse in 1 study (128); and null in 1 study (175).
TABLE 5.
Risk of bias assessment of prospective studies examining dietary sodium and its association with clinical cardiovascular disease indicators1
| Reference | Association | Control of key confounding | Selection unrelated to sodium/outcome | Coinciding follow-up/baseline exam | Departures from intended intervention | Potential for systematic error | Potential for random error | Adequate follow-up | Indicator assessors blinded to exposure group |
|---|---|---|---|---|---|---|---|---|---|
| Lamelas et al. (136) | + | H | M | U | U | H | H | U | U |
| Liu H et al. (143) | + | L | L | H | U | H | H | L | U |
| Mente et al. (153) | + | H | U | H | U | H | H | U | U |
| Merino et al. (154) | + | L | U | L | U | H | H | L | U |
| Mills et al. (156) | + | L | H | L | U | L | L | L | U |
| Polonia et al. (174) | + | H | H | U | U | L | H | U | U |
| Saleh et al. (186) | + | H | H | U | H | L | H | U | U |
| Zhao et al. (224) | + | H | H | U | U | L | H | U | U |
| Doukky et al. (96) | 0 | H | H | U | U | H | L | U | L |
| Kalogeropoulos et al. (123) | 0 | H | H | H | U | H | L | U | U |
| Lelli et al. (138) | 0 | L | M | H | U | L | H | U | U |
| Prentice et al. (175) | 0 | U | H | U | U | H | L | U | L |
| Singer et al. (192) | 0 | H | H | H | H | H | H | U | U |
| Welsh et al. (216) | 0 | L | L | L | U | H | H | U | U |
| Kieneker et al. (128) | − | L | M | U | U | L | L | U | L |
| Saulnier et al. (189) | − | H | H | U | U | H | H | U | L |
| Mente et al. (152) | U-shaped | H | H | U | U | H | H | U | U |
| Total high rankings | 10 | 10 | 5 | 2 | 11 | 12 | 0 | 0 |
n = 17. H, high; L, low; M, moderate; U, unclear.
The most common biases for studies examining clinical CVD events were potential for systematic and random measurement error in sodium assessment (11 and 12 studies, respectively), selection bias due to recruiting sick participants (10 studies), and confounding (10 studies) (Table 5). Most studies were judged to be at high ROB for ≥2 of the 8 criteria. One study was judged to be at low ROB, with the exception of a high risk for potential selection bias (because people with a history of CVD were not excluded from the study or the analyses of clinical CVD events), an unclear ROB related to unknown departures from the intended exposure (after the first 2 y of assessment), and blinding of indicator/outcome assessors (156). If people with a history of CVD were lowering their sodium intake, one might expect a higher percentage of participants with a history of CVD to be in the lowest quartile of intake, but the opposite was observed. In this study (156), higher urinary sodium excretion was associated with increased risk of combined CVD events, HF, and stroke among patients with CKD, and adjustment for history of CVD did not change the direction of the association. One study at mostly high/unclear ROB found that higher sodium intake increased the risk of cardiovascular events [separately and in combination with all-cause hospitalizations (n = 18 persons)] among persons with HF and comorbid diabetes mellitus (DM) (186). However, this study lacked control of key confounding, had departures from the intended exposure status, and was at a high risk for random error.
Meta-analyses evaluating the effects/associations of dietary sodium on CVD events varied in their results. One review of RCTs conducted in adults with HF lacked sufficient data to evaluate the effects of reduced dietary sodium intake on CVD-related mortality (241). Another review indicated that sodium intake <3000 mg/d, but not 3000–5000 mg/d or >5000 mg/d, was associated with increased risk of cardiac death in an analysis of 7 cohort studies (251). The results of this meta-analysis were largely driven and limited by 3 studies at high risk of reverse causality. Participants in the lowest sodium group had higher prevalence of CVD factors in 2 studies (254, 255) and had more severe disease status and/or concurrent illness in 1 study of persons with DM (256). Limitations of 1 study have been discussed previously and include possible confounding, concurrent illness of participants, or under-collection of 24-h urine samples, which may explain the inverse association found (257). Two reviews that examined the association of dietary sodium intake and CVD mortality, among observational studies of generally healthy adults with no chronic or acute illnesses, found direct, positive associations (243, 250). Both reviews had high levels of heterogeneity between included studies and were limited by disagreements in methods to assess sodium intake and control for confounding factors in included studies. Lastly, a review by Jayedi et al. (236) found that higher sodium intake was associated with higher risk of stroke among 16 observational studies conducted in generally healthy adults. Results from this review were mainly driven by 2 large-scale cohort studies of Japanese adults and results may not be generalizable to other populations. Further, high levels of heterogeneity and disagreements in sodium assessments and control of confounding factors were found between studies.
Subclinical CVD measures
From 2015 to 2019, PWV was the most common subclinical CVD measure examined (n = 6 studies and 2 reviews), followed by β-type natriuretic peptide (BNP) concentrations (n = 4 studies and 2 reviews), heart rate (n = 4 studies and 1 review), cardiac baroflex sensitivity (n = 2 studies), and pulse augmentation index (n = 2 studies). Other subclinical CVD measures evaluated in single studies included QT-interval, C-reactive protein concentrations, carotid intima media thickness (cIMT), microvascular density, and cardiac function and geometry measures.
Of the 4 trials that evaluated PWV as the outcome, the reported effect of sodium intake among middle-aged adults was null in 3 crossover trials (36, 58, 64) and positive in 1 (50) (Tables 6, 7). In the 1 trial that included adults aged <30 y (50), the effect was null (Table 6). Two trials conducted in adults with prehypertension (pre-HTN) and DM with null results (36, 58) were judged to be at low ROB across all domains assessed, although adherence to the intervention was low (<90%) and the difference in intake between groups was >1000 mg/d in both trials (Tables 1 and 6). For the 2 cohort studies that evaluated PWV, the reported associations with sodium were positive (122, 163) (Table 8). However, both studies were judged to be at high ROB for ≥2 criteria, lacked control of key confounding, and had a high potential for systematic error in assessment of sodium intake (Table 8).
TABLE 6.
Risk of bias assessment of randomized intervention trials examining dietary sodium and its effect on subclinical cardiovascular disease measures1
| Reference | Indicator | Effect | Intervention randomly allocated | Concealed allocation process | Participants/staff blinded to intervention | Adequate follow-up | Indicator assessors blinded to intervention | Prespecified indicator |
|---|---|---|---|---|---|---|---|---|
| Parallel RCT | ||||||||
| Colin-Ramirez et al. (31) | BNP concentration | 0 | L | U | NA | L | L | U |
| Hummel et al. (42) | BNP concentration; C-reactive protein; troponin | 0 | U | U | NA | H | L | L |
| Fabricio et al. (33) | BNP concentration; HR | 0 | L | L | L | L | L | L |
| Crossover RCT | ||||||||
| Riphagen et al. (54) | BNP concentration | + | L | L | NA | L | L | L |
| Babcock et al. (23) | Cardiovagal baroflex sensitivity; HR | + | U | U | NA | U | L | U |
| Baqar et al. (25) | Cardiac baroflex sensitivity; AIX; HR | +; 0; 0 | U | U | L | L | L | U |
| Gijsbers et al. (36) | PWV; HR; AIX | 0 | L | L | L | L | L | L |
| Muth et al. (50) | PWV | + (A); 0 (C) | U | U | NA | U | U | U |
| Suckling et al. (58) | PWV | 0 | L | L | L | L | L | L |
| Rorije et al. (55) | Microvascular density | 0 | L | L | NA | H | L | L |
| Total high rankings | 0 | 0 | 0 | 0 | 2 | 0 | 0 | |
n = 7. A, adults; AIX, Augmentation Index; BNP, β-type natriuretic peptide; C, children; H, high; HR, heart rate; L, low; NA, not applicable; PWV, pulse wave velocity; RCT, randomized controlled trial; U, unclear.
TABLE 7.
Risk of bias assessment of crossover nonrandomized intervention trials examining dietary sodium and its effect on subclinical cardiovascular disease measures1
| Reference | Indicator | Association | Selection unrelated to sodium/outcome | Coinciding follow-up/baseline exam | Departures from intended intervention | Adequate follow-up | Indicator assessors blinded to intervention group | Prespecified indicator |
|---|---|---|---|---|---|---|---|---|
| He M et al. (39) | QT-interval | + | L | L | L | L | L | U |
| Wang et al. (64) | PWV | 0 | U | L | L | L | U | U |
| Total high rankings | 0 | 0 | 0 | 0 | 0 | 0 |
n = 2. L, low; PWV, pulse wave velocity; U, unclear.
TABLE 8.
Risk of bias assessment of prospective studies examining the association between dietary sodium and subclinical cardiovascular disease measures1
| Reference | Indicator | Association | Control of key confounding | Selection unrelated to sodium/outcome | Coinciding follow-up/baseline exam | Departures from intended intervention | Potential for systematic error | Potential for random error | Adequate follow-up | Indicator assessors blinded to intervention group | Prespecified indicator |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nerbass et al. (163) | PWV | + | H | H | H | H | H | H | U | U | L |
| Jung et al. (122) | PWV; cIMT | + | H | L | U | L | H | L | U | L | L |
| Haring et al. (108) | LV cardiac geometry and function | 0 | H | U | L | U | U | L | U | U | L |
| Total high rankings | 3 | 1 | 1 | 1 | 2 | 1 | 0 | 0 | 0 |
n = 3. cIMT, carotid intima-media thickness; H, high; L, low; LV, left ventricular; PWV, pulse wave velocity; U, unclear.
Of the trials that evaluated BNP as an outcome, 3 trials among persons with HF found a null association (31, 33, 42), whereas 1 trial among persons with pre-HTN found a positive association (54) (Table 6). In 2 of the trials, ROB was unclear or high for ≥2 criteria and both had significant differences in sodium intake between intervention groups (31, 42), whereas the other 2 trials were at mostly low ROB (33, 54). Results for heart rate variability were mixed: in 3 trials at mostly low ROB the association was null (25, 33, 36), whereas in 1 trial at mostly unclear ROB, the association was positive (23). In 2 trials with unclear ROB for ≥2 criteria, conducted in normotensive adults (23) and adults with DM (25), sodium supplementation increased cardiac baroflex sensitivity, whereas in 2 trials at mostly low ROB the augmentation index was not significantly different by sodium intake (25, 36). In 1 RCT at mostly low ROB, higher sodium intake had no effect on microvascular density (without nitroglycerin) among healthy adult males; however, the authors did not disclose reasons for losses to follow-up and there was no mention of an intent-to-treat (55). In 1 non-RCT with mostly low ROB (39), higher sodium intake increased the QT-interval; however, this outcome was not prespecified (Table 7). For the remaining subclinical CVD indicators [C-reactive protein (42), cIMT (122), and left ventricular cardiac geometry and function measures (108)], the ROB of the published studies was judged to be uncertain or high for ≥2 indicators.
Two meta-analyses of trials evaluated the effect of sodium reduction on PWV. In 1 review of RCTs or non-RCTs in generally healthy adults, PWV was similar across dietary sodium interventions of ≥4 wk duration (237), whereas in another review of RCTs in adults with no specifications on disease status or intervention, a positive association was reported (228). In 2 reviews the effect of sodium on BNP concentrations was evaluated. One review identified only 1 trial that met their criteria with results that suggested a positive association in adults with CKD (242), whereas the other review had 7 trials that met their criteria with results that varied based on HF classification (New York Heart Association Functional Classification I–VI) (238). In 1 review of 72 RCTs among generally healthy or hypertensive adults, sodium reduction, assessed using 8-h or 24-h urine collections, significantly increased heart rate by ∼2% (234).
BP
Roughly 60% of RCTs indicated that higher sodium intake increased systolic blood pressure (SBP) and diastolic blood pressure (DBP) (Table 9). In 3 trials judged to be low ROB on the criteria examined, higher sodium intake increased both SBP and DBP among persons with DM or pre-HTN (36, 58), whereas no effect was found on SBP or DBP among persons with HF (33). In contrast, among the cohort studies that evaluated BP as an outcome, the reported association with sodium intake was more variable and all studies evaluated were judged to be at high or uncertain ROB for at least half of the criteria examined (Table 10). The most common biases for the 12 cohort studies examining BP were potential for systematic and random measurement error in sodium assessment (10 and 9 studies, respectively), confounding (10 studies), and selection bias due to recruiting sick participants (4 studies) (Table 10).
TABLE 9.
Risk of bias assessment of RCTs examining dietary sodium and its effect on blood pressure1
| Association2 | Intervention randomly allocated | Concealed allocation process | Participants/staff blinded to intervention | Adequate follow-up | Indicator assessors blinded to intervention | Prespecified indicator | ||
|---|---|---|---|---|---|---|---|---|
| Reference | SBP | DBP | ||||||
| Parallel RCT | ||||||||
| Nakano et al. (51)3 | + | + | L | U | NA | L | H | L |
| He FJ et al. (37)2 | + (A); 0 (C) | 0 | L | L | NA | L | U | L |
| Takada et al. (59) | + | 0 | L | U | NA | L | L | L |
| Pinjuh Markota et al. (48) | 0 | 0 | U | L | NA | L | U | U |
| Meuleman et al. (49) | 0 | 0 | L | L | NA | L | H | L |
| Fabricio et al. (33) | 0 | 0 | L | L | L | L | L | L |
| Crossover RCT | ||||||||
| Gijsbers et al. (36)3 | + | + | L | L | L | L | L | L |
| Muth et al. (50) | + | + | U | U | NA | U | U | U |
| Saran et al. (56) | + | + | U | L | NA | H | L | L |
| Juraschek et al. (44) | + | + | U | U | NA | U | L | L |
| Suckling et al. (58)3 | + | + | L | L | L | L | L | L |
| Brian et al. (27)2,3 | + (F); 0 (M) | 0 | U | U | NA | L | U | U |
| Cashman et al. (28) | + | 0 | U | U | NA | L | U | L |
| Babcock et al. (24) | 0 | 0 | U | U | NA | U | L | L |
| Baqar et al. (25) | 0 | 0 | U | U | L | L | L | U |
| Total high rankings | 0 | 0 | 0 | 1 | 2 | 0 | ||
n = 14. A, adults; BP, blood pressure; C, children; DBP, diastolic blood pressure; H, high; L, low; NA, not applicable; RCT, randomized controlled trial; SBP, systolic blood pressure; U, unclear.
If the authors stratified results to examine specific subpopulations (e.g., by age group or gender), criteria selections are presented for each with the specific subpopulation noted in parentheses.
Results presented are for overall 24-h SBP/DBP (mm Hg), because the authors also evaluated clinic BP and other measures of 24-h BP measurements (e.g., morning 24 h).
TABLE 10.
Risk of bias assessment of prospective studies examining the association between dietary sodium and blood pressure1
| Association | Control of key confounding | Selection unrelated to sodium/outcome | Coinciding follow-up/baseline exam | Departures from intended intervention | Potential for systematic error | Potential for random error | Adequate follow-up | Indicator assessors blinded to intervention group | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Reference | SBP | DBP | ||||||||
| Prentice et al. (175) | + (HTN) | H | H | U | U | H | L | U | L | |
| Zhao et al. (224) | + (HTN) | H | H | U | U | L | H | U | U | |
| Welsh et al. (216) | + (MABP) | H | L | L | U | H | H | U | U | |
| Lamelas et al. (136) | + | + | H | M | U | U | H | H | U | U |
| Nerbass et al. (163) | + | + | H | H | H | H | H | H | U | U |
| Nguyen et al. (164) | + | + | L | M | L | H | H | H | U | U |
| Takase et al. (201) | + | NA | H | U | L | L | H | H | U | U |
| Krupp et al. (133)2 | + (B); 0 (G) | 0 | H | L | L | U | L | L | U | U |
| Umesawa et al. (205)2 | + (OV-WT); 0 (N) | 0 | H | M | L | L | H | H | U | U |
| Buendia et al. (76) | 0 | 0 | L | L | L | U | H | H | U | U |
| Morgenstern et al. (158) | 0 | 0 | H | H | L | U | H | L | U | U |
| Setayeshgar et al. (190) | 0 | + | H | U | H | U | H | H | H | U |
| Total high rankings | 10 | 4 | 2 | 2 | 10 | 9 | 1 | 0 | ||
n = 12. B, boy; DBP, diastolic blood pressure; G, girl; H, high; HTN, hypertension; L, low; M, moderate; MABP, mean arterial blood pressure; N, normal weight; NA, not applicable; OV-WT, overweight; SBP, systolic blood pressure; U, unclear.
The authors stratified results to examine specific subpopulations (e.g., by gender, weight, or HTN status). Criteria selections are presented for each with the specific subpopulation noted in parentheses.
Of the 8 meta-analyses of trials examining the effect of sodium on SBP and DBP, 6 indicated a positive effect—4 among persons with normotension, pre-HTN, or hypertension (HTN) (9, 232, 233, 235) and 2 among persons with CKD (231, 242); 1 indicated no significant change among generally healthy adults (237); and in 1 among persons with HF, investigators concluded evidence was insufficient (241). Two reviews indicated a positive relation between sodium intake and SBP in experimental and observational studies among generally healthy children and children with clinical conditions (239, 249). Lastly, in 1 review of observational studies a positive association was reported with risk of HTN in both urban and rural populations of lower- to middle-income countries (250).
Discussion
Since January 2015, the majority of the evidence on the relation between sodium intake and health, among the general population and specific subgroups, was based on results from observational studies or analyses, rather than RCTs, and thus subject to potential bias from error in assessment of sodium intake and confounding. Most of the published evidence from observational studies was based on cross-sectional surveys or analyses, which are subject to reverse causality. In addition, most of the recently published studies on sodium intake and health focused on CVD or renal risk. The number of studies varied by region and some studies, such as observational studies on sodium intake and gastric cancer conducted in Asia, may not apply to other regions, because the sources and distribution of sodium intake differ. For CVD risk indicators, the direction of sodium intake effects and associations varied; however, results of studies with low ROB confirmed higher sodium intake increased risk of mortality, CVD events, and BP, but did not affect PWV.
Most studies published since 2015 did not address the research gaps or meet recommendations for research methods published in the 2013 IOM report (2), suggesting lack of knowledge about the recommendations, resources necessary, or time for implementation and reporting. For instance, only a few studies used recruitment strategies to specifically enroll African Americans (n = 1 trial) (30), adults aged 51–70 y (n = 4 trials and 9 observational studies), 70 y or older (n = 4 observational studies), or other high-risk subgroups such as persons with DM (n = 3 trials, 4 observational studies) or CKD (n = 4 trials, 9 observational studies, 2 reviews). Most observational studies did not apply the recommended methods for assessment of sodium intake exposure and about one-third of RCTs included interventions with <4 wk duration, with few trials or studies focused on children or people with CHF. A minority of studies evaluated dietary sodium intake levels corresponding to levels in current guidelines (i.e., 1500–2300 mg) (3). During the time frame of this review, no trials evaluated risk of CVD events, stroke, or mortality, and in 1 review of adults with HF investigators concluded data were insufficient to evaluate the effects of reduced dietary sodium intake on cardiovascular-associated mortality (241). Further, few trials were conducted among patients with HF and examined measures related to symptoms of the disease (31, 33, 42). Lastly, gastric cancer was examined in 2 observational studies among Asian adults and results from both studies indicated that higher dietary sodium was associated with higher risk of gastric cancer but may not be generalizable to US adults (195, 206).
The results of this review are difficult to compare with previous reviews because the objectives differed. This review did not encompass the totality of evidence, but rather evidence published since 2015, and the selection criteria for included studies were broad. Unlike in previous reviews (3, 258), cross-sectional surveys, case-control studies, and nonrandomized trials were included. In addition, we did not exclude studies based on outcomes evaluated or inclusion of study methods that might bias results. This allowed us to understand the extent to which the 2013 IOM research recommendations (2) were applied in recent published studies and the extent to which different ROB criteria might affect the assessment of evidence. Two recent RCTs, for example, were judged to be at low ROB based on the criteria in this review but were excluded from the Agency for Healthcare Research and Quality (AHRQ) review (243) and the NASEM DRI (3) because 1 trial did not include a washout period between sodium intervention levels (36) and 1 trial enrolled persons with diabetes (58). Since the 2019 NASEM and AHRQ reviews (3, 243), 2 recent RCTs meeting NASEM inclusion criteria have been published (24, 59). Both examined the effect of sodium reduction on BP and were judged to be at mostly unclear or low ROB (24, 59). Further, 3 cohort studies examining clinical CVD events at moderate to high ROB were published since these reviews (128, 143, 216), although only 1 met the NASEM/AHRQ inclusion criteria (128). Despite these differences, the results of this review support the findings of previous reviews that conclude that lowering population salt intake would be beneficial for health.
Multiple studies in this review examined associations/effects of sodium intake on health indicators other than those discussed in the previous 2013 IOM report or in other systematic reviews (2). Systematic reviews may be warranted to assess outcomes other than CVD or renal risk, especially for endothelial and vascular function, to better characterize mechanisms underlying CVD risk independent of BP (3). Further, for outcomes or indicators with ≥2 recently published studies, further review and evaluation of ROB may be warranted to update previously published systematic reviews. Such indicators include body fatness, insulin/glucose intolerance, RAAS, metabolic syndrome, bone measures, blood lipids, rheumatoid arthritis, nonalcoholic fatty liver disease, cataracts, inflammation, cognition, and muscle function.
Among recent published trials, crossover research designs were most commonly used when examining the effects of sodium intake and health indicators because these designs allow for smaller sample sizes and reduced resources (259). Trials published since 2015 and included in this review were short in duration (<4 wk) with large between-group differences in sodium intake close to or exceeding public health recommendations, particularly among feeding trials. Although the majority of trials used appropriate and standard measures for assessing mean group intake (i.e., using the mean of more than one 24-h urine collection per participant) (15), noncompliance with the intervention was observed in the majority of trials included in this ongoing review, a well-known problem with dietary studies (19).
Cohort studies suffered from methodological limitations inherent to observational studies including selection, information, and confounding biases. For example, reverse causality due to the recruitment of sick participants, inadequate follow-up/data reporting, or lack of adjustment for key sociodemographic characteristics or pre-existing conditions were common methodological issues with potential to alter the direction of the association (21). In addition, most cohort studies evaluated in the ROB assessment had potential for systematic and random error due to methodological errors in measurement of sodium intake. Roughly 30% of cohort studies used spot urine samples, an inaccurate and unreliable method, whereas <1% collected three or more 24-h urine collections on nonconsecutive days, i.e., the gold standard for assessment of long-term individual intake (260). Missing or unclear reporting of evidence made it difficult to determine biases relevant to our assessment, causing uncertainty around the conduct of studies and reported results.
Our results indicate there remains a paucity of recent RCT research examining the effects of sodium on CVD outcomes (including stroke and mortality) among the general population and for specific populations that are at higher risk, including CHF patients. Long-term sodium reduction trials are required to evaluate the effects of sodium intake on chronic disease and are difficult to conduct owing to logistic, financial, and ethical constraints (19, 261, 262). Specifically, issues related to compliance, blinding, the nature of the food supply (i.e., >70% of sodium is consumed from processed food in the United States), and the interaction and aggregation of effects across other dietary components and health systems, all limit the feasibility of sustaining and achieving sodium modifications over a long duration (19, 262). To overcome these challenges, researchers have proposed conducting such a trial in a fully or partially institutionalized population (e.g., military personnel, nursing home residents/retirement home communities, prison population) (2, 263). Other proposed solutions are to monitor individuals as part of a natural experiment in areas where sodium policies are in effect or to conduct trials in geographic areas or communities where there is greater potential for sustained sodium reduction (e.g., tribal population or countries where the main source of sodium is discretionary) (2, 262).
Our review has several strengths. We describe recent studies related to any health indicator/outcome to understand whether current recommendations for research were applied. We identified domains of bias from existing risk assessment tools specific to study design (21, 264), defined and extended essential criteria to concepts/challenges inherent in nutritional epidemiology, and systematically applied and assessed the quality of evidence across multiple interventions and numerous outcomes. Through review from a native speaker, we were able to review and make decisions based on our eligibility criteria on 3 non-English full-text articles. We also identified 86 new studies published since the recent systematic reviews included here, as well as in the AHRQ review and 2019 NASEM report (3, 258).
This systematic review also has limitations. Given the objective of the review to examine recent evidence and provide directions for future research, evidence published before 2015 was excluded. Whereas terminology and tools for assessing ROB in individual RCTs have been validated and used consistently throughout the literature (264), a similar tool is unavailable for non-RCTs and observational studies (265). Because no such tool has been recommended to assess risk of bias in nonrandomized studies, we identified the ROBINS-I tool developed by the Cochrane Collaboration which assesses domains through which bias may be introduced into a nonrandomized study, and modified it to evaluate potential issues related to outcomes examined, nutritional epidemiology, and methodological challenges related to measuring sodium intake (21, 22, 265). Although the quality review applied systematic, uniform methods and standards, this approach required numerous judgments which can be subjective (265). Our evaluation to determine if RCT research was conducted among high-risk groups was limited to trials that specifically enrolled these populations; consequently, trials that conducted stratified analyses using these subgroups were not considered. Lastly, a considerable amount of information to assess ROB criteria was missing, limiting our ability to assess validity in some studies.
Conclusions
This systematic review summarizes the literature of dietary sodium and health published between 2015 and 2019. Most of the published evidence on sodium and CVD risk during this time period was observational rather than interventional and although almost all studies assessed for ROB were subject to some bias, cohort studies suffered more bias because of methodological limitations inherent to their design. Our assessment on sodium and health was complicated by differences in the methods used to measure sodium intake. In addition, trial evidence was limited and measured differences in sodium intake were largely not applicable to population sodium reduction recommendations. However, the results of this review support the findings of previous reviews concluding that lowering population salt intake would be beneficial for health. Overall, data and method gaps remain in studies on sodium and health consistent with those identified by the IOM in 2013. The 2013 IOM review addresses the need for studies to standardize methodological approaches to measure sodium intake and report results consistently and thoroughly, points which were re-emphasized in the 2019 NASEM report as well as other reports (2, 3). In light of studies on new health indicators, broader systematic reviews to update the total body of evidence, including that published before 2015, may be warranted to assess the evidence surrounding the effects and associations of sodium intake with outcomes not identified in the previous reviews.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Peter Yang, Sandra Jackson, Lixia Zhao, Carma Ayala, Mia Donley, and Alexa Morse for their assistance. The authors’ responsibilities were as follows—MEC and RKM: conceived and designed the ongoing literature search; KJO, ZSQ, JM, and MB: independently assessed titles/abstracts in the monthly reviews, ordered full-text articles, and assessed for inclusion/exclusion based on PICOTS criteria; MEC: resolved disagreements in assessments through discussion; KJO: transcribed information from included articles into tables specific to study design, wrote the manuscript, and has final responsibility for the final content; MB and KJO: independently assessed each study in the ROB analysis and any discrepancies were resolved through discussion with MEC; JW, MGG, RKM, MB, JM, ZSQ, and MEC: provided subject matter expertise, reviewed, and provided feedback on the review; and all authors: read and approved the final manuscript.
Notes
Supported by the CDC, Division for Heart Disease and Stroke Prevention and by National Heart, Lung, and Blood Institute grant T32HL130025 (to ZSQ).
Author disclosures: The authors report no conflicts of interest.
The findings and conclusions presented in this article are those of the authors and do not necessarily represent the official position of the CDC.
Supplemental Tables 1–10 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: AHRQ, Agency for Healthcare Research and Quality; AI, Adequate Intake; BNP, β-type natriuretic peptide; BP, blood pressure; CDRR, Chronic Disease Risk Reduction; CHF, chronic heart failure; cIMT, carotid intima media thickness; CKD, chronic kidney disease; CVD, cardiovascular disease; DASH, Dietary Approaches to Stop Hypertension; DBP, diastolic blood pressure; DM, diabetes mellitus; HF, heart failure; HTN, hypertension; IOM, Institute of Medicine; LS, low sodium; NASEM, National Academies of Science, Engineering, and Medicine; PICOTS, population, intervention, comparison, outcome, time, setting/study design; pre-HTN, prehypertension; PWV, pulse wave velocity; RAAS, renin-angiotensin-aldosterone system; RCT, randomized controlled trial; ROBINS-I, Risk of Bias in Non-Randomised Studies; SBP, systolic blood pressure.
References
- 1. US Department of Health and Human Services (HHS) and USDA. 2015–2020 Dietary Guidelines for Americans. [Internet] 8th ed Washington (DC): US HHS and USDA; 2015; [accessed May 5, 2020]. Available from: http://health.gov/dietaryguidelines/2015/guidelines/. [Google Scholar]
- 2. Institute of Medicine (IOM). Sodium intake in populations: assessment of evidence. Washington (DC): The National Academies Press; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. National Academies of Sciences, Engineering, and Medicine. Dietary Reference Intakes for sodium and potassium. Washington (DC): The National Academies Press; 2019. [PubMed] [Google Scholar]
- 4. Institute of Medicine (IOM). Strategies to reduce sodium intake in the United States. Washington (DC): The National Academies Press; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW et al.. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):e13–115. [DOI] [PubMed] [Google Scholar]
- 6. World Health Organization. Guideline: sodium intake for adults and children. Geneva, Switzerland: WHO; 2012. [PubMed] [Google Scholar]
- 7. Aburto NJ, Ziolkovska A, Hooper L, Elliott P, Cappuccio FP, Meerphol JJ. Effect of lower sodium intake on health: systematic review and meta-analysis. BMJ. 2013;346:f1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. He FJ, Li J, MacGregor GA. Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomised trials. BMJ. 2013;346:f1325. [DOI] [PubMed] [Google Scholar]
- 9. Graudal NA, Hubeck-Graudal T, Jurgens G. Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride. Cochrane Database Syst Rev. 2017;4:CD004022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Arcand J, Webster J, Johnson C, Raj TS, Neal B, McLean R, Trieu K, Wong MMY, Leung AA, Campbell NRC. Announcing “Up to date in the science of sodium”. J Clin Hypertens (Greenwich). 2016;18(2):85–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. WHO Collaborating Centre on Population Salt Reduction and The George Institute for Global Health. Science of Salt weekly. [Internet] Newtown, Australia: The George Institute for Global Health; [accessed May 6, 2020]. Available from: https://www.whoccsaltreduction.org/portfolio/science-of-salt-weekly/. [Google Scholar]
- 12. Moher D, Liberati A, Tetzlaff J, Altman DG; The PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. World Health Organization. WHO regional offices. [Internet] Geneva, Switzerland: WHO; [updated2019; cited 18 October, 2019]. Available from: https://www.who.int/about/who-we-are/regional-offices. [Google Scholar]
- 14. Centers for Disease Control and Prevention. Salt home: sodium reduction toolkit: a global opportunity to reduce population-level sodium intake. [Internet] Atlanta, GA: CDC; [updated2016; cited 18 October, 2019]. Available from: https://www.cdc.gov/salt/sodium_toolkit.htm. [Google Scholar]
- 15. Thompson FE, Kirkpatrick SI, Subar AF, Reedy J, Schap TE, Wilson MM, Krebs-Smith SM. The National Cancer Institute's Dietary Assessment Primer: a resource for diet research. J Acad Nutr Diet. 2015;115(12):1986–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sun Q, Bertrand KA, Franke AA, Rosner B, Curhan GC, Willett WC. Reproducibility of urinary biomarkers in multiple 24-h urine samples. Am J Clin Nutr. 2017;105(1):159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Olde Engeberink RHG, van den Hoek TC, van Noordenne ND, van den Born B-JH, Peters-Sengers H, Vogt L. Use of a single baseline versus multiyear 24-hour urine collection for estimation of long-term sodium intake and associated cardiovascular and renal risk. Circulation. 2017;136(10):917–26. [DOI] [PubMed] [Google Scholar]
- 18. Cogswell ME, Maalouf J, Elliott P, Loria CM, Patel S, Bowman BA. Use of urine biomarkers to assess sodium intake: challenges and opportunities. Annu Rev Nutr. 2015;35:349–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Satija A, Yu E, Willett WC, Hu FB. Understanding nutritional epidemiology and its role in policy. Adv Nutr. 2015;6(1):5–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Institute of Medicine (IOM). Dietary Reference Intakes for water, potassium, sodium, chloride, and sulfate. Washington (DC): The National Academies Press; 2005. [Google Scholar]
- 21. Cobb LK, Anderson CAM, Elliott P, Hu FB, Liu K, Neaton JD, Whelton PK, Woodward M, Appel LJ; American Heart Association Council on Lifestyle and Metabolic Health . Methodological issues in cohort studies that relate sodium intake to cardiovascular disease outcomes: a science advisory from the American Heart Association. Circulation. 2014;129(10):1173–86. [DOI] [PubMed] [Google Scholar]
- 22. Sterne JAC, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I et al.. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Babcock MC, Brian MS, Watso JC, Edwards DG, Stocker SD, Wenner MM, Farquhar WB. Alterations in dietary sodium intake affect cardiovagal baroflex sensitivity. Am J Physiol Regul Integr Comp Physiol. 2018;315(4):R688–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Babcock MC, Robinson AT, Migdal KU, Watso JC, Wenner MM, Stocker SD, Farquhar WB. Reducing dietary sodium to 1000 mg per day reduces neurovascular transduction without stimulating sympathetic outflow. Hypertension. 2019;73(3):587–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baqar S, Kong YW, Chen AX, O'Callaghan C, MacIsaac RJ, Bouterakos M, Lambert GW, Jerums G, Lambert EE, Ekinci EI. Effect of salt supplementation on sympathetic activity and endothelial function in salt-sensitive type 2 diabetes. J Clin Endocrinol Metab. 2020;105(4):dgz219. [DOI] [PubMed] [Google Scholar]
- 26. Barić L, Drenjančević I, Matić A, Stupin M, Kolar L, Mihaljević Z, Lenasi H, Šerić V, Stupin A. Seven-day salt loading impairs microvascular endothelium-dependent vasodilation without changes in blood pressure, body composition and fluid status in healthy young humans. Kidney Blood Press Res. 2019;44(4):835–47. [DOI] [PubMed] [Google Scholar]
- 27. Brian MS, Dalpiaz A, Matthews EL, Lennon-Edwards S, Edwards DG, Farquhar WB. Dietary sodium and nocturnal blood pressure dipping in normotensive men and women. J Hum Hypertens. 2017;31(2):145–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cashman KD, Kenny S, Kerry JP, Leenhardt F, Arendt EK. ‘Low salt’ bread as an important component of a pragmatic reduced-salt diet for lowering blood pressure in adults with elevated blood pressure. Nutrients. 2019;11(8):1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen L, Zhang Z, Chen W, Whelton PK, Appel LJ. Lower sodium intake and risk of headaches: results from the Trial of Nonpharmacologic Interventions in the Elderly. Am J Public Health. 2016;106(7):1270–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen L, He FJ, Dong Y, Huang Y, Harshfield GA, Zhu H. Sodium reduction, metabolomic profiling, and cardiovascular disease risk in untreated black hypertensives: a randomized, double-blind, placebo-controlled trial. Hypertension. 2019;74(1):194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Colin-Ramirez E, McAlister FA, Zheng Y, Sharma S, Armstrong PW, Ezekowitz JA. The long-term effects of dietary sodium restriction on clinical outcomes in patients with heart failure. The SODIUM-HF (Study of Dietary Intervention Under 100 mmol in Heart Failure): a pilot study. Am Heart J. 2015;169(2):274–81..e1. [DOI] [PubMed] [Google Scholar]
- 32. Derkach A, Sampson J, Joseph J, Playdon MC, Stolzenberg-Solomon RZ. Effects of dietary sodium on metabolites: the Dietary Approaches to Stop Hypertension (DASH)–Sodium Feeding Study. Am J Clin Nutr. 2017;106(4):1131–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fabricio CG, Tanaka DM, Rodrigues de Souza Gentil J, Ferreira Amato CA, Marques F, Schwartzmann PV, Schmidt A, Simões MV. A normal sodium diet preserves serum sodium levels during treatment of acute decompensated heart failure: a prospective, blind and randomized trial. Clin Nutr ESPEN. 2019;32:145–52. [DOI] [PubMed] [Google Scholar]
- 34. Foo M, Coppack SW, Denver AE, Bulmer K, Yudkin JS. Lack of impact of angiotensin-converting enzyme gene polymorphism and salt intake on insulin resistance and limb blood flow. Clin Endocrinol (Oxf). 2015;82(1):76–83. [DOI] [PubMed] [Google Scholar]
- 35. Gant CM, Laverman GD, Vogt L, Slagman MCJ, Heerspink HJL, Waanders F, Hemmelder MH, Navis G; Holland Nephrology Study (HONEST) Network . Renoprotective RAAS inhibition does not affect the association between worse renal function and higher plasma aldosterone levels. BMC Nephrol. 2017;18(1):370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gijsbers L, Dower JI, Mensink M, Siebelink E, Bakker SJL, Geleijnse JM. Effects of sodium and potassium supplementation on blood pressure and arterial stiffness: a fully controlled dietary intervention study. J Hum Hypertens. 2015;29(10):592–8. [DOI] [PubMed] [Google Scholar]
- 37. He FJ, Wu Y, Feng X-X, Ma J, Ma Y, Wang H, Zhang J, Yuan J, Lin C-P, Nowson C et al.. School based education programme to reduce salt intake in children and their families (School Edu-Salt): cluster randomised controlled trial. BMJ. 2015;350:h770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. He FJ, Ma Y, Feng X, Zhang W, Lin L, Guo X, Zhang J, Niu W, Wu Y, MacGregor GA. Effect of salt reduction on iodine status assessed by 24 hour urinary iodine excretion in children and their families in northern China: a substudy of a cluster randomised controlled trial. BMJ Open. 2016;6(9):e011168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. He M, Mu J, Liu F, Ren K, Wang Y, Guo T, Wang D. Effects of a high salt intake and potassium supplementation on QT interval dispersion in normotensive healthy subjects. Intern Med. 2015;54(3):295–301. [DOI] [PubMed] [Google Scholar]
- 40. Hu J-W, Wang Y, Chu C, Mu J-J. Effect of salt intervention on serum levels of fibroblast growth factor 23 (FGF23) in Chinese adults: an intervention study. Med Sci Monit. 2018;24:1948–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hu J-W, Wang Y, Chu C, Wang K, Yan Y, Zheng W, Ma Q, Mu J-J. The responses of the inflammatory marker, pentraxin 3, to dietary sodium and potassium interventions. J Clin Hypertens (Greenwich). 2018;20(5):925–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hummel SL, Karmally W, Gillespie BW, Helmke S, Teruya S, Wells J, Trumble E, Jimenez O, Marolt C, Wessler JD et al.. Home-delivered meals postdischarge from heart failure hospitalization: the GOURMET-HF pilot study. Circ Heart Fail. 2018;11(8):e004886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jablonski KL, Klawitter J, Chonchol M, Bassett CJ, Racine ML, Seals DR. Effect of dietary sodium restriction on human urinary metabolomic profiles. Clin J Am Soc Nephrol. 2015;10(7):1227–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Juraschek SP, Gelber AC, Choi HK, Appel LJ, Miller ER III. Effects of the Dietary Approaches to Stop Hypertension (DASH) diet and sodium intake on serum uric acid. Arthritis Rheumatol. 2016;68(12):3002–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kang HJ, Jun DW, Lee SM, Jang EC, Cho YK. Low salt and low calorie diet does not reduce more body fat than same calorie diet: a randomized controlled study. Oncotarget. 2018;9(9):8521–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Keyzer CA, van Breda GF, Vervloet MG, de Jong MA, Laverman GD, Hemmelder MH, Janssen WMT, Lambers Heerspink HJ, Kwakernaak AJ, Bakker SJL et al.. Effects of vitamin D receptor activation and dietary sodium restriction on residual albuminuria in CKD: the ViRTUE-CKD trial. J Am Soc Nephrol. 2017;28(4):1296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Liu F-Q, Liu S-Q, Zhang Y, Wang Y, Chu C, Wang D, Pan S, Wang J-K, Yu Q, Mu J-J. Effects of salt loading on plasma osteoprotegerin levels and protective role of potassium supplement in normotensive subjects. Circ J. 2016;81(1):77–81. [DOI] [PubMed] [Google Scholar]
- 48. Pinjuh Markota N, Rumboldt M, Rumboldt Z. Emphasized warning reduces salt intake: a randomized controlled trial. J Am Soc Hypertens. 2015;9(3):214–20. [DOI] [PubMed] [Google Scholar]
- 49. Meuleman Y, Hoekstra T, Dekker FW, Navis G, Vogt L, van der Boog PJM, Bos WJW, van Montfrans GA, van Dijk S; ESMO Study Group . Sodium restriction in patients with CKD: a randomized controlled trial of self-management support. Am J Kidney Dis. 2017;69(5):576–86. [DOI] [PubMed] [Google Scholar]
- 50. Muth BJ, Brian MS, Chirinos JA, Lennon SL, Farquhar WB, Edwards DG. Central systolic blood pressure and aortic stiffness response to dietary sodium in young and middle-aged adults. J Am Soc Hypertens. 2017;11(10):627–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nakano M, Eguchi K, Sato T, Onoguchi A, Hoshide S, Kario K. Effect of intensive salt-restriction education on clinic, home, and ambulatory blood pressure levels in treated hypertensive patients during a 3-month education period. J Clin Hypertens (Greenwich). 2016;18(5):385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Parvanova A, Trillini M, Podestà MA, Petrov Iliev I, Ruggiero B, Abbate M, Perna A, Peraro F, Diadei O, Rubis N et al.. Moderate salt restriction with or without paricalcitol in type 2 diabetes and losartan-resistant macroalbuminuria (PROCEED): a randomised, double-blind, placebo-controlled, crossover trial. Lancet Diabetes Endocrinol. 2018;6(1):27–40. [DOI] [PubMed] [Google Scholar]
- 53. Peng AW, Appel LJ, Mueller NT, Tang O, Miller ER III, Juraschek SP. Effects of sodium intake on postural lightheadedness: results from the DASH-sodium trial. J Clin Hypertens (Greenwich). 2019;21(3):355–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Riphagen IJ, Gijsbers L, van Gastel MDA, Kema IP, Gansevoort RT, Navis G, Bakker SJL, Geleijnse JM. Effects of potassium supplementation on markers of osmoregulation and volume regulation: results of a fully controlled dietary intervention study. J Hypertens. 2016;34(2):215–20. [DOI] [PubMed] [Google Scholar]
- 55. Rorije NMG, Rademaker E, Schrooten EM, Wouda RD, Homan Van Der Heide JJ, Van Den Born B-JH, Vogt L. High-salt intake affects sublingual microcirculation and is linked to body weight change in healthy volunteers: a randomized cross-over trial. J Hypertens. 2019;37(6):1254–61. [DOI] [PubMed] [Google Scholar]
- 56. Saran R, Padilla RL, Gillespie BW, Heung M, Hummel SL, Derebail VK, Pitt B, Levin NW, Zhu F, Abbas SR et al.. A randomized crossover trial of dietary sodium restriction in stage 3–4 CKD. Clin J Am Soc Nephrol. 2017;12(3):399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Serizawa N, Nishimuta M, Kodama N, Shimada M, Yoshitake Y, Hongu N, Ota M, Yano T. Salt restriction affects the excretions of minerals (Na, K, Ca, Mg, P and Zn) in the second voided fasting early morning urine. J Nutr Sci Vitaminol (Tokyo). 2019;65(2):142–7. [DOI] [PubMed] [Google Scholar]
- 58. Suckling RJ, He FJ, Markandu ND, MacGregor GA. Modest salt reduction lowers blood pressure and albumin excretion in impaired glucose tolerance and type 2 diabetes mellitus: a randomized double-blind trial. Hypertension. 2016;67(6):1189–95. [DOI] [PubMed] [Google Scholar]
- 59. Takada T, Imamoto M, Sasaki S, Azuma T, Miyashita J, Hayashi M, Fukuma S, Fukuhara S. Effects of self-monitoring of daily salt intake estimated by a simple electrical device for salt reduction: a cluster randomized trial. Hypertens Res. 2018;41(7):524–30. [DOI] [PubMed] [Google Scholar]
- 60. Todd AS, Walker RJ, MacGinley RJ, Kelly J, Merriman TR, Major TJ, Johnson RJ. Dietary sodium modifies serum uric acid concentrations in humans. Am J Hypertens. 2017;30(12):1196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Toering TJ, Gant CM, Visser FW, van der Graaf AM, Laverman GD, Danser AHJ, Faas MM, Navis G, Lely AT. Differences in renin-angiotensin-aldosterone system affect extracellular volume in healthy subjects. Am J Physiol Renal Phsyiol. 2018;314(5):F873–8. [DOI] [PubMed] [Google Scholar]
- 62. Wan Z, Wen W, Ren K, Zhou D, Liu J, Wu Y, Zhou J, Mu J, Yuan Z. Involvement of NLRP3 inflammasome in the impacts of sodium and potassium on insulin resistance in normotensive Asians. Br J Nutr. 2018;119(2):228–37. [DOI] [PubMed] [Google Scholar]
- 63. Wang K, Chu C, Hu J, Wang Y, Zheng W, Lv Y, Yan Y, Ma Q, Mu J. Effect of salt intake on the serum cardiotrophin-1 levels in Chinese adults. Ann Nutr Metab. 2018;73(4):302–9. [DOI] [PubMed] [Google Scholar]
- 64. Wang Y, Mu JJ, Geng LK, Wang D, Ren KY, Guo TS, Chu C, Xie BQ, Liu FQ, Yuan ZY. Effect of salt intake and potassium supplementation on brachial-ankle pulse wave velocity in Chinese subjects: an interventional study. Braz J Med Biol Res. 2015;48(1):83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wang Y, Wang D, Chu C, Mu J-J, Wang M, Liu F-Q, Xie B-Q, Yang F, Dong Z-Z, Yuan Z-Y. Effect of salt intake and potassium supplementation on urinary renalase and serum dopamine levels in Chinese adults. Cardiology. 2015;130(4):242–8. [DOI] [PubMed] [Google Scholar]
- 66. Wang Y, Chu C, Wang K-K, Hu J-W, Yan Y, Lv Y-B, Cao Y-M, Zheng W-L, Dang X-L, Xu J-T et al.. Effect of salt intake on plasma and urinary uric acid levels in Chinese adults: an interventional trial. Sci Rep. 2018;8(1):1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wang Y-Y, He W-W, Liu Y-C, Lin Y-F, Hong L-F. The effect of salt intake and potassium supplementation on serum gastrin levels in Chinese adults: a randomized trial. Nutrients. 2017;9(4):389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhang J, Yin Y, Chen L, Chu C, Wang Y, Lv Y, He M, Martin M, Huang P-H, Mu J-J et al.. Short-term high-salt diet increases corin level to regulate the salt–water balance in humans and rodents. Am J Hypertens. 2018;31(2):253–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhang Y, Li F, Liu F-Q, Chu C, Wang Y, Wang D, Guo T-S, Wang J-K, Guan G-C, Ren K-Y et al.. Elevation of fasting ghrelin in healthy human subjects consuming a high-salt diet: a novel mechanism of obesity?. Nutrients. 2016;8(6):323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Afsar B, Elsurer R, Kirkpantur A, Kanbay M. Urinary sodium excretion and ambulatory blood pressure findings in patients with hypertension. J Clin Hypertens (Greenwich). 2015;17(3):200–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ahn SY, Kim DK, Park JH, Shin SJ, Lee SH, Choi BS, Lim CS, Lee A, Jung H, Chin HJ. Long-term effects of intensive low-salt diet education on deterioration of glomerular filtration rate among non-diabetic hypertensive patients with chronic kidney disease. Kidney Blood Press Res. 2019;44(5):1101–14. [DOI] [PubMed] [Google Scholar]
- 72. Anderson J, Couper JJ, Toome S, Mpundu-Kaambwa C, Giles LC, Gent R, Coppin B, Peña AS. Dietary sodium intake relates to vascular health in children with type 1 diabetes. Pediatr Diabetes. 2018;19(1):138–42. [DOI] [PubMed] [Google Scholar]
- 73. Baldo MP, Brant LCC, Cunha RS, Molina MdCB, Griep RH, Barreto SM, Lotufo PAL, Bensenor IM, Mill JG. The association between salt intake and arterial stiffness is influenced by a sex-specific mediating effect through blood pressure in normotensive adults: the ELSA-Brasil study. J Clin Hypertens (Greenwich). 2019;21(12):1771–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Baqar S, Liu D, Lincz LF, Kong YW, Jerums G, Ekinci EI. The relationship between habitual dietary sodium intake and RAAS blockade on circulating microparticle levels in type two diabetes. Clin Sci (Lond). 2018;132(20):2207–20. [DOI] [PubMed] [Google Scholar]
- 75. Braga D, Rosa MLG, Gismondi RA, Lugon JR, Torres K, Nalin B, Kang H, Alcoforado V, Martínez Cerón DM. Uric acid and salt intake as predictors of incident hypertension in a primary care setting. Rev Colomb Cardiol. 2019; (Epub ahead of print; DOI: 10.1016/j.rccar.2019.07.011). [Google Scholar]
- 76. Buendia JR, Bradlee ML, Daniels SR, Singer MR, Moore LL. Longitudinal effects of dietary sodium and potassium on blood pressure in adolescent girls. JAMA Pediatr. 2015;169(6):560–8. [DOI] [PubMed] [Google Scholar]
- 77. Campanozzi A, Avallone S, Barbato A, Iacone R, Russo O, de Filippo G, D'Angelo G, Pensabene L, Malamisura B, Cecere G et al.. High sodium and low potassium intake among Italian children: relationship with age, body mass and blood pressure. PLoS One. 2015;10(4):e0121183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Campino C, Baudrand R, Valdivia CA, Carvajal C, Vecchiola A, Tapia-Castillo A, Martinez-Aguayo A, Garcia H, Garcia L, Allende F et al.. Sodium intake is associated with endothelial damage biomarkers and metabolic dysregulation. Am J Hypertens. 2018;31(10):1127–32. [DOI] [PubMed] [Google Scholar]
- 79. Cao WT, He J, Chen GD, Wang C, Qiu R, Chen YM. The association between urinary sodium to potassium ratio and bone density in middle-aged Chinese adults. Osteoporos Int. 2017;28(3):1077–86. [DOI] [PubMed] [Google Scholar]
- 80. Carranza-Leon D, Octaria R, Ormseth MJ, Oeser A, Solus JF, Zhang Y, Okafor CR, Titze J, Stein CM, Chung CP. Association between urinary sodium and potassium excretion and blood pressure and inflammation in patients with rheumatoid arthritis. Clin Rheumatol. 2018;37(4):895–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Carbone L, Johnson KC, Huang Y, Pettinger M, Thomas F, Cauley J, Crandall C, Tinker L, LeBoff MS, Wactawski-Wende J et al.. Sodium intake and osteoporosis: findings from the Women's Health Initiative. J Clin Endocrinol Metab. 2016;101(4):1414–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Chakma T, Kavishwar A, Sharma RK, Rao PV. High prevalence of hypertension and its selected risk factors among adult tribal population in Central India. Pathog Glob Health. 2017;111(7):343–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Charlton K, Ware LJ, Baumgartner J, Cockeran M, Schutte AE, Naidoo N, Kowal P. How will South Africa's mandatory salt reduction policy affect its salt iodisation programme? A cross-sectional analysis from the WHO-SAGE Wave 2 Salt & Tobacco Study. BMJ Open. 2018;8(3):e020404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Chen X, Guo X, Ma J, Zhang J, Tang J, Yan L, Xu C, Zhang X, Ren J, Lu Z et al.. Urinary sodium or potassium excretion and blood pressure in adults of Shandong province, China: preliminary results of the SMASH project. J Am Soc Hypertens. 2015;9(10):754–62. [retracted]. [DOI] [PubMed] [Google Scholar]
- 85. Chmielewski J, Carmody JB. Dietary sodium, dietary potassium, and systolic blood pressure in US adolescents. J Clin Hypertens (Greenwich). 2017;19(9):904–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Choi HM, Lee K-B, Kim H, Hyun YY. Sodium excretion and health-related quality of life: the results from the Korea National Health and Nutrition Examination Survey 2010–2011. Eur J Clin Nutr. 2018;72(11):1490–6. [DOI] [PubMed] [Google Scholar]
- 87. Choi HS, Chang JH, Kim JH, Kang JW. Is high sodium intake associated with hearing impairment? The association between spot urine sodium concentration and hearing threshold in Korean adolescents. Asia Pac J Clin Nutr. 2018;27(3):646–8. [DOI] [PubMed] [Google Scholar]
- 88. Choi J-H, Heo Y-R. The association between dietary sodium intake and adiposity, inflammation, and hormone markers: a preliminary study. J Nutr Health. 2017;50(6):578–84. [Google Scholar]
- 89. Choi J-H, Heo Y-R. The association between dietary sodium intake and the risk of cataract: data from Korean National Health and Nutrition Examination Survey 2012. J Nutr Health. 2019;52(3):277–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Chun YH, Han K, Kim D, Park YG, Cho KH, Choi YS, Kim SM, Kim YH, Nam GE. Association of urinary sodium excretion with insulin resistance in Korean adolescents: results from the Korea National Health and Nutrition Examination Survey 2009–2010. Medicine (Baltimore). 2016;95(17):e3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Cook NR, Appel LJ, Whelton PK. Sodium intake and all-cause mortality over 20 years in the Trial of Hypertension Prevention. J Am Coll Cardiol. 2016;68(15):1609–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Correia-Costa L, Cosme D, Nogueira-Silva L, Morato M, Sousa T, Moura C, Mota C, Guerra A, Albino-Teixeira A, Areias JC et al.. Gender and obesity modify the impact of salt intake on blood pressure in children. Pediatr Nephrol. 2016;31(2):279–88. [DOI] [PubMed] [Google Scholar]
- 93. Cortese M, Yuan C, Chitnis T, Ascherio A, Munger KL. No association between dietary sodium intake and the risk of multiple sclerosis. Neurology. 2017;89(13):1322–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Crouch SH, Ware LJ, Gafane-Matemane LF, Kruger HS, Van Zyl T, Van der Westhuizen B, Schutte AE. Dietary sodium intake and its relationship to adiposity in young black and white adults: the African-PREDICT study. J Clin Hypertens (Greenwich). 2018;20(8):1193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Deriaz D, Guessous I, Vollenweider P, Devuyst O, Burnier M, Bochud M, Ponte B. Estimated 24-h urinary sodium and sodium-to-potassium ratio are predictors of kidney function decline in a population-based study. J Hypertens. 2019;37(9):1853–60. [DOI] [PubMed] [Google Scholar]
- 96. Doukky R, Avery E, Mangla A, Collado FM, Ibrahim Z, Poulin M-F, Richardson D, Powell LH. Impact of dietary sodium restriction on heart failure outcomes. JACC Heart Fail. 2016;4(1):24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Elfassy T, Mossavar-Rahmani Y, van Horn L, Gellman M, Sotres-Alvarez D, Schneiderman N, Daviglus M, Beasley JM, Llabre MM, Shaw PA et al.. Associations of sodium and potassium with obesity measures among diverse US Hispanic/Latino adults: results from the Hispanic Community Health Study/Study of Latinos. Obesity (Silver Spring). 2018;26(2):442–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Farhadnejad H, Asghari G, Mirmiran P, Yuzbashian E, Azizi F. Micronutrient intakes and incidence of chronic kidney disease in adults: Tehran Lipid and Glucose Study. Nutrients. 2016;8(4):217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Gamage AU, De Alwis Seneviratne R, Hanna FS. Salt intake, blood pressure, and socioeconomic disparities among government employees in Sri Lanka: a cross-sectional study. J Public Health Policy. 2017;38(3):327–44. [DOI] [PubMed] [Google Scholar]
- 100. Ge Z, Guo X, Chen X, Tang J, Yan L, Ren J, Zhang J, Lu Z, Dong J, Xu J et al.. Association between 24 h urinary sodium and potassium excretion and the metabolic syndrome in Chinese adults: the Shandong and Ministry of Health Action on Salt and Hypertension (SMASH) study. Br J Nutr. 2015;113(6):996–1002. [DOI] [PubMed] [Google Scholar]
- 101. Grimes CA, Riddell LJ, Campbell KJ, He FJ, Nowson CA. 24-h urinary sodium excretion is associated with obesity in a cross-sectional sample of Australian schoolchildren. Br J Nutr. 2016;115(6):1071–9. [DOI] [PubMed] [Google Scholar]
- 102. Gruppen EG, Connelly MA, Vart P, Otvos JD, Bakker SJL, Dullaart RPF. GlycA, a novel proinflammatory glycoprotein biomarker, and high sensitivity C-reactive protein are inversely associated with sodium intake after controlling for adiposity: the Prevention of Renal and Vascular End-Stage Disease study. Am J Clin Nutr. 2016;104(2):415–22. [DOI] [PubMed] [Google Scholar]
- 103. Hallvass AEC, Claro LM, Gonçalves S, Olandoski M, Nerbass FB, Aita CAM, de Moraes TP, Pecoits-Filho R. Evaluation of salt intake, urinary sodium excretion and their relationship to overhydration in chronic kidney disease patients. Blood Purif. 2015;40(1):59–65. [DOI] [PubMed] [Google Scholar]
- 104. Han SY, Kim NH, Kim DH, Han K, Kim SM. Relationship between urinary sodium-creatinine ratios and insulin resistance in Korean children and adolescents with obesity. J Pediatr Endocrinol Metab. 2018;31(4):375–83. [DOI] [PubMed] [Google Scholar]
- 105. Han W, Hu Y, Tang Y, Xue F, Hou L, Liang S, Zhang B, Wang W, Asaiti K, Pang H et al.. Relationship between urinary sodium with blood pressure and hypertension among a Kazakh community population in Xinjiang, China. J Hum Hypertens. 2017;31(5):333–40. [DOI] [PubMed] [Google Scholar]
- 106. Han W, Han X, Sun N, Chen Y, Jiang S, Li M. Relationships between urinary electrolytes excretion and central hemodynamics, and arterial stiffness in hypertensive patients. Hypertens Res. 2017;40(8):746–51. [DOI] [PubMed] [Google Scholar]
- 107. Han W, Wang W, Sun N, Li M, Chen L, Jiang S, Chen Y, Han X. Relationship between 24-hour urinary sodium excretion and blood pressure in the adult population in Shandong, China. J Clin Hypertens (Greenwich). 2019;21(9):1370–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Haring B, Wang W, Lee ET, Jhamnani S, Howard BV, Devereux RB. Effect of dietary sodium and potassium intake on left ventricular diastolic function and mass in adults ≤40 years (from the Strong Heart Study). Am J Cardiol. 2015;115(9):1244–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Haring B, Wu C, Coker LH, Seth A, Snetselaar L, Manson JE, Rossouw JE, Wassertheil-Smoller S. Hypertension, dietary sodium, and cognitive decline: results from the Women's Health Initiative Memory Study. Am J Hypertens. 2016;29(2):202–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Hassan NE, El Shebini SM, El-Masry SA, Ahmed NH, Ali MM, El-Saeed GSM, El-Lebedy D. Association between dietary sodium, calcium, saturated fat and blood pressure in obese Egyptian adolescents. Egyptian Pediatr Assoc Gazette. 2019;67:6. [Google Scholar]
- 111. He J, Mills KT, Appel LJ, Yang W, Chen J, Lee BT, Rosas SE, Porter A, Makos G, Weir MR et al.. Urinary sodium and potassium excretion and CKD progression. J Am Soc Nephrol. 2016;27(4):1202–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. He J, Zhou X. Association between 24-h urine sodium and proteinuria among hospitalized patients with type 2 diabetes. J Diabetes Complications. 2020;34(3):107498. [DOI] [PubMed] [Google Scholar]
- 113. Hou L, Zhang M, Han W, Tang Y, Xue F, Liang S, Zhang B, Wang W, Asaiti K, Wang Y et al.. Influence of salt intake on association of blood uric acid with hypertension and related cardiovascular risk. PloS One. 2016;11(4):e0150451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Huang F, Yu P, Yuan Y, Li Q, Lin F, Gao Z, Chen F, Zhu P. The relationship between sodium excretion and blood pressure, urine albumin, central retinal arteriolar equivalent. BMC Cardiovasc Disord. 2016;16(1):194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Huh JH, Lim JS, Lee MY, Chung CH, Shin JY. Gender-specific association between urinary sodium excretion and body composition: analysis of the 2008–2010 Korean National Health and Nutrition Examination Surveys. Metabolism. 2015;64(7):837–44. [DOI] [PubMed] [Google Scholar]
- 116. Huh JH, Lee KJ, Lim JS, Lee MY, Park HJ, Kim MY, Kim JW, Chung CH, Shin JY, Kim H-S et al.. High dietary sodium intake assessed by estimated 24-h urinary sodium excretion is associated with NAFLD and hepatic fibrosis. PLoS One. 2015;10(11):e0143222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Iida H, Kurita N, Takahashi S, Sasaki S, Nishiwaki H, Omae K, Yajima N, Fukuma S, Hasegawa T, Fukuhara S et al.. Salt intake and body weight correlate with higher blood pressure in the very elderly population: the Sukagawa study. J Clin Hypertens (Greenwich). 2019;21(7):942–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Imaizumi Y, Eguchi K, Murakami T, Arakawa K, Tsuchihashi T, Kario K. High salt is independently associated with hypertensive target organ damage. J Clin Hypertens (Greenwich). 2016;18(4):315–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Ito T, Takeda M, Hamano T, Kijima T, Yamasaki M, Isomura M, Yano S, Shiwaku K, Nabika T. Effect of salt intake on blood pressure in patients receiving antihypertensive therapy: Shimane CoHRE Study. Eur J Intern Med. 2016;28:70–3. [DOI] [PubMed] [Google Scholar]
- 120. Jackson SL, Cogswell ME, Zhao L, Terry AL, Wang C-Y, Wright J, Coleman King SM, Bowman B, Chen T-C, Merritt R et al.. Association between urinary sodium and potassium excretion and blood pressure among adults in the United States: National Health and Nutrition Examination Survey, 2014. Circulation. 2018;137(3):237–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Jensen PN, Bao TQ, Huong TTT, Heckbert SR, Fitzpatrick AL, LoGerfo JP, van Ngoc TL, Mokdad AH. The association of estimated salt intake with blood pressure in a Viet Nam national survey. PLoS One. 2018;13(1):e0191437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Jung S, Kim MK, Shin J, Choi BY, Lee Y-H, Shin DH, Shin M-H. High sodium intake and sodium to potassium ratio may be linked to subsequent increase in vascular damage in adults aged 40 years and older: the Korean multi-rural communities cohort (MRCohort). Eur J Nutr. 2019;58(4):1659–71. [DOI] [PubMed] [Google Scholar]
- 123. Kalogeropoulos AP, Georgiopoulou VV, Murphy RA, Newman AB, Bauer DC, Harris TB, Yang Z, Applegate WB, Kritchevsky SB. Dietary sodium content, mortality, and risk for cardiovascular events in older adults: the Health, Aging, and Body Composition (Health ABC) Study. JAMA Intern Med. 2015;175(3):410–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Kang MS, Kim CH, Jeong SJ, Park TS. Dietary sodium intake in people with diabetes in Korea: the Korean National Health and Nutrition Examination Survey for 2008 to 2010. Diabetes Metab J. 2016;40(4):290–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Kapoor K, Fashanu O, Post WS, Lutsey PL, Michos ED, deFilippi CR, McEvoy JW. Relation of dietary sodium intake with subclinical markers of cardiovascular disease (from MESA). Am J Cardiol. 2019;124(4):636–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Khalili H, Malik S, Ananthakrishnan AN, Garber JJ, Higuchi LM, Joshi A, Peloquin J, Richter JM, Stewart KO, Curhan GC et al.. Identification and characterization of a novel association between dietary potassium and risk of Crohn's disease and ulcerative colitis. Front Immunol. 2016;7:554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Kieneker LM, Bakker SJL, de Boer RA, Navis GJ, Gansevoort RT, Joosten MM. Low potassium excretion but not high sodium excretion is associated with increased risk of developing chronic kidney disease. Kidney Int. 2016;90(4):888–96. [DOI] [PubMed] [Google Scholar]
- 128. Kieneker LM, Eisenga MF, Gansevoort RT, de Boer RA, Navis G, Dullaart RPF, Joosten MM, Bakker SJL. Association of low urinary sodium excretion with increased risk of stroke. Mayo Clin Proc. 2018;93(12):1803–9. [DOI] [PubMed] [Google Scholar]
- 129. Kim J, Park E. Comparisons of cardiometabolic biomarkers, lifestyle behaviors, and dietary sodium and potassium intake in a representative sample of Korean adults with and without cardio-cerebrovascular diseases. Asian Nursing Res (Korean Soc Nurs Sci). 2017;11(3):223–9. [DOI] [PubMed] [Google Scholar]
- 130. Kim J, Lee J, Kim K-N, Oh K-H, Ahn C, Lee J, Kang D, Park SK. Association between dietary mineral intake and chronic kidney disease: the Health Examinees (HEXA) study. Int J Environ Res Public Health. 2018;15(6):1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Kim S, Kim M, Min J, Yoo J, Kim M, Kang J, Won CW. How much intake of sodium is good for frailty?: the Korean Frailty and Aging Cohort Study (KFACS). J Nutr Health Aging. 2019;23(6):503–8. [DOI] [PubMed] [Google Scholar]
- 132. Kim YM, Kim SH, Shim YS. Association of sodium intake with insulin resistance in Korean children and adolescents: the Korea National Health and Nutrition Examination Survey 2010. J Pediatr Endocrinol Metab. 2018;31(2):117–25. [DOI] [PubMed] [Google Scholar]
- 133. Krupp D, Shi L, Egert S, Wudy SA, Remer T. Prospective relevance of fruit and vegetable consumption and salt intake during adolescence for blood pressure in young adulthood. Eur J Nutr. 2015;54(8):1269–79. [DOI] [PubMed] [Google Scholar]
- 134. Kwon S-J, Ha Y-C, Park Y. High dietary sodium intake is associated with low bone mass in postmenopausal women: Korea National Health and Nutrition Examination Survey, 2008–2011. Osteoporosis Int. 2017;28(4):1445–52. [DOI] [PubMed] [Google Scholar]
- 135. Kyung Kim M, Kwon M, Rhee M-Y, Kim K-I, Nah D-Y, Kim S-W, Gu N, Sung K-C, Hong K-S, Cho E-J et al.. Dose–response association of 24-hour urine sodium and sodium to potassium ratio with nighttime blood pressure at older ages. Eur J Prev Cardiol. 2019;26(9):952–60. [DOI] [PubMed] [Google Scholar]
- 136. Lamelas PM, Mente A, Diaz R, Orlandini A, Avezum A, Oliveira G, Lanas F, Seron P, Lopez-Jaramillo P, Camacho-Lopez P et al.. Association of urinary sodium excretion with blood pressure and cardiovascular clinical events in 17,033 Latin Americans. Am J Hypertens. 2016;29(7):796–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Lee SK, Kim J-S, Kim SH, Kim YH, Lim HE, Kim EJ, Park CG, Cho G-Y, Kim J, Baik I et al.. Sodium excretion and cardiovascular structure and function in the nonhypertensive population: the Korean Genome and Epidemiology Study. Am J Hypertens. 2015;28(8):1010–16. [DOI] [PubMed] [Google Scholar]
- 138. Lelli D, Antonelli-Incalzi R, Bandinelli S, Ferrucci L, Pedone C. Association between sodium excretion and cardiovascular disease and mortality in the elderly: a cohort study. J Am Med Dir Assoc. 2018;19(3):229–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Lemogoum D, Ngatchou W, Lele CB, Okalla C, Leeman M, Degaute J-P, van de Borne P. Association of urinary sodium excretion with blood pressure and risk factors associated with hypertension among Cameroonian pygmies and bantus: a cross-sectional study. BMC Cardiovasc Disord. 2018;18:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Li C-L, Wang H-J, Si Q-J, Zhou J, Li K-L, Ding Y. Association between urinary sodium excretion and coronary heart disease in hospitalized elderly patients in China. J Int Med Res. 2018;46(8):3078–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Li M, Yan S, Li X, Jiang S, Ma X, Zhao H, Li J, Sun C, Jin L, Yao Y et al.. Association between blood pressure and dietary intakes of sodium and potassium among US adults using quantile regression analysis NHANES 2007–2014. J Hum Hypertens. 2019; (Epub ahead of print; DOI: 10.1038/s41371-019-0224-9). [DOI] [PubMed] [Google Scholar]
- 142. Li R-Q, Chen J-C, He H-B, Zhao Z-G, Zhong J, Chen J, Zhu Z-M. Effects of obesity and salt intake on blood pressure in hypertensive patients. Chinese J Practical Int Med. 2015;35(4):338–41. [Google Scholar]
- 143. Liu H, Gao X, Zhou L, Wu Y, Li Y, Mai J, Nie Z, Wu Y, Liu X, Zhao L. Urinary sodium excretion and risk of cardiovascular disease in the Chinese population: a prospective study. Hypertens Res. 2018;41(10):849–55. [DOI] [PubMed] [Google Scholar]
- 144. Ma Y, He FJ, MacGregor GA. High salt intake: independent risk factor for obesity?. Hypertension. 2015;66(4):843–9. [DOI] [PubMed] [Google Scholar]
- 145. Marouen S, du Cailar G, Audo R, Lukas C, Vial G, Tournadre A, Barrat E, Ribstein J, Combe B, Morel J et al.. Sodium excretion is higher in patients with rheumatoid arthritis than in matched controls. PLoS One. 2017;12(10):e0186157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Martinez MG, Dos Santos Silva V, do Valle AP, de Oliveira RC, Banin VB, Hokama NK, Martin LC. Association between sodium intake and urinary fractional albumin and immunoglobulin G excretion in chronic nondialytic renal disease: a prospective longitudinal study. Nephron. 2019;143(1):62–7. [DOI] [PubMed] [Google Scholar]
- 147. Maseko M, Mashao M, Bawa-Allah A, Phukubje E, Miambo B, Nyundu T. Obesity masks the relationship between dietary salt intake and blood pressure in people of African ancestry: the impact of obesity on the relationship between sodium and blood pressure. Cardiovasc J Afr. 2018;29(3):172–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Matsuo T, Miyata Y, Sakai H. Daily salt intake is an independent risk factor for pollakiuria and nocturia. Int J Urol. 2017;24(5):384–9. [DOI] [PubMed] [Google Scholar]
- 149. Mazarova A, Molnar AO, Akbari A, Sood MM, Hiremath S, Burns KD, Ramsay TO, Mallick R, Knoll GA, Ruzicka M. The association of urinary sodium excretion and the need for renal replacement therapy in advanced chronic kidney disease: a cohort study. BMC Nephrol. 2016;17(1):123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. McDonald J, Graves J, Waldman A, Lotze T, Schreiner T, Belman A, Greenberg B, Weinstock-Guttman B, Aaen G, Tillema J-M et al.. A case-control study of dietary salt intake in pediatric-onset multiple sclerosis. Mult Scler Relat Disord. 2016;6:87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Mente A, Dagenias G, Wielgosz A, Lear SA, McQueen MJ, Zeidler J, Fu L, DeJesus J, Rangarajan S, Bourlaud A-S et al.. Assessment of dietary sodium and potassium in Canadians using 24-hour urinary collection. Can J Cardiol. 2016;32(3):319–26. [DOI] [PubMed] [Google Scholar]
- 152. Mente A, O'Donnell M, Rangarajan S, Dagenais G, Lear S, McQueen M, Diaz R, Avezum A, Lopez-Jaramillo P, Lanas F et al.. Associations of urinary sodium excretion with cardiovascular events in individuals with and without hypertension: a pooled analysis of data from four studies. Lancet. 2016;388(10043):465–75. [DOI] [PubMed] [Google Scholar]
- 153. Mente A, O'Donnell M, Rangarajan S, McQueen M, Dagenais G, Wielgosz A, Lear S, Lap Ah ST, Wei L, Diaz R et al.. Urinary sodium excretion, blood pressure, cardiovascular disease, and mortality: a community-level prospective epidemiological cohort study. Lancet. 2018;392(10146):496–506. [DOI] [PubMed] [Google Scholar]
- 154. Merino J, Guasch-Ferré M, Martínez-González MA, Corella D, Estruch R, Fitó M, Ros E, Arós F, Bulló M, Gómez-Gracia E et al.. Is complying with the recommendations of sodium intake beneficial for health in individuals at high cardiovascular risk? Findings from the PREDIMED study. Am J Clin Nutr. 2015;101(3):440–8. [DOI] [PubMed] [Google Scholar]
- 155. Mill JG, Baldo MP, Molina MdCB, Schmidt MI, Barreto SM, Chor D, Griep RH, Matos SM, Ribeiro ALP, Duncan BB et al.. Sex-specific patterns in the association between salt intake and blood pressure: the ELSA-Brasil study. J Clin Hypertens. 2019;21(4):502–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Mills KT, Chen J, Yang W, Appel LJ, Kusek JW, Alper A, Delafontaine P, Keane MG, Mohler E, Ojo A et al.. Sodium excretion and the risk of cardiovascular disease in patients with chronic kidney disease. JAMA. 2016;315(20):2200–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Monteiro C, Costa AR, Peleteiro B. Sodium intake and Helicobacter pylori infection in the early stages of life. Porto Biomed J. 2016;1(2):52–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Morgenstern LB, Sánchez BN, Conley KM, Morgenstern MC, Sais E, Skolarus LE, Levine DA, Brown DL. The association between changes in behavioral risk factors for stroke and changes in blood pressure. J Stroke Cerebrovasc Dis. 2016;25(9):2116–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Mrug S, Orihuela C, Mrug M, Sanders PW. Sodium and potassium excretion predict increased depression in urban adolescents. Physiol Rep. 2019;7(16):e14213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Murao S, Takata Y, Yasuda M, Osawa H, Kohi F. The influence of sodium and potassium intake and insulin resistance on blood pressure in normotensive individuals is more evident in women. Am J Hypertens. 2018;31(8):876–85. [DOI] [PubMed] [Google Scholar]
- 161. Ndanuko RN, Tapsell LC, Charlton KE, Neale EP, O'Donnell KM, Batterham MJ. Relationship between sodium and potassium intake and blood pressure in a sample of overweight adults. Nutrition. 2017;33:285–90. [DOI] [PubMed] [Google Scholar]
- 162. Nerbass FB, Pecoits-Filho R, McIntyre NJ, McIntyre CW, Taal MW. High sodium intake is associated with important risk factors in a large cohort of chronic kidney disease patients. Eur J Clin Nutr. 2015;69(7):786–90. [DOI] [PubMed] [Google Scholar]
- 163. Nerbass FB, Pecoits-Filho R, McIntyre NJ, Shardlow A, McIntyre CW, Taal MW. Reduction in sodium intake is independently associated with improved blood pressure control in people with chronic kidney disease in primary care. Br J Nutr. 2015;114(6):936–42. [DOI] [PubMed] [Google Scholar]
- 164. Nguyen TTM, Miura K, Tanaka-Mizuno S, Tanaka T, Nakamura Y, Fujiyoshi A, Kadota A, Tamaki J, Takebayashi T, Okamura T et al.. Association of blood pressure with estimates of 24-h urinary sodium and potassium excretion from repeated single-spot urine samples. Hypertens Res. 2019;42(3):411–18. [DOI] [PubMed] [Google Scholar]
- 165. Nowak KL, Fried L, Jovanovich A, Ix J, Yaffe K, You Z, Chonchol M. Dietary sodium/potassium intake does not affect cognitive function or brain imaging indices. Am J Nephrol. 2018;47(1):57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Oak MG, Ghugre P. Consumption of high sodium foods, salt and fat and its association with obesity and blood pressure. Indian J Public Health Res Dev. 2018;9(2):129–34. [Google Scholar]
- 167. Oh SW, Han KH, Han SY, Koo HS, Kim S, Chin HJ. Association of sodium excretion with metabolic syndrome, insulin resistance, and body fat. Medicine (Baltimore). 2015;94(39):e1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Oh SW, Koo HS, Han KH, Han SY, Chin HJ. Associations of sodium intake with obesity, metabolic disorder, and albuminuria according to age. PLoS One. 2017;12(12):e0188770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Overwyk KJ, Zhao L, Zhang Z, Wiltz JL, Dunford EK, Cogswell ME. Trends in blood pressure and usual dietary sodium intake among children and adolescents, National Health and Nutrition Examination Survey 2003 to 2016. Hypertension. 2019;74(2):260–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Ozkayar N, Dede F, Ates I, Akyel F, Yildirim T, Altun B. The relationship between dietary salt intake and ambulatory blood pressure variability in non-diabetic hypertensive patients. Nefrologia. 2016;36(6):694–700. [DOI] [PubMed] [Google Scholar]
- 171. Padilha BM, Ferreira RC, Bueno NB, Tassitano RM, de Souza Holanda L, Vasconcelos SML, Cabral PC. Association between blood cholesterol and sodium intake in hypertensive women with excess weight. Medicine (Baltimore). 2018;97(15):e0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Park SM, Joung JY, Cho YY, Sohn SY, Hur KY, Kim JH, Chung JH, Lee MK, Min Y-K. Effect of high dietary sodium on bone turnover markers and urinary calcium excretion in Korean postmenopausal women with low bone mass. Eur J Clin Nutr. 2015;69(3):361–6. [DOI] [PubMed] [Google Scholar]
- 173. Park Y, Kwon SJ, Ha YC. Association between urinary sodium excretion and bone health in male and female adults. Ann Nutr Metab. 2016;68(3):189–96. [DOI] [PubMed] [Google Scholar]
- 174. Polonia J, Monteiro J, Almeida J, Silva JA, Bertoquini S. High salt intake is associated with a higher risk of cardiovascular events: a 7.2-year evaluation of a cohort of hypertensive patients. Blood Press Monit. 2016;21(5):301–6. [DOI] [PubMed] [Google Scholar]
- 175. Prentice RL, Huang Y, Neuhouser ML, Manson JE, Mossavar-Rahmani Y, Thomas F, Tinker LF, Allison M, Johnson KC, Wassertheil-Smoller S et al.. Associations of biomarker-calibrated sodium and potassium intakes with cardiovascular disease risk among postmenopausal women. Am J Epidemiol. 2017;186(9):1035–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Rafie N, Mohammadifard N, Khosravi A, Feizi A, Safavi SM. Relationship of sodium intake with obesity among Iranian children and adolescents. ARYA Atheroscler. 2017;13(1):1–6. [PMC free article] [PubMed] [Google Scholar]
- 177. Ravi S, Bermudez OI, Harivanzan V, Chui KHK, Vasudevan P, Must A, Thanikachalam S, Thanikachalam M. Sodium intake, blood pressure, and dietary sources of sodium in an adult South Indian population. Ann Glob Health. 2016;82(2):234–42. [DOI] [PubMed] [Google Scholar]
- 178. Rebholz CM, Anderson CAM, Grams ME, Bazzano LA, Crews DC, Chang AR, Coresh J, Appel LJ. Relationship of the American Heart Association's Impact Goals (Life's Simple 7) with risk of chronic kidney disease: results from the Atherosclerosis Risk in Communities (ARIC) cohort study. J Am Heart Assoc. 2016;5(4):e003192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Rebholz CM, Crews DC, Grams ME, Steffen LM, Levey AS, Miller ER III, Appel LJ, Coresh J. DASH (Dietary Approaches to Stop Hypertension) diet and risk of subsequent kidney disease. Am J Kidney Dis. 2016;68(6):853–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Rhee M-Y, Shin S-J, Gu N, Nah D-Y, Kim B-K, Hong K-S, Cho E-J, Sung K-C, Lee S-Y, Kim K-I. Relationship between 24-h urine sodium/potassium ratio and central aortic systolic blood pressure in hypertensive patients. Hypertens Res. 2017;40(4):405–10. [DOI] [PubMed] [Google Scholar]
- 181. Rhee M-Y, Jo S-H, Kim J-H, Kim K-I, Nah D-Y, Kim S-W, Gu N, Sung K-C, Hong K-S, Cho E-J et al.. Difference in 24-hour urine sodium excretion between controlled and uncontrolled patients on antihypertensive drug treatment. J Clin Hypertens (Greenwich). 2019;21(8):1057–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Rhee OJ, Rhee MY, Oh SW, Shin SJ, Gu N, Nah DY, Kim SW, Lee JH. Effect of sodium intake on renin level: analysis of general population and meta-analysis of randomized controlled trials. Int J Cardiol. 2016;215:120–6. [DOI] [PubMed] [Google Scholar]
- 183. Rodrigues SL, Souza PR Jr, Pimentel EB, Baldo MP, Malta DC, Mill JG, Szwarcwald CL. Relationship between salt consumption measured by 24-h urine collection and blood pressure in the adult population of Vitória (Brazil). Braz J Med Biol Res. 2015;48(8):728–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Rush TM, Kritz-Silverstein D, Laughlin GA, Fung TT, Barrett-Connor EL, McEvoy LK. Association between dietary sodium intake and cognitive function in older adults. J Nutr Health Aging. 2017;21(3):276–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Sadanaga T, Hirota S, Enomoto K, Kohsaka S, Tsujita K, Ito M, Mitamura H, Fukuda K. Evaluation of sodium intake for the prediction of cardiovascular events in Japanese high-risk patients: the ESPRIT study. Hypertens Res. 2019;42(2):233–40. [DOI] [PubMed] [Google Scholar]
- 186. Saleh ZT, Lennie TA, Alhurani AS, Almansour IM, Alduraidi H, Moser DK. High dietary sodium intake is associated with shorter event-free survival in patients with heart failure and comorbid diabetes. Clin Nurs Res. 2019; (Epub ahead of print; DOI: 10.1177/1054773819888743). [DOI] [PubMed] [Google Scholar]
- 187. Salgado E, Bes-Rastrollo M, de Irala J, Carmona L, Gomez-Reino JJ. High sodium intake is associated with self-reported rheumatoid arthritis: a cross sectional and case control analysis within the SUN cohort. Medicine (Baltimore). 2015;94(37):e924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. dos Santos EM, de Araújo Brito DJ, da Cunha Teixeira França AK, Lages JS, dos Santos AM, Filho NS. Association between estimated glomerular filtration rate and sodium excretion in urine of African descendants in Brazil: a population-based study. J Bras Nefrol. 2018;40(3):248–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Saulnier P-J, Gand E, Ragot S, Bankir L, Piguel X, Fumeron F, Rigalleau V, Halimi J-M, Marechaud R, Roussel R et al.. Urinary sodium concentration is an independent predictor of all-cause and cardiovascular mortality in a type 2 diabetes cohort population. J Diabetes Res. 2017;2017:5327352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Setayeshgar S, Ekwaru JP, Maximova K, Majumdar SR, Storey KE, McGavock J, Veugelers PJ. Dietary intake and prospective changes in cardiometabolic risk factors in children and youth. Appl Physiol Nutr Metab. 2017;42(1):39–45. [DOI] [PubMed] [Google Scholar]
- 191. Shimizu Y, Kadota K, Koyamatsu J, Yamanashi H, Nagayoshi M, Noda M, Nishimura T, Tayama J, Nagata Y, Maeda T. Salt intake and mental distress among rural community-dwelling Japanese men. J Physiol Anthropol. 2015;34:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Singer P, Cohen H, Alderman M. Assessing the associations of sodium intake with long-term all-cause and cardiovascular mortality in a hypertensive cohort. Am J Hypertens. 2015;28(3):335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Siriopol D, Covic A, Iliescu R, Kanbay M, Tautu O, Radulescu L, Mitu O, Salaru D, Dorobantu M. Arterial stiffness mediates the effect of salt intake on systolic blood pressure. J Clin Hypertens (Greenwich). 2018;20(11):1587–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Smyth A, Griffin M, Yusuf S, Mann JFE, Reddan D, Canavan M, Newell J, O'Donnell M. Diet and major renal outcomes: a prospective cohort study. The NIH-AARP Diet and Health Study. J Renal Nutr. 2016;26(5):288–98. [DOI] [PubMed] [Google Scholar]
- 195. Song JH, Kim YS, Heo NJ, Lim JH, Yang SY, Chung GE, Kim JS. High salt intake is associated with atrophic gastritis with intestinal metaplasia. Cancer Epidemiol Biomarkers Prev. 2017;26(7):1133–8. [DOI] [PubMed] [Google Scholar]
- 196. Sougawa Y, Miyai N, Morioka I, Utsumi M, Takeda S, Miyashita K, Arita M. The combination of obesity and high salt intake are associated with blood pressure elevation among healthy Japanese adolescents. J Hum Hypertens. 2020;34(2):117–24. [DOI] [PubMed] [Google Scholar]
- 197. Strauss M, Smith W, Kruger R, van der Westhuizen B, Schutte AE. Large artery stiffness is associated with salt intake in young healthy black but not white adults: the African-PREDICT study. Eur J Nutr. 2018;57(7):2649–56. [DOI] [PubMed] [Google Scholar]
- 198. Sugiura T, Takase H, Ohte N, Dohi Y. Dietary salt intake is a significant determinant of impaired kidney function in the general population. Kidney Blood Press Res. 2018;43(4):1245–54. [DOI] [PubMed] [Google Scholar]
- 199. Sun N, Xi Y, Han W, Zhao L, Wang H, Chen Y. Relationship of 24-h urinary sodium excretion with blood pressure, arterial distensibility, and urine albumin in Chinese hypertensive patients. Eur Heart J Suppl. 2015;17(suppl_F):F37–43. [Google Scholar]
- 200. Sundström B, Johansson I, Rantapää-Dahlqvist S. Interaction between dietary sodium and smoking increases the risk for rheumatoid arthritis: results from a nested case-control study. Rheumatology (Oxford). 2015;54(3):487–93. [DOI] [PubMed] [Google Scholar]
- 201. Takase H, Sugiura T, Kimura G, Ohte N, Dohi Y. Dietary sodium consumption predicts future blood pressure and incident hypertension in the Japanese normotensive general population. J Am Heart Assoc. 2015;4(8):e001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Thuesen BH, Toft U, Buhelt LP, Linneberg A, Friedrich N, Nauck M, Wallaschofski H, Jørgensen T. Estimated daily salt intake in relation to blood pressure and blood lipids: the role of obesity. Eur J Prev Cardiol. 2015;22(12):1567–74. [DOI] [PubMed] [Google Scholar]
- 203. Torres SJ, Grimes C, Nowson CA, Jayasinghe SU, Bruce CR, Mason SA, He FJ, Turner AI. Urinary sodium is positively associated with urinary free cortisol and total cortisol metabolites in a cross-sectional sample of Australian schoolchildren aged 5–12 years and their mothers. Br J Nutr. 2019;121(2):164–71. [DOI] [PubMed] [Google Scholar]
- 204. Uchiyama K, Yanai A, Ishibashi Y. Spot urine-guided salt reduction in chronic kidney disease patients. J Ren Nutr. 2017;27(5):311–16. [DOI] [PubMed] [Google Scholar]
- 205. Umesawa M, Yamagashi K, Noda H, Ikeda A, Sawachi S, Muraki I, Chei C-L, Cui R, Nagao M, Ohira T et al.. The relationship between sodium concentrations in spot urine and blood pressure increases: a prospective study of Japanese general population: the Circulatory Risk in Communities Study (CIRCS). BMC Cardiovasc Disord. 2016;16:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Umesawa M, Iso H, Fujino Y, Kikuchi S, Tamakoshi A; JACC Study Group . Salty food preference and intake and risk of gastric cancer: the JACC study. J Epidemiol. 2016;26(2):92–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Ustundag S, Yilmaz G, Sevinc C, Akpinar S, Temizoz O, Sut N, Ustundag A. Carotid intima media thickness is independently associated with urinary sodium excretion in patients with chronic kidney disease. Ren Fail. 2015;37(8):1285–92. [DOI] [PubMed] [Google Scholar]
- 208. van den Berg EH, Gruppen EG, Blokzijl H, Bakker SJL, Dullaart RPF. Higher sodium intake assessed by 24 hour urinary sodium excretion is associated with non-alcoholic fatty liver disease: the PREVEND cohort study. J Clin Med. 2019;8(12):2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. van der Westhuizen B, Schutte AE, Gafane-Matemane LF, Kruger R. Left ventricular mass independently associates with 24-hour sodium excretion in young masked hypertensive adults: the African-PREDICT study. Int J Cardiol. 2019;276:218–23. [DOI] [PubMed] [Google Scholar]
- 210. Vega-Vega O, Fonseca-Correa JI, Mendoza-de la Garza A, Rincón-Pedrero R, Espinosa-Cuevas A, Baeza-Arias Y, Dary O, Herrero-Bervera B, Nieves-Anaya I, Correa-Rotter R. Contemporary dietary intake: too much sodium, not enough potassium, yet sufficient iodine: the SALMEX cohort results. Nutrients. 2018;10(7):816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Vijayalakshmi A, Sravya G, Pavithra D. A prospective study on the effect of sodium intake on renal function in hypertensive patients. Drug Invent Today. 2018;10(3):356–60. [Google Scholar]
- 212. Watanabe S, Konta T, Ichikawa K, Watanabe M, Ishizawa K, Ueno Y, Yamashita H, Kayama T, Kubota I. The association between urinary sodium excretion and blood pressure in a community-based population: the Yamagata (Takahata) study. Clin Exp Nephrol. 2019;23(3):380–6. [DOI] [PubMed] [Google Scholar]
- 213. Wang X, Kim D, Tucker KL, Weisskopf MG, Sparrow D, Hu H, Park SK. Effect of dietary sodium and potassium intake on the mobilization of bone lead among middle-aged and older men: the Veterans Affairs Normative Aging Study. Nutrients. 2019;11(11):2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Wang Y, Hu J-W, Qu P-F, Wang K-K, Yan Y, Chu C, Zheng W-L, Xu X-J, Lv Y-B, Ma Q et al.. Association between urinary sodium excretion and uric acid, and its interaction on the risk of prehypertension among Chinese young adults. Sci Rep. 2018;8(1):7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Weaver CM, Bailey RL, McCabe LD, Moshfegh AJ, Rhodes DG, Goldman JD, Lobene AJ, McCabe GP. Mineral intake ratios are a weak but significant factor in blood pressure variability in US adults. J Nutr. 2018;148(11):1845–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Welsh CE, Welsh P, Jhund P, Delles C, Celis-Morales C, Lewsey JD, Gray S, Lyall D, Iliodromiti S, Gill JMR et al.. Urinary sodium excretion, blood pressure, and risk of future cardiovascular disease and mortality in subjects without prior cardiovascular disease. Hypertension. 2019;73(6):1202–9. [DOI] [PubMed] [Google Scholar]
- 217. Won JC, Hong JW, Noh JH, Kim D-J. Association between estimated 24-h urinary sodium excretion and metabolic syndrome in Korean adults: the 2009 to 2011 Korean National Health and Nutrition Examination Survey. Medicine (Baltimore). 2016;95(15):e3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Yan L, Guo X, Wang H, Zhang J, Tang J, Lu Z, Cai X, Liu L, Gracely EJ, Ma J. Population-based association between urinary excretion of sodium, potassium and its ratio with albuminuria in Chinese. Asia Pac J Clin Nutr. 2016;25(4):785–97. [DOI] [PubMed] [Google Scholar]
- 219. Yi SS, Firestone MJ, Beasley JM. Independent associations of sodium intake with measures of body size and predictive body fatness. Obesity (Silver Spring). 2015;23(1):20–3. [DOI] [PubMed] [Google Scholar]
- 220. Yin L, Deng G, Mente A, Sun Y, Liu X, Zhang X, Wang X, Wang Y, Bo J, Chen H et al.. Association patterns of urinary sodium, potassium, and their ratio with blood pressure across various levels of salt-diet regions in China. Sci Rep. 2018;8(1):6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Yoon C-Y, Noh J, Lee J, Kee YK, Seo C, Lee M, Cha M-U, Kim H, Park S, Yun H-R et al.. High and low sodium intakes are associated with incident chronic kidney disease in patients with normal renal function and hypertension. Kidney Int. 2018;93(4):921–31. [DOI] [PubMed] [Google Scholar]
- 222. Zhang X, Wang J, Li J, Yu Y, Song Y. A positive association between dietary sodium intake and obesity and central obesity: results from the National Health and Nutrition Examination Survey 1999–2006. Nutr Res. 2018;55:33–44. [DOI] [PubMed] [Google Scholar]
- 223. Zhao L, Cogswell ME, Yang Q, Zhang Z, Onufrak S, Jackson SL, Chen T-C, Loria CM, Wang C-Y, Wright JD et al.. Association of usual 24-h sodium excretion with measures of adiposity among adults in the United States: NHANES, 2014. Am J Clin Nutr. 2019;109(1):139–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Zhao X, Zhang Y, Zhang X, Kang Y, Tian X, Wang X, Peng J, Zhu Z, Han Y. Associations of urinary sodium and sodium to potassium ratio with hypertension prevalence and the risk of cardiovascular events in patients with prehypertension. J Clin Hypertens (Greenwich). 2017;19(12):1231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Zhou L, Stamler J, Chan Q, Van Horn L, Daviglus ML, Dyer AR, Miura K, Okuda N, Wu Y, Ueshima H et al.. Salt intake and prevalence of overweight/obesity in Japan, China, the United Kingdom, and the United States: the INTERMAP study. Am J Clin Nutr. 2019;110(1):34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Zhu H, Bhagatwala J, Pollock NK, Parikh S, Gutin B, Stallmann-Jorgensen I, Thomas J, Harshfield GA, Dong Y. High sodium intake is associated with short leukocyte telomere length in overweight and obese adolescents. Int J Obes (Lond). 2015;39(8):1249–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. D'Elia L, Rossi G, di Cola MS, Savino I, Galletti F, Strazzullo P. Meta-analysis of the effect of dietary sodium restriction with or without concomitant renin-angiotensin-aldosterone system–inhibiting treatment on albuminuria. Clin J Am Soc Nephrol. 2015;10(9):1542–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. D'Elia L, Galletti F, La Fata E, Sabino P, Strazzullo P. Effect of dietary sodium restriction on arterial stiffness: systematic review and meta-analysis of the randomized controlled trials. J Hypertens. 2018;36(4):734–43. [DOI] [PubMed] [Google Scholar]
- 229. Fang Z, Wang J, Chen Y, Kong L. Sodium intake and chronic kidney disease risk: a meta-analysis of prospective studies. Int J Clin Exp Med. 2016;9(2):3104–10. [Google Scholar]
- 230. Fatahi S, Namazi N, Larijani B, Azadbakht L. The association of dietary and urinary sodium with bone mineral density and risk of osteoporosis: a systematic review and meta-analysis. J Am Coll Nutr. 2018;37(6):522–32. [DOI] [PubMed] [Google Scholar]
- 231. Garofalo C, Borrelli S, Provenzano M, de Stefano T, Vita C, Chiodini P, Minutolo R, De Nicola L, Conte G. Dietary salt restriction in chronic kidney disease: a meta-analysis of randomized clinical trials. Nutrients. 2018;10(6):732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Graudal N, Hubeck-Graudal T, Jürgens G, McCarron DA. The significance of duration and amount of sodium reduction intervention in normotensive and hypertensive individuals: a meta-analysis. Adv Nutr. 2015;6(2):169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Graudal N, Jürgens G. The blood pressure sensitivity to changes in sodium intake is similar in Asians, blacks, and whites. An analysis of 92 randomized controlled trials. Front Physiol. 2015;6:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Graudal NA, Hubeck-Graudal T, Jürgens G. Reduced dietary sodium intake increases heart rate. A meta-analysis of 63 randomized controlled trials including 72 study populations. Front Physiol. 2016;7:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Graudal N, Hubeck-Graudal T, Jürgens G, Taylor RS. Dose-response relation between dietary sodium and blood pressure: a meta-regression analysis of 133 randomized controlled trials. Am J Clin Nutr. 2019;109(5):1273–8. [DOI] [PubMed] [Google Scholar]
- 236. Jayedi A, Ghomashi F, Zargar MS, Shab-Bidar S. Dietary sodium, sodium-to-potassium ratio, and risk of stroke: a systematic review and nonlinear dose-response meta-analysis. Clin Nutr. 2019;38(3):1092–100. [DOI] [PubMed] [Google Scholar]
- 237. Kelly J, Khalesi S, Dickinson K, Hines S, Coombes JS, Todd AS. The effect of dietary sodium modification on blood pressure in adults with systolic blood pressure less than 140 mmHg: a systematic review. JBI Database System Rev Implement Rep. 2016;14(6):196–237. [DOI] [PubMed] [Google Scholar]
- 238. Lee Y-W, Huang L-H, Ku C-H. Use of dietary sodium intervention effect on neurohormonal and fluid overload in heart failure patients: review of select research based literature. Appl Nurs Res. 2018;42:17–21. [DOI] [PubMed] [Google Scholar]
- 239. Leyvraz M, Chatelan A, da Costa BR, Taffé P, Paradis G, Bovet P, Bochud M, Chiolero A. Sodium intake and blood pressure in children and adolescents: a systematic review and meta-analysis of experimental and observational studies. Int J Epidemiol. 2018;47(6):1796–810. [DOI] [PubMed] [Google Scholar]
- 240. Liu N, Sun W, Xing Z, Ma F, Sun T, Wu H, Dong Y, Xu Z, Fu Y, Yuan H. Association between sodium intakes with the risk of chronic kidney disease: evidence from a meta-analysis. Int J Clin Exp Med. 2015;8(11):20939–45. [PMC free article] [PubMed] [Google Scholar]
- 241. Mahtani KR, Heneghan C, Onakpoya I, Tierney S, Aronson JK, Roberts N, Hobbs FDR, Nunan D. Reduced salt intake for heart failure: a systematic review. JAMA Intern Med. 2018;178(12):1693–700. [DOI] [PubMed] [Google Scholar]
- 242. McMahon EJ, Campbell KL, Bauer JD, Mudge DW. Altered dietary salt intake for people with chronic kidney disease. Cochrane Database Syst Rev. 2015;(2):CD010070. [DOI] [PubMed] [Google Scholar]
- 243. Milajerdi A, Djafarian K, Shab-Bidar S. Dose–response association of dietary sodium intake with all-cause and cardiovascular mortality: a systematic review and meta-analysis of prospective studies. Public Health Nutr. 2019;22(2):295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Moosavian SP, Haghighatdoost F, Surkan PJ, Azadbakht L. Salt and obesity: a systematic review and meta-analysis of observational studies. Int J Food Sci Nutr. 2017;68(3):265–77. [DOI] [PubMed] [Google Scholar]
- 245. Nomura K, Asayama K, Jacobs L, Thijs L, Staessen JA. Renal function in relation to sodium intake: a quantitative review of the literature. Kidney Int. 2017;92(1):67–78. [DOI] [PubMed] [Google Scholar]
- 246. Oh H, Lee HY, Jun DW, Lee SM. Low salt diet and insulin resistance. Clin Nutr Res. 2016;5(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Patel SM, Cobb P, Saydah S, Zhang X, de Jesus JM, Cogswell ME. Dietary sodium reduction does not affect circulating glucose concentrations in fasting children or adults: findings from a systematic review and meta-analysis. J Nutr. 2015;145(3):505–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Poggio R, Gutierrez L, Matta MG, Elorriaga N, Irazola V, Rubinstein A. Daily sodium consumption and CVD mortality in the general population: systematic review and meta-analysis of prospective studies. Public Health Nutr. 2015;18(4):695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Rios-Leyvraz M, Bloetzer C, Chatelan A, Bochud M, Burnier M, Santschi V, Paradis G, Tabin R, Bovet P, Chiolero A. Sodium intake and blood pressure in children with clinical conditions: a systematic review with meta-analysis. J Clin Hypertens (Greenwich). 2019;21(1):118–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Subasinghe AK, Arabshahi S, Busingye D, Evans RG, Walker KZ, Riddell MA, Thrift AG. Association between salt and hypertension in rural and urban populations of low to middle income countries: a systematic review and meta-analysis of population based studies. Asia Pac J Clin Nutr. 2016;25(2):402–13. [DOI] [PubMed] [Google Scholar]
- 251. Zhu Y, Zhang J, Li Z, Liu Y, Fan X, Zhang Y, Zhang Y. Association of sodium intake and major cardiovascular outcomes: a dose-response meta-analysis of prospective cohort studies. BMC Cardiovasc Disord. 2018;18(1):192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Svetkey LP, Sacks FM, Obarzanek E, Vollmer WM, Appel LJ, Lin P-H, Karanja NM, Harsha DW, Bray GA, Aickin M et al.. The DASH diet, sodium intake and blood pressure trial (DASH-Sodium): rationale and design. J Am Diet Assoc. 1999;99(8 Suppl):S96–104. [DOI] [PubMed] [Google Scholar]
- 253. Kumae T, Kogure H, Nishimuta M, Kodama N, Yoshitake Y. Effects of a 21 day metabolic study on serum opsonic activity in female college students, assessed by a chemiluminescence technique. Luminescence. 2006;21(4):256–61. [DOI] [PubMed] [Google Scholar]
- 254. Stolarz-Skrzypek K, Kuznetsova T, Thijs L, Tikhonoff V, Seidlerová J, Richart T, Jin Y, Olszanecka A, Malyutina S, Casiglia E et al.. Fatal and nonfatal outcomes, incidence of hypertension, and blood pressure changes in relation to urinary sodium excretion. JAMA. 2011;305(17):1777–85. [DOI] [PubMed] [Google Scholar]
- 255. Cohen HW, Hailpern SM, Alderman MH. Sodium intake and mortality follow-up in the third National Health and Nutrition Examination Survey (NHANES III). J Gen Intern Med. 2008;23(9):1297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Ekinci EI, Clarke S, Thomas MC, Moran JL, Cheong K, MacIsaac RJ, Jerums G. Dietary salt intake and mortality in patients with type 2 diabetes. Diabetes Care. 2011;34(3):703–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Campbell NRC, Cappuccio FP, Tobe SW. Viewpoint: unnecessary controversy regarding dietary sodium: a lot about a little. Can J Cardiol. 2011;27(4):404–6. [DOI] [PubMed] [Google Scholar]
- 258. Newberry SJ, Chung M, Anderson CAM, Chen C, Fu Z, Tang A, Zhao N, Booth M, Marks J, Hollands S et al.. Sodium and potassium intake: effects on chronic disease outcomes and risks. Comparative Effectiveness Review No. 206. AHRQ Publication No. 18-EHC009-EF Rockville, MD: Agency for Healthcare Research and Quality; June2018. [PubMed] [Google Scholar]
- 259. Harris JE, Raynor HA. Crossover designs in nutrition and dietetics research. J Acad Nutr Diet. 2017;117(7):1023–30. [DOI] [PubMed] [Google Scholar]
- 260. Campbell NRC, He FJ, Tan M, Cappuccio FP, Neal B, Woodward M, Cogswell ME, McLean R, Arcand J, MacGregor G et al.. The International Consortium for Quality Research on Dietary Sodium/Salt (TRUE) position statement on the use of 24-hour, spot, and short duration (<24 hours) timed urine collections to assess dietary sodium intake. J Clin Hypertens (Greenwich). 2019;21(6):700–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. He FJ, MacGregor GA. Salt reduction lowers cardiovascular risk: meta-analysis of outcome trials. Lancet. 2011;378(9789):380–2. [DOI] [PubMed] [Google Scholar]
- 262. Whelton PK, Appel LJ, Sacco RL, Anderson CAM, Antman EM, Campbell N, Dunbar SB, Frohlich ED, Hall JE, Jessup M et al.. Sodium, blood pressure, and cardiovascular disease: further evidence supporting the American Heart Association sodium reduction recommendations. Circulation. 2012;126(24):2880–9. [DOI] [PubMed] [Google Scholar]
- 263. Jones DW, Luft FC, Whelton PK, Alderman MH, Hall JE, Peterson ED, Califf RM, McCarron DA. Can we end the salt wars with a randomized clinical trial in a controlled environment?. Hypertension. 2018;72(1):10–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JAC et al.. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Institute of Medicine (IOM). Finding what works in health care: standards for systematic reviews. Washington (DC): The National Academies Press; 2011. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.