ABSTRACT
The relation between meal frequency and measures of obesity is inconclusive. Therefore, this systematic review and network meta-analysis (NMA) set out to compare the isocaloric effects of different meal frequencies on anthropometric outcomes and energy intake (EI). A systematic literature search was conducted in 3 electronic databases (Medline, Cochrane Library, Web of Science; search date, 11 March 2019). Randomized controlled trials (RCTs) were included with ≥2 wk intervention duration comparing any 2 of the eligible isocaloric meal frequencies (i.e., 1 to ≥8 meals/d). Random-effects NMA was performed for 4 outcomes [body weight (BW), waist circumference (WC), fat mass (FM), and EI], and surface under the cumulative ranking curve (SUCRA) was estimated using a frequentist approach (P-score: value is between 0 and 1). Twenty-two RCTs with 647 participants were included. Our results suggest that 2 meals/d probably slightly reduces BW compared with 3 meals/d [mean difference (MD): −1.02 kg; 95% CI: −1.70, −0.35 kg) or 6 meals/d (MD: −1.29 kg; 95% CI: −1.74, −0.84 kg; moderate certainty of evidence). We are uncertain whether 1 or 2 meals/d reduces BW compared with ≥8 meals/d (MD1 meal/d vs. ≥8 meals/d: −2.25 kg; 95% CI: −5.13, 0.63 kg; MD2 meals/d vs. ≥8 meals/d: −1.32 kg; 95% CI: −2.19, −0.45 kg) and whether 1 meal/d probably reduces FM compared with 3 meals/d (MD: −1.84 kg; 95% CI: −3.72, 0.05 kg; very low certainty of evidence). Two meals per day compared with 6 meals/d probably reduce WC (MD: −3.77 cm; 95% CI: −4.68, −2.86 cm; moderate certainty of evidence). One meal per day was ranked as the best frequency for reducing BW (P-score: 0.81), followed by 2 meals/d (P-score: 0.74), whereas 2 meals/d performed best for WC (P-score: 0.96). EI was not affected by meal frequency. In conclusion, our findings indicate that there is little robust evidence that reducing meal frequency is beneficial.
Keywords: network meta-analysis, meal frequency, snacking, obesity, weight loss
Introduction
Across the globe, dietary habits include the consumption of foods (snacking) and calorie-containing beverages between main meals (1). It has been hypothesized that eating small, frequent meals instead of few larger meals enhances fat loss and helps to achieve better weight maintenance (2). A number of observational studies provided support for this hypothesis, with an inverse relation noted between the frequency of eating and obesity (3, 4). For example in the Malmö Diet and Cancer Study, lower meal frequencies (≤3 meals/d) were associated with a higher risk of (abdominal) obesity compared with a higher meal frequency (≥6 meals/d) (4). These findings are in line with a meta-analysis of randomized controlled trials (RCTs), indicating that a higher meal frequency was effective in reducing fat mass (5). Controversially, another prospective cohort study indicated that increasing the number of eating occasions beyond 3 meals/d is associated with a higher risk for weight gain (6). Similarly, a recent pairwise meta-analysis of 13 RCTs suggested that lowering meal frequency through skipping breakfast and thereby prolonging fasting times may help to reduce weight in adults (7).
Given these contradictions, it is also still unclear whether an increased meal frequency through snacking between main meals influences anthropometric outcomes. Generally, there are healthy (e.g., fruit, salads, or nuts) and unhealthy (e.g., sweets or crisps) snacks that have a different effect on health and anthropometric outcomes as well (1). Yet, no consensus on the relation between meal frequency—by spreading main meals over more eating occasions or through healthy snacking—and obesity has been reached (8).
Compared with the above-described pairwise meta-analyses, the methodological approach of network meta-analysis (NMA) offers the possibility to combine direct (i.e., from trials comparing 2 meal frequency interventions directly: e.g., 3 meals/d vs. 6 meals/d) and indirect (i.e., from a connected root via ≥1 intermediate comparators) evidence in a network of trials. To the best of our knowledge, no NMA has been conducted to date that simultaneously compared the isocaloric effects of different meal frequencies on anthropometric outcomes and energy intake. Therefore, the aim of the present research project was to investigate the impact of different meal frequencies, combine the direct and indirect evidence, and rank the different dietary approaches for effects on anthropometric outcomes (body weight, waist circumference, fat mass) and energy intake using NMA methodology.
Methods
This NMA was registered in PROSPERO (International Prospective Register of Systematic Reviews; www.crd.york.ac.uk/prospero/index.asp, identifier CRD42019138572) and is being reported in adherence to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) standards for reporting NMAs (9, 10).
Search strategy
The systematic literature search was performed in the electronic databases Medline (Supplemental Appendix 1), Web of Science, and the Cochrane Central Register of Controlled Trials (CENTRAL) up until 11 March 2019, with no restriction of language and calendar date. Furthermore, the reference lists from eligible studies were screened to identify additional relevant research. Screening and study selection were conducted by 2 authors independently (LS, KB).
Selection of studies
Studies were included in the NMA if they met the following criteria:
RCTs (with a parallel or crossover design) examining ≥2 dietary meal frequency interventions per day: 1 meal/d vs. 2 vs. 3 vs. 4 vs. 5 vs. 6 vs. 7 vs. ≥8.
-
Isocaloric comparison (11):
-
If the amount of energy intake was exchanged with an equal amount of energy intake for the different meal frequency interventions within a trial.
Hypo vs. hypocaloric energy intake within a trial
Eucaloric vs. eucaloric energy intake within a trial
-
If the RCT involved overfeeding, such that excess energy was supplied, resulting in a positive energy balance, then the comparison was still considered isocaloric as long as the comparator was matched for the excess energy, resulting in the same positive energy balance.
Hyper vs. hypercaloric energy intake within a trial
-
Minimum duration of the intervention: ≥2 wk, in line with a recent meta-analysis on breakfast skipping (7).
Participants with a mean age ≥18 y.
The following studies were excluded:
RCTs of acute (single meal) postprandial effects only.
RCTs with critically ill and hospitalized patients, patients undergoing bariatric surgery, patients with eating disorders.
RCTs based solely on liquid/formula diets, meal replacement interventions, or dietary supplements.
Co-intervention (e.g., drugs, supplements, diet, or physical activity) not applied in all intervention arms.
Data extraction
For included studies, 2 reviewers independently (KB, JZ) extracted the following characteristics to a piloted data extraction form: name of first author, year of publication, study origin (country), study design (RCT: parallel or crossover, including duration of washout period), comparison of meal frequency type, sample size, disease status (i.e., healthy, obese, type 2 diabetic, hypercholesterolemia), mean age, mean BMI, presence of type 2 diabetes (T2D; %), gender, duration of intervention (weeks), specification of the type of isocaloric comparison within an RCT (i.e., hypocaloric, eucaloric, hypercaloric), provision of food (yes/no), meals consumed in research center (yes/no), energy intake and macronutrient composition, outcomes extracted for the present NMA, and dietary adherence.
The preferred outcome data were change scores adjusted for baseline measurements with corresponding SDs, followed by postintervention values and change scores not adjusted for baseline measurements, as reported previously (12).
Risk-of-bias assessment
Risk of bias (RoB) was assessed by 2 authors independently (SL, GT) according to the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (13). The following domains were considered: selection bias (random-sequence generation and allocation concealment), performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessment), attrition bias (incomplete outcome data), reporting bias (selective reporting), and other bias (carryover effect in crossover RCTs and potential conflict of interest).
Data synthesis
Statistical analysis
Available direct comparisons between dietary interventions were illustrated using a network plot (14) for the following outcomes: body weight (kilograms), waist circumference (centimeters), fat mass (kilograms), and energy intake (kilocalories per day). Of note, in NMAs the size of the nodes is proportional to the sample size of each dietary intervention and the thickness of the lines proportional to the number of studies available.
Afterwards, the direct and indirect treatment effects across the RCTs were pooled, and weighted mean differences (MDs) for the outcome measures were calculated.
We performed NMAs in a contrast-based framework using the R package netmeta, version 6.6–6 (15). Treatments were ranked by P-scores, which are a frequentist version of the surface under the cumulative ranking curve (SUCRA) (16, 17). P-scores are values between 0 and 1, where a value of 1 means that a treatment ranks always best and a value of 0 means that a treatment ranks always worst.
Assessment of intransitivity
To evaluate the assumption of transitivity (18), we compared the similarity of the included populations and study settings in terms of age, BMI, disease status, and study length for the available direct comparisons.
Assessment of inconsistency
The effect estimate for each comparison was split into the contribution of direct and indirect evidence to see whether they differed and, therefore, we were able to assess potential inconsistency. We created a net heat plot by applying a full treatment-design interaction model (19). This model separates effects within and between different designs (19). A design is defined as the subset of treatments that are compared in a trial.
Secondary analyses and sensitivity analyses
We planned a priori to conduct secondary analyses for gender, study duration (<6 vs. ≥6 mo), anthropometric status (e.g., lean vs. overweight vs. obese), provision of food (yes/no), meal consumed in research center (yes/no), energy intake (hypo- vs. eucaloric), breakfast skipping (yes/no), and by excluding RCTs rated for high RoB.
Dissemination bias
To evaluate dissemination bias, a comparison-adjusted funnel plot (20) was created for each direct pairwise comparison, and Egger's linear regression test for funnel plot asymmetry was conducted to investigate small study effects (21).
Grading of recommendations assessment, development, and evaluation (certainty of the evidence)
We followed the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach to rate the certainty of evidence derived from NMA. For all outcomes, 2 authors independently (LS, CS) rated the certainty of evidence in each of the direct, indirect, and network estimates (22). Direct estimates were evaluated with the following GRADE criteria: RoB, indirectness, inconsistency, and publication bias. As suggested recently by the GRADE working group, consideration of imprecision is not necessary when rating the direct and indirect estimates to inform the rating of NMA estimates (22). The indirect estimate assessments were based on the direct estimate certainty and were rated down if intransitivity was judged as serious (i.e., disease status). The NMA certainty estimates were based on the direct and indirect estimates certainty (specifically, the higher certainty between direct and indirect was chosen as the certainty of the NMA estimate), and rated down if incoherence or imprecision were present (22). Overall, GRADE specifies 4levels of certainty of evidence: high, moderate, low, and very low.
Results
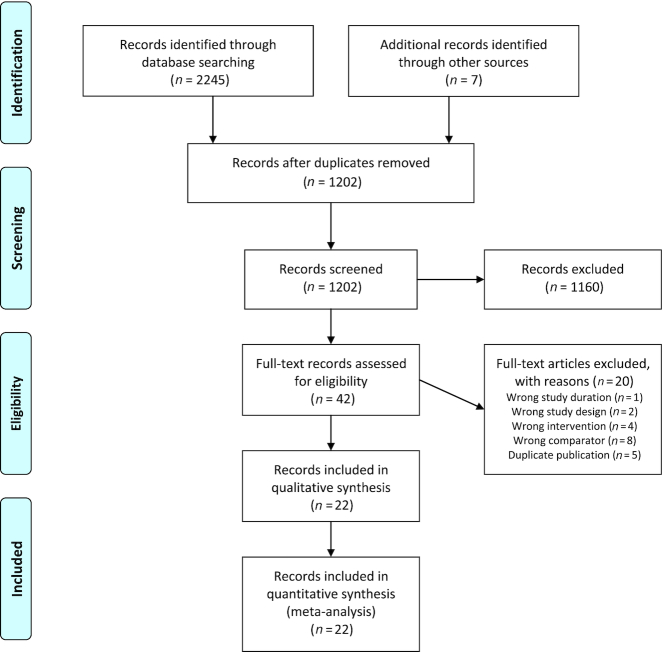
Out of 1202 records identified by the literature searches, 42 records were assessed as full texts (Figure 1, Supplemental Appendix 2). Finally, 22 RCTs with a total of 647 participants published between 1971 and 2018 were included in the systematic review and NMA (23–44).
FIGURE 1.
Flow diagram showing study selection process.
Eight RCTs were conducted in the United States (23, 25, 29, 32, 40–42, 44); 3 in New Zealand (26–28); 2 each in the Netherlands (36, 43), Canada (31, 34), and Greece (38, 39); and 1 each in France (24), Sweden (30), Japan (33), Czech Republic (35), and United Kingdom (37). The study duration ranged from 2 to 52 wk; the mean age of the participants ranged from 20 to 70 y, their BMI (kg/m2) ranged from 20.9 to 38.4. In 10 RCTs, the mean BMI of the included participants was ≥30 (23, 24, 29–31, 36, 39, 41, 43, 44), while in 5 RCTs (23, 24, 30, 31, 41) only participants with a BMI ≥30 were included and 3 RCTs included participants with T2D or impaired glucose tolerance, respectively (28, 35, 39). The study sample of 5 RCTs was described as healthy with no major diseases (26, 33, 36, 37, 42). The most common type of frequency comparison was 3 vs. 6 meals/d, assessed in 9 RCTs, followed by 2 vs. 6 meals/d and 3 vs. 9 meals/d evaluated in 3 RCTs, respectively. In 13 RCTs the intervention of different meal frequencies aimed to reduce body weight (prescription of a hypocaloric diet) (23–25, 29–33, 35, 36, 41, 43, 44). Nine of the 22 included RCTs (41%) provided information on the macronutrient distribution: the ranges were 15–35% for protein, 20–40% for fat, and 40–60% for carbohydrates (23, 25, 29, 32, 33, 35, 41, 42, 44). Eleven RCTs provided information on caloric intake, but not macronutrient distribution for single meals (26–28, 30, 34, 37–40, 43). Study and participant characteristics are summarized in Tables 1 and 2, respectively.
TABLE 1.
Study characteristics of the included RCTs1
| Author, year, reference | Study origin (country) | Study design (RCT: parallel or crossover) | Study length, wk | Washout (length) | Comparison of types of meal frequency | Sample size, n | Primary disease status | Mean age, y | Mean BMI, kg/m2 | T2D, % | Female, % |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Alencar et al., 2015 (23) | USA | RCT, crossover | 2 | 2 wk | 6 vs. 2 | 11 | Obesity | 52 | 39.1 | 0 | 100 |
| Antoine et al., 1984 (24) | France | RCT, crossover | 2 | None | 6 vs. 3 | 10 | Obesity | 40.9 | 31.8 | NR | 100 |
| Arciero et al., 2013 (25) | USA | RCT, parallel | 4 | — | 6 vs. 3 | 30 | Overweight or obesity | 46 | 29.9 | 0 | 86 |
| Arnold et al., 1993 (26) | New Zealand | RCT, crossover | 2 | 1–7 d | 9 vs. 3 | 19 | Healthy | 32.1 | 23.1 | 0 | 50 |
| Arnold et al., 1994 (27) | New Zealand | RCT, crossover | 4 | None | 9 vs. 3 | 16 | Hypercholesterolemia | 49.9 | 26.5 | 0 | 31 |
| Arnold et al., 1997 (28) | New Zealand | RCT, crossover | 4 | None | 9 vs. 3 | 13 | T2D, impaired glucose tolerance | 46–70 (range) | 29.9 | 85 | 69 |
| Bachman and Raynor, 2012 (29) | USA | RCT, parallel | 24 | — | 7–9 vs. 3 | 51 | Overweight or obesity | 51 | 35.5 | 0 | 58 |
| Berteus Forslund et al., 2008 (30) | Sweden | RCT, parallel | 52 | — | 6 vs. 3 | 140 | Obesity | 39.5 | 38.4 | 0 | 74 |
| Cameron et al., 2010 (31) | Canada | RCT, parallel | 8 | — | 6 vs. 3 | 18 | Obesity | 35.5 | 36 | 0 | 50 |
| Finkelstein and Fryer, 1971 (32) | USA | RCT, parallel | 9 | — | 6 vs. 3 | 8 | Overweight | 20–22 | 26.9 | 0 | 100 |
| Iwao et al., 1996 (33) | Japan | RCT, parallel | 2 | — | 6 vs. 2 | 12 | Healthy | 20 | 20.9 | 0 | NR |
| Jenkins et al., 1995 (34) | Canada | RCT, crossover | 2 | 3 wk | 17 vs. 3 | 7 | Normal weight and overweight | 39.6 | NR | 0 | 0 |
| Kahleova et al., 2014 (35) | Czech Republic | RCT, crossover | 12 | None | 6 vs. 2 | 54 | T2D | 59.4 | 32.6 | 100 | 46 |
| Koopman et al., 2014 (36) | Netherlands | RCT, parallel | 6 | — | 6 vs. 3 | 36 | Healthy | 22 | 22.5 | 0 | 0 |
| Murphy et al., 1996 (37) | UK | RCT, crossover | 2 | 3 wk | 12 vs. 2 | 11 | Healthy | 22 | 23.6 | 0 | 100 |
| Papakonstantinou et al., 2016 (38) | Greece | RCT, crossover | 12 | None | 6 vs. 3 | 45 | PCOS | 27 | 27 | 0 | 100 |
| Papakonstantinou et al., 2018 (39) | Greece | RCT, crossover | 12 | None | 6 vs. 3 | 53 | Impaired glucose tolerance or T2D | 49.3 | 32.4 | 26 | 53 |
| Perrigue et al., 2017 (40) | USA | RCT, crossover | 3 | 2 wk | 8 vs. 3 | 15 | Healthy and obesity | 27.1 | 23.7 | 0 | 67 |
| Schlundt et al., 1992 (41) | USA | RCT, parallel | 12 | — | 3 vs. 2 | 52 | Obesity | 18–55 (range) | 30.6 | 0 | 100 |
| Stote et al., 2007 (42) | USA | RCT, crossover | 8 | 11 wk | 3 vs. 1 | 21 | Healthy | 45 | 23.4 | 0 | 67 |
| Verboeket-van de Venne and Westerterp, 1993 (43) | Netherlands | RCT, parallel | 4 | — | 4 vs. 2 | 14 | Overweight and obesity | 46.1 | 30.2 | NR | 100 |
| Young et al., 1971 (44) | USA | RCT, crossover | 5 | None | 6 vs. 3 vs. 1 | 11 | Overweight and obesity | 22.2 | 33.5 | NR | 0 |
1NR, not reported; PCOS, polycystic ovary syndrome; RCT, randomized controlled trial; T2D, type 2 diabetes.
TABLE 2.
Specific study characteristics of the included randomized controlled trials1
| Author, year, reference | Specification of the intervention arms (meals/d) | Specification of the intervention arms (meals/d) | Provision of food | Meals consumed in research center | Energy intake during/after intervention, kcal/d | Adherence (method) |
|---|---|---|---|---|---|---|
| Alencar et al., 2015 (23) | 6 meals/d:Hypocaloric, 1200 kcal/d with ∼75 g/d of protein. Daily participant dietary macronutrients averaged per day 52% carbohydrates, 27% protein, and 21% fat, every 2 to 3 h. | 2 meals/d:Hypocaloric, 1200 kcal/d with ∼75 g/d of protein. Daily participant dietary macronutrients averaged per day 52% carbohydrates, 27% protein, and 21% fat, every 5 to 6 h. | Yes | No | NR | Assessed but not reported (reported during weekly meetings) |
| Antoine et al., 1984 (24) | 6 meals/d:Hypocaloric, 1200 kcal/d (feeding study) | 3 meals/d:Hypocaloric 1200 kcal/d (feeding study) | Yes | Yes | NR | Assessed but not reported (supervision during meals in clinic) |
| Arciero et al., 2013 (25) | 6 meals/d:Eucaloric (28 d), hypocaloric (28 d);35% protein, 45% carbohydrates, and 20% fat/d | 3 meals/d:Eucaloric (28 d), hypocaloric (28 d);35% protein, 45% carbohydrates, and 20% fat/d | Partly | No | 6 meals/d: 1541;3 meals/d: 1559 | Assessed but not reported (monitored through daily subject-researcher contact) |
| Arnold et al., 1993 (26) | 9 meals/d:Eucaloric; daily energy allowance was divided into early morning 8.3%, breakfast 8.3%, midmorning 8.3%, lunch 8.3%, midafternoon 8.3%, late afternoon 8.3%, dinner 16.6%, midevening 16.6%, and late evening 16.6%. Guidelines about how to allocate food into the meal or snack category were given and the nine meals were spaced between 1 and 2 h apart depending on the subject's schedule. | 3 meals/d:Eucaloric; breakfast 25%, lunch 25%, dinner 50%, and a single small snack of 150 kcal to be consumed when desired | No | No | 9 meals/d: 1995;3 meals/d: 1934 | 9 meals/d: 8.3;3 meals/d: 3.2 (3-d dietary records) |
| Arnold et al., 1994 (27) | 9 meals/d:Eucaloric; daily energy allowance was divided into early morning 8.3%, breakfast 8.3%, midmorning 8.3%, lunch 8.3%, midafternoon 8.3%, late afternoon 8.3%, dinner 16.6%, midevening 16.6%, and late evening 16.6%. Guidelines about how to allocate food into the meal or snack category were given and the 9 meals were spaced between 1 and 2 h apart depending on the subject's schedule. | 3 meals/d:Eucaloric; breakfast 25%, lunch 25%, dinner 50%, and a single small snack of 150 kcal to be consumed when desired | No | No | 9 meals/d: 1972;3 meals/d: 1898 | 9 meals/d: 7.9;3 meals/d: 3.1 (dietary records) |
| Arnold et al., 1997 (28) | 9 meals/d:Eucaloric; daily energy allowance was divided into early morning 8.3%, breakfast 8.3%, midmorning 8.3%, lunch 8.3%, midafternoon 8.3%, late afternoon 8.3%, dinner 16.6%, midevening 16.6%, and late evening 16.6%. Guidelines about how to allocate food into the meal or snack category were given and the nine meals were spaced between 1 and 2 h apart depending on the subject's schedule. | 3 meals/d:Eucaloric; breakfast 25%, lunch 25%, dinner 50%, and a single small snack of 150 kcal to be consumed when desired | No | No | NR | Assessed but not reported (regular contact between researchers and participants + dietary records) |
| Bachman and Raynor, 2012 (29) | 7–9 meals/d:Hypocaloric (1200–1500 kcal/d, <30 % from fat/d), consume at least 100 kcal every 2–3 h | 3 meals/d:Hypocaloric (1200–1500 kcal/d, <30% of kcal from fat/d) | No | No | 7–9 meals/d: 1314;3 meals/d: 1217 | Assessed but not reported (3-d food records) |
| Berteus Forslund et al., 2008 (30) | 6 meals/d:Hypocaloric (from the estimated total energy expenditure 30% was subtracted to get the prescribed energy intake). The minimum energy level prescribed was 1400 kcal/d. The prescribed energy level was divided into 3 meals and 3 snacks, daily energy intake was divided in breakfast 20%, lunch 25%, dinner 25% and 3 snacks, each snack 10% of daily energy; breakfast 20% + lunch 25% + dinner 25% + 3×10% = 100%. | 3 meals/d:Hypocaloric (from the estimated total energy expenditure 30% was subtracted to get the prescribed energy intake. The minimum energy level prescribed was 1400 kcal/d). Recommended energy intake in the group of 3 meals was divided in breakfast, 30% of daily energy intake, lunch 35%, and dinner 35% and no snacks with the exception of limited fruit intake and calorie-free drinks. | No | No | 6 meals/d: 2150;3 meals/d: 2098 | Assessed but not reported (questionnaires, telephone interviews) |
| Cameron et al., 2010 (31) | 6 meals/d:Hypocaloric: −700 kcal/d | 3 meals/d:Hypocaloric: −700 kcal/d | No | No | NR | Data not collected |
| Finkelstein and Fryer, 1971 (32) | 6 meals/d:Hypocaloric (period 1: 1700 kcal/d;period 2: 1400 kcal/d). All meals containing similar amounts of protein, carbohydrate, and fat. | 3 meals/d:Hypocaloric (period 1: 1700 kcal/d; period 2: 1400 kcal/d). All meals containing similar amounts of protein, carbohydrate, and fat. | Yes | Partly | NR | Data not collected |
| Iwao et al., 1996 (33) | 6 meals/d:Hypocaloric (1200 kcal/d), intervals of 2 h, daily percentages of carbohydrates, protein and lipids in total sources of energy were 60%, 20%, and 20% | 2 meals/d:Hypocaloric (1200 kcal/d), intervals of 12 h, daily percentages of carbohydrates, protein, and lipids in total sources of energy were 60%, 20%, and 20% | Yes | No | NR | Data not collected |
| Jenkins et al., 1995 (34) | 17 meals/d: Eucaloric (2730 kcal/d).Equal macronutrient and caloric content per meal. | 3 meals/d:Eucaloric (2730 kcal/d).Daily energy distribution: 30% breakfast, 30% lunch, and 40% dinner; 33% fat, 15% protein, and 52% carbohydrate. | Yes | Partly (for the 17-meals/d intervention: at least 1 portion/d was eaten in the study kitchen; for 3-meals/d intervention: at least 1 meal/d was eaten in the study kitchen) | NR | Assessed but not reported (food diary) |
| Kahleova et al., 2014 (35) | 6 meals/d:Hypocaloric, energy requirements were based on the formula: (resting energy expenditure × 1.5) − 500 kcal.Macronutrient distribution per day: 50–55% carbohydrates, 20–25% protein, and <30% from fat. | 2 meals/d:Hypocaloric, energy requirements were based on the formula: (resting energy expenditure × 1.5) – 500 kcal.Macronutrient distribution per day: 50–55% carbohydrates, 20–25% protein, and <30% from fat. | Partly (for one-half of the participants, equally in both groups) | No | 6 meals/d: −380;2 meals/d: −420 | Assessed but not reported (dietary records) |
| Koopman et al., 2014 (36) | 6 meals/d:Hypercaloric (40% caloric surplus on top of the ad libitum weight-maintaining diet) | 3 meals/d:Hypercaloric (40% caloric surplus on top of the ad libitum weight-maintaining diet) | Partly (additional meal, liquid meal) | No | 6 meals/d: 3614;2 meals/d: 3474 | Assessed but not reported (diet monitoring) |
| Murphy et al., 1996 (37) | 12 meals/d:Eucaloric, ∼2000 kcal/d, with a daily average 40% fat, 20% protein, and 40% carbohydrate; meals were eaten at hourly intervals from 8 h to 20 h. | 2 meals/d:Eucaloric, ∼2000 kcal/d, with a daily average 40% fat, 20% protein, and 40% carbohydrate; meals were split into 2 meals/d to be eaten at 12 h and 19:30 h. | No | No | 12 meals/d: 2020;2 meals/d: 2082 | 2 meals: 90%;12 meals: 78% |
| Papakonstantinou et al., 2016 (38) | 6 meals/d:Eucaloric, weight-maintenance diet; daily distribution: 40% carbohydrates, 25% protein, and 35% fat | 3 meals/d:Eucaloric, weight-maintenance diet; daily distribution: 40% carbohydrates, 25% protein, and 35% fat | No | No | 6 meals/d: 1916;3 meals/d: 1859 | Assessed but not reported (food records) |
| Papakonstantinou et al., 2018 (39) | 6 meals/d:Eucaloric, weight-maintenance diet; daily distribution: 40% carbohydrates, 25% protein, and 35% fat | 3 meals/d:Eucaloric, weight-maintenance diet; daily distribution: 40% carbohydrates, 25% protein, and 35% fat | No | No | 6 meals/d: 2081;3 meals/d: 1917 | Adherence to Mediterranean diet score: 32.1 from 55 |
| Perrigue et al., 2017 (40) | 8 meals/d:Eucaloric (providing all energy as evenly spaced eating occasions per day) | 3 meals/d:Eucaloric (providing all energy as evenly spaced eating occasions per day) | No | No | NR | 8 meals: 100%;3 meals: 96% |
| Schlundt et al., 1992 (41) | 3 meals/d:Breakfast; hypocaloric 1200 kcal/d.Daily distribution: 55% of energy from carbohydrates, 15–20% from protein, and 25–30% from fats. | 2 meals/d:No breakfast; hypocaloric 1200 kcal/d.Daily distribution: 55% of energy from carbohydrates, 15–20% from protein, and 25–30% from fats. | No | No | NR | Whole study group: 71% |
| Stote et al., 2007 (42) | 3 meals/d:Eucaloric (weight maintenance). Daily macronutrient distribution: 50% carbohydrates, 15% protein, 35% fats. | 1 meal/d:Eucaloric (weight maintenance; minimum fast of 20 h/d). Daily macronutrient distribution: 50% carbohydrates, 15% protein, 35% fats. | Yes | Partly (only dinner in study center) | 3 meals/d: 2429;1 meals/d: 2364 | Assessed but not reported (daily questionnaires) |
| Verboeket-van de Venne and Westerterp, 1993 (43) | 4 meals/d:Hypocaloric, 1000 kcal/d; 300 kcal/d breakfast, 300 kcal/d lunch (with option of 100 kcal/d snack), 400 kcal/d dinner (with option 100 kcal/d snack) | 2 meals/d:Hypocaloric, 1000 kcal/d, no breakfast, 400 kcal lunch, 600 kcal dinner. | No | No | 4 meals/d: 1018;2 meals/d: 1090 | Data not collected |
| Young et al., 1971 (44) | 6 meals/d:Hypocaloric, 1800 kcal/d. Daily macronutrient distribution: 115 g protein, 104 g carbohydrates, 103 g fat. | 3 meals/d:Hypocaloric, 1800 kcal/d. Daily macronutrient distribution: 115 g protein, 104 g carbohydrates, 103 g fat.1 meal/d:Hypocaloric, 1800 kcal/d. Daily macronutrient distribution: 115 g protein, 104 g carbohydrates, 103 g fat. | Yes | No | NR | Data not collected |
NR, not reported.
RoB
The results of the RoB assessment are provided in Supplemental Figure 1. No study was judged to have a low risk of selection bias. No RCT adequately performed blinding of participants and personnel; no RCT was judged as low RoB for blinding of outcome assessment; however, 13 RCTs (59%) were judged as low RoB for incomplete data outcome (24, 25, 27, 29, 31, 32, 34–39, 41). Twenty RCTs (91%) were judged to have a low RoB for selective reporting (23–39, 41–43), and 6 RCTs (26%) showed a low risk of other bias (Supplemental Figure 1) (23, 25, 29–31, 34). Overall, 15 RCTs (68%) were rated as high RoB for ≥1 domain (23–30, 33, 35, 36, 38, 39, 42, 44).
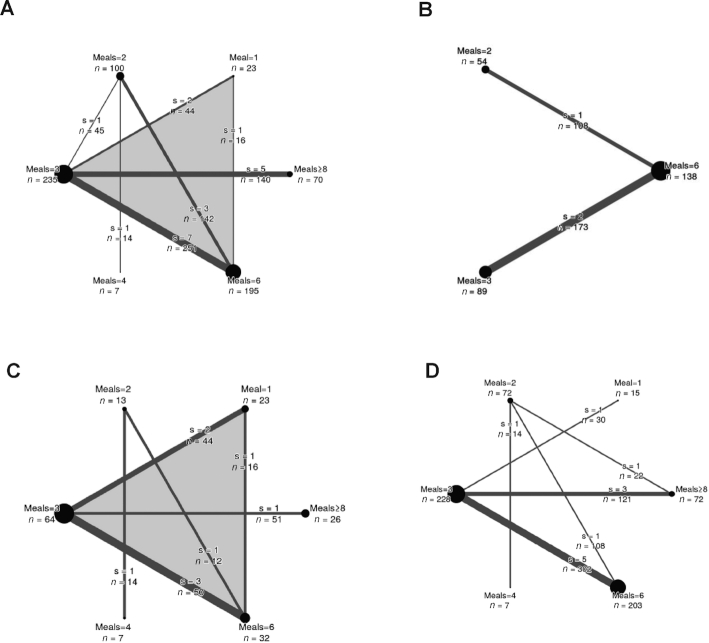
Figure 2 shows the network diagrams for body weight (Figure 2A), waist circumference (Figure 2B), fat mass (Figure 2C), and energy intake (Figure 2D).
FIGURE 2.
Network diagrams for body weight (A), waist circumference (B), fat mass (C), and energy intake (D). The size of the nodes is proportional to the total number of participants allocated to the intervention and the thickness of the lines proportional to the number of studies evaluating each direct comparison. Moreover, the total number of participants for each comparison is displayed (e.g., the body-weight comparison of 3 meals/d and 6 meals/d included 7 studies with a total of 251 participants). The number of multiarm studies for the interventions are indicated by the shade of gray of the background (white for none). s, studies.
Body weight
We are uncertain whether 1 meal/d (MD: −2.25 kg; 95% CI: −5.13, 0.63 kg) or 2 meals/d (MD: −1.32 kg; 95% CI: −2.19, −0.45 kg) reduce body weight compared with ≥8 meals/d (very low certainty of evidence) (Figure 3A). Moreover, our results suggest that 2 meals/d probably slightly reduces body weight compared with 3 meals/d (MD: −1.02 kg; 95% CI: −1.70, −0.35 kg) or 6 meals/d (MD: −1.29 kg; 95% CI: −1.74, −0.84 kg; moderate certainty of evidence) (Supplemental Figure 2). No effects were observed when 3, 4, or 6 meals/d were compared with ≥8 meals/d.
FIGURE 3.
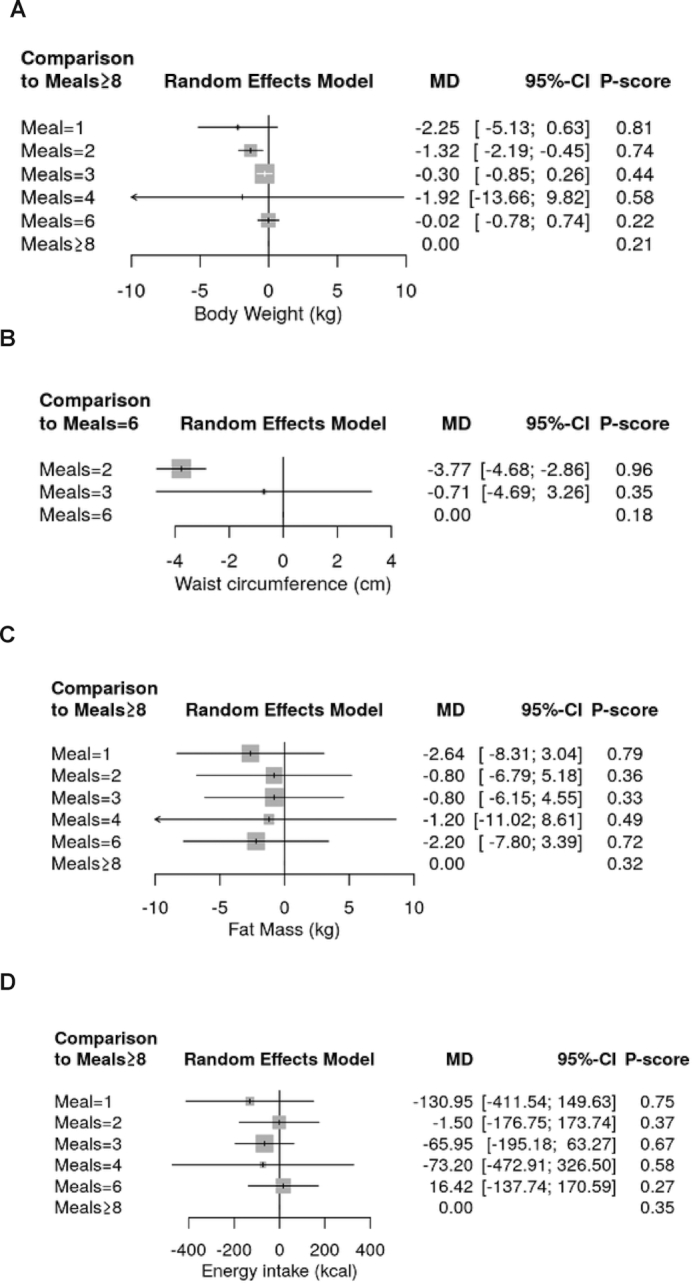
Summary effect estimates of different dietary approaches on body weight (A), waist circumference (B), fat mass (C), and energy intake (D). “≥8” Meals (if available) is defined as the reference treatment. P-scores are defined, such that they are between 0 and 1, where 0 means that a treatment is always worst and 1 means that a treatment is always best compared with the other treatments in the network. For example, 2 meals/d (P-score: 0.96) was ranked best to improve waist circumference, followed by 3 meals/d (0.35), and 6 meals/d (0.18). MD, mean difference.
Waist circumference
Two meals per day probably improve waist circumference compared with 6 meals/d (MD: −3.77 cm; 95% CI: −4.68, −2.86 cm; moderate certainty of evidence) (Figure 3B), whereas no effects were detected comparing 3 vs. 6 meals/d (MD: −0.71 cm; 95% CI: −4.69, 3.26 cm; low certainty of evidence).
Fat mass
Neither 1, 2, 3, 4, nor 6 meals/d were more effective in reducing fat mass compared with ≥8 meals/d (Figure 3C). We are uncertain whether 1 meal/d is more effective than 3 meals/d in reducing fat mass (MD: −1.84 kg; 95% CI: −3.72, 0.05 kg; very low certainty of evidence) (Supplemental Figure 3).
Energy intake
No significant differences were observed comparing the different meal frequencies on energy intake (mainly very low certainty of evidence) (Figure 3D, Supplemental Figure 4).
Table 3 and Supplemental Tables 1–3 show the certainty of evidence assessment for the direct evidence, indirect evidence, and network estimates for all outcomes.
TABLE 3.
GRADE evaluation for body weight (kg) and all comparisons1
| Direct evidence | Indirect evidence | Network meta-analysis | |||||
|---|---|---|---|---|---|---|---|
| Comparison (meals/d) | No. of studies | MD (95% CI) | Certainty of evidence | MD (95% CI) | Certainty of evidence | MD (95% CI) | Certainty of evidence |
| 1 vs. 2 | 0 | — | — | −0.93 (−3.80, 1.94) | ⊕⊕◯◯2 | −0.93 (−3.80, 1.94) | ⊕◯◯◯3 |
| 1 vs. 3 | 2 | −3.35 (−6.60, −0.09) | ⊕⊕⊕◯4 | 2.28 (−3.40, 7.96) | ⊕⊕◯◯ | −1.95 (−4.78, 0.87) | ⊕⊕◯◯ 3, 5, 6 |
| 1 vs. 4 | 0 | — | — | −0.33 (−12.39, 11.73) | ⊕⊕◯◯2 | −0.33 (−12.39, 11.73) | ⊕◯◯◯3(↓↓) |
| 1 vs. 6 | 1 | 0.01 (−3.94, 3.96) | ⊕⊕⊕◯4 | −4.61 (−8.68, −0.53) | ⊕⊕◯◯2 | −2.22 (−5.06, 0.61) | ⊕⊕◯◯3, 5, 6 |
| 1 vs. ≥8 | 0 | — | — | −2.25 (−5.13, 0.63) | ⊕⊕◯◯2 | −2.25 (−5.13, 0.63) | ⊕◯◯◯3 |
| 2 vs. 3 | 1 | −1.13 (−3.36, 1.10) | ⊕⊕⊕⊕ | −1.01 (−1.72, −0.31) | ⊕⊕⊕◯2 | −1.02 (−1.70, −0.35) | ⊕⊕⊕◯ |
| 2 vs. 4 | 1 | 0.60 (−11.11, 12.31) | ⊕⊕⊕⊕ | — | — | 0.60 (−11.11, 12.31) | ⊕⊕◯◯3(↓↓) |
| 2 vs. 6 | 3 | −1.29 (−1.75, −0.83) | ⊕⊕⊕◯4 | −1.41 (−3.70, 0.89) | ⊕⊕⊕◯2 | −1.29 (−1.74, −0.84) | ⊕⊕⊕◯5 |
| 2 vs. ≥8 | 0 | — | — | −1.32 (−2.19, −0.45) | ⊕⊕◯◯2 | −1.32 (−2.19, −0.45) | ⊕◯◯◯3 |
| 3 vs. 4 | 0 | — | — | 1.62 (−10.11, 13.35) | ⊕⊕◯◯2 | 1.62 (−10.11, 13.35) | ⊕◯◯◯3(↓↓) |
| 3 vs. 6 | 7 | −0.28 (−0.81, 0.26) | ⊕⊕⊕◯4 | −0.18 (−2.44, 2.09) | ⊕⊕⊕◯2 | −0.27 (−0.79, 0.25) | ⊕⊕⊕◯5 |
| 3 vs. ≥8 | 5 | −0.30(−0.85, 0.26) | ⊕⊕⊕⊕ | — | — | −0.30(−0.85, 0.26) | ⊕⊕⊕◯7 |
| 4 vs. 6 | 0 | — | — | −1.89 (−13.61, 9.83) | ⊕⊕◯◯2 | −1.89 (−13.61, 9.83) | ⊕◯◯◯3(↓↓) |
| 4 vs. ≥8 | 0 | — | — | −1.92 (−13.66, 9.82) | ⊕⊕◯◯2 | −1.92 (−13.66, 9.82) | ⊕◯◯◯3(↓↓) |
| 6 vs. ≥8 | 0 | — | — | −0.02 (−0.78, 0.74) | ⊕⊕◯◯2 | −0.02 (−0.78, 0.74) | ⊕⊕◯◯ |
Direct estimates were evaluated with the following GRADE criteria: risk of bias, indirectness, inconsistency, and publication bias. As suggested recently by the GRADE working group, consideration of imprecision is not necessary when rating the direct and indirect estimates to inform the rating of NMA estimates. GRADE, Grading of Recommendations Assessment, Development, and Evaluation; MD, mean difference; NMA, network meta-analysis; ⊕⊕⊕⊕, high; ⊕⊕⊕, moderate; ⊕⊕◯◯, low; ⊕◯◯◯, very low; ↓↓, downgraded twice due to very serious imprecision.
2Downgraded due to intransitivity (i.e., patients with obesity and healthy participants included).
Downgraded due to imprecision (95% CI overlaps important benefit: −2 kg; or important harm: +2 kg).
4Downgraded due to risk of bias (≥1 RCT with high risk of bias).
5Direct evidence contributing more to the NMA estimate (>50%).
6Not downgraded due to incoherence (dominant estimate similar to network estimate).
7Downgraded due to imprecision (sample size: <400).
P-score and rankings
One meal per day was ranked as the best treatment for body weight (P-score: 0.81), followed by 2 meals/d (P: 0.74), whereas 2 meals/d performed best for waist circumference (P: 0.96). Rankings for fat mass and energy intake were inconclusive since none of the different meal frequencies investigated were “superior” when compared with each other (Figure 3).
Inconsistency
The net heat plots are shown in 9. No relevant inconsistency between designs was observed for body weight and fat mass. Due to the low number of comparisons available for the outcome waist circumference, it was not possible to investigate inconsistency.
Secondary and sensitivity analysis
In sensitivity analyses including only patients with obesity, both 1 and 2 meals/d were slightly more effective than 6 meals/d (effect ranged between −1.31 and −2.31 kg) in reducing body weight. Considering only overweight participants, no further effects of different meal frequencies were observed (Supplemental Figures 8–9).
Sensitivity analyses for provision of food (yes/no), meals consumed in research center (yes/no), energy intake (hypo- vs. eucaloric), and breakfast skipping (yes/no) indicated no influence on the primary analysis (Supplemental Figures 10–31).
Due to the low number of studies it was not possible to conduct the additional a priori planned sensitivity analyses for gender differences (e.g., 3 RCTs including men only), study length (e.g., only 2 RCTs with an intervention duration ≥6 mo), and by excluding high RoB RCTs, since ∼70% of the included studies were rated as high RoB.
Dietary adherence
Dietary adherence was only reported in 6 RCTs (22%) (26, 27, 37, 39–41), however assessed but not reported in 11 of the included RCTs through regular interviews and questionnaires (see Table 2). Especially where food was provided or even served in research facilities, adherence was considered as good. Moreover, as shown above, no significant differences were observed comparing the different meal frequencies on energy intake, which indicated substantial adherence.
Dissemination biasm
For none of the outcomes, the funnel plots appeared obviously asymmetric, and the Egger's test showed no evidence for small study effects (Supplemental Figures 32–35).
Discussion
This is the first NMA to evaluate the isocaloric effects of different meal frequencies on anthropometric outcomes (body weight, waist circumference, and fat mass) and energy intake in 647 participants.
In summary, a lower eating frequency (2 meals/d) seems to reduce body weight and waist circumference as compared with 6 meals/d in the short term. We are uncertain whether 1 or 2 meals/d reduce body weight compared with ≥8 meals/d, and whether 1 meal/d may reduce fat mass compared with 3 meals/d as the certainty of the evidence has been assessed as very low. No significant effects were observed for energy intake. Generally, for most of the comparisons, the present NMA yielded no important effects and the certainty of evidence was mainly rated as low or very low.
Comparison with other systematic reviews and possible mechanisms
Contrary to our findings, in a recent pairwise meta-analysis of 15 RCTs, ≥5 meals/d resulted in greater loss of fat mass (by −1.25 kg) than did 1–2 meals/d (5). However, these findings were highly driven by 1 RCT, and in a sensitivity analysis the observed effect became nonsignificant. For the outcome body weight, no significant effects were observed comparing 1–2 meals/d vs. 3–4 meals/d or vs. ≥5 meals/d. The authors concluded from their findings a potential benefit of increased meal frequencies for enhancing body composition (5). In addition, observational studies reported an inverse association between the frequency of eating and BMI; however, underreporting of energy intake and eating frequency could be an important bias for this observation (45, 46). In contrast, our findings are more in line with a recent pairwise meta-analysis of RCTs, which examined weight change and energy intake in adults consuming or skipping breakfast and therefore reducing meal frequency (7). The meta-analysis found no evidence to support the notion that breakfast consumption (i.e., a higher meal frequency) promotes weight loss or that a lower meal frequency leads to weight gain (7). Moreover, the lower meal frequency group had a higher daily energy intake (∼250 kcal/d) compared with the higher meal frequency group (7). Specifically in this meta-analysis, consumption of breakfast was associated with a clinically nonrelevant body weight increase of 0.5 kg (time frame up to 16 wk). Of note, all included RCTs in this systematic review were considered as high RoB. Discordance between studies could be driven by mixing isocaloric and nonisocaloric comparisons in the recent meta-analysis of RCTs (i.e., breakfast consumption leads to higher energy intake and therefore weight gain) (7). More than 20 y ago, Bellisle et al. (45) claimed that the reason for weight loss is not to be found in the frequency of meals but in general hypocaloric energy intake. To avoid this bias, we included only isocaloric comparisons in our NMA.
However, dietary adherence was not reported in all RCTs, but no differences were observed for energy intake comparing the different meal frequencies. Thus, if energy intake was similar between the comparison groups, the question arises if other mechanisms could explain these observations. There is no consensus yet, and we can only speculate about the mechanisms. The meal frequency could have different effects on metabolic rates, including energy expenditure and carbohydrate and fat oxidation (35, 42). In addition, in combination with timing of the meal (e.g., skipping of late-night meals), the frequency could have an influence on satiety hormones (ghrelin and leptin), circadian rhythms, and a beneficial effect on oxidative damage (47).
Relevance of our findings
Research has shown that, in adults with overweight and obesity, a reduction in body weight of 5–10% of initial body weight was associated with improvements in health risk factors (48). In our NMA, weight loss was not of sufficient magnitude to likely be associated with clinical benefits following lower meal frequencies compared with higher meal frequencies (−0.30 to −2.31 kg) in patients with obesity. However 2 meals/d reduced waist circumference by ∼4 cm compared with 6 meals/d. This effect is considered clinically relevant, since evidence from observational studies has shown, that a 1-cm increase in waist circumference is associated with a 2% increased risk of cardiovascular disease (49). Again, the role of the length of fasting periods has to be taken into consideration as longer fasting periods are inversely associated with fat-free mass (50).
Implications
In a recent scoping review, nearly 50 countries and 7 organization were identified that referred to snacking between main meals (i.e., higher meal frequencies) (1). Most of them established quantitative recommendations most often related to frequency of meals (i.e., snacking throughout the day), often with a special focus on children. Of all of the recommendations, only 2 suggested avoidance of small meals between main meals. The findings of our NMA have important implications with respect to the prevailing recommendation that eating small, frequent meals is helpful for optimizing weight management in the general (adult) population. Increasing meal frequency is often promoted as a beneficial strategy for anthropometric outcomes (2). The results of our NMA do not support these recommendations, but rather point to a slight contrary effect. Our findings are more in line with a recent statement by the American Heart Association, where the authors concluded that altering meal frequency under eucaloric conditions may not be effective for decreasing body weight (51).
Strength and limitations
Our systematic review and NMA has several strengths and limitations that need to be considered. Among the strengths are the application of the NMA methodology, the a priori–deposited protocol, the comprehensive search strategy, RoB assessment, sensitivity analyses, and the GRADE certainty of evidence judgment.
Limitations of the current NMA include the absence of long-term RCTs (>52 wk); therefore, we were not able to provide data on the persistence of observed effects. Overall, only 2 out of 22 RCTs (9%) had a study duration >12 wk. Moreover, the certainty of evidence was rated as mainly low or very low. This was mainly driven by RoB and imprecision: overall, 15 RCTs (68%) were rated with a high RoB for ≥1 domain. Imprecision was driven by the low sample sizes of the various study arms; hence, the studies and also the meta-analyses may have lacked power to detect differences within or between groups. Due to the low number of RCTs, it was not possible to conduct several a priori–planned sensitivity analyses. Adherence was assessed and reported in only 6 RCTs. Moreover, only very few studies implemented a controlled-feeding protocol.
Conclusions
In conclusion, our findings indicate that there is little robust evidence that reduced meal frequency is a beneficial strategy for anthropometric outcomes. In addition, no significant effects were observed for energy intake. The currently available evidence is of low certainty and does not support current recommendations for increased meal frequencies. The findings should be, however, interpreted with caution. Further high-quality RCTs are needed to substantiate whether individuals seeking to lose weight should skip or consume meals.
Supplementary Material
ACKNOWLEDGEMENTS
All authors substantially contributed to the concept, data collection and analysis, or preparation of the manuscript, and read and approved the final manuscript.
Notes
The authors reported no funding received for this work. SL participated in this project during her research stay at the Institute for Evidence in Medicine, University of Freiburg, supported by the Alexander von Humboldt Foundation, Germany.
Author disclosures: LS is a member of the Editorial Board of Advances in Nutrition. The other authors report no conflicts of interest.
Supplemental Appendixes 1 and 2, Supplemental Figures 1–35, and Supplemental Tables 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances.
Abbreviations used: BW, body weight; EI, energy intake; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; MD, mean difference; NMA, network meta-analysis; RCT, randomized controlled trial; RoB, risk of bias; T2D, type 2 diabetes; WC, waist circumference.
References
- 1. Potter M, Vlassopoulos A, Lehmann U. Snacking recommendations worldwide: a scoping review. Adv Nutr. 2018;9(2):86–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Louis-Sylvestre J, Lluch A, Neant F, Blundell JE. Highlighting the positive impact of increasing feeding frequency on metabolism and weight management. Forum Nutr. 2003;56:126–8. [PubMed] [Google Scholar]
- 3. Ma Y, Bertone ER, Stanek EJ 3rd, Reed GW, Hebert JR, Cohen NL, Merriam PA, Ockene IS. Association between eating patterns and obesity in a free-living US adult population. Am J Epidemiol. 2003;158(1):85–92. [DOI] [PubMed] [Google Scholar]
- 4. Holmback I, Ericson U, Gullberg B, Wirfalt E. A high eating frequency is associated with an overall healthy lifestyle in middle-aged men and women and reduced likelihood of general and central obesity in men. Br J Nutr. 2010;104(7):1065–73. [DOI] [PubMed] [Google Scholar]
- 5. Schoenfeld BJ, Aragon AA, Krieger JW. Effects of meal frequency on weight loss and body composition: a meta-analysis. Nutr Rev. 2015;73(2):69–82. [DOI] [PubMed] [Google Scholar]
- 6. van der Heijden AA, Hu FB, Rimm EB, van Dam RM. A prospective study of breakfast consumption and weight gain among U.S. men. Obesity. 2007;15(10):2463–9. [DOI] [PubMed] [Google Scholar]
- 7. Sievert K, Hussain SM, Page MJ, Wang Y, Hughes HJ, Malek M, Cicuttini FM. Effect of breakfast on weight and energy intake: systematic review and meta-analysis of randomised controlled trials. BMJ (Clin Res Ed). 2019;364:l42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hess JM, Jonnalagadda SS, Slavin JL. What is a snack, why do we snack, and how can we choose better snacks? a review of the definitions of snacking, motivations to snack, contributions to dietary intake, and recommendations for improvement. Adv Nutr. 2016;7(3):466–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, Ioannidis JP, Straus S, Thorlund K, Jansen JP et al.. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–84. [DOI] [PubMed] [Google Scholar]
- 10. Chaimani A, Caldwell DM, Li T, Higgins JPT, Salanti G. Additional considerations are required when preparing a protocol for a systematic review with multiple interventions. J Clin Epidemiol. 2017;83:65–74. [DOI] [PubMed] [Google Scholar]
- 11. Schwingshackl L, Neuenschwander M, Hoffmann G, Buyken AE, Schlesinger S. Dietary sugars and cardiometabolic risk factors: a network meta-analysis on isocaloric substitution interventions. Am J Clin Nutr. 2019;111(1):187–96. [DOI] [PubMed] [Google Scholar]
- 12. Schwingshackl L, Krause M, Schmucker C, Hoffmann G, Rucker G, Meerpohl JJ. Impact of different types of olive oil on cardiovascular risk factors: a systematic review and network meta-analysis. Nutr Metab Cardiovasc Dis. 2019;29(10):1030–9. [DOI] [PubMed] [Google Scholar]
- 13. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ (Clin Res Ed). 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLoS One. 2013;8(10):e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rücker G, Krahn U, König J, Efthimiou O, Schwarzer G. netmeta: network meta-analysis using frequentist methods. R package version 1.1-0. 2019; [Internet]. [Accessed 2019 Nov 22]. Available from: https://CRAN.R-project.org/package = netmeta. [Google Scholar]
- 16. Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64(2):163–71. [DOI] [PubMed] [Google Scholar]
- 17. Rücker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Methodol. 2015;15:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schwingshackl L, Schwarzer G, Rucker G, Meerpohl JJ. Perspective: network meta-analysis reaches nutrition research: current status, scientific concepts, and future directions. Adv Nutr. 2019;10(5):739–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Krahn U, Binder H, Konig J. A graphical tool for locating inconsistency in network meta-analyses. BMC Med Res Methodol. 2013;13:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chaimani A, Salanti G. Using network meta-analysis to evaluate the existence of small-study effects in a network of interventions. Res Syn Meth. 2012;3(2):161–76. [DOI] [PubMed] [Google Scholar]
- 21. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clin Res Ed). 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brignardello-Petersen R, Bonner A, Alexander PE, Siemieniuk RA, Furukawa TA, Rochwerg B, Hazlewood GS, Alhazzani W, Mustafa RA, Murad MH et al.. Advances in the GRADE approach to rate the certainty in estimates from a network meta-analysis. J Clin Epidemiol. 2018;93:36–44. [DOI] [PubMed] [Google Scholar]
- 23. Alencar MK, Beam JR, McCormick JJ, White AC, Salgado RM, Kravitz LR, Mermier CM, Gibson AL, Conn CA, Kolkmeyer D et al.. Increased meal frequency attenuates fat-free mass losses and some markers of health status with a portion-controlled weight loss diet. Nutr Res. 2015;35(5):375–83. [DOI] [PubMed] [Google Scholar]
- 24. Antoine JM, Rohr R, Gagey MJ, Bleyer RE, Debry G. Feeding frequency and nitrogen balance in weight-reducing obese women. Hum Nutr Clin Nutr. 1984;38(1):31–8. [PubMed] [Google Scholar]
- 25. Arciero PJ, Ormsbee MJ, Gentile CL, Nindl BC, Brestoff JR, Ruby M. Increased protein intake and meal frequency reduces abdominal fat during energy balance and energy deficit. Obesity (Silver Spring). 2013;21(7):1357–66. [DOI] [PubMed] [Google Scholar]
- 26. Arnold LM, Ball MJ, Duncan AW, Mann J. Effect of isoenergetic intake of three or nine meals on plasma lipoproteins and glucose metabolism. Am J Clin Nutr. 1993;57(3):446–51. [DOI] [PubMed] [Google Scholar]
- 27. Arnold L, Ball M, Mann J. Metabolic effects of alterations in meal frequency in hypercholesterolaemic individuals. Atherosclerosis. 1994;108(2):167–74. [DOI] [PubMed] [Google Scholar]
- 28. Arnold L, Mann JI, Ball MJ. Metabolic effects of alterations in meal frequency in type 2 diabetes. Diabetes Care. 1997;20(11):1651–4. [DOI] [PubMed] [Google Scholar]
- 29. Bachman JL, Raynor HA. Effects of manipulating eating frequency during a behavioral weight loss intervention: a pilot randomized controlled trial. Obesity (Silver Spring). 2012;20(5):985–92. [DOI] [PubMed] [Google Scholar]
- 30. Berteus Forslund H, Klingstrom S, Hagberg H, Londahl M, Torgerson JS, Lindroos AK. Should snacks be recommended in obesity treatment? A 1-year randomized clinical trial. Eur J Clin Nutr. 2008;62(11):1308–17. [DOI] [PubMed] [Google Scholar]
- 31. Cameron JD, Cyr MJ, Doucet E. Increased meal frequency does not promote greater weight loss in subjects who were prescribed an 8-week equi-energetic energy-restricted diet. Br J Nutr. 2010;103(8):1098–101. [DOI] [PubMed] [Google Scholar]
- 32. Finkelstein B, Fryer BA. Meal frequency and weight reduction of young women. Am J Clin Nutr. 1971;24(4):465–8. [DOI] [PubMed] [Google Scholar]
- 33. Iwao S, Mori K, Sato Y. Effects of meal frequency on body composition during weight control in boxers. Scand J Med Sci Sports. 1996;6(5):265–72. [DOI] [PubMed] [Google Scholar]
- 34. Jenkins DJ, Khan A, Jenkins AL, Illingworth R, Pappu AS, Wolever TM, Vuksan V, Buckley G, Rao AV, Cunnane SC et al.. Effect of nibbling versus gorging on cardiovascular risk factors: serum uric acid and blood lipids. Metabolism. 1995;44(4):549–55. [DOI] [PubMed] [Google Scholar]
- 35. Kahleova H, Belinova L, Malinska H, Oliyarnyk O, Trnovska J, Skop V, Kazdova L, Dezortova M, Hajek M, Tura A et al.. Eating two larger meals a day (breakfast and lunch) is more effective than six smaller meals in a reduced-energy regimen for patients with type 2 diabetes: a randomised crossover study. Diabetologia. 2014;57(8):1552–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Koopman KE, Caan MW, Nederveen AJ, Pels A, Ackermans MT, Fliers E, la Fleur SE, Serlie MJ. Hypercaloric diets with increased meal frequency, but not meal size, increase intrahepatic triglycerides: a randomized controlled trial. Hepatology. 2014;60(2):545–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Murphy MC, Chapman C, Lovegrove JA, Isherwood SG, Morgan LM, Wright JW, Williams CM. Meal frequency; does it determine postprandial lipaemia? Eur J Clin Nutr. 1996;50(8):491–7. [PubMed] [Google Scholar]
- 38. Papakonstantinou E, Kechribari I, Mitrou P, Trakakis E, Vassiliadi D, Georgousopoulou E, Zampelas A, Kontogianni MD, Dimitriadis G. Effect of meal frequency on glucose and insulin levels in women with polycystic ovary syndrome: a randomised trial. Eur J Clin Nutr. 2016;70(5):588–94. [DOI] [PubMed] [Google Scholar]
- 39. Papakonstantinou E, Kontogianni MD, Mitrou P, Magriplis E, Vassiliadi D, Nomikos T, Lambadiari V, Georgousopoulou E, Dimitriadis G. Effects of 6 vs. 3 eucaloric meal patterns on glycaemic control and satiety in people with impaired glucose tolerance or overt type 2 diabetes: a randomized trial. Diabetes Metab. 2018;44(3):226–34. [DOI] [PubMed] [Google Scholar]
- 40. Perrigue MM, Drewnowski A, Wang CY, Song X, Kratz M, Neuhouser ML. Randomized trial testing the effects of eating frequency on two hormonal biomarkers of metabolism and energy balance. Nutr Cancer. 2017;69(1):56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schlundt DG, Hill JO, Sbrocco T, Pope-Cordle J, Sharp T. The role of breakfast in the treatment of obesity: a randomized clinical trial. Am J Clin Nutr. 1992;55(3):645–51. [DOI] [PubMed] [Google Scholar]
- 42. Stote KS, Baer DJ, Spears K, Paul DR, Harris GK, Rumpler WV, Strycula P, Najjar SS, Ferrucci L, Ingram DK et al.. A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults. Am J Clin Nutr. 2007;85(4):981–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Verboeket-van de Venne WP, Westerterp KR. Frequency of feeding, weight reduction and energy metabolism. Int J Obes Relat Metab Disord. 1993;17(1):31–6. [PubMed] [Google Scholar]
- 44. Young CM, Scanlan SS, Topping CM, Simko V, Lutwak L. Frequency of feeding, weight reduction, and body composition. J Am Diet Assoc. 1971;59(5):466–72. [PubMed] [Google Scholar]
- 45. Bellisle F, McDevitt R, Prentice AM. Meal frequency and energy balance. Br J Nutr. 1997;77(Suppl 1):S57–70. [DOI] [PubMed] [Google Scholar]
- 46. Canuto R, da Silva Garcez A, Kac G, de Lira PIC, Olinto MTA. Eating frequency and weight and body composition: a systematic review of observational studies. Public Health Nutr. 2017;20(12):2079–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Paoli A, Tinsley G, Bianco A, Moro T. The influence of meal frequency and timing on health in humans: the role of fasting. Nutrients. 2019;11(4):719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yumuk V, Tsigos C, Fried M, Schindler K, Busetto L, Micic D, Toplak H. European guidelines for obesity management in adults. Obes Facts. 2015;8(6):402–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. de Koning L, Merchant AT, Pogue J, Anand SS. Waist circumference and waist-to-hip ratio as predictors of cardiovascular events: meta-regression analysis of prospective studies. Eur Heart J. 2007;28(7):850–6. [DOI] [PubMed] [Google Scholar]
- 50. Pellegrini M,Cioffi I,Evangelista A, PonzoV,Goitre I,Ciccone G,Ghigo E,Bo S. Effects of time-restricted feeding on body weight and metabolism: a systematic review and meta-analysis. Rev Endocr Metab Disord. 2020;21(1):17–33. [DOI] [PubMed] [Google Scholar]
- 51. St-Onge MP, Ard J, Baskin ML, Chiuve SE, Johnson HM, Kris-Etherton P, Varady K. Meal timing and frequency: implications for cardiovascular disease prevention: a scientific statement from the American Heart Association. Circulation. 2017;135(9):e96–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.