ABSTRACT
The Dietary Approaches to Stop Hypertension (DASH) diet is recognized as an effective dietary intervention to reduce blood pressure (BP). However, among randomized controlled trials (RCTs) investigating the DASH diet–mediated BP reduction, there are significant methodological and clinical differences. The purpose of this study was to comprehensively assess the DASH diet effect on BP in adults with and without hypertension, accounting for underlying methodological and clinical confounders. We systematically searched Medline and the Cochrane Collaboration Library databases and identified 30 RCTs (n = 5545 participants) that investigated the BP effects of the DASH diet compared with a control diet in hypertensive and nonhypertensive adults. Both random-effects and fixed-effect models were used to calculate the mean attained systolic BP (SBP) and diastolic BP (DBP) differences during follow-up. Subgroup and meta-regression analyses were also conducted. Compared with a control diet, the DASH diet reduced both SBP and DBP (difference in means: −3.2 mm Hg; 95% CI: −4.2, −2.3 mm Hg; P < 0.001, and −2.5 mm Hg; 95% CI: −3.5, −1.5 mm Hg; P < 0.001, respectively). Hypertension status did not modify the effect on BP reduction. The DASH diet compared with a control diet reduced SBP levels to a higher extent in trials with sodium intake >2400 mg/d than in trials with sodium intake ≤2400 mg/d, whereas both SBP and DBP were reduced more in trials with mean age <50 y than in trials of older participants. The quality of evidence was rated as moderate for both outcomes according to the Grading of Recommendations, Assessment, Development, and Evaluation approach. The adoption of the DASH diet was accompanied by significant BP reduction in adults with and without hypertension, although higher daily sodium intake and younger age enhanced the BP-lowering effect of the intervention.
This meta-analysis was registered at www.crd.york.ac.uk/prospero as CRD42019128120.
Keywords: Dietary Approaches to Stop Hypertension, DASH, diet, blood pressure, hypertension, systematic review, meta-analysis, randomized controlled trials
Introduction
Hypertension remains one of the most significant causes of premature morbidity and mortality worldwide (1), because elevated blood pressure (BP) influences adversely cardiovascular and renal outcomes (2, 3). Raised BP levels result from complex interactions between genetic and environmental factors (4). Consistent evidence suggests that individual nutrients, such as sodium and potassium, but also different dietary patterns such as the Dietary Approaches to Stop Hypertension (DASH) diet, are directly associated with BP reduction (5). For the reduction of hypertension burden and BP control within the goal, the current hypertension management guidelines recommend as an integral part of ongoing treatment the adoption of lifestyle modifications, including a healthy diet, independently of the underlying antihypertensive drug treatment (6, 7).
The BP-lowering effect of the DASH diet was first noted >20 y ago, when the first DASH clinical trial, which was a controlled feeding trial, tested the effects of 3 different diets on BP levels. The “combination” diet, which was rich in fruits, vegetables, and low-fat dairy products, currently named the “DASH” diet, reduced systolic BP (SBP) and diastolic BP (DBP) compared with both the control and fruits-and-vegetables diets (8). Since then, different clinical trials have suggested that the DASH diet alone or in combination with other lifestyle changes, such as sodium restriction, weight loss, or physical exercise, is effective for BP reduction across a wide range of BP levels (9).
The existing clinical trials examining the effect of the DASH diet on BP levels vary in terms of study design, implemented dietary protocol, and clinical characteristics of the recruited patients. Consequently, methodological and clinical differences, such as sodium and/or energy restriction or hypertensive compared with normotensive baseline BP levels, can differently modulate the achieved BP reduction. Previous pairwise meta-analyses of randomized controlled trials (RCTs) investigating the effect of the DASH diet on BP demonstrated that the DASH diet significantly reduced both SBP and DBP (10–13). However, all these meta-analyses presented the absolute mean BP difference from baseline levels, a measure that is related to outcome-related bias, because baseline BP levels may vary across studies and the randomized arms of each study demonstrate different baseline BP levels.
Thus, in the present systematic review and meta-analysis we aimed to estimate the effect of the DASH diet compared with a control diet on the attained BP reduction (i.e., independent of the baseline BP) by considering all available RCTs in hypertensive and nonhypertensive adults and accounting for underlying methodological and clinical confounders not previously considered.
Methods
Protocol and registration
We undertook a systematic review and meta-analysis (CRD42019128120), assessing the effect of the DASH diet on BP levels in hypertensive and nonhypertensive adults, according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (14).
Eligibility criteria
RCTs examining the effect of the DASH diet compared with a control diet on BP levels in hypertensive or nonhypertensive adults, irrespective of accompanying comorbidities (e.g., metabolic syndrome, type 2 diabetes mellitus, obesity), were eligible for inclusion, whether they considered BP changes either as a primary or as a secondary outcome. Studies examining the DASH diet in combination with other lifestyle changes, such as sodium restriction, weight loss, or physical exercise, were also included, whether or not the control group underwent equal lifestyle changes. Eligible studies were evaluated for potentially overlapping populations. In the case of multiple publications from the same population, the study with the largest sample size was retained.
Nonrandomized trials, studies with no control group, studies not reporting outcomes about BP, studies with internet-based interventions, and studies conducted either in children/adolescents or in pregnant women were excluded. We also excluded head-to-head RCTs between the DASH diet and other active comparator “DASH-like” diets, such as the modified DASH diet with altered macronutrient content.
Information sources and search
In the first week of March 2019, 2 independent reviewers (CDF and CGT) performed a systematic literature search in Medline and the Cochrane Collaboration Library databases for publications up until 28 February, 2019, to select eligible RCTs.
The search strategy was organized around the PICO approach (Problem: hypertension; Intervention: DASH diet–mediated BP reduction; Comparison: Control diet; Outcomes: attained SBP and DBP reduction). Appropriate keywords were combined based on the following search algorithm: (DASH diet OR dietary approaches to stop hypertension) AND (hypertension OR blood pressure OR high blood pressure OR office blood pressure OR ambulatory blood pressure OR cardiovascular disease). The filter “clinical trial” was activated. No language or time restriction was applied. In addition, references of the included studies and previous relevant meta-analyses in the field were searched to identify any missing articles.
Study selection
Titles and/or abstracts of studies retrieved through the database searching and those from additional sources were screened by 2 independent reviewers (CDF and CGT) to identify studies potentially meeting the inclusion criteria. The full texts of these studies were retrieved and independently assessed for eligibility by the same 2 reviewers. Any disagreement was resolved through discussion.
Data collection process
A standardized, prepiloted form was used to extract data from the included studies. Two authors (CDF and CGT) extracted data independently; discrepancies were identified and resolved through discussion.
Data items
For each trial the following information was extracted: publication details (first author's name, journal, year of publication); study characteristics (country of conduct, type of study—i.e., single or multicenter, mean age of participants, male sex prevalence, baseline antihypertensive medication, hypertension prevalence, mean BMI, type of BP measurement—i.e., office or home or ambulatory, number of participants); study design (parallel or crossover); type of implemented data analysis (intention-to-treat or per-protocol); study quality; dropout rate; dietary adherence rate; follow-up period; baseline mean SBP and DBP; attained mean SBP and DBP during follow-up; attained difference in means of SBP and DBP between the 2 randomized arms during follow-up; attained difference in weight change during follow-up; and change in 24-h urinary excretion of sodium during follow-up. We also considered the overall context in which the dietary intervention was administered in terms of controlled or noncontrolled feeding, as well as sodium restriction and/or energy restriction along with the implemented diet. Although the main outcome of the study was the difference in the means of office SBP and DBP during follow-up, the difference in the means of daytime or home SBP and DBP was also used for studies exclusively reporting ambulatory or home BP measurements, respectively.
Risk of bias in individual studies and quality of evidence rating
Two independent reviewers (CDF and CGT) assessed the risk of bias of the included studies using the Cochrane Collaboration risk of bias tool for RCTs (15) to reflect the overall quality. The evaluation of each of the 7 criteria was categorically reported as high, low, or unclear risk of bias. Studies with >2 high or unclear risk of bias elements were considered of lower quality. The same 2 reviewers rated the quality of evidence for each outcome separately, using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach (16). Disagreements between the 2 review authors over the risk of bias in studies and the quality assessment of the evidence were resolved by discussion, with the involvement of a third author (CPT) whenever necessary.
Summary measures
Because the BP-lowering effects of any pharmacological or nonpharmacological antihypertensive intervention are related to the mean attained BP difference between the randomized arms during follow-up (i.e., mean postintervention BP difference) and not to the extent of the absolute mean BP difference from baseline levels (17), the main outcome explored in the present meta-analysis was the attained mean SBP and DBP difference (i.e., the ongoing BP reduction) between randomized arms.
Statistical analysis
Data analysis was done using Comprehensive Meta-Analysis version 3 (Biostat). Outcome variables were pooled as mean differences with 95% CIs, using tabulated data from the original publications. The SD of the BP difference between the 2 independent randomized arms during follow-up was computed according to the formula: SDdiff = square root (SD12/2 + SD22/2), where SDdiff is the SD of the BP difference and SD1 and SD2 are the individual SDs of the 2 randomized arms (18). The proportion of inconsistency across studies not explained by chance was assessed by the I2 index. Whenever no significant heterogeneity was detected by the χ2 Cochran Q statistic (P > 0.1), a fixed-effect model was implemented; otherwise, a random-effects model was used. The influence of individual RCTs on the pooled effect sizes was tested by excluding 1 trial at a time: if the point estimate of the combined effect size with a given trial excluded lay outside the 95% CI of the overall BP estimate with all available trials, the trial in question was considered to have excessive influence. Quality-guided sensitivity analysis including only studies of higher quality was also performed. Additional sensitivity analyses were performed and limited to, first, studies reporting intention-to-treat analysis to control for investigator bias or protocol violation; second, studies with office BP measurements to control for ecological bias resulting from out-of-clinic BP evaluation; and third, studies with a parallel design to control for bias of the carryover effects related to crossover design. Different subgroup analyses were conducted according to specific clinical characteristics of the participants and study design variables (hypertension status, underlying antihypertensive drug treatment, baseline SBP and DBP, age, BMI, type of feeding, daily sodium intake, energy restriction along with the implemented diet, duration of the follow-up period, and type of device used for office BP measurements), whereas separate univariate meta-regression analyses of the mean difference against SE (random-effects plotting) were performed for relevant clinical variables (age, BMI, baseline SBP and DBP, duration of the follow-up period, weight change, and change in 24-h urinary sodium during follow-up) to explain heterogeneity across studies. Owing to the relatively small number of the included studies, a multivariate meta-regression analysis with a limited and selective number of covariates (i.e., baseline SBP and DBP, age, male sex, BMI, duration of the follow-up period) was conducted to determine whether the modulating effect of clinical variables remained significant. The presence of publication bias was graphically investigated by funnel plots (random-effects model) and by using Egger's and Begg's tests. A P value < 0.05 (2-tailed) was considered statistically significant.
Results
Study selection and study characteristics
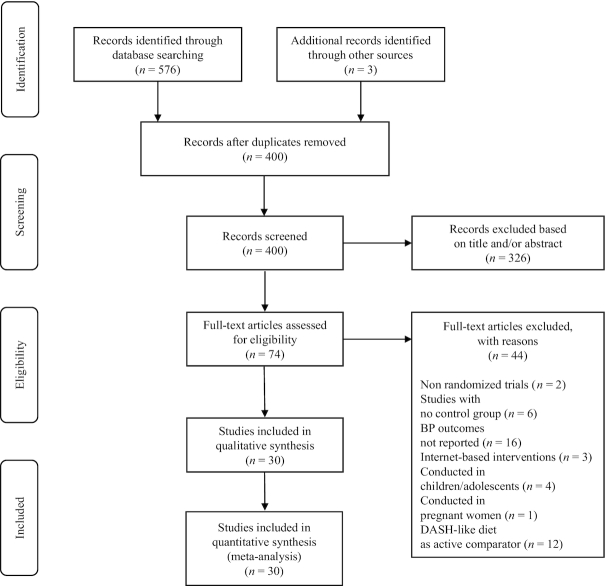
Figure 1 indicates the flow diagram of the study selection process. A total of 576 records were identified through the database searching, whereas 3 additional records were identified through other sources. After duplicates were removed (n = 179), 400 records were screened. Among them, 326 records were excluded based on the title and/or abstract evaluation, whereas 74 articles were assessed for eligibility at the full-text level. Supplemental Table 1 lists the excluded articles (n = 44) along with the reasons for exclusion and their references. Therefore, 30 studies (n = 5545 participants, 45% men, mean age 51 y, mean BMI 29.2 kg/m2, mean baseline SBP/DBP 134.3/84.9 mm Hg, mean follow-up period 15.3 wk) were selected. Table 1 summarizes the baseline and follow-up characteristics of the included studies (19–48).
FIGURE 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram of the study identification and selection process for eligible RCTs. DASH, Dietary Approaches to Stop Hypertension; RCT, randomized controlled trial.
TABLE 1.
Baseline and follow-up characteristics of the included studies1
| Study | Design | Analysis | Subjects, n | HTN prevalence, % | Anti-HTN treatment, % | Mean age, y | Male sex, % | Mean BMI, kg/m2 | CF | ER | FU, wk | Office BP methodology | Sodium intake, mg/d | Dietary adherence, % | Mean baseline SBP/DBP, mm Hg | Mean ± SD attained SBP difference, mm Hg | Mean ± SD attained DBP difference, mm Hg |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al-Solaiman et al. (19) | CO | PP | 60 | 50.02 | No | 38.5 | 20.0 | 28.7 | No | No | 3 | M | 3000 | NR | 123.4/79.3 | −4.8 ± 6.0 | −3.3 ± 5.5 |
| Appel et al. (20) | P | ITT | 305 | 33.02 | No | 44.0 | 50.8 | 28.3 | Yes | No | 8 | M | 3000 | 93.2 | 131.6/85.2 | −5.5 ± 16.8 | −3.0 ± 11.5 |
| Appel et al. (21) | P | ITT | 537 | 37.22 | No | 50.0 | 38.0 | 33.2 | No | Yes | 24 | M | 2400 | NR | 135.2/84.8 | −0.6 ± 19.0 | −0.9 ± 13.0 |
| Azadbakht et al. (22) | P | ITT | 76 | NR | No | 38.0 | 29.0 | 29.8 | No | Yes | 24 | M | 2400 | NR | 143.5/86.0 | −3.5 ± 10.0 | −5.0 ± 12.0 |
| Azadbakht et al. (23) | CO | PP | 62 | NR | No | NR | 42.0 | NR | No | No | 8 | NR | 2400 | NR | 136.0/81.9 | −13.4 ± 17.8 | −9.0 ± 15.0 |
| Azadbakht et al. (24) | P | ITT | 60 | 0.0 | No | 31.0 | 46.6 | 26.9 | No | Yes | 10 | NR | 2400 | NR | 123.7/78.7 | −4.6 ± 19.7 | −2.8 ± 10.4 |
| Blumenthal et al. (25) | P | ITT | 95 | NR | No | 51.8 | 34.0 | 32.9 | No | No | 16 | M | 2400 | NR | 137.8/85.8 | −8.0 ± 20.5 | −3.7 ± 13.7 |
| Burke et al. (26) | P | PP | 204 | 100 | Yes | 56.2 | 44.0 | 30.1 | No | Yes | 16 | NR | NR | NR | 131.5/81.0 | −1.0 ± 13.0 | −2.0 ± 13.0 |
| Chiu et al. (27) | CO | PP | 72 | NR | No | 47.5 | 58.3 | 27.5 | Yes | No | 3 | A | 2700 | NR | 134.5/84.5 | −3.4 ± 8.0 | −2.9 ± 4.2 |
| Conlin et al. (28) | P | ITT | 55 | 100 | No | 52.0 | 45.0 | 31.0 | Yes | No | 4 | M | 3000 | NR | 149.4/94.1 | −5.0 ± 21.6 | −4.0 ± 11.1 |
| Edwards et al. (29) | P | ITT | 37 | NR | No | 47.3 | 50.0 | 30.6 | No | Yes | 12 | NR | NR | NR | 140.3/87.5 | −6.1 ± 11.3 | −7.6 ± 9.8 |
| Green et al. (30) | P | ITT | 200 | 30.0 | Yes | 47.2 | 28.0 | 38.3 | No | Yes | 24 | NR | NR | NR | 119.0/79.5 | −1.6 ± 17.5 | −1.2 ± 12.2 |
| Kirpizidis et al. (31) | P | ITT | 201 | 100 | Yes | 53.8 | 67.7 | NR | No | No | 16 | M | 2400 | NR | 149.0/99.0 | −4.6 ± 38.0 | −3.3 ± 40.0 |
| Kucharska et al. (32) | P | PP | 126 | 100 | Yes | 59.8 | 40.0 | 32.8 | No | Yes | 12 | M | NR | NR | 130.4/84.5 | −3.0 ± 5.7 | −2.0 ± 3.5 |
| Lima et al. (33) | P | ITT | 206 | 100 | Yes | 50.0 | 22.4 | NR | No | No | 24 | A | NR | NR | 144.0/84.0 | −4.9 ± 19.0 | −1.7 ± 13.0 |
| Lin et al. (34) | P | ITT | 20 | 100 | No | 44.3 | 35.0 | 33.9 | Yes | No | 2 | A | 3400 | NR | 144.2/88.5 | −8.7 ± 12.5 | −10.2 ± 10.4 |
| Lopes et al. (35) | CO | ITT | 48 | 50.02 | No | 37.0 | 50.0 | 28.4 | No | No | 4 | M | 3000 | NR | 120.5/82.0 | −4.5 ± 7.7 | −0.9 ± 6.9 |
| Ma et al. (36) | P | ITT | 90 | 0.0 | No | 51.8 | 33.0 | 27.9 | No | No | 24 | M | 2300 | NR | 117.4/74.9 | −2.0 ± 15.6 | −1.1 ± 8.7 |
| Malloy-McFall et al. (37) | P | ITT | 20 | 16.5 | No | 38.3 | 60.0 | 31.5 | No | No | 4 | M | 3300 | NR | 137.4/87.2 | −1.2 ± 9.0 | 0.7 ± 7.0 |
| Márquez-Celedonio et al. (38) | P | PP | 92 | 0.0 | No | 43.2 | NR | 31.2 | No | Yes | 24 | NR | NR | NR | 132.8/86.6 | −10.5 ± 9.7 | −7.4 ± 6.1 |
| Miller et al. (39) | P | PP | 45 | 100 | Yes | 54.0 | 38.0 | 33.6 | Yes | Yes | 9 | M | 2400 | 97.0 | 137.4/84.4 | −7.4 ± 19.8 | −5.7 ± 9.7 |
| Naseem et al. (40) | P | PP | 1492 | 100 | Yes | 53.3 | 50.0 | 27.6 | No | No | 5 | M | 1500 | NR | 128.3/84.4 | −2.1 ± 3.4 | 0.3 ± 3.0 |
| Nowson et al. (41) | CO | PP | 188 | 45.0 | Yes | 55.6 | 60.0 | 29.0 | No | No | 4 | NR | 2300 | NR | 129.4/80.6 | −1.9 ± 10.0 | −0.7 ± 8.2 |
| Nowson et al. (42) | P | PP | 54 | 33.0 | Yes | 47.1 | 100 | 29.9 | No | Yes | 12 | NR | 2000 | NR | 134.8/88.4 | −3.0 ± 14.5 | −6.1 ± 11.0 |
| Nowson et al. (43) | P | ITT | 95 | 37.02 | Yes | 59.2 | 0.0 | 29.6 | No | No | 14 | A | 1000 | NR | 127.5/81.0 | −1.5 ± 11.7 | −1.8 ± 7.8 |
| Paula et al. (44) | P | ITT | 40 | 100 | Yes | 62.2 | 48.0 | 29.4 | No | Yes | 4 | A | NR | NR | 160.3/81.5 | −6.6 ± 14.5 | −3.9 ± 9.5 |
| Roussell et al. (45) | CO | PP | 72 | 0.0 | No | 50.0 | 41.7 | 25.8 | Yes | No | 5 | A | 3000 | 93.0 | 117.2/68.5 | −2.8 ± 11.4 | −0.7 ± 9.0 |
| Sacks et al. (46) | P | ITT | 412 | 41.02 | No | 48.0 | 43.5 | 29.5 | Yes | No | 4 | M | 1200 | NR | 134.5/86.0 | −4.4 ± 23.7 | −2.1 ± 13.5 |
| Sacks et al. (46) | P | ITT | 412 | 41.02 | No | 48.0 | 43.5 | 29.5 | Yes | No | 4 | M | 2400 | NR | 134.5/86.0 | −4.4 ± 23.7 | −2.1 ± 13.5 |
| Sacks et al. (46) | P | ITT | 412 | 41.02 | No | 48.0 | 43.5 | 29.5 | Yes | No | 4 | M | 3600 | NR | 134.5/86.0 | −4.4 ± 23.7 | −2.1 ± 13.5 |
| Whitt-Glover et al. (47) | P | ITT | 25 | 75.0 | Yes | 50.7 | 12.0 | 35.9 | No | No | 12 | NR | 2300 | NR | 130.0/78.4 | −3.1 ± 18.0 | −3.9 ± 11.5 |
| Wong et al. (48) | P | PP | 556 | 100 | No | 55.1 | 49.0 | 24.2 | No | No | 52 | A | NR | NR | 145.0/90.2 | −0.2 ± 13.0 | −0.9 ± 10.0 |
A, automated; BP, blood pressure; CF, controlled feeding; CO, crossover; DBP, diastolic blood pressure; ER, energy restriction; FU, follow-up; HTN, hypertension; ITT, intention-to-treat; M, manual; NR, not reported; P, parallel; PP, per-protocol; SBP, systolic blood pressure.
Separate data for hypertensive and normotensive patients are provided.
Risk of bias within studies and rating of evidence quality (GRADE)
As Supplemental Table 2 indicates, according to the quality procedure assessment, 50% (n = 15) of the included studies (20–22, 24, 25, 27, 30, 33, 34, 36–39, 44, 46) were of higher quality, whereas the remaining studies demonstrated >2 elements that were judged either as unclear or as high risk of bias. According to the GRADE approach, the quality of evidence was rated as moderate, for both outcomes. The level of evidence was downgraded because of either inappropriate blinding of participants and personnel or incomplete outcome data (Supplemental Tables 3 and 4).
Effect of the DASH diet on BP levels
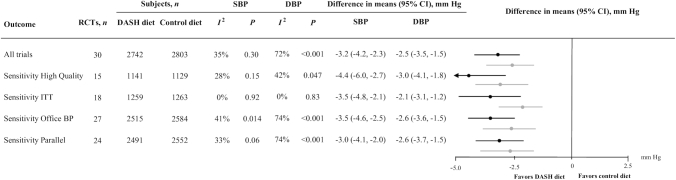
When all selected RCTs (n = 30) (19–48) were analyzed together, the mean difference of SBP and DBP between the 2 randomized arms during follow-up was −3.2 mm Hg; 95% CI: −4.2, −2.3 mm Hg; P < 0.001 and −2.5 mm Hg; 95% CI: −3.5, −1.5 mm Hg; P < 0.001, respectively. By excluding 1 trial at a time, no trial demonstrated an excessive influence on the overall BP estimate (data not shown). Limiting our analysis to studies of higher quality (n = 15) (20–22, 24, 25, 27, 30, 33, 34, 36–39, 44, 46), the overall interventional effect on BP did not change, whereas between-study heterogeneity was reduced. When only intention-to-treat trials were considered (n = 18) (20–22, 24, 25, 28–31, 33–37, 43, 44, 46, 47), the obtained BP estimates were not different from the primary analysis and the heterogeneity across trials faded away. Whenever either only trials with office BP measurements (n = 27) (19–25, 27–40, 43–48) or only trials with a parallel design (n = 24) (20–22, 24–26, 28–34, 36–40, 42–44, 46–48) were considered, again the extent of BP reduction was not different from the primary analysis in both cases (Figure 2).
FIGURE 2.
BP-lowering effect of the DASH diet in adults with and without hypertension: total analysis for SBP and DBP outcomes and sensitivity analyses, according to clinical and methodological characteristics of the selected trials. Difference in means of attained SBP and DBP difference in trials investigating the effect of the DASH diet compared with a control diet. From left to right, the columns indicate the type of analysis, the number of trials analyzed, the number of subjects per randomized arm in each separate analysis for both BP outcomes, the heterogeneity in each analysis for each outcome, the difference in means and 95% CIs for each outcome under the appropriate model based on heterogeneity (the minus sign indicates a lower BP value in the first group), and the forest plots of the difference in means and 95% CIs (black = SBP reduction and gray = DBP reduction). BP, blood pressure; DASH, Dietary Approaches to Stop Hypertension; DBP, diastolic blood pressure; ITT, intention-to-treat; RCT, randomized controlled trial; SBP, systolic blood pressure.
The role of hypertension, antihypertensive drug treatment, and baseline BP levels
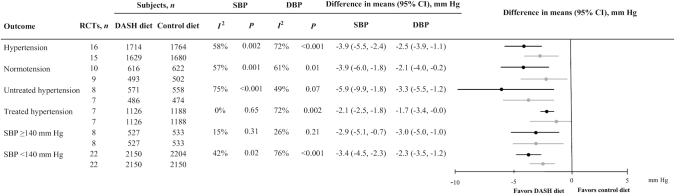
Ten trials or subgroups of trials (19–21, 24, 35, 36, 38, 43, 45, 46) included patients without hypertension and 16 trials or subgroups of trials (19–21, 26, 28, 31–35, 39, 40, 43, 44, 46, 48) included only hypertensive patients. The observed SBP and DBP reductions between the 2 randomized arms were not different between the hypertensive and the nonhypertensive subgroups (P = 0.96 and P = 0.70 for SBP and DBP reduction, respectively) and heterogeneity was of the same extent in both hypertensive and nonhypertensive populations for BP outcomes. In trials or subgroups of trials conducted in hypertensive patients without underlying antihypertensive treatment (n = 8) (19–21, 28, 34, 35, 46, 48), the extent of the mean SBP and DBP reduction was by absolute means higher than that observed for the entire group of hypertensive patients (by 2.0 mm Hg and 0.8 mm Hg, respectively). In comparison with trials conducted in hypertensive patients with underlying antihypertensive treatment (n = 7) (26, 31–33, 39, 40, 44), the result pointed in the same direction, but it did not reach significance regarding both SBP and DBP reduction (P = 0.07 and P = 0.23, respectively). Stratification of trials by a baseline SBP threshold of ≥140 mm Hg (n = 8) (22, 28, 29, 31, 33, 34, 44, 48) compared with <140 mm Hg (n = 22) (19–21, 23–27, 30, 32, 35–43, 45–47) was not accompanied by a differential DASH-diet effect on SBP and DBP difference (P = 0.70 and P = 0.56, respectively) (Figure 3). Whenever trials were stratified by the threshold of 80 mm Hg for baseline DBP, again no differential effect of the DASH diet was observed (data not shown).
FIGURE 3.
BP-lowering effect of the DASH diet in adults with and without hypertension: subgroup analyses for SBP and DBP outcomes, according to clinical and methodological characteristics of the selected trials. Difference in means of attained SBP and DBP difference in trials investigating the effect of the DASH diet compared with control diet in different conditions: hypertension, normotension, untreated hypertension, treated hypertension, and stratified by the SBP threshold of 140 mm Hg. From left to right, the columns indicate condition, the number of trials analyzed, the number of subjects per randomized arm in each separate analysis for both BP outcomes, the heterogeneity in each analysis for each outcome, the difference in means and 95% CIs for each outcome under the appropriate model based on heterogeneity (the minus sign indicates a lower BP value in the first group), and the forest plots of the difference in means and 95% CIs (black = SBP reduction and gray = DBP reduction). BP, blood pressure; DASH, Dietary Approaches to Stop Hypertension; DBP, diastolic blood pressure; RCT, randomized controlled trial; SBP, systolic blood pressure.
Subgroup and meta-regression analyses by relevant variables
We tested different variables as effect modifiers in our analysis. Age, BMI, controlled feeding, daily sodium intake, energy restriction along with the implemented diet, duration of the follow-up period, and type of device used to measure office BP were separately considered as dichotomous variables. The treatment effect of the DASH diet was more pronounced regarding SBP reduction in trials or subgroups of trials with sodium intake >2400 mg/d (n = 9) (19, 20, 27, 28, 34, 35, 37, 45, 46) than in trials or subgroups of trials with sodium intake ≤2400 mg/d (n = 14) (21–25, 31, 36, 39–43, 46, 47) (P = 0.003). Also, the treatment effect of the DASH diet was more pronounced regarding both SBP and DBP reduction in trials with mean age <50 y (n = 13) (19, 20, 22, 24, 27, 29, 30, 34, 35, 37, 38, 42, 46) than in the trials with mean age ≥50 y (n = 16) (21, 25, 26, 28, 31–33, 36, 39–41, 43–45, 47, 48) (P < 0.001 and P = 0.009 for SBP and DBP reduction, respectively). The remaining effect modifiers had no differential effect on either of the 2 BP components (Table 2). To examine the potential modifying effect of continuous variables (age, BMI, baseline SBP and DBP, duration of the follow-up period, attained weight change during follow up, and change in 24-h urinary sodium during follow-up) on the association between the DASH diet and SBP/DBP reduction, we ran meta-regression analyses. Only age was negatively associated with the extent of SBP-lowering between the 2 arms (P = 0.002), whereas the remaining variables had no significant modifying effect on SBP and DBP reduction (Supplemental Figure 1). The results from the multivariate meta-regression analysis showed that among selective effect modifiers (baseline SBP and DBP, age, male sex, BMI, duration of the follow-up period), duration of the DASH diet treatment and marginally age determined SBP reduction. Although age positively determined DBP reduction, baseline SBP and BMI were negative determinants of the same outcome (Supplemental Table 5).
TABLE 2.
BP-lowering effect of the Dietary Approaches to Stop Hypertension diet in adults with and without hypertension: subgroup analyses for SBP and DBP outcomes, according to clinical and methodological characteristics of the selected trials1
| Subgroups | RCTs, n | Subjects, n | Difference in means for SBP difference (95%, CI), mm Hg | P between-groups | I 2 | P-heterogeneity | Difference in means for DBP difference (95% CI), mm Hg | P between-groups | I 2 | P-heterogeneity |
|---|---|---|---|---|---|---|---|---|---|---|
| Age, y | < 0.001 | 0.009 | ||||||||
| ≥50 | 16 | 4027 | −2.0 (−2.4, −1.8) | 0% | 0.79 | −1.3 (−2.2, −0.4) | 50% | 0.01 | ||
| <50 | 13 | 1396 | −4.9 (−6.2, −3.5) | 2% | 0.43 | −3.5 (−4.8, −2.1) | 43% | 0.05 | ||
| BMI, kg/m2 | 0.12 | 0.14 | ||||||||
| ≥30 | 12 | 1456 | −3.9 (−6.1, −1.7) | 48% | 0.03 | −3.3 (−5.0, −1.7) | 57% | 0.01 | ||
| <30 | 15 | 3620 | −2.1 (−2.5, −1.8) | 0% | 0.49 | −1.8 (−2.9, −0.7) | 64% | < 0.001 | ||
| Controlled feeding | 0.23 | 0.52 | ||||||||
| No | 23 | 4564 | −3.1 (−4.1, −2.0) | 43% | 0.02 | −2.3 (−3.4, −1.2) | 73% | < 0.001 | ||
| Yes | 7 | 981 | −4.4 (−6.4, −2.4) | 0% | 0.94 | −2.8 (−4.1, −1.7) | 0% | 0.55 | ||
| Daily sodium intake, mg | 0.003 | 0.39 | ||||||||
| >2400 | 9 | 790 | −4.5 (−6.1, −3.0) | 0% | 0.95 | −2.7 (−3.8, −1.6) | 0% | 0.65 | ||
| ≤2400 | 14 | 3292 | −2.1 (−2.5, −1.8) | 0% | 0.59 | −1.9 (−3.3, −0.6) | 54% | 0.009 | ||
| Energy restriction | 0.48 | 0.09 | ||||||||
| No | 19 | 4074 | −2.9 (−3.9, −1.9) | 21% | 0.19 | −1.8 (−2.8, −0.8) | 59% | < 0.001 | ||
| Yes | 11 | 1471 | −3.7 (−5.7, −1.7) | 48% | 0.04 | −3.5 (−5.1, −1.8) | 57% | 0.009 | ||
| Follow-up period, mo | 0.67 | 0.75 | ||||||||
| ≥3 | 15 | 2594 | −3.0 (−4.6, −1.4) | 47% | 0.02 | −2.6 (−3.8, −1.4) | 48% | 0.01 | ||
| <3 | 15 | 1428 | −3.5 (−4.6, −2.3) | 23% | 0.19 | −2.3 (−3.7, −0.9) | 69% | < 0.001 | ||
| Office BP methodology | 0.95 | 0.98 | ||||||||
| Manual | 14 | 3562 | −2.3 (−2.7, −1.8) | 1% | 0.44 | −1.9 (−3.1, −0.7) | 67% | < 0.001 | ||
| Automated | 7 | 1061 | −2.2 (−4.0, −0.4) | 14% | 0.32 | −1.9 (−3.0, −0.7) | 6% | 0.38 |
Trials or subgroups of trials entered in subgroup analyses: age (21, 25, 26, 28, 31–33, 36, 39–41, 43–45, 47, 48 compared with 19, 20, 22, 24, 27, 29, 30, 34, 35, 37, 38, 42, 46); BMI (21, 25, 26, 28–30, 32, 34, 37–39, 47 compared with 19, 20, 22, 24, 27, 35, 36, 40–46, 48); controlled feeding (19, 21, 22–26, 29–33, 35–38, 40–44, 47, 48 compared with 20, 27, 28, 34, 39, 45, 46); daily sodium intake (19, 20, 27, 28, 34, 35, 37, 45, 46 compared with 21–25, 31, 36, 39–43, 46, 47); energy restriction (19, 20, 23, 25, 27, 28, 31, 33–37, 40, 41, 43, 45–48 compared with 21, 22, 24, 26, 29, 30, 32, 38, 39, 42, 44); follow-up period (21, 22, 25, 26, 29–33, 36, 38, 42, 43, 47, 48 compared with 19, 20, 23, 24, 27, 28, 34, 35, 37, 39–41, 44–46); and office BP methodology (19–22, 25, 28, 31, 32, 35–37, 39, 40, 46 compared with 27, 33, 34, 43–45, 48). BP, blood pressure; DBP, diastolic blood pressure; SBP, systolic blood pressure.
Publication bias
As presented in the funnel plots, no publication bias was produced for both SBP and DBP under the random-effects plotting, a finding further suggested by the Egger's and Begg's tests (Supplemental Figure 2).
Discussion
Summary of evidence
Our updated analysis confirmed that the adoption of the DASH diet results in a significant SBP and DBP reduction, and to our knowledge for the first time provided summary evidence that this effect is independent of baseline BP levels. Although no differential BP effect was noticed between hypertensive and nonhypertensive patients, the extent of SBP and DBP reduction was higher by absolute means in hypertensive patients without underlying antihypertensive treatment than that observed in all hypertensive patients. The BP effect of the DASH diet was consistent across a wide range of baseline SBP and DBP levels, i.e., from normotensive BP levels to grade 1 hypertension, according to the Joint National Committee 7 definition of hypertension (49), and it was independent of the concomitant energy restriction. However, regarding sodium intake, it was found that higher amounts of daily sodium intake enhance the DASH diet BP-lowering effect. Moreover, our study raised the hypothesis that age may be an inverse modulator of the DASH diet–mediated effect on BP reduction.
Because the extent of BP reduction is semilogarithmically related to the incidence of cardiovascular outcomes (17), even a small reduction of BP levels, such as the one suggested by the present meta-analysis, may result in nonpharmacological treatment benefits. We resisted estimating the change of SBP and DBP from baseline in each randomized trial, because the extent of BP reduction is largely related to Wilder's principle (i.e., different baseline BP levels across studies) (50) and also baseline BP levels were not equal between the randomized arms in individual studies. Therefore, our analysis was based exclusively on the attained BP difference between the 2 randomized arms during follow-up not only to overcome these methodological drawbacks, but also to parallel with the estimated BP reductions observed in drug BP-lowering RCTs (7).
Interpretation of findings
We were unable to demonstrate any modulating role of hypertension status on BP reduction, potentially because of the underlying “regression to the mean” phenomenon, which operates in a bidirectional fashion, i.e., from higher to lower BP levels in case of hypertension trials and from lower to higher BP levels in case of normotension trials (51). In addition, different operating pathophysiological pathways in hypertension, such as endothelial dysfunction and increased sympathetic tone, may limit the BP-lowering effect of the DASH diet at variance with normotension or prehypertension in which vascular integrity is less impaired (52, 53). The implementation of the DASH diet at baseline SBP levels above and below 140 mm Hg was not associated with differential mean SBP and DBP reduction, but this type of analysis should be interpreted with caution because it includes patients with treated hypertension. Our finding that untreated hypertension was associated with greater BP reduction after the DASH diet intervention supports the hypothesis that lifestyle measures can result in a larger BP change in untreated individuals, who usually demonstrate higher baseline BP levels than hypertensive individuals already treated with antihypertensive agents. Beyond the accelerated vascular aging in hypertension compared with either prehypertension or normotension, the higher the age the lower the BP reduction after the DASH diet intervention (54). However, age-increase represents per se a nonmodifiable trigger of vascular damage that may result in reduced responsiveness to antihypertensive interventions, including the DASH diet.
The DASH diet seems to exert natriuretic action (55) and to interact with the renin–angiotensin–aldosterone system, resulting in vascular and hormonal responses which induce a hypotensive effect (56, 57). Our finding that the DASH diet was accompanied by a greater BP reduction in those with higher sodium intake than in their lower sodium intake counterparts is not without precedents. Indeed, Sacks et al. (46) suggested that the BP-lowering effect of the DASH diet compared with the control diet was almost 3-fold higher for those at higher than for those at lower sodium intake. It was assumed that low amounts of dietary sodium attenuated the hypotensive effects of potassium or, inversely, the high potassium or calcium content of the DASH diet attenuated the effects of low amounts of sodium. We did not observe any modulating role of energy restriction on BP levels, meaning that the DASH diet may result in BP reduction with or without concomitant energy restriction.
In studies with controlled feeding, dietary adherence to the prescribed diets was assessed by direct observation of the participants during on-site meals, participants’ reports about the consumption of the “take-home” study foods, and urine collections. In studies with noncontrolled feeding, dietary adherence was assessed by different methods, such as 3-d food records, 24-h dietary recalls, photographs of food and beverages consumed, urine collections, and attendance at the dietary sessions. Although the vast majority of studies did not provide data about dietary adherence rates, studies with controlled feeding are expected to demonstrate higher adherence rates than studies conducted in free-living environments.
Comparison with previous evidence
The effect of the DASH diet on BP levels has been investigated in previous pairwise meta-analyses by Saneei et al. (10) (17 studies; 2561 participants), Ndanuko et al. (10 studies; 2798 participants) (11), Siervo et al. (12) (16 studies; 1581 participants), and Gay et al. (13) (4 studies; 668 participants). Overall, the results in these meta-analyses showed that the DASH diet reduced SBP/DBP by −6.7/−3.5 mm Hg, −4.9/−3.6 mm Hg, −5.2/−2.6 mm Hg, and −7.6/−4.2 mm Hg, respectively. However, the BP estimates in all these meta-analyses represented the mean difference as a change from baseline BP, a measure that is dependent on both the baseline BP levels and the unequal baseline BP between arms. Consequently, the previously reported BP reduction introduces outcome-related bias and, therefore, is hardly comparable with our BP estimates. At variance with the previous evidence, we provided a more comprehensive and updated meta-analysis, because we considered a larger number of RCTs (30 studies; 5545 participants). Also, we considered the attained mean BP difference between the randomized arms (i.e., mean postintervention BP difference) to overcome outcome-related bias and we accounted for methodological and clinical confounders not previously approached. A network meta-analysis which considered the postintervention SBP/DBP difference similarly to our setting found that the DASH diet reduced SBP/DBP by −7.4/−4.4 mm Hg compared with a control diet (58). However, this analysis did not consider trials with duration <12 wk and with baseline BP <130/85 mm Hg; moreover, owing to its network nature the control diet arm was disproportionally larger than the DASH diet arm, suggesting loss of randomization. Consequently, these results are not comparable with traditional pairwise meta-analysis results in which randomization is preserved.
Limitations
In this comprehensive and updated meta-analysis, we acknowledge several limitations. First, the quality of the included studies was suboptimal, because 50% of them had >2 elements scored either as unclear or as high risk of bias. The unblinded nature of the randomized trials considered here introduces investigator-related bias and discloses that the Hawthorne effect (59) at the participant level might be increased compared with blinded studies. Also, the moderate grade of evidence observed in the present synthesis indicates that the true effect of the DASH diet on BP levels is likely to be close to our mean estimate, but the possibility that this effect might be substantially different cannot be excluded. Moreover, the measurement of office BP was problematic or unclear in all the selected trials (outcome-based bias). Although subgroup analyses were conducted across mean clinical thresholds in each separate trial, it is undetermined whether individual trial patients were above or below this preselected threshold. No subgroup analysis was attempted by sex because of the very limited available data and lack of statistical power (20, 22, 26, 32). Also, meta-regression analyses should be interpreted with a critical view, because they represent cross-sectional tools without a prospective potential. The multivariable regression analysis included only a small number of confounders to preserve enough statistical power, but this power was still suboptimal because of the limited number of the included studies. The mean follow-up time was relatively small (almost 15 wk), thus it cannot be suggested that the BP-lowering effect of the DASH diet is extended to longer periods. The heterogeneity across studies was overall moderate and partly downgraded by explanatory analyses (i.e., subgroup and meta-regression analyses based on different clinical and methodological characteristics). Lastly, both the recommended DASH and control diets in each trial were not identical in terms of macro- and micronutrient content; moreover, some of the included trials were designed with a different purpose to that of exploring the BP-lowering effect of the DASH diet.
Conclusions
In conclusion, this synthesis of moderate quality according to the GRADE system demonstrated that the adoption of the DASH diet reduces BP in subjects with or without hypertension, irrespective of baseline BP levels or ongoing antihypertensive treatment, whereas the extent of BP-lowering is greater in those with higher sodium intake and younger individuals. Our findings give further support to the recommendations of current hypertension guidelines, supporting that the DASH diet should be pursued to delay hypertension onset and increase the effectiveness of antihypertensive drug treatment.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—CDF, CGT, and CCM: designed the research; CDF and CGT: performed the systematic literature search, identified the studies meeting the inclusion criteria, extracted data from the included studies, and wrote the paper; CDF, CGT, and CPT: assessed the risk of bias of the included studies; CGT: performed the statistical analysis; CPT, CCM, KSD, LIS, CC, PIN, and DMT: assisted in the interpretation of the results and the revision of the manuscript; and all authors: had primary responsibility for the final content and read and approved the final manuscript.
Notes
The authors reported no funding received for this study.
Author disclosures: The authors report no conflicts of interest.
Supplemental Tables 1–5 and Supplemental Figures 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: BP, blood pressure; DASH, Dietary Approaches to Stop Hypertension; DBP, diastolic blood pressure; GRADE, Grading of Recommendations, Assessment, Development, and Evaluation; RCT, randomized controlled trial; SBP, systolic blood pressure.
References
- 1. WHO. Global Health Observatory (GHO) data: raised blood pressure: situation and trends. [Internet] Geneva: WHO; [cited February, 2016]. Available from: http://www.who.int/gho/ncd/risk_factors/blood_pressure_prevalence_text/en/. [Google Scholar]
- 2. Mule G, Castiglia A, Cusumano C, Scaduto E, Geraci G, Altieri D, Di Natale E, Cacciatore O, Cerasola G, Cottone S. Subclinical kidney damage in hypertensive patients: a renal window opened on the cardiovascular system. Focus on microalbuminuria. Adv Exp Med Biol. 2017;956:279–306. [DOI] [PubMed] [Google Scholar]
- 3. Kjeldsen SE. Hypertension and cardiovascular risk: general aspects. Pharmacol Res. 2018;129:95–9. [DOI] [PubMed] [Google Scholar]
- 4. Bazzano LA, Green T, Harrison TN, Reynolds K. Dietary approaches to prevent hypertension. Curr Hypertens Rep. 2013;15:694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Savica V, Bellinghieri G, Kopple JD. The effect of nutrition on blood pressure. Annu Rev Nutr. 2010;30:365–401. [DOI] [PubMed] [Google Scholar]
- 6. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A et al.. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–104. [DOI] [PubMed] [Google Scholar]
- 7. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW et al.. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:2199–269. [DOI] [PubMed] [Google Scholar]
- 8. Appel LJ. The effects of dietary factors on blood pressure. Cardiol Clin. 2017;35:197–212. [DOI] [PubMed] [Google Scholar]
- 9. Chiavaroli L, Viguiliouk E, Nishi SK, Blanco Mejia S, Rahelić D, Kahleová H, Salas-Salvadó J, Kendall CW, Sievenpiper JL. DASH dietary pattern and cardiometabolic outcomes: an umbrella review of systematic reviews and meta-analyses. Nutrients. 2019;11:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Saneei P, Salehi-Abargouei A, Esmaillzadeh A, Azadbakht L. Influence of Dietary Approaches to Stop Hypertension (DASH) diet on blood pressure: a systematic review and meta-analysis on randomized controlled trials. Nutr Metab Cardiovasc Dis. 2014;24:1253–61. [DOI] [PubMed] [Google Scholar]
- 11. Ndanuko RN, Tapsell LC, Charlton KE, Neale EP, Batterham MJ. Dietary patterns and blood pressure in adults: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. 2016;7:76–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Siervo M, Lara J, Chowdhury S, Ashor A, Oggioni C, Mathers JC. Effects of the Dietary Approach to Stop Hypertension (DASH) diet on cardiovascular risk factors: a systematic review and meta-analysis. Br J Nutr. 2015;113:1–15. [DOI] [PubMed] [Google Scholar]
- 13. Gay HC, Rao SG, Vaccarino V, Ali MK. Effects of different dietary interventions on blood pressure: systematic review and meta-analysis of randomized controlled trials. Hypertension. 2016;67:733–9. [DOI] [PubMed] [Google Scholar]
- 14. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA et al.. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schünemann H, Brożek J, Guyatt G, Oxman A; The GRADE Working Group . GRADE handbook for grading quality of evidence and strength of recommendations. [Internet] [cited October, 2013] Canada;2000 [last updated 4 December, 2015]. Available from: https://gdt.guidelinedevelopment.org/app/handbook/handbook.html. [Google Scholar]
- 17. Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension: 7. Effects of more vs. less intensive blood pressure lowering and different achieved blood pressure levels – updated overview and meta-analyses of randomized trials. J Hypertens. 2016;34:613–22. [DOI] [PubMed] [Google Scholar]
- 18. Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed Hillsdale, NJ: Lawrence Erlbaum Associates; 1998. [Google Scholar]
- 19. Al-Solaiman Y, Jesri A, Mountford WK, Lackland DT, Zhao Y, Egan BM. DASH lowers blood pressure in obese hypertensives beyond potassium, magnesium and fibre. J Hum Hypertens. 2010;24:237–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM et al.. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336:1117–24. [DOI] [PubMed] [Google Scholar]
- 21. Appel LJ, Champagne CM, Harsha DW, Cooper LS, Obarzanek E, Elmer PJ, Stevens VJ, Vollmer WM, Lin PH, Svetkey LP et al.. Effects of comprehensive lifestyle modification on blood pressure control: main results of the PREMIER clinical trial. JAMA. 2003;289:2083–93. [DOI] [PubMed] [Google Scholar]
- 22. Azadbakht L, Mirmiran P, Esmaillzadeh A, Azizi T, Azizi F. Beneficial effects of a Dietary Approaches to Stop Hypertension eating plan on features of the metabolic syndrome. Diabetes Care. 2005;28:2823–31. [DOI] [PubMed] [Google Scholar]
- 23. Azadbakht L, Fard NR, Karimi M, Baghaei MH, Surkan PJ, Rahimi M, Esmaillzadeh A, Willett WC. Effects of the Dietary Approaches to Stop Hypertension (DASH) eating plan on cardiovascular risks among type 2 diabetic patients: a randomized crossover clinical trial. Diabetes Care. 2011;34:55–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Azadbakht L, Izadi V, Ehsani S, Esmaillzadeh A. Effects of the Dietary Approaches to Stop Hypertension (DASH) eating plan on the metabolic side effects of corticosteroid medications. J Am Coll Nutr. 2016;35:285–90. [DOI] [PubMed] [Google Scholar]
- 25. Blumenthal JA, Babyak MA, Hinderliter A, Watkins LL, Craighead L, Lin PH, Caccia C, Johnson J, Waugh R, Sherwood A. Effects of the DASH diet alone and in combination with exercise and weight loss on blood pressure and cardiovascular biomarkers in men and women with high blood pressure: the ENCORE study. Arch Intern Med. 2010;170:126–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Burke V, Beilin LJ, Cutt HE, Mansour J, Wilson A, Mori TA. Effects of a lifestyle programme on ambulatory blood pressure and drug dosage in treated hypertensive patients: a randomized controlled trial. J Hypertens. 2005;23:1241–9. [DOI] [PubMed] [Google Scholar]
- 27. Chiu S, Bergeron N, Williams PT, Bray GA, Sutherland B, Krauss RM. Comparison of the DASH (Dietary Approaches to Stop Hypertension) diet and a higher-fat DASH diet on blood pressure and lipids and lipoproteins: a randomized controlled trial. Am J Clin Nutr. 2016;103:341–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Conlin PR, Erlinger TP, Bohannon A, Miller ER 3rd, Appel LJ, Svetkey LP, Moore TJ. The DASH diet enhances the blood pressure response to losartan in hypertensive patients. Am J Hypertens. 2003;16:337–42. [DOI] [PubMed] [Google Scholar]
- 29. Edwards KM, Wilson KL, Sadja J, Ziegler MG, Mills PJ. Effects on blood pressure and autonomic nervous system function of a 12-week exercise or exercise plus DASH-diet intervention in individuals with elevated blood pressure. Acta Physiol (Oxf). 2011;203:343–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Green CA, Yarborough BJ, Leo MC, Yarborough MT, Stumbo SP, Janoff SL, Perrin NA, Nichols GA, Stevens VJ. The STRIDE weight loss and lifestyle intervention for individuals taking antipsychotic medications: a randomized trial. Am J Psychiatry. 2015;172:71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kirpizidis H, Stavrati A, Geleris P. Assessment of quality of life in a randomized clinical trial of candesartan only or in combination with DASH diet for hypertensive patients. J Cardiol. 2005;46:177–82. [PubMed] [Google Scholar]
- 32. Kucharska A, Gajewska D, Kiedrowski M, Sinska B, Juszczyk G, Czerw A, Augustynowicz A, Bobinski K, Deptala A, Niegowska J. The impact of individualised nutritional therapy according to DASH diet on blood pressure, body mass, and selected biochemical parameters in overweight/obese patients with primary arterial hypertension: a prospective randomised study. Kardiol Pol. 2018;76:158–65. [DOI] [PubMed] [Google Scholar]
- 33. Lima ST, da Silva Nalin de Souza B, França AK, Salgado Filho N, Sichieri R. Dietary approach to hypertension based on low glycaemic index and principles of DASH (Dietary Approaches to Stop Hypertension): a randomised trial in a primary care service. Br J Nutr. 2013;110:1472–9. [DOI] [PubMed] [Google Scholar]
- 34. Lin P-H, Allen JD, Li Y-J, Yu M, Lien LF, Svetkey LP. Blood pressure-lowering mechanisms of the DASH dietary pattern. J Nutr Metab. 2012:472396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lopes HF, Martin KL, Nashar K, Morrow JD, Goodfriend TL, Egan BM. DASH diet lowers blood pressure and lipid-induced oxidative stress in obesity. Hypertension. 2003;41:422–30. [DOI] [PubMed] [Google Scholar]
- 36. Ma J, Strub P, Lv N, Xiao L, Camargo CA Jr, Buist AS, Lavori PW, Wilson SR, Nadeau KC, Rosas LG. Pilot randomised trial of a healthy eating behavioural intervention in uncontrolled asthma. Eur Respir J. 2016;47:122–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Malloy-McFall J, Barkley JE, Gordon KL, Burzminski N, Glickman EL. Effect of the DASH diet on pre- and stage 1 hypertensive individuals in a free-living environment. Nutr Metab Insights. 2010;3:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Márquez-Celedonio FG, Téxon-Fernández O, Chávez-Negrete A, Hernández-López S, Marín-Rendón S, Berlín-Lascurain S. [Clinical effect of lifestyle modification on cardiovascular risk in prehypertensives: PREHIPER I study]. Rev Esp Cardiol. 2009;62:86–90. [PubMed] [Google Scholar]
- 39. Miller ER 3rd, Erlinger TP, Young DR, Jehn M, Charleston J, Rhodes D, Wasan SK, Appel LJ. Results of the Diet, Exercise, and Weight Loss Intervention Trial (DEW-IT). Hypertension. 2002;40:612–18. [DOI] [PubMed] [Google Scholar]
- 40. Naseem S, Ghazanfar H, Assad S, Ghazanfar A. Role of sodium-restricted dietary approaches to control blood pressure in Pakistani hypertensive population. J Pak Med Assoc. 2016;66:837–42. [PubMed] [Google Scholar]
- 41. Nowson CA, Worsley A, Margerison C, Jorna MK, Frame AG, Torres SJ, Godfrey SJ. Blood pressure response to dietary modifications in free-living individuals. J Nutr. 2004;134:2322–9. [DOI] [PubMed] [Google Scholar]
- 42. Nowson CA, Worsley A, Margerison C, Jorna MK, Godfrey SJ, Booth A. Blood pressure change with weight loss is affected by diet type in men. Am J Clin Nutr. 2005;81:983–9. [DOI] [PubMed] [Google Scholar]
- 43. Nowson CA, Wattanapenpaiboon N, Pachett A. Low-sodium Dietary Approaches to Stop Hypertension–type diet including lean red meat lowers blood pressure in postmenopausal women. Nutr Res. 2009;29:8–18. [DOI] [PubMed] [Google Scholar]
- 44. Paula TP, Viana LV, Neto AT, Leitao CB, Gross JL, Azevedo MJ. Effects of the DASH diet and walking on blood pressure in patients with type 2 diabetes and uncontrolled hypertension: a randomized controlled trial. J Clin Hypertens (Greenwich). 2015;17:895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Roussell MA, Hill AM, Gaugler TL, West SG, Ulbrecht JS, Vanden Heuvel JP, Gillies PJ, Kris-Etherton PM. Effects of a DASH-like diet containing lean beef on vascular health. J Hum Hypertens. 2014;28:600–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER 3rd, Simons-Morton DG et al.. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344:3–10. [DOI] [PubMed] [Google Scholar]
- 47. Whitt-Glover MC, Hunter JC, Foy CG, Quandt SA, Vitolins MZ, Leng I, Hornbuckle LM, Sanya KA, Bertoni AG. Translating the Dietary Approaches to Stop Hypertension (DASH) diet for use in underresourced, urban African American communities, 2010. Prev Chronic Dis. 2013;10:120088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wong MC, Wang HH, Kwan MW, Fong BC, Chan WM, Zhang DX, Li ST, Yan BP, Coats AJ, Griffiths SM. Dietary counselling has no effect on cardiovascular risk factors among Chinese grade 1 hypertensive patients: a randomized controlled trial. Eur Heart J. 2015;36:2598–607. [DOI] [PubMed] [Google Scholar]
- 49. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr et al.. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–72. [DOI] [PubMed] [Google Scholar]
- 50. Messerli FH, Bangalore S, Schmieder RE. Wilder's principle: pre-treatment value determines post-treatment response. Eur Heart J. 2015;36:576–9. [DOI] [PubMed] [Google Scholar]
- 51. Salam A, Atkins E, Sundström J, Hirakawa Y, Ettehad D, Emdin C, Neal B, Woodward M, Chalmers J, Berge E et al.. Effects of blood pressure lowering on cardiovascular events, in the context of regression to the mean: a systematic review of randomized trials. J Hypertens. 2019;37:16–23. [DOI] [PubMed] [Google Scholar]
- 52. Mancia G, Grassi G. The autonomic nervous system and hypertension. Circ Res. 2014;114:1804–14. [DOI] [PubMed] [Google Scholar]
- 53. Mordi I, Mordi N, Delles C, Tzemos N. Endothelial dysfunction in human essential hypertension. J Hypertens. 2016;34:1464–72. [DOI] [PubMed] [Google Scholar]
- 54. Kotsis V, Antza C, Doundoulakis I, Stabouli S. Markers of early vascular ageing. Curr Pharm Des. 2017;23:3200–4. [DOI] [PubMed] [Google Scholar]
- 55. Akita S, Sacks FM, Svetkey LP, Conlin PR, Kimura G; DASH-Sodium Trial Collaborative Research Group . Effects of the Dietary Approaches to Stop Hypertension (DASH) diet on the pressure-natriuresis relationship. Hypertension. 2003;42:8–13. [DOI] [PubMed] [Google Scholar]
- 56. Svetkey LP, Moore TJ, Simons-Morton DG, Appel LJ, Bray GA, Sacks FM, Ard JD, Mortensen RM, Mitchell SR, Conlin PR et al.. Angiotensinogen genotype and blood pressure response in the Dietary Approaches to Stop Hypertension (DASH) study. J Hypertens. 2001;19:1949–56. [DOI] [PubMed] [Google Scholar]
- 57. Maris SA, Williams JS, Sun B, Brown S, Mitchell GF, Conlin PR. Interactions of the DASH diet with the renin-angiotensin-aldosterone system. Curr Dev Nutr. 2019;3(9):nzz091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Schwingshackl L, Chaimani A, Schwedhelm C, Toledo E, Punsch M, Hoffmann G, Boeing H. Comparative effects of different dietary approaches on blood pressure in hypertensive and pre-hypertensive patients: a systematic review and network meta-analysis. Crit Rev Food Sci Nutr. 2019;59(16):2674–87. [DOI] [PubMed] [Google Scholar]
- 59. Nahler G. Hawthorne effect. In: Dictionary of pharmaceutical medicine. 4th ed Vienna: Springer; 2009. p. 84. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.