ABSTRACT
People living with HIV (PLWHIV) are at high risk of anemia due to inadequate iron intake, HIV and opportunistic infections, and inflammation, and as a side effect of antiretroviral therapy. Though iron supplementation can reduce iron deficiency anemia (IDA) in the general population, its role in anemia and in the health of PLWHIV is unclear due to concerns that iron supplementation may increase HIV replication and risk of opportunistic infections. We systematically reviewed the evidence on indicators of iron status, iron intake, and clinical outcomes among adults and children with HIV. The evidence suggests that anemia is associated with an increased risk of all-cause mortality and incident tuberculosis among HIV-infected individuals, regardless of anemia type, and the magnitude of the risk is greater with more severe anemia. High serum ferritin is associated with adverse clinical outcomes, although it is unclear if this is due to high iron or inflammation from disease progression. One large observational study found an increased risk of all-cause mortality among HIV-infected adults if they received iron supplementation. Published randomized controlled trials of iron supplementation among PLWHIV tend to have small sample sizes and have been inconclusive in terms of effectiveness and safety. Large randomized trials exploring approaches to safely and effectively provide iron supplementation to PLWHIV are warranted.
Keywords: anemia, iron, HIV, mortality, viral load
This review examined the evidence regarding the relation of anemia, iron status, iron supplementation, and the risk of mortality and HIV-related outcomes among people living with HIV.
Introduction
The global burden of anemia is huge. The primary causes of anemia are poor diet quality, infections, and genetic disorders (1). Heavy menstrual losses and obstetric hemorrhage are also important contributing factors among women of reproductive age. The global burden of HIV infection is substantial (2) and anemia is one of the most common indices of the severity of HIV disease, often predicting mortality and disease progression among people living with HIV (PLWHIV) (3, 4). The prevalence of anemia among PLWHIV is as high as 80–90% in some settings (4, 5). In the context of HIV infection, anemia is commonly caused by iron deficiency (ID), anemia of chronic disease (ACD), or coexisting infections, or as a side effect of antiretroviral therapy (ART) (6–8).
Iron supplementation is well known to effectively prevent and treat iron deficiency anemia (IDA) and is therefore recommended by the WHO as part of standard prenatal care and as prophylaxis among children in high–anemia burden contexts, regardless of HIV status (9, 10). However, the effectiveness of iron supplementation in PLWHIV remains unclear since a large proportion of anemia may be due to ACD and coexisting infections rather than IDA alone. The appropriate treatment for ACD is unclear, although it is believed that ART may ameliorate it (11). It is also unclear whether ART reduces ACD and thereby unmasks IDA if these variables coexist. Furthermore, iron may be harmful in HIV infection, a supposition based partly on studies from the pre-ART era with findings suggesting that elevated iron status may be associated with an increased risk of disease progression (12). More recent studies also suggest that replication of the virus is an iron-dependent process and that increased concentrations of non–transferrin bound iron (NTBI) from supplementation may be associated with the risk of opportunistic infections (13). The extent to which ART modifies this risk is unclear.
In this paper we review the evidence linking hematologic status, iron intake, and important clinical outcomes in the context of HIV infection. Specific objectives include the following: 1) to examine the relation of hematologic status and clinical outcomes, including mortality, tuberculosis (TB), CD4 counts, and viral load; 2) to evaluate the effectiveness and safety of iron supplementation in the prevention and treatment of anemia among HIV-infected adults; and 3) to assess the extent to which ART may modify the relation of hematologic status, iron supplement use, and clinical outcomes. In this review, we aimed to synthesize the available evidence regarding the utility of iron supplementation in the context of ART among PLWHIV and therefore all possible causes of anemia are not considered in detail.
Methods
Systematic literature search and selection of studies
We searched the medical literature databases of Medline and EMBASE for original research articles published as of July 2019. Medical subject headings and keywords representing HIV, TB, iron, or hematologic status were employed (Supplementary Table 1). No restrictions by age, year, or language of publication were applied. In addition, we did handsearching of the references of included papers and published systematic reviews (4, 14, 15). The titles and abstracts of identified studies were screened, and full texts were examined in duplicate (AIA and CTA). Discrepancies were resolved by discussion among authors. We included studies that were of observational and experimental design that were carried out among HIV-infected or TB-infected populations, or populations at risk of these outcomes for studies in which the incidence of HIV or TB was a study outcome. Studies were included if they considered iron biomarker status, iron supplementation, or dietary iron intake as the exposure and HIV- or TB-related outcomes (e.g., incidence, disease progression, opportunistic infections, or hematologic status) as the outcomes. Studies were excluded if they were in vitro studies, reported cases, or case series only or included both HIV-infected and HIV-uninfected patients (except longitudinal studies of HIV incidence or studies that presented subgroup findings for HIV-infected patients alone). Studies of TB in the absence of HIV were also excluded.
Data on study design, population characteristics, exposures, outcomes, covariates, and findings were extracted from selected studies, including point estimates with CIs, SEs, or P values. For continuous measures, these included means, medians, and mean differences, whereas for binary or categorical outcomes, we noted RRs, ORs, and HRs.
Meta-analysis
We pooled estimates for the association of exposure–outcome relations reported by ≥4 studies. For studies that reported >1 estimate, the estimate with the greatest degree of adjustment for confounding was included. If the study reported anemia as a categorical exposure (for instance mild, moderate, or severe), the most extreme estimate (for instance, severe anemia) was included. Two eligible studies were published from the same cohort (16, 17), and the study with the larger sample size was included in the meta-analysis.
Random effects meta-analysis was conducted based on the Dersimonian–Laird method (18). Heterogeneity was formally assessed with the Higgins I2 statistic, a measure of the total variability due to between-study variation. I2 was regarded as substantial if 50–90% and considerable if >90% (19). Too few studies were included in the meta-analyses, and heterogeneity was not further assessed using meta-regression or subgroup analyses. Publication bias was evaluated with Egger's tests and visual inspection of funnel plots. P values are 2 sided and significance was set at P < 0.05. Statistical analyses were conducted using Stata version 15.0.
Anemia and iron status indicators
Before presenting the results of the systematic review, we summarize the major iron status indicators. Anemia is a commonly used measure of ID, particularly in low- and middle-income countries (LMICs) due to cost and laboratory capacity. Prolonged inadequate iron intake or high iron loss is almost certain to result in anemia. However, anemia can also be caused by other factors, including other nutritional deficiencies, infection, and genetic disorders. Anemia can also occur due to chronic inflammation, as in the case of ACD, in which iron is sequestered from hematopoiesis. ACD is also known as anemia of inflammation (AI) and iron-restricted anemia (IRA).
Ferritin is the storage form of iron in the cells, and serum ferritin is strongly associated with body iron stores. However, ferritin concentrations also rise during inflammation. In the context of HIV, ferritin concentrations are not certain to reflect true ID, and adjustment for markers of inflammation may be warranted for optimal interpretation (20). In a fasting state, serum iron reflects iron in transit from the reticuloendothelial system to the bone marrow, and low concentrations of serum iron indicate ID. However, serum iron also decreases during inflammation, and a patient with inflammation may incorrectly appear to be iron deficient.
Transferrin, total iron-binding capacity (TIBC), transferrin saturation, and soluble transferrin receptor (sTfR) are 4 other related measures of iron status that have been used in the selected studies. Transferrin is the main protein responsible for the transport of iron in blood and is also an acute-phase reactant that decreases during inflammation (21). Total iron-binding capacity measures the amount of iron that can be bound by proteins in the blood and as such is an indirect measure of transferrin. Concentrations of serum transferrin and TIBC rise during ID (21). Transferrin saturation is an estimate of the proportion of transferrin iron-binding sites that are occupied, and this value is obtained by dividing the serum iron concentration by the TIBC (22). Transferrin saturation is low during ID. Transferrin receptors facilitate the entry of iron-bound transferrin through cell membranes, and concentrations of the truncated soluble form, soluble transferrin receptor (sTfR), may rise in serum during ID (21). The key advantage of sTfR is that sTfR concentration is influenced to a lesser extent in the context of inflammation, thereby aiding in a differential diagnosis between ACD and anemia resulting from ID (23).
Hepcidin is the master regulator of iron homeostasis (24). It determines the rate of intestinal absorption of iron. It also determines the rate of release of iron from macrophages in the reticuloendothelial system. When hepcidin concentrations rise, iron bioavailability tends to reduce, and vice versa. Hepcidin concentrations are low during ID and high during iron repletion. Other factors that may influence hepcidin concentrations include inflammation, erythropoiesis, and hypoxia (21).
Results
Summary of included studies
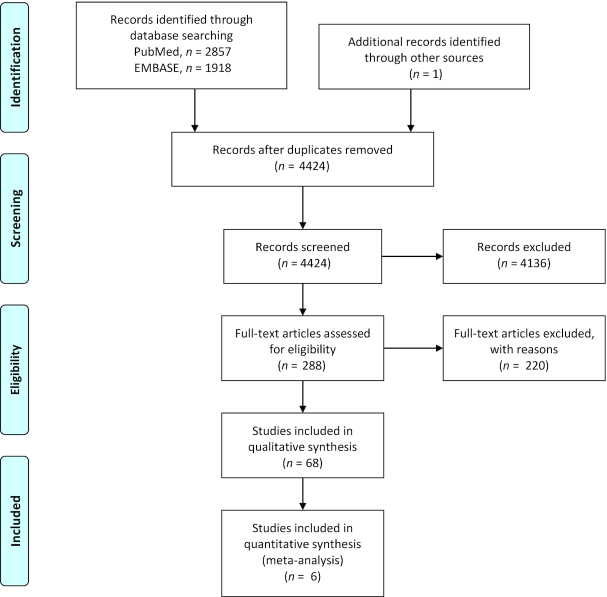
We identified 68 studies (Figure 1) that examined the relation of hematologic status and important clinical outcomes among 58,886 PLWHIV (5, 11, 16, 17, 25–94). There were 36 cross-sectional studies, 32 cohort studies, and 3 randomized controlled trials (RCT). Most studies recruited adults only (85%); 9 were pediatric studies and 1 study was limited to adolescents (46). There were 23 studies from high-income countries, 42 studies from LMICs, and 3 multicountry studies from both settings. The studies were conducted in Asia (n = 8), Europe (n = 10), North America (n = 13), South America (n = 1), Sub-Saharan Africa (n = 33), and multiple regions (n = 3). Participants were 48% female, on average. Some studies included females (27, 49, 68, 69, 81, 82, 90, 91, 95) or males alone (25, 29, 48, 61, 71, 77, 83, 84, 96).
FIGURE 1.
Flow chart of selected studies conducted among people living with HIV.
Cross-sectional studies
Thirty-three of the 36 cross-sectional studies were conducted among adults, and 3 in children (Supplementary Table 2). Hemoglobin concentrations and anemia were among the most commonly reported indicators among cross-sectional studies (12 of 34 studies). Overall, these studies tended to find that low hemoglobin concentrations were associated with indicators of HIV disease severity and progression, such as being symptomatic (33, 34), having a higher viral load (56), having opportunistic infections (50), and being immunosuppressed (34)—including lower CD4 T-cell counts (33, 51, 52, 55, 56). Four studies found no association between hemoglobin and CD4 count (41, 44, 57) or viral load (41, 57, 81).
Serum ferritin was the most commonly reported indicator of iron status among cross-sectional studies (12 of 34 studies). In unadjusted comparisons, higher concentrations of serum ferritin were found among patients with advanced stage disease (29, 35, 38, 47, 54, 96), higher viral loads (47), opportunistic infections (35), and those with lower CD4 T-cell counts (47, 57). Kupka and colleagues found that serum ferritin was significantly correlated with viral load (ρ = 0.29) (81). Higher ferritin concentrations were also associated with AIDS–dementia complex (53) and insulin resistance (48). However, 3 studies found no association between ferritin concentrations and indicators of disease severity (50, 51, 57). A study among pregnant women in Zimbabwe not receiving ART, the only study of the 3 to control for potential confounding factors, found that HIV viral loads were 3.7 times higher among those with serum ferritin >24 μg/L than those with serum ferritin <6 μg/L.
Three studies assessed the relation of serum iron and clinical outcomes. While 2 studies found that serum iron was associated with low CD4 T-cell counts (51) and symptomatic disease progression (29), 1 study found that lower serum iron concentrations were correlated with higher CD4 counts (28). The plurality of studies, however, showed no association between serum iron and HIV disease indicators, including CD4 counts (36, 52) and opportunistic infections (91), and none included adjustment for confounders.
Two studies support the notion that transferrin concentrations should be lower during infection and inflammation (29, 91). However, 1 study had contradictory findings that low transferrin was associated with higher CD4 cell counts (51). Two studies in West Africa were conducted on TIBC, and 1 of these studies found that higher TIBC was associated with lower CD4 T-cell counts (51). There was no association with CD4 concentrations in the other study (52). Two studies of transferrin saturation found no difference in CD4 counts (52) or opportunistic infections (91), but 1 of these studies indicated that lower saturation was associated with lower CD4 counts (51). Kupka and colleagues found that viral load was poorly correlated with sTfR and the sTfR–ferritin index (81). These studies of transferrin, TIBC, and transferrin saturation all lacked adjustment for covariates.
Overall, a large number of cross-sectional studies have been conducted to evaluate the relation between indicators of iron status and HIV disease progression. However, these studies are greatly limited by the fact that the majority do not control for confounding, and inherently the temporality between iron status and disease progression cannot be resolved.
Prospective cohort studies of children
We identified 3 prospective cohort studies among children living with HIV (Table 1). In 1 study of a small sample of children (n = 24) in the United States who were followed for 5 y, it was found that serum iron at baseline was not associated mortality (58). Another study of children aged 0–2 y in Tanzania suggested that IDA was associated with twice the risk of mortality, though this finding was not statistically significant (P = 0.07) (59). In a cohort study of Indian HIV-infected children aged 2–12 y who were followed for 1 y (n = 240), routine iron supplementation did not improve inflammation or CD4 parameters, although the prevalence of advanced disease stage declined faster in the iron-supplemented group than in those who did not receive iron (89). In a study of 247 perinatally infected children in Dar es Salaam, Tanzania, during the pre-ART era, maternal severe anemia was found to be associated with a 2.49 times greater risk of child mortality than no anemia, and a 2.47 times greater risk of mortality associated with hypochromic microcytosis compared with normochromic normocytic cells (65). No association was noted between maternal anemia or hypochromic microcytosis and child CD4 count.
TABLE 1.
Prospective studies of anemia, iron status, and HIV-related outcomes among children with HIV infection 1
| Study | Study type | Sample size | Female, % | Mean baseline age | Country | Baseline anemia, % | ART, % | Exposures | Outcomes | Categories | Result estimate (95% CI) | Confounders adjusted |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Campa, 1999 (58) | Cohort | 24 | 58 | 50 mo | USA | Unclear | 83 | Serum iron, μg/100mL | Time to death | Continuous | RR: 1.49 (0.3, 7.4) | Baseline CD4 < 200 (binary) |
| Chatterjee, 2010 (59) | Cohort | 829; 3404 child-mo at risk | Unclear | Unclear | Tanzania | Unclear | 0 | Hemoglobin, g/L | Time to death | Hemoglobin ≥85/<85 g/L | HR: 1.00/1.38 (0.95, 2.01) | Maternal regimen, pregnancy CD4 count, education level, per capita food expenditure/d; child birth wt, age started semisolid food, time-varying CD4 counts, malaria parasitemia |
| Isanaka, 2012 (65) | Cohort | 247 | Unclear | 6 wk | Tanzania | Unclear | 0 | Maternal anemia | Child mortality at complete follow-up | No anemia/moderate/severe | HR: 1.00/1.81 (1.27, 2.57)/2.58 (1.66, 4.01) | Maternal age; baseline BMI, viral load, CD4, WHO HIV stage, malaria; parity, prenatal iron, folic acid supplement regimen and compliance |
| Child mortality, 0–11 mo | No anemia/moderate/severe | HR: 1.00/2.22 (1.20, 4.08)/2.97 (1.50, 5.87) | ||||||||||
| Child mortality, 12–23 mo | No anemia/moderate/severe | HR: 1.00/1.57 (0.84, 2.93)/3.78 (1.60, 8.93) | ||||||||||
| Maternal hypochromic microcytosis | Child mortality during follow-up | Absent/mild/moderate/severe | HR: 1.00/1.58 (1.12, 2.33)/1.45 (0.80, 2.62)/2.36 (1.27, 4.38)/1.90 (0.83, 4.37) | |||||||||
| Child mortality, 0–11 mo | Absent/mild/moderate/severe | HR: 1.00/2.02 (1.26, 3.24)/3.45 (1.50, 7.93)/1.90 (0.83, 4.37) | ||||||||||
| Child mortality, 12–23 mo | Absent/mild/moderate/severe | HR: 1.00/0.93 (0.41, 2.12)/1.05 (0.30, 3.62)/3.02 (0.67, 13.6)/1.46 (0.91, 2.33) | ||||||||||
| Maternal anemia | HIV transmission to child | No anemia/moderate/severe | HR: 1.00/1.43 (1.00, 2.04) | |||||||||
| HIV transmission to child via breastfeeding | No anemia/moderate/severe | HR: 1.00/1.29 (0.84, 1.98)/1.30 (0.66, 2.53) | ||||||||||
| Total HIV transmission or death | No anemia/moderate/severe | HR: 1.00/1.43 (1.05, 1.94)/1.48 (0.99, 2.22) | ||||||||||
| Maternal hypochromic microcytosis | Transmission of HIV to child | Absent/mild/moderate or severe | HR: 1.00/1.17 (0.84, 1.62)/1.07 (0.63, 1.82) | |||||||||
| HIV transmission to child via breastfeeding | Absent/mild/moderate or severe | HR: 1.00/1.03 (0.64, 1.66)/0.84 (0.36, 1.99) | ||||||||||
| Total HIV transmission or death | Absent/mild/moderate or severe | HR: 1.00/1.14 (0.85, 1.52)/1.32 0.73, 2.42) |
Excludes iron supplementation studies. AFB, acid-fast bacteria; ART, antiretroviral therapy.
Prospective studies among adults
Anemia and all-cause mortality
The preponderance of the evidence from prospective studies among adults (Table 2) suggests that anemia is associated with an increased risk of all-cause mortality among PLWHIV (61, 69, 94). Three studies examined the association of hemoglobin as a continuous variable with all-cause mortality, and found a 7–27% lower risk for each additional 10 g/L of hemoglobin. In a study of 155 South African adults newly diagnosed with HIV, each 10 g/L increase in hemoglobin was associated with a 7% lower odds of all-cause mortality (94). In a study in Mozambique, each 10 g/L increase in hemoglobin at baseline was associated with a 27% reduced odds of death or hospitalization during follow-up in unadjusted analysis (61). Among 643 female participants of the ZVITAMBO (Zimbabwe Vitamin A for Mothers and Babies) study in Harare, Zimbabwe, enrolled with moderate anemia within 96 h postpartum, for every 10 g/L increase in hemoglobin concentration, the risk of all-cause mortality was decreased by 20% (69). Further, Brentlinger and colleagues measured hemoglobin at multiple timepoints and found that for every 10 g/L increase in hemoglobin concentration above the lowest measured value (nadir), there was a 57% decrease in the odds of hospitalization or mortality (61).
TABLE 2.
Prospective studies of anemia, iron status and mortality among HIV-infected adults1
| Study | Sample size | Female, % | Mean age | Country | Baseline anemia, % | Antiretroviral use, % | Outcome | Exposures | Categories | Result estimate (95% CI)2 | Confounders adjusted for |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Brentlinger, 2016 (61) | 324 | 0 | NR | Mozambique | 100 moderate anemia | NR | Hospitalization or death | Hemoglobin at baseline, g/L | Continuous | OR: 0.73 (0.53, 1.00) | None |
| Hemoglobin at nadir, g/L | Continuous | OR: 0.43 (0.25, 0.73) | Study site, CD4, cotrimoxazole use, bacteremia, any bleeding | ||||||||
| De Monye, 1999 (62) | 348 | NR | NR | USA | NR | NR | Time from bone marrow aspirate to death | Bone marrow iron stores | Grade 0–2/4–5 | HR: 1.00/2.1 (1.3, 3.5) | Lymphocyte count, blood transfused, hemoglobin, neutrophil count, year of bone aspiration, history of infection with MAI |
| Time from HIV diagnosis to death | Bone marrow iron stores | Grade 0–2/4–5 | HR: 1.00/2.8 (1.5, 4.9) | ||||||||
| Ezeamama, 2018 (63) | 398 | 69 | NR | Uganda | 49 | Baseline: 50Follow-up: 100 | Hospitalizaton or death | Hemoglobin, g/L at baseline | No anemiaAnemia | HR: 1.002.03 (1.18, 3.56) | Age, female sex, wealth, baseline CRP, HAART at enrollment, multivitamin use history, baseline vitamin D, smoking status, baseline BMI, baseline CD4 |
| No anemia/mild/moderate to severe | HR: 1.00/3.9 (2.1, 7.2)/6.7 (3.6, 12.7) | ||||||||||
| Hemoglobin, g/L at baseline and follow-up | Group 1. No anemia | HR: 1.00 | |||||||||
| Group 2. Baseline anemia, resolved | 1.11 (0.4, 2.9) | ||||||||||
| Group 3. Baseline anemia, not resolved | 2.1 (0.8, 5.5) | ||||||||||
| Group 4. Incident moderate/severe or persistent mild | 2.27 (1.01, 5.1) | ||||||||||
| Group 5. Baseline mild or single mild episode | 4.70 (2.0, 10.9) | ||||||||||
| Group 6. Baseline moderate or single moderate/severe | 3.1 (1.4, 6.5) | ||||||||||
| Ferritin, μg/L | Normal3/low/high | HR: 1.00/1.86 (0.85, 4.1)/1.75 (0.92, 3.3) | |||||||||
| Ezeamama, 2019 (11) | 400 | 69 | NR | Uganda | 49 | Baseline: 50Follow-up: 100 | Hospitalizaton or death | Baseline anemia type | No anemia Microcytic anemia | HR: 1.000.73 (0.33, 1.62) | Age, female sex, wealth, baseline CRP, HAART at enrollment, multivitamin use history, alcohol use, smoking status |
| Macrocytic anemia | 1.25 (0.64, 2.46) | ||||||||||
| ACD | 1.10 (0.68, 1.78) | ||||||||||
| Gordeuk, 2006 (64) | 302 | 100 | NR | USA | NR | 85 | All-cause mortality | Log10 Ferritin | Continuous | OR: 1.67 (0.98, 2.86) | Ethnicity, baseline ART use, and smoking status, and all baseline characteristics associated with mortality |
| Log10 sTfR | Continuous | OR: 0.52 (0.12, 2.22) | |||||||||
| Log10 sTfR–ferritin index | Continuous | OR: 0.89 (0.81, 0.99) | |||||||||
| Ferritin, μg/L | Cases/controls | Med (IQR): 392 (161–736)/330 (84–625) | None | ||||||||
| sTfR, mg/L | Cases/controls | Med (IQR): 6.5 (4.8–9.4)/5.5 (4.4–8.3) | |||||||||
| sTfR–ferritin index | Cases/controls | Med (IQR): 2.6 (1.9–3.8)/2.7 (1.8–3.9) | |||||||||
| Haider, 2019 (5) | 40,6574 | 66 | 37 | Tanzania | 85 | 100 | Early all-cause mortality | Baseline hemoglobin, g/L | ≥1205/100 to <120/70 to <100/<70 | RR: 1.00/1.26 (1.09, 1.45)/1.90 (1.65, 1.45)/3.32 (2.86, 3.86) | Sex, age, facility, BMI, CD4 cell count, WHO disease stage, ALT, HAART regimen, iron use, TB history, TB treatment, oral candidiasis, diarrhea, and calendar year of HAART initiation, and season of visit |
| Baseline hemoglobin, g/L and MCV | No anemia or ID | RR: 1.00 | |||||||||
| ID without anemia | 1.23 (0.90, 1.69) | ||||||||||
| Anemia without ID | 1.74 (1.44, 2.10) | ||||||||||
| IDA | 2.16 (1.79, 2.61) | ||||||||||
| Overall all-cause mortality | Hemoglobin during follow-up, g/L | ≥120/100 to <120/70 to <100/<70 | RR: 1.00/1.28 (1.17, 1.40)/2.00 (1.82, 2.19)/3.90 (3.51, 4.32) | ||||||||
| Hemoglobin during follow-up, g/L and MCV | No anemia or ID | RR: 1.00 | |||||||||
| ID without anemia | 1.02 (0.85, 1.24) | ||||||||||
| Anemia without ID | 1.63 (1.46, 1.82) | ||||||||||
| IDA | 1.87 (1.68, 2.09) | ||||||||||
| Iron supplements across anemia categories | All-cause mortality | No iron + no anemia | HR: 1.00 | ||||||||
| + mild anemia | 1.33 (1.17, 1.53) | ||||||||||
| + moderate | 2.02 (1.76, 2.31) | ||||||||||
| + severe | 4.06 (3.46, 4.77) | ||||||||||
| Iron + no anemia | 3.84 (2.43, 6.06) | ||||||||||
| + mild | 4.14 (3.25, 5.28) | ||||||||||
| + moderate | 4.10 (3.43, 4.91) | ||||||||||
| + severe | 6.33 (5.23, 7.65) | ||||||||||
| Isanaka, 2012 (60) | 362 | NR | NR | Tanzania | NR | NR | All-cause mortalityDisease progression or death | Ferritin, μg/LFerritin, μg/L | <30/30–150/>150<30/30–150/>150 | RR: 0.82 (0.33, 2.05)/1.00/2.70 (1.72, 4.24)RR: 0.82 (0.37, 1.84)/1.00/1.34 (0.87, 2.06) | Sex, age, food expenses, number of colonies in AFB culture, Karnofsky score, BMI, history of TB disease, HIV infection status, CD4 T-cell count (cells/μL) and log HIV RNA (copies/mL), trial regimen and CRP (mg/L). |
| Isanaka, 2012 (66) | 417 | NR | NR | Tanzania | NR | NR | All-cause mortality | Hemoglobin, g/L and ferritin, μg/L | No anemia, no ID/ID, no anemia/anemia, no ID/IDA | RR: 1.00/2.78 (1.33, 5.81)/2.53 (1.36, 4.68)/1.99 (1.01, 3.93) | Sex, age, food expense, number of colonies in AFB culture, Karnofsky score, BMI, prior TB, HIV, CD4, log HIV RNA, malaria. Trial regimen |
| Kerkhoff, 2016 (94) | 155 | 77 | 34 | South Africa | NR | 44 | All-cause mortality | Hemoglobin, g/L | Continuous | HR: 0.93 (0.69, 1.25) | Age, CD4+ T-cell count, HIV load, hemoglobin, eGFR, and CRP |
| Hepcidin, per 10 ng/mL | HR: 1.09 (1.05, 1.14) | ||||||||||
| McDermid, 2007 (17) | 1362 | 57 | 33 | Gambia | NR | 0 | All-cause mortality | Transferrin | >1.89/1.26–1.89/<1.26 | HR: 1.00/1.64 (1.19, 2.26)/2.18 (1.51, 3.14) | Sex, age, BMI, plasma α1-antichymotrypsin, HIV type, CD4 count, hemoglobin |
| Ferritin | <12/12–300/>300 to 1000/>1000 | HR: 1.00/1.54 (0.96, 2.48)/1.96 (1.17, 3.29)/2.98 (1.73, 5.12) | |||||||||
| sTfR-ferritin index | >−0.55 | HR: 1.00 | |||||||||
| −1.22 to −0.55 | 1.11 (0.85, 1.46) | ||||||||||
| Min to <-1.22 | 1.60 (1.18, 2.16) | ||||||||||
| TSAT, % | ≤55/>55 | HR: 1.00/1.38 (0.99, 1.90) | |||||||||
| Serum iron | <6.6/6.6–11/>11 | HR: 1.00/1.18 (0.92, 1.51)/1.20 (0.90, 1.59) | |||||||||
| sTfR | >80/>29.9 to 80/10.6–29.9/<10.6 | HR: 1.00/1.07 (0.57, 2.02)/1.19 (0.63, 2.24)/1.15 (0.54, 2.45) | |||||||||
| Hemoglobin, g/L | <90/90–110/>110 | HR: 1.00/1.04 (0.82, 1.32)/0.72 (0.54, 0.97) | |||||||||
| Minchella, 2015 (16) | 196 | 55 | 34.3 y | Gambia | 79 | 0 | All-cause mortality | Hepcidin, μg/L | ≤7.8/>7.8 to <57.6/≥57.6 | HR: 1.00/0.97 (0.56, 1.67)/1.11 (0.59, 2.08) | Age, sex, BMI, CD4, α1 chymotrypsin |
| Ferritin, μg/L | <12/12 to < 2006/200 to <1000/≥1000 | HR: 0.58 (0.28, 1.21)/1.00/1.90 (1.14, 3.18)/2.78 (1.49, 5.17) | |||||||||
| Transferrin, g/L | 2.0–3.6/<2.0 | HR: 1.00/1/03 (0.57, 1.88) | |||||||||
| Transferrin, g/L | ≥1.89/>1.47 to <1.89/≤1.47 | HR: 1.00/0.79 (0.40, 1.58)/2.13 (1.21, 3.75) | |||||||||
| Iron, μmol/L | <20/20–55/>55 | HR: 1.02 (0.68, 1.54)/1.00/0.78 (0.18, 3.27) | |||||||||
| sTfR, nmol/L | <10.6/10.6–29.9/>29.9 | HR: 0.52 (0.20, 1.35)/1.00/0.90 (0.58, 1.38) | |||||||||
| Hemoglobin, g/L | Nonanemic | HR:1.00 | |||||||||
| Anemic | 2.72 (1.29, 5.72) | ||||||||||
| O'Brien, 2005 (68) | 1078 | 100 | NR | Tanzania | 83 | 0 | All cause mortality | Hemoglobin, g/L | ≥110/85 to <110/<85 | HR: 1.00/2.06 (1.52, 2.79)/2.06 (1.52, 2.79) | CD4 count, WHO clinical stage, age, pregnancy, treatment, BMI |
| Erythrocyte morphology | Normal | HR: 1.00 | |||||||||
| Hypochromasia, microcytosis | 2.90 (2.12, 3.96) | ||||||||||
| Hypochromasia, no microcytosis | 2.39 (1.79, 3.19) | ||||||||||
| Other abnormality | 2.17 (0.53, 8.94) | ||||||||||
| Rawat, 2009 (69) | 643 | 100 | NR | Zimbabwe | 100 | 0 | All-cause mortality | Hemoglobin, g/L | Continuous | HR: 0.80 (0.65, 1.00) | Age, marital status, maternal education, AGP, only crude sTfR was presented |
| Log10 ferritin, μg/L | Continuous | HR: 4.10 (1.64, 10.23) | |||||||||
| Log10 sTfR, mg/L | Continuous | HR: 0.46 (0.07, 3.08) | |||||||||
| Sun, 2016 (71) | 32 | 0 | 41 | China | NR | 9 | Death | Ferritin, μg/L | Nonsurvivors/survivors | Mean (SD): 1790 (1879)/871 (702) | None |
| Wisaksana, 2011 (70) | 6117 | 32 | 28 | Indonesia | NR | 0 | All-cause mortality | Hemoglobin, g/L | >1208/105 to <120/≤105 | HR: 1.00/2.2 (0.6, 7.6)/6.5 (2.0, 21.2) | Sex, age, BMI, injection drug use, ART at baseline |
| Ferritin, μg/L | <1509 | HR: 1.00 | |||||||||
| >150 | 1.3 (0.4, 4.6) | ||||||||||
| sTfR, kU/L | >1870 | HR: 1.00 | |||||||||
| <1870 | 1.7 (0.5, 5.3) |
ACD, anemia of chronic disease; AGP, acid glycoprotein; ART, antiretroviral therapy; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; HAART, highly active antiretroviral therapy; ID, iron deficiency; IDA, iron deficiency anemia; MAI, Mycobacterium avium-intracellulare infection; MCV = mean corpuscular volume; NR, not reported; sTfR, soluble transferrin receptor; TB, tuberculosis; TSat, transferrin saturation.
Estimates are HRs or ORs with 95% CIs. One study (64) instead reported estimates that were medians with interquartile range (IQR).
High ferritin was defined as >200 μg/L for men and >150 μg/L for women.
The sample size for analyses involving MCV was 27,569.
The upper limit of the range of normal ferritin was defined as 200 in men aged 18–44 y and women aged ≥45 y and 150 in women aged 18–44 y and 300 in men aged ≥45 y.
Serum ferritin was only available among 95 participants.
Threshold for high ferritin was defined as 400 μg/L for men (70).
Studies that examined the association of anemia at ART initiation with all-cause mortality found that the presence and severity of anemia were associated with a substantially higher risk of mortality (5, 16, 17, 70). Using data and samples from the pre-ART era among 1362 ART-naïve adult participants of the Medical Research Council (MRC) Clinical Cohort in Gambia, individuals with moderate to severe anemia (<90 g/L) had a 28% lower risk of all-cause mortality than nonanemic individuals, while mild anemia was not associated with a substantial increase in the risk of death (17). Among 196 adults from the same cohort who had blood samples collected within 3 mo of diagnosis with HIV, baseline anemia was associated with a 2.72-fold greater risk of mortality (16). Among ART-naïve injection drug users in West Java, Indonesia, mild and moderate anemia were associated with 2.2- and 6.5-fold increased risks of all-cause mortality, respectively (70).
In addition, a recent analysis among 40,657 adults on ART in Tanzania found a similar trend (5); in this study, mild, moderate, and severe anemia at ART initiation were associated with 1.26-, 1.90-, and 3.32-fold increased risks of all-cause mortality, respectively. Although the strength of the association varied among the studies (1.4–6.5), the findings were consistent that anemia increases the risk of mortality, regardless of ART use and degree of adjustment for confounding. The authors also considered the interaction of anemia and iron use on mortality risk. Compared with the risk in nonanemic individuals who did not receive iron, the risks of all-cause mortality were 1.3, 2.0, and 4.1 times greater among individuals with mild, moderate, or severe anemia, respectively. If these individuals received iron, however, the relative risks of mortality were 3.8, 4.1, 4.1, and 6.3 times greater among individuals with no anemia or mild, moderate, or severe anemia, respectively, compared with nonanemic individuals who did not receive iron. Therefore, both iron use and anemia were independently associated with increased mortality risk.
Results of studies reporting estimates of the association of anemia and anemia categories with the risk of all-cause mortality were pooled (5, 17, 63, 68, 70). The pooled hazard ratio of all-cause mortality was 3.50-fold (95% CI: 2.48, 4.94) greater among individuals with anemia than among their nonanemic counterparts (Figure 2). There was no heterogeneity in the estimates (I2 = 0%) and no evidence of publication bias (P value = 0.66).
FIGURE 2.
Pooled estimates of anemia and all-cause mortality from studies conducted among people living with HIV.
Most cohort studies evaluate the exposure as time-invariant as defined at the time of entry into the study. However, exposures such as hemoglobin concentration and anemia status may change over time for any participant in a cohort study. The HR obtained from analyzing time-varying exposures explicitly considers the value of the exposure at the time the outcome occurs (98). This is therefore likely to be a more detailed representation of the true exposure. Among the 40,657 Tanzanian adults on ART, time-varying mild, moderate, and severe anemia were associated with 1.3-, 2.0-, and 3.9-fold increased risks of mortality, respectively (5). The increased risk of mortality as severity of anemia worsened was consistent with the findings for anemia at ART initiation.
Among 398 HIV-infected adults in Uganda who were initiating or on highly active antiretroviral therapy (HAART), anemia type was not associated with a significant risk of hospitalization or mortality. Anemia types considered were microcytic, macrocytic, and ACD. The investigators also examined the influence of change in anemia type on the risk of hospitalization and mortality, and they found that individuals with either microcytic anemia or ACD that was present at baseline, persistent through follow-up, or incident had an ∼40% greater risk of mortality, although the CIs included the null (11). While the prevalence of ACD and microcytic anemia declined considerably during the 18-mo follow-up, by 82% and 71%, respectively, the prevalence of macrocytic anemia increased by >3-fold.
Iron deficiency and all-cause mortality
Studies of ID without anemia have yielded conflicting results. One Tanzanian study in the pre-ART era defined ID using MCV <70 fL and found that ID was associated with a 2.8-fold increased risk of death among 417 HIV-infected adults (66). Another Tanzanian study among individuals receiving HAART reported no association of ID (defined using MCV) with the risk of death (99).
Three studies have defined ID using different ferritin cutoffs, without considering hemoglobin concentrations. Their findings were not consistent. Low serum ferritin (<30 μg/L) was not associated with all-cause mortality among 362 Tanzanian adults with HIV (60) or 398 Ugandan adults with HIV (63), compared with counterparts with normal serum ferritin concentrations (30–150 μg/L). Low serum ferritin concentration (<12 μg/L) was also not associated with all-cause mortality relative to normal ferritin concentration (12–300 μg/L) among 1139 ART-naïve adults in The Gambia (17). In contrast, among 196 ART-naïve adults from the same cohort in The Gambia, recruited within the first 3 mo of diagnosis with HIV, ID (ferritin <12 μg/L) was associated with a 42% lower risk of mortality relative to normal ferritin concentrations (16).
Elevated sTfR is reflective of depleted iron stores, which precede iron-deficient erythropoiesis and IDA (100). Studies evaluating the association of sTfR as a continuous variable observed an inverse association with all-cause mortality risk, suggesting a protective relation for iron depletion [P = 0.048 (64)], or no significant association [P = 0.43 (69)]. Specifically, a log10 increase in sTfR concentration was associated with a 48% decrease in mortality risk among 302 US women, most of whom were on ART (64). Among 643 Zimbabwean women, a log10 increase in sTfR was associated with a 54% decrease in the hazard of all-cause mortality (69). Measurement of sTfR may be influenced by inflammation, and the sTfR-ferritin index is often estimated as an alternative that is less influenced by inflammation. Among 302 US women, a log10 increase in the sTfR-ferritin index was associated with an 11% lower risk of all-cause mortality (64).
Elevated iron stores and all-cause mortality
Although ID with or without anemia may be harmful, increases in iron stores, defined using different biomarkers, appear to lead to increased risk of all-cause mortality. Three studies examining the association of continuous ferritin concentration and mortality suggest that elevated iron status is associated with harm. Among 643 Zimbabwean postpartum women with moderate anemia at baseline, for every log10 increase in serum ferritin concentration, the hazard of death during the following year of follow-up was 4.10-fold higher (69). Among 302 US women, most of whom were on ART, a log10 increase in serum ferritin concentration was associated with a 1.67-fold increase in the odds of death during follow-up (64). Among 32 male Chinese patients with Pneumocystis pneumonia, baseline serum ferritin was substantially lower in those who died. These studies assume that the association of serum ferritin and all-cause mortality is linear, which may not necessarily be correct, and it is plausible that the relation between iron status and mortality is U-shaped, with high risk notable with IDA, as reviewed below, as well as with elevated iron load. Two recent studies also evaluated the association of serum hepcidin concentrations with the risk of all-cause mortality. Among 155 patients with HIV and TB coinfection in South Africa, 44% of whom were on ART at baseline, a 10 μg/L increase in hepcidin was associated with an 8% increase in the hazard of mortality (94). However, there was no significant association of hepcidin tertiles with the risk of mortality after adjusting for age, sex, CD4, and the inflammatory biomarker α1-chymotrypsin among 196 patients enrolled in the pre-ART Gambia MRC Clinical Cohort (16).
A number of studies have also evaluated elevated iron as a dichotomous exposure. Stainable bone marrow iron is the gold standard index of the overall amount of body iron. Among 348 US adults with HIV infection, high bone marrow iron stores, represented by macrophage iron concentrations, were associated with 2.1 times greater risk of mortality (62). Sequestration of iron in bone marrow macrophages could be a reflection of elevated overall iron stores, or redistribution of body iron as part of the infectious process, a phenomenon that underlies ACD. Assessing bone marrow iron, however, is an invasive procedure and is not routinely done in practice.
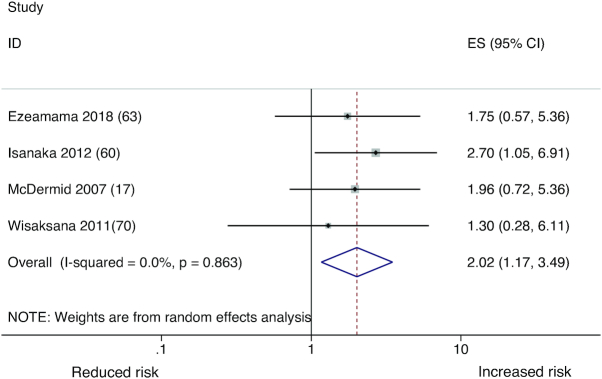
Elevated serum ferritin may reflect elevated overall iron store, or may occur as part of an acute-phase reaction to inflammatory insult or infection. Studies that considered conventional or WHO-recommended thresholds (101) to evaluate the association of elevated serum ferritin and all-cause mortality indicated that ferritin is associated with increased risk of all-cause mortality. High serum ferritin (>200 μg/L in males and >150 μg/L in females) was associated with a 2.7-fold increased risk of all-cause mortality among 362 adults in Tanzania with HIV and TB coinfection (60). Among 196 adults in The Gambia within 3 mo of HIV diagnosis, serum ferritin categories of 200 to <1000 μg/L and ≥1000 μg/L were associated with a 1.9- and 2.8-fold greater risk of all-cause mortality, respectively (16). Among 95 injection drug users in Indonesia, serum ferritin >150 μg/L was associated with a 30% greater risk of all-cause mortality, though the CIs were wide and crossed the null. These studies had incorporated statistical adjustment for confounding by inflammation to different extents, by controlling ≥1 of the following: of C-reactive protein (60), α1-acid glycoprotein (69), and α1-antichymotrypsin (16, 17), or none (70). Taken together, these studies suggest that elevated iron stores may be associated with an increased risk of all-cause mortality. The pooled hazard ratio of all-cause mortality from these studies was 2.02 (95% CI: 1.17, 3.49) (Figure 3). Heterogeneity in the estimates was limited (I2 = 0%) and there was no evidence of publication bias (P value = 0.12).
FIGURE 3.
Pooled estimates of elevated serum ferritin and all-cause mortality from studies conducted among people living with HIV.
Studies evaluating the association of elevated iron stores defined as very low sTfR did not find significant relations, although the direction was similar to that reported in most ferritin studies. These were prospective cohort studies and had been conducted in The Gambia (16, 17), Indonesia (70), and the United States (64). One study (17) also examined the sTfR–ferritin index as a measure of iron stores, accounting for inflammation. Among 1139 ART-naïve adults in The Gambia, individuals with the lowest sTfR–ferritin index, indicative of higher iron stores, had a 60% increased risk of all-cause mortality relative to those with the highest sTfR–ferritin index (17).
Hematologic status and incident TB
Few studies have examined the association of hematologic status with the prospective risk of tuberculosis among patients coinfected with HIV (Table 3). The overall direction of the association is similar to those reported with respect to all-cause mortality: anemia is harmful regardless of anemia type, increasing hemoglobin is protective, but increasing ferritin may be harmful. A nested case-control study among 332 ART-naïve adults participating in a multicountry study found that anemia at ART initiation was associated with a 2.2-fold increased risk of incident TB during 96 wk of follow-up (75). Among 1139 adults in The Gambia, for every additional 1 g/L of hemoglobin, the incidence rate ratio of TB was lower by 12%. Only 10% of the participants in this study were iron deficient at baseline (17).
TABLE 3.
Prospective observational studies of anemia, iron status, and TB-related outcomes among adults with HIV infection1
| Study | Sample size | Female, % | Mean age | Country | Baseline anemia, % | ART use, % | Outcome | Exposures | Exposure categories | Result estimate (95% CI) | Confounders adjusted for |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Isanaka, 2012 (60) | 362 | NR | NR | Tanzania | NR | NR | TB treatment failureTB recurrence | Ferritin, μg/LFerritin, μg/L | <30/30–150/>150<30/30–150/>150 | RR: 1.95 (1.07, 3.52)/1.00/1.54 (1.00, 2.39)RR: 4.21 (1.22, 14.6) 1.00/2.43 (0.93, 6.34) | Sex, age, money spent on food, number of colonies in AFB culture, Karnofsky score, BMI, history of TB disease, HIV infection status, CD4 T-cell count (cells/μL) and log HIV RNA (copies/mL), trial regimen and CRP (mg/L). |
| Isanaka, 2012 (66) | 417 | NR | NR | Tanzania | NR | NR | TB treatment failure | Hemoglobin, g/L and ferritin, μg/L | No anemia, no ID | RR: 1.00 | Sex, age, food expense, number of colonies in AFB culture, Karnofsky score, BMI, previous TB disease, HIV, CD4, log HIV RNA, malaria infection, and trial regimen. |
| ID, no anemia | 3.28 (0.91, 11.9) | ||||||||||
| Anemia, no ID | 2.51 (0.78, 8.05) | ||||||||||
| IDA | 1.78 (0.52, 6.13) | ||||||||||
| TB recurrence | Hemoglobin, g/L and ferritin, μg/L | No anemia, no ID | RR: 1.00 | ||||||||
| ID, no anemia | 2.11 (0.36, 12.5) | ||||||||||
| Anemia, no ID | 6.68 (2.50, 17.8) | ||||||||||
| IDA | 3.81 (1.26, 11.6) | ||||||||||
| McDermid, 2013 (72) | 1139 | 57 | 34.7 | Gambia | NR | 0 | Incident TB | Transferrin, g/L | Continuous | IRR: 0.53 (0.33, 0.84) | Sex, age, CD4, BMI, ethnicity |
| TSat, % | Continuous | IRR: 1.00 (0.99, 1.01) | |||||||||
| Ferritin, μg/L | Continuous | IRR: 1.26 (1.05, 1.51) | |||||||||
| Hemoglobin, g/L | Continuous | IRR: 0.88 (0.79, 0.98) | |||||||||
| Iron, μmol/L | Continuous | IRR: 0.96 (0.92, 1.01) | |||||||||
| sTfR, nmol/L | Continuous | IRR: 1.00 (0.99, 1.01) | |||||||||
| Minchella, 2014 (73) | 196 | 55 | 34.3 y | Gambia | 79 | 0 | Incident TB | Hepcidin | Continuous | IRR: 1.93 (0.67, 5.54) | Age, sex, BMI, CD4, α1 chymotrypsin |
| Mupfumi, 2018 (74) | 300 | 64 | 36 y | Botswana | NR | Baseline: 0 | Incident TB | Hemoglobin, g/L | ≥80 g/L | HR: 1.00 | Age, sex, BMI, CD4 |
| Initiating ART | <80 g/L | 6.88 (1.78, 26.6) | |||||||||
| Tenforde, 2015 (75) | 332 | 47 | 35 y | Nine countries – including Malawi, India, Zimbabwe | NR | 100 | Incident TB, 96 wk follow-up | Hemoglobin, g/L | No anemia | OR: 1.00 | Age, sex, treatment, study site, baseline CD4, viral load |
| Anemia | 2.16 (1.07, 4.35) | ||||||||||
| Ferritin, μg/L | ≤150 | OR: 1.00 | |||||||||
| >150 | 1.72 (0.92, 3.21) | ||||||||||
| Wisaksana, 2013 (76) | 127 | 9 | 29 y | Indonesia | 38 | 67% | Incident TB | Hepcidin, nM | Cases, mean | 9.9 (5.0, 18.2) | Matched controls for age, sex, and CD4 count |
| Controls, mean | 7.2 (2.9, 10.2) | ||||||||||
| Ferritin, μg/L | Cases, mean | 681 (245, 1439) | |||||||||
| Controls, mean | 297 (175, 462) | ||||||||||
| sTfR, mg/L | Cases, mean | 1.4 (1.1, 2.2) | |||||||||
| Controls, mean | 1.4 (1.2, 1.9) | ||||||||||
| Iron, μmol/L | Cases, mean | 7.0 (5,0, 11.0) | |||||||||
| Controls, mean | 10.5 (6.3, 17.5) | ||||||||||
| TIBC, μmol/L | Cases, mean | 39.0 (38.0, 54.0) | |||||||||
| Controls, mean | 51.0 (43.3, 59.8) | ||||||||||
| TSat, % | Cases, mean | 16.0 (12.0, 27.0) | |||||||||
| Controls, mean | 24.0 (14.5, 31.3) | ||||||||||
| MCV, fL | Cases, mean | 83.5 (80.3, 92.5) | |||||||||
| Controls, mean | 85.5 (82.0, 93.8) | ||||||||||
| MCH, pg | Cases, mean | 29.0 (26.3, 32.0) | |||||||||
| Controls, mean | 29.0 (28.0, 32.0) |
AFB, acid-fast bacteria; ART, antiretroviral therapy; CRP, C-reactive protein; ID, iron deficiency; IDA, iron deficiency anemia; IRR, incidence rate ratio; MCH, mean corpuscular hemoglobin; MCV, mean corpuscular volume; NR, not reported; sTfR, soluble transferrin receptor; TB, tuberculosis; TIBC, total iron binding capacity; TSat, transferrin saturation.
As mentioned earlier, anemia is a risk factor for TB incidence, and anemia type is likely an important secondary consideration. Isanaka and colleagues found that anemia without ID (likely ACD) was associated with a 6.7-fold increased risk of TB recurrence compared with absence of anemia and ID in a cohort of 417 ART-naïve adults with HIV infection in Tanzania (66). IDA was also associated with a 3.8-fold increased risk of TB recurrence in the same population (66). Anemia with and without ID were also related to an increased risk of TB treatment failure, although the association was not significant (66). Among 300 HIV-infected adults initiating combination ART (cART) in Botswana, baseline hemoglobin <80 g/L was associated with a 6.9-fold increased risk of incident TB during follow-up.
As with all-cause mortality, elevated iron stores may actually be harmful. Among 362 adults in Tanzania with HIV and TB coinfection, the associations of ferritin concentrations with TB treatment failure and TB recurrence were U-shaped. For instance, the risk of TB treatment failure was increased by 95% and 54% among those with ferritin concentrations of <30 μg/L and >150 μg/L, respectively, compared with those with ferritin concentrations of 30–150 μg/L (60). In a cohort of Indonesian adults with injection drug use, however, the concentration of serum ferritin was considerably greater in those who were later diagnosed with TB than in their counterparts (76). For every 1 μg/L increase in serum ferritin, the incidence of TB among 1139 participants in the Gambian MRC Clinical Cohort increased by 26% (72). Among 332 ART-naïve adults who participated in an international multisite study, there was a 72% increased odds of TB infection if serum ferritin concentration was >150 μg/L compared with <150 μg/L during the course of a 96-wk follow-up period (75).
Two studies evaluated the relation of hepcidin concentrations with the incidence of TB, and reported positive associations, unlike with all-cause mortality. Among 196 patients in the MRC Clinical Cohort, Minchella and colleagues reported a 96% increase in the incidence of TB infection during follow-up for every 1 μg/L increase in hepcidin concentration, although the association was not significant (73). In a matched case-control study nested within a prospective cohort among injected drug users in Indonesia, the hepcidin concentration was significantly greater in HIV-infected patients with incident TB infection than in HIV-infected patients without incident TB infection. Participants were matched for age, gender, and CD4 (76).
Studies also examined the association of other hematologic biomarkers with the incidence of TB, with inconsistent or negative findings. For instance, the concentration of sTfR was not related to the incidence rate of TB among 1139 patients in an 11-year cohort in The Gambia (72). The concentration of sTfR also did not significantly differ among patients with incident TB compared with those without incident TB in a matched case-control study among injection drug users in Indonesia (76). Studies that evaluated the association of serum iron with the incidence of TB also did not find a significant relation (72, 76).
Hematologic status and disease progression (clinical, immunologic, and viral)
Clinical progression
Very few studies have examined the association of clinical disease progression, including the occurence of AIDS-defining illness or AIDS-related mortality, with hematologic status (Table 4). The findings with respect to anemia are consistent with those reported with respect to TB and all-cause mortality. Both anemia without ID and ID with or without anemia were associated with increased risk of disease progression among ART-naïve Tanzanian patients (66). Individuals with severe anemia (hemoglobin <85 g/L) had a 3.5-fold increased risk of AIDS-related mortality compared with those who were nonanemic (66). Individuals with moderate anemia had a 2.2-fold increased risk of AIDS-related mortality (66). Hypochromasia with microcytosis, suggestive of iron deficient erythropoiesis, was also associated with a 2.9-fold increased risk of AIDS-related mortality compared with normal erythrocyte morphology in the same population (68). A study among 799 US adults in the HAART era also examined the association of anemia and the risk of HIV-associated neurocognitive disorder (HAND), and found a 55% increased risk of neurocognitive disorder among those with anemia, compared with nonanemic counterparts (80). Although 70% of patients in the US cohort were on HAART, no information on adherence to HAART was provided.
TABLE 4.
Prospective observational studies of anemia, iron status and HIV disease progression among adults1
| Study | Sample size | Female % | Mean age | Country | Baseline anemia, % | ART use, % | Outcome | Exposures | Categories | Result estimate (95% CI) | Confounders adjusted for |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abrams, 1993 (77) | 296 | 0 | 35 y | USA | NR | 0 | Time to development of AIDS | Dietary iron intake (FFQ) | Continuous, for every 100% increase | HR: 0.78 (0.65, 0.95) | Age, smoking, energy intake, CD4 count, symptoms |
| Armitage, 2014 (78) | 19 | NR | NR | China and UK | NR | 0 | Set-point viral load | Hepcidin, μg/L | Continuous | Correlation: 0.49 | None |
| Boom, 2007 (79) | 138 | 40 | NR | Thailand | NR | 100 | Time to achieving CD4 > 350 | Ferritin, μg/L | >200/30–200/<30 | RR: 1.00/0.93 (0.63, 1.37)/1.81 (1.05, 3.14) | None |
| Hemoglobin, g/L | ≥110/<100 | RR: 100/0.66 (0.43, 1.01) | |||||||||
| Ezeamama, 2018 (63) | 398 | 69 | NR | Uganda | 49 | Baseline: 50 | Absolute CD4 count | Hemoglobin, g/L at baseline | 0 mo: | Age, female sex, wealth, baseline CRP, HAART at enrollment, multivitamin use history, alcohol use, smoking status, baseline BMI | |
| Follow-up: 100 | No anemia | Mean: 148 ± 7 | |||||||||
| Mild | Diff: 15 (−9, 38) | ||||||||||
| Moderate/severe | Diff: −14 (−36, 9) | ||||||||||
| 6 mo: | |||||||||||
| Mean: 227 ± 8 | |||||||||||
| Diff: 20 (−8, 39) | |||||||||||
| Diff: −9 (−37, 9) | |||||||||||
| 12 mo: | |||||||||||
| Mean: 261 ± 9 | |||||||||||
| Diff: −1 (−31, 30) | |||||||||||
| Diff: −7 (−41, 28) | |||||||||||
| 18 mo: | |||||||||||
| Mean: 293 ± 11 | |||||||||||
| Diff: −8 (−44, 27) | |||||||||||
| Diff: 9 (−34, 52) | |||||||||||
| Ferritin, μg/L | Normal2 | 0 mo: | |||||||||
| Low | Mean: 153 ± 7 | ||||||||||
| High | Diff: −7 (−29, 14) | ||||||||||
| Diff: −7 (−35, 21) | |||||||||||
| 6 mo: | |||||||||||
| Mean: 231 ± 8 | |||||||||||
| Diff: 0.3 (−16, 17) | |||||||||||
| Diff: −7 (−30, 17) | |||||||||||
| 12 mo: | |||||||||||
| Mean: 251 ± 9 | |||||||||||
| Diff: 15 (−5, 35) | |||||||||||
| Diff: −8 (−19, 35) | |||||||||||
| 18 mo: | |||||||||||
| Mean: 287 ± 11 | |||||||||||
| Diff: 16 (−13, 46) | |||||||||||
| Diff: −4 (−37, 30) | |||||||||||
| Hemoglobin, g/L at baseline and follow-up | 18 mo: | ||||||||||
| Group 1. No anemia | Mean: 309 ± 12 | ||||||||||
| Group 2. Baseline anemia, resolved | Diff: 27 (−17, 72) | ||||||||||
| Group 3. Baseline anemia, not resolved | Diff: −35 (−80, 21) | ||||||||||
| Group 4. Incident moderate/severe or persistent mild | Diff: −46 (−91, −1) | ||||||||||
| Group 5. Baseline mild or single mild episode | Diff: −49 (−119, 21) | ||||||||||
| Group 6. Baseline moderate or single moderate/severe | Diff: −73 (−126, −20) | ||||||||||
| Ezeamama, 2019 (11) | 400 | 69 | NR | Uganda | 49 | Baseline: 50Follow-up: 100 | Absolute CD4 count | Baseline anemia type | No anemia | 0 mo:Ref. | Age, female sex, wealth, baseline CRP, HAART at enrollment, multivitamin use history, alcohol use, smoking status |
| Microcytic anemia | Diff: −7 (−38, 25) | ||||||||||
| Macrocytic anemia | Diff: 41 (9, 73) | ||||||||||
| ACD | Diff: 4 (−19, 26) | ||||||||||
| 6 mo: | |||||||||||
| No anemia | Ref. | ||||||||||
| Microcytic anemia | Diff: −6 (−47, 35) | ||||||||||
| Macrocytic anemia | Diff: −24 (−56, 8) | ||||||||||
| ACD | Diff: 10 (−19, 40) | ||||||||||
| 12 mo: | |||||||||||
| No anemia | Ref. | ||||||||||
| Microcytic anemia | Diff: 16 (−32, 63) | ||||||||||
| Macrocytic anemia | Diff: −4 (−40, 32) | ||||||||||
| ACD | Diff: −0.3 (−26, 25) | ||||||||||
| 18 mo: | |||||||||||
| No anemia | Ref. | ||||||||||
| Microcytic anemia | Diff: −14 (−54, 25) | ||||||||||
| Macrocytic anemia | Diff: −5 (−50, 40) | ||||||||||
| ACD | Diff: −7 (−36, 21) | ||||||||||
| Absolute CD4 count | Current anemia type | 0 mo: | Age, female sex, wealth, baseline CRP, HAART at enrollment, multivitamin use | ||||||||
| No anemia | Ref. | ||||||||||
| Microcytic anemia | Diff: −5 (−33, 21) | ||||||||||
| Macrocytic anemia | Diff: 53 (26, 80) | ||||||||||
| ACD | Diff: 1.2 (−18, 20) | ||||||||||
| 6 mo: | History, alcohol use, smoking status | ||||||||||
| No anemia | Ref. | ||||||||||
| Microcytic anemia | Diff: −12 (−55, 30) | ||||||||||
| Macrocytic anemia | Diff: 13 (−6, 31) | ||||||||||
| ACD | Diff: 11 (19, 41) | ||||||||||
| 12 mo: | |||||||||||
| No anemia | Ref. | ||||||||||
| Microcytic anemia | Diff: −4 (−62, 54) | ||||||||||
| Macrocytic anemia | Diff: 13 (−9, 34) | ||||||||||
| ACD | Diff: −9 (−33, 15) | ||||||||||
| 18 mo: | |||||||||||
| No anemia | Ref. | ||||||||||
| Microcytic anemia | Diff: 58 (−4119) | ||||||||||
| Macrocytic anemia | Diff: 13 (−9, 34) | ||||||||||
| ACD | Diff: −20 (−49, 9) | ||||||||||
| Time to postenrollment gain of > 100 cells/L | Baseline anemia type | No anemia | HR: 1.00 | ||||||||
| Microcytic anemia | 1.16 (0.77, 1.76) | ||||||||||
| Macrocytic anemia | 0.57 (0.36, 0.89) | ||||||||||
| ACD | 1.00 (0.76, 1.32) | ||||||||||
| Change in anemia type | No anemia | HR: 1.00 | |||||||||
| 1. Persistent ACD | 0.62 (0.40, 0.95) | ||||||||||
| 2. Baseline ACD, resolved | 0.92 (0.60, 1.41) | ||||||||||
| 3. Incident ACD/microcytic anemia | 0.43 (0.25, 0.73) | ||||||||||
| 4. Persistent macrocytosis | 0.52 (0.25, 1.08) | ||||||||||
| 5. Incident macrocytosis | 0.77 (0.52, 1.14) | ||||||||||
| Isanaka, 2012 (60) | 362 | NR | NR | Tanzania | NR | NR | All-cause mortality | Ferritin, μg/L | RR: | Sex, age, money spent on food, number of colonies in AFB culture, Karnofsky score, BMI, history of TB disease, HIV infection status, CD4 T cell count (cells/μL) and log HIV RNA (copies/mL), trial regimen and C-reactive protein (mg/L) | |
| <30 | 0.82 (0.33, 2.05) | ||||||||||
| 30–150 | 1.00 | ||||||||||
| >150 | 2.70 (1.72, 4.24) | ||||||||||
| TB treatment failure | Ferritin, μg/L | RR: | |||||||||
| <30 | 1.95 (1.07, 3.52) | ||||||||||
| 30–150 | 1.00 | ||||||||||
| >150 | 1.54 (1.00, 2.39) | ||||||||||
| TB recurrence | Ferritin, μg/L | RR: | |||||||||
| <30 | 4.21 (1.22, 14.6) | ||||||||||
| 30–150 | 1.00 | ||||||||||
| >150 | 2.43 (0.93, 6.34) | ||||||||||
| Disease progression | Ferritin, μg/L | RR: | |||||||||
| <30 | 1.70 (0.71, 4.08) | ||||||||||
| 30–150 | 1.00 | ||||||||||
| >150 | 1.25 (0.73, 2.14) | ||||||||||
| Disease progression or death | Ferritin, μg/L | RR: | |||||||||
| <30 | 0.82 (0.37, 1.84) | ||||||||||
| 30–150 | 1.00 | ||||||||||
| >150 | 1.34 (0.87, 2.06) | ||||||||||
| Isanaka, 2012 (66) | 417 | NR | NR | Tanzania | NR | NR | Disease progression3 | Hemoglobin, g/L and Ferritin, μg/L | No anemia, no ID | RR: 1.00 | Sex, age, food expense, number of colonies in AFB culture, Karnofsky score, BMI, previous TB disease, HIV, CD4, log HIV RNA, malaria infection, and trial regimen |
| ID, no anemia | 3.72 (1.04, 13.8) | ||||||||||
| Anemia, no ID | 6.19 (1.03, 37.1) | ||||||||||
| IDA | 3.81 (0.64, 22.6) | ||||||||||
| Kallianpur, 2016 (80) | 799 | NR | NR | USA | NR | 70 | Neurocognitive impairment | Hemoglobin, g/L | Anemia | HR: 1.55 (1.13, 2.12) | Age, sex, race/ethnicity, HAART, nadir CD4+ T-cell count, ZDV use, MCV at baseline, premorbid IQ, and comorbid conditions |
| Kupka, 2007 (81) | 584 | 100 | 26 | Tanzania | 48 | NR | AIDS-related mortality | Ferritin, μg/L | <12 | RR: 1.00 | Maternal age, trial treatment group, anemia, CRP, CD4 count, viral load, BMI, and MUAC |
| 12.0–29.9 | 0.90 (0.53, 1.50) | ||||||||||
| 30.0–89.9 | 0.69 (0.41, 1.13) | ||||||||||
| 90.0–150 | 0.22 (0.65, 2.28 | ||||||||||
| >150 | 0.87 (0.47, 1.61) | ||||||||||
| Progression to stage 4 | Ferritin, μg/L | <12 | RR: 1.00 | ||||||||
| 12.0–29.9 | 1.32 (0.58, 3.00) | ||||||||||
| 30.0–89.9 | 1.28 (0.59, 2.77) | ||||||||||
| 90.0–150 | 1.04 (0.31, 3.45) | ||||||||||
| >150 | 1.78 (0.68, 4.64) | ||||||||||
| AIDS-related mortality or progression to stage 4 | Ferritin, μg/L | <12 | RR: 1.00 | ||||||||
| 12.0–29.9 | 0.99 (0.61, 1.59) | ||||||||||
| 30.0–89.9 | 0.91 (0.58, 1.42) | ||||||||||
| 90.0–150 | 1.20 (0.66, 2.15) | ||||||||||
| >150 | 1.29 (0.75, 2.21) | ||||||||||
| O'Brien, 2005 (68) | 1078 | 100 | NR | Tanzania | 83 | 0 | AIDS-related mortality | Hemoglobin, g/L | ≥110 | HR: 1.00 | CD4 count, WHO clinical stage, age, pregnancy, treatment arm, BMI |
| 85 to <100 | 2.21 (1.53, 3.19) | ||||||||||
| <85 | 3.47 (2.25, 5.33) | ||||||||||
| Erythrocyte morphology | Normal | HR: 1.00 | |||||||||
| Hypochromasia, microcytosis | 2.94 (2.01, 4.29) | ||||||||||
| Hypochromasia, no microcytosis | 2.85 (2.02, 4.03) | ||||||||||
| Other abnormality | 1.45 (0.20, 10.7) | ||||||||||
| Phiri, 2006 (82) | 135 | 100 | 25.9 | Zambia | NR | 0 | Plasma viral load at week 6 | Hemoglobin on day 7, g/L | Continuous | 0.20 (−0.9, 0.14) | None |
| Quiros-Roldan, 2017 (83) | 18 | 0 | 42.8 | Brescia, Italy | 100 | Baseline: 0 | Hemoglobin, g/L | cART | All patients | None | |
| Initiating ART: 100 | Med (min, max): | ||||||||||
| Baseline | 124 (95, 129) | ||||||||||
| 12 mo | 133 (108, 157) | ||||||||||
| Baseline, anemia, resolved (n = 10) | |||||||||||
| Med (min, max): | |||||||||||
| Baseline | 127 (109, 129) | ||||||||||
| 12 mo | 143 (132, 157) | ||||||||||
| No anemia (Nn = 8) | |||||||||||
| Med (min, max): | |||||||||||
| Baseline | 119 (95, 128) | ||||||||||
| 12 mo | 120 (108, 129) | ||||||||||
| Ferritin, μg/L | cART | All patients | None | ||||||||
| Med (min, max): | |||||||||||
| Baseline | 171 (9, 415) | ||||||||||
| 12 mo | 90 (6, 180) | ||||||||||
| Baseline, anemia, resolved (n = 10) | |||||||||||
| Med (min, max): | |||||||||||
| Baseline | 193 (9, 415) | ||||||||||
| 12 mo | 80 (6, 220) | ||||||||||
| No anemia (n = 8) | |||||||||||
| Med (min, max): | |||||||||||
| Baseline | 149 (16, 303) | ||||||||||
| 12 mo | 10 (58, 280) | ||||||||||
| Hepcidin, μg/L | cART | All patients | None | ||||||||
| Med (min, max): | |||||||||||
| Baseline | 2.1 (05, 22.9) | ||||||||||
| 12 mo | 2.67 (0.3, 11.4) | ||||||||||
| Baseline, anemia, resolved (n = 10) | |||||||||||
| Med (min, max): | |||||||||||
| Baseline | 4.4 (0.5, 22.9) | ||||||||||
| 12 mo | 2.7 (0.3, 11.4) | ||||||||||
| No anemia (n = 8) | |||||||||||
| Med (min, max): | |||||||||||
| Baseline | 1.8 (0.5, 3.9) | ||||||||||
| 12 mo | 2.5 (0.7, 6.9) | ||||||||||
| Iron, μg/dl | cART | All patients | None | ||||||||
| Med (min, max): | |||||||||||
| Baseline | 73 (32, 112) | ||||||||||
| 12 mo | 71 (24, 160) | ||||||||||
| Baseline, anemia, resolved (n = 10) | |||||||||||
| Med (min, max): | |||||||||||
| Baseline | 54 (32, 91) | ||||||||||
| 12 mo | 71 (24, 160) | ||||||||||
| No anemia (n = 8) | |||||||||||
| Med (min, max): | |||||||||||
| Baseline | 86 (42, 112) | ||||||||||
| 12 mo | 71 (42, 156) | ||||||||||
| Log10 viral load | cART | All patients | None | ||||||||
| Med (min, max): | |||||||||||
| Baseline | 4.37 (1.40, 5.94) | ||||||||||
| 12 mo | <1.57 | ||||||||||
| Baseline, anemia, resolved (n = 10) | |||||||||||
| Med (min, max): | |||||||||||
| Baseline | 4.54 (3.62, 5.13) | ||||||||||
| 12 mo | <1.57 | ||||||||||
| No anemia (n = 8) | |||||||||||
| Med (min, max): | |||||||||||
| Baseline | 4.09 (1.40, 5.94) | ||||||||||
| 12 mo | <1.57 | ||||||||||
| CD4, cells/μL | cART | All patients | None | ||||||||
| Med (min, max): | |||||||||||
| Baseline | 410 (10, 831) | ||||||||||
| 12 mo | 566 (252, 1026) | ||||||||||
| Baseline, anemia, resolved (n = 10) | |||||||||||
| Med (min, max): | |||||||||||
| Baseline | 410 (10, 831) | ||||||||||
| 12 mo | 673 (334, 1026) | ||||||||||
| No anemia (n = 8) | |||||||||||
| Med (min, max): | |||||||||||
| Baseline | 430 (106, 697) | ||||||||||
| 12 mo | 551 (252, 733) | ||||||||||
| Sarcletti, 2003 (84) | 35 | 0 | 39 y | Austria | NR | 100 | CD4 count | Increase in hemoglobin during the first 6 mo | Continuous | OR: 4.17 (0.84, 20.6) | None |
| Shivakotti, 2018 (85) | 270 | 50 | 35 y | 8 LMICs and USA | 51 | Baseline: 0Initiating ART: 100 | CD4 count | Ferritin, μg/L | Iron deficient4 | Mean difference: −12.4 (−46.0, 11.2) | Time-varying viral load, baseline CD4, age, sex, BMI, country, treatment regimen, anemia, hypoalbuminemia |
ACD, anemia of chronic disease; AFB, acid-fast bacteria; ART, antiretroviral therapy; cART, combination antiretroviral therapy; CRP, C-reactive protein; ID, iron deficiency; IDA, iron deficiency anemia; LMIC, low and middle income countries; max, maximum; min, minimum; MUAC, mid–upper arm circumference; NR, not reported; ZDV, zidovudine.
High ferritin was defined as >200 μg/L for men and >150 μg/L for women.
Among patients classified as stage 3 at baseline.
ID was defined based on either sTfR >8.3 mg/L, ferritin <12 μg/L if CRP ≤5 mg/L or ferritin <30 μg/L if CRP >5 mg/L.
Two studies in Tanzania evaluated associations with ferritin concentrations but did not report any significant associations. For instance, compared with normal ferritin concentrations, neither high nor low ferritin concentration was associated with advanced disease among 362 ART-naïve Tanzanian patients with TB and HIV (60). Among 584 Tanzanian women, Kupka and colleagues did not find any significant association of serum ferritin across multiple categories with the risk of progression to stage 4 AIDS, AIDS-related mortality, or both (81). Of note, there was a 78% increased risk of progression to stage 4 disease but the CIs were wide. Both studies used the higher ferritin cutoff approach to define ID (20). This approach entails the use of a ferritin concentration of <30 μg/L rather than <15 μg/L. This approach does not correct for the influence of inflammation on serum ferritin concentration at higher ferritin concentrations.
Among 296 ART-naïve homosexual men in San Francisco, a 100% higher dietary iron intake (measured by FFQ) was associated with a 22% reduced risk of progression to AIDS (77). Nutrient intake had been assessed before HIV diagnosis.
CD4 count and viral load
Findings from studies evaluating the relation of hematologic status and CD4 count may partly explain the results obtained from assessments with respect all-cause mortality and clinical disease progresion. In the context of initiation of ART based on CD4 <350 cells/μL, Boom and colleagues evaluated whether hematologic status was prospectively related to the immunologic response among 138 ART-naïve participants in Thailand who were randomly assigned to either CD4-guided ART or continuous ART. CD4-guided patients discontinued ART if CD4 was > 350 after 6 mo. Participants who were anemic had a 34% lower probability of achieving the CD4 target compared with nonanemic individuals (79). However, the probability of achieving the CD4 target was 81% greater in individuals with low ferritin concentrations (<30 μg/L) than in those with high ferritin concentrations (>200 μg/L) (79), suggesting that elevated iron stores may lead to poor immunologic response. In Austria, 35 men who were ART naïve or who had commenced ART <1 year prevviously were enrolled in a prospective cohort study and followed for 6 mo. The study evaluated the influence of increased hemoglobin concentrations during the first 6 mo on improvements in CD4 counts and found a 4.17-fold increased odds of CD4 <53 cells/μL among those with compared with those without an increased hemoglobin concentration (84). The association was, however, not significant, likely due to the small sample size.
The concentrations of hematologic, immunologic, and viral biomarkers were measured in anemic compared with nonanemic subgroups at the initiation of ART and 12 mo later among 18 patients initiating cART in Italy (83). CD4 increased and viral load decreased considerably overall, as well as in both subgroups. Hemoglobin concentrations improved among those who were anemic at baseline, but not among nonanemic counterparts. While ferritin concentration worsened similarly in both groups, the changes in serum iron, hepcidin, and transferrin did not significantly differ by group. In a larger study among 398 patients initiating or continuing HAART in Uganda, CD4 counts increased considerably over 18 mo of follow-up, and the improvement did not differ by baseline ferritin or anemia status (63). The investigators, however, examined the influence of change in anemia status during follow-up on CD4 counts, and found that CD4 counts substantially decreased over time among patients with persistent mild anemia, as well as those with moderate/severe anemia at baseline or during follow-up. The influence of anemia type on the time to postenrollment gain of ≥100 cells/μL was also examined, as an index of immune recovery (11). Individuals with anemia of different types (macrocytic, microcytic, or ACD) that were present at baseline and did not resolve were 38–57% less likely to gain ≥100 cells/μL after 18 mo of follow-up, suggesting slow immune recovery. In a random subcohort of a multicountry study, the change in CD4 counts was also examined among individuals with ID and HIV infection compared with iron-replete counterparts, and no significant change was found after 96 wk on cART.
Few other prospective studies have evaluated the association of hematologic biomarkers with viral load among HIV-infected patients, and those that did were of short duration. No significant association of hemoglobin concentration with viral load was noted in a cohort study among 135 women in their immediate postpartum period (82). In another study using samples from China and the UK, hepcidin concentrations within the first 60 d of HIV infection were positively correlated with a mean of multiple viral load measurements from 3–12 mo among 19 newly HIV-infected adults (ρ = 0.49) (78). Hepcidin concentration increased considerably following HIV infection and remained high during follow-up, although viral load had become undetectable.
A small cohort study (n = 59) in Zambia examined the influence of interventional postpartum iron supplementation on milk and plasma viral load. Iron supplements were recommended to women with hemoglobin <90 g/L, per standard of care. Among pregnant women with hemoglobin <100 g/L at childbirth, milk and plasma viral load at 6 wk did not differ significantly whether they took iron supplements in the first 2 wk postpartum or not. The hemoglobin concentration in those who received iron was significantly lower compared with the concentration in their counterparts (P < 0.001).
Prospective studies among women during pregnancy
We identified 1 prospective study conducted among pregnant women (68). It was a secondary analysis of a multivitamin trial among 1078 ART-naïve pregnant women in Tanzania. The prevalence of anemia at baseline among these women was 83%. The study showed that moderate and severe anemia were associated with a 2.1-fold and 3.2-fold increased risk, respectively, of all-cause mortality during follow-up. Similarly, there was a significant dose-related association of anemia level with the risk of AIDS-related mortality. Hypochromasia with microcytosis, suggestive of ID, was associated with a 2.9-fold increased risk of all-cause mortality compared with normal erythrocyte morphology. The findings with respect to mortality were consistent with those in nonpregnant adults.
Iron supplementation studies
Three iron supplementation trials have been conducted among HIV-infected individuals (Table 5). One of these was in a pediatric population. The pediatric trial was conducted in Malawi and enrolled 209 HIV-infected children aged 6–59 mo presenting with moderate anemia (hemogobin 70–90 g/L). The subjects were randomly assigned to receive 3 mo of iron (3 mg/kg daily) plus multivitamins (vitamins A, C, and D) or multivitamins alone and followed for 6 mo (n = 196). Iron-supplemented children had increased hemoglobin (mean difference: 6 g/L; 95% CI: 0.6, 11.3), and reduced risk of persistent anemia (prevalence ratio: 0.6; 95% CI: 0.4, 0.9) for 6 mo of follow-up. At 3 mo follow-up, the risk of ID was lower by 72% (95% CI: 51, 85%) and the CD4 % was greater by 6% (95% CI: 1.8, 10.2), but these outcomes were not significant at 6 mo. The incidence of malaria was also greater in the iron group at 3 mo (incidence rate ratio: 2.7; 95% CI: 1.1, 6.6) but not at 6 mo (86). The dose of iron supplements (3 mg/kg) exceeded the current recommendation of 1–2 mg/kg, and only 36% of subjects received antiretroviral therapy. Iron supplementation effectively improved hematologic and immunologic status in this trial, but malaria incidence was more common.
TABLE 5.
Intervention studies of iron supplementation among children and adults with HIV infection1
| Study | Study design | Sample size | Female, % | Age | Country | Baseline anemia, % | ART use, % | Exposures | Outcome | Categories | Results (estimate, 95% CI) | Confounders adjusted for |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Esan, 2013 (86) | RCT | 209 | 53 | Range: 6–59 mo | Malawi | 100 | 36 | Iron supplement Trt: 3 mg/kg/d | Change in hemoglobin g/L | Mean diff: | ||
| 3 mo/6 mo | 0.62 (0.20, 1.04)/0.60 (0.06, 1.13) | |||||||||||
| Ctrl: placebo | Anemia, % | Baseline/3 mo/6 mo | PR: 1.00/0.71 (0.51, 0.97)/0.59 (0.38, 0.92) | |||||||||
| Change in CD4 % | 3 mo/6 mo | Mean diff: 6.00 (1.84, 10.2)/0.80 (−2.90, 4.50) | ||||||||||
| ID, % | Baseline/3 mo/6 mo | PR: 1.00/0.28 (0.15, 0.49)/0.85 (0.49, 1.50) | ||||||||||
| Olsen, 2004 (87) | RCT | 45 | 73 | 27 y | Kenya | NR | 0 | Trt: parenteral iron (iron dextran); control: placebo | Log10 viral load at 4-mo follow-up, geq/mL | Continuous | Mean diff: −0.04, 95% CI: −0.62, 0.55 | Baseline log viral load, hookworm infection, baseline variables |
| Phiri, 2006 (82) | Cohort | 59 | 100 | 25.9 y | Zambia | 0 | 0 | Trt: iron supplements in 1st 2 wk postpartum; control: no iron | Plasma viral load at 6 wk | Continuous | 0.09 (−0.42, 0.59) | None |
| Semba, 2007 (90) | RCT | 138 | 100 | 40 y | USA | 32 | Unclear | Trt: Micronutrients with iron | Log10 HIV RNA, copies/mL | Enrollment | Trt: 3.8 (1.2), Ctrl: 3.8 (1.0), P = 0.74 | Baseline characteristics similar among treated and untreated |
| 6 mo | Trt: 3.8 (1.1), Ctrl: 3.7 (1.0), P = 0.75 | |||||||||||
| 12 mo | Trt: 3.7 (1.2), Ctrl: 4.1 (1.0), P = 0.19 | |||||||||||
| Ctrl: micronutrients, no iron | CD4 count, cells/μL | Enrollment | Trt: 313 (204), Ctrl: 264 (155), P = 0.18 | |||||||||
| 6 mo | Trt: 388 (227), Ctrl: 260 (151), P = 0.01 | |||||||||||
| 12 mo | Trt: 313 (181), Ctrl: 278 (244), P = 0.57 | |||||||||||
| Shet, 2015 (89) | Cohort | 240 | 45 | 7.7 y | India | 47 | 43 | Trt | Hemoglobin, g/L | Baseline | Iron: 104 vs. no iron: 120, P = 0.003 | |
| Iron supplementation 3 mg/kg/day | 3 mo | Iron: 109 vs. no iron: 120, P = 0.04 | ||||||||||
| Ctrl: no iron | 1 y | Iron: 113 vs. no iron: 119, P = 0.2 | ||||||||||
| CD4% | Baseline | Iron: 21 vs. no iron: 20.5, P = 0.6 | ||||||||||
| 1 y | Iron: 26.5 vs. no iron: 26, P = 0.7 | |||||||||||
| WHO stage 3–4 | Baseline | Iron: 25.7 vs. no iron: 16.7, P = 0.04 | ||||||||||
| 1 y | Iron: 10.9 vs. no iron: 12.5, P = 1.0 | |||||||||||
| Prevalence of ID | Baseline | Iron: 68.1 vs. no iron: 62.7, P = 0.4 | ||||||||||
| 1 y | Iron: 68.1 vs. no iron: 58, P = 1.0 |
ART, antiretroviral therapy; Ctrl, control; diff, difference; ID, iron deficiency; NR, not reported; PR, prevalence ratio; Trt, treatment.
We did not find any RCTs providing oral iron supplements to adults at a dose of 60 mg elemental iron daily. One US study examined low-dose iron supplements (18 mg daily) as part of a double-blind RCT of micronutrients with iron or micronutrients alone (90). Adult female injection drug users (n = 458) infected with hepatitis C virus were randomly assigned to either treatment arm and supplements were provided for 12 mo. Of these subjects, 138 were HIV positive as well. Exclusion criteria included pregnancy and menopause. Participants with serum ferritin ≥200 μg/L were also excluded. CD4 was significantly higher in the iron group at the 6-mo follow-up (388 compared with 260 cells/μL, P = 0.01). Hematologic status findings were not reported in the HIV-positive subgroup alone. Log10 viral load (copies/mL) did not substantially differ in both groups at 6 and 12 mo of follow-up. Iron supplementation therefore improved immunologic response in this study, without exacerbating viral replication.
One study in western Kenya provided iron parenterally (87). Male and female adults (n = 181) were randomly assigned to receive 60 mg iron as ferrous dextran twice weekly. Of these, 45 were HIV-positive and ART-naïve, and follow-up was only complete for 32 participants—16 in each of the iron and control groups. The log10 viral load was greater in the iron group at baseline (5.6 compared with 4.9 geq/mL, P = 0.02) as well as at the 4-mo follow-up (4.7 compared with 4.2 geq/mL, P < 0.01). Adjusting for baseline log viral load, hookworm infection, and baseline variables did not yield any significant differences in viral load during follow-up (difference: −0.04 geq/mL; 95% CI: −0.62, 0.55).
Taken together, these were small studies with short duration of follow-up or considerable loss to follow-up that were conducted in the era before access to ART became optimal and do not note evidence of harm from providing iron, but also provide no conclusive evidence of the safety or efficacy of iron supplementation in the context of HIV infection.
Discussion
This review of 68 epidemiologic studies with varying contexts and study designs was performed to examine the influence of hematologic status on the risk of important clinical outcomes among children and adults with HIV. Anemia at ART initiation and during follow-up was associated with a substantially increased risk of mortality, TB, and disease progression. These findings were consistent regardless of the severity or etiology of anemia. Even mild anemia was associated with a ≥26% greater risk of all-cause mortality. Both ID and elevated iron status were associated with increased risk of adverse outcomes, though iron status measures were not often adjusted for inflammation. Only 3 iron supplementation studies have been conducted and their findings with respect to the safety of supplementation have been inconsistent. In this review we have explored these findings and attempted to explain the general findings and identify gaps in published studies.
Whether iron supplementation may be beneficial in patients with HIV is unclear for 2 reasons. First, the burden of anemia in HIV infection is huge. The overall prevalence of anemia among HIV-infected patients ranges from 21% to 71% (76, 81, 102–105) and may be higher in those with advanced disease and in pregnant women (5, 68). While ACD accounts for 41–47% of anemia among HIV-infected adults (5, 11, 89), IDA accounts for 20–44% (5, 63, 81, 106). Inadequate intake and poor bioavailability of iron underlie both major causes of anemia. Regardless of etiology, we found that anemia is consistently associated with elevated risk of mortality, incident TB, and AIDS-related mortality, and iron supplementation is well known to prevent and treat anemia in the general population.
Second, iron is an essential constituent of numerous enzymes important in biological processes, such as mitochondrial respiration, DNA synthesis, hormone production, and immune function (107). Heme oxygenases, present in the vascular system or expressed in response to infection or injury, catalyze the degradation of heme, and thereby function as anti-inflammatory and cytoprotective enzymes, with significant antiviral activity against HIV (108, 109). Levels of heme oxygenase rise in acute HIV infection, as part of the immune response to limit the infection (110, 111). Heme oxygenase function is protective against TB infection, especially if the immune system is still intact (112, 113). In fact, heme oxygenase upregulation may be required to successfully mount resistance to mycobacteria infection (112). When iron stores are depleted, the upregulation of heme oxygenase is impaired (114), likely favoring unrestricted viral growth, worsening disease progression, and increasing mortality risk. We found that iron deficient erythropoiesis, but not iron depletion alone (66), may be associated with clinical progression of HIV, suggesting that the extent of ID is an important determinant of the impact on the immune system.
There are concerns that iron supplementation may not be optimally effective to prevent or treat anemia among HIV patients and may even be unsafe. This concern arises because ACD accounts for a substantial proportion of the anemia burden among HIV-infected patients. Elevated concentrations of proinflammatory mediators in AI—especially IL-6 upregulate hepcidin production. Hepcidin binds to ferroportin at numerous sites—including the gastrointestinal tract and reticuloendothelial system—and limits the degree to which ferroportin is available to transport iron across the plasma membrane, rendering iron supplementation ineffective (115, 116). Unabsorbed iron could therefore reach the colon in large quantities—both free and sequestered in enterocytes. This could lead to gut inflammation, promote the growth of pathogenic microbes in the colon, and increase the risk of diarrhea, with potential systemic effects. If these concerns are confirmed in population studies, additional testing to exclude ACD may be required before commencing iron supplementation.
Importantly, there are also concerns that iron repletion may accelerate viral replication inside CD4 T-cells, worsening the progression of the HIV disease. Iron is a component of the host's cellular enzymes required as part of the life cycle of the HIV virus, such as for generating viral particles (117). A few in vitro studies have examined this question (118–121). While increased cellular iron leads to increased viral replication in CD4 T-cells, iron chelation led to decreased viral replication in circulating lymphocytes (119–121). Population studies examining the effect of iron on viral load are warranted, including studies comparing the relation of iron and viral load in the context of viral suppression and optimal adherence to antiretroviral therapy.
There are additional concerns that iron supplementation may lead to increased concentrations of non–transferrin bound iron (NTBI) in iron-replete HIV-infected individuals (122). Levels of NTBIs have been reported to be 6-fold greater among patients with Pneumocystis carinii pneumonia (PCP) (123). Unbound iron is potentially more available for pathogens to access for their homeostasis, and multiple in vitro studies have demonstrated increases in intracellular and extracellular growth of Mycobacterium TB and other bacteria when hemoglobin or ferritin iron is increased (124, 125). It could be argued that there is a threshold beyond which increasing iron stores through supplementation would be harmful. In a mouse model of Mycobacterium bovis infection, intake of an iron-rich diet was found to lead to decreased mycobacterial growth, lower concentrations of proinflammatory cytokines, and higher T-cell recruitment, if the concentrations of iron in the liver were not excessive (126). In a Tanzanian cohort, those who received iron had increased mortality risk regardless of anemia categories (99). Epidemiologic studies do not provide satisfactory explanations for these findings since prospective studies and RCTs either find iron supplementation may protect against worsening virologic (87) or immunologic (86) status, or find no significant relation of iron supplement use with viral load (82, 90). Interrupted treatment studies that guide the provision of iron supplementation using common hematologic biomarkers may also help clarify the appropriateness, costs, and effectiveness of alternative supplementation approaches.
As mentioned above, ACD is also important in the context of other chronic diseases such as TB, schistosomiasis, chronic kidney disease, and cancers. IDA is also common in these conditions, often due to poor dietary intake, and the balance of risks and benefits of iron supplementation has been considered (127, 128). Iron supplementation is increasingly common among patients with some of these conditions due to studies demonstrating its superior efficacy relative to other treatment modalities (129, 130). Recent meta-analyses conducted among cancer patients found that including iron with erythropoiesis-stimulating agents (ESAs) led to 17% better hematopoietic response and reduced the need for transfusions, with no increased risk of adverse events, compared with ESAs alone, suggesting a role for iron supplementation in these and similar conditions (130). Among 131 patients with pulmonary TB in India, those who were randomly assigned to iron supplements had considerably greater hemoglobin and ferritin concentrations and larger mean corpuscular volume (MCV) after 1 mo of treatment, compared with those assigned to placebo (131). The iron concentrations remained higher by 6 mo of follow-up, but the difference was no longer statistically significant. This trial among TB patients did not report on adverse events. These findings in other chronic diseases, and the limited evidence from the iron supplementation studies among HIV patients (82, 132), together support the argument that iron supplementation is likely to be beneficial among HIV patients with anemia.
Studies have also considered which route of administration of iron may be optimal in chronic diseases. Recent meta-analyses and RCTs among patients with chronic kidney disease (CKD) (133), inflammatory bowel disease, and cancer (134, 135) have observed during follow-up that hemoglobin, ferritin, and transferrin saturation concentrations are higher with intravenous (IV) iron supplementation than with oral supplementation. The need for blood transfusion did not differ in the oral and IV iron groups (133, 134, 136). Among patients with inflammatory bowel disease, there was a 5-fold higher risk of serious adverse events in those who received IV iron than in those who received oral iron (137). In patients with CKD and cancer, however, there was no increased risk of adverse events and no difference in survival (133, 136). It is likely that the effectiveness and safety of oral iron depends on the specific iron compound. Most studies reviewed above provided oral iron in the form of ferrous sulfate. One study among cancer patients compared IV iron to oral sucrosomial iron and found no difference in the hemoglobin response (136). Recent placebo-controlled studies demonstrated the efficacy of oral ferric citrate capsules among patients with CKD (138, 139), and studies comparing oral ferric citrate to IV iron may further clarify its effectiveness. In the context of HIV infection, parenteral administration of iron could potentially complicate efforts to improve adherence to antiretroviral medication, and randomized trials examining the effectiveness and safety of different oral formulations of iron are warranted.
As mentioned above, ACD results from chronic inflammation in the context of HIV. It occurs as part of the innate immune response to restrict bioavailable iron accessible to pathogens. Duodenal iron absorption is reduced, and absorbed iron is sequestered within enterocytes and eventually passed out in feces when enterocytes slough off. Iron is also sequestered within macrophages in the reticuloendothelial system in response to the elaboration of IL-2 and -6 in chronic inflammation. Increased hepcidin concentration underlies the sequestration in both cases, and hepcidin is therefore described as the master regulator of iron metabolism (24). Hepcidin binds to ferroportin, a cellular iron transport protein expressed on the surface of cells (140). Hepcidin binding to ferroportin leads to its disintegration, thereby limiting iron transport across the cell membrane (115, 116). The adverse consequences of ACD likely result from chronic inflammation, poor iron bioavailability, or both.
In light of the findings from this review, as well as current practices in the context of chronic noninfectious diseases, we conclude that there appears to be equipoise with respect to the balance of safety and efficacy of iron supplementation among HIV patients. Many of the studies on this subject are observational studies, with varying degrees of adjustment for confounding variables. There have been substantial improvements in access to ART, and now in most countries all HIV-infected patients receive HAART as soon as they have been diagnosed. Many of the primary studies in this review were, however, conducted in the pre-ART days, or in settings where HAART was only provided to patients with more advanced disease or based on clinical immunologic criteria, or both, limiting the relevance of the inferences from these studies to current practice. Even in the ART era, many studies do not provide information on the extent of adherence to ART. The major limitation of this review, therefore, is the paucity of primary studies, the limited generalizability of many included studies in the light of changing treatment guidelines, and the inadequate consideration of the impact of use of and adherence to ART on findings.
None of the RCTs reviewed had examined iron supplementation in the groups of HIV-infected individuals most likely to benefit from supplementation, such as pregnant women and adults and children with ID. Testing for iron depletion (ferritin <15 μg/L) at baseline and during follow-up could guide delayed or interrupted treatment approaches, with potential to prevent anemia, without worsening the risk of adverse events. These approaches require evaluation in randomized controlled trials. Increased understanding of the interaction of HAART and iron supplementation would also strengthen the evidence for and against recommending supplementation in the context of universal HAART use. RCTs in the pre-ART era (141, 142) and a recent large observational study (143) among patients initiating HAART have shown that multiple micronutrient supplements (MMS) lower the risk of anemia and mortality. MMS contain antioxidants, improve the immune response, and slow viral replication (143). Multiple micronutrient supplementation may therefore be a suitable alternative if iron supplementation is unsafe or ineffective, and a complement if otherwise. The appropriate dosage of iron in MMS for use among PLWHIV is unclear, since the previous studies in this setting included a range of doses from 0 to 30 mg (141, 144–148). The findings from RCTs may inform the composition of MMS, with respect to iron.
We conclude that there is consistent evidence that PLWHIV who are anemic are at increased risk of all-cause mortality, incident TB, and disease progression. However, there are also potential adverse consequences of supplementing iron. Trials of efficacy and safety of iron supplementation in diverse populations, including pregnant women who may be taking iron as standard prenatal care, are warranted.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—AIA, CTA, WF: drafted the paper; CRS: contributed to drafting the paper; WF: designed the study; AIA, CTA: reviewed titles, abstracts, and full texts and extracted data as necessary; WF: had full access to all of the data in the study and takes responsibility for the integrity of the review; all authors: contributed to the development of the manuscript; and all authors: read and approved the final version of the manuscript.
Notes
The authors report no funding received for this study.
Author disclosures: The authors report no conflicts of interest.
Supplementary Tables 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: ACD, anemia of chronic disease; AFB, acid fast bacilli; AI, anemia of inflammation; ART, antiretroviral therapy; cART, combination ART; CBC, complete blood count; CKD, chronic kidney disease; CRP, C-reactive protein; ESA, erythropoietin-stimulating agent; HAART, highly active antiretroviral therapy; HAND, HIV-associated neurocognitive disorder; ID, iron deficiency; IDA, iron deficiency anemia; IRA, iron-restricted anemia; IV, intravenous; LMIC, low and middle income countries; MRC, Medical Research Council; NR, Not reported; NTBI, non–transferrin bound iron; PCP, Pneumocystic cariniipneumonia; PLWHIV, people living with HIV; RCT, randomized controlled trial; SGA, small for gestational age; sTfR, soluble transferrin receptor; TB, tuberculosis; TIBC, total iron binding capacity; ZPP, zinc protoporphyrin.
References
- 1. Kassebaum NJ. The global burden of anemia. Hematol Oncol Clin North Am. 2016;30(2):247–308. [DOI] [PubMed] [Google Scholar]
- 2. GBD 2015 HIV Collaborators. Estimates of global, regional, and national incidence, prevalence, and mortality of HIV, 1980–2015: the Global Burden of Disease Study 2015. Lancet HIV. 2016;3(8):e361–e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Makubi A, Okuma J, Spiegelman D, Hawkins C, Darling AM, Jackson E, Mugusi F, Chalamilla G, Fawzi W. Burden and determinants of severe anemia among HIV-infected adults: results from a large urban HIV program in Tanzania, East Africa. J Int Assoc Provid AIDS Care. 2015;14(2):148–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Belperio PS, Rhew DC. Prevalence and outcomes of anemia in individuals with human immunodeficiency virus: a systematic review of the literature. Am J Med. 2004;116(7):27–43. [DOI] [PubMed] [Google Scholar]
- 5. Haider BA, Spiegelman D, Hertzmark E, Sando D, Duggan C, Makubi A, Sudfeld C, Aris E, Chalamilla GE, Fawzi WW. Anemia, iron deficiency, and iron supplementation in relation to mortality among HIV-infected patients receiving highly active antiretroviral therapy in Tanzania. Am J Trop Med Hyg. 2019;100(6):1512–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Redig AJ, Berliner N. Pathogenesis and clinical implications of HIV-related anemia in 2013. J Int Assoc Provid AIDS Care. 2013;2013(1):377–81. [DOI] [PubMed] [Google Scholar]
- 7. Moyle G, Sawyer W, Law M, Amin J, Hill A. Changes in hematologic parameters and efficacy of thymidine analogue-based, highly active antiretroviral therapy: a meta-analysis of six prospective, randomized, comparative studies. Clin Ther. 2004;26(1):92–7. [DOI] [PubMed] [Google Scholar]
- 8. Parkes-Ratanshi R, Katende D, Levin J, Wakeham K, Heiner G, Kamali A, Lalloo DG. Development of severe anemia and changes in hemoglobin in a cohort of HIV-infected Ugandan adults receiving zidovudine-, stavudine-, and tenofovir-containing antiretroviral regimens. J Int Assoc Provid AIDS Care. 2015;14(5):455–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. World Health Organization. Nutrient requirements for people living with HIV. [Internet] 2004; [accessed December 5, 2019]. Available from:https://www.who.int/nutrition/publications/Content_nutrient_requirements.pdf. [Google Scholar]
- 10. World Health Organization. Daily iron supplementation in infants and children. [Internet]. [accessed December 5, 2019]. Available from: https://www.who.int/nutrition/publications/micronutrients/guidelines/daily_iron_supp_childrens/en/. [PubMed] [Google Scholar]
- 11. Ezeamama AE, Sikorskii A, Bajwa RK, Tuke R, Kyeyune RB, Fenton JI, Guwatudde D, Fawzi WW. Evolution of anemia types during antiretroviral therapy—implications for treatment outcomes and quality of life among HIV-infected adults. Nutrients. 2019;11(4):755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Clark T, Semba R. Iron supplementation during human immunodeficiency virus infection: a double-edged sword? Med Hypotheses. 2001;57(4):476–9. [DOI] [PubMed] [Google Scholar]
- 13. Drakesmith H, Prentice AM. Hepcidin and the iron-infection axis. Science. 2012;338(6108):768–72. [DOI] [PubMed] [Google Scholar]
- 14. Adetifa I, Okomo U. Iron supplementation for reducing morbidity and mortality in children with HIV. Cochrane Database Syst Rev. 2009;(1):CD006736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Calis JC, van Hensbroek MB, de Haan RJ, Moons P, Brabin BJ, Bates I. HIV-associated anemia in children: a systematic review from a global perspective. AIDS (London, England). 2008;22(10):1099–112. [DOI] [PubMed] [Google Scholar]
- 16. Minchella PA, Armitage AE, Darboe B, Jallow MW, Drakesmith H, Jaye A, Prentice AM, McDermid JM. Elevated hepcidin is part of a complex relation that links mortality with iron homeostasis and anemia in men and women with HIV infection. J Nutr. 2015;145(6):1194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McDermid JM, Jaye A, Schim van der Loeff MF, Todd J, Bates C, Austin S, Jeffries D, Awasana AA, Whittlex AA, Prentice A. Elevated iron status strongly predicts mortality in West African adults with HIV infection. J Acquir Immune Defic Syndr. 2007;46(4):498–507. [DOI] [PubMed] [Google Scholar]
- 18. DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28(2):105–14. [DOI] [PubMed] [Google Scholar]
- 19. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savović J, Schulz KF, Weeks L, Sterne JA. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Namaste SM, Rohner F, Huang J, Bhushan NL, Flores-Ayala R, Kupka R, Mei Z, Rawat R, Williams AM, Raiten DJ. Adjusting ferritin concentrations for inflammation: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am J Clin Nutr. 2017;106(suppl_1):359S–71S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lynch S, Pfeiffer CM, Georgieff MK, Brittenham G, Fairweather-Tait S, Hurrell RF, McArdle HJ, Raiten DJ. Biomarkers of Nutrition for Development (BOND)-iron review. J Nutr. 2018;148(suppl_1):1001s–67s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wish JB. Assessing iron status: beyond serum ferritin and transferrin saturation. Clin J Am Soc Nephrol. 2006;1(suppl_1):S4–8. [DOI] [PubMed] [Google Scholar]
- 23. Stoltzfus RJ, Klemm R. Research, policy, and programmatic considerations from the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am J Clin Nutr. 2017;106(suppl_1):428S–34S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ganz T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood. 2003;102(3):783–8. [DOI] [PubMed] [Google Scholar]
- 25. Abuye C, Tsegaye A, West CE, Versloot P, Sanders EJ, Wolday D, Hamann D, De Wit TF, Fontanet AL. Determinants of CD4 counts among HIV-negative Ethiopians: role of body mass index, gender, cigarette smoking, khat (Catha edulis) chewing, and possibly altitude?. J Clin Immunol. 2005;25(2):127–33. [DOI] [PubMed] [Google Scholar]
- 26. al-Khafaji B, Kralovic S, Smith RD. Increased hepatic iron in the acquired immunodeficiency syndrome: an autopsy study. Mod Pathol. 1997;10(5):474–80. [PubMed] [Google Scholar]
- 27. Antelman G, Msamanga GI, Spiegelman D, Urassa EJ, Narh R, Hunter DJ, Fawzi WW. Nutritional factors and infectious disease contribute to anemia among pregnant women with human immunodeficiency virus in Tanzania. J Nutr. 2000;130(8):1950–7. [DOI] [PubMed] [Google Scholar]
- 28. Banjoko SO, Oseni FA, Togun RA, Onayemi O, Emma-Okon BO, Fakunle JB. Iron status in HIV-1 infection: implications in disease pathology. BMC Clin Pathol. 2012;12:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Blumberg BS, Hann HW, Mildvan D, Mathur U, Lustbader E, London WT. Iron and iron binding proteins in persistent generalised lymphadenopathy and AIDS. Lancet (London, England). 1984;1(8372):347. [DOI] [PubMed] [Google Scholar]
- 30. Crist MB, Melekhin VV, Bian A, Shintani A, Milne GL, Kallianpur AR, Dageforde LA, Haas DW, Hulgan T. Higher serum iron is associated with increased oxidant stress in HIV-infected men. J Acquir Immune Defic Syndr. 2013;64(4):367–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dannhauser A, van Staden AM, van der Ryst E, Nel M, Marais N, Erasmus E, Attwood EM, Barnard HC, le Roux GD. Nutritional status of HIV-1 seropositive patients in the Free State Province of South Africa: anthropometric and dietary profile. Eur J Clin Nutr. 1999;53(3):165–73. [DOI] [PubMed] [Google Scholar]
- 32. Deisenhammer F, Miller RF, Brink NS, Harrison MJ, Thompson EJ. Cerebrospinal fluid ferritin in HIV infected patients with acute neurological episodes. Genitourin Med. 1997;73(3):181–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dikshit B, Wanchu A, Sachdeva RK, Sharma A, Das R. Profile of hematological abnormalities of Indian HIV infected individuals. BMC Blood Disord. 2009;9:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Eley BS, Sive AA, Shuttleworth M, Hussey GD. A prospective, cross-sectional study of anaemia and peripheral iron status in antiretroviral naive, HIV-1 infected children in Cape Town, South Africa. BMC Infect Dis. 2002;2:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ellaurie M, Rubinstein A. Ferritin levels in pediatric HIV-1 infection. Acta Paediatr. 1994;83(10):1035–7. [DOI] [PubMed] [Google Scholar]
- 36. Eshetu A, Tsegaye A, Petros B. Selected micronutrient levels and response to highly active antiretroviral therapy (HAART) among HIV/AIDS patients attending a teaching Hospital in Addis Ababa, Ethiopia. Biol Trace Elem Res. 2014;162(1-3):106–12. [DOI] [PubMed] [Google Scholar]
- 37. Friis H, Gomo E, Nyazema N, Ndhlovu P, Krarup H, Madsen PH, Michaelsen KF. Iron, haptoglobin phenotype, and HIV-1 viral load: a cross-sectional study among pregnant Zimbabwean women. J Acquir Immune Defic Syndr. 2003;33(1):74–81. [DOI] [PubMed] [Google Scholar]
- 38. Fuchs D, Zangerle R, Artner-Dworzak E, Weiss G, Fritsch P, Tilz GP, Dierich MP, Wachter H. Association between immune activation, changes of iron metabolism and anaemia in patients with HIV infection. Eur J Haematol. 1993;50(2):90–4. [DOI] [PubMed] [Google Scholar]
- 39. Gherardi RK, Mhiri C, Baudrimont M, Roullet E, Berry JP, Poirier J. Iron pigment deposits, small vessel vasculitis, and erythrophagocytosis in the muscle of human immunodeficiency virus-infected patients. Hum Pathol. 1991;22(12):1187–94. [DOI] [PubMed] [Google Scholar]
- 40. Gupta S. Study of activated T cells in man. II. Interleukin 2 receptor and transferrin receptor expression on T cells and production of interleukin 2 in patients with acquired immune deficiency syndrome (AIDS) and AIDS-related complex. Clin Immunol Immunopathol. 1986;38(1):93–100. [DOI] [PubMed] [Google Scholar]
- 41. James EB, Harmatz P, Lee M, Kennedy C, Petru A, Wara D, Miaskowski C. Altered iron metabolism in children with human immunodeficiency virus disease. Pediatr Hematol Oncol. 2009;26(2):104–19. [DOI] [PubMed] [Google Scholar]
- 42. Kagu MB, Khalil MI, Ahmed SG. Bone marrow macrophage iron stores in patients with HIV infection and AIDS-associated Kaposi's sarcoma. Afr J Med Med Sci. 2007;36(2):125–8. [PubMed] [Google Scholar]
- 43. Karcher DS, Frost AR. The bone marrow in human immunodeficiency virus (HIV)-related disease. Morphology and clinical correlation. Am J Clin Pathol. 1991;95(1):63–71. [DOI] [PubMed] [Google Scholar]
- 44. Kotwal J, Singh V, Kotwal A, Dutta V, Nair V. A study of haematological and bone marrow changes in symptomatic patients with human immune deficiency virus infection with special mention of functional iron deficiency, anaemia of critically ill and haemophagocytic lymphohistiocytosis. Med J Armed Forces India. 2013;69(4):319–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kruger HS, Balk LJ, Viljoen M, Meyers TM. Positive association between dietary iron intake and iron status in HIV-infected children in Johannesburg, South Africa. Nutr Rese. 2013;33(1):50–8. [DOI] [PubMed] [Google Scholar]
- 46. Kruzich LA, Marquis GS, Carriquiry AL, Wilson CM, Stephensen CB. US youths in the early stages of HIV disease have low intakes of some micronutrients important for optimal immune function. J Am Diet Assoc. 2004;104(7):1095–101. [DOI] [PubMed] [Google Scholar]
- 47. Lopez-Calderon C, Palacios R, Cobo A, Nuno E, Ruiz J, Marquez M, Santos J. Serum ferritin in HIV-positive patients is related to immune deficiency and inflammatory activity. Int J STD AIDS. 2015;26(6):393–7. [DOI] [PubMed] [Google Scholar]
- 48. Malvoisin E, Livrozet JM, Ducluzeau PH, Makloufi D, Cohen R, Vincent N. Hyperglycosylated ferritin in sera of HIV-1-infected patients treated with highly active antiretroviral therapy. AIDS (London, England). 2006;20(3):457–9. [DOI] [PubMed] [Google Scholar]
- 49. Malvoisin E, Makhloufi D, Livrozet JM. Serum hepcidin levels in women infected with HIV-1 under antiviral therapy. J Med Virol. 2014;86(10):1656–60. [DOI] [PubMed] [Google Scholar]
- 50. Masaisa F, Breman C, Gahutu JB, Mukiibi J, Delanghe J, Philippe J. Ferroportin (SLC40A1) Q248H mutation is associated with lower circulating serum hepcidin levels in Rwandese HIV-positive women. Ann Hematol. 2012;91(6):911–16. [DOI] [PubMed] [Google Scholar]
- 51. Obirikorang C, Issahaku RG, Osakunor DN, Osei-Yeboah J. Anaemia and iron homeostasis in a cohort of HIV-infected patients: a cross-sectional study in Ghana. AIDS Res Treat. 2016;2016:1623094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ogbe PJ, Idoko OA, Ezimah AC, Digban KA, Oguntayo BO. Evaluation of iron status in anemia of chronic disease among patients with HIV infection. Clin Lab Sci. 2012;25(1):7–12. [PubMed] [Google Scholar]
- 53. Perrella O, Finelli L, Munno I, Perrella A, Soscia E, Carrieri PB. Cerebrospinal fluid ferritin in human immunodeficiency virus infection: a marker of neurologic involvement?. J Infect Dis. 1993;168(4):1079–80. [DOI] [PubMed] [Google Scholar]
- 54. Riera A, Gimferrer E, Cadafalch J, Remacha A, Martin S. Prevalence of high serum and red cell ferritin levels in HIV-infected patients. Haematologica. 1994;79(2):165–7. [PubMed] [Google Scholar]
- 55. Salome MA, Grotto HZ. Human immunodeficiency virus-related anemia of chronic disease: relationship to hematologic, immune, and iron metabolism parameters, and lack of association with serum interferon-gamma levels. AIDS Patient Care STDs. 2002;16(8):361–5. [DOI] [PubMed] [Google Scholar]
- 56. Shet A, Arumugam K, Rajagopalan N, Dinakar C, Krishnamurthy S, Mehta S, Shet AS. The prevalence and etiology of anemia among HIV-infected children in India. Eur J Pediatr. 2012;171(3):531–40. [DOI] [PubMed] [Google Scholar]
- 57. Totin D, Ndugwa C, Mmiro F, Perry RT, Jackson JB, Semba RD. Iron deficiency anemia is highly prevalent among human immunodeficiency virus-infected and uninfected infants in Uganda. J Nutr. 2002;132(3):423–9. [DOI] [PubMed] [Google Scholar]
- 58. Campa A, Shor-Posner G, Indacochea F, Zhang G, Lai H, Asthana D, Scott GB, Baum MK. Mortality risk in selenium-deficient HIV-positive children. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20(5):508–13. [DOI] [PubMed] [Google Scholar]
- 59. Chatterjee A, Bosch RJ, Kupka R, Hunter DJ, Msamanga GI, Fawzi WW. Predictors and consequences of anaemia among antiretroviral-naive HIV-infected and HIV-uninfected children in Tanzania. Public Health Nutr. 2010;13(2):289–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Isanaka S, Aboud S, Mugusi F, Bosch RJ, Willett WC, Spiegelman D, Duggan C, Fawzi WW. Iron status predicts treatment failure and mortality in tuberculosis patients: a prospective cohort study from Dar es Salaam, Tanzania. PLoS One. 2012;7(5):e37350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Brentlinger PE, Silva WP, Vermund SH, Valverde E, Buene M, Moon TD. Practical management of HIV-associated anemia in resource-limited settings: prospective observational evaluation of a new Mozambican guideline. AIDS Res Hum Retroviruses. 2016;32(1):12–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. de Monye C, Karcher DS, Boelaert JR, Gordeuk VR. Bone marrow macrophage iron grade and survival of HIV-seropositive patients. AIDS (London, England). 1999;13(3):375–80. [DOI] [PubMed] [Google Scholar]
- 63. Ezeamama A, Guwatudde D, Sikorskii A, Kabagambe E, Spelts R, Vahey G, Fenton J, Fawzi W. Impaired hematologic status in relation to clinical outcomes among HIV-infected adults from Uganda: a prospective cohort study. Nutrients. 2018;10(4):475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gordeuk VR, Onojobi G, Schneider MF, Dawkins FW, Delapenha R, Voloshin Y, von Wyl V, Bacon M, Minkoff H, Levine A et al.. The association of serum ferritin and transferrin receptor concentrations with mortality in women with human immunodeficiency virus infection. Haematologica. 2006;91(6):739–43. [PubMed] [Google Scholar]
- 65. Isanaka S, Spiegelman D, Aboud S, Manji KP, Msamanga GI, Willet WC, Duggan C, Fawzi WW. Post-natal anaemia and iron deficiency in HIV-infected women and the health and survival of their children. Matern Child Nutr. 2012;8(3):287–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Isanaka S, Mugusi F, Urassa W, Willett WC, Bosch RJ, Villamor E, Spiegelman D, Duggan C, Fawzi WW. Iron deficiency and anemia predict mortality in patients with tuberculosis. J Nutr. 2012;142(2):350–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kerkhoff AD, Meintjes G, Opie J, Vogt M, Jhilmeet N, Wood R, Lawn SD. Anaemia in patients with HIV-associated TB: relative contributions of anaemia of chronic disease and iron deficiency. Int J Tuberc Lung Dis. 2016;20(2):193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. O'Brien ME, Kupka R, Msamanga GI, Saathoff E, Hunter DJ, Fawzi WW. Anemia is an independent predictor of mortality and immunologic progression of disease among women with HIV in Tanzania. J Acquir Immune Defic Syndr. 2005;40(2):219–25. [DOI] [PubMed] [Google Scholar]
- 69. Rawat R, Humphrey JH, Ntozini R, Mutasa K, Iliff PJ, Stoltzfus RJ. Elevated iron stores are associated with HIV disease severity and mortality among postpartum women in Zimbabwe. Public Health Nutr. 2009;12(9):1321–9. [DOI] [PubMed] [Google Scholar]
- 70. Wisaksana R, Sumantri R, Indrati AR, Zwitser A, Jusuf H, de Mast Q, van Crevel R, van der Ven A. Anemia and iron homeostasis in a cohort of HIV-infected patients in Indonesia. BMC Infect Dis. 2011;11:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sun J, Su J, Xie Y, Yin MT, Huang Y, Xu L, Zhou Q, Zhu B. Plasma IL-6/IL-10 ratio and IL-8, LDH, and HBDH level predict the severity and the risk of death in AIDS patients with pneumocystis pneumonia. J Immunol Res. 2016;2016:1583951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. McDermid JM, Hennig BJ, van der Sande M, Hill AV, Whittle HC, Jaye A, Prentice AM. Host iron redistribution as a risk factor for incident tuberculosis in HIV infection: an 11-year retrospective cohort study. BMC Infect Dis. 2013;13:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Minchella PA, Armitage AE, Darboe B, Jallow MW, Drakesmith H, Jaye A, Prentice AM, McDermid JM. Elevated hepcidin at HIV diagnosis is associated with incident tuberculosis in a retrospective cohort study. Int J Tuberc Lung Dis. 2014;18(11):1337–9. [DOI] [PubMed] [Google Scholar]
- 74. Mupfumi L, Moyo S, Molebatsi K, Thami PK, Anderson M, Mogashoa T, Iketleng T, Makhema J, Marlink R, Kasvosve I et al.. Immunological non-response and low hemoglobin levels are predictors of incident tuberculosis among HIV-infected individuals on Truvada-based therapy in Botswana. PLoS One. 2018;13(1):e0192030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tenforde MW, Gupte N, Dowdy DW, Asmuth DM, Balagopal A, Pollard RB, Sugandhavesa P, Lama JR, Pillay S, Cardoso SW et al.. C-reactive protein (CRP), interferon gamma-inducible protein 10 (IP-10), and lipopolysaccharide (LPS) are associated with risk of tuberculosis after initiation of antiretroviral therapy in resource-limited settings. PLoS One. 2015;10(2):e0117424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wisaksana R, de Mast Q, Alisjahbana B, Jusuf H, Sudjana P, Indrati AR, Sumantri R, Swinkels D, van Crevel R, van der Ven A. Inverse relationship of serum hepcidin levels with CD4 cell counts in HIV-infected patients selected from an Indonesian prospective cohort study. PLoS One. 2013;8(11):e79904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Abrams B, Duncan D, Hertz-Picciotto I. A prospective study of dietary intake and acquired immune deficiency syndrome in HIV-seropositive homosexual men. J Acquir Immune Defic Syndr. 1993;6(8):949–58. [PubMed] [Google Scholar]
- 78. Armitage AE, Stacey AR, Giannoulatou E, Marshall E, Sturges P, Chatha K, Smith NM, Huang X, Xu X, Pasricha SR et al.. Distinct patterns of hepcidin and iron regulation during HIV-1, HBV, and HCV infections. Proc Natl Acad Sci. 2014;111(33):12187–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Boom J, Kosters E, Duncombe C, Kerr S, Hirschel B, Ruxrungtham K, de Mast Q, Kosalaraksa P, Ulbolyam S, Jupimai T et al.. Ferritin levels during structured treatment interruption of highly active antiretroviral therapy. HIV Med. 2007;8(6):388–95. [DOI] [PubMed] [Google Scholar]
- 80. Kallianpur AR, Wang Q, Jia P, Hulgan T, Zhao Z, Letendre SL, Ellis RJ, Heaton RK, Franklin DR, Barnholtz-Sloan J et al.. Anemia and red blood cell indices predict HIV-associated neurocognitive impairment in the highly active antiretroviral therapy era. J Infect Dis. 2016;213(7):1065–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kupka R, Msamanga GI, Mugusi F, Petraro P, Hunter DJ, Fawzi WW. Iron status is an important cause of anemia in HIV-infected Tanzanian women but is not related to accelerated HIV disease progression. J Nutr. 2007;137(10):2317–23. [DOI] [PubMed] [Google Scholar]
- 82. Phiri W, Kasonka L, Collin S, Makasa M, Sinkala M, Chintu C, Kasolo F, Kaseba C, Tomkins AM, Filteau SM. Factors influencing breast milk HIV RNA viral load among Zambian women. AIDS Res Hum Retroviruses. 2006;22(7):607–14. [DOI] [PubMed] [Google Scholar]
- 83. Quiros-Roldan E, Castelli F, Lanza P, Pezzoli C, Vezzoli M, Biasiotto G, Zanella I. The impact of antiretroviral therapy on iron homeostasis and inflammation markers in HIV-infected patients with mild anemia. J Transl Med. 2017;15(1):256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Sarcletti M, Quirchmair G, Weiss G, Fuchs D, Zangerle R. Increase of haemoglobin levels by anti-retroviral therapy is associated with a decrease in immune activation. Eur J Haematol. 2003;70(1):17–25. [DOI] [PubMed] [Google Scholar]
- 85. Shivakoti R, Ewald ER, Gupte N, Yang WT, Kanyama C, Cardoso SW, Santos B, Supparatpinyo K, Badal-Faesen S, Lama JR et al.. Effect of baseline micronutrient and inflammation status on CD4 recovery post-cART initiation in the multinational PEARLS trial. Clin Nutr. 2019;38(3):1303–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Esan MO, van Hensbroek MB, Nkhoma E, Musicha C, White SA, Ter Kuile FO, Phiri KS. Iron supplementation in HIV-infected Malawian children with anemia: a double-blind, randomized, controlled trial. Clin Infect Dis. 2013;57(11):1626–34. [DOI] [PubMed] [Google Scholar]
- 87. Olsen A, Mwaniki D, Krarup H, Friis H. Low-dose iron supplementation does not increase HIV-1 load. J Acquir Immune Defic Syndr. 2004;36(1):637–8. [DOI] [PubMed] [Google Scholar]
- 88. Ray A, Ndugwa C, Mmirot F, Ricks MO, Semba RD. Soluble transferrin receptor as an indicator of iron deficiency in HIV-infected infants. Ann Trop Paediatr. 2007;27(1):11–16. [DOI] [PubMed] [Google Scholar]
- 89. Shet A, Bhavani PK, Kumarasamy N, Arumugam K, Poongulali S, Elumalai S, Swaminathan S. Anemia, diet and therapeutic iron among children living with HIV: a prospective cohort study. BMC Pediatr. 2015;15:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Semba RD, Ricketts EP, Mehta S, Netski D, Thomas D, Kirk G, Wu AW, Vlahov D. Effect of micronutrients and iron supplementation on hemoglobin, iron status, and plasma hepatitis C and HIV RNA levels in female injection drug users: a controlled clinical trial. J Acquir Immune Defic Syndr. 2007;45(3):298–303. [DOI] [PubMed] [Google Scholar]
- 91. Masaisa F, Gahutu JB, Mukiibi J, Delanghe J, Philippe J. Transferrin polymorphism and opportunistic infections in HIV-infected women in Rwanda. Acta Haematol. 2012;128(2):100–6. [DOI] [PubMed] [Google Scholar]
- 92. Perrella O, Finelli E, Perrella A, Tartaglia G, Scognamiglio P, Scalera G. Combined therapy with zidovudine, recombinant granulocyte colony stimulating factors and erythropoietin in asymptomatic HIV patients. J Chemother (Florence, Italy). 1996;8(1):63–6. [DOI] [PubMed] [Google Scholar]
- 93. Shet A, Mehta S, Rajagopalan N, Dinakar C, Ramesh E, Samuel NM, Indumathi CK, Fawzi WW, Kurpad AV. Anemia and growth failure among HIV-infected children in India: a retrospective analysis. BMC Pediatr. 2009;9:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kerkhoff AD, Meintjes G, Burton R, Vogt M, Wood R, Lawn SD. Relationship between blood concentrations of hepcidin and anemia severity, mycobacterial burden, and mortality among patients with HIV-associated tuberculosis. J Infect Dis. 2016;213(1):61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. James P, Friis H, Woodd S, Rehman AM, PrayGod G, Kelly P, Koethe JR, Filteau S. Minimal impact of an iron-fortified lipid-based nutrient supplement on Hb and iron status: a randomised controlled trial in malnourished HIV-positive African adults starting antiretroviral therapy. Br J Nutr. 2015;114(3):387–97. [DOI] [PubMed] [Google Scholar]
- 96. Gupta S, Imam A, Licorish K. Serum ferritin in acquired immune deficiency syndrome. J Clin Lab Immunol. 1986;20(1):11–13. [PubMed] [Google Scholar]
- 97. World Health Organization (WHO). Haemoglobin concentration for the diagnosis of anaemia and assessment of severity. Vitamin and mineral nutrition information system. Geneva: WHO; 2011. [Google Scholar]
- 98. Kleinbaum DG, Klein M. Extension of the Cox proportional hazards model for time-dependent variables. Survival analysis. Statistics for biology and health. New York: Springer; 2012, p. 241–88. [Google Scholar]
- 99. Haider BA, Spiegelman D, Hertzmark E, Sando D, Duggan C, Makubi A, Sudfeld C, Aris E, Chalamilla GE, Fawzi WW. Anemia, iron deficiency, and iron supplementation in relation 1 to mortality among HIV-infected patients receiving highly active antiretroviral therapy in Tanzania. Am J Trop Med Hyg. 2019;100(6):1512–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Bothwell TH, Charlton R, Cook J, Finch CA. Iron metabolism in man. Iron metabolism in man. Oxford: Blackwell Scientific Publications; 1979. [Google Scholar]
- 101. World Health Organization (WHO). Serum ferritin concentrations for the assessment of iron status and iron deficiency in populations. Geneva: WHO; 2011. [Google Scholar]
- 102. Tesfaye Z, Enawgaw B. Prevalence of anemia before and after initiation of highly active antiretroviral therapy among HIV positive patients in Northwest Ethiopia: a retrospective study. BMC Res Notes. 2014;7:745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Levine AM, Berhane K, Masri-Lavine L, Sanchez M, Young M, Augenbraun M, Cohen M, Anastos K, Newman M, Gange SJ. Prevalence and correlates of anemia in a large cohort of HIV-infected women: Women's Interagency HIV Study. J Acquir Immune Defic Syndr. 2001;26(1):28–35. [DOI] [PubMed] [Google Scholar]
- 104. Jin Y, Li Q, Meng X, Xu Q, Yuan J, Li Z, Guo H, Liu Z. Prevalence of anaemia among HIV patients in rural China during the HAART era. Int J STD AIDS. 2017;28(1):63–8. [DOI] [PubMed] [Google Scholar]
- 105. Meidani M, Rezaei F, Maracy MR, Avijgan M, Tayeri K. Prevalence, severity, and related factors of anemia in HIV/AIDS patients. J Res Med Sci. 2012;17(2):138. [PMC free article] [PubMed] [Google Scholar]
- 106. Frosch AEP, Ayodo G, Odhiambo EO, Ireland K, Vulule J, Cusick SE. Iron deficiency is prevalent among HIV-infected Kenyan adults and is better measured by soluble transferrin receptor than ferritin. Am J Trop Med Hyg. 2018;99(2):439–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Weiss G, Carver PL. Role of divalent metals in infectious disease susceptibility and outcome. Clin Microbiol Infect. 2018;24(1):16–23. [DOI] [PubMed] [Google Scholar]
- 108. Espinoza JA, Gonzalez PA, Kalergis AM. Modulation of antiviral immunity by heme oxygenase-1. Am J Pathol. 2017;187(3):487–93. [DOI] [PubMed] [Google Scholar]
- 109. Zhu Y, Luo S, Sabo Y, Wang C, Tong L, Goff SP. Heme oxygenase 2 binds myristate to regulate retrovirus assembly and TLR4 signaling. Cell Host Microbe. 2017;21(2):220–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Angin M, Fathi A, King M, Ledoux MB, Piechocka-Trocha A, Altfeld M, Addo MM. Acute HIV-1 infection is associated with increased plasma levels of heme oxygenase-1 and presence of heme oxygenase-1-specific regulatory T cells. AIDS (London, England). 2017;31(5):635–41. [DOI] [PubMed] [Google Scholar]
- 111. Zhou ZH, Kumari N, Nekhai S, Clouse KA, Wahl LM, Yamada KM, Dhawan S. Heme oxygenase-1 induction alters chemokine regulation and ameliorates human immunodeficiency virus-type-1 infection in lipopolysaccharide-stimulated macrophages. Biochem Biophys Res Commun. 2013;435(3):373–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Silva-Gomes S, Appelberg R, Larsen R, Soares MP, Gomes MS. Heme catabolism by heme oxygenase-1 confers host resistance to Mycobacterium infection. Infect Immun. 2013;81(7):2536–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Costa DL, Namasivayam S, Amaral EP, Arora K, Chao A, Mittereder LR, Maiga M, Boshoff HI, Barry CE 3rd, Goulding CW et al.. Pharmacological inhibition of host heme oxygenase-1 suppresses mycobacterium tuberculosis infection in vivo by a mechanism dependent on T lymphocytes. mBio. 2016;7(5):e01675–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Bolloskis MP, Carvalho FP, Loo G. Iron depletion in HCT116 cells diminishes the upregulatory effect of phenethyl isothiocyanate on heme oxygenase-1. Toxicol Appl Pharmacol. 2016;297:22–31. [DOI] [PubMed] [Google Scholar]
- 115. Kroot JJ, Tjalsma H, Fleming RE, Swinkels DW. Hepcidin in human iron disorders: diagnostic implications. Clin Chem. 2011;57(12):1650–69. [DOI] [PubMed] [Google Scholar]
- 116. Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science (New York, NY). 2004;306(5704):2090–3. [DOI] [PubMed] [Google Scholar]
- 117. Mancone C, Grimaldi A, Refolo G, Abbate I, Rozera G, Benelli D, Fimia GM, Barnaba V, Tripodi M, Piacentini M. Iron overload down-regulates the expression of the HIV-1 Rev cofactor eIF5A in infected T lymphocytes. Proteome Sci. 2017;15(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Chang HC, Bayeva M, Taiwo B, Palella FJ Jr., Hope TJ, Ardehali H. Short communication: high cellular iron levels are associated with increased HIV infection and replication. AIDS Res Hum Retroviruses. 2015;31(3):305–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Traoré HN, Meyer D. The effect of iron overload on in vitro HIV-1 infection. J Clin Virol. 2004;31:92–8. [DOI] [PubMed] [Google Scholar]
- 120. Georgiou NA, van der Bruggen T, Oudshoorn M, Nottet HH, Marx JJ, van Asbeck BS. Combining iron chelators with the nucleoside analog didanosine in anti-HIV therapy. Transfus Sci. 2000;23(3):249–50. [DOI] [PubMed] [Google Scholar]
- 121. Van Asbeck B, Georgiou N, Van der Bruggen T, Oudshoorn M, Nottet H, Marx J. Anti-HIV effect of iron chelators: different mechanisms involved. J Clin Virol. 2001;20(3):141–7. [DOI] [PubMed] [Google Scholar]
- 122. Schumann K, Solomons NW, Romero-Abal ME, Orozco M, Weiss G, Marx J. Oral administration of ferrous sulfate, but not of iron polymaltose or sodium iron ethylenediaminetetraacetic acid (NaFeEDTA), results in a substantial increase of non-transferrin-bound iron in healthy iron-adequate men. Food Nutr Bull. 2012;33(2):128–36. [DOI] [PubMed] [Google Scholar]
- 123. Mateos F, Gonzalez C, Dominguez C, Losa JE, Jimenez A, Perez-Arellano JL. Elevated non-transferrin bound iron in the lungs of patients with Pneumocystis carinii pneumonia. J Infect. 1999;38(1):18–21. [DOI] [PubMed] [Google Scholar]
- 124. Tanner R, O'Shea MK, White AD, Muller J, Harrington-Kandt R, Matsumiya M, Dennis MJ, Parizotto EA, Harris S, Stylianou E et al.. The influence of haemoglobin and iron on in vitro mycobacterial growth inhibition assays. Sci Rep. 2017;7:43478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Ellermann M, Arthur JC. Siderophore-mediated iron acquisition and modulation of host-bacterial interactions. Free Radic Biol Med. 2017;105:68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Agoro R, Benmerzoug S, Rose S, Bouyer M, Gozzelino R, Garcia I, Ryffel B, Quesniaux VFJ, Mura C. An iron-rich diet decreases the mycobacterial burden and correlates with hepcidin upregulation, lower levels of proinflammatory mediators, and increased T-cell recruitment in a model of Mycobacterium bovis Bacille Calmette-Guerin infection. J Infect Dis. 2017;216(7):907–18. [DOI] [PubMed] [Google Scholar]
- 127. Spivak JL. Iron and the anemia of chronic disease: vindication for the non-essential role of iron supplementation. Oncology (Williston Park). 2011;25(5):421–3. [PubMed] [Google Scholar]
- 128. Slotki I, Cabantchik ZI. The labile side of iron supplementation in CKD. J Am Soc Nephrol. 2015;26(11):2612–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Fraenkel PG. Anemia of inflammation: A review. Med Clin North Am. 2017;101(2):285–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Mhaskar R, Wao H, Miladinovic B, Kumar A, Djulbegovic B. The role of iron in the management of chemotherapy-induced anemia in cancer patients receiving erythropoiesis-stimulating agents. Cochrane Database Syst Rev. 2016;2:Cd009624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Das BS, Devi U, Mohan Rao C, Srivastava VK, Rath PK, Das BS. Effect of iron supplementation on mild to moderate anaemia in pulmonary tuberculosis. Br J Nutr. 2003;90(3):541–50. [DOI] [PubMed] [Google Scholar]
- 132. Esan MO, Jonker FA, Hensbroek MB, Calis JC, Phiri KS. Iron deficiency in children with HIV-associated anaemia: a systematic review and meta-analysis. Trans R Soc Trop Med Hyg. 2012;106(10):579–87. [DOI] [PubMed] [Google Scholar]
- 133. O'Lone EL, Hodson EM, Nistor I, Bolignano D, Webster AC, Craig JC. Parenteral versus oral iron therapy for adults and children with chronic kidney disease. Cochrane Database Syst Rev. 2019;2:CD007857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Keeler B, Simpson J, Ng O, Padmanabhan H, Brookes M, Acheson A, Group IT, Banerjea A, Walter C, Maxwell‐Armstrong C. Randomized clinical trial of preoperative oral versus intravenous iron in anaemic patients with colorectal cancer. Br J Surg. 2017;104(3):214–21. [DOI] [PubMed] [Google Scholar]
- 135. Henry DH, Dahl NV, Auerbach M, Tchekmedyian S, Laufman LR. Intravenous ferric gluconate significantly improves response to epoetin alfa versus oral iron or no iron in anemic patients with cancer receiving chemotherapy. Oncologist. 2007;12(2):231–42. [DOI] [PubMed] [Google Scholar]
- 136. Mafodda A, Giuffrida D, Prestifilippo A, Azzarello D, Giannicola R, Mare M, Maisano R. Oral sucrosomial iron versus intravenous iron in anemic cancer patients without iron deficiency receiving darbepoetin alfa: a pilot study. Support Care Cancer. 2017;25(9):2779–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Bonovas S, Fiorino G, Allocca M, Lytras T, Tsantes A, Peyrin-Biroulet L, Danese S. Intravenous versus oral iron for the treatment of anemia in inflammatory bowel disease: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2016;95(2):e2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. McCullough PA, Uhlig K, Neylan JF, Pergola PE, Fishbane S. Usefulness of oral ferric citrate in patients with iron-deficiency anemia and chronic kidney disease with or without heart failure. Am J Cardiol. 2018;122(4):683–8. [DOI] [PubMed] [Google Scholar]
- 139. Block GA, Fishbane S, Rodriguez M, Smits G, Shemesh S, Pergola PE, Wolf M, Chertow GM. A 12-week, double-blind, placebo-controlled trial of ferric citrate for the treatment of iron deficiency anemia and reduction of serum phosphate in patients with CKD stages 3–5. Am J Kidney Dis. 2015;65(5):728–36. [DOI] [PubMed] [Google Scholar]
- 140. Donovan A, Lima CA, Pinkus JL, Pinkus GS, Zon LI, Robine S, Andrews NC. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 2005;1(3):191–200. [DOI] [PubMed] [Google Scholar]
- 141. Fawzi WW, Msamanga GI, Kupka R, Spiegelman D, Villamor E, Mugusi F, Wei R, Hunter D. Multivitamin supplementation improves hematologic status in HIV-infected women and their children in Tanzania. Am J Clin Nutr. 2007;85(5):1335–43. [DOI] [PubMed] [Google Scholar]
- 142. Jiang S, He J, Zhao X, Li H. The effect of multiple micronutrient supplementation on mortality and morbidity of HIV-infected adults: a meta-analysis of randomized controlled trials. J Nutr Sci Vitaminol. 2012;58(2):105–12. [DOI] [PubMed] [Google Scholar]
- 143. Sudfeld CR, Buchanan A, Ulenga N, Spiegelman D, Mtisi E, Hertzmark E, Muya AN, Sando D, Mungure E, Mizinduko M. Effectiveness of a multivitamin supplementation program among HIV-infected adults in Tanzania. AIDS (London, England). 2019;33(1):93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Range N, Changalucha J, Krarup H, Magnussen P, Andersen AB, Friis H. The effect of multi-vitamin/mineral supplementation on mortality during treatment of pulmonary tuberculosis: a randomised two-by-two factorial trial in Mwanza, Tanzania. Br J Nutr. 2006;95(4):762–70. [DOI] [PubMed] [Google Scholar]
- 145. Semba RD, Kumwenda J, Zijlstra E, Ricks M, Van Lettow M, Whalen C, Clark T, Jorgensen L, Kohler J, Kumwenda N. Micronutrient supplements and mortality of HIV-infected adults with pulmonary TB: a controlled clinical trial. Int J Tuberc Lung Dis. 2007;11(8):854–9. [PubMed] [Google Scholar]
- 146. Villamor E, Mugusi F, Urassa W, Bosch RJ, Saathoff E, Matsumoto K, Meydani SN, Fawzi WW. A trial of the effect of micronutrient supplementation on treatment outcome, T cell counts, morbidity, and mortality in adults with pulmonary tuberculosis. J Infect Dis. 2008;197(11):1499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Jiamton S, Pepin J, Suttent R, Filteau S, Mahakkanukrauh B, Hanshaoworakul W, Chaisilwattana P, Suthipinittharm P, Shetty P, Jaffar S. A randomized trial of the impact of multiple micronutrient supplementation on mortality among HIV-infected individuals living in Bangkok. AIDS (London, England). 2003;17(17):2461–9. [DOI] [PubMed] [Google Scholar]
- 148. Kelly P, Katubulushi M, Todd J, Banda R, Yambayamba V, Fwoloshi M, Zulu I, Kafwembe E, Yavwa F, Sanderson IR. Micronutrient supplementation has limited effects on intestinal infectious disease and mortality in a Zambian population of mixed HIV status: a cluster randomized trial. Am J Clin Nutr. 2008;88(4):1010–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.