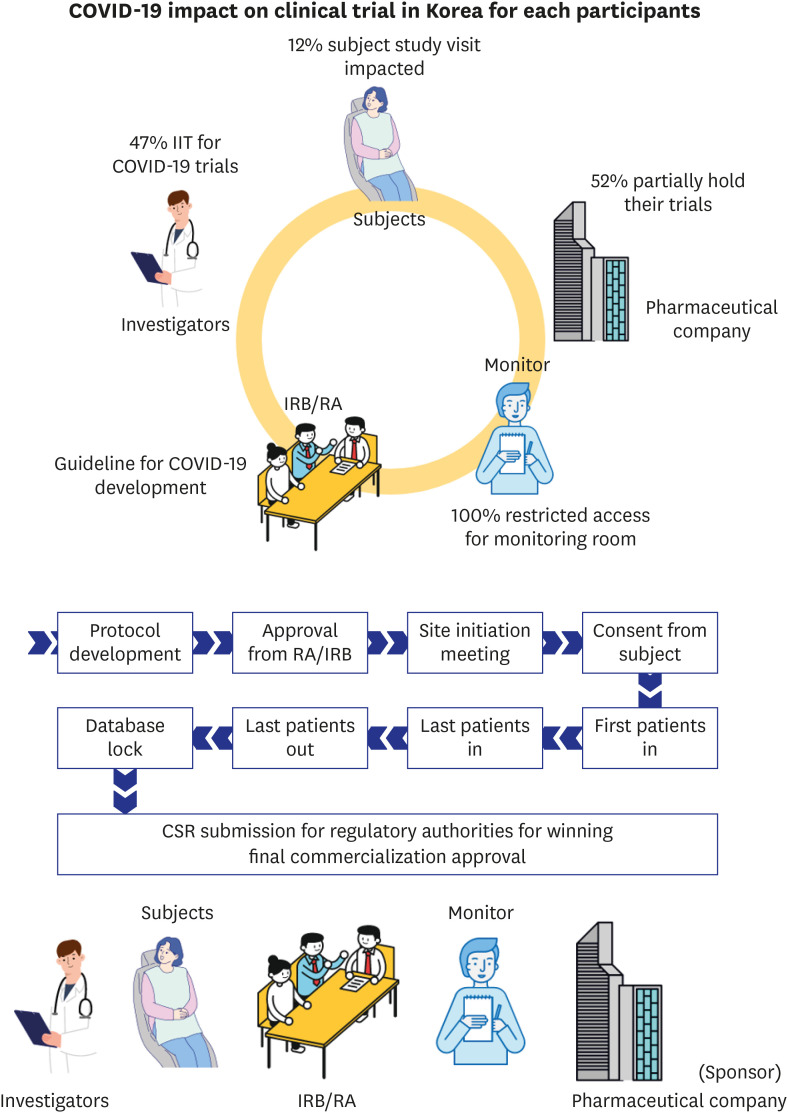
Abstract
Background
The number of clinical trials conducted in Korea continues to increase and an increasing proportion focus on severe and rare incurable diseases. After the start of the severe acute respiratory syndrome, coronavirus disease 2019 (COVID-19), Korea Centers for Disease Control and Prevention (KCDC) developed guidelines to prevent the spread of infection. This study evaluated the impact of COVID-19 and the KCDC guideline on the conduct of clinical research in Korea. The purpose was to develop recommendations on how to minimize the risk of infection while enabling subjects to take part in the trials if no better alternative treatment options were available.
Methods
The impact on subject's scheduled visits and major milestones of clinical trials in Korea were measured by conducting a survey among clinical project manager (CPMs) working at global clinical research organization. The policy on monitor's access to hospital and site initiation meetings was investigated through correspondence with clinical trial center of 39 hospitals. The Top 25 pharmaceutical companies' official press and public clinical trial registry database were used to analyze companies' trial strategy during the pandemic and COVID-19 clinical research status, respectively.
Results
Of 85 CPMs, 12% reported that trial subjects' scheduled visits had been affected in their project. Monitors' access to hospital for source data verification was restricted at all sites in February 2020. Accordingly, 43% of 105 CPMs reported that the COVID-19 epidemic had an effect on study major milestones and data cleaning and database lock accounted for > 60% of milestones affected. In addition, 87% sites advised not to have site initiation meetings and 52% pharmaceutical companies suspended recruitment or new study start-up due to the pandemic. On the other hands, the number of COVID-19 related clinical trials increased rapidly in Korea and worldwide, with investigator-initiated trials accounting for 47% and 63% of all trials locally and globally, respectively. Most trials were phase 2 and were in the recruitment stage.
Conclusion
The COVID-19 and the KCDC guideline influenced all parties involved in clinical trials in Korea. In order to ensure the safety and well-being of trial subjects during the pandemic, new approaches are required for clinical trials to respond to the impact actively. Method of non-contact is developed to replace and supplement the face-to-face contact and alternatives to reduce the travel is introduced to decrease the risk of infection for all trial participants in whole trial process. The relevant regulations should be developed and the guidelines for foreign countries need to be adopted in accordance with the situation in Korea. COVID-19 trial is rapidly increasing worldwide and continuous support of health authorities, regulation, and facilities is required for developing the treatments with protecting all trial participants.
Keywords: COVID-19, Clinical Trial, Korea
Graphical Abstract

INTRODUCTION
In terms of the clinical trial share, Korea ranks eighth globally and is in third place in a single country clinical trial basis.1 The number of approved clinical trials for medical product conducted in Korea increased from 143 cases in 2003 to 679 in 2018.2 The Ministry of Food and Drug Safety (MFDS) has reported an increasing trend of trials for severe and rare incurable diseases.2 Anti-cancer clinical trials account for 36.4% of all clinical trials, and have been the most common indication of clinical trial for 3 consecutive years.2 The conduct of clinical trials consists of a series of processes and multiple parties are involved in the overall trial process.3 Accordingly, face to face contact, meetings and travel to designated places are required (Fig. 1).
Fig. 1. Series of process and involved parties for conduct of clinical trial for medical products.
During this process, face-to-face contact and travel occurs for reasons such as meeting of Regulatory Authority and Institutional Review Board committee for protocol review, subjects' travel to hospital for protocol scheduled visits and written informed consents, while touch base with investigator and delegated hospital staff and monitor's visits for source data verification, triggering meeting with trial staff in hospital.
COVID-19 = coronavirus disease 2019, CSR = clinical study report, IRB = Institutional Review Board, RA = regulatory authority.
Coronavirus disease 2019 (COVID-19) has spread globally since the first outbreak in December 2019 from Wuhan in Hubei Province, China.4 The first case in Korea was confirmed on January 20, 2020, in an individual who entered Korea from Wuhan.4 Compared to previous respiratory syndrome, Middle East respiratory syndrome (MERS) epidemic that occurred in 2012-2015, COVID-19 had greater community spread.5 The first case not linked with epidemiologic evidence, identified on February 16, 2020, accelerated increase in the number of confirmed cases due to community transmission.4 The Korea Centers for Disease Control and Prevention (KCDC) raised the disease alert to the highest level on February 23, 2020 to heighten the response (Fig. 2).4 As there was no available treatment approved for COVID-19 in Korea yet, the government emphasized prevention, to avoid the spread of infection. This strategy influenced not only personal lifestyles but also the conduct of clinical trials of medical products.
Fig. 2. The number of cumulative confirmed cases of MERS and COVID-19 every five days since the discovery of the first confirmed cases in Korea, May 20, 2015 for MERS and January 20, 2020 for COVID-19.
This graph shows a change in the number of confirmed cases every five days since the discovery of the first confirmed cases in Korea, May 20, 2015 for MERS and January 20, 2020 for COVID-19. Final total confirmed number was 186 for MERS in Korea. In case of COVID-19, since February 2020, community transmission led to significant increase of confirmed cases. As of April 15, 2020, a total number of 10,591 confirmed cases and 225 death cases were reported in Korea and 1,926,410 cases globally from 216 countries, including 124,754 deaths
MERS = Middle East respiratory syndrome, COVID-19 = coronavirus disease 2019.
There were no exceptions made to compliance with KCDC guideline when conducting clinical trials, because of the concern about infection during the operation of trial. The guideline called on all citizens to maintain social distancing, refrain from visiting crowded places and reduce gatherings as well as outdoor activities.6 Conducting clinical trials results in an increased number of subject visits to hospitals, face to face contact and meetings. There was thus a conflict between being compliant with the KCDC guideline firmly and clinical trials activities, including encouraging subjects' continuous participation. New approaches were necessary in clinical trials to eliminate the risk of infection by complying with the guideline and enable subjects to continue to participate in trials if no better alternative treatment options were available, for protecting the subjects' safety and well-being.
The impact of the COVID-19 pandemic has not been addressed on clinical trials in Korea. The study evaluated the impact of the COVID-19 epidemic and the KCDC disease control guideline on the conduct of clinical research in Korea, on subjects, investigators, monitor, pharmaceutical companies, Institutional Review Boards (IRBs) and regulatory authorities (RAs), in order to suggest recommendations for conducting clinical trials during the pandemic.
METHODS
To measure the impact of COVID-19, the study selected individual indicators for each involved party in clinical trials. To evaluate the impact on subjects' study visits and study major milestones, a questionnaire was administered. The survey was distributed to total 140 clinical project manager (CPMs) who were working at global clinical research organization and responsible for trials performed in Korea, according to method of simple random sampling from February 24, 2020 to March 7, 2020. The questionnaire included questions on whether COVID-19 had an effect on trial subjects' scheduled study visits and whether there was any major milestone influenced or expected to be delayed by COVID-19. The milestones could be described by the CPMs and in the process of data analysis, they were classified as site initiation, close out, first patients in/last patient in/last patient out, data cleaning and database lock. If a CPM reported that there was an impact on the milestone however did not provide details, they were contacted to try to obtain further information. If response was not received, the impact was classified as not specified.
The impact of the policy by trial sites on monitor's access to monitoring room and site initiation was investigated by contacting clinical trial center in 39 hospitals. Majority of hospitals (95%) were selected in Seoul and Gyeonggi-do where clinical trials were actively performed in Korea and Gyeongsangbuk-do and Daegu where high infection rate was shown. They were contacted for the week of February 24, 2020 to March 1, 2020, when the confirmed cases increased dramatically and the week of April 23 to 29, 2020, when the daily confirmed cases decreased less than 10 in Korea. The response on the access of monitor was classified as total closure, partially available and available, depending on the degree of access that monitors were permitted.
The official press statement of the top 25 pharmaceutical companies on the conduct of their clinical trials during COVID-19 pandemic were reviewed in April 2020 and their decision were classified.7 Clinical trials for COVID-19 listed in local and worldwide public clinical trial registry databases, provided by MFDS and the United States (US) National Institutes of Health were analyzed, during the week of June 22 to 28, 2020, according to the used investigational products, study status, phase and initiating parties, using the pivot table function.8,9 For investigational products-based analysis, drug intervention studies for COVID-19 from both database were included. The used drugs in each arm of individual trial were classified and if 2 or more products were being administered in the same arm as a combination, each product was counted separately.
RESULTS
Impact of COVID-19 on subjects' scheduled study visits
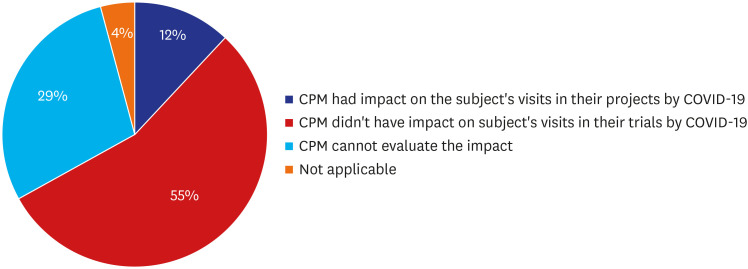
Of 140 CPMs, survey was retrieved from total 105 (75%). Of 105 respondents, 84 CPMs provided valid answer on the question about the impact of COVID-19 on subject study visits in Korea and others left it blank. Of 84 CPMs, 12% reported that they had an impact of COVID-19 in their project, 55% reported there was no impact on the subject visits, 29% reported that they had no planned subject's visit during the survey period so the impact could not be estimated, and 4% reported that they didn't have active subjects in Korea at the time of survey, because recruitment was not initiated, or all subject visits were already completed (Fig. 3). The confirmed reason was that hospitals started restricting patient's physical visit from 2 local cluster, Daegu and Gyeongsangbuk-do, with the highest infection rates, 89.7% in Korea, while Seoul had a 1.8% infection rate at the time of the survey.10 The number of outpatients visiting hospitals was lower during the COVID-19 epidemic that in the corresponding period in 2019 and there were some trial subjects who requested to postpone hospitals visits because of concern about COVID-19.11
Fig. 3. Impact of COVID-19 on subjects' scheduled study visits (%).
COVID-19 = coronavirus disease 2019.
Impact of COVID-19 on monitor's access to hospital for source data verification
Of total 39 sites contacted, during the week of February 24, 2020 to March 1, 2020, all sites (100%) restricted external visitor's access to the hospital, including that of monitors. Four of the sites (10%) allowed only urgent monitoring to comply with the KCDC guideline. For the week of April 24 to 30, 2020, as the number of daily confirmed cases decreased, sites started opening the monitoring rooms again. Of 39 sites, 20 (51%) partially opened the room with only limited seats available to maintain social distancing, 9 (23%) made the room available, and 10 (26%) remained closed the room but planned to reopen on May 1, 2020 (Fig. 4). Even the sites that had reopened the room, applied the guidance for preventing spread of infection. Monitors who had visited countries with a high incidence of COVID-19 or lived with someone who had visited a high incidence country within 14 days were still restricted from visiting the monitoring room. Some sites requested non-contact communication with site staff.
Fig. 4. Impact of COVID-19 on monitors' access to monitoring rooms at 39 hospitals in Korea (%). (A) Feb 24 to March 1, 2020, (B) Apr 24 to 30, 2020.
COVID-19 = coronavirus disease 2019.
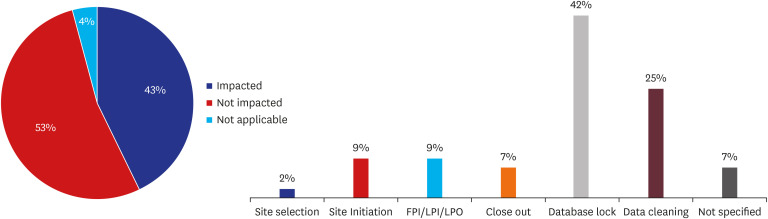
Of 140 CPMs, a total 105 CPMs (75%) answered the question of survey about impact on study milestone by COVID-19 in Korea. Of 105 CPMs, 43% reported that they expected the impact in their project. 53% reported that study milestone was not affected, and 4% reported that the study had been completed so the question was not applicable. Data cleaning and database lock were the most common milestone affected (> 60%), as a result of restriction of monitor's access to the sites which resulted in delayed source data verification (Fig. 5).
Fig. 5. Impact of COVID-19 on study major milestones and specific milestones affected (%).
COVID-19 = coronavirus disease 2019, FPI/LPI/LPO = first patient in/last patient in/last patient out.
Impact of COVID-19 on the conduct of trials by investigators
The site initiation meeting is an important milestone that marks the trial start at the site. Thirty-three (85%) out of 39 sites advised not to have site initiation meetings in February 2020, considering the meeting brings external visitors to the hospital and entail a gathering for the meeting. For the critical trials which should be initiated urgently, such as COVID-19 trials, in the site where onsite initiation meeting is not recommended, teleconference or web-based site initiation meeting is the option which could be utilized, considering MFDS allow the teleconference IRB meeting and web-based training for clinical trial workers.12,13
Some trial sites prohibited visits from subjects living in area with high infection rates, and subjects who were exposed to infectious agents were required to be quarantined according to the KCDC guideline, so some subjects were unable to attend scheduled study visits.6 Although clinical trial drugs could be delivered in accordance with exceptional regulation permit, appropriate examination should be conducted for subject's safety by investigators.12 Through temporally allowed remote assessment by MFDS for trial subjects, it is required for investigator to evaluate the subjects' condition even during their isolation whether it is suitable to prescribe same investigational products repeatedly and whether laboratory tests could be omitted.12
Impact of COVID-19 on pharmaceutical companies' (Sponsors') management of their clinical trials.
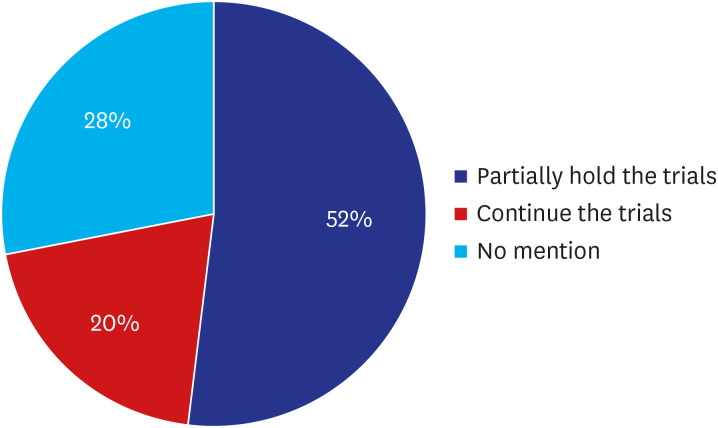
As a final decision-making authority on continuation and suspension of their clinical trials, the limitation and reluctance of subject to visit hospitals and medical profession's burden for COVID-19 control are the factors to consider when deciding whether to proceed with trials, based on risk and benefit assessments.3,11 RA of United Kingdom, Medicines and Healthcare products Regulatory Agency, advised for the early phase healthy volunteer trials, where there is no therapeutic benefit to the volunteer but taking part in the trial dose pose a risk of infection.14 To avoid adding to demands on healthcare system during this unprecedented crisis with COVID-19 epidemic and to reduce the possibility of infection of trial participants, 13 (52%) of the 25 top pharmaceutical companies declared to hold recruitment or activation of the new trials at least partially.7 Five (20%) companies continued ongoing trials in close discussion with the trial investigators in all the participating countries. Seven (28%) pharmaceutical companies did not mention a change in their conduct of trials on their official website or in press release (Fig. 6).
Fig. 6. Impact of COVID-19 on pharmaceutical companies' (Sponsors') management of their clinical trials (%).
COVID-19 = coronavirus disease 2019.
Multiple countries are turning to pharmaceutical companies to develop coronavirus treatments, so accordingly, the number of COVID-19 related clinical trials is growing very rapidly worldwide.15 A notable feature of the global COVID-19 treatment clinical study is that the existing licensed treatment or new drug candidate material is being evaluated to determine whether they can be repurposed for treating COVID-19.16 In Korea, the MFDS approved a total of 15 COVID-19 clinical trials, 6 in March 2020 and 3 in each of the following 3 months.8 Two trials (13%) were for vaccine development and 13 (87%) were for treatment development. A total 10 COVID-19 studies (66%) were recruiting patients or preparing for enrollment. Of the 15 trials, investigator-initiated trials and sponsor-initiated trials accounted for almost the same percentage, 7 trial (47%) and 8 trials (53%) each. Among the sponsor-initiated trials, phase 2 occupied highest portion (27%) (Fig. 7). Remdesivir was the most commonly used investigational drug (3 trials), followed by hydroxychloroquine and nafamostat mesylate with 2 trials. Kyungpook National University Hospital, located in Daegu, and Seoul Medical Center in Seoul, which operated a professional negative-pressure isolation ward took part in the largest number of COVID-19 clinical trials in Korea, with 4 trials each.
Fig. 7. COVID-19 clinical trial status in Korea (%). (A) Treatment/vaccine, (B) phase, (C) status, and (D) initiating party.
COVID-19 = coronavirus disease 2019, IIT = investigator initiated trial, MFDS = Ministry of Food and Drug Safety, SIT = sponsor initiated tral.
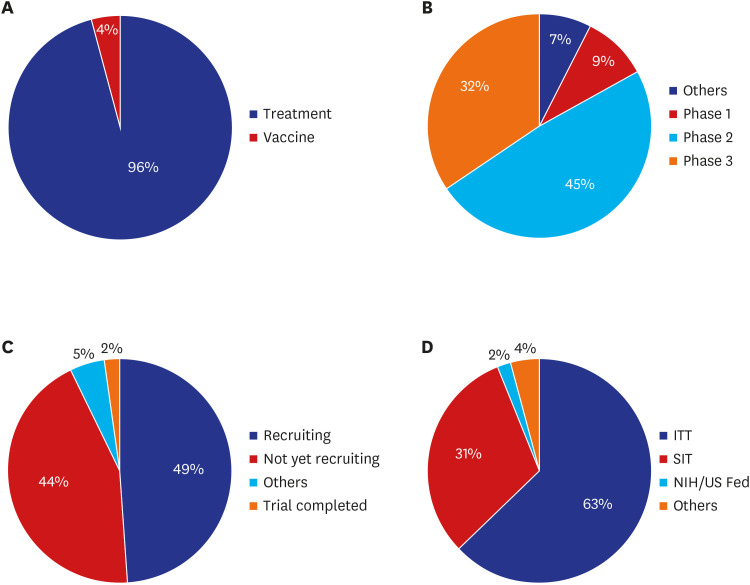
Globally, a total 963 drug or biological agent intervention clinical trials were registered for COVID-19.9 In terms of study status, almost half (49%) were recruitment phase. Regarding initiating parties, 591 trials (63%) were investigator-initiated, 297 (31%) were sponsor initiated and 17 (2%) were sponsored by the US National Institutes of Health or US Federal Reserve, as of June 22, 2020.15 The majority of studies were phase 2 trials (45%) and treatment development trials (96%) (Fig. 8). Hydroxychloroquine was the most commonly used investigational drug (Table 1).
Fig. 8. COVID-19 clinical trial status worldwide (%). (A) Treatment/vaccine, (B) phase, (C) status, and (D) initiating party.
COVID-19 = coronavirus disease 2019, IIT = investigator initiated trial, NIH/US Feb = US National Institutes of Health or US Federal Reserve, SIT = sponsor initiated trial.
Table 1. Total number of trials worldwide using each investigational product according to ClinicalTrials.gov.
| Investigational productsa | Total number of clinical trials using the products | Percentage in total clinical trials,b (%) |
|---|---|---|
| Hydroxychloroquine | 167 | 17.3 |
| Tocilizumab | 37 | 3.8 |
| Azithromycin | 32 | 3.3 |
| Convalescent plasma | 30 | 3.1 |
| Lopinavir | 28 | 2.9 |
| Ivermectin | 27 | 2.8 |
| Ritonavir | 26 | 2.7 |
| Favipiravir | 24 | 2.5 |
| Chloroquine | 20 | 2.1 |
| Ruxolitinib | 17 | 1.8 |
| Remdesivir | 16 | 1.7 |
| Enoxaparin | 15 | 1.6 |
| Colchicine | 14 | 1.4 |
| Baricitinib | 13 | 1.3 |
| Methylprednisolone | 13 | 1.3 |
| Anakinra | 13 | 1.3 |
| Nitric oxide | 11 | 1.1 |
aOnly products that were being investigated in more than 10 trials are shown; bTotal number of clinical trials searched in ClinicalTrials.gov for COVID-19 = 967.
Response of IRBs and RAs
Several national authorities issued guidance for clinical trials during the COVID-19 emergency.12,14,17,18 In common, they provided detailed guidance on how to respond to unexpected changes caused by the COVID-19 pandemic and focused on the health care of the trial subjects, protecting their safety and well-being during the COVID-19 pandemic. The Korea RA, MFDS released guidance, Considerations for clinical trials in a situation of COVID-19 on March 20, 2020.12 They proposed IRB expedite review for COVID-19 trials and suggested IRBs work cooperatively to ensure prompt review.12 If the trial is conducted in multiple sites, they recommended that a joint review committee be established to review each study protocol and make a joint decision.12 To prevent the spread of COVID-19 during review meetings, it was suggested that the review process be modified by having non-contact review meetings, and that this be documented.12 In order to support COVID-19 drug development, the MFDS applied for expedited review of COVID-19 trial protocols. A trial of remdesivir for treatment of COVID-19, sponsored by Gilead Sciences, Inc was submitted to the MFDS for RA approval on February 27, 2020 and approved on March 2, 2020.
The US Food and Drug Administration suggested that sponsors and clinical investigators document the reason for any contingency measures implemented, how restrictions related to COVID-19 led to changes in the study conduct, which trial participants were affected and how these participants were affected in the Clinical Study Report.17 The European Medicine Agency guided for regularities to put priority on any clinical trial application for the treatment or prevention of COVID-19 infection, and the approval of applications for substantial amendments to existing clinical trials if necessary as a result of the COVID-19 pandemic.
DISCUSSION
The COVID-19 pandemic is having a global impact. Clinical trials are one of the areas that have been affected. Clinical trials are meaningful in that they provide patients with novel treatment options during the process, and if they lead to marketing of new treatments, they may be of benefit to many future patients. Moreover, considering that clinical trials are required for developing COVID-19 treatments, conditions should be established in which necessary clinical trials can be carried out smoothly even during the pandemic, while protecting all trial participants' safety. In Korea, all parties involved in the conduct of trials have been affected by COVID-19 pandemic. Various guidelines for conducting trials during the COVID-19 pandemic have been released both locally and globally for supporting the operation of trials. Guidelines developed by foreign countries should be considered and adopted in accordance with the situation in Korea if they are judged to be appropriate.
Twelve percent CPMs responded that subject study visits were not performed as planned according to the protocol. Although the proportion of trials not affected (55%) was higher, given that the provision of treatment is contingent on assuring the safety of subject, 12% is a sizeable proportion that cannot be overlooked. If the treatment that subjects receive through clinical trial participation cannot be replaced by the standard of care, action should be taken to maintain the treatment while complying with the KCDC guideline. Under the current Article 50 (1) of the Pharmaceutical Affairs Act, the sale of medicines outside pharmacies is prohibited in Korea, which makes it impossible to deliver medicines by courier.19 However, MFDS released a guideline that allows investigational products to be delivered to subjects directly on a temporary basis, to enable them to receive the products while reducing the risk of SARS-CoV-2 infection that they could face on their way to hospital.12 The guideline stipulates that the products be stored according to protocol requirements during the delivery process by a certified carrier.12
The US FDA guidance advises investigators to confirm that subjects voluntarily consent to receive investigational products via courier. The Italy RA, decrees of the Italian President of the Council of Ministers allow healthcare providers to provide an increased amount of investigational product, to cover an extended period of treatment if it is beneficial and safe for the subjects. For the products that are administered in a health care setting, the US FDA permits utilize home nursing services or healthcare professionals to serve as alternative sites, in order to keep access to medical products from being cut off even if the products needs to be administered by professionals with specialized skills.17 The guidance of the European Medicines Agency (EMA) states that it is acceptable for laboratory, imaging or other diagnostic tests to be conducted at a local laboratory or relevant clinical facility certified to perform such tests routinely.18 For the introduction of these foreign guidelines in Korea, policies on the supply and demand of necessary personnel or the designation criteria of alternative institutions must first be in place.
Comparing monitoring room availability for source data verification in February and April 2020, it is evident that it is affected by the number of COVID-19 confirmed cases per day. Given the possibility of worsening of the pandemic situation, monitoring rooms may be forced to close again and considering the limited seats available and the high demand on monitor to verify cumulative clinical trial data, measures should be introduced to verify clinical data efficiently with fewer site visits or the methods of obtaining clinical data that require less verification should be considered.
Risk-based monitoring can be recommended. It enables earlier detection of issues through focused centralized and remote monitoring activities, and targeted on-site visit only for key risk indicators. This can reduce the total number of on-site monitoring visits required. Association of clinical research organizations has suggested that even after routine monitoring is resumed, it is not necessary to perform 100% source data verification if remote monitoring was closely performed during the closure of monitoring room.20 Regulations in Italy have allowed remote source data verification exceptionally, limited to COVID-19 pandemic once these methods are described in a specific guideline by the sponsor and clinical research organization and approved by the Personal Data Protection Officer of the trial site.21 Once the research is equipped with a mechanism for collecting data remotely and directly from the patient or vendor, by using methods such as electronic questionnaires, centralized laboratories or electronic image data, remote verification can supplement on-site monitoring.
The increasing number of investigator-initiated trials and trials conducted under the auspices of US Federal Reserve and the US National Institutes of Health indicates that all parties are making concerted efforts to develop COVID-19 treatments and vaccines.9 Considering that the number of clinical trials of COVID-19 related products is rapidly increasing and that most patients are being recruited, measures are needed to ensure an ongoing supply of clinical trial drugs. It is necessary to diversify supply and demand channels for raw materials and to establish regular import and export channels between countries.
Given that the studies target patients with SARS-CoV-2 infection, relevant regulations should be implemented and facilities should be supported to use non-contact methods in order to protect investigators and trial staff. As one of non-contact approaches, telephone consent process is allowed for patients during isolation.12 Guidance on the management of clinical trials during the COVID-19 pandemic by EMA advises the presence of an impartial witness, if written consent by the trial participant is not possible.18 To enable trial facilities to maintain the non-contact between investigators and trial subjects, they should be well equipped for COVID-19 trials with capabilities such as electronic informed consent or device to electronically scan and save the signed informed consent without bringing the form out of the subjects' ward .
This study has some limitations. Although a single indicator was used to identify the impact of the COVID-19 pandemic on each party, the effects of more diverse areas needs to be assessed using a greater diversity of indicators. The analysis of COVID-19 related trials was based on a search of ClinicalTrials.gov, so the trials not registered in this database were not included in the analysis. We only consider the guidance from RAs in the US, Korea, European Union and the United Kingdom, so referring to the guidance of other RA could help to suggest more various measures.
All RA guidelines put patient safety first, and MFDS is making continuous efforts to conduct clinical trials that are beneficial to participants. Health authorities and policymaker should refer to various guidelines and consider alternative measures to conduct trials during this pandemic situation, taking the scope of the application into consideration while prioritizing subject safety, so that the most appropriate method can be implemented and adopted in Korea, in a timely manner. At this time, the impact of the COVID-19 pandemic on clinical trials in Korea has not been as marked as in some other countries. However, restrictions on subject study visits to hospital can directly impact on subject safety and monitor's restricted access to site caused a delayed safety review for subjects. Therefore all the lessons learned and policies implemented internally and externally should be evaluated, in order to be prepared for persistence of the COVID-19 pandemic or future pandemics. Development of the required regulations, strategies, facilities, and platform could help Korea not only adapt to the rapidly changed environments and protection of trial participants, but also emerge as a contributing country in the field of trials. In order to increase in the number of clinical trials for treatment of serious diseases, consideration should be given to developing regulations that will enable patients who have difficulty with visiting hospital physically even after pandemic is controlled, so that varying methods can be applied to patients with different clinical conditions.
ACKNOWLEDGMENTS
We express our sincere appreciation for all clinical project managers who conducted the survey and all clinical trial participants who contributed new drug developments.
Footnotes
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Jeon JY, Yu KS.
- Methodology: Jeon JY.
- Writing - original draft: Jeon JY.
- Writing - review & editing: Kim HK, Yu KS.
References
- 1.Korea Clinical Trials Information Center. [Updated 2020]. [Accessed June 29, 2020]. https://www.koreaclinicaltrials.org/kr/contents/datainfo_data_01_tab03/view.do.
- 2.Ministry of Food and Drug Safety. Presentation of the results of approval of clinical trials for medicines in Korea in 2018. [Updated 2019]. [Accessed May 4, 2020]. https://www.mfds.go.kr/brd/m_99/view.do?seq=43284.
- 3.ICH GCP. ICH harmonized guideline integrated addendum to ICH E6(R1): Guideline for Good Clinical Practice ICH E6(R2) ICH Consensus Guideline. [Updated 2017]. [Accessed June 29, 2020]. https://ichgcp.net/
- 4.Korean Society of Infectious Diseases; Korean Society of Pediatric Infectious Diseases; Korean Society of Epidemiology; Korean Society for Antimicrobial Therapy; Korean Society for Healthcare-associated Infection Control and Prevention; Korea Centers for Disease Control and Prevention. Report on the epidemiological features of coronavirus disease 2019 (COVID-19) outbreak in the Republic of Korea from January 19 to March 2, 2020. J Korean Med Sci. 2020;35(10):e112. doi: 10.3346/jkms.2020.35.e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ministry of Health. Daily status of Middle East Respiratory Syndrome, MERS. [Updated 2018]. [Accessed June 29, 2020]. http://www.mohw.go.kr/react/al/sal0301ls.jsp?PAR_MENU_ID=04&MENU_ID=0403.
- 6.Korea Centers for Disease Control and Prevention. COVID-19 response guideline 9th edition by Central Disaster Management Headquarters · Central Disease Control Headquarters. [Updated 2020]. [Accessed June 29, 2020]. https://www.cdc.go.kr/board/board.es?mid=a20507020000&bid=0019.
- 7.Korea Pharmaceutical and Bio-Pharma Manufacturers' Association. 2019 Pharmaceutical Industry Database Book Statistical Information. [Updated 2019]. [Accessed June 29, 2020]. http://www.kpbma.or.kr.
- 8.Ministry of Food and Drug Safety. Integrated drug information system. [Updated 2020]. [Accessed June 24, 2020]. https://nedrug.mfds.go.kr/searchClinic.
- 9.U.S National Library of Medicine. ClinicalTrials.gov. [Updated 2020]. [Accessed June 28, 2020]. https://clinicaltrials.gov/
- 10.Ministry Health and Welfare. Daily status of COVID-19. [Updated 2020]. [Accessed March 20, 2020]. http://ncov.mohw.go.kr/bdBoardList_Real.do.
- 11.Korean Hospital Association. [Updated 2020]. [Accessed June 28, 2020]. https://www.kha.or.kr.
- 12.Ministry of Food and Drug Safety. Considerations for clinical trials in a situation of COVID-19. [Updated 2020]. [Accessed March 27, 2020]. https://www.mfds.go.kr/index.do.
- 13.Ministry of Food and Drug Safety. Notice of training considerations for clinical trial workers according to corona 19 situation. [Updated 2020]. [Accessed June 28, 2020]. https://www.mfds.go.kr/index.do.
- 14.United Kingdom Medicines and Healthcare Products Regulatory Agency. Synopsis of MHRA advice on management of clinical trials in relation to coronavirus. [Updated 2020]. [Accessed March 27, 2020]. https://www.gov.uk/government/collections/mhra-guidance-on-coronavirus-covid-19.
- 15.Korea National Enterprise for Clinical Trials. Analysis of domestic and international clinical trials. [Updated 2020]. [Accessed June 28, 2020]. https://www.koreaclinicaltrials.org/kr/board/covid19_smry/boardView.do?bbsIdx=888&pageIndex=1&searchCondition=&searchKeyword=
- 16.Korea National Enterprise for Clinical Trials. KoNECT brief. Ed1. [Updated 2020]. [Accessed March 27, 2020]. https://www.konect.or.kr/
- 17.Food and Drug Administration. Guidance on Conduct of Clinical Trials of Medical Products during COVID-19 Public Health Emergency. [Updated 2020]. [Accessed June 28, 2020]. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/fda-guidance-conduct-clinical-trials-medical-products-during-covid-19-public-health-emergency.
- 18.European Medicines Agency. Guidance on the management of clinical trials during the COVID-19 Pandemic. [Updated 2020]. [Accessed June 28, 2020]. https://ec.europa.eu/health/sites/health/files/files/eudralex/vol-10/guidanceclinicaltrials_covid19_en.pdf.
- 19.Korea Ministry of Government Legislation. Korea Pharmaceutical Affairs Act. [Updated 2020]. [Accessed June 28, 2020]. http://law.go.kr/%EB%B2%95%EB%A0%B9/%EC%95%BD%EC%82%AC%EB%B2%95/%EC%A0%9C50%EC%A1%B0.
- 20.Association of Clinical Research Organizations. ACRO's considerations on monitoring during COVID-19. [Updated 2020]. [Accessed June 28, 2020]. https://www.acrohealth.org/acro-considerations-on-monitoring-during-covid-19/
- 21.The Italian Medicines Agency (AIFA) Clinical trials' management in Italy during the COVID-19 (coronavirus disease 19) emergency. [Updated 2020]. [Accessed June 28, 2020]. https://www.aifa.gov.it/documents/20142/871583/Comunicato_gestione_studi_clinici_in_emergenza_COVID-19_EN_12.03.2020.pdf/ee1f33e3-bb3e-9ce9-2a93-b33e88eea94d.