Abstract
Background
Coronavirus disease 2019 (COVID-19) was first reported in December 2019 in China, and then it has disseminated worldwide. In Korea, a religious group-related super-spreading event triggered a sudden outbreak in Daegu city and Gyeongsangbuk-do in southeast Korea. This study was undertaken to document the clinical characteristics of patients hospitalized in Gyeongsangbuk-do.
Methods
Three hundred and fifty-two patients with confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection hospitalized at Dongguk University Gyeongju Hospital or at the Andong Medical Center between February 18th and June 30th were enrolled in this study. Medical records were reviewed and demographic and clinical features, including comorbidities, symptoms, radiological and laboratory findings on admission were analyzed. In addition, we sought to identify risk factors of mortality.
Results
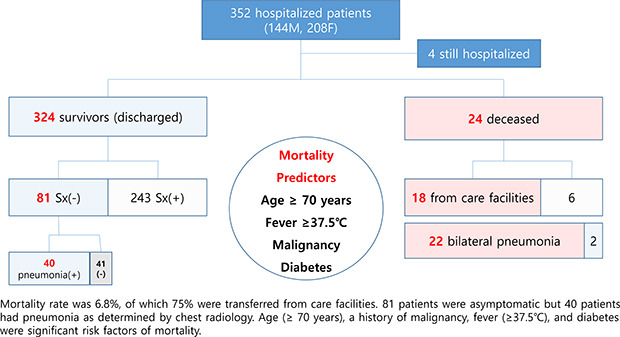
Mean age of the 352 study subjects was 56 years (range, 14–95). The mortality rate was 6.8% and mean age at death was 81 years (range, 57–91). The most common symptom was cough (31.8%) followed by a febrile sensation (28.4%), sputum (17.0%), sore throat (15.6%), and myalgia (13.1%). Eighty-one (23.0%) patients were asymptomatic, but a half of these patients exhibited pneumonic infiltration at presentation. Chest radiology showed no active lesion in 41.8% of the study subjects, bilateral pneumonia in 46.9%, and unilateral pneumonic infiltration in 11.4%. Among 24 patients that died, 18 subjects were transferred from a care facility. An age of ≥ 70 years, previous history of malignancy or diabetes, and fever (≥ 37.5°C) on admission were found to be significant risk factors of mortality.
Conclusion
Patients aged ≥ 70 years, those with fever on admission, and patients with an underlying malignancy or diabetes were found to be more likely to succumb to COVID-19. Elderly in care facilities or hospitalized patients with an underlying disease should receive more attention and be considered for preventive quarantine.
Keywords: Coronavirus, Mortality, Pandemics, Hospitals, Korea
Graphical Abstract
INTRODUCTION
Since the first case of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection was identified in December 2019 in Wuhan city, Hubei Province, China,1,2 the disease has spread explosively.3 On January 30, 2020, the World Health Organization (WHO) declared the outbreak constituted a public health emergency of international concern,4 and at the time of writing, the coronavirus disease 2019 (COVID-19) pandemic presented not only a global threat to health but had also resulted in devastating social and economic consequences worldwide. The disease remains uncontrolled and is responsible for several thousands of casualties daily,3 despite the efforts of pharmaceutical companies and governments to develop effective vaccines and therapeutics.5,6,7 Only 30 cases of COVID-19 were confirmed in Korea before an outbreak associated with a religious group occurred in Daegu city and Gyeongsangbuk-do, in southeast Korea on February 20, 2020.8,9 From the beginning of the epidemic, the Korea Center for Disease Control and Prevention (KCDC) instituted active tracing and tested all identified individuals exposed to cases.10 COVID-19 patients were hospitalized at several local hospitals in negative-pressure rooms, which were specifically designated for COVID-19 at the beginning of the epidemic. However, as the number of cases exponentially increased, patients with no or mild symptoms were also accommodated in therapeutic living centers after triage due to limited hospital capacity.11 In Gyeongsangbuk-do, seven hospitals including Dongguk University Gyeongju Hospital (DUGH) and the Andong Medical Center (AMC) were designated special hospitals for the isolation and treatment of COVID-19 patients, although extremely severe cases were transferred to higher-level hospitals for intensive care. As of July 18, 2020, 13,711 patients had been diagnosed and 294 deaths attributed to COVID-19 in Korea, and of these cases, 10.3% occurred in Gyeongsangbuk-do.12 Unfortunately, the COVID-19 crisis is rapidly evolving, and thus, medical personnel need to be prepared for another infection surge before the advent of any game-changer, like an effective vaccine. In the present situation, understanding the characteristics of hospitalized patients is crucial.
Here, we investigated the demographic and clinical characteristics of COVID-19 patients hospitalized at DUGH and the AMC, Gyeongsangbuk-do, Korea, and sought to identify predictors of COVID-19-related mortality.
METHODS
Study subjects
Three hundred and fifty-two patients (144 males and 208 females) with a confirmed diagnosis of COVID-19 and hospitalized at DUGH or the AMC from the 18th February to the 30th of June 2020, were included in the present study. For patients transferred to DUGH from the AMC, initial AMC records were included to prevent duplication. Information on demographic and clinical characteristics, including chest radiologic (X-ray or computerized tomography) and laboratory findings were collected. Initial symptoms at admission, pre-existing diseases, vital signs (i.e., blood pressure, heart rate, respiratory rate, and body temperature) and pulse oximetry oxygen saturation in room air were obtained. Laboratory findings included complete blood cell (CBC) counts, aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LDH), blood urea nitrogen (BUN), creatinine, serum albumin, C-reactive protein (CRP) levels, and erythrocyte sedimentation rate (ESR). Blood was sampled at time of admission. Therapeutics administered included lopinavir/ritonavir, chloroquine, antibiotics, macrolides, pegylated interferon-α, and oxygen supplementation.
Statistical analysis
Nominal variables are presented as numbers of cases and percentages, and continuous variables as median and interquartile ranges. Treatment outcomes were classified as; ‘discharged,’ ‘discharged to a therapeutic living center,’ ‘transferred to a higher-level hospital’ for intensive care, ‘expired,’ and ‘hospitalized.’ Survival statuses were dichotomized as ‘non-survivors’ or ‘survivors’; ‘survivors’ included patients ‘discharged’ or ‘discharged to a therapeutic living center.’ Five patients that died after transfer to a higher-level hospital were classified as ‘non-survivors.’ Four subjects that remained in hospital were excluded from the comparative analysis of ‘non-survivors’ and ‘survivors.’ The Student's t-test or Mann-Whitney U test was used to determine the significance of differences between these two groups. χ2 analysis or Fisher's exact test was used to determine the significances of differences between categorical variables. Laboratory parameters were classified as ‘above normal limit’ or ‘normal’ or ‘below normal limit’ or ‘normal’ using the following reference ranges; high AST, > 39 U/L (male) and > 31 U/L (female); high ALT, > 40 U/L (male) and > 32 U/L (female); high LDH, > 225 U/L(male) and > 214 U/L (female); high creatinine, 1.3 mg/dL (male) and 1.1 mg/dL (female); anemia, < 14 g/dL(male) and < 12 g/dL(female); low albumin, < 3.5 g/dL; high ESR, > 20 mm/hour; high CRP, > 0.5 mg/dL. Univariate and multivariate logistic regression analyses were performed to investigate associations between clinical and laboratory parameters and risk of death. Potential risk factors included in the univariate analysis were: age ≥ 70 years, care facility resident, presence of a comorbidity (i.e., hypertension, diabetes, cardiovascular disease, dementia, or malignancy), symptoms present at admission (i.e., dyspnea, febrile sensation, and diarrhea), physical examination findings (i.e., body temperature ≥ 37.5°C, systolic blood pressure ≤ 90 mmHg, and pulse oximetry oxygen saturation in room air ≤ 90 mmHg), bilateral pneumonia, and laboratory findings (i.e., leukocytosis, lymphopenia, high AST, high LDH, high creatinine, anemia, high ESR, and CRP). Multivariate logistic regression analysis was used to determine the natures of associations between potential risk factors identified by univariate analysis. All tests were two-sided and P values of < 0.05 were considered to indicate statistical significance. All statistical analyses were performed using the Statistical Package for Social Sciences ver. 20.0 (SPSS, Chicago, IL, USA).
Ethics statement
This study protocol was exempted from ethical review by the Institutional Review Board of Dongguk University Gyeongju Hospital because the analyzed data did not contain personally identifiable information (IRB registration No. 110757-202006-HR-04-02).
RESULTS
General characteristics of the study subjects
Patient characteristics are provided in Tables 1 and 2. Of the 352 study subjects (144 males and 208 females), 292 patients recovered, 37 were transferred to a higher-level hospital, and 19 expired. However, 5 of the 37 transferred died. Thus, in total there were 24 deaths and the mortality rate was 6.8%. Mean age of the study subjects was 56 years (range, 14–95), and notably, 101 (28.7%) were transferred from care facilities (e.g., nursing homes and geriatric nursing hospitals). Mean days spent in hospital was 19.2 (range, 1–98), and the percentages of patients with underlying hypertension, diabetes, dementia, cardiovascular disease, and malignancy were 24.1%, 16.2%, 9.9%, 5.7%, and 4.3%, respectively. At the time of admission, respiratory infection-related symptoms such as cough, fever, sputum, sore throat, and myalgia were the most common manifestations, though 81 patients were asymptomatic. Other symptoms included gastrointestinal symptoms like diarrhea and nausea/vomiting, gustatory and olfactory dysfunction, and fatigue. Twenty-seven (7.7%) patients presented with fever (≥ 37.5°C) on admission. As shown in Table 2, 41.8% of the study subjects showed no pneumonic infiltration, but 46.9% had bilateral pneumonia by chest radiology. Of note, 40 (49.4%) asymptomatic patients exhibited pneumonic infiltration by radiologic examination (data not shown). As for inflammatory markers, 45.7% and 39.2% of the study subjects showed elevated CRP and elevated ESR, respectively. Lopinavir/ritonavir was the most commonly used drug followed by antibiotics and antivirals.
Table 1. General characteristics of the study subjects.
| Variables | Total subjects | Deceased | Survivors | P value | |
|---|---|---|---|---|---|
| Number of cases | 352 | 24 | 324 | ||
| Age, yr | 57 (38–72) | 84 (78–88) | 56 (37–69) | < 0.001 | |
| 10–29 | 61 (17.3) | 0 | 60 (18.5) | ||
| 30–49 | 68 (19.3) | 0 | 67 (20.7) | ||
| 50–69 | 126 (35.8) | 3 (12.5) | 122 (37.7) | ||
| 70–89 | 86 (24.4) | 19 (79.2) | 66 (20.4) | ||
| ≥ 90 | 11 (3.1) | 2 (8.3) | 9 (2.8) | ||
| Sex | 0.154 | ||||
| Male | 144 (40.9) | 12 (50.0) | 128 (39.5) | ||
| Female | 208 (59.1) | 12 (50.0) | 196 (60.5) | ||
| Resident in care facility | 101 (28.7) | 18 (75.0) | 82 (25.3) | < 0.001 | |
| Foreign national | 9 (2.6) | 0 (0.0) | 7 (2.2) | 0.583 | |
| Days of hospitalization | 15 (10–25) | 9 (4–13) | 16 (10–25) | < 0.001 | |
| Outcome | |||||
| Discharged | 239 (67.9) | NA | 239 (73.8) | ||
| Discharged to therapeutic living center | 53 (15.1) | NA | 53 (16.4) | ||
| Transferred to a higher-level hospital | 37 (10.5) | 5 (20.9) | 32 (10.0) | ||
| Death | 19 (5.4) | 19 (79.1) | NA | ||
| Hospitalization | 4 (1.1) | NA | NA | ||
| Rehospitalization | 24 (6.8) | 0 | 24 (7.4) | NA | |
| Comorbidity | |||||
| Hypertension | 85 (24.1) | 14 (58.3) | 70 (21.6) | < 0.001 | |
| Diabetes mellitus | 57 (16.2) | 11 (45.8) | 46 (14.2) | < 0.001 | |
| Dementia | 35 (9.9) | 7 (29.2) | 27 (8.3) | 0.020 | |
| Cardiovascular disease | 20 (5.7) | 4 (16.6) | 16 (5.0) | 0.034 | |
| Malignancy | 15 (4.3) | 4 (16.7) | 11 (3.4) | 0.020 | |
| Symptoms on admission | |||||
| Asymptomatic | 81 (23.0) | NA | 76 (23.5) | ||
| Cough | 112 (31.8) | 5 (20.8) | 105 (32.4) | 0.581 | |
| Sputum | 60 (17.0) | 1 (4.2) | 59 (18.2) | 0.076 | |
| Rhinorrhea | 15 (4.3) | 1 (4.2) | 14 (4.3) | 0.963 | |
| Sore throat | 55 (15.6) | 1 (4.2) | 54 (16.7) | 0.076 | |
| Dyspnea | 32 (9.1) | 5 (20.8) | 27 (8.3) | 0.019 | |
| Gustatory dysfunction | 4 (1.1) | 0 | 4 (1.2) | 0.582 | |
| Olfactory dysfunction | 11 (3.1) | 0 | 11 (3.4) | 0.356 | |
| Febrile sensation | 100 (28.4) | 13 (54.2) | 87 (26.9) | 0.001 | |
| Headache | 43 (12.2) | 1 (4.2) | 42 (13.0) | 0.207 | |
| Myalgia | 46 (13.1) | 1 (4.2) | 45 (13.9) | 0.235 | |
| Fatigue | 17 (4.8) | 2 (8.3) | 15 (4.6) | 0.271 | |
| Chest pain/discomfort | 25 (7.0) | 0 | 25 (7.1) | 0.163 | |
| Nausea/vomiting | 4 (1.1) | 0 | 4 (1.4) | 0.698 | |
| Diarrhea | 13 (3.7) | 3 (12.5) | 10 (3.1) | 0.012 | |
| Physical examination | |||||
| Body temperature, ≥ 37.5°C | 27 (7.7) | 8 (33.3) | 18 (5.6) | < 0.001 | |
| Systolic blood pressure, ≤ 90 mmHg | 14 (4.0) | 5 (20.8) | 9 (2.8) | < 0.001 | |
| Tachypnea, > 20 breaths/min | 272 (77.3) | 22 (91.9) | 246 (75.9) | 0.062 | |
| O2 saturation, ≤ 90 mmHg | 13 (3.7) | 10 (41.7) | 3 (0.9) | < 0.001 | |
Results are presented as median (interquartile ranges) or numbers (%).
NA = non-applicable.
Table 2. Chest imaging findings, laboratory characteristics, and treatments administered.
| Variables | Total subjects | Deceased | Survivors | P value | |
|---|---|---|---|---|---|
| Chest imaging | < 0.001 | ||||
| None | 147 (41.8) | 0 | 145 (44.8) | ||
| Bilateral infiltration | 165 (46.9) | 22 (91.7) | 142 (43.8) | ||
| Unilateral infiltration | 40 (11.4) | 2 (8.3) | 37 (11.4) | ||
| Laboratory findings | |||||
| Leukocyte, > 10.7 × 109/L | 21 (6.0) | 6 (25.0) | 15 (4.6) | < 0.001 | |
| Lymphocyte, < 1.0 × 109/L | 63 (17.9) | 12 (50.0) | 51 (15.7) | < 0.001 | |
| High aspartate aminotransferase | 89 (25.3) | 14 (58.3) | 74 (22.8) | < 0.001 | |
| High alanine aminotransferase | 56 (15.9) | 3 (12.5) | 52 (16.0) | 0.409 | |
| High lactate dehydrogenase | 63 (17.9) | 12 (50.0) | 51 (15.7) | < 0.001 | |
| High creatinine | 24 (6.8) | 6 (25.0) | 17 (5.2) | < 0.001 | |
| Anemia | 73 (20.7) | 18 (75.0) | 55 (17.0) | < 0.001 | |
| Albumin, < 3.5 g/dL | 31 (8.8) | 13 (54.2) | 18 (5.6) | < 0.001 | |
| Erythrocyte sedimentation rate, > 20 mm/hr | 138 (39.2) | 20 (83.3) | 117 (36.1) | < 0.001 | |
| C-reactive protein, > 0.5 mg/dL | 161 (45.7) | 24 (100) | 135 (41.7) | < 0.001 | |
| Treatments | |||||
| Lopinavir/ritonavir | 190 (54.0) | 21 (87.5) | 167 (51.5) | < 0.001 | |
| Chloroquine | 57 (16.2) | 4 (16.7) | 53 (16.4) | 0.888 | |
| Antibiotics | 149 (42.3) | 22 (91.7) | 126 (38.9) | < 0.001 | |
| Macrolide | 88 (25.0) | 10 (41.7) | 78 (24.1) | 0.036 | |
| Pegylated interferon-α | 57 (16.2) | 12 (50.0) | 45 (13.9) | < 0.001 | |
| O2 supplement | 62 (17.6) | 21 (87.5) | 41 (12.7) | < 0.001 | |
Results are presented as numbers (%).
NA = non-applicable.
Characteristics of non-survivors
As shown in Tables 1 and 2, 24 patients (mean age, 80.8 ± 9.1 years) died, and 75% of these were residents or patients at a nursing facility. The most common comorbidity among those that succumbed was hypertension (58.3%), which was followed by diabetes (45.8%). Among the deceased, 29.8% and 16.7% of subjects had dementia or a malignancy, respectively. A febrile sensation (54.2%) was the most common symptom on admission followed by cough and dyspnea (20.8%). On physical examination, 91.9% of the deceased showed tachypnea (> 20 breaths/min). Chest radiology showed 91.7% of the non-survivors had bilateral pneumonia and that the remainder had unilateral pneumonia. CRP levels in all non-survivors at admission were > 0.5 mg/dL.
Risk factors of mortality in COVID-19 patients
Univariate regression analysis showed that non-survivors were older (≥ 70 years) and had a higher prevalence of diabetes, and previous malignancy (Table 3). Physical examination results showed that on admission non-survivors were more likely to have fever, low systolic blood pressure, and low oxygen saturation by pulse oximetry. Bilateral pneumonia was also more common among non-survivors. At admission, laboratory findings revealed that non-survivors more frequently had abnormal leukocyte, lymphocyte, AST, LDH, creatinine, anemia, albumin, ESR, and CRP levels than survivors. Multivariate regression analysis showed age (≥ 70 years), a history of malignancy, fever (≥ 37.5°C), and diabetes were significant risk factors of mortality (Table 4).
Table 3. Univariate regression analysis for mortality risk factorsa .
| Variables | OR (95% CI) | P value | |
|---|---|---|---|
| Age, ≥ 70 yr | 17.217 (6.746–80.064) | < 0.001 | |
| Resident in care facility | 2.819 (0.978–8.127) | 0.055 | |
| Comorbidity | |||
| Hypertension | 1.902 (0.747–4.844) | 0.178 | |
| Diabetes mellitus | 2.687 (1.054–6.846) | 0.038 | |
| Cardiovascular disease | 1.775 (0.311–10.131) | 0.518 | |
| Dementia | 1.125 (0.405–3.128) | 0.821 | |
| Malignancy | 5.767 (1.296–25.661) | 0.021 | |
| Symptoms on admission | |||
| Dyspnea | 2.439 (0.746–7.979) | 0.140 | |
| Febrile sensation | 2.133 (0.864–5.266) | 0.101 | |
| Diarrhea | 3.318 (0.696–15.812) | 0.132 | |
| Physical examination | |||
| Body temperature, > 37.5°C | 18.549 (4.488–76.659) | < 0.001 | |
| Systolic blood pressure, ≤ 90 mmHg | 5.454 (1.248–23.839) | 0.024 | |
| O2 saturation, ≤ 90 mmHg | 7.810 (1.842–33.112) | 0.005 | |
| Chest imaging | |||
| Bilateral infiltration | 5.635 (1.223–25.974) | 0.027 | |
| Laboratory findings | |||
| Leukocyte, > 10.7 × 109/L | 4.561 (1.363–15.263) | 0.014 | |
| Lymphocyte, < 1.0 × 109/L | 3.883 (1.526–9.880) | 0.004 | |
| High aspartate aminotransferase | 4.550 (1.796–11.577) | 0.001 | |
| High lactate dehydrogenase | 4.412 (1.720–11.316) | 0.002 | |
| High creatinine | 2.691 (0.863–8.389) | 0.088 | |
| Anemia | 7.181 (2.571–20.060) | < 0.001 | |
| Albumin, < 3.5 g/dL | 7.488 (2.720–20.614) | < 0.001 | |
| Erythrocyte sedimentation rate, > 20 mm/hr | 4.357 (1.377–13.790) | 0.012 | |
| C-reactive protein | 1.241 (1.132–1.360) | < 0.001 | |
OR = odds ratio, CI = confidence interval.
aAdjusted for age.
Table 4. Multivariate regression analysis for mortality risk factors.
| Variables | OR (95% CI) | P value |
|---|---|---|
| Age, ≥ 70 yr | 18.264 (3.419–97.573) | 0.001 |
| Malignancy | 8.789 (1.436–53.788) | 0.019 |
| Body temperature, > 37.5°C | 21.883 (3.813–125.584) | 0.001 |
| Diabetes mellitus | 5.451 (1.544–19.253) | 0.001 |
OR = odds ratio, CI = confidence interval.
DISCUSSION
COVID-19 triggered a global pandemic unprecedented in modern times, and currently, the worldwide death toll attributed to COVID-19 sets new records daily. As of July 18, 2020, 593,087 deaths had been reported.3 This study describes the clinical characteristics of patients hospitalized with COVID-19 in Gyeongsangbuk-do, Korea based on the medical records of DUGH and the AMC, which are COVID-19 designated hospitals. The ages of the 352 patients included in the study varied from 14 to 95 years, which demonstrates SARS-CoV-2 affects all age groups. As of July 18, confirmed cases and deaths in Korea totaled 13,711 and 294, respectively, a mortality rate of 2.1%.12 Current country mortality rates vary from 0% in Vietnam to 15.4% in the United Kingdom.13 The reason for this wide range of mortality rates among countries is not clear although the effectiveness of quarantine, accessibility to medical treatment, hospital capacities, and racial and genetic differences are considered important reasons. The mortality rate in the present study was higher than for all Korean cases (6.8% vs. 2.1%), which we believed was due to higher percentages of elderly patients (≥ 70 years) (27.5% vs. 10.7%)14 and the hospitalization of more severe cases after triage.
Notably, 29% of our study subjects were from care facilities. The Korean government performed a comprehensive inspection of residents at care facilities in Gyeongsangbuk-do, and as a result, many cases were detected and isolated during the early disease stage. In fact, the majority of fatal cases in the present study involved residents at a care facility. It is evident that special attention and quarantine arrangements are required for this population.
Twenty-four patients were re-hospitalized soon after being discharged from hospitals or therapeutic living centers because their reverse transcriptase-polymerase chain reaction (RT-PCR) results changed from negative to positive. Twelve of the 24 had no symptoms and the others presented respiratory symptoms. All finally recovered their health and were discharged. Readmission cases after discharge have been reported in several studies.15,16 The reasons for readmissions are unclear, though viral reactivation, reinfection, a chronic carrier status, viral gene fragments without activity and false-negative test results have been suggested. The possibility of infectivity in this patient group requires attention.
In this study, 81 patients were asymptomatic but 40 had pneumonia as determined by chest radiology, which is consistent with previous studies.9,17 Furthermore, asymptomatic patients may be continuous sources of community infection. Among the study subjects, 28% and 7% presented with a febrile sensation or had fever (≥ 37.5°C) on admission, respectively. The most common manifestations were similar to those of common cold and included fever, cough, sputum, sore throat, and myalgia. Patients with COVID-19 may not realize they had been infected in the early prodromal phase, which means it is crucial that all exposed people should be traced, tested, and isolated before they spread the virus inadvertently. Furthermore, social distancing and mask wearing are recommended to prevent infection from unidentified carriers.
Sixteen patients presented gastrointestinal symptoms such as nausea, vomiting, diarrhea, and abdominal pain. Only 4 of these patients had gastrointestinal manifestations without common respiratory symptoms such as cough, sputum, rhinorrhea, and fever (data not shown). It has been established that SARS-CoV-2 uses angiotensin-converting enzyme 2 (ACE2), which is widely expressed by gastrointestinal epithelial cells, as a cellular entry receptor.18
Several types of therapeutics have been tried in COVID-19 patients, such as antivirals (lopinavir/ritonavir and pegylated interferon-α), antibiotics, macrolides, and chloroquine. However, in the present study, we could not determine whether these drugs affect disease course, although some have suggested single or combination therapies based on these drugs are effective against SARS-CoV-2.19,20 Drug repositioning approaches are being used to identify effective treatments.21
Several predictors of COVID-19-related mortality have been suggested.22,23 In our study, mean age of non-survivors was greater than that of survivors. In fact, all non-survivors, except two, were ≥ 69 years old. The two exceptions were patients at Daenam Hospital in Cheongdo-gun, which was at the epicenter of the religious group-related outbreak in Gyeongsangbuk-do. One of these patients had schizophrenia and the other had hypertension, diabetes, and dyslipidemia. The KCDC has reported COVID-19-related mortality rates increase sharply from age 70 (25.3% in ≥ 80 year, 9.4% 70 to 79 year, and 2.29% in 60 to 69 year).12 In the current study, non-survivors had a higher prevalence of pre-existing diseases (e.g., hypertension, diabetes, cardiovascular disease, dementia, and malignancy), which is consistent with the results of previous studies.22,23,24 We found diabetes and history of malignancy were risk factors of death. Mortality among patients with diabetes was 19.3%, whereas previous studies have reported rates from 7.3% to 81.3%.25,26,27,28,29 Several factors such as an impaired immune system, a pre-existing proinflammatory state, direct pancreatic damage, and dysregulation of ACE2 signaling have been suggested to contribute to the association between diabetes and COVID-19.30,31 Cancer patients tend to be immunocompromised and at increased risk of serious COVID-19-related events.32,33 In an Italian study on 3,000 COVID-19 cases, 20% of patients that died had a history of malignancy during the previous 5 years.34 In our study, notably, the majority of these patients contracted COVID-19 within a care facility. It is apparent that elderly at care facilities and hospitalized patients with underlying diseases are extremely vulnerable to SARS-CoV-2 infection and require special attention and strict quarantine.
This study has several limitations. First, it was based on the results of retrospective analysis of medical records, which may have been incomplete in terms of data regarding symptoms and preexisting diseases. However, the data recorded in electronic medical records, a Picture Archiving Communication System, and Order Communication System were thoroughly reviewed by medical staff, including three doctors. Second, the study cohort did not include all patients hospitalized at seven COVID-19 designated hospitals in Gyeongsangbuk-do although this study included the majority of hospitalized patients in Gyeongsangbuk-do area.
The present study shows SARS-CoV-2 affects all age groups and that elderly patients with fever on admission, diabetes, or a malignancy are more likely to succumb to the disease. Furthermore, our findings caution that elderly in care facilities and hospitalized patients with another underlying disease require close monitoring for COVID-19.
ACKNOWLEDGMENTS
The authors acknowledge the support of the Gyeongsangbuk-do Medical Association and Gyeongsangbuk-do Provincial Government and the assistance of members of Gyeongsangbuk-do Center for Infectious Disease Control and Prevention. Also, we especially thank the staff of the Andong Medical Centers for the kind help for this study.
Footnotes
Funding: This research was supported by the Gyeongsanbuk-do Medical Association and Dongguk University research fund (2020).
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Moon SS, Lee K, Lee DS.
- Data curation: Moon SS, Park J.
- Formal analysis: Moon SS.
- Investigation: Moon SS, Park J, Yun S, Lee YS.
- Methodology: Moon SS, Yun S, Lee YS.
- Software: Moon SS.
- Validation: Moon SS, Lee K, Lee DS.
- Writing - original draft: Moon SS.
- Writing - review & editing: Moon SS, Lee K, Lee DS.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. [Updated 2020]. [Accessed July 19, 2020]. https://covid19.who.int.
- 4.World Health Organization. Statement on the second meeting of the international health regulations (2005) emergency committee regarding the outbreak of novel coronavirus (2019-nCoV) [Update 2020]. [Accessed July 19, 2020]. https://www.who.int/news-room/detail/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov)
- 5.Thanh Le T, Andreadakis Z, Kumar A, Gómez Román R, Tollefsen S, Saville M, et al. The COVID-19 vaccine development landscape. Nat Rev Drug Discov. 2020;19(5):305–306. doi: 10.1038/d41573-020-00073-5. [DOI] [PubMed] [Google Scholar]
- 6.Ahn DG, Shin HJ, Kim MH, Lee S, Kim HS, Myoung J, et al. Current status of epidemiology, diagnosis, therapeutics, and vaccines for novel coronavirus disease 2019 (COVID-19) J Microbiol Biotechnol. 2020;30(3):313–324. doi: 10.4014/jmb.2003.03011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020;38(1):1–9. doi: 10.12932/AP-200220-0772. [DOI] [PubMed] [Google Scholar]
- 8.Lim J, Jeon S, Shin HY, Kim MJ, Seong YM, Lee WJ, et al. Case of the index patient who caused tertiary transmission of COVID-19 infection in Korea: the application of lopinavir/ritonavir for the treatment of COVID-19 infected pneumonia monitored by quantitative RT-PCR. J Korean Med Sci. 2020;35(6):e79. doi: 10.3346/jkms.2020.35.e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim ES, Chin BS, Kang CH, Kim NJ, Kang YM, Choi JP, et al. Clinical course and outcomes of patients with severe acute respiratory syndrome coronavirus 2 infection: a preliminary report of the first 28 patients from the Korean cohort study on COVID-19. J Korean Med Sci. 2020;35(13):e142. doi: 10.3346/jkms.2020.35.e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korea Center of Disease Control and Prevention. COVID-19 response: Korean government's response system (as of February 25, 2020) [Update 2020]. [Accessed July 19, 2020]. http://ncov.mohw.go.kr/en/baroView.do?brdId=11&brdGubun=111&dataGubun=&ncvContSeq=&contSeq=&board_id=
- 11.Kim SW, Lee KS, Kim K, Lee JJ, Kim JY Daegu Medical Association. A brief telephone severity scoring system and therapeutic living centers solved acute hospital-bed shortage during the COVID-19 outbreak in Daegu, Korea. J Korean Med Sci. 2020;35(15):e152. doi: 10.3346/jkms.2020.35.e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korea Center of Disease Control and Prevention. Coronavirus disease-2019, Republic of Korea. [Update 2020]. [Accessed July 19, 2020]. http://ncov.mohw.go.kr/en/
- 13.Korea Center of Disease Control and Prevention. Global locations with COVID-19. [Update 2020]. [Accessed July 19, 2020]. http://ncov.mohw.go.kr/en/bdBoardList.do?brdId=16&brdGubun=163&dataGubun=&ncvContSeq=&contSeq=&board_id=
- 14.Korea Center of Disease Control and Prevention. Cases in Korea. [Update 2020]. [Accessed August 24, 2020]. http://ncov.mohw.go.kr/bdBoardList_Real.do?brdId=1&brdGubun=11&ncvContSeq=&contSeq=&board_id=&gubun=
- 15.Lan L, Xu D, Ye G, Xia C, Wang S, Li Y, et al. Positive RT-PCR test results in patients recovered from COVID-19. JAMA. 2020;323(15):1502–1503. doi: 10.1001/jama.2020.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen M, An W, Xia F, Yang P, Li K, Zhou Q, et al. Clinical characteristics of rehospitalized patients with COVID-19 in China. J Med Virol. 2020 doi: 10.1002/jmv.26002. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim JY, Choe PG, Oh Y, Oh KJ, Kim J, Park SJ, et al. The first case of 2019 novel coronavirus pneumonia imported into Korea from Wuhan, China: implication for infection prevention and control measures. J Korean Med Sci. 2020;35(5):e61. doi: 10.3346/jkms.2020.35.e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Obukhov AG, Stevens BR, Prasad R, Li Calzi S, Boulton ME, Raizada MK, et al. SARS-CoV-2 infections and ACE2: clinical outcomes linked with increased morbidity and mortality in individuals with diabetes. Diabetes. 2020;69(9):1875–1886. doi: 10.2337/dbi20-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cortegiani A, Ingoglia G, Ippolito M, Giarratano A, Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020;57:279–283. doi: 10.1016/j.jcrc.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Lababidi RM, Mooty M, Bonilla MF, Salem NM. Treatment of severe pneumonia due to COVID-19 with peginterferon alfa 2a. IDCases. 2020;21:e00837. doi: 10.1016/j.idcr.2020.e00837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosa SGV, Santos WC. Clinical trials on drug repositioning for COVID-19 treatment. Rev Panam Salud Publica. 2020;44:e40. doi: 10.26633/RPSP.2020.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Du RH, Liang LR, Yang CQ, Wang W, Cao TZ, Li M, et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 2020;55(5):2000524. doi: 10.1183/13993003.00524-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun Y, Koh V, Marimuthu K, Ng OT, Young B, Vasoo S, et al. Epidemiological and clinical predictors of COVID-19. Clin Infect Dis. 2020;71(15):786–792. doi: 10.1093/cid/ciaa322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo W, Li M, Dong Y, Zhou H, Zhang Z, Tian C, et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020;31:e3319. doi: 10.1002/dmrr.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan Y, Yang Y, Wang F, Ren H, Zhang S, Shi X, et al. Clinical characteristics and outcomes of patients with severe covid-19 with diabetes. BMJ Open Diabetes Res Care. 2020;8(1):e001343. doi: 10.1136/bmjdrc-2020-001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chung SM, Lee YY, Ha E, Yoon JS, Won KC, Lee HW, et al. The risk of diabetes on clinical outcomes in patients with coronavirus disease 2019: a retrospective cohort study. Diabetes Metab J. 2020;44(3):405–413. doi: 10.4093/dmj.2020.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team. Vital surveillances: the epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19): China, 2020. China CDC Wkly. 2020;2(8):113–122. [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu L, She ZG, Cheng X, Qin JJ, Zhang XJ, Cai J, et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31(6):1068–1077.e3. doi: 10.1016/j.cmet.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hussain A, Bhowmik B, do Vale Moreira NC. COVID-19 and diabetes: knowledge in progress. Diabetes Res Clin Pract. 2020;162:108142. doi: 10.1016/j.diabres.2020.108142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Noh J, Chang HH, Jeong IK, Yoon KH. Coronavirus disease 2019 and diabetes: the epidemic and the Korean Diabetes Association perspective. Diabetes Metab J. 2020;44(3):372–381. doi: 10.4093/dmj.2020.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ueda M, Martins R, Hendrie PC, McDonnell T, Crews JR, Wong TL, et al. Managing cancer care during the COVID-19 pandemic: agility and collaboration toward a common goal. J Natl Compr Canc Netw. 2020;18(4):366–369. doi: 10.6004/jnccn.2020.7560. [DOI] [PubMed] [Google Scholar]
- 33.Dariya B, Nagaraju GP. Understanding novel COVID-19: Its impact on organ failure and risk assessment for diabetic and cancer patients. Cytokine Growth Factor Rev. 2020;53(53):43–52. doi: 10.1016/j.cytogfr.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323(18):1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]


