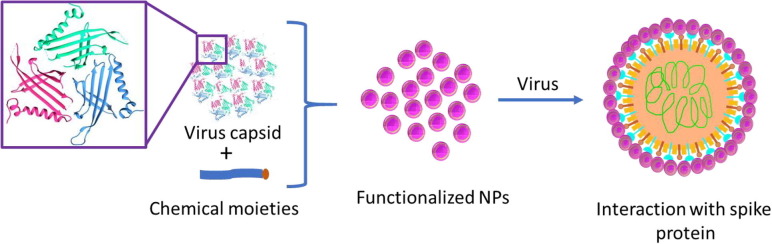
In the present time, coronavirus disease COVID-19 has become prevalent across the globe and declared as a pandemic by the world health organization (WHO) [1]. As of now, many vaccines and medicines are in the approval process or at different stages of preclinical investigations to coup with the COVID-19 [2]. Coronavirus has a spike protein, which tends to bind with receptors present in the lung tissues, and that helps in its entry to the cells and replication inside [3]. Therefore, we are in need to design the multivalent nanomaterials, which can act as receptors to bind with spike protein of virus and stop its replication (Fig. 1 ).
Fig. 1.
(Color online) Preparation of multivalent nanoparticles for blocking the spike proteins of SARS-CoV-2.
In the last two decades, nanotechnology has transformed the drug delivery approach for cancer, bacterial and viral diseases by minimizing the off-target deleterious effects and releases the drug at the desired site of the target [4], [5], [6]. Several approaches can be used to design the nanomaterials for the required set of targets, and designed multivalent nanoparticles are more fascinating because they mimic the natural systems. In this approach, surfaces of nanoparticles are functionalized with specific ligands to achieve the recognition for different cellular receptors, which helps to cure the disease. The binding capability of nanoparticles can be tremendously increased by using the ligands with multivalent binding sites [7]. It is unfortunate so far that the nanomaterials have not been able to achieve the optimal targets because of lacking in the distinct features of the design for the multivalent binding interactions. This can be achieved by improving the design strategies of nanoparticles through tuning the ligand density and length of linker, which are the keys pillars of the multivalent interaction of nanoparticle and receptors [8]. Poly (ethylene glycol), RGD inclusive peptides and proteins, and polymers have been used as ligands for the modification of nanoparticles via various chemistry-based synthetic techniques. Along with factors, longer ligands can enhance the interaction capability (avidity) of nanoparticles with the receptors.
The multivalence phenomenon is based on the weak non-covalent interaction between partner entities and mainly reversible. Multi- and polyvalent ligands have a high tendency of binding and shielding the virus surface as compared to monovalent ones, which significantly contributes to the inhibition of virus adhesion. Multivalent interactions play a determining role for recognition, adhesion, and signal processing in the biological systems. Besides this, these non-covalent interactions act as a significant player in the binding of pathogens and the host cell, that first lead to adhesion, following with the engulfment to the cellular membrane, and thus taken up by the endocytosis process [9]. Therefore, polyvalent interaction can inhibit the infection at its beginning. The blockage in the binding interaction/sites can be the choice of treatment against pathogens to contain the viral disease.
The successful design of nanoparticles based on the multivalent approach can be the point of reference for the upcoming nanomaterial design strategies for COVID-19 [10]. Recently, Lauster et al. [11] reported the interaction of multivalent phage capsid nanoparticles with the influenza virus to inhibit its replication. They exploited the concept of multivalent interaction, which resulted in the natural recognition and mediation in the cell-cell adherence process [12]. The trimeric haemagglutinin (HA) of the influenza A virus (IAV) has three binding sites for the sialic acid (Sia) residue at the surface glycans of the host cell [13]. As nowadays, the spatial design of nanomaterials usually is supported by the statistical model to assess the design and its efficacy; therefore, they concluded that the bacteriophage capsid (Qβ) is the best candidate to show the interaction with sialic acid residues. The K16 amino acid residue in the coat protein of capsid provided the interacting sites with anchoring different reagents. It is a well-adopted approach to functionalize the ligand with a functional group such as -SH, -NH2, -N3 to create the non-covalent interacting sites [14]. One of the several bioconjugation techniques, azide-alkyne cycloaddition chemistry, was used to make them functionalized for the interaction with the spike proteins [15]. The electron microscopy at high-resolution mode and tomography images revealed that the phage capsid nanoparticles completely covered the virus due to the intrinsic interacting sites for the spike proteins (Fig. 2 ). These phage capsid nanoparticles generated the hypoxic type environment for the virus to stop its binding with the host, which lead to its multiplication and spread. The inhibition in the growth of the virus was also confirmed by the cytokine reduction in the host cells. Phage capsid Qβ-Sia nanoparticles significantly reduced the virus titer in the ex-vivo study at human lung tissue. They managed to encapsulate the flu viruses in the scaffold, and no infection was observed in their host cells. Finally, they found the effectiveness of phage capsid nanoparticles for acute pneumonia of BALB/c mice, as evident from the reduction in weight loss, reduced cytokine production, and low morbidity rate [11]. Thus, a multivalent approach can overcome several problems in the rational design of nanomaterials for countering viral diseases.
Fig. 2.
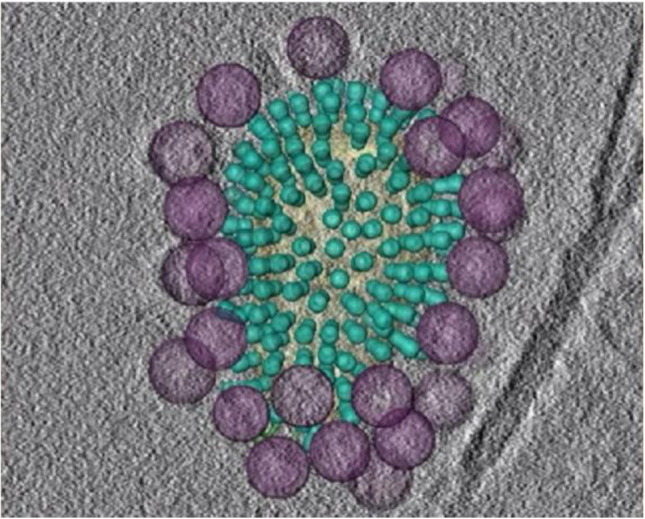
(Color online) The phage capsid multivalent nanoparticles surrounded the influenza virus due to intrinsic interaction with the spike proteins and created the suffocation for the virus. Reproduced with permission from Ref. [11]. Copyright 2020, Springer Nature.
In summary, multivalency is an approach that can be manipulated in the rational design of nanomaterials to counter viral diseases, and it is a definite hope where we have too many disappointments. Such concerns also make people more prone to the virus. Still, it is indispensable with a strong collaboration of diverse research areas like physics, chemistry, chemical biology, and statistics. Such partnerships can effectively execute the research projects at their required urgency, for example, against the COVID-19, as it is need for the current situation. The binding sites of multivalent nanomaterials play decisive role in the biological systems, especially in receptor-ligand interactions and signal transduction. Besides this, it is also commonly used in the self-organization of matter to create diverse kinds of nanomaterials. Phage capsid nanoparticles are biological and biomimetic and, especially when designed via a multivalent approach, can make them potent to use for inhibition of viral infections.
In response to the pandemic of COVID-19, we highlighted the role of multivalency to design the potential nanomaterials as alternative medicines and vaccine adjuvants. The enhanced binding capacity of phage capsid nanoparticles or the biological nanomaterials with polyvalence binding sites can provide the solution to contain viral infections, including COVID-19. On the other hand, the selectivity in the binding tendency of multivalent nanomaterials can play a role in the prevention of viral infections. Therefore, we suggest the scientific community to use the multivalent approach for designing the nanomaterials as it is a facile method with more productive capabilities to inhibit the virus multiplications due to intrinsically known activity for binding.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgments
Manzar Abbas acknowledges financial support from the European Union’s Horizon 2020 program under grant 839177 (PEPREP). Chunying Chen acknowledges financial support from the Ministry of Science and Technology of China (2016YFA0201600, 2016YFE0133100) and the Science Fund for Creative Research Groups of the National Natural Science Foundation of China (11621505). The authors thank Dr. Muhammad Saleem (Centre for Biomolecular Sciences, University of Nottingham NG7 2RD, UK) for helpful discussion and proof-reading of the article.
Biographies

Manzar Abbas completed his Ph.D. degree with distinction from the Institute of Process Engineering, Chinese Academy of Sciences (CAS), in 2017. After graduation, he worked as a post-doctoral researcher in Biological and Environmental Science and Engineering Division (BESE), King Abdullah University of Science and Technology (KAUST), Kingdom of Saudi Arabia (KSA). Currently, he is working as Marie Curie Individual Fellowship in Coacervates and Soft Interfaces group at Radboud University, The Netherlands. His research interest is focused on the design of functional short peptide building blocks, self-assembly, and applications in nanomedicine, protocells-coacervates, and bio-catalysis.

Muhammad Ovais obtained his B.S. degree in Biotechnology from the University of Peshawar in 2015 and M.S. degree in 2017 from Quaid-i-Azam University. The latter year he joined Professor Chen’s group as a Ph.D. candidate at CAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety, National Center for Nanoscience and Technology of China. His research interest lies in the development of advanced nano/bio-materials for cancer theranostic, immuno-engineering, and nanocatalytic applications.

Chunying Chen is a principal investigator at the CAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety in the National Center for Nanoscience and Technology of China. She received her Bachelor’s degree in Chemistry (1991) and Ph.D. degree in Biomedical Engineering from Huazhong University of Science and Technology of China in 1996. Her research interests include potential toxicity of nanoparticles, therapies for malignant tumors using theranostic nanomedicine systems, and vaccine nanoadjuvants using nanomaterials.
References
- 1.Mahase E. Covid-19: WHO declares pandemic because of “alarming levels” of spread, severity, and inaction. BMJ. 2020;368 doi: 10.1136/bmj.m1036. [DOI] [PubMed] [Google Scholar]
- 2.Shin M.D., Shukla S., Chung Y.H. COVID-19 vaccine development and a potential nanomaterial path forward. Nat Nanotechnol. 2020;15:646–655. doi: 10.1038/s41565-020-0737-y. [DOI] [PubMed] [Google Scholar]
- 3.Zhao Z, Wang Y, Qiu L. New insights from chemical biology: molecular basis for transmission, diagnosis and therapy of SARS-CoV-2. CCS Chem. 2020;2:1–30. [Google Scholar]
- 4.Weiss C., Carriere M., Fusco L. Toward nanotechnology-enabled approaches against the COVID-19 pandemic. ACS Nano. 2020;14:6383–6406. doi: 10.1021/acsnano.0c03697. [DOI] [PubMed] [Google Scholar]
- 5.Gupta A., Mumtaz S., Li C.-H. Combatting antibiotic-resistant bacteria using nanomaterials. Chem Soc Rev. 2019;48:415–427. doi: 10.1039/c7cs00748e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiehe A., O’Brien J.M., Senge M.O. Trends and targets in antiviral phototherapy. Photochem Photobiol Sci. 2019;18:2565–2612. doi: 10.1039/c9pp00211a. [DOI] [PubMed] [Google Scholar]
- 7.Hong S., Leroueil P.R., Majoros I.J. The binding avidity of a nanoparticle-based multivalent targeted drug delivery platform. Chem Biol. 2007;14:107–115. doi: 10.1016/j.chembiol.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 8.Abstiens K., Gregoritza M., Goepferich A.M. Ligand density and linker length are critical factors for multivalent nanoparticle–receptor interactions. ACS Appl Mater Interfaces. 2019;11:1311–1320. doi: 10.1021/acsami.8b18843. [DOI] [PubMed] [Google Scholar]
- 9.Fasting C., Schalley C.A., Weber M. Multivalency as a chemical organization and action principle. Angew Chem Int Ed. 2012;51:10472–10498. doi: 10.1002/anie.201201114. [DOI] [PubMed] [Google Scholar]
- 10.Talebian S., Wallace G.G., Schroeder A. Nanotechnology-based disinfectants and sensors for SARS-CoV-2. Nat Nanotechnol. 2020;15:618–621. doi: 10.1038/s41565-020-0751-0. [DOI] [PubMed] [Google Scholar]
- 11.Lauster D., Klenk S., Ludwig K. Phage capsid nanoparticles with defined ligand arrangement block influenza virus entry. Nat Nanotechnol. 2020;15:373–379. doi: 10.1038/s41565-020-0660-2. [DOI] [PubMed] [Google Scholar]
- 12.Kiessling L.L., Gestwicki J.E., Strong L.E. Synthetic multivalent ligands in the exploration of cell-surface interactions. Curr Opin Chem Biol. 2000;4:696–703. doi: 10.1016/s1367-5931(00)00153-8. [DOI] [PubMed] [Google Scholar]
- 13.Sauter N.K., Bednarski M.D., Wurzburg B.A. Hemagglutinins from two influenza virus variants bind to sialic acid derivatives with millimolar dissociation constants: a 500-MHz proton nuclear magnetic resonance study. Biochemistry. 1989;28:8388–8396. doi: 10.1021/bi00447a018. [DOI] [PubMed] [Google Scholar]
- 14.Arsiwala A., Castro A., Frey S. Designing multivalent ligands to control biological interactions: from vaccines and cellular effectors to targeted drug delivery. Chem Asian J. 2019;14:244–255. doi: 10.1002/asia.201801677. [DOI] [PubMed] [Google Scholar]
- 15.Crich D., Li W. α-Selective sialylations at −78 °C in nitrile solvents with a 1-adamantanyl thiosialoside. J Org Chem. 2007;72:7794–7797. doi: 10.1021/jo7012912. [DOI] [PMC free article] [PubMed] [Google Scholar]