Abstract
This review focuses on the hypothetical mechanisms for enhanced vulnerability of African Americans to SARS-CoV-2 infection, COVID-19 severity, and increased deaths. A disproportionately higher number of African Americans are afflicted with autoimmune and inflammatory diseases (e.g., diabetes, hypertension, obesity), and SARS-CoV-2 has helped expose these health disparities. Several factors including socioeconomic status, inferior health care, and work circumstances contribute to these disparities. Identifying potential inflammatory biomarkers and decreasing basal levels in high-risk individuals with comorbidities through preventive measures is critical. Immune cells, particularly neutrophils, protect us against pathogens (bacteria, fungi, and viruses) through increased generation of free radicals or oxidants and neutrophil extracellular traps (NETs) that ensnare pathogens, killing them extracellularly. However, continued generation of NETs coupled with the lack of prompt removal pose danger to host cells. NET levels are increased during pro-inflammatory diseases. COVID-19 patients exhibit elevated NET levels, depending upon disease severity. Conceivably, high-risk individuals with elevated basal NET levels would exhibit hyper-inflammation when infected with SARS-CoV-2, amplifying disease severity and deaths. Drugs inhibiting oxidant formation and vitamin supplements decreased NET formation in mice models of inflammation. Thus, it is conceivable that preventive treatments lowering NET levels and inflammation in high-risk individuals could mitigate SARS-CoV-2-induced complications and decrease mortality.
Keywords: Coronavirus, COVID-19, Oxidants, Neutrophils, African Americans, Black Americans
Graphical abstract
Highlights
-
•
COVID-19 severity is magnified in people with underlying medical conditions.
-
•
African Americans are disproportionately affected by COVID-19.
-
•
Decreasing inflammatory & immune-system-related markers might mitigate COVID-19 severity.
“Health disparities have always existed for the African American community … [coronavirus is] shining a bright light on how unacceptable that is because, yet again, when you have a situation like the coronavirus, they are suffering disproportionately.” –Dr. Anthony S. Fauci [1].
1. Introduction
A disproportionately higher number of African Americans, Hispanics, and other ethnic minorities from various age groups suffer from inflammatory diseases such as hypertension, diabetes, coronary artery diseases, obesity, lupus, sickle cell disease, asthma, and other autoimmune disorders [[2], [3], [4]]. When individuals with these underlying medical conditions are infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the severity of the disease it causes, coronavirus disease 19 (COVID-19), is greatly magnified, resulting in lengthy hospitalization and dire consequences. Studies reveal that African Americans, Hispanics, and other ethnic minority groups are disproportionately affected by SARS-CoV-2 [[5], [6], [7], [8]]. Importantly and disturbingly, their death rate from COVID-19 is much higher. Several social determinants contribute to enhanced health disparities, including lower income, substandard living conditions with a lack of physical separation, less-than-ideal work circumstances, decreased nutrition, and inferior health care. To understand and mitigate the disproportionate impact of SARS-CoV-2 or related viruses on the health and morbidity of African Americans, Hispanics, and other ethnic minority groups, a concerted and systematic effort that addresses the fundamental mechanisms responsible for disparate underlying conditions must be undertaken. What is the mechanistic basis for linking increased oxidative damage and pro-inflammatory conditions in high-risk individuals to enhanced susceptibility to COVID-19 severity? Might any preventive measures help mitigate disease severity? Although most COVID-19 cases result in mild symptoms, some progress to respiratory failure and death. Reports indicate that elevated levels of neutrophil extracellular traps (NETs) are produced in the lungs of COVID-19 patients, leading to hyperinflammation and respiratory failure [9]. Increased levels of NETs in the serum could be used as a biomarker that might predict the long-term risk for enhanced hyperinflammatory and deadly effects caused by SARS-CoV-2. Targeting NETs is therefore a promising strategy to mitigate inflammation induced by SARS-CoV-2 [9,10]. Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases and a member of the White House coronavirus task force, said that the COVID-19 pandemic has exposed the underlying health disparities in the African American community [1]. To that end, this review focuses on NET biology, its mechanisms of formation, and potential interventional approaches to decrease NET levels in African Americans with underlying medical conditions and higher risk of mortality against SARS-CoV-2.
2. Neutrophils and NET formation: a defense mechanism and a double-edged sword
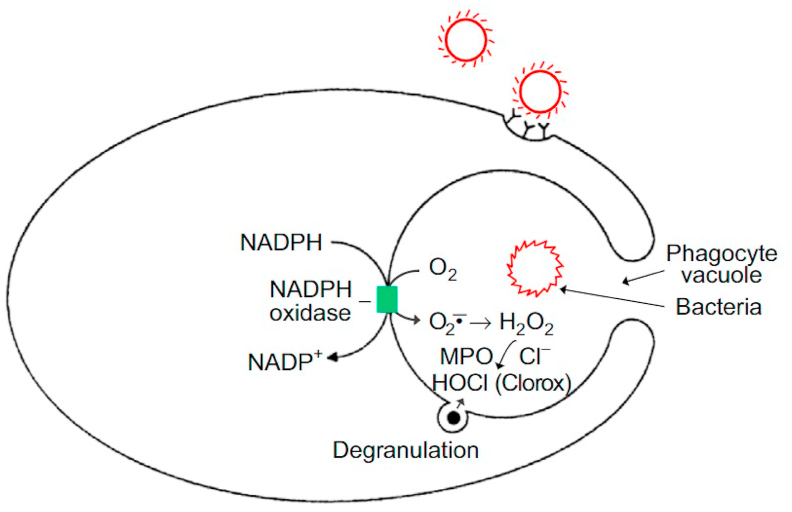
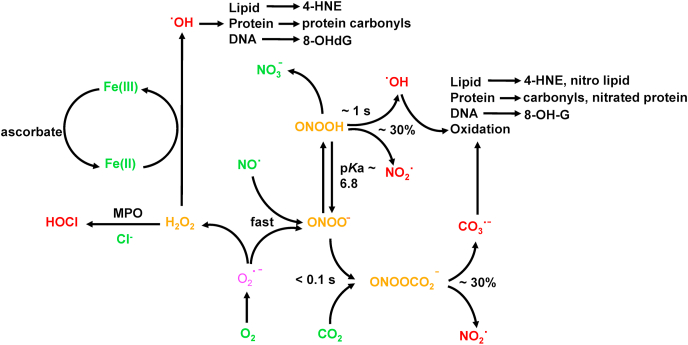
Neutrophils, belonging to the family of polymorphonuclear leukocytes, are the most abundant type of white blood cell circulating in our bodies and are responsible for providing innate immunity. Neutrophils are the first immune cell responders for infection. In response to bacterial, fungal, and viral infections, neutrophils activate other immune cells, engulf pathogens by phagocytosis, and destroy the invading pathogens in the style of the Pac-Man video game (Fig. 1). This is known as the respiratory, or oxidative, burst pathway and is dependent upon the activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase enzymes (NADPH oxidase 2 [Nox2]) whose main function is to generate the superoxide anion (O2•–) using NADPH as a cofactor via a one-electron reduction of oxygen as part of their antimicrobial mechanism [11]. Dismutation of O2•– forms additional oxidants (hydrogen peroxide [H2O2]) that, in the presence of myeloperoxidase (MPO) and the chloride ion generate, hypochlorous acid (HOCl) or bleach as a potent antibacterial agent. Other potent oxidants such as peroxynitrite (ONOO−) and hydroxyl radicals (•OH) are formed from the reaction between O2•– and nitric oxide (NO•) formed from the nitric oxide synthase (NOS)-catalyzed oxidation of l-arginine (Fig. 2) [12]. Neutrophils use these highly potent oxidants and free radicals (also described as cellular atomic bombs) to destroy phagocytized pathogens intracellularly (Fig. 1). In 2004, it was proposed that in addition to the intracellular antimicrobial killing mechanism, neutrophils trap and kill pathogens by casting a toxic extracellular net [13,14].
Fig. 1.
Conventional host defense involving the stimulation of oxidative or respiratory burst in neutrophils during phagocytosis of pathogens, including bacteria, fungi, or viruses. Activation of the enzyme, NADPH oxidase, stimulates O2•– and H2O2 formation. Degranulation releases the enzyme MPO that in the presence of H2O2 oxidizes the chloride anion to HOCl or bleach, a strong antimicrobial agent. Reprinted from Redox Biology, 1, Kalyanaraman B, Teaching the basics of redox biology to medical and graduate students: Oxidants, antioxidants and disease mechanisms, 244–257, Copyright 2013, with permission from Elsevier.
Fig. 2.
Oxidants cascading from one-electron reduction of oxygen to O2•–. Several potent oxidants are generated from H2O2 formed from dismutation of O2•–. One of the most potent antimicrobial oxidants, HOCl, is formed from MPO-catalyzed oxidation of chloride ion. ONOO−, another potent oxidant, is formed from the reaction between O2•– and nitric oxide and results in the oxidation of lipid, proteins, and DNA.
3. Neutrophils, extracellular traps, or NETosis, a novel form of cell death: dying to kill?
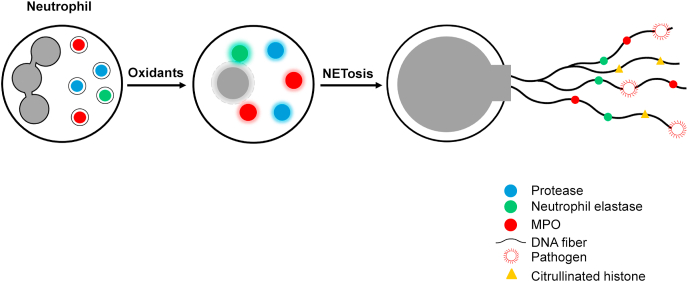
NETosis is a form of cell death that is distinctly different from apoptosis or necrosis [15,16]. Following an intracellular oxidative burst in response to a bacterial, fungal, or viral infection, neutrophils extrude DNA and antimicrobial proteins during NETosis into the extracellular space, forming a net-like structure, or NETs (similar to a spiderweb), to trap and kill invading pathogens, including bacteria, fungi, and viruses (Fig. 3) [[15], [16], [17]]. Structurally, NETs consist of extracellular DNA decorated with histones, cytosolic proteins, and granular proteins; neutrophil elastase; and oxidative enzymes, Nox2, MPO, and NOS [18,19] (Fig. 3). Histones are positively charged and form a nucleosome with chromatin. Neutrophil elastase, a serine protease, translocates to the nucleus and instigates NET formation. NET formation is triggered by innate immunity receptors activating Nox2 and/or mitochondria that subsequently activate MPO, neutrophil elastase, and a calcium-dependent protein-arginine deiminase type 4 (PAD4). PAD4 citrullinates histones and decreases the net positive charge by converting the positively charged arginine to the neutral citrulline, promoting chromatin decondensation [20]. Neutrophils derived from patients with chronic granulomatous disease with mutations in Nox2, which does not generate O2•–, failed to induce NET formation [21]. Both Nox2 and MPO are implicated in NET formation in plasma isolated from patients with autoimmune conditions [22]. The 8-hydroxy deoxyguanosine (8-OHdG)-enriched DNA present in NETs binds to a transmembrane protein on tumor cells and facilitates their metastatic potential, as discussed later [23,24].
Fig. 3.
NETosis and NET formation as an extracellular antimicrobial mechanism. Stimulation of oxidants during the phagocytic killing of microbes causes rupture of nuclear membranes extruding chromatin, MPO, neutrophil elastase, histones, and proteolytic enzymes into the extracellular space forming a net-like structure that traps and kills pathogens.
4. Imbalance between NET formation and NET clearance or degradation
Despite its antimicrobial effects, the excessive accumulation of NET causes damage to the host by inducing pro-inflammatory mechanisms [25,26]. The timely removal of NETs is crucial for preventing self-antigens [27]. Degradation of NETs in ischemia/reperfusion-challenged intestinal tissue with deoxyribonuclease 1 (DNase 1) is proposed as an effective treatment against intestinal ischemia/reperfusion injury [28]. Acute and chronic inflammation and autoimmune disorders are associated with enhanced NET levels [17]. A decreased ability to degrade NETs is pronounced in systemic lupus erythematosus (SLE) patients with antiphospholipid syndrome [[29], [30], [31], [32]] and nephritis [27], and degradation of NETs is impaired by the presence of autoantibodies, increasing the risk of thrombosis.
5. Enhanced NET formation in COVID-19 patients
Enhanced release of NETs was reported to occur in severe cases of COVID-19 [33,34]. Sera from COVID-19 patients revealed elevated levels of extracellular DNA (cell-free DNA), and two specific markers of NETs: MPO-DNA and citrullinated histone H3. The levels of cell-free DNA showed a strong correlation with other markers of inflammation (e.g., C-reactive protein, D-dimer). Both cell-free DNA and MPO-DNA were much higher in patients on mechanical ventilation as compared with patients breathing room air. Another interesting finding of this study [17] is that sera from COVID-19 patents triggered NET release from control neutrophils in vitro, indicating that circulating NETs could be used to predict the extent of disease severity. NETs stimulated immunothrombosis in COVID-19 patients and were suggested as suitable therapeutic targets for mitigating prothrombotic complications in COVID-19 patients [35].
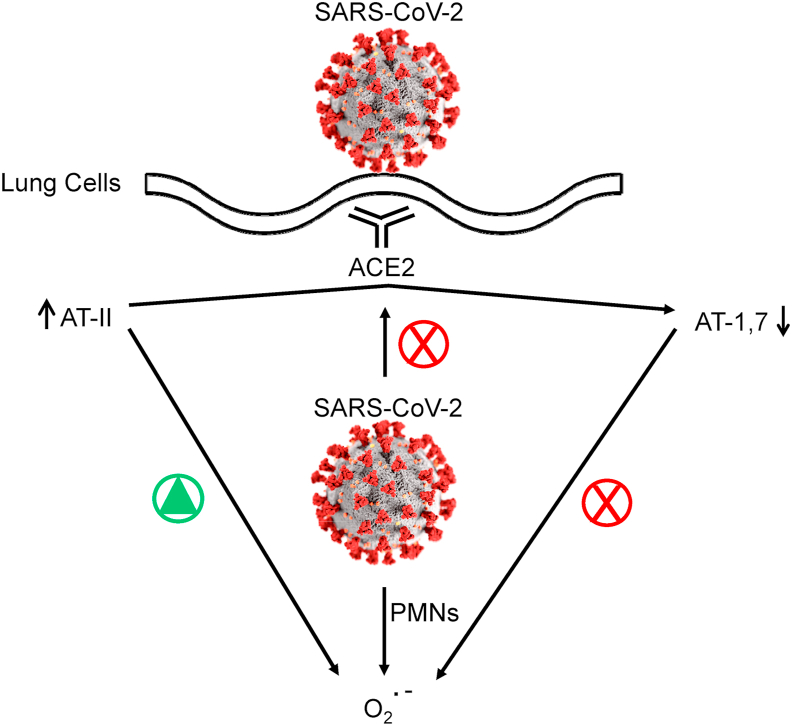
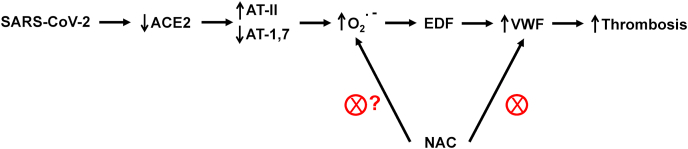
After entering the respiratory tract, SARS-CoV-2 uses a spike protein and latches onto the angiotensin-converting enzyme 2 (ACE2) receptor present on the surface of the cell membrane, fuses into the lung cell membrane, and begins replicating (Fig. 4) [36]. A major function of ACE2 is to convert angiotensin II (AT-II) that is vasoconstrictive to angiotensin 1,7 (AT-1,7) that exerts vasodilatory effects. AT-II activates O2•– whereas AT-1,7 decreases O2•– in vascular cells [37,38]. Lung inflammation is enhanced by AT-II and diminished in the presence of AT-1,7 [39]. SARS-CoV-2 decreases ACE2 receptors; as a result, AT-II levels are elevated, resulting in enhanced oxidant formation, oxidative stress, and inflammation [40,41]. ACE2 deficiency increases Nox2-mediated oxidant formation [42,43]. Recent reports indicate that neither ACE inhibitors nor angiotensin receptor blockers were associated with increased mortality in COVID-19 patients [44,45].
Fig. 4.
SARS-CoV-2 from the respiratory tract enters lung cells through the ACE2 receptor. The virus inactivates the ability of ACE2 to convert AT-II to AT-1,-7. SARS-CoV-2 stimulates PMN (polymorphonuclear neutrophils-mediated) O2•–.
6. NET and the cytokine storm
COVID-19 patients who were admitted into the intensive care unit showed elevated levels of cytokines as compared with those who were not admitted. During the early stages of this pandemic, many patients on ventilators developed respiratory illness and died. More recently, due to antiviral drugs (e.g., remdesivir) and anti-inflammatory steroids (e.g., dexamethasone), respiratory-illness-related deaths have dramatically decreased. Remdesivir, a nucleoside analog that mimics a viral RNA component, inhibits the RNA polymerase enzyme that SARS-CoV-2 uses for replication [46]. Dexamethasone, an FDA-approved immunosuppressive steroid that has been used in the clinic for over 50 years, prevented mortality in seriously ill COVID-19 patients [47]. In response to SARS-CoV-2 infection, the immune system is activated through recruitment of T-cells, macrophages, and dendritic cells. These immune cells secrete molecules, cytokines and chemokines, to combat the viral infection. The “cytokine storm” refers to an uncontrolled, hyper-activation of immune cells, cytokines (IL-6), and chemokines (CXCL10). NETs induce pro-inflammatory cytokines that further stimulate NET and cause a “feedback” snowballing effect [48]. Drugs decreasing the NET levels have been shown to prevent COVID-19 associated cytokine release [49].
7. African Americans are disproportionately affected by inflammatory diseases: NET hypothesis
COVID-19 patients with underlying medical conditions (e.g., diabetes, hypertension, obesity, and immunosuppression) with increased basal levels of oxidative stress need hospitalization and frequently develop more severe complications [50]. Because COVID-19 patients have increased NET levels accompanied by increased severity, it is conceivable that people with elevated basal levels of NET caused by underlying medical conditions (as described below) would be more vulnerable to life-threatening complications induced by SARS-CoV-2. Whether enhanced NET induction is the cause or consequence of underlying medical conditions, decreasing NETs with drugs or nutritional and vitamin supplements has been shown to be beneficial [51]. African Americans are disproportionately affected by several chronic medical conditions as discussed in the following sections.
7.1. Obesity
The link between oxidative stress and endothelial dysfunction is well established [52]. In an animal model of obesity, increased NET formation was shown to be associated with inflammation and endothelial dysfunction [53]. Prevention of NET formation with 2-chloroamidine, an inhibitor of peptidylarginine deaminase 4, that inhibits the citrullination of histone decreased obesity-related endothelial dysfunction and inflammation [53]. In another study, it was reported that NET levels did not affect the onset of obesity or adipose tissue inflammation but may affect obesity-induced pathologies [54]. Severe obesity in patients was shown to be associated with enhanced NET generation [55]. Obesity is proposed as a major risk factor for increased prevalence, severity, and lethality of COVID-19 [56]. Inhibiting NET in obese individuals would be a promising therapeutic strategy.
7.2. Hypertension
Hypertension (increased blood pressure) is more prevalent in African Americans, and obesity is a major contributing factor [57]. Emerging research reveals a new role for neutrophils in hypertension [58]. The presence of a salt-sensitive gene that regulates vascular resistance and renal sodium transport in African Americans increases the risk of developing high blood pressure [59]. This particular gene is a variant of the angiotensin-converting enzyme that enhances the release of AT-II, a vasoconstrictor. Enhanced formation of oxidants and NETs were shown to contribute to elevated blood pressure in spontaneously hypertensive rats [60]. Studies implicate a role for enhanced angiotensin II and activation of Nox2 and NETosis in arterial hypertension [[60], [61], [62]]. African Americans with hypertension exhibit decreased activity of antioxidant enzymes [63].
7.3. Cardiovascular disorders
In contrast to initial thinking that COVID-19 predominantly damages the lungs, recent research shows that the virus causes inflammation in the heart, affects heart function, and diminishes oxygen supply to the heart muscle [64]. People with preexisting heart conditions are at increased risk for developing severe heart problems. SARS-CoV-2 presumably enters through the ACE2 receptors present in the heart and directly attacks the heart cells, causing an extreme inflammatory reaction that induces a cytokine storm (e.g., IL-6,7,22 and CXCL10) and can even trigger a heart attack [65]. NETs were proposed to play a primary role in acute myocardial infarction caused by the rupture of a coronary atherosclerotic plaque followed by a thrombus artery occlusion [66,67]. Impaired DNase 1-mediated degradation of NET is associated with excessive microvascular thrombosis [68]. Lack of removal of NETs over a long period of time induces collagen deposition, increased fibrosis, and heart failure [69].
7.4. Diabetes
Diabetes is a key risk factor for developing severe COVID-19, and COVID-19 patients with this underlying condition are more likely to die of respiratory and cardiovascular complications [70,71]. Diabetes is marked by chronic inflammation, endothelial dysfunction, and other cardiovascular complications. The connection between high glucose or hyperglycemia-induced NET formation and the NADPH oxidase pathway and diabetic retinopathy is strong [[72], [73], [74], [75]]. NET levels and biomarkers of NETosis were increased in the serum of diabetics [72]. Increased NETs and pro-inflammatory cytokines were detected in type 2 diabetes mellitus patients [[73], [74], [75]]. Impaired wound healing in patients with type 2 diabetes was attributed to increased NET levels [76]. SARS-CoV-2 has been found to destroy insulin-producing cells, inducing conditions in COVID-19 patients similar to those in patients with type 1 diabetes [77,78]. A recent report suggests that elevated glucose levels enhanced SARS-CoV-2 infection and viral load in patients with uncontrolled diabetes via a glycolytic mechanism [79]. Enhanced generation of mitochondrial O2•– was thought to be responsible for SARS-CoV-2 replication and for elevated cytokine production in monocytes present in diabetic patients.
7.5. Sickle cell disease
Sickle cell disease (SCD) is an inherited autosomal recessive disorder that is predominantly seen in African Americans [50]. The distorted shape of sickle red blood cells makes them more prone to hemolysis. SCD is characterized by chronic hemolysis with elevated levels of heme and cell-free hemoglobin. Neutrophilia is a major characteristic of SCD. In a humanized SCD mouse model, enhanced levels of NETs were detected in the lungs and in plasma [80]. This study also revealed that heme present in the plasma stimulates in vitro and in vivo NET formation in SCD mice [81]. Increasing or decreasing plasma heme concentrations was shown to induce or prevent NETosis in SCD, respectively. Treating SCD mice with hemopexin to scavenge free heme reduced NET formation [81]. Results from these studies show that free iron is involved in NET formation and treatment of SCD plasma, with the iron chelator deferoxamine or the iron binding apotransferrin effectively inhibiting NET release [82]. The commonly used and FDA-approved drug hydroxyurea, which induces fetal hemoglobin and reduces neutrophil count, did not decrease the NET activity and pro-inflammatory effects associated with SCD [83].
7.6. Autoimmune diseases: systemic lupus erythematosus
SLE is an autoimmune disease characterized by generation of autoantibodies directed against one's own DNA. SLE is three times more prevalent in African American women than in Caucasian women. NETs act as autoantigens, and SLE patients develop autoantibodies to modified histones, ubiquitinated MPO, and other neutrophil proteins [84]. NET-associated proteins are recognized by autoantibodies in systemic autoimmune diseases [84]. AntiNET antibodies prevent DNase I access to NETs. Timely removal of NETs is crucial to avoid self-antigen formation [27,85]. It was reported that NET levels increased with worsening SLE several months in advance, and this allows for preventative intervention. Progressive damage to kidneys, the vasculature, skin tissues, and other organs occurs in SLE patients. Understanding the mechanism of NET formation and NET removal is critical for developing precise therapeutic intervention. Whether SLE-induced NETs are mediated by O2•–/H2O2-independent hyper-citrullination or Nox2-or mitochondria-dependent oxidants remains debatable; however, reports suggest that mitochondria-targeted antioxidants (Mito-Quinone [Mito-Q] and Mito-TEMPOL) attenuated the severity of lupus in the mouse model [86,87]. It was suggested that Mito-Q could inhibit type 1 interferon (IFN-1), upstream of O2•– production [88]. Mito-Q also inhibited IFN-1 that is a byproduct of NETosis [88]. Immune system activation by mitochondria was recently reported [89]. Post-translational protein citrullination mediated by the nuclear enzyme PAD4 has also been implicated in autoimmune disorders (e.g., rheumatoid arthritis) in African Americans [90].
7.7. Airway diseases: COPD and asthma
African Americans are at higher risk of developing chronic obstructive pulmonary disease (COPD) and asthma, diseases that obstruct airways and make breathing difficult. African American women (including non-smokers) in particular are vulnerable to developing COPD [91]. Enhanced airway neutrophilic inflammation is a characteristic hallmark of COPD. Stable COPD patients exhibit enhanced NET formation and extracellular DNA in sputum [92]. Reports suggest that oxidants enhance NET formation in airway neutrophils; in addition, it was reported that chemokine CXCL8 regulates NET formation in COPD patients [48]. CXCR2 antagonist AZD5069 decreased NETosis and NET levels in COPD-derived neutrophils [48]. Studies show that NET formation correlates with the severity of airflow limitation [91]. Decreasing NET levels in COPD and asthmatic patients may be beneficial to mitigate chronic airway inflammation.
7.8. Alzheimer's disease and related dementias
The prevalence of Alzheimer's disease (AD) in African Americans is two-to threefold higher than in Caucasians, and older African Americans are disproportionately affected by the disease [[93], [94], [95]]. The inflammatory network is a characteristic feature of AD [96]. Recent studies show that increased levels of NETs are released in AD [[97], [98], [99]]. Pro-inflammatory cytokines generated in AD brain microvessels further elevate intravascular NETosis in AD brains [98]. The beta-amyloid deposits that accumulate during AD enhanced Nox2-mediated O2•–, promoting intraparenchymal NET formation [100]. The discovery of NETs in mouse models of AD and in Alzheimer's patients reveals a novel role of neutrophils in neuroinflammation. Whether NETs are related to early biomarkers of AD is not known; however, therapeutic targeting of NETs through preventive approaches in the early stages of AD could be a promising therapeutic strategy to mitigate neuroinflammation.
7.9. Cerebrovascular disease: ischemic stroke
Stroke is the third leading cause of death in African Americans [101]. Ischemic stroke is caused by occlusive thrombus (blockage of blood flow due to blood clot). NETs (e.g., extracellular DNA-histone complexes, citrullinated histones colocalizing with NET) were identified in all stroke thrombi in ischemic stroke patients [[102], [103], [104], [105]]. Neutrophils support thrombosis through formation of NETs. Ex vivo addition of DNase 1 improved tissue plasminogen (tPA)-mediated dissolution of thrombi isolated from stroke patients [104]. Further research on the use of DNase 1, an FDA-approved drug for cystic fibrosis, as a prothrombolytic drug alone or in combination with conventional drugs (e.g., tPA) is clearly warranted.
7.10. Cancer metastasis
Metastasis of breast cancer, a leading cause of death, occurs at high rates in African American women [106]. Increased levels of NETs were identified in the metastatic lesions [23,24]. A recent study showed that NETosis and enhanced NET formation in the distant organs preceded cancer metastasis, suggesting that NETs in blood could be used as a predictive biomarker of metastasis [23]. 8-OHdG, a characteristic hallmark of NET-DNA, is an extracellular DNA sensor. The transmembrane protein CDC25 interacts with NET-DNA, supporting the proliferation of metastatic cells [23,24]. Other markers of NETosis and serum NET levels (e.g., MPO-DNA) were found to be higher in breast cancer patients with liver metastases [23]. Therapeutic targeting CDC25 and mitigating NETs in cancer patients may be an effective approach for preventing cancer metastasis [23,24]. Targeting NETosis in the tumor immune microenvironment was proposed as a promising strategy to inhibit metastatic progression [23,24,107].
7.11. Thrombosis or blood clots in COVID-19 patients
Early on, COVID-19 was thought to be primarily a lung-related problem resulting in acute respiratory disease syndrome. Elevated levels of D-dimer and procoagulants such as von Willebrand factor (VWF), a multimeric glycoprotein, and coagulation factor VIII (FVIII) were detected in COVID-19 patients after stroke. These patients had enhanced blood clots or microthrombosis mediated by VWF and FVIII and were treated with unfractured heparin and other VWF-modifying agents.
The presence of enhanced levels of high-molecular-weight multimers of VWF is an established risk factor for arterial thrombosis [108]. The virus binds to endothelial cells and induces inflammation through inactivation of the ACE2 enzyme. ACE2 inactivation increases AT-II, a vasoconstrictor, as it is metabolized to AT-1,7, which causes vasodilation (Fig. 4). ACE2 inactivation and an increase in AT-II results in enhanced O2•– formation from Nox2 and inflammation of the sub-endothelium. During this process, procoagulants (VWF and FVIII) are released.
8. NETs as potential therapeutic targets
8.1. FDA-approved drugs
Basic research strongly supports that drugs mitigating NET levels in diseases decrease inflammation [109]. We hypothesize that mitigation of NETs in African Americans with underlying medical conditions would improve their health and decrease the severity of COVID-19, thus saving lives.
FDA-approved drugs decrease NET and inflammatory biomarkers [110]. Metformin, one of the most widely used antidiabetic drugs, decreased NET formation in SLE patients [111]. 5-aminiosalicylic acid (mesalamine), which is widely used to treat inflammatory bowel diseases, inhibits NET formation [112]. Dipyridamole is an FDA-approved drug that activates adenosine A2A receptors and inhibits NETs [113]. Recently, an antibody therapy using the anti-citrullinated protein antibody against NET was reported [114].
Hydroxychloroquine and chloroquine are FDA-approved drugs for treating malaria, lupus, and rheumatoid arthritis. Hydroxychloroquine and chloroquine inhibit NET formation in murine models of pancreatitis and ischemia/reperfusion injury [[115], [116], [117]]. However, use of hydroxychloroquine and chloroquine in COVID-19 patients causes serious heart rhythm problems [118]. Recent studies have concluded that neither hydroxychloroquine nor chloroquine, either alone or in combination with azithromycin, is an effective antiviral drug against SARS-CoV-2 [[118], [119], [120]]. A randomized, double-blind, placebo-controlled trial showed that the prophylactic use of hydroxychloroquine did not prevent symptomatic infection after SARS-CoV-2 exposure [121]. Investigators also reported that hydroxychloroquine decreased COVID-19 associated mortality in a multi-center retrospective study [122]. Additional randomized, placebo-controlled studies that are presently ongoing should provide definitive answers as to whether the use of hydroxychloroquine mitigates COVID-19 severity. Regardless of whether or not it is protective as an antiviral drug for COVID-19, the use of hydroxychloroquine is contraindicated in COVID-19 patients with preexisting cardiovascular, pulmonary, and other complications. Recently, the FDA issued a warning cautioning against the use of hydroxychloroquine and chloroquine for COVID-19 patients outside of the hospital setting or in a clinical trial due to the elevated risk of cardiovascular complications.
8.2. Nutritional supplements and natural products
8.2.1. Glutathione
Glutathione (GSH), a tripeptide consisting of cysteine, glycine, and glutamate, is one of the most abundant cellular antioxidants involved in the removal of H2O2 and activation of key enzymes (thioredoxins, peroxiredoxins, and glutathione peroxidases). N-acetylcysteine (NAC) is an intracellular precursor of GSH, and its protective mechanism is linked to reduction of disulfides and regeneration of GSH. Recent clinical reports hypothesized that GSH deficiency is a plausible cause of increased susceptibility to SARS-CoV-2 infection in older people and in those with preexisting medical conditions (e.g., diabetes and cardiovascular and respiratory diseases) [123]. GSH lowers viral load and viral infection; inhibits oxidative stress, inflammation, pro-inflammatory cytokines (e.g., IL-6, IL-8, and TNF-α), and thrombosis; and potentially boosts immune function [123,124]. Inadequate nutrition and insufficient consumption of fresh fruits and vegetables could contribute to endogenous GSH deficiency. GSH therapy has been effectively used to alleviate dyspnea in COVID-19 patients [125]. Prior to supplementation therapy, the basal levels of GSH should be determined.
8.2.2. N-acetylcysteine
NAC, an FDA-approved mucolytic drug for chronic obstructive lung disease and acetaminophen toxicity, is currently undergoing clinical trials in COVID-19 patients [126]. NET formation induced by O2•– promotes thrombosis [127]. NAC can act as an anticoagulant and exert a thrombolytic effect [127]. NAC was found to block NETosis in vitro [128]. Although a multitude of publications claim that NAC acts as an antioxidant by scavenging the O2•– anion, the collective opinion of experts in the areas of oxidative biology and free radical chemistry is that NAC does not exert protection against oxidative stress by directly scavenging O2•– or H2O2 [129]. NAC reduces the disulfide bonds (-S-S-) to sulfhydryl groups (-SH). Thus, NAC can increase intracellular GSH levels by reducing glutathione disulfide. The VWF protein forms large multimers through disulfide cross-linking, and microvascular thrombosis is caused by aggregation of platelets due to binding to VWF multimers [130]. NAC alters VWF binding through a reduction of the intrachain disulfide bond and a decrease in the number of VWF multimers [130,131]. Fig. 5 illustrates the plausible mechanism by which NAC might induce platelet disaggregation by reducing the -S-S- bond and decreasing VWF multimers.
Fig. 5.
Potential thrombolytic effect of NAC. Increased formation of O2•– as shown in Fig. 4 results in enhanced VWF and thrombosis. Reduction of disulfide (-S-S-) to sulfhydryl groups (-SH) in VWF cross linked to proteins is proposed as the likely mechanism for the vasoprotective effects of NAC.
8.2.3. l-glutamine
l-glutamine, one of the most abundant amino acids in the bloodstream, is approved by the FDA as an antioxidant drug for treatment of SCD. l-glutamine enhances intracellular GSH and NAD+ (nicotinamide adenine dinucleotide) levels. The immunomodulatory aspects of l-glutamine supplementation and its beneficial effects in immunocompromised people have recently been discussed [51]. Clearly, the appropriate clinical trials are required to establish the efficacy of l-glutamine in immunocompromised COVID-19 patients.
8.2.4. Vitamin C
Vitamin C (ascorbic acid) is an essential nutrient that is acquired from intake of fruits and vegetables or from nutritional supplements. Vitamin C deficiency results in oxidative stress, inflammation, and compromised immune function. Intracellular concentration of ascorbate can be significantly increased when supplemented with dehydroascorbate, the oxidized form of ascorbate [132]. The antioxidant mechanism of ascorbic acid is important for the antimicrobial function of neutrophils [132,133]. Ascorbate supplementation decreased NET formation in activated neutrophils. Ascorbate supplementation protects against enhanced oxidative stress induced by inflammatory and autoimmune conditions [133].
8.2.5. Vitamin D
Vitamin D deficiency, also known as hypovitaminosis D, is associated with several chronic diseases (e.g., asthma and respiratory disorders) and negatively affects immune function and response. Under these conditions, it is essential to increase the levels of vitamin D through appropriate supplementation. Vitamin D supplementation enhances the innate immune system and reportedly prevents immune system overactivation. Investigators reported a significant correlation between vitamin D deficiency, cytokine storm, and increased COVID-19 mortality [[134], [135], [136]]. Vitamin D deficiency was suggested to be a predictor of poor prognosis in COVID-19 patients with acute respiratory distress syndrome [137]. More rigorous studies linking vitamin D and SARS-CoV-2 need to be performed. Dietary factors, sunlight, and vitamin D supplementation enhance endogenous vitamin D levels. African Americans and other individuals with darker skin pigmentation have decreased vitamin D levels because of UVB light absorption by the melanin pigment [135]. UVB present in the sunlight is needed for vitamin D synthesis. Elderly people also often have vitamin D deficiency. Because an optimal level of vitamin D is essential for proper functioning of the immune system and for inhibiting the pro-inflammatory cytokines, any perceived deficiency in high-risk individuals should be rectified by supplementation with vitamin D.
8.2.6. Vanilloids
Recent high-throughput studies discovered a novel class of vanilloids as effective inhibitors of NET formation [138]. The vanilloids capsaicin, dihydrocapsaicin, and resiniferatoxin are natural products that contain the vanillyl moiety (4-hydroxy-3-methoxybenzyl). Capsaicin and dihydrocapsaicin, well known agonists of the TRPV1 (transient receptor potential vanilloid 1) receptor, are believed to exert their effects via downregulation of NF-κB signaling [138].
9. Role of mitochondria in NET: repurposing mitochondria-targeted drugs in COVID-19 treatment?
Published reports suggest that compounds inhibiting the mitochondrial respiratory chain inhibit neutrophil activation and oxidative burst [139,140]. Mitochondrial complex I inhibitors, rotenone and metformin, significantly inhibit the recruitment of neutrophils in a lipopolysaccharide-induced lung inflammation mouse model [141]. Using a neutrophil-specific knockout zebrafish model, the first in vivo evidence was provided for mitochondrial regulation of neutrophil function [142]. Primary neutrophils depend on mitochondrial membrane potential for chemotaxis. Proper functioning of the mitochondrial electron transport chain is required for neutrophil motility to occur [142]. Perturbation of mitochondrial function was shown to greatly decrease the antimicrobial potency of inflammatory neutrophils [140]. However, the role of mitochondria in oxidative burst induced by neutrophils is questionable; it was previously demonstrated that cyanide inhibited the macrophage/monocyte respiratory burst but not the neutrophil-generated O2•– and H2O2 [143,144].
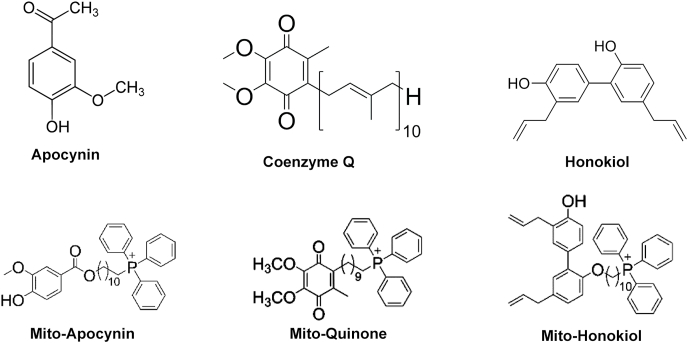
Mitochondria-targeted drugs inhibited Nox2 levels, oxidative damage, and inflammation in a mouse model of Parkinson's disease [145]. Mito-apocynin synthesized from apocynin, a non-specific inhibitor of Nox2, inhibited Nox2 and oxidative/nitrative modification of proteins and inflammation in microglia [145]. Mito-Q, co-enzyme Q conjugated to the triphenylphosphonium moiety, decreased NET formation in a lupus mouse model [88]. Mito-Q treatment of SARS-CoV-2-infected monocytes from diabetics decreased viral replication and cytokine upregulation [79]. Fig. 6 shows the chemical structures of selected drugs that target mitochondria and inhibit Nox2 activity and inflammation. These and related drugs are promising candidates for mitigation of NETs [146].
Fig. 6.
Structures of drugs that target mitochondria and inhibit Nox2 activity.
Other mechanisms contribute to oxidative stress in COVID-19 that have not been discussed here. One of the major pathways regulating cellular oxidant balance is the transcription factor, nuclear factor erythroid 2-related factor (Nrf2) [147,148]. Nrf2 regulates the expression of antioxidant proteins that protect against oxidative damage induced by inflammation. Several FDA-approved drugs (ursodiol, dimethyl fumarate) and natural compounds (sulforaphane, curcumin, resveratrol, quercetin) are known to activate Nrf2 [149]. It was suggested that Nrf2 activation may significantly decrease the intensity of the cytokine storm in COVID-19 [150,151]. Recent reports suggest that the Nrf2 activating agent may be a potential therapeutic for COVID-19 [150,152].
10. Conclusions
NETs are a double-edged sword—they kill pathogens and viruses by oxidative damage and microbicidal activities, but they also cause collateral damage in surrounding tissues due to a cascade of inflammatory reactions. Recently, a group of medical research organizations (i.e., the NETwork) including the Cold Spring Harbor Laboratory began investigating whether overactive immune cells that produce NETs are responsible for lung inflammation and, if so, whether drugs that decrease NET formation would decrease the severity of lung disease in COVID-19 patients. Several therapeutic approaches targeting the cytokine storm, neutrophil elastase, PAD4, and oxidative enzymes Nox2 and MPO have been proposed.
African Americans are disproportionately affected by many inflammatory diseases in which increased NETs induce inflammation and thrombosis. SARS-CoV-2 infection induces a double whammy in individuals with preexisting inflammatory medical conditions, causing a hyperactive immune response with deleterious consequences. Drugs that decrease NETs in high-risk groups, including African Americans, Hispanics, and other ethnic minorities, as well as in individuals from other groups with underlying medical conditions (e.g., hypertension, diabetes, and cardiovascular disorders), could potentially decrease the severity and mortality of SARS-CoV-2 and future pandemic viruses.
Declaration of competing interest
No potential competing interest was reported by the author.
Acknowledgements
This research was supported by the Harry R. and Angeline E. Quadracci Professor in Parkinson's Research Endowment. Thanks to Lydia Washechek for preparing and editing the manuscript and to Kathleen Schmainda, PhD, for alerting me to the importance of oxidative stress in COVID-19 and directing me to a didactic lecture series by Roger Seheult, MD, at MedCram.com.
References
- 1.Fauci A.S. White House Coronavirus Task Force Press Briefing; 2020. April 7, 2020. [Google Scholar]
- 2.Thames A.D., Irwin M.R., Breen E.C., Cole S.W. Experienced discrimination and racial differences in leukocyte gene expression. Psychoneuroendocrinology. 2019;106:277–283. doi: 10.1016/j.psyneuen.2019.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wortham J.M., Lee J.T., Althomsons S., Latash J., Davidson A., Guerra K., Murray K., McGibbon E., Pichardo C., Toro B., Li L., Paladini M., Eddy M.L., Reilly K.H., McHugh L., Thomas D., Tsai S., Ojo M., Rolland S., Bhat M., Hutchinson K., Sabel J., Eckel S., Collins J., Donovan C., Cope A., Kawasaki B., McLafferty S., Alden N., Herlihy R., Barbeau B., Dunn A.C., Clark C., Pontones P., McLafferty M.L., Sidelinger D.E., Krueger A., Kollmann L., Larson L., Holzbauer S., Lynfield R., Westergaard R., Crawford R., Zhao L., Bressler J.M., Read J.S., Dunn J., Lewis A., Richardson G., Hand J., Sokol T., Adkins S.H., Leitgeb B., Pindyck T., Eure T., Wong K., Datta D., Appiah G.D., Brown J., Traxler R., Koumans E.H., Reagan-Steiner S. Characteristics of persons who died with COVID-19 - United States, february 12-may 18, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:923–929. doi: 10.15585/mmwr.mm6928e1. [DOI] [PubMed] [Google Scholar]
- 4.Williams D.R. Race, socioeconomic status, and health. The added effects of racism and discrimination. Ann. N. Y. Acad. Sci. 1999;896:173–188. doi: 10.1111/j.1749-6632.1999.tb08114.x. [DOI] [PubMed] [Google Scholar]
- 5.Yancy C.W. COVID-19 and african Americans. J. Am. Med. Assoc. 2020;323:1891–1892. doi: 10.1001/jama.2020.6548. [DOI] [PubMed] [Google Scholar]
- 6.Dorn A.V., Cooney R.E., Sabin M.L. COVID-19 exacerbating inequalities in the US. Lancet. 2020;395:1243–1244. doi: 10.1016/s0140-6736(20)30893-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Czeisler M.E., Lane R.I., Petrosky E., Wiley J.F., Christensen A., Njai R., Weaver M.D., Robbins R., Facer-Childs E.R., Barger L.K., Czeisler C.A., Howard M.E., Rajaratnam S.M.W. Mental health, substance use, and suicidal ideation during the COVID-19 pandemic — United States, june 24–30, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:1049–1057. doi: 10.15585/mmwr.mm6932a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore J.T., Ricaldi N.N., Rose C.E., Fuld J., Parise M., Kang G.J., Driscoll A.K., Norris T., Wilson N., Rainisch G., Valverde E., Beresovsky V., Agnew Brune C., Oussayef N.L., Rose D.A., Adams L.E., Awel S., Villanueva J., Meaney-Delman D., Honein M.A., COVID-19 State, T., Local, and Territorial Response Team Disparities in incidence of COVID-19 among underrepresented racial/ethnic groups in counties identified as hotspots during june 5–18, 2020 — 22 states, february–june 2020. MMWR morb mortal wkly rep. 2020. [DOI] [PMC free article] [PubMed]
- 9.Barnes B.J., Adrover J.M., Baxter-Stoltzfus A., Borczuk A., Cools-Lartigue J., Crawford J.M., Daßler-Plenker J., Guerci P., Huynh C., Knight J.S., Loda M., Looney M.R., McAllister F., Rayes R., Renaud S., Rousseau S., Salvatore S., Schwartz R.E., Spicer J.D., Yost C.C., Weber A., Zuo Y., Egeblad M. Targeting potential drivers of COVID-19: neutrophil extracellular traps. J. Exp. Med. 2020;217 doi: 10.1084/jem.20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thierry A.R. Does the newly observed inflammatory syndrome in children demonstrate a link between uncontrolled neutrophil extracellular traps formation and COVID-19? Pediatr. Res. 2020 doi: 10.1038/s41390-020-0996-1. [DOI] [PubMed] [Google Scholar]
- 11.Babior B.M. Oxygen-dependent microbial killing by phagocytes (first of two parts) N. Engl. J. Med. 1978;298:659–668. doi: 10.1056/nejm197803232981205. [DOI] [PubMed] [Google Scholar]
- 12.Beckman J.S., Beckman T.W., Chen J., Marshall P.A., Freeman B.A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. U. S. A. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D.S., Weinrauch Y., Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 14.Brinkmann V., Zychlinsky A. Beneficial suicide: why neutrophils die to make NETs. Nat. Rev. Microbiol. 2007;5:577–582. doi: 10.1038/nrmicro1710. [DOI] [PubMed] [Google Scholar]
- 15.Yipp B.G., Kubes P. NETosis: how vital is it? Blood. 2013;122:2784–2794. doi: 10.1182/blood-2013-04-457671. [DOI] [PubMed] [Google Scholar]
- 16.Sorvillo N., Cherpokova D., Martinod K., Wagner D.D. Extracellular DNA NET-works with dire consequences for health. Circ. Res. 2019;125:470–488. doi: 10.1161/circresaha.119.314581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delgado-Rizo V., Martínez-Guzmán M.A., Iñiguez-Gutierrez L., García-Orozco A., Alvarado-Navarro A., Fafutis-Morris M. Neutrophil extracellular traps and its implications in inflammation: an overview. Front. Immunol. 2017;8:81. doi: 10.3389/fimmu.2017.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papayannopoulos V., Metzler K.D., Hakkim A., Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol. 2010;191:677–691. doi: 10.1083/jcb.201006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dąbrowska D., Jabłońska E., Garley M., Ratajczak-Wrona W., Iwaniuk A. New aspects of the biology of neutrophil extracellular traps. Scand. J. Immunol. 2016;84:317–322. doi: 10.1111/sji.12494. [DOI] [PubMed] [Google Scholar]
- 20.Neeli I., Khan S.N., Radic M. Histone deimination as a response to inflammatory stimuli in neutrophils. J. Immunol. 2008;180:1895–1902. doi: 10.4049/jimmunol.180.3.1895. [DOI] [PubMed] [Google Scholar]
- 21.Hule G.P., Bargir U.A., Kulkarni M., Kambli P., Taur P., Desai M., Madkaikar M.R. Does pioglitazone lead to neutrophil extracellular traps formation in chronic granulomatous disease patients? Front. Immunol. 2019;10:1739. doi: 10.3389/fimmu.2019.01739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McHugh J. Distinguishing NET subtypes. Nat. Rev. Rheumatol. 2018;14:560. doi: 10.1038/s41584-018-0088-3. [DOI] [PubMed] [Google Scholar]
- 23.Yang L., Liu Q., Zhang X., Liu X., Zhou B., Chen J., Huang D., Li J., Li H., Chen F., Liu J., Xing Y., Chen X., Su S., Song E. DNA of neutrophil extracellular traps promotes cancer metastasis via CCDC25. Nature. 2020;583:133–138. doi: 10.1038/s41586-020-2394-6. [DOI] [PubMed] [Google Scholar]
- 24.Rayes R.F., Mouhanna J.G., Nicolau I., Bourdeau F., Giannias B., Rousseau S., Quail D., Walsh L., Sangwan V., Bertos N., Cools-Lartigue J., Ferri L.E., Spicer J.D. Primary tumors induce neutrophil extracellular traps with targetable metastasis promoting effects. JCI Insight. 2019;5 doi: 10.1172/jci.insight.128008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018;18:134–147. doi: 10.1038/nri.2017.105. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan M.J., Radic M. Neutrophil extracellular traps: double-edged swords of innate immunity. J. Immunol. 2012;189:2689–2695. doi: 10.4049/jimmunol.1201719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hakkim A., Fürnrohr B.G., Amann K., Laube B., Abed U.A., Brinkmann V., Herrmann M., Voll R.E., Zychlinsky A. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc. Natl. Acad. Sci. Unit. States Am. 2010;107:9813–9818. doi: 10.1073/pnas.0909927107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang S., Xie T., Sun S., Wang K., Liu B., Wu X., Ding W. DNase-1 treatment exerts protective effects in a rat model of intestinal ischemia-reperfusion injury. Sci. Rep. 2018;8:17788. doi: 10.1038/s41598-018-36198-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leffler J., Stojanovich L., Shoenfeld Y., Bogdanovic G., Hesselstrand R., Blom A.M. Degradation of neutrophil extracellular traps is decreased in patients with antiphospholipid syndrome. Clin. Exp. Rheumatol. 2014;32:66–70. [PubMed] [Google Scholar]
- 30.Bruschi M., Bonanni A., Petretto A., Vaglio A., Pratesi F., Santucci L., Migliorini P., Bertelli R., Galetti M., Belletti S., Cavagna L., Moroni G., Franceschini F., Fredi M., Pazzola G., Allegri L., Sinico R.A., Pesce G., Bagnasco M., Manfredi A., Ramirez G.A., Ramoino P., Bianchini P., Puppo F., Pupo F., Negrini S., Mattana F., Emmi G., Garibotto G., Santoro D., Scolari F., Ravelli A., Tincani A., Cravedi P., Volpi S., Candiano G., Ghiggeri G.M. Neutrophil extracellular traps profiles in patients with incident systemic lupus erythematosus and lupus nephritis. J. Rheumatol. 2020;47:377–386. doi: 10.3899/jrheum.181232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salemme R., Peralta L.N., Meka S.H., Pushpanathan N., Alexander J.J. The role of NETosis in systemic lupus erythematosus. J Cell Immunol. 2019;1:33–42. doi: 10.33696/immunology.1.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McHugh J. NET formation differs in SLE and vasculitis. Nat. Rev. Rheumatol. 2019;15:512. doi: 10.1038/s41584-019-0284-9. [DOI] [PubMed] [Google Scholar]
- 33.Zuo Y., Yalavarthi S., Shi H., Gockman K., Zuo M., Madison J.A., Blair C., Weber A., Barnes B.J., Egeblad M., Woods R.J., Kanthi Y., Knight J.S. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020;5 doi: 10.1172/jci.insight.138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mozzini C., Girelli D. The role of Neutrophil Extracellular Traps in Covid-19: only an hypothesis or a potential new field of research? Thromb. Res. 2020;191:26–27. doi: 10.1016/j.thromres.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Middleton E.A., He X.Y., Denorme F., Campbell R.A., Ng D., Salvatore S.P., Mostyka M., Baxter-Stoltzfus A., Borczuk A.C., Loda M., Cody M.J., Manne B.K., Portier I., Harris E., Petrey A.C., Beswick E.J., Caulin A.F., Iovino A., Abegglen L.M., Weyrich A.S., Rondina M.T., Egeblad M., Schiffman J.D., Yost C.C. Neutrophil extracellular traps (NETs) contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020 doi: 10.1182/blood.2020007008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Welch W.J. Angiotensin II-dependent superoxide: effects on hypertension and vascular dysfunction. Hypertension. 2008;52:51–56. doi: 10.1161/hypertensionaha.107.090472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang F., Ren X., Zhao M., Zhou B., Han Y. Angiotensin-(1-7) abrogates angiotensin II-induced proliferation, migration and inflammation in VSMCs through inactivation of ROS-mediated PI3K/Akt and MAPK/ERK signaling pathways. Sci. Rep. 2016;6:34621. doi: 10.1038/srep34621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Papinska A.M., Soto M., Meeks C.J., Rodgers K.E. Long-term administration of angiotensin (1-7) prevents heart and lung dysfunction in a mouse model of type 2 diabetes (db/db) by reducing oxidative stress, inflammation and pathological remodeling. Pharmacol. Res. 2016;107:372–380. doi: 10.1016/j.phrs.2016.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Derouiche S. Oxidative stress associated with SARS-cov-2 (COVID-19) increases the severity of the lung disease - a systematic review. J Infect Dis Epidemiol. 2020;6:121. doi: 10.23937/2474-3658/1510121. [DOI] [Google Scholar]
- 41.Gheblawi M., Wang K., Viveiros A., Nguyen Q., Zhong J.C., Turner A.J., Raizada M.K., Grant M.B., Oudit G.Y. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ. Res. 2020;126:1456–1474. doi: 10.1161/circresaha.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wysocki J., Ortiz-Melo D.I., Mattocks N.K., Xu K., Prescott J., Evora K., Ye M., Sparks M.A., Haque S.K., Batlle D., Gurley S.B. ACE2 deficiency increases NADPH-mediated oxidative stress in the kidney. Phys. Rep. 2014;2 doi: 10.1002/phy2.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rabelo L.A., Alenina N., Bader M. ACE2-angiotensin-(1-7)-Mas axis and oxidative stress in cardiovascular disease. Hypertens. Res. 2011;34:154–160. doi: 10.1038/hr.2010.235. [DOI] [PubMed] [Google Scholar]
- 44.Mancia G., Rea F., Ludergnani M., Apolone G., Corrao G. Renin-angiotensin-aldosterone system blockers and the risk of covid-19. N. Engl. J. Med. 2020;382:2431–2440. doi: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fosbøl E.L., Butt J.H., Østergaard L., Andersson C., Selmer C., Kragholm K., Schou M., Phelps M., Gislason G.H., Gerds T.A., Torp-Pedersen C., Køber L. Association of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use with COVID-19 diagnosis and mortality. Jama. 2020 doi: 10.1001/jama.2020.11301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saha A., Sharma A.R., Bhattacharya M., Sharma G., Lee S.S., Chakraborty C. Probable molecular mechanism of remdesivir for the treatment of COVID-19: need to know more. Arch. Med. Res. 2020 doi: 10.1016/j.arcmed.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ledford H. Coronavirus breakthrough: dexamethasone is first drug shown to save lives. Nature. 2020;582:469. doi: 10.1038/d41586-020-01824-5. [DOI] [PubMed] [Google Scholar]
- 48.Pedersen F., Waschki B., Marwitz S., Goldmann T., Kirsten A., Malmgren A., Rabe K.F., Uddin M., Watz H. Neutrophil extracellular trap formation is regulated by CXCR2 in COPD neutrophils. Eur. Respir. J. 2018;51:1700970. doi: 10.1183/13993003.00970-2017. [DOI] [PubMed] [Google Scholar]
- 49.Assimakopoulos S.F., Marangos M. N-acetyl-cysteine may prevent COVID-19-associated cytokine storm and acute respiratory distress syndrome. Med. Hypotheses. 2020;140:109778. doi: 10.1016/j.mehy.2020.109778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Center for Disease Control and Prevention . 2019. Data & Statistics on Sickle Cell Disease.https://www.cdc.gov/ncbddd/sicklecell/data.html [Google Scholar]
- 51.Cruzat V., Macedo Rogero M., Noel Keane K., Curi R., Newsholme P. Glutamine: metabolism and immune function, supplementation and clinical translation. Nutrients. 2018;10 doi: 10.3390/nu10111564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gori T., Münzel T. Oxidative stress and endothelial dysfunction: therapeutic implications. Ann. Med. 2011;43:259–272. doi: 10.3109/07853890.2010.543920. [DOI] [PubMed] [Google Scholar]
- 53.Wang H., Wang Q., Venugopal J., Wang J., Kleiman K., Guo C., Eitzman D.T. Obesity-induced endothelial dysfunction is prevented by neutrophil extracellular trap inhibition. Sci. Rep. 2018;8:4881. doi: 10.1038/s41598-018-23256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Braster Q., Silvestre Roig C., Hartwig H., Beckers L., den Toom M., Döring Y., Daemen M.J., Lutgens E., Soehnlein O. Inhibition of NET release fails to reduce adipose tissue inflammation in mice. PloS One. 2016;11 doi: 10.1371/journal.pone.0163922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.D'Abbondanza M., Martorelli E.E., Ricci M.A., De Vuono S., Migliola E.N., Godino C., Corradetti S., Siepi D., Paganelli M.T., Maugeri N., Lupattelli G. Increased plasmatic NETs by-products in patients in severe obesity. Sci. Rep. 2019;9:14678. doi: 10.1038/s41598-019-51220-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Petrakis D., Margină D., Tsarouhas K., Tekos F., Stan M., Nikitovic D., Kouretas D., Spandidos D.A., Tsatsakis A. Obesity - a risk factor for increased COVID-19 prevalence, severity and lethality (Review) Mol. Med. Rep. 2020;22:9–19. doi: 10.3892/mmr.2020.11127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nesbitt S.D. Management of hypertension in african-Americans. US Cardiol. 2009;6:59–62. [Google Scholar]
- 58.Obama T., Scalia R., Eguchi S. Targeting neutrophil: new approach against hypertensive cardiac remodeling? Hypertension. 2014;63:1171–1172. doi: 10.1161/hypertensionaha.114.03288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zilbermint M., Hannah-Shmouni F., Stratakis C.A. Genetics of hypertension in african Americans and others of african descent. Int. J. Mol. Sci. 2019;20:1081. doi: 10.3390/ijms20051081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vijay-Kumar M., Saha P., Yeoh B.S., Golonka R., McCarthy C., Spegele A., Abokor A., Chakraborty S., Mell B., Koch L., Joe B. Abstract P1126: neutrophil extracellular traps: new players in hypertension. Hypertension. 2019;74 doi: 10.1161/hyp.74.suppl_1.P1126. AP1126-AP1126. [DOI] [Google Scholar]
- 61.Abais-Battad J.M., Lund H., Dasinger J.H., Fehrenbach D.J., Cowley A.W., Jr., Mattson D.L. NOX2-derived reactive oxygen species in immune cells exacerbates salt-sensitive hypertension. Free Radic. Biol. Med. 2020;146:333–339. doi: 10.1016/j.freeradbiomed.2019.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hofbauer T.M., Scherz T., Ondracek A., Mueller J., Mangold A., Lang I.M. P149Angiotensin-II enhances neutrophil extracellular trap formation in an AT1R and NADPH oxidase-dependent manner. Cardiovasc. Res. 2018;114 doi: 10.1093/cvr/cvy060.110. S39-S39. [DOI] [Google Scholar]
- 63.Zhou L., Xiang W., Potts J., Floyd M., Sharan C., Yang H., Ross J., Nyanda A.M., Guo Z. Reduction in extracellular superoxide dismutase activity in African-American patients with hypertension. Free Radic. Biol. Med. 2006;41:1384–1391. doi: 10.1016/j.freeradbiomed.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 64.Döring Y., Libby P., Soehnlein O. Neutrophil extracellular traps participate in cardiovascular diseases. Circ. Res. 2020;126:1228–1241. doi: 10.1161/CIRCRESAHA.120.315931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Clerkin K.J., Fried J.A., Raikhelkar J., Sayer G., Griffin J.M., Masoumi A., Jain S.S., Burkhoff D., Kumaraiah D., Rabbani L., Schwartz A., Uriel N. COVID-19 and cardiovascular disease. Circulation. 2020;141:1648–1655. doi: 10.1161/circulationaha.120.046941. [DOI] [PubMed] [Google Scholar]
- 66.Bonaventura A., Vecchié A., Abbate A., Montecucco F. Neutrophil extracellular traps and cardiovascular diseases: an update. Cells. 2020;9:231. doi: 10.3390/cells9010231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mangold A., Hofbauer T.M., Ondracek A.S., Artner T., Scherz T., Speidl W.S., Krychtiuk K.A., Sadushi-Kolici R., Jakowitsch J., Lang I.M. Neutrophil extracellular traps and monocyte subsets at the culprit lesion site of myocardial infarction patients. Sci. Rep. 2019;9:16304. doi: 10.1038/s41598-019-52671-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jiménez-Alcázar M., Napirei M., Panda R., Köhler E.C., Kremer Hovinga J.A., Mannherz H.G., Peine S., Renné T., Lämmle B., Fuchs T.A. Impaired DNase1-mediated degradation of neutrophil extracellular traps is associated with acute thrombotic microangiopathies. J. Thromb. Haemostasis. 2015;13:732–742. doi: 10.1111/jth.12796. [DOI] [PubMed] [Google Scholar]
- 69.Liu P.P., Blet A., Smyth D., Li H. The science underlying COVID-19: implications for the cardiovascular system. Circulation. 2020 doi: 10.1161/circulationaha.120.047549. [DOI] [PubMed] [Google Scholar]
- 70.Zhu L., She Z.G., Cheng X., Qin J.J., Zhang X.J., Cai J., Lei F., Wang H., Xie J., Wang W., Li H., Zhang P., Song X., Chen X., Xiang M., Zhang C., Bai L., Xiang D., Chen M.M., Liu Y., Yan Y., Liu M., Mao W., Zou J., Liu L., Chen G., Luo P., Xiao B., Zhang C., Zhang Z., Lu Z., Wang J., Lu H., Xia X., Wang D., Liao X., Peng G., Ye P., Yang J., Yuan Y., Huang X., Guo J., Zhang B.H., Li H. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metabol. 2020;31:1068–1077. doi: 10.1016/j.cmet.2020.04.021. e1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Berezin A. Challenging role of neutrophil extracellular traps in vascular complications of diabetes mellitus. Int. J. Mol. Med. 2018;5:1–5. doi: 10.15761/IMM.1000330. [DOI] [Google Scholar]
- 72.Wang L., Zhou X., Yin Y., Mai Y., Wang D., Zhang X. Hyperglycemia induces neutrophil extracellular traps formation through an NADPH oxidase-dependent pathway in diabetic retinopathy. Front. Immunol. 2018;9:3076. doi: 10.3389/fimmu.2018.03076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Menegazzo L., Ciciliot S., Poncina N., Mazzucato M., Persano M., Bonora B., Albiero M., Vigili de Kreutzenberg S., Avogaro A., Fadini G.P. NETosis is induced by high glucose and associated with type 2 diabetes. Acta Diabetol. 2015;52:497–503. doi: 10.1007/s00592-014-0676-x. [DOI] [PubMed] [Google Scholar]
- 74.Park J.H., Kim J.E., Gu J.Y., Yoo H.J., Park S.H., Kim Y.I., Nam-Goong I.S., Kim E.S., Kim H.K. Evaluation of circulating markers of neutrophil extracellular trap (NET) formation as risk factors for diabetic retinopathy in a case-control association study. Exp. Clin. Endocrinol. Diabetes. 2016;124:557–561. doi: 10.1055/s-0042-101792. [DOI] [PubMed] [Google Scholar]
- 75.Carestia A., Frechtel G., Cerrone G., Linari M.A., Gonzalez C.D., Casais P., Schattner M. NETosis before and after hyperglycemic control in type 2 diabetes mellitus patients. PloS One. 2016;11 doi: 10.1371/journal.pone.0168647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wong S.L., Demers M., Martinod K., Gallant M., Wang Y., Goldfine A.B., Kahn C.R., Wagner D.D. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat. Med. 2015;21:815–819. doi: 10.1038/nm.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Docherty A.B., Harrison E.M., Green C.A., Hardwick H.E., Pius R., Norman L., Holden K.A., Read J.M., Dondelinger F., Carson G., Merson L., Lee J., Plotkin D., Sigfrid L., Halpin S., Jackson C., Gamble C., Horby P.W., Nguyen-Van-Tam J.S., Dunning J., Openshaw P.J., Baillie J.K., Semple M.G. Features of 16,749 hospitalised UK patients with COVID-19 using the ISARIC WHO clinical characterisation protocol. medRxiv. 2020 doi: 10.1101/2020.04.23.20076042. 2020.2004.2023.20076042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rubino F., Amiel S.A., Zimmet P., Alberti G., Bornstein S., Eckel R.H., Mingrone G., Boehm B., Cooper M.E., Chai Z., Del Prato S., Ji L., Hopkins D., Herman W.H., Khunti K., Mbanya J.-C., Renard E. New-onset diabetes in covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2018688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Codo A.C., Davanzo G.G., de Brito Monteiro L., Fabiano de Souza G., Muraro S.P., Virgilio-da-Silva J.V., Prodonoff J.S., Carregari V.C., Oliveira de Biagi Junior C.A., Crunfli F., Jimenez Restrepo J.L., Vendramini P.H., Reis-de-Oliveira G., Bispo dos Santos K., Augusto de Toledo Teixeira D., Parise P.L., Martini M.C., Marques R.E., Carmo H.R., Borin A., Coimbra L.D., Boldrini V.O., Brunetti N.S., Vieira A.S., Mansour E., Ulaf R.G., Bernardes A.F., Nunes T.A., Ribeiro L.C., Palma A.C., Agrela M.V., Moretti M.L., Sposito A.C., Pereira F.B., Velloso L.A., Ramirez Vinolo M.A., Damasio A., Proença-Módena J.L., Carvalho R.F., Mori M.A., Martins-de-Souza D., Nakaya H.I., Farias A.S., Moraes-Vieira P.M.M. Elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a HIF-1 beta/glycolysis dependent axis. Cell Metabol. 2020 doi: 10.1016/j.cmet.2020.07.007. [preprint] [DOI] [Google Scholar]
- 80.Chen G., Zhang D., Fuchs T.A., Manwani D., Wagner D.D., Frenette P.S. Heme-induced neutrophil extracellular traps contribute to the pathogenesis of sickle cell disease. Blood. 2014;123:3818–3827. doi: 10.1182/blood-2013-10-529982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen G., Fuchs T.A., Wagner D.D., Frenette P.S. Heme-induced neutrophil extracellular traps (NETs) formation contributes to sickle cell disease pathogenesis. Blood. 2013;122 doi: 10.1182/blood.V122.21.184.184. 184-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Van Avondt K., Schimmel M., Bulder I., Bruggen R.v., Biemond B.J., Luken B.M., Zeerleder S.S. Free iron in sera of patients with sickle cell disease contributes to the release of neutrophil extracellular traps. Blood. 2016;128 doi: 10.1182/blood.V128.22.161.161. 161-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Barbu E.A., Dominical V.M., Mendelsohn L., Thein S.L. Neutrophils remain detrimentally active in hydroxyurea-treated patients with sickle cell disease. PloS One. 2019;14 doi: 10.1371/journal.pone.0226583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu C.L., Tangsombatvisit S., Rosenberg J.M., Mandelbaum G., Gillespie E.C., Gozani O.P., Alizadeh A.A., Utz P.J. Specific post-translational histone modifications of neutrophil extracellular traps as immunogens and potential targets of lupus autoantibodies. Arthritis Res. Ther. 2012;14:R25. doi: 10.1186/ar3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gocho T., Mori H., Islam M.M., Maruchi Y., Takenaka N., Tomino A., Tsuda M., Kano H., Takeyama N. Removal of circulating neutrophil extracellular trap components with an immobilized polymyxin B filter: a preliminary study. Shock. 2020;54:44–49. doi: 10.1097/shk.0000000000001476. [DOI] [PubMed] [Google Scholar]
- 86.Lood C., Blanco L.P., Purmalek M.M., Carmona-Rivera C., De Ravin S.S., Smith C.K., Malech H.L., Ledbetter J.A., Elkon K.B., Kaplan M.J. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat. Med. 2016;22:146–153. doi: 10.1038/nm.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Barnado A., Crofford L.J., Oates J.C. At the Bedside: neutrophil extracellular traps (NETs) as targets for biomarkers and therapies in autoimmune diseases. J. Leukoc. Biol. 2016;99:265–278. doi: 10.1189/jlb.5BT0615-234R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fortner K.A., Blanco L.P., Buskiewicz I., Huang N., Gibson P.C., Cook D.L., Pedersen H.L., Yuen P.S.T., Murphy M.P., Perl A., Kaplan M.J., Budd R.C. Targeting mitochondrial oxidative stress with MitoQ reduces NET formation and kidney disease in lupus-prone MRL-lpr mice. Lupus Sci Med. 2020;7 doi: 10.1136/lupus-2020-000387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Iwasaki Y., Takeshima Y., Fujio K. Basic mechanism of immune system activation by mitochondria. Immunol Med. 2020:1–6. doi: 10.1080/25785826.2020.1756609. [DOI] [PubMed] [Google Scholar]
- 90.Navarro-Millán I., Darrah E., Westfall A.O., Mikuls T.R., Reynolds R.J., Danila M.I., Curtis J.R., Rosen A., Bridges S.L., Jr. Association of anti-peptidyl arginine deiminase antibodies with radiographic severity of rheumatoid arthritis in African Americans. Arthritis Res. Ther. 2016;18:241. doi: 10.1186/s13075-016-1126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Grabcanovic-Musija F., Obermayer A., Stoiber W., Krautgartner W.-D., Steinbacher P., Winterberg N., Bathke A.C., Klappacher M., Studnicka M. Neutrophil extracellular trap (NET) formation characterises stable and exacerbated COPD and correlates with airflow limitation. Respir. Res. 2015;16:59. doi: 10.1186/s12931-015-0221-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dicker A.J., Crichton M.L., Pumphrey E.G., Cassidy A.J., Suarez-Cuartin G., Sibila O., Furrie E., Fong C.J., Ibrahim W., Brady G., Einarsson G.G., Elborn J.S., Schembri S., Marshall S.E., Palmer C.N.A., Chalmers J.D. Neutrophil extracellular traps are associated with disease severity and microbiota diversity in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2018;141:117–127. doi: 10.1016/j.jaci.2017.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Alzheimer's Association . 2002. African-Americans and Alzheimer's Disease: the Silent Epidemic; pp. 1–5. [Google Scholar]
- 94.Alzheimer's Association 2020 alzheimer's disease facts and figures. Alzheimers Dement. 2020;16:391. [Google Scholar]
- 95.Barnes L.L., Bennett D.A. Alzheimer's disease in African Americans: risk factors and challenges for the future. Health Aff. 2014;33:580–586. doi: 10.1377/hlthaff.2013.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stock A.J., Kasus-Jacobi A., Pereira H.A. The role of neutrophil granule proteins in neuroinflammation and Alzheimer's disease. J. Neuroinflammation. 2018;15:240. doi: 10.1186/s12974-018-1284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zenaro E., Pietronigro E., Della Bianca V., Piacentino G., Marongiu L., Budui S., Turano E., Rossi B., Angiari S., Dusi S., Montresor A., Carlucci T., Nanì S., Tosadori G., Calciano L., Catalucci D., Berton G., Bonetti B., Constantin G. Neutrophils promote Alzheimer's disease-like pathology and cognitive decline via LFA-1 integrin. Nat. Med. 2015;21:880–886. doi: 10.1038/nm.3913. [DOI] [PubMed] [Google Scholar]
- 98.Pietronigro E.C., Della Bianca V., Zenaro E., Constantin G. NETosis in Alzheimer's disease. Front. Immunol. 2017;8 doi: 10.3389/fimmu.2017.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Manda-Handzlik A., Demkow U. The brain entangled: the contribution of neutrophil extracellular traps to the diseases of the central nervous system. Cells. 2019;8:1477. doi: 10.3390/cells8121477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Azevedo E.P., Guimarães-Costa A.B., Torezani G.S., Braga C.A., Palhano F.L., Kelly J.W., Saraiva E.M., Foguel D. Amyloid fibrils trigger the release of neutrophil extracellular traps (NETs), causing fibril fragmentation by NET-associated elastase. J. Biol. Chem. 2012;287:37206–37218. doi: 10.1074/jbc.M112.369942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sharrief A.Z., Johnson B., Abada S., Urrutia V.C. Stroke knowledge in african Americans: a narrative review. Ethn. Dis. 2016;26:255–262. doi: 10.18865/ed.26.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Laridan E., Denorme F., Desender L., François O., Andersson T., Deckmyn H., Vanhoorelbeke K., De Meyer S.F. Neutrophil extracellular traps in ischemic stroke thrombi. Ann. Neurol. 2017;82:223–232. doi: 10.1002/ana.24993. [DOI] [PubMed] [Google Scholar]
- 103.Vallés J., Lago A., Santos M.T., Latorre A.M., Tembl J.I., Salom J.B., Nieves C., Moscardó A. Neutrophil extracellular traps are increased in patients with acute ischemic stroke: prognostic significance. Thromb. Haemostasis. 2017;117:1919–1929. doi: 10.1160/th17-02-0130. [DOI] [PubMed] [Google Scholar]
- 104.Ducroux C., Di Meglio L., Loyau S., Delbosc S., Boisseau W., Deschildre C., Ben Maacha M., Blanc R., Redjem H., Ciccio G., Smajda S., Fahed R., Michel J.B., Piotin M., Salomon L., Mazighi M., Ho-Tin-Noe B., Desilles J.P. Thrombus neutrophil extracellular traps content impair tPA-induced thrombolysis in acute ischemic stroke. Stroke. 2018;49:754–757. doi: 10.1161/strokeaha.117.019896. [DOI] [PubMed] [Google Scholar]
- 105.Acta Neuropathologica C. Publisher correction to: acta neuropathologica communications, volume 7. Acta Neuropathologica Communications. 2019;7:131. doi: 10.1186/s40478-019-0784-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schootman M., Jeffe D.B., Gillanders W.E., Aft R. Racial disparities in the development of breast cancer metastases among older women: a multilevel study. Cancer. 2009;115:731–740. doi: 10.1002/cncr.24087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Binnewies M., Roberts E.W., Kersten K., Chan V., Fearon D.F., Merad M., Coussens L.M., Gabrilovich D.I., Ostrand-Rosenberg S., Hedrick C.C., Vonderheide R.H., Pittet M.J., Jain R.K., Zou W., Howcroft T.K., Woodhouse E.C., Weinberg R.A., Krummel M.F. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018;24:541–550. doi: 10.1038/s41591-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lillicrap D. Thrombolytic potential of N-acetylcysteine. Circulation. 2017;136:661–663. doi: 10.1161/CIRCULATIONAHA.117.029313. [DOI] [PubMed] [Google Scholar]
- 109.Van Avondt K., Hartl D. Mechanisms and disease relevance of neutrophil extracellular trap formation. Eur. J. Clin. Invest. 2018;48(Suppl 2) doi: 10.1111/eci.12919. [DOI] [PubMed] [Google Scholar]
- 110.van Dam L.S., Rabelink T.J., van Kooten C., Teng Y.K.O. Clinical implications of excessive neutrophil extracellular trap formation in renal autoimmune diseases. Kidney Int Rep. 2019;4:196–211. doi: 10.1016/j.ekir.2018.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang H., Li T., Chen S., Gu Y., Ye S. Neutrophil extracellular trap mitochondrial DNA and its autoantibody in systemic lupus erythematosus and a proof-of-concept trial of metformin. Arthritis Rheum. 2015;67:3190–3200. doi: 10.1002/art.39296. [DOI] [PubMed] [Google Scholar]
- 112.Kirchner T., Hermann E., Möller S., Klinger M., Solbach W., Laskay T., Behnen M. Flavonoids and 5-aminosalicylic acid inhibit the formation of neutrophil extracellular traps. Mediat. Inflamm. 2013:710239. doi: 10.1155/2013/710239. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ali R.A., Gandhi A.A., Meng H., Yalavarthi S., Vreede A.P., Estes S.K., Palmer O.R., Bockenstedt P.L., Pinsky D.J., Greve J.M., Diaz J.A., Kanthi Y., Knight J.S. Adenosine receptor agonism protects against NETosis and thrombosis in antiphospholipid syndrome. Nat. Commun. 2019;10:1916. doi: 10.1038/s41467-019-09801-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chirivi R.G.S., van Rosmalen J.W.G., van der Linden M., Euler M., Schmets G., Bogatkevich G., Kambas K., Hahn J., Braster Q., Soehnlein O., Hoffmann M.H., Es H.H. G.v., Raats J.M.H. Therapeutic ACPA inhibits NET formation: a potential therapy for neutrophil-mediated inflammatory diseases. Cell. Mol. Immunol. 2020;10 doi: 10.1038/s41423-020-0381-3. 1038/s41423-020-0381-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Murthy P., Singhi A.D., Ross M.A., Loughran P., Paragomi P., Papachristou G.I., Whitcomb D.C., Zureikat A.H., Lotze M.T., Zeh H.J., III, Boone B.A. Enhanced neutrophil extracellular trap formation in acute pancreatitis contributes to disease severity and is reduced by chloroquine. Front. Immunol. 2019;10 doi: 10.3389/fimmu.2019.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang S., Zhang Q., Wang F., Guo X., Liu T., Zhao Y., Gu B., Chen H., Li Y. Hydroxychloroquine inhibiting neutrophil extracellular trap formation alleviates hepatic ischemia/reperfusion injury by blocking TLR9 in mice. Clin. Immunol. 2020;216:108461. doi: 10.1016/j.clim.2020.108461. [DOI] [PubMed] [Google Scholar]
- 117.Boone B.A., Murthy P., Miller-Ocuin J., Doerfler W.R., Ellis J.T., Liang X., Ross M.A., Wallace C.T., Sperry J.L., Lotze M.T., Neal M.D., Zeh H.J., 3rd Chloroquine reduces hypercoagulability in pancreatic cancer through inhibition of neutrophil extracellular traps. BMC Canc. 2018;18:678. doi: 10.1186/s12885-018-4584-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cavalcanti A.B., Zampieri F.G., Rosa R.G., Azevedo L.C.P., Veiga V.C., Avezum A., Damiani L.P., Marcadenti A., Kawano-Dourado L., Lisboa T., Junqueira D.L.M., de Barros e Silva P.G.M., Tramujas L., Abreu-Silva E.O., Laranjeira L.N., Soares A.T., Echenique L.S., Pereira A.J., Freitas F.G.R., Gebara O.C.E., Dantas V.C.S., Furtado R.H.M., Milan E.P., Golin N.A., Cardoso F.F., Maia I.S., Hoffmann Filho C.R., Kormann A.P.M., Amazonas R.B., Bocchi de Oliveira M.F., Serpa-Neto A., Falavigna M., Lopes R.D., Machado F.R., Berwanger O. Hydroxychloroquine with or without azithromycin in mild-to-moderate covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2019014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hoffmann M., Mösbauer K., Hofmann-Winkler H., Kaul A., Kleine-Weber H., Krüger N., Gassen N.C., Müller M.A., Drosten C., Pöhlmann S. Chloroquine does not inhibit infection of human lung cells with SARS-CoV-2. Nature. 2020 doi: 10.1038/s41586-020-2575-3. [DOI] [PubMed] [Google Scholar]
- 120.Maisonnasse P., Guedj J., Contreras V., Behillil S., Solas C., Marlin R., Naninck T., Pizzorno A., Lemaitre J., Gonçalves A., Kahlaoui N., Terrier O., Fang R.H.T., Enouf V., Dereuddre-Bosquet N., Brisebarre A., Touret F., Chapon C., Hoen B., Lina B., Calatrava M.R., van der Werf S., de Lamballerie X., Le Grand R. Hydroxychloroquine use against SARS-CoV-2 infection in non-human primates. Nature. 2020 doi: 10.1038/s41586-020-2558-4. [DOI] [PubMed] [Google Scholar]
- 121.Boulware D.R., Pullen M.F., Bangdiwala A.S., Pastick K.A., Lofgren S.M., Okafor E.C., Skipper C.P., Nascene A.A., Nicol M.R., Abassi M., Engen N.W., Cheng M.P., LaBar D., Lother S.A., MacKenzie L.J., Drobot G., Marten N., Zarychanski R., Kelly L.E., Schwartz I.S., McDonald E.G., Rajasingham R., Lee T.C., Hullsiek K.H. A randomized trial of hydroxychloroquine as postexposure prophylaxis for covid-19. N. Engl. J. Med. 2020;383:517–525. doi: 10.1056/NEJMoa2016638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Arshad S., Kilgore P., Chaudhry Z.S., Jacobsen G., Wang D.D., Huitsing K., Brar I., Alangaden G.J., Ramesh M.S., McKinnon J.E., O'Neill W., Zervos M. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int. J. Infect. Dis. 2020;97:396–403. doi: 10.1016/j.ijid.2020.06.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Polonikov A. Endogenous deficiency of glutathione as the most likely cause of serious manifestations and death in COVID-19 patients. ACS Infect. Dis. 2020 doi: 10.1021/acsinfecdis.0c00288. [DOI] [PubMed] [Google Scholar]
- 124.Ghezzi P. Role of glutathione in immunity and inflammation in the lung. Int. J. Gen. Med. 2011;4:105–113. doi: 10.2147/ijgm.S15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Horowitz R.I., Freeman P.R., Bruzzese J. Efficacy of glutathione therapy in relieving dyspnea associated with COVID-19 pneumonia: a report of 2 cases. Respir Med Case Rep. 2020;30:101063. doi: 10.1016/j.rmcr.2020.101063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Van Hecke O., Lee J. Center for Evidence-Based Medicine; 2020. N-Acetylcysteine: A Rapid Review of the Evidence for Effectiveness in Treating COVID-19. [Google Scholar]
- 127.Fuchs T.A., Brill A., Duerschmied D., Schatzberg D., Monestier M., Myers D.D., Wrobleski S.K., Wakefield T.W., Hartwig J.H., Wagner D.D. Extracellular DNA traps promote thrombosis. Proc. Natl. Acad. Sci. Unit. States Am. 2010;107:15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Craver B.M., Ramanathan G., Hoang S., Chang X., Mendez Luque L.F., Brooks S., Lai H.Y., Fleischman A.G. N-acetylcysteine inhibits thrombosis in a murine model of myeloproliferative neoplasm. Blood Adv. 2020;4:312–321. doi: 10.1182/bloodadvances.2019000967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Murphy M.P., Holmgren A., Larsson N.G., Halliwell B., Chang C.J., Kalyanaraman B., Rhee S.G., Thornalley P.J., Partridge L., Gems D., Nystrom T., Belousov V., Schumacker P.T., Winterbourn C.C. Unraveling the biological roles of reactive oxygen species. Cell Metabol. 2011;13:361–366. doi: 10.1016/j.cmet.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Martinez de Lizarrondo S., Gakuba C., Herbig B.A., Repessé Y., Ali C., Denis C.V., Lenting P.J., Touzé E., Diamond S.L., Vivien D., Gauberti M. Potent thrombolytic effect of N-acetylcysteine on arterial thrombi. Circulation. 2017;136:646–660. doi: 10.1161/circulationaha.117.027290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chen J., Reheman A., Gushiken F.C., Nolasco L., Fu X., Moake J.L., Ni H., López J.A. N-acetylcysteine reduces the size and activity of von Willebrand factor in human plasma and mice. J. Clin. Invest. 2011;121:593–603. doi: 10.1172/jci41062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bozonet S.M., Carr A.C. The role of physiological vitamin C concentrations on key functions of neutrophils isolated from healthy individuals. Nutrients. 2019;11:1363. doi: 10.3390/nu11061363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lee K.H., Kronbichler A., Park D.D., Park Y., Moon H., Kim H., Choi J.H., Choi Y., Shim S., Lyu I.S., Yun B.H., Han Y., Lee D., Lee S.Y., Yoo B.H., Lee K.H., Kim T.L., Kim H., Shim J.S., Nam W., So H., Choi S., Lee S., Shin J.I. Neutrophil extracellular traps (NETs) in autoimmune diseases: a comprehensive review. Autoimmun. Rev. 2017;16:1160–1173. doi: 10.1016/j.autrev.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 134.Daneshkhah A., Agrawal V., Eshein A., Subramanian H., Roy H.K., Backman V. 2020. The Possible Role of Vitamin D in Suppressing Cytokine Storm and Associated Mortality in COVID-19 Patients. medRxiv [preprint], 2020.2004.2008.20058578. [DOI] [Google Scholar]
- 135.GlobalData Healthcare Vitamin D affects Covid-19 mortality. 2020. https://www.pharmaceutical-technology.com/comment/vitamin-d-covid-19/
- 136.Rhodes J.M., Subramanian S., Laird E., Griffin G., Kenny R.A. Perspective: vitamin D deficiency and COVID-19 severity – plausibly linked by latitude, ethnicity, impacts on cytokines, ACE2, and thrombosis (R1) J. Intern. Med. 2020 doi: 10.1111/joim.13149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Carpagnano G.E., Di Lecce V., Quaranta V.N., Zito A., Buonamico E., Capozza E., Palumbo A., Di Gioia G., Valerio V.N., Resta O. Vitamin D deficiency as a predictor of poor prognosis in patients with acute respiratory failure due to COVID-19. J. Endocrinol. Invest. 2020:1–7. doi: 10.1007/s40618-020-01370-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sondo E., Bertelli R., Pesce E., Ghiggeri G.M., Pedemonte N. High-content screening identifies vanilloids as a novel class of inhibitors of NET formation. Front. Immunol. 2019;10:963. doi: 10.3389/fimmu.2019.00963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bao Y., Ledderose C., Seier T., Graf A.F., Brix B., Chong E., Junger W.G. Mitochondria regulate neutrophil activation by generating ATP for autocrine purinergic signaling. J. Biol. Chem. 2014;289:26794–26803. doi: 10.1074/jbc.M114.572495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fossati G., Moulding D.A., Spiller D.G., Moots R.J., White M.R., Edwards S.W. The mitochondrial network of human neutrophils: role in chemotaxis, phagocytosis, respiratory burst activation, and commitment to apoptosis. J. Immunol. 2003;170:1964–1972. doi: 10.4049/jimmunol.170.4.1964. [DOI] [PubMed] [Google Scholar]
- 141.Zmijewski J.W., Lorne E., Zhao X., Tsuruta Y., Sha Y., Liu G., Siegal G.P., Abraham E. Mitochondrial respiratory complex I regulates neutrophil activation and severity of lung injury. Am. J. Respir. Crit. Care Med. 2008;178:168–179. doi: 10.1164/rccm.200710-1602OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhou W., Cao L., Jeffries J., Zhu X., Staiger C.J., Deng Q. Neutrophil-specific knockout demonstrates a role for mitochondria in regulating neutrophil motility in zebrafish. Dis Model Mech. 2018;11 doi: 10.1242/dmm.033027. dmm033027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sbarra A.J., Karnovsky M.L. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J. Biol. Chem. 1959;234:1355–1362. [PubMed] [Google Scholar]
- 144.Bannister J.V., Bannister W.H. Production of oxygen-centered radicals by neutrophils and macrophages as studied by electron spin resonance (ESR) Environ. Health Perspect. 1985;64:37–43. doi: 10.1289/ehp.856437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Langley M., Ghosh A., Charli A., Sarkar S., Ay M., Luo J., Zielonka J., Brenza T., Bennett B., Jin H., Ghaisas S., Schlichtmann B., Kim D., Anantharam V., Kanthasamy A., Narasimhan B., Kalyanaraman B., Kanthasamy A.G. Mito-apocynin prevents mitochondrial dysfunction, microglial activation, oxidative damage, and progressive neurodegeneration in MitoPark transgenic mice. Antioxidants Redox Signal. 2017;27:1048–1066. doi: 10.1089/ars.2016.6905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Schönrich G., Raftery M.J., Samstag Y. Devilishly radical NETwork in COVID-19: oxidative stress, neutrophil extracellular traps (NETs), and T cell suppression. Advances in Biological Regulation. 2020;77:100741. doi: 10.1016/j.jbior.2020.100741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.da Costa R.M., Rodrigues D., Pereira C.A., Silva J.F., Alves J.V., Lobato N.S., Tostes R.C. Nrf2 as a potential mediator of cardiovascular risk in metabolic diseases. Front. Pharmacol. 2019;10:382. doi: 10.3389/fphar.2019.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Robledinos-Antón N., Fernández-Ginés R., Manda G., Cuadrado A. Activators and inhibitors of NRF2: a review of their potential for clinical development. Oxid Med Cell Longev. 2019:9372182. doi: 10.1155/2019/9372182. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.McCord J.M., Hybertson B.M., Cota-Gomez A., Geraci K.P., Gao B. Nrf2 activator PB125(®) as a potential therapeutic agent against COVID-19. Antioxidants. 2020;9 doi: 10.3390/antiox9060518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zinovkin R.A., Grebenchikov O.A. Transcription factor Nrf2 as a potential therapeutic target for prevention of cytokine storm in COVID-19 patients. Biochemistry. Biokhimiia. 2020;85:833–837. doi: 10.1134/S0006297920070111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cuadrado A., Pajares M., Benito C., Jiménez-Villegas J., Escoll M., Fernández-Ginés R., Garcia Yagüe A.J., Lastra D., Manda G., Rojo A.I., Dinkova-Kostova A.T. Can activation of NRF2 Be a strategy against COVID-19? Trends Pharmacol. Sci. 2020;41:598–610. doi: 10.1016/j.tips.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]