Abstract
Background
Asymptomatic carriers were positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) without developing symptoms, which might be a potential source of infection outbreak. Here, we aim to clarify the epidemiologic and influencing factors of asymptomatic carriers in the general population.
Methods
In our hospital, all hospital staff have received throat swab RT-PCR test, plasma COVID-19 IgM/IgG antibodies test and chest CT examination. We analyzed the correlation between infection rates and gender, age, job position, work place and COVID-19 knowledge training of the staff. After that, all asymptomatic staff were re-examined weekly for 3 weeks.
Findings
A total of 3764 hospital staff were included in this single-center cross-sectional study. Among them, 126 hospital staff had abnormal findings, and the proportion of asymptomatic infection accounted for 0.76% (28/3674). There were 26 staff with IgM+, 73 with IgG+, and 40 with ground glass shadow of chest CT. Of all staff with abnormal findings, the older they are, the more likely they are to be the staff with abnormal results, regardless of their gender. Of 3674 hospital staff, the positive rate of labor staff is obviously higher than that of health care workers (HCWs) and administrative staff (P<0.05). In the course of participating in the treatment of COVID-19, there was no statistically significant difference in positive rates between high-risk departments and low-risk departments (P>0.05). The positive rate of HCWs who participated in the COVID-19 knowledge training was lower than those did not participate in early training (P <0.01). Importantly, it was found that there was no statistical difference between the titers of IgM antibody of asymptomatic infections and confirmed patients with COVID-19 in recovery period (P>0.05). During 3 weeks follow-up, all asymptomatic patients did not present the development of clinical symptoms or radiographic abnormalities after active intervention in isolation point.
Interpretation
To ensure the safety of resumption of work, institutions should conduct COVID-19 prevention training for staff and screening for asymptomatic patients, and take quarantine measures as soon as possible in areas with high density of population.
Funding
The Key Project for Anti-2019 novel Coronavirus Pneumonia from the Ministry of Science and Technology, China; Wuhan Emergency Technology Project of COVID-19 epidemic, China.
Keywords: COVID-19, SARS-CoV-2, Asymptomatic, Hospital staff, Immune antibody
1. Introduction
Currently, novel coronavirus disease (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has become a global pandemic [1,2,3,4], which has been declared a global public health emergency by World Health Organization (WHO). In China, tens of thousands of medical workers participated in this battle against COVID-19. While saving the lives of patients, thousands of medical workers were unfortunately infected with COVID-19 [5], [6], [7]. Our hospital, the Zhongnan Hospital of Wuhan University in China, has also made great efforts to fight the epidemic, including setting up 2000 isolation beds in our hospital and taking over the management of three designated hospitals (Wuhan No.7 Hospital, the Wuhan living room cabin Hospital and Wuhan Leishenshan Hospital), with a total of 5400 beds for emergency treatment of COVID-19 patients. Most of the medical staff in our hospital participated in the first-line battlefield of fighting against COVID-19.
Nosocomial infection is an important way of COVID-19 transmission. Studies have shown that asymptomatic cases have a similar viral load as symptomatic cases [8], a higher contagion to transmission more frequently [9]. A study has been reported that the role of asymptomatic SARS-CoV-2 transmission in the propagation of a large superspreading event, which led to at least two further generations [10]. A mathematical model incorporating asymptomatic carriers indicated that asymptomatic individuals are major drivers for the growth of COVID-19 pandemic [11]. Cases without symptoms suggest silent chains of transmission [12], [13], it is needed to prevent spread of infection to vulnerable populations from asymptomatic carriers. Therefore, it is particularly important to find, isolate, test, and treat every case to break the chains of COVID-19 transmission.
Recently, in order to avoid further nosocomial infection, all staff without clinical symptoms in our hospital participated in the physical examination before resumption of ordinary job, including chest CT, throat swab RT-PCR test and plasma COVID-19 IgM/IgG antibodies test. This study aims to analyze the examination results, understand the infection status of staff, track the infection related risk factors, as well as tracing of asymptomatic infection individual, so as to provide effective suggestions for other hospitals and non-medical institution in Wuhan, ensuring scientific and safe return to work.
2. Methods
2.1. Study design and participants
Screening of clinically asymptomatic, we enrolled hospital staff at Zhongnan Hospital of Wuhan University in this cross-sectional study, from March 16, 2020, to March 25, 2020. Inclusion criteria: hospital staff without clinical symptoms related to COVID-19. Exclusion criteria: hospital staff who had been infected SARS-CoV-2. The staff carries on the chest CT inspection, COVID-19 IgM/IgG test for antibodies against SARS-CoV-2, and reverse transcription-polymerase chain reaction (RT-PCR) test for COVID-19 nucleic acid using throat swabs samples. Among them, the main outcome were IgM/IgG and RT-PCR test, the secondary outcome was chest computerized tomography (CT) result. All patients met the diagnostic criteria of “Diagnosis and Treatment Scheme of Novel Coronavirus–Infected Pneumonia (Tentative 7th Edition)” formulated by the General Office of the National Health Committee of China [14]. Thereafter, asymptomatic staff were re-examined weekly for 3 weeks until April 16, 2020. In our study, asymptomatic carrier refers to patients who have mild or non-symptoms but with positive test for viral nucleic acid of SARS-CoV-2 or with positive test for serum specific IgM antibody. Four physicians (M.X.L., J.F.L., X.B.Z. and C.H.) extracted the following data using data collection form from electronic medical records. The study was approved by the institutional ethics board at Zhongnan Hospital of Wuhan University (No. 2020074) and all participants signed their consent.
We define hospital staff as health care workers (HCWs, including doctors and nurses), administrative staff and clinical support staff (including drug delivery workers, toll collectors, sterilizers, technician assistants, caregivers, pharmacists, drivers, feeders, registrars, auxiliary workers, hygienists, blood collectors, distribution workers, security guard, and logistics staff) according to different workplaces. All HCWs without clinical symptoms distributed in 46 clinical medical technology departments during the treatment of COVID-19. We divided the departments of HCWs into high-risk departments (HRDs) and low-risk departments (LRDs). HRDs included the department of anesthesiology, department of pneumology and critical care medicine, emergency center, intensive care unit and department of infectious disease; all other clinical departments were identified as LRDs. During the epidemic, most HCWs were trained on the prevention and control of the outbreak, and training content included to standardize the flow of medical personnel entering and leaving the isolation ward, strengthen the training of nurses in and out of isolation clothes/protective clothing, and strengthen the self-protection consciousness.
2.2. RT-PCR test for SARS-CoV-2 virus RNA
Clinical specimens’ collection and RT-PCR test for SARS-CoV-2 were previously described [15]. In brief, oropharyngeal swabs were collected by trained nurses or physicians wearing proper personal protection equipment. The Viral Nucleic Acid Kit (Daan gene biotechnologies) was used to extract nucleic acids from clinical samples according to the kit instructions. RT-PCR tests for SARS-CoV-2 were performed to detect the ORF1ab gene (nCovORF1ab) and the N gene (nCoV-NP) according to the manufacturer's instructions [16]. ORF1ab gene: forward primer CCCTGTGGGTTTTACACTTAA; reverse primer ACGATTGTGCATCAGCTGA; and the probe 5′-VIC—CCGTCTGCGGTATGTGGAAAGGTTATGG-BHQ1–3′. N gene: forward primer GGGGAACTTCTCCTGCTAGAAT; reverse primer CAGACATTTTGCTCTCAAGCTG; and the probe 5′-FAM- TTGCTGCTGCTTGACAGATT-TAMRA-3′. The diagnostic criteria for positive and negative RT-PCR results were based on the recommendation by the National Institute for Viral Disease Control and Prevention (China) [17].
2.3. COVID-19 IGM/IGG test for antibodies against SARS-CoV-2
Serum samples from these people were collected. Serum IgM and IgG antibodies to SARS-CoV-2 were measured with COVID-19 IgM/IgG chemiluminescence test kit (Shenzhen Yahuilong Biotechnology Co., Ltd., China; product code: C86095M/C86095G). The test kit contained recombinant SARS-CoV-2 antigen (spike protein and nucleocapsid protein) labelled with magnetic beads, anti-human IgM monoclonal antibody, and anti-human IgG monoclonal antibody. Test results were showed as relative light unit (RLU). The fully‑automated chemiluminescence immunoassay analyzer automatically calculated serum COVID-19 IgM and IgG levels (AU/ml) by comparing RLU to the internal calibration curve, as RLU correlated well with serum COVID-19 IgM and IgG levels. After 20 μl of serum was diluted at a ratio of 1:3, the color development of the test card was observed within 10 min. Tests were performed on iFlash 3000 automated analyzer and an IgM or IgG level ≥10.0 AU/ml was designated as positive.
2.4. CT scan for COVID-19 patients
The image data acquisition requires both lung window and mediastinal window images, and the image quality meets the evaluation requirements. The patient routinely takes the supine position with his arms raised above his head, and breathes quickly after holding a deep spiral. All radiologic images were reviewed by two radiologists
2.5. Statistical analysis
Continuous variables such as titers of COVID-19 IgM/IgG antibodies were reported using mean and 95% confidence interval (CI) if normally distributed or median and interquartile if nonnormally distributed. Categorical variables were described as frequency rates and percentages. Means for normally-distributed continuous variables were compared using ANOVA test, following by Tukey test for adjusting for multiple comparisons. Mann-Whitney test was used for assessing differences of nonnormal-distributed variables. The χ2 test was used for the comparison of categorical variables and Fisher's exact test was used when frequency was too low. Statistical analysis was performed with SPSS 22.0. All figures were performed by using GraphPad Prism (GraphPad Company, San Diego, CA, USA) version 8 software. P-value < 0.05 was considered as statistical significance.
2.6. Role of the funding source
The funder had no role in the design and conduct of this study; collection, management, analysis, and interpretation of the data; preparation, review or approval of this manuscript; and decision to submit the manuscript for publication.
3. Results
3.1. Sociodemographic characteristics
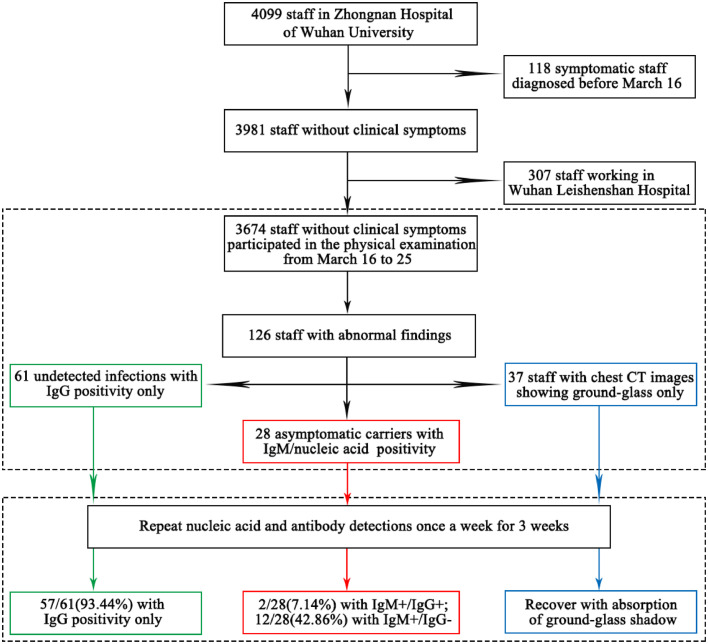
The work flow diagram of 3674 hospital staff participated in the physical examination from Zhongnan Hospital of Wuhan University were shown in the Fig. 1. Of all hospital staff without clinical symptoms, there are 2406 health care workers (HCWs, doctors or nurses), 505 administrative logistics staff and 763 labor staff. Among all the hospital staff without clinical symptoms, 67.7% (2486/3674) are female, and 82.6% (3034/3674) with age ranging from 18 to 50 years old. (see Table 1).
Fig. 1.
Work flow diagram of staff selection for this study.
Table 1.
Gender, age and work classification for abnormal findings among 3674 staff in Zhongnan Hospital of Wuhan University.
| Characteristics | COVID-19 antibodies |
Nucleic acid positive | CT positive | Number of positive cases/Number of physical examination cases (%) | P Value | ||
|---|---|---|---|---|---|---|---|
| IgM+/ IgG+ | IgM+/ IgG- | IgM-/ IgG+ | |||||
| Gender | |||||||
| Male | 2 | 4 | 25 | 2 | 15 | 45/1188(3.79) | 0.409 |
| Female | 8 | 12 | 38 | 0 | 25 | 81/2486(3.26) | |
| Age (years) | |||||||
| 18–30 | 2 | 3 | 22 | 0 | 8 | 35/1378(2.54) | 0.003 |
| 31–50 | 4 | 9 | 24 | 1 | 20 | 55/1656(3.32) | |
| >50 | 4 | 4 | 17 | 1 | 12 | 36/640(5.63) | |
| Position | |||||||
| HCWs | 5 | 9 | 33 | 1 | 27 | 73/2406(3.03) | 0.007 |
| administrative staff | 1 | 3 | 7 | 0 | 1 | 12/505(2.37) | |
| clinical support staff | 4 | 4 | 23 | 1 | 12 | 41/763(5.37) | |
| Total | 10 | 16 | 63 | 2 | 40 | 126/3674(3.43) | … |
Abbreviations: HCWs, health care workers; clinical support staff including drug delivery workers, toll collectors, sterilizers, technician assistants, caregivers, pharmacists, drivers, feeders, clinical assistants (registrars, auxiliary workers, hygienists, blood collectors, distribution workers), security guard and logistics staff.
A total of 126 hospital staff were accompanied by abnormal findings: 61 staff only had abnormal IgG results (IgG positivity), 37 only had abnormal chest CT images (ground glass shadow), and 28 were asymptomatic carriers (IgM/nucleic acid positive). Of the 3674 hospital staff, there is no statistical difference between the proportions of male staff and female staff with abnormal findings (P>0.05). However, the positive rate of physical examination results for staff of different ages is statistically significant (P<0.05). The older they are, the more likely they are to be the staff with abnormal results, regardless of their gender (see Table 1).
3.2. Laboratory and radiologic findings
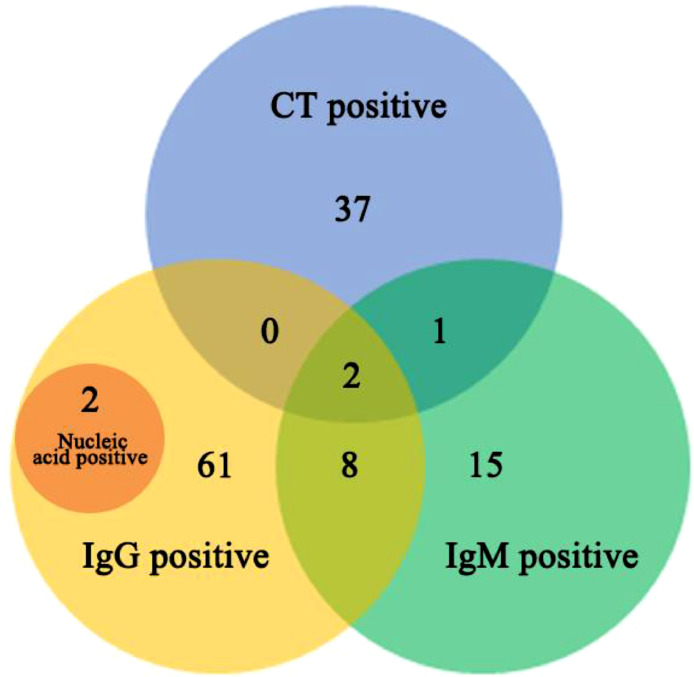
In Fig. 2, there were 126 hospital staff with abnormal findings. The percentage of COVID-19 IgM+/ IgG+ positive was 0.27% (10/3674). There were 26 staff with IgM+, 73 with IgG+, and 40 with ground glass shadow of chest CT. (Fig. 5). Of these 126 staff, 2 cases were positive in the results of throat swab RT-PCR test, and their results of COVID-19 IgG antibody were also positive.
Fig. 2.
Venn diagrams showing overlap of IgM/IgG test and chest CT among 3674 patients. Venn diagram depicts the proportion of indicators detected based on IgM/IgG test, chest CT, and PCR results.
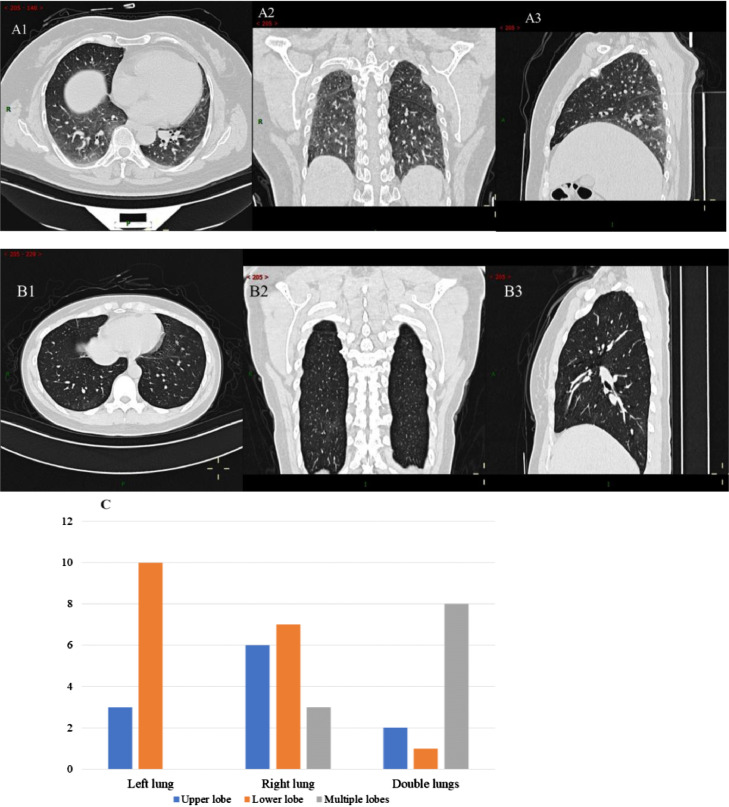
Fig. 5.
The chest CT imaging manifestation of asymptomatic infected persons in Zhongnan Hospital of Wuhan University. Figures A and B are the CT manifestations of two asymptomatic patients. (A patient's CT results show: multiple lung ground glass density patch shadows in the lungs, considering the possibility of COVID-19; B patient's CT results show that there was a little ground glass density shadow in the posterior basal segment of the right lower lobe, and micro nodules in the posterior segment of the upper left lobe of the left lung and the outer segment of the middle right lobe of the right lung.) Figure C is the proportion of affected lung positions in 40 asymptomatic patients with CT ground glass.
According to the COVID-19 Diagnosis and Treatment plan (Tentative 7th Edition) in China [18], the number of asymptomatic infections reached 28, and the proportion of asymptomatic infection is 0.76% (28/3674) in Zhongnan Hospital of Wuhan University. These asymptomatic infection carriers were isolated and followed up regularly, without medication administration. Asymptomatic infections from HCWs, administrative staff, clinical support staff accounted for 0.62% (15/2406), 0.79% (4/505), and 1.18% (9/763), respectively. The positive rate of clinical support staff is obviously higher than that of HCWs and administrative staff (P<0.05) (see Table 1). According to the tracking, no obvious infection transmission occurred in the later period. After 3 weeks follow-up, the positive nuclear acid in 2 patients became negative, of which one patient was discharged within 14 days after isolation, and the other patient was discharged 21 days after treatment. Although RT-PCR tests were all negative after 3 weeks, it showed 2/28(7.14%) with IgM+/IgG+ and 12/28(42.86%) with IgM+/IgG- from IgM/IgG antibodies test. However, all staff with abnormal CT findings recovered with absorption of ground-glass shadow after 3 weeks follow-up (see Fig. 1).
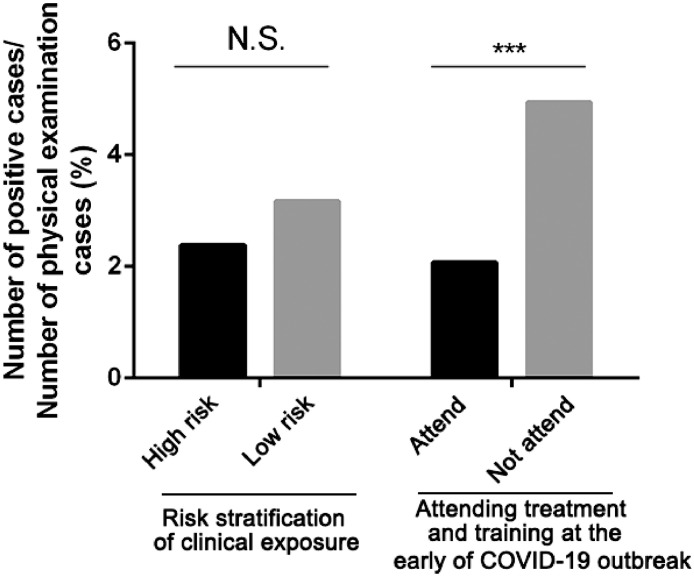
However, there was no statistically significant difference in positive rates between high-risk departments and low-risk departments (P = 0.386). In addition, the positive rate of HCWs who participated in the treatment of COVID-19 was lower than those of not participate in early training (P <0.01) (see Fig. 3), which showed that early training could help us to avoid getting infected.
Fig. 3.
The results of HCWs comparison. In the course of participating in the treatment of COVID-19, there was no statistically significant difference in positive rates between high-risk departments and low-risk departments (P = 0.386>0.05). The positive rate of HCWs who participated in the training of treatment of COVID-19 in the early stage was lower than those of not participate in training (P <0.01), which showed that early training could help us to avoid getting infected. (high-risk departments: department of anesthesiology, department of pneumology and critical care medicine, emergency center, intensive care unit and department of infectious disease; low-risk departments: other departments). ***Significant p<0.001.
3.3. Comparison of the titers of antibodies of asymptomatic infection population
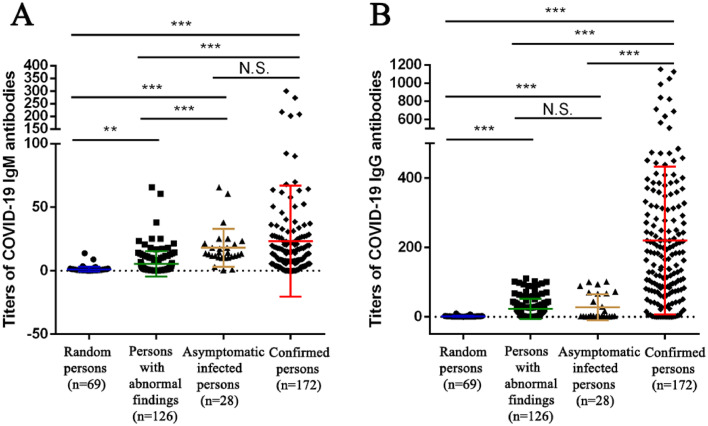
We compared the titers of COVID-19 IgM/IgG antibodies of 28 asymptomatic infections and 126 staff with abnormal findings screened from 3674 asymptomatic staff with 69 random healthy individuals and 172 confirmed patients with COVID-19 in recovery period at same time. It was found that there was no statistical difference between the titers of IgM antibody of asymptomatic infections and confirmed patients with COVID-19 in recovery period (P>0.05), but IgM antibody of asymptomatic infections was statistically significant compared to that of the other two groups (P<0.05, see Fig. 4A). However, there was no significant difference between the titers of IgG antibody of asymptomatic infections and the staff with abnormal findings (P>0.05), but IgG antibody of asymptomatic infections was statistically significant compared with that of the other two groups (P<0.05, see Fig. 4B). In addition, it was found that there was no significant difference among different ages and different gender in the titers of IgM/IgG antibodies of asymptomatic infections and the staff with abnormal findings (P>0.05, see Figure S1). The results showed that the titers of IgM/IgG antibodies test could help us to screen and identify asymptomatic infections.
Fig. 4.
Titers of COVID-19 IgM(A)/IgG(B) antibodies in asymptomatic infected persons and persons with abnormal findings in Zhongnan Hospital of Wuhan University (normal value: < 10). **Significant p<0.05, ***Significant p<0.001.
4. Discussion
With the further understanding of the COVID-19, China has quickly taken effective measures to prevent and control it [19], [20], [21]. The epidemic situation has been initially controlled in Wuhan, China. In the next stage to promote the resumption of work and production in Wuhan, the prevention and control of the epidemic should focus on screening asymptomatic individuals who may not only progress to symptomatic infection patients[22,23], but more importantly, are potential infectious sources [24].
Our hospital had completed COVID-19 screening of 3674 staff without clinical symptoms, the results showed the proportion of asymptomatic infected staff was 0.76%. The proportion of asymptomatic individuals was 0.79% in administrative staff and 1.18% in labor staff. Considering the asymptomatic transmission, the high proportion of asymptomatic infected people is a huge hidden danger [25], which may bring difficulties to the resumption of work and production. Asymptomatic carriers have been reported in several studies, Rivett L et al. [26] reported that 3% of 1032 asymptomatic HCWs from a large UK teaching hospital tested positive for SARS-CoV-2, indicating that it is necessary to screen asymptomatic carriers among the hospital staff, which may be important to prevent the secondary outbreak of the epidemic.
There are several suggestions to reduce COVID-19 transmission risk in this study. Firstly, given the high cost and the radiation damage to human body by CT scan, COVID-19 IgM/IgG antibodies and nucleic acid is more suitable for scanning asymptomatic carriers in general population, especially in densely populated work areas in Wuhan, which may be helpful to confirm asymptomatic infection as early as possible. Meanwhile, our results found that after isolation of asymptomatic patients for 3 weeks, although the nucleic acid test has turned negative, half of the patients are still positive for IgM+ (with virus status). It is very essential to take rapid identification, registration, reporting, surveillance and epidemiological tracking of asymptomatic infected individuals. Secondly, it should make full consideration to the possibility of long-term epidemic prevention and control. In particular, priority should be given to screening populations that have migrated due to the need to resume work and production to prevent the occurrence of “import cases” between regions. We also need to formulate differentiated regional management measures, strengthen the management of asymptomatic infection cases, take strict isolation measures, focus on high risk groups and special places, and do a good job in the prevention and control of the epidemic in crowded and enclosed environment. Only in this way, we can ensure the prevention and control work is effective and sustainable.
The safety of medical institutions is the key to ensure the health of average citizen. In our study, the positive rate of HCWs who participated in the training was lower than those of not participate in training. In the early stages of the outbreak, due to lack of protected awareness or personal protective equipment, some medical workers were infected after contacting COVID-19 patients, which may become the infection source in the department [6]. It demonstrated that good protection awareness and protective measures are the key for self-protection. Therefore, it is necessary to promote protection knowledge among HCWs, supervise the strict implementation of protection measures at the early stage of the outbreak, to ensure the safety of HCWs and prevent the spread of the epidemic [27].
Our findings have several important clinical and public health implications. In this single-center cross-sectional study, very few hospital staff had positive IgM/IgG tests. Both serum IgM and IgG levels were higher in recovered confirmed patients than hospital staff (P<0.001 for both). It is possible that some people may live with SARS-CoV-2 for a long period of time. Whether those people are still infectious warrant further studies. Unlike severe acute respiratory syndrome (SARS) [28], herd immunity will not develop for SARS-CoV-2 in the general population and seasonal outbreaks in the next few years may still be possible [29]. Importantly, there would be a big question mark for the success of a vaccine against SARS-CoV-2 [30]. In our study, most of the healthcare providers without a confirmed COVID-19 diagnosis have been exposed to a highly contagious environment with the wild-type SARS-CoV-2 virus. These findings raise the concern if the inactivated virus, subunit, and recombinant vaccines currently under development will be able to induce effective immune protections against SARS-CoV-2.
In our study, all asymptomatic patients did not present the development of clinical symptoms or radiographic abnormalities after 3 weeks follow-up. The positive nuclear acid in 2 patients became negative for 2 to 3 weeks after active intervention in isolation point. It is clear that highly effective contact tracing and case isolation is necessary to control the progression of COVID-19. Although the PCR test were all negative after 3 weeks, most of them showed positive IgM antibody. Asymptomatic infection still carries the SARS-CoV-2, which means the risk of transmission and present a new challenge to isolation. We need to do SARS-CoV-2 detection to figure out what's going on for asymptomatic population with epidemiological contact history. Meanwhile, treatment of the underlying disease may also help stop transmission of COVID-19.
There are several limitations to our study. First, our study only included the results of one hospital in Wuhan, which may not be sufficient to represent the situation of other hospitals, whether it is from other hospitals in Wuhan or other regions of China. Therefore, it has certain limitations for the promotion of the results. Second, we did not explore the relationship between clinical indicators and asymptomatic infection. Other studies would be designed to explore the correlation between clinical parameters and asymptomatic carriers in the future, to link the asymptomatic state with differences in biochemical, immune, viral load and other factors to provide deeper insights.
Our work underscores the need for measures to limit transmission by individuals who become ill. These findings can support evidence-based policy to combat the spread of COVID-19, and prospective planning to mitigate future emerging pathogens.
Contributors
L.C., X.H.W. and F.L.Z. designed the project. F.L.Z. and J.F.L. wrote the manuscript, M.X.L., J.F.L., X.B.Z. and C.H. collected the data. L.L.M., X.Y.L, Y.B.P., L.J.C., Y.W., S.Y.W., Y.C.W., Y.R.L. and H.B.X. analyzed the data. L.C. and X.H.W. provided professional guidance and revised the manuscript.
Declaration of Competing Interest
We declare no competing interests.
Acknowledgments
Acknowledgments
We acknowledge all hospital staff participated in the diagnosis and treatment of COVID-19 patients in Zhongnan Hospital of Wuhan University.
Data sharing statement
The raw data used in the study will accessible on contacting the corresponding authors.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2020.100510.
Contributor Information
Xinghuan Wang, Email: wangxinghuan@whu.edu.cn.
Lin Cai, Email: orthopedics@whu.edu.cn.
Appendix. Supplementary materials
References
- 1.Zhu N., Zhang D., Wang W. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu R., Zhao X., Li J. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5(4):536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The Lancet COVID-19: protecting health-care workers. Lancet. 2020;395(10228):922. doi: 10.1016/S0140-6736(20)30644-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou P., Huang Z., Xiao Y., Huang X., Fan X.G. Protecting Chinese healthcare workers while combating the 2019 novel coronavirus. Infect Control Hosp Epidemiol. 2020;41(6):745–746. doi: 10.1017/ice.2020.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chu J., Yang N., Wei Y. Clinical characteristics of 54 medical staff with COVID-19: A retrospective study in a single center in Wuhan, China. J Med Virol. 2020;92(7):807–813. doi: 10.1002/jmv.25793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huff H.V., Singh A. Asymptomatic transmission during the COVID-19 pandemic and implications for public health strategies [published online ahead of print, 2020 May 28] Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa654. ciaa654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishiura H., Linton N.M., Akhmetzhanov A.R. Serial interval of novel coronavirus (COVID-19) infections. Int J Infect Dis. 2020;93:284–286. doi: 10.1016/j.ijid.2020.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong J., Jamaludin S.A., Alikhan M.F., Chaw L. Asymptomatic transmission of SARS-CoV-2 and implications for mass gatherings [published online ahead of print, 2020 May 30] Influenza Other Respir Viruses. 2020 doi: 10.1111/irv.12767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aguilar J.B., Faust J.S., Westafer L.M., Gutierrez J.B. Investigating the Impact of Asymptomatic Carriers on COVID-19 Transmission. medRxiv. 2020 doi: 10.1101/2020.03.18.20037994. [DOI] [Google Scholar]
- 12.Kelvin A.A., Halperin S. COVID-19 in children: the link in the transmission chain. Lancet Infect Dis. 2020;20(6):633–634. doi: 10.1016/S1473-3099(20)30236-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qiu H., Wu J., Hong L., Luo Y., Song Q., Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020;20(6):689–696. doi: 10.1016/S1473-3099(20)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin Y.H., Cai L., Cheng Z.S. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version) Mil Med Res. 2020;7(1):4. doi: 10.1186/s40779-020-0233-6. Published 2020 Feb 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang D., Hu B., Hu C. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China [published online ahead of print, 2020 Feb 7] JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kapitulav D.S., Jiang Z., Jiang Z., Zhu J., Chen X., Lin C.Q. Performance & quality evaluation of marketed COVID-19 RNA detection kits. medRxiv. 2020 doi: 10.1101/2020.04.25.20080002. [DOI] [Google Scholar]
- 17.The National Institute for Viral Disease Control and Prevention (China). Specific primers and probes for detection 2019 novel coronavirus. Accessed from: http://ivdc.chinacdc.cn/kyjz/202001/t20200121_211337.html. Accessed date: 2020-01-21.
- 18.National Health Commission of the People's Republic of China. [Diagnosis and Treatment guideline for COVID-19 (Trial Seventh Ed.)]. 2020; http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml. Accessed June 24, 2020.
- 19.Zhang J.F., Yan K., Ye H.H., Lin J., Zheng J.J., Cai T. SARS-CoV-2 turned positive in a discharged patient with COVID-19 arouses concern regarding the present standards for discharge. Int J Infect Dis. 2020;97:212–214. doi: 10.1016/j.ijid.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Y.C., Chen C.S., Chan Y.J. The outbreak of COVID-19: An overview. J Chin Med Assoc. 2020;83(3):217–220. doi: 10.1097/JCMA.0000000000000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo Y.R., Cao Q.D., Hong Z.S. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil Med Res. 2020;7(1):11. doi: 10.1186/s40779-020-00240-0. Published 2020 Mar 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu Z., Song C., Xu C. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci China Life Sci. 2020;63(5):706–711. doi: 10.1007/s11427-020-1661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin C., Ding Y., Xie B. Asymptomatic novel coronavirus pneumonia patient outside Wuhan: The value of CT images in the course of the disease. Clin Imaging. 2020;63:7–9. doi: 10.1016/j.clinimag.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu S., Lin J., Zhang Z. Alert for non-respiratory symptoms of Coronavirus Disease 2019 (COVID-19) patients in epidemic period: A case report of familial cluster with three asymptomatic COVID-19 patients [published online ahead of print, 2020 Mar 19] J Med Virol. 2020 doi: 10.1002/jmv.25776. [DOI] [PubMed] [Google Scholar]
- 25.Lai C.C., Liu Y.H., Wang C.Y. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): Facts and myths. J Microbiol Immunol Infect. 2020;53(3):404–412. doi: 10.1016/j.jmii.2020.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rivett L., Sridhar S., Sparkes D. Screening of healthcare workers for SARS-CoV-2 highlights the role of asymptomatic carriage in COVID-19 transmission. Elife. 2020;9:e58728. doi: 10.7554/eLife.58728. Published 2020 May 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huh S. How to train health personnel to protect themselves from SARS-CoV-2 (novel coronavirus) infection when caring for a patient or suspected case. J Educ Eval Health Prof. 2020;17:10. doi: 10.3352/jeehp.2020.17.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prompetchara E., Ketloy C., Palaga T. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020;38(1):1–9. doi: 10.12932/AP-200220-0772. [DOI] [PubMed] [Google Scholar]
- 29.Neher R.A., Dyrdak R., Druelle V., Hodcroft E.B., Albert J. Potential impact of seasonal forcing on a SARS-CoV-2 pandemic. Swiss Med Wkly. 2020;150:w20224. doi: 10.4414/smw.2020.20224. Published 2020 Mar 16. [DOI] [PubMed] [Google Scholar]
- 30.Zhang J., Zeng H., Gu J., Li H., Zheng L., Zou Q. Progress and Prospects on Vaccine Development against SARS-CoV-2. Vaccines (Basel) 2020;8(2):153. doi: 10.3390/vaccines8020153. Published 2020 Mar 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.