Abstract
Exploring photocatalysts to promote CO2 photoreduction into solar fuels is of great significance. We develop TiO2/perovskite (CsPbBr3) S-scheme heterojunctions synthesized by a facile electrostatic-driven self-assembling approach. Density functional theory calculation combined with experimental studies proves the electron transfer from CsPbBr3 quantum dots (QDs) to TiO2, resulting in the construction of internal electric field (IEF) directing from CsPbBr3 to TiO2 upon hybridization. The IEF drives the photoexcited electrons in TiO2 to CsPbBr3 upon light irradiation as revealed by in-situ X-ray photoelectron spectroscopy analysis, suggesting the formation of an S-scheme heterojunction in the TiO2/CsPbBr3 nanohybrids which greatly promotes the separation of electron-hole pairs to foster efficient CO2 photoreduction. The hybrid nanofibers unveil a higher CO2-reduction rate (9.02 μmol g–1 h–1) comparing with pristine TiO2 nanofibers (4.68 μmol g–1 h–1). Isotope (13CO2) tracer results confirm that the reduction products originate from CO2 source.
Subject terms: Photocatalysis, Photocatalysis, Quantum dots
Rational design and fabrication of high-performance photocatalyst is of great importance for CO2 reduction into solar fuel. Here, the authors demonstrate that S-scheme heterojunction TiO2/CsPbBr3 photocatalyst exhibits enhanced CO2 photoreduction activity.
Introduction
The depletion of fossil fuels and continuous CO2 emissions have caused emerging global energy and environmental crises1–5. The photoreduction of CO2 into renewable fuels with solar energy is recognized as a potential solution to solve above issues6–10. As a chemically inert, nontoxic and earth-abundant photocatalyst, TiO2 is supposed to be proverbially utilized for CO2 photoreduction11–13. However, like the majority of unitary photocatalysts, the photocatalytic efficiency of TiO2 is still far away from the practical requirements largely due to its rapid electron–hole recombination14,15. Hybridizing TiO2 with another semiconductor with a suitable band structure is a widely adopted strategy to tackle this issue owing to the efficient separation of photoinduced electron–hole pairs16–20. Therefore, it is of significance to explore or design a TiO2-based heterojunction to improve the photocatalytic CO2 reduction performance.
CsPbBr3, a typical material of halide perovskites, has attracted significant scientific interest in optoelectronic applications owing to its outstanding properties, including narrow photoemission, high photoluminescence quantum yield, tunable bandgap, and competing optoelectronic properties21–24. Inspired from the achievements in optoelectronic applications, CsPbBr3 is a potential candidate for conducting efficient photocatalysis25,26. CsPbBr3 quantum dots (QDs) have recently been hybridized with 2D graphene oxide27 and porous g-C3N428 for CO2 photoreduction. Nevertheless, in these cases, the electrons in the conduction band of CsPbBr3 transferred into graphene and g-C3N4, forming Schottky and type-II heterojunctions, respectively, sacrificing the reduction ability of the photoinduced electrons despite achieving better charge separation. Very recently, an S-scheme heterojunction composed of two n-type semiconductors has been proposed29,30. The transfer path of photogenerated charge carriers at interfaces is like an “S” figure, enabling the heterojunctions to have the highest redox ability. The S-type charge transportation correlates with the band bending and internal electric field (IEF) at the junction. The n-type nature and remarkably different work functions of TiO2 and CsPbBr3 suggest a high possibility of forming S-scheme TiO2/CsPbBr3 heterojunctions. Up to now, however, constructing perovskite CsPbBr3 with TiO2, an emerging photoactive material and the most widespread photocatalyst, for efficient CO2 photoreduction has not yet been reported.
Herein, we report on a unique TiO2/CsPbBr3 S-scheme heterojunction built by electrostatic self-assembly of TiO2 nanofibers and CsPbBr3 QDs for boosted photocatalytic CO2 reduction. TiO2 nanofibers show no aggregation upon dispersion in solution and thereby retain their phototactically active sites exposed on the surface. Meanwhile, randomly stacked TiO2 nanofibres readily form a loose network, facilitating the adsorption–desorption and transportation of reactants and products. More importantly, the TiO2 nanofibres are composed of small nanocrystals, possessing interparticle voids and rough surface, which make TiO2 nanofibres an ideal host to anchor CsPbBr3 QDs. Experimental study and density functional theory (DFT) calculation verify the presence of IEF in the unique TiO2/CsPbBr3 heterojunction, which separate photoinduced charge carriers more efficiently. We argue the formation of the S-scheme charge transfer route at TiO2/CsPbBr3 interfaces upon light irradiation. The obtained TiO2/CsPbBr3 heterojunction shows a superior activity for reducing CO2 into solar fuels under UV–visible-light irradiation. This work provides a point of view in TiO2-based photocatalyst for efficient CO2 photoreduction driven by the S-scheme electron transfer route.
Results and discussion
Characterization of as-prepared CsPbBr3 QDs
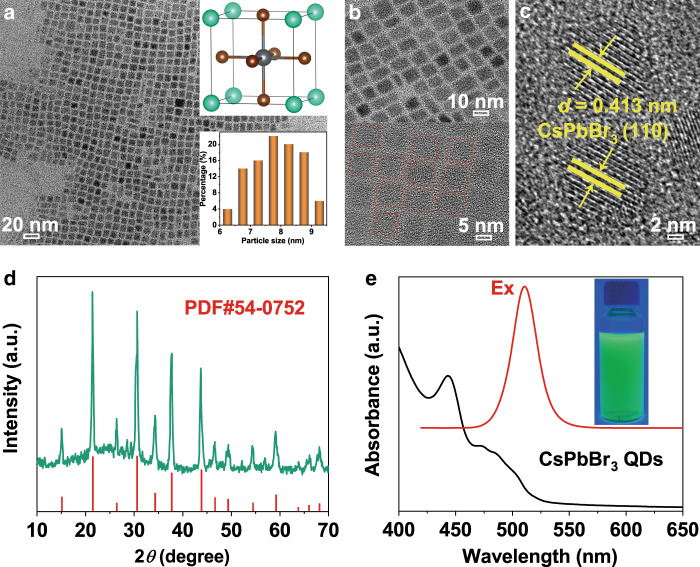
Transmission electron microscopy (TEM) images with different magnifications are shown in Fig. 1a, b. The CsPbBr3 QDs were of nanocubes with a size of 6–9 nm (inset in Fig. 1a). High-resolution TEM (HRTEM) image (Fig. 1c) showed lattice spacings of 0.413 nm, corresponding to the (110) facets of CsPbBr3. As-prepared CsPbBr3 QDs were of cubic phase (JCPDS No. 54-0752) as revealed by X-ray diffraction (XRD) pattern (Fig. 1d). The UV–vis absorption spectrum of CsPbBr3 QDs revealed strong bands at 450 and 500 nm (Fig. 1e). The corresponding photoluminescence (PL) spectrum unfolded a narrow emission at 520 nm, agreeing with previous reports21,31. Accordingly, the QDs solution showed a bright green fluorescence under 365 nm UV light (inset of Fig. 1e).
Fig. 1. Characterization of CsPbBr3 QDs.
a, b Transmission electron microscopy (TEM) image and corresponding size distribution (lower right inset of panel a), the geometrical structure (upper right inset of panel a), c high-resolution TEM (HRTEM) image, d X-ray diffraction (XRD) pattern, and e UV–vis absorption (black line) and PL emission (red line). Inset shows the photograph of CsPbBr3 QDs colloidal solutions in hexane under UV light of 365 nm.
Characterization of TiO2/CsPbBr3 heterojunction
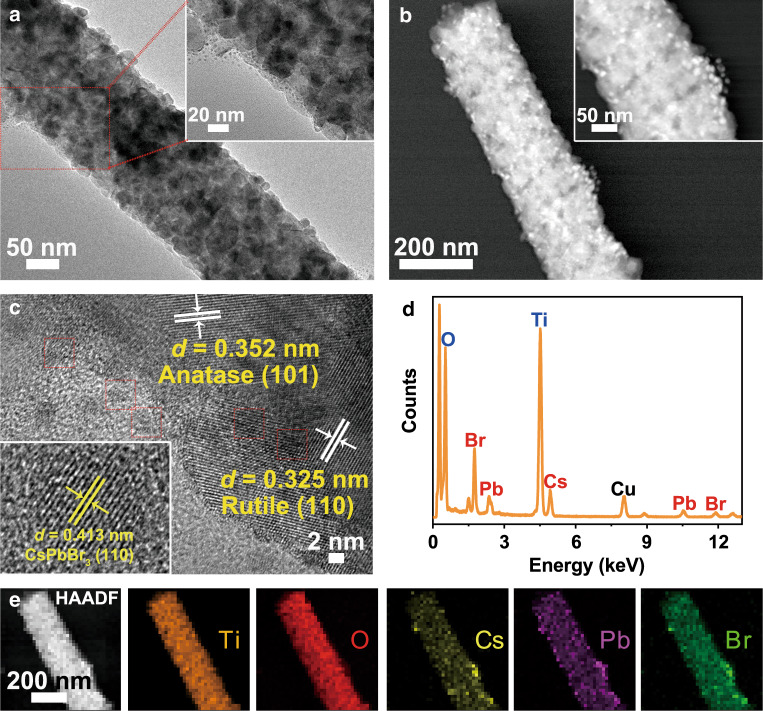
The TiO2/CsPbBr3 heterojunction was synthesized via electrostatic self-assembly of TiO2 nanofibers and CsPbBr3 QDs. Moreover, the minimization of the surface energy of the QDs should also be responsible for their adsorption to the TiO2 nanofibers. The TiO2/CsPbBr3 hybrids were denoted as TCx, where T and C denote TiO2 and CsPbBr3 QDs, respectively; x represents the weight percentage of CsPbBr3 with respect to TiO2. The phase structures of TiO2, TC2, and TC4 were determined via XRD analysis (Supplementary Fig. 1). TiO2 nanofibers showed intensive reflections belonging to anatase (JCPDS No. 21-1272) and rutile (JCPDS No. 21-1276) phases. TC2 showed a similar XRD pattern with pristine TiO2, where the reflections of CsPbBr3 QDs cannot be distinguished due to their low content. Apart from the characteristic reflections of TiO2, TC4 showed additional reflections at 21.5° and 30.6°, which corresponded to the (110) and (200) planes of CsPbBr3 QDs, confirming the formation of TiO2/CsPbBr3 nanohybrids. The morphology and crystalline phase of pristine TiO2 (Supplementary Fig. 2a) exhibited a porous nanofibrous shape with an average diameter of 200 nm. The porous feature was further revealed by the N2 sorption isotherms of TCx (Supplementary Fig. 3). All the TCx samples showed similar pore size distributions with a wide range of 10–20 nm, much larger than the size of CsPbBr3 QDs (6–9 nm). The resultant specific surface areas (SBET), pore volumes (Vp), and average pore sizes (dp) presented a volcano shape with increasing the loading of CsPbBr3 QDs (Supplementary Table 1). At a low QDs loading (<2 wt.%), TCx showed an increased SBET and reached the maximum value at TC2 because the low filling enables QDs to deposit onto the inner wall of TiO2 mesopores. Such island-like QDs on the inner wall contribute additional specific surface area for the hybrid. When the QDs loading was further increased, QDs would aggregate in TiO2 mesopores and the island-like distribution vanished, which thereby resulted in a decrease of SBET. The HRTEM image (Supplementary Fig. 2b) showed clear lattice spacings of 0.352 and 0.325 nm, corresponding to anatase (101) and rutile (110) d-spacings, respectively. After the assembling process, the QDs were uniformly deposited on the TiO2 nanofibers (Fig. 2a, b). The lattice spacings of anatase and rutile phase TiO2, as well as CsPbBr3 QDs, appeared in the HRTEM image, as shown in Fig. 2c, confirming the formation of TiO2/CsPbBr3 nanohybrids. The energy-dispersive X-ray spectroscopy (EDX) spectrum of TC2 (Fig. 2d) revealed the existence of Cs, Pb, and Br apart from the dominant Ti and O elements. All the elemental mappings overlapped perfectly (Fig. 2e). Fourier-transform infrared (FTIR) spectra showed the presence of (Ti)–OH on TiO2 and organic residues on QDs (Supplementary Fig. 4a, b)32. The (Ti)–OH signal weakened upon QDs deposition owing to the shielding effect of QDs. All the results confirmed the successful electrostatic assembly of TiO2 nanofibers and CsPbBr3 QDs.
Fig. 2. Morphology and structure of TiO2/CsPbBr3 heterojunction.
a–c Transmission electron microscopy (TEM), STEM, and high-resolution TEM (HRTEM) images of TC2, d EDX spectrum of TC2, and e high-angle annular dark-field (HAADF) image and EDX elemental mappings of Ti, O, Cs, Pb, and Br elements in TC2.
The optical absorption of the samples was investigated by UV–vis diffuse reflectance spectrometer (DRS) (Supplementary Fig. 5a). The absorption edges of pristine TiO2 nanofibers and CsPbBr3 QDs were located at 400 and 550 nm, corresponding to the bandgap energy of 3.10 and 2.24 eV, respectively (Supplementary Fig. 5b). In comparison with pristine TiO2, TCx showed two obvious absorption edges belonging to TiO2 and CsPbBr3 QDs, and exhibited slightly enhanced UV and visible-light harvesting when increasing the amount of CsPbBr3 QDs owing to the strong light-harvesting capability of perovskite QDs. Note that the calculated bandgap energy of TiO2 and CsPbBr3 in TC4 was different from their intrinsic bandgap, implying that there exist electrostatic attraction and interaction between TiO2 and CsPbBr3 during the hybridization.
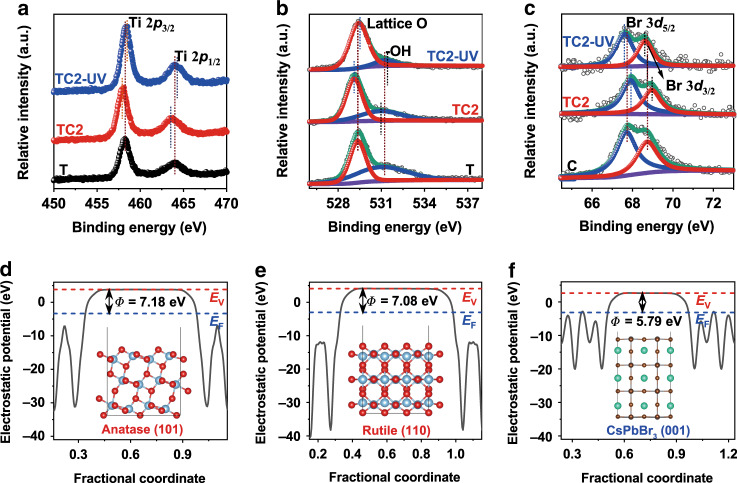
X-ray photoelectron spectroscopy (XPS) was further performed to explore the chemical states of the resultant samples. The survey XPS spectrum (Supplementary Fig. 6a) showed the presence of Cs, Pb, and Br elements within TC2, as well as Ti and O. The ex-situ Ti 2p XPS spectra of TiO2 and TC2 (Fig. 3a) showed symmetrical Ti 2p doublets of Ti4+ ions. The O 1s XPS spectra (Fig. 3b) revealed the presence of lattice oxygen (529.3 eV) and –OH surface group (531.2 eV). Interestingly, TC2 showed a weaker XPS signal of –OH than pristine TiO2, which was also attributed to an increase of QDs over TiO2 nanofiber surface and was in agreement with the above FTIR results. The Br 3d-binding energies (BEs) of CsPbBr3 QDs were 67.8 and 69.8 eV, corresponding to Br 3d5/2 and Br 3d3/2, respectively (Fig. 3c). Noticeably, the BEs of Ti 2p and O 1s in TC2 were shifted by 0.2 eV toward a lower BE in comparison with those of pristine TiO2, while the Cs 3d, Pb 4f (Supplementary Fig. 6c, d) and Br 3d BEs of TC2 became more positive as compared with those of QDs, indicating that the electrons transferred from CsPbBr3 QDs to TiO2 upon hybridization due to the difference of their work functions. Such electron transfer created an IEF at interfaces pointing from QDs to TiO2, facilitating the construction of S-scheme TiO2/CsPbBr3 heterojunction without any redox mediator, which would efficiently separate the charge carriers and thus promote the CO2 photoreduction33–35.
Fig. 3. Electron transfer between TiO2 and CsPbBr3 quantum dots (QDs).
In-situ and ex-situ X-ray photoelectron spectroscopy (XPS) spectra of a Ti 2p, b O 1s, and c Br 3d of TiO2, CsPbBr3, and TC2. In-situ XPS spectra were collected under UV–vis light irradiation. The electrostatic potentials of d anatase TiO2 (101), e rutile TiO2 (110), and f CsPbBr3 (001) facets. The blue, red, green, gray, and brown spheres stand for Ti, O, Cs, Pb, and Br atoms, respectively. Blue and red dashed lines indicate the Fermi and vacuum energy levels.
Work function (Φ), as another important parameter to study the electron transfer within duplicate semiconductor heterostructures, can be estimated from the energy difference of vacuum and Fermi levels according to the electrostatic potential of a material. As shown in Fig. 3d–f, the work function of anatase TiO2 (101), rutile TiO2 (110), and CsPbBr3 QDs (001) were 7.18, 7.08, and 5.79 eV, respectively, indicating that both anatase and rutile TiO2 have lower Fermi levels than CsPbBr3 QDs. When they contacted with each other, electrons would flow from CsPbBr3 to anatase and/or rutile TiO2 to enable the phases at the same Fermi level and definitely created an IEF at TiO2/CsPbBr3 interfaces. These results were absolutely consistent with above ex-situ XPS results and beneficial to the charge separation and CO2 photoreduction activity.
CO2 photoreduction activity of TiO2/CsPbBr3 hybrids
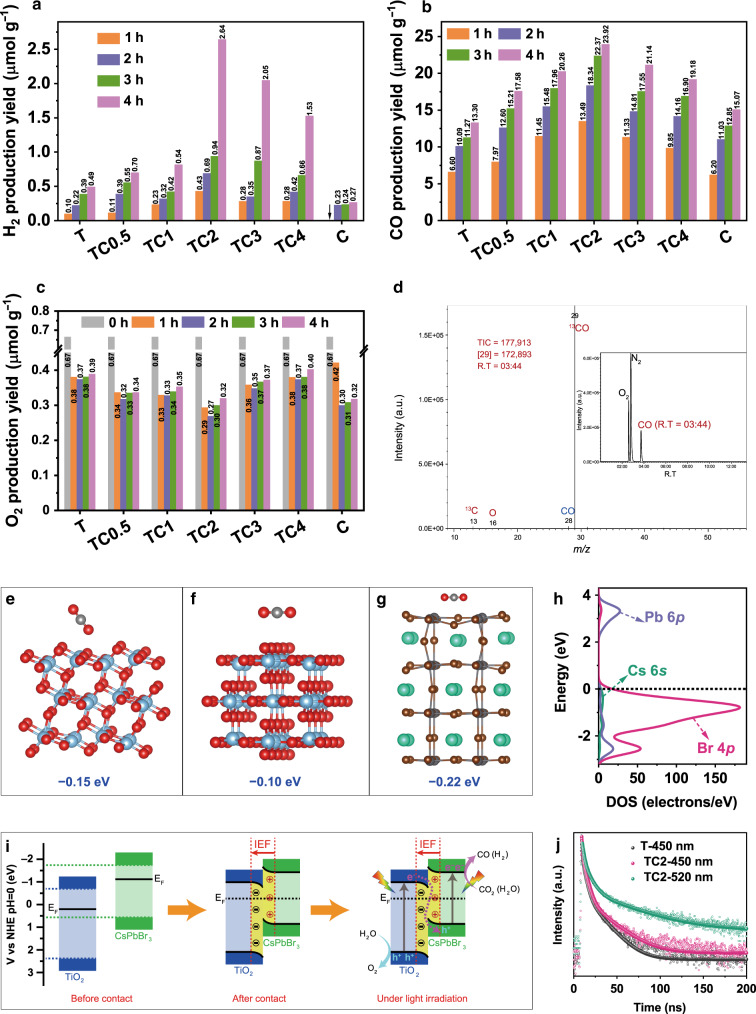
The CO2 photoreduction activity of resultant samples was measured in a closed gas-circulation system (Supplementary Fig. 7) with a Quartz and Pyrex glass hybrid reaction cell (Supplementary Fig. 8) and the photocatalytic reduction products consisted of a majority of CO and a small amount of H2. The original chromatograms for the reduction of CO2 on sample TC2 are shown in Supplementary Fig. 9. Control experiments (Supplementary Fig. 10 and Table 2) showed that neither H2 nor CO was detected in the dark or in the absence of CO2, suggesting that the light irradiation and input CO2 were indispensable for the photocatalytic reaction. As shown in Fig. 4a, b, pristine TiO2 and CsPbBr3 QDs exhibited relatively lower production rates of H2 (0.12 and 0.06 μmol g–1 h–1, respectively) and CO (4.68 and 4.94 μmol g–1 h–1, respectively), resulting from the rapid charge recombination. Note that the H2 and CO productions were greatly enhanced with increased loading of QDs, and the generation of CO reached a maximum rate (9.02 μmol g–1 h–1) with a relatively high selectivity (95%) over TC2, due to the efficient charge separation of TiO2/CsPbBr3 heterostructure. Further increasing CsPbBr3 QDs amount would be detrimental to the photocatalytic activity (e.g., TC3 and TC4), because the overloading of CsPbBr3 could shield the light absorption of TiO2 and decrease SBET of the nanohybrids. Interestingly, with the reaction time went on, the amount of O2 decreased first and then increased, as shown in Fig. 4c. The initial O2 in the system came from the input high-purity CO2. In the first two hours of photocatalytic CO2 reduction, the fresh materials exhibit relatively strong reactivity of photoreduction. As a competitive reaction to CO2 reduction, the consumption rate of O2 (O2 + e– → ·O2–) was much higher than the production rate at the initial 2 h, while in the following 2 h, the production rate of O2 was higher than the consumption rate, the total amount of oxygen and the ratio of oxygen:nitrogen have increased to a certain extent.
Fig. 4. CO2 photoreduction performance and the photocatalytic mechanism of S-scheme heterojunction.
Photocatalytic activities of CO2 reduction over TiO2, TCx, and CsPbBr3 quantum dots (QDs) during 4-h experiment performed under UV–vis light irradiation: time course of a H2, b CO, and c O2 production yields. The initial O2 concentrations were normalized. d Mass spectra of 13CO and total ion chromatography (inset) over TC2 in the photocatalytic reduction of 13CO2. Optimized structures of CO2 molecule adsorbed on e anatase TiO2 (101), f rutile TiO2 (110), and g CsPbBr3 (001) facets. The blue, red, green, gray, and brown spheres stand for Ti, O, Cs, Pb, and Br atoms, respectively. h The DOS of CsPbBr3. i Schematic illustration of TiO2/CsPbBr3 heterojunction: internal electric field (IEF)-induced charge transfer, separation, and the formation of S-scheme heterojunction under UV–visible-light irradiation for CO2 photoreduction. j Time-resolved photoluminescence (TRPL) spectra of TiO2 (T) and TC2 at emission wavelengths of 450 and 520 nm, respectively.
The recyclability and stability of TC2 for CO2 photoreduction were investigated (Supplementary Fig. 11). After four times cycles, the decay of photocatalytic production yields of H2 and CO were hardly perceptible. To evaluate the photostability of the nanohybrids, we have characterized the recycled photocatalyst using XRD, TEM, XPS, and FTIR. As shown in the XRD pattern (Supplementary Fig. 12a), the used photocatalyst showed no detectable phase change. The TEM image confirms that the QDs did not show obvious aggregation after cycled photocatalytic reactions, and the morphology was well maintained (Supplementary Fig. 12b). The chemical states of the used photocatalyst were also consistent with those of the fresh one, as examined by XPS (Supplementary Fig. 13). The FTIR spectra of TC2 before and after reaction were presented in Supplementary Fig. 14. The characteristic absorbance bands of the aliphatic species from QDs showed no obvious variation, implying that the capping agent of QDs was stable and was not decomposed during the photocatalytic CO2 reduction.
To determine the origin of CO2 photoreduction products, we performed an isotope-labeled carbon dioxide (13CO2) photocatalytic reduction over TC2. Since the amount of products without photosensitizer and hole sacrificial agent was beyond the detection limit of mass spectrometry detector, we added tris(2,2’-bipyridyl)ruthenium(II) chloride hexahydrate ([RuII(bpy)3]Cl2·6H2O)36 and 1,3-dimethyl-2-phenyl-2,3-dihydro-1H-benzo[d]imidazole (BIH)37 into the system to promote the photocatalytic activity, which behaved as the photosensitizer and hole sacrificial agent, respectively. In this case, the production yields of H2 and CO were significantly enhanced (Supplementary Fig. 15 and Table 2) and readily detected by gas chromatography–mass spectrometer (GC-MS). As shown in Fig. 4d, the total ion chromatographic peak ~3.44 min corresponded to CO, which produced three signals in the mass spectra. The main MS signal at m/z = 29 belonged to 13CO and the others (13C at m/z = 13 and O at m/z = 16) corresponded to the fragments of 13CO, confirming that the CO product exactly originated from the CO2 photoreduction over TiO2/CsPbBr338,39. In addition, the total ion chromatographic peaks ~2.36 and 2.48 min can be assigned to the O2 and N2, respectively (Supplementary Fig. 16).
The CO2 adsorption of a photocatalyst is an essential step for CO2 photoreduction40. Figure 4e–g compared the optimized models of one CO2 molecule adsorbed on anatase TiO2 (101), rutile TiO2 (110), and CsPbBr3 (001) surfaces. Clearly, the adsorption energy (Eads) of CO2 onto CsPbBr3 (−0.22 eV) was more negative than that onto anatase and rutile TiO2 (−0.15 and −0.10 eV), which suggests that CO2 molecules adsorbed on CsPbBr3 is more stable than on TiO2. The results also indicate that CsPbBr3 QDs were in favor of the adsorption of CO2 molecules and the photocatalytic CO2 reduction.
To further explore the photocatalytic mechanism, the band structures of TiO2 and CsPbBr3 QDs were investigated. The valence band (VB) potential was obtained by analyzing the VB XPS spectra. As shown in Supplementary Fig. 17a, b, the energy level of valence band maximum (VBM) of TiO2 and CsPbBr3 is 2.39 and 1.03 eV, respectively. Mott–Schottky (M–S) curves showed that TiO2 and CsPbBr3 were of n-type semiconductors and had flat-band potentials of 0.01 eV and −0.51 eV (vs. NHE), respectively (Supplementary Fig. 17c, d). Thus, the band structures of TiO2 and CsPbBr3 QDs can be derived, and the positions of VBM and conduction band minimum (CBM) of TiO2 and CsPbBr3 are shown in Supplementary Fig. 17f.
Photocatalytic mechanism of S-scheme heterojunction
From the above analysis, the superior photoreduction activity was ascribed to the stronger CO2 adsorption of CsPbBr3 QDs and the formation of S-scheme heterojunction between TiO2 and CsPbBr3 QDs. As revealed by the above ex-situ XPS and DFT analyses, TiO2 has a lower Fermi level than CsPbBr3 QDs before they contact. Upon hybridization, the electrons preferred to flow from CsPbBr3 QDs to TiO2, which created an IEF at TiO2/CsPbBr3 interfaces pointing from CsPbBr3 to TiO2 and bent the energy bands of TiO2 and CsPbBr3. Upon photoexcitation, the VB electrons of TiO2 and CsPbBr3 jumped to their CBs. Driven by the interfacial IEF and bent bands, the photogenerated electrons in TiO2 CB spontaneously slid toward CsPbBr3 and recombined with the holes in CsPbBr3 VB. The electron-rich CsPbBr3 QDs then acted as active sites and donated electrons to activated CO2 molecules for producing H2 and CO. Noted that Pb was the active site for CO2 photoreduction since the CB of CsPbBr3 was mainly consisted of Pb 6p orbitals as evidenced by the density of states (DOS) of CsPbBr3 (Fig. 4h). Clearly, the transportation of photoinduced charge carriers follows a slide-like pathway, which implies the presence of S-scheme heterojunction between TiO2 and CsPbBr3 QDs. This unique S-scheme charge transfer efficiently separated the photoinduced electron–hole pairs and meanwhile remained the high redox ability of electrons in CsPbBr3 CB and holes in TiO2 VB, respectively. The S-scheme heterostructure of TiO2/CsPbBr3 QDs along with the charge transfer and separation is illustrated in Fig. 4i. Such an S-scheme charge transfer route was strongly evidenced by the in-situ XPS spectra measured under light irradiation. As revealed in Fig. 3a–c and Supplementary Fig. 6c, d, the BEs of Ti 2p and O 1s for TC2 under light irradiation shifted positively by 0.3 eV with reference to those in the corresponding ex-situ spectra. Conversely, the BEs of Cs 3d, Pb 4f, and Br 3d of TC2 shifted negatively by 0.5 eV. The BE shifts unequivocally proved that the photoexcited electrons in TiO2 CB transferred to CsPbBr3 QDs VB under light irradiation, following an S-scheme pathway, which supported the proposed photocatalytic mechanism.
It is worth mentioning that the TiO2 we used consisted of both anatase and rutile phases, and the charge transfer between the two phases may take place as a result of forming homojunction. As evidenced by DFT results (Fig. 3d, e), the work function of anatase TiO2 (101) was larger than that of rutile TiO2 (110), indicating that electrons would flow from rutile to anatase and created an IEF at anatase/rutile TiO2 interfaces. Driven by the interfacial IEF, the photogenerated electrons in anatase TiO2 CB spontaneously slid toward rutile TiO2 VB and recombined with the holes in the rutile TiO2 VB. Such transportation of photoinduced charge carriers follows an S-like pathway (S-scheme homojunction) between anatase and rutile TiO2 (Supplementary Fig. 18), which is consistent with our previous work41. When CsPbBr3 QDs deposited on TiO2 nanofibers, all possible schematic illustrations between anatase TiO2, rutile TiO2, and CsPbBr3 QDs are shown in Supplementary Fig. 19.
To further prove the efficient charge separation of TiO2/CsPbBr3 S-scheme heterojunction, photoluminescence (PL) emission spectra of the samples were collected (Supplementary Fig. 20). TC2 and TC4 showed a marginally lower PL intensity than TiO2, implying that the presence of CsPbBr3 QDs efficiently retarded the electron–hole recombination in TiO2. To gain a deeper insight into the charge transfer dynamics, the time-resolved photoluminescence (TRPL) spectra of TiO2 and TC2 were recorded at emission wavelengths (EW) of 450 nm and 520 nm (Fig. 4j), corresponding to the maximum fluorescence emissions of TiO2 and QDs, respectively. The fitted decay curves disclose the lifetime (τ) and percentage (Rel.%) of charge carriers (Supplementary Table 3). The short lifetime (τ1) corresponds to radiative recombination of the carriers (denoted as τ1-carriers), while the long lifetimes (τ2 and τ3) correspond to non-radiative recombination and energy-transfer process42. Note that the un-recombined τ1-carriers will participate in surface photocatalytic reaction. Thus, the decrease of τ1-carrier percentage implies radiative recombination inhibited. At EW = 450 nm, only TiO2 showed a fluorescence emission signal. As shown in Supplementary Table 3, TC2 had a lower percentage (36.27%, 450 nm) of τ1-carriers than pristine TiO2 (37.98%, 450 nm), suggesting the radiative recombination over TiO2 was inhibited upon QDs deposition due to the formation of S-scheme heterojunction43,44. Further, a similar decrease in τ1-carrier percentage was also observed at EW = 520 nm. Notably, TC2 showed longer lifetime than pristine TiO2 due to the transfer of the electrons in TiO2 CB to QDs VB. Therefore, it is not surprising that the TC2 composite sample exhibited enhanced photocatalytic CO2 reduction performance.
The electrochemical impedance spectra (EIS) (Supplementary Fig. 21a) showed the samples with CsPbBr3 QDs exhibited smaller semicircle compared to pure TiO2 and revealed lower charge-transfer resistance. The polarization curves of TiO2 and TC2 under light irradiation (Supplementary Fig. 21b) showed that the overpotential for TC2 was much lower than that of TiO2, indicating that TiO2/CsPbBr3 hybrids presented better reduction capability than that of TiO2. These results proved that CsPbBr3 QDs, as an emerging semiconductor, could form S-scheme heterojunction with TiO2 to promote the electron transfer and separate the electron–hole pairs for efficient CO2 photoreduction.
In summary, an S-scheme TiO2/CsPbBr3 heterojunction synthesizes through an electrostatic assembly method. The resulting TiO2/CsPbBr3 heterojunction reveals an enhanced activity toward CO2 photoreduction under UV–visible-light irradiation due to the IEF-induced, more efficient charge separation between TiO2 and CsPbBr3. DFT calculations reveal the work function of TiO2 was greater than that of CsPbBr3, implying electrons transfer from CsPbBr3 to TiO2 upon hybridization and thus created an IEF at interfaces. The IEF drives photoinduced electrons in TiO2 CB to immigrate to CsPbBr3 VB as evidenced by in-situ XPS analysis, confirming an S-path of charge transfer. Isotope (13CO2) tracer results confirm that the reduction products originate from CO2 source, instead of any contaminant carbon species. This work provides a point of view in the design of photocatalysts with distinct heterojunctions for efficient photocatalytic CO2 reduction.
Methods
Synthesis of electrospun TiO2 nanofibers
All the chemicals were of analytic grade and purchased from Shanghai Chemical Company. Typically, tetrabutyl titanate (TBT, 2.0 g) and poly(vinyl pyrrolidone) (PVP, 0.75 g, MW = 1,300,000) were mixed with ethanol (10.0 g) and acetic acid (2.0 g) to form a transparent pale-yellow solution after magnetic stirring for 5 h. Afterward, the solution was transferred into a 10-mL syringe in an electrospinning setup with a voltage of 20 kV and a solution feeding rate of 2.5 mL h–1. The needle-to-collector distance was 10 cm. The collected TiO2 precursor was annealed at 550 °C for 2 h with a heating rate of 2 °C min–1 in air.
Synthesis of perovskite CsPbBr3 QDs
Briefly, 130 mg of Cs2CO3 (0.4 mmol) were mixed with octadecylene (ODE, 6 mL) and oleic acid (OA, 0.5 mL) under stirring in a three-neck flask (25 mL). The mixture was dried at 120 °C for 1 h under vacuum and heated to 150 °C under N2 gas to form Cs(oleate) solution, which was stored at room temperature and preheated to 140 °C prior to use. Then 72 mg of PbBr2 (0.196 mmol) was mixed with ODE (5.0 mL), oleylamine (0.5 mL), and OA (0.5 mL) in another flask (25 mL), and was dried under vacuum at 105 °C for 0.5 h. The mixture was heated to 170 °C, and Cs(oleate) (0.45 mL) was rapidly injected under vigorously stirring for 5 s. The reaction was quenched by immersing the flask into an ice-water bath. The obtained product was mixed with 3 mL of hexane and centrifuged at 1208 × g for 2 min to remove aggregates and large particles. The supernatant was precipitated with acetone and centrifuged at 3355 × g for 5 min. As-collected CsPbBr3 QDs were re-dispersed in hexane for further use.
Preparation of TiO2/CsPbBr3 heterostructures
Typically, 200 mg of TiO2 nanofibers were dispersed into 20 mL of hexane. A certain amount of CsPbBr3 QDs solution was added into TiO2 suspension under vigorous stirring for 2 h. TiO2 and CsPbBr3 QDs were assembled by electrostatic self-assembly. The mixture was then vacuum-dried at 50 °C for 2 h to form TiO2/CsPbBr3 heterostructures. The products are labeled as TCx, where T and C denote TiO2 and CsPbBr3 QDs, respectively; x is the mass percentage of CsPbBr3 QDs.
Characterization
XRD was performed on a D/Max-RB X-ray diffractometer (Rigaku, Japan) with Cu Kα radiation. TEM images were observed on a Titan G2 60-300 electron microscope equipped with an EDX spectrometer. UV–visible DRS was collected on a Shimadzu UV-2600 UV–visible spectrophotometer (Japan). XPS was performed on a Thermo ESCALAB 250Xi instrument with Al Kα X-ray radiation. In-situ XPS was conducted under the same condition, except that UV–visible-light irradiation was introduced. FTIR spectra were recorded with an attenuated total reflectance (ATR) mode on Nicolet iS 50 (Thermo Fisher, USA). The PL emission spectra were collected on a fluorescence spectrophotometer (F-7000, Hitachi, Japan). TRPL spectra were recorded on a fluorescence lifetime spectrophotometer (FLS 1000, Edinburgh, UK) at an excitation wavelength of 325 nm. Electrochemical measurements were conducted on an electrochemical analyzer (CHI660C, CH Instruments, Shanghai). Pt wire, Ag/AgCl (saturated KCl), and 0.5 M Na2SO4 solution functioned as the counter electrode, reference electrode, and electrolyte, respectively. For the working electrode, 20 mg of TCx was ground in 1.0 mL of ethanol and 10 μL of Nafion solution to make a slurry, which was coated onto F-doped SnO2-coated (FTO) glass with an exposed area of 1 cm2. The FTO electrode was then vacuum-dried at 60 °C for 1 h.
Photocatalytic CO2 reduction
The photocatalytic CO2 reduction was performed in a gas-closed system equipped with a gas-circulated pump. The apparatus of the system is shown in Supplementary Fig. 7. Typically, 10 mg of photocatalysts, 30 mL of acetonitrile, and 100 μL of water were added in a Quartz and Pyrex glass hybrid reaction cell (Supplementary Fig. 8). The airtight system was completely evacuated by using a vacuum pump. Then ~80 kPa of high-purity CO2 (99.999%) gas was injected. After adsorption equilibrium, the photocatalytic cell was irradiated with a 300 W Xe arc lamp (PLS-SXE300D, Beijing Perfectlight, China), and the reaction system was kept at 10 °C as controlled by cooling water. The CO2-reduction products were analyzed on a gas chromatograph (GC-2030, Shimadzu Corp., Japan) equipped with a barrier discharge ionization detector (BID) and a capillary column (Carboxen 1010 PLOT Capillary, 60 m × 0.53 mm). The column was maintained at 35 °C for 15 min. It was then heated to 180 °C at 20 °C min–1, and maintained for another 5 min. Helium was the carrier gas with pressure set to 70 kPa. The temperatures of the injector and BID were set to be 150 and 280 °C, respectively. For comparison, 2 mM of tris(2,2′-bipyridyl)ruthenium(II) chloride hexahydrate ([RuII(bpy)3]Cl2·6H2O) and 10 mM of 1,3-dimethyl-2-phenyl-2,3-dihydro-1H-benzo[d]imidazole (BIH) were added into the photocatalytic system (other parameters were unchanged), which behaved as the photosensitizer and hole sacrificial agent, respectively. A series of control experiments were also conducted, and the results are summarized in Supplementary Table 2.
Isotope-labeling measurement
The isotope-labeling experiment was conducted by using 13CO2 (isotope purity, 99% and chemical purity, 99.9%, Tokyo Gas Chemicals Co., Ltd.) as the carbon source. Typically, 10 mg of photocatalysts, 2 mM of [RuII(bpy)3]Cl2·6H2O, 10 mM of BIH, 30 mL of acetonitrile and 100 μL of water were loaded into the reaction cell. The protocol of 13CO2 photoreduction was the same as that mentioned above. The gas products were analyzed by gas chromatography–mass spectrometry (JMS-K9, JEOL-GCQMS, Japan and 6890 N Network GC system, Agilent Technologies, USA) equipped with the column for detecting the products of 13CO (HP-MOLESIEVE, 30 m × 0.32 mm × 25 μm). Helium was used as carrier gas. The column was maintained at 60 °C for 20 min, and the flow of the carrier was 0.5 ml L–1. The temperatures of the injector, EI source, and the GCITF were set to be 200, 200, and 250 °C, respectively.
Supplementary information
Acknowledgements
This work was supported by NSFC (51932007, 21573170, U1705251, 51961135303, and 51902121), the National Key Research and Development Program of China (2018YFB1502001), National Postdoctoral Program for Innovative Talents (BX20190259), China Postdoctoral Science Foundation (2019M660189), and the Fundamental Research Funds for the Central Universities (WUT: 2019IVA111). J.X. is grateful to the financial support by the Australian Research Council. The project is also supported by the State Key Laboratory of Advanced Technology for Materials Synthesis and Processing (Wuhan University of Technology) (2018-KF-17).
Source data
Author contributions
F.X., B.C., and J.Y. conceived and designed the experiments. F.X. and K.M. carried out the synthesis of the materials and the characterizations of the materials. F.X. and S.W. carried out the photocatalytic test. F.X., S.W., J.X., and J.Y. contributed to data analysis. J.Y. and J.X. supervised the project. F.X. wrote the paper. J.Y., S.W., and J.X. revised and reviewed the paper. All authors discussed the results and commented on the paper.
Data availability
All data are available from the corresponding author on request. Source data are provided with this paper. Source data are also available in figshare with the identifier 10.6084/m9.figshare.12715484.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks Laura Schelhas and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shengyao Wang, Email: wangshengyao@mail.hzau.edu.cn.
Jingsan Xu, Email: jingsan.xu@qut.edu.au.
Jiaguo Yu, Email: jiaguoyu93@whut.edu.cn.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41467-020-18350-7.
References
- 1.El-Khoulya ME, El-Mohsnawy E, Fukuzumi S. Solar energy conversion: from natural to artificial photosynthesis. J. Photochem. Photobiol. C. 2017;31:36–83. [Google Scholar]
- 2.Crake A. Metal-organic frameworks based materials for photocatalytic CO2 reduction. Mater. Sci. Technol. 2017;33:1737–1749. [Google Scholar]
- 3.Collado L, et al. Unravelling the effect of charge dynamics at the plasmonic metal/semiconductor interface for CO2 photoreduction. Nat. Commun. 2018;9:4986. doi: 10.1038/s41467-018-07397-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu Q, et al. Direct Z-scheme photocatalysts: principles, synthesis, and applications. Mater. Today. 2018;21:1042–1063. [Google Scholar]
- 5.Yan Z-H, et al. Photo-generated dinuclear {Eu(II)}2 active sites for selective CO2 reduction in a photosensitizing metal-organic framework. Nat. Commun. 2018;9:3353. doi: 10.1038/s41467-018-05659-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li X, Wen J, Low J, Fang Y, Yu J. Design and fabrication of semiconductor photocatalyst for photocatalytic reduction of CO2 to solar fuel. Sci. China Mater. 2014;57:70–100. [Google Scholar]
- 7.Ran J, Jaroniec M, Qiao S-Z. Cocatalysts in semiconductor-based photocatalytic CO2 reduction: achievements, challenges, and opportunities. Adv. Mater. 2018;30:1704649. doi: 10.1002/adma.201704649. [DOI] [PubMed] [Google Scholar]
- 8.Nahar S, Zain MFM, Kadhum AAH, Hasan HA, Hasan MR. Advances in photocatalytic CO2 reduction with water: a review. Materials. 2017;10:629. doi: 10.3390/ma10060629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu W, Xu D, Peng T. Enhanced photocatalytic activity of g-C3N4 for selective CO2 reduction to CH3OH via facile coupling of ZnO: a direct Z-scheme mechanism. J. Mater. Chem. A. 2015;3:19936–19947. [Google Scholar]
- 10.Singh AK, Montoya JH, Gregoire JM, Persson KA. Robust and synthesizable photocatalysts for CO2 reduction: a data-driven materials discovery. Nat. Commun. 2019;10:443. doi: 10.1038/s41467-019-08356-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ola O, Maroto-Valer MM. Review of material design and reactor engineering on TiO2 photocatalysis for CO2 reduction. J. Photochem. Photobiol., C. 2015;24:16–42. [Google Scholar]
- 12.Xu F, Meng K, Cheng B, Yu J, Ho W. Enhanced photocatalytic activity and selectivity for CO2 reduction over a TiO2 nanofibre mat using Ag and MgO as Bi-cocatalyst. ChemCatChem. 2018;10:465–472. [Google Scholar]
- 13.Wang S, et al. Porous hypercrosslinked polymer-TiO2-graphene composite photocatalysts for visible-light-driven CO2 conversion. Nat. Commun. 2019;10:676. doi: 10.1038/s41467-019-08651-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdullah, H., Khan, M. M. R., Ong, H. R. & Yaakob, Z. Modified TiO2 photocatalyst for CO2 photocatalytic reduction: an overview. J. CO2Util.22, 15–32 (2017).
- 15.Fang W, Xing M, Zhang J. Modifications on reduced titanium dioxide photocatalysts: a review. J. Photochem. Photobiol., C. 2017;32:21–39. [Google Scholar]
- 16.Low J, Cheng B, Yu J. Surface modification and enhanced photocatalytic CO2 reduction performance of TiO2: a review. Appl. Surf. Sci. 2017;392:658–686. [Google Scholar]
- 17.Edelmannová M, et al. Photocatalytic hydrogenation and reduction of CO2 over CuO/TiO2 photocatalysts. Appl. Surf. Sci. 2018;454:313–318. [Google Scholar]
- 18.Shehzad, N., Tahir, M., Johari, K., Murugesan, T. & Hussaind, M. A critical review on TiO2 based photocatalytic CO2 reduction system: strategies to improve efficiency. J. CO2Util.26, 98–122 (2018).
- 19.Xu F, Zhang J, Zhu B, Yu J, Xu J. CuInS2 sensitized TiO2 hybrid nanofibers for improved photocatalytic CO2 reduction. Appl. Catal., B. 2018;230:194–202. [Google Scholar]
- 20.Yuan L, Lu K-Q, Zhang F, Fu X, Xu Y-J. Unveiling the interplay between light-driven CO2 photocatalytic reduction and carbonaceous residues decomposition: a case study of Bi2WO6-TiO2 binanosheets. Appl. Catal., B. 2018;237:424–431. [Google Scholar]
- 21.Hu H, et al. Interfacial synthesis of highly stable CsPbX3/oxide Janus nanoparticles. J. Am. Chem. Soc. 2018;140:406–412. doi: 10.1021/jacs.7b11003. [DOI] [PubMed] [Google Scholar]
- 22.Li M, Zhang X, Matras-Postolek K, Chen H-S, Yang P. An anion-driven Sn2+ exchange reaction in CsPbBr3 nanocrystals towards tunable and high photoluminescence. J. Mater. Chem. C. 2018;6:5506–5513. [Google Scholar]
- 23.Li X, Wang Y, Sun H, Zeng H. Amino-mediated anchoring perovskite quantum dots for stable and low-threshold random lasing. Adv. Mater. 2017;29:1701185. doi: 10.1002/adma.201701185. [DOI] [PubMed] [Google Scholar]
- 24.Pan A, et al. Nanorod suprastructures from a ternary graphene oxide-polymer-CsPbX3 perovskite nanocrystal composite that display high environmental stability. Nano Lett. 2017;17:6759–6765. doi: 10.1021/acs.nanolett.7b02959. [DOI] [PubMed] [Google Scholar]
- 25.Protesescu L, et al. Nanocrystals of cesium lead halide perovskites (CsPbX(3), X = Cl, Br, and I): novel optoelectronic materials showing bright emission with wide color gamut. Nano Lett. 2015;15:3692–3696. doi: 10.1021/nl5048779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu Y-F, et al. Enhanced solar-driven gaseous CO2 conversion by CsPbBr3 nanocrystal/Pd nanosheet Schottky-junction photocatalyst. ACS Appl. Energy Mater. 2018;1:5083–5089. [Google Scholar]
- 27.Xu Y-F, et al. A CsPbBr3 perovskite quantum dot/graphene oxide composite for photocatalytic CO2 reduction. J. Am. Chem. Soc. 2017;139:5660–5663. doi: 10.1021/jacs.7b00489. [DOI] [PubMed] [Google Scholar]
- 28.Ou M, et al. Amino-assisted anchoring of CsPbBr3 perovskite quantum dots on porous g-C3N4 for enhanced photocatalytic CO2 reduction. Angew. Chem. Int. Ed. 2018;57:13570–13574. doi: 10.1002/anie.201808930. [DOI] [PubMed] [Google Scholar]
- 29.Fu J, Xu Q, Low J, Jiang C, Yu J. Ultrathin 2D/2D WO3/g-C3N4 step-scheme H2-production photocatalyst. Appl. Catal., B. 2019;243:556–565. [Google Scholar]
- 30.Xu Q, Zhang L, Cheng B, Fan J, Yu J. S-scheme heterojunction photocatalyst. Chem. 2020;6:1543–1559. [Google Scholar]
- 31.Pavliuk MV, Abdellah M, Sá J. Hydrogen evolution with CsPbBr3 perovskite nanocrystals under visible light in solution. Mater. Today Commun. 2018;16:90–96. [Google Scholar]
- 32.Cai Z, et al. Preparation and characterization of a bi-layered nano-filtration membrane from a chitosan hydrogel and bacterial cellulose nanofiber for dye removal. Cellulose. 2018;25:5123–5137. [Google Scholar]
- 33.Low J, Dai B, Tong T, Jiang C, Yu J. In situ irradiated X-ray photoelectron spectroscopy investigation on direct Z-scheme TiO2/CdS composite film photocatalyst. Adv. Mater. 2019;30:1802981. doi: 10.1002/adma.201802981. [DOI] [PubMed] [Google Scholar]
- 34.Xu F, Zhang L, Cheng B, Yu J. Direct Z-scheme TiO2/NiS core–shell hybrid nanofibers with enhanced photocatalytic H2-production activity. ACS Sustain. Chem. Eng. 2018;6:12291–12298. [Google Scholar]
- 35.Wang S, et al. Direct Z-scheme ZnO/CdS hierarchical photocatalyst for enhanced photocatalytic H2-production activity. Appl. Catal., B. 2019;243:19–26. [Google Scholar]
- 36.Zhang S, et al. An artificial photosynthesis system comprising a covalent triazine framework as an electron relay facilitator for photochemical carbon dioxide reduction. J. Mater. Chem. C. 2020;8:192–200. [Google Scholar]
- 37.Hu Y, et al. Tracking mechanistic pathway of photocatalytic CO2 reaction at Ni sites using operando, time-resolved spectroscopy. J. Am. Chem. Soc. 2020;142:5618–5626. doi: 10.1021/jacs.9b12443. [DOI] [PubMed] [Google Scholar]
- 38.Wang S, et al. Intermolecular cascaded pi-conjugation channels for electron delivery powering CO2 photoreduction. Nat. Commun. 2020;11:1149. doi: 10.1038/s41467-020-14851-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang X, et al. Oxygen vacancies induced special CO2 adsorption modes on Bi2MoO6 for highly selective conversion to CH4. Appl. Catal., B. 2019;259:118088. [Google Scholar]
- 40.Peng C, Reid G, Wang H, Hu P. Perspective: photocatalytic reduction of CO2 to solar fuels over semiconductors. J. Chem. Phys. 2017;147:030901. doi: 10.1063/1.4985624. [DOI] [PubMed] [Google Scholar]
- 41.Xu F, Xiao W, Cheng B, Yu J. Direct Z-scheme anatase/rutile bi-phase nanocomposite TiO2 nanofiber photocatalyst with enhanced photocatalytic H2-production activity. Int. J. Hydrog. Energ. 2014;39:15394–15402. [Google Scholar]
- 42.Das K, Sharma SN, Kumar M, De SK. Morphology dependent luminescence properties of Co doped TiO2 nanostructures. J. Phys. Chem. C. 2009;113:14783–14792. [Google Scholar]
- 43.Xia P, Liu M, Cheng B, Yu J, Zhang L. Dopamine modified g-C3N4 and its enhanced visible-light photocatalytic H2-production activity. ACS Sustain. Chem. Eng. 2018;6:8945–8953. [Google Scholar]
- 44.Xia P, Zhu B, Yu J, Cao S, Jaroniec M. Ultra-thin nanosheet assemblies of graphitic carbon nitride for enhanced photocatalytic CO2 reduction. J. Mater. Chem. A. 2017;5:3230–3238. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available from the corresponding author on request. Source data are provided with this paper. Source data are also available in figshare with the identifier 10.6084/m9.figshare.12715484.