Abstract
Background
Laparoscopic procedures under certain pressure have the potential to cause intra-abdominal adhesions. However, the pathomechanism of this disorder is unknown. Release of mast cell mediators due to mast cell degranulation is thought to be the cause.
Materials and methods
Thirty male Sprague-Dawley rats were grouped into five groups (n = 6 per group): one control group and four intervention groups to which 60 min insufflation was performed using carbon dioxide at 5, 8, 10 and 12 mmHg. Seven days after laparoscopy, we euthanized and evaluated the levels of histamine, tryptase, and chymase of peritoneal fluid, the thickness of ECM of peritoneal tissue, and intraabdominal adhesion scoring system.
Results
Histamine and tryptase levels in peritoneal fluid were significantly higher at the 10- and 12 mm Hg intervention compared to control (histamine: 0.50 ± 0.35 vs. 0.41 ± 0.41 vs. 0.04 ± 0.02 ng/mL, respectively; and tryptase: 0.69 ± 0.11 vs. 0.65 ± 0.05 vs. 0.48 ± 0.02 ng/ml respectively). The ECM was significantly thicker in the intervention groups at 10- and 12-mm Hg compared to control (71.3 [66.7–85.2] vs. 48.4 [34.5–50.3] vs. 10.25 [8.7–12.1] μm, respectively). Moreover, the intra-abdominal scoring was also significantly higher in the intervention groups at 10- and 12 mm Hg compared to control (4 [0–4] vs. 4.5 [4–5], vs. 0, respectively).
Conclusions
Laparoscopic procedures increase the release of mast cell mediators in peritoneal fluid, the thickness of ECM and intraabdominal adhesion scoring in rats, implying that it might increase the possibility of intrabdominal adhesion in humans.
Keywords: Laparoscopy, Mast cell mediators, Histamine, Protease, Extracellular matrix thickness, Intra-abdominal adhesion
Abbreviations: ATP, Adenosine triphosphate; CRAC, Calcium release-activated channels; CO2, Carbon dioxide; DAMPs, Damage Associated Molecular Patterns; DNA, Deoxyribonucleic acid; ELISA, Enzyme-linked-immunosorbent-assay; ECM, Extracellular matrix; GPCR, G Protein-Coupled Receptors; pro-MMP9, pro Matrix metallopeptidase 9; PAR-2, protease-activated receptor 2; ROS, Reactive Oxygen Species; TGF-β, Transforming growth factor-beta; TRPC, Transient receptor potential canonical; TRPV4, Transient receptor potential vanilloid 4; tPA, tissue plasminogen activator; uPA, urokinase plasminogen activator; VDAC, Voltage-dependent anion channel
Highlights
-
•
Laparoscopic procedures at specific pressures potentially cause intra-abdominal adhesion, however, its pathomechanism is still challenging to understand.
-
•
Laparoscopic procedures increase the release of mast cell mediators in peritoneal fluid, the thickness of ECM and intraabdominal adhesion scoring in rats.
-
•
Our findings imply that laparoscopic procedures might increase the possibility of intrabdominal adhesion in humans.
1. Introduction
Carbon dioxide (CO2) insufflation in laparoscopy procedures causes mesothelial morphological changes [1], structure damage [2], and the risk of intra-abdominal adhesion [3]. Tissue damage triggers the inflammatory response, mast cell infiltration, and degranulation that are believed to stimulate adhesion. The study about the effect of mast cell mediators on the intra-abdominal adhesion pathomechanism is still rarely conducted.
Mast cells are specific [4], mature in the tissues, and form 10% of the mesothelium immune cell population [5]. Laparoscopy procedures cause mast cell infiltration and degranulation [6]. The release of histamine, tryptase, and chymase due to mast cell degranulation [7] are presumed to play a role in intra-abdominal adhesion. Our objective was to determine the impact of the laparoscopic procedure on 1. mast cell mediators’ level, including histamine, tryptase, and chymase; 2. the thickness of the extracellular matrix (ECM) of peritoneal tissue; and 3. intraabdominal adhesion scoring system.
2. Materials and methods
2.1. Animal subjects
This study was conducted according to the 3R5F principles of experimental animal studies [8,9]. Thirty males [10], 200–250 g, and 20–25 weeks old Sprague-Dawley rats (Rattus norvegicus) were randomly computerize divided into a control group and four intervention groups. The rats were kept in standard breeding-housing (maintained 20 ± 2 °C temperature, 12 h light/dark cycle), standard food, mineral water, health monitor, and 7 days of acclimation) [11]. The sick and dead rats were excluded from the study and replaced with healthy. The control group (n = 6) did not receive pneumoperitoneum. The intervention groups of P-5 mmHg, P-8 mmHg, P-10 mmHg, and P-12 mmHg (all n = 6) were given 5, 8, 10, and 12 mmHg CO2 pneumoperitoneum, respectively [6,12]. Our study strictly followed the ethical and euthanasia guidelines for animal research (http://risetcenterfk.ulm.ac.id/euthanasia/). The Animal Experimentation Ethical Committee, Research Center, Faculty of Medicine, Universitas Lambung Mangkurat, Banjarmasin, Indonesia, had approved our research (No.282/KEPK-FK.UNLAM/EC/VII/2019). The experiments were conducted in the Chemical/Biochemical Laboratory, the Anatomical Pathology Laboratory, Faculty of Medicine, Universitas Lambung Mangkurat, Banjarmasin, Indonesia.
2.2. Laparoscopy procedures
According to the previous study [6], 60-min laparoscopy was performed in a sterile area after shaving and povidone-iodine application. Ten mg/kg BW intramuscular injections of ketamine hydrochloride (KTM-10; PT Guardian Pharmatama, No. Reg. DKL0408013443B1) were used for anesthesia. Pneumoperitoneum used standard CO2 and CO2 automatic-insufflators (Gimmi, Gimmi®GmbH, Germany, 2000).
2.3. Sample collection
Decapitation was performed to euthanize the rats on the 7th-day after laparoscopy [13]. The peritoneum was stained with Masson trichrome to evaluate the ECM thickness in 40x magnification [14].
2.4. Histamine, tryptase and chymase analysis
The peritoneal fluid, mast cells, histamine and protease levels were measured using a commercial kit, the enzyme-linked-immunosorbent-assay (ELISA). Histamine and protease levels used Cloud-clone Corp. ELISA Kit for Histamine (HA) for pan-species CEA927Ge [15], Tryptase (TPS) for Rat SEB070Ra [16], and Chymase-1 Mast Cell (CMA1) for Rat SEG515Ra [17].
2.5. Extracellular matrix thickness and intra-abdominal scoring evaluation
The ECM thickness was measured by using the Masson trichrome stain, based on Skytec TRM-1-IFU's collagen Trichrome Stain (Connective Tissue Stain) [18] collagen deposition and quantified with ImageJ version 1.51j8 RRID: SCR_003070 [19]. Modified intra-abdominal adhesion scoring for laparoscopy [2] was used (Table 1).
Table 1.
Modified intra-abdominal adhesion after laparoscopic surgery.
| Variables | Score |
|---|---|
| Extension of adhesion | |
|
0 |
|
1 |
|
2 |
|
3 |
| Severity of adhesion | |
|
0 |
|
1 |
|
2 |
|
3 |
| Bleeding level during dissection of adhesion | |
|
0 |
|
1 |
|
2 |
|
3 |
2.6. Statistical analysis
Our study results were presented as numbers, percentages, mean ± standard deviation (SD), and median (range, minimum-maximum). Data were analyzed for normality (using Kolmogorov–Smirnov, and Shapiro–Wilk tests), homogeneity using Levine's test, and underwent data transformation methods (power > 1, inverse, log 10, and square root). One-way ANOVA and the post-hoc LSD tests were used for normally and homogeneously distributed data. One-way test of Equality of Means and the post-hoc Games-Howell test were used for normally but non-homogeneously distributed data. Kruskal-Wallis and post-hoc Mann-Whitney tests were used for non-normally distributed data. With a confidence interval of 95% (α = 0.05), the analysis used IBM SPSS version 23.0 and Microsoft Excel 2010.
3. Results
3.1. Mast cell mediators’ level after laparoscopic procedures
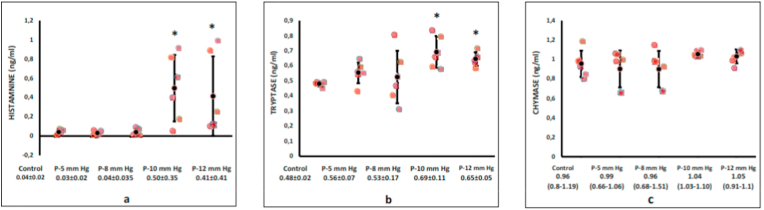
Histamine and tryptase levels in peritoneal fluid were significantly higher in the 10 and 12 mm Hg intervention groups than the control group (histamine: 0.04 ± 0.02 vs. 0.03 ± 0.02 vs. 0.04 ± 0.035 vs. 0.50 ± 0.35 vs. 0.41 ± 0.41 ng/mL for control, 5-, 8-, 10-, and 12-mmHg, respectively, p < 0.05; and tryptase: 0.48 ± 0.02 vs. 0.56 ± 0.07 vs. 0.53 ± 0.17 vs. 0.69 ± 0.11 vs. 0.65 ± 0.05 ng/ml for control, 5-, 8-, 10-, and 12-mmHg, respectively, p < 0.05). Chymase levels were similar among groups (0.96 [range, 0.8–1.19] vs. 0.99 [range, 0.66–1.06] vs. 0.96 [range, 0.68–1.51] vs. 1.04 [range, 1.03–1.10] vs. 1.05 [rage, 0.91–1.1] ng/ml, for control, 5-, 8-, 10-, and 12-mmHg, respectively, p > 0.05). (Fig. 1a–c).
Fig. 1.
The mast cell mediators' level after laparoscopic surgery. Histamine (a) and tryptase (b) levels in peritoneal fluid were a significantly higher in the 10 and 12 mmHg intervention groups than the control group (p < 0.05), while chymase (c) levels were similar in both groups (p > 0.05).
3.2. Extracellular matrix thickness following surgery
The ECM was significantly thicker in the intervention groups at 10- and 12-mm Hg than in the control group (10.25 [range, 8.7–12.1] vs. 37.15 [range, 31.3–43.7] vs. 40.05 [range, 33.2–44.4] vs. 71.3 [range, 66.7–85.2] vs. 48.4 [range, 34.5–50.3] μm, for control, 5-, 8-, 10-, and 12-mmHg, respectively, p < 0.05 (Fig. 2, Fig. 3).
Fig. 2.
Histopathological findings of extracellular matrix thickness after laparoscopic procedures. The increasing insufflation pressure increases ECM thickness of the parietal peritoneum tissue (Masson trichrome staining, 40x magnifications).
Fig. 3.
The thickness of the extra-cellular matrix was strongly increased after the laparoscopic procedures compared to the control group (p < 0.05).
3.3. Intra-abdominal scoring system after procedure
The intra-abdominal scoring was significantly higher in the intervention groups at 10- and 12- mm Hg than control group (0 vs. 3.5 [range, 0–4] vs. 4 [range, 0–5] vs. 4 [range, 0–4] vs. 4.5 [range, 4–5], for control, 5-, 8-, 10-, and 12-mmHg, respectively, p < 0.05 (Fig. 4).
Fig. 4.
The intra-abdominal scoring was significantly different between laparoscopic and control groups (p < 0.05).
4. Discussion
Laparoscopic pneumo-peritoneum causes hypoxia and ischemia-reperfusion injury (especially during desufflation), oxidative stress, and cell damage [20,21]. Cell damage triggers the production of Damage Associated Molecular Patterns (DAMPs) and inflammatory responses [22,23]. Mast cells and other innate immune cells will become active for homeostasis [20]. Mast cells have unique features compared to other inflammatory cells [24], becoming mature in tissue, with longer life, and a role in the fibrosis process [25]. These pathological conditions cause excessive mast cell infiltration and degranulation [[26], [27], [28]]. Our study identified an increase in histamine and tryptase levels in laparoscopic procedure pressures of 10- and 12-mm Hg. The pneumoperitoneum procedure involves non-immunological (physical stimulation) [29] and causes mast cell degranulation [17]. Hypoxia triggers anaerobic respiration, and adenosine triphosphate (ATP) deficiency, which results in interference of the ATPase dependent on mast cell membrane channel. This mechanism disrupts water, ion, and cellular homeostasis [30]. Hypoxia causes the activation of the C3a and C5a molecules and activates the G Protein-Coupled Receptors (GPCR) receptors resulting in degranulation [31]. The pressure and cold of CO2 pneumoperitoneum cause interference to the Ca2+ channel of mast cells [29]. Lipid, protein, and deoxyribonucleic acid (DNA) peroxidation due to Reactive Oxygen Species (ROS) also cause mast cell degranulation [32]. The mast cell is a non-excitable immunological cell that is sensitive to physical trauma [33,34]. Transient receptor potential canonical (TRPC) Ca2+ channel is sensitive to temperature changes. Calcium release-activated channels (CRAC) [35] and transient receptor potential vanilloid (TRPV4) [36] are mechanosensitive (MS) channels that are sensitive to pressure. The voltage-dependent anion channel (VDAC) mitochondria Ca2+ channel regulates cytoplasmic levels and causes mast cell degranulation if the VDAC function has interfered [29].
Mast cell degranulation releases histamine and proteases. Mast cell histamine and proteases are high in fibrosis areas [37]. Histamine causes vascular vasodilation, and increases molecular cell adhesion, and modulates the migration and proliferation of fibroblasts [25]. Mast cell tryptase and chymase increase transforming growth factor-beta (TGF-β) activity, decrease the cell tight junction affinity and become a pro-fibrotic protein [25,38]. TGF-β triggers mesothelial-transformation increases the ECM thickness [39] and leads to fibrosis [40]. Tryptase and chymase are the angiogenic factors [25] and trigger ECM thickness. Chymase results in the degradation of the enzymes' vitronectin and fibronectin, transforming the matrix metallopeptidase 9 (pro-MMP9) into active forms and modulate the thickening of ECM [7]. Tryptase causes degradation of type 4 collagen as the main structure of the basement membrane [41]. Tryptase and chymase inhibit the fibrinolysis enzymes (tissue plasminogen activator/tPA and urokinase plasminogen activator/uPA), increasing fibrin [25]. They activate the protease-activated receptor 2 (PAR-2) receptors, causing degradation of the cell junction components, which causes mesothelial release from the basement membrane [42]. Different from research conducted by Berdun et al. [17], our study found no significant increase in chymase levels. It was suspected that the mast cell chymase population is lower than tryptase in mesothelial tissue. This finding is most likely related to the specific trauma of laparoscopy.
Our study found an increase in the ECM and intra-abdominal scoring in laparoscopy over 10 mm Hg. The ECM is a 3-dimensional structure consisting of collagen, enzymes, glycoproteins (proteoglycans), and extracellular vesicles (Deoxyribonucleic acid/DNA, Ribonucleic acid/RNA, and Matrix-bound Nano vesicles/MBVs) [43,44]. The effect of laparoscopy is multi-factorial on the 3-dimensional structure of ECM, including mast cell degranulation [45]. Laparoscopy procedures trigger proliferation, differentiation, migration, and ECM formation towards fibrosis, due to an imbalance of the coagulation and fibrinolysis process [46].
Although good and simple to apply clinically, the intraabdominal scoring system should be done in more studies, particularly in humans. Future research is needed on mast cell stabilizers to prevent intra-abdominal adhesion to further confirm our findings.
5. Conclusions
Laparoscopic procedures increase the release of mast cell mediators in peritoneal fluid, the thickness of extracellular matrix and intraabdominal adhesion scoring in rats, implying that it might increase the possibility of intrabdominal adhesion in humans.
Conflicts of interest
All authors declare that they have no conflict of interest.
Consent
Not applicable.
Acknowledgments
We thank all who offered excellent assistance during this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2020.08.043.
Contributor Information
Hery Poerwosusanta, Email: herpoerwo@ulm.ac.id.
Gunadi, Email: drgunadi@ugm.ac.id.
Ika Kustiyah Oktaviyanti, Email: ikaoktaviyanti@ymail.com.
Nia Kania, Email: kania9008@gmail.com.
Zairin Noor, Email: noorzairin@gmail.com.
Funding
Personal funding.
Provenance and peer review
Not commissioned, externally peer reviewed.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Papparella A., Nino F., Coppola S., Noviello C., Paciello O., Papparella S. Peritoneal morphological changes due to pneumoperitoneum: the effect of intra-abdominal pressure. Eur. J. Pediatr. Surg. 2014;24(4):322–327. doi: 10.1016/j.ijsu.2015.06.071. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro L., Holste J.L., Muench T., diZerega G. Rapid reperitonealization and wound healing in a preclinical model of abdominal trauma repair with a composite mesh. Int. J. Surg. 2015;22:86–91. doi: 10.1016/j.ijsu.2015.06.071. [DOI] [PubMed] [Google Scholar]
- 3.Okabayashi K., Ashrafian H., Zacharakis E., Hasegawa H., Kitagawa Y., Athanasiou T., Darzi A. Adhesions after abdominal surgery: a systematic review of the incidence, distribution and severity. Surg. Today. 2014;44(3):405–420. doi: 10.1007/s00595-013-0591-8. [DOI] [PubMed] [Google Scholar]
- 4.Galli S.J., Tsai M. Mast cells in allergy and infection: versatile effector and regulatory cells in innate and adaptive immunity. Eur. J. Immunol. 2010;40(7):1843–1851. doi: 10.1002/eji.201040559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sammour T., Kahokehr A., Soop M., Hill A.G. Peritoneal damage: the inflammatory response and clinical implications of the neuro-immuno-humoral axis. World J. Surg. 2010;34(4):704–720. doi: 10.1007/s00268-009-0382-y. [DOI] [PubMed] [Google Scholar]
- 6.Poerwosusanta H., Gunadi Noor Z., Oktaviyanti I.K., Mintaroem K., Pardjianto B., Widodo M.A., Widjajanto E. The effect of laparoscopy on mast cell degranulation and mesothelium thickness in rats. BMC Surg. 2020 Dec;20(1):1–10. doi: 10.1186/s12893-020-00775-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pejler G., Rönnberg E., Waern I., Wernersson S. Mast cell proteases: multifaceted regulators of inflammatory disease. Blood. 2010;115(24):4981–4990. doi: 10.1182/blood-2010-01-257287. [DOI] [PubMed] [Google Scholar]
- 8.Ferdowsian H., Johnson L.S.M., Johnson J., Fenton A., Shriver A., Gluck J. A belmont report for animals? Camb. Q. Healthc. Ethics. 2020;29(1):19–37. doi: 10.1017/S0963180119000732. [DOI] [PubMed] [Google Scholar]
- 9.Lu Sneddon, Halsey L.G., Bury N.R. Considering aspects of the 3Rs principles within experimental animal biology. J. Exp. Biol. 2017;220(17):3007–3016. doi: 10.1242/jeb.147058. [DOI] [PubMed] [Google Scholar]
- 10.Federer W.T. Randomization and sample size in experimentation. Food Drug Adm Stat Semin. 1966;1–14 [Google Scholar]
- 11.Ishida Y., Hino S., Morita T., Ikeda S., Nishimura N. Hydrogen produced in rat colon improves in vivo redox balance due to induced regeneration of α-tocopherol. Br. J. Nutr. 2019;123(5):537–544. doi: 10.1017/S0007114519003118. [DOI] [PubMed] [Google Scholar]
- 12.Kilkenny C., Browne W.J., Cuthill I.C., Emerson M., Altman D.G. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010 Jun 29;8(6) doi: 10.1371/journal.pbio.1000412. https://www.nc3rs.org.uk/arrive-guidelines [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leary S., Underwood W., Anthony R., Cartner S., Grandin T., Greenacre C. AVMA guidelines for the euthanasia of animals: 2020 edition [internet] 2020. https://www.avma.org/sites/default/files/2020-02/Guidelines-on-Euthanasia-2020.pdf 82-91p. Available from:
- 14.Ozawa A., Sakaue M. New decolorization method produces more information from tissue sections stained with hematoxylin and eosin stain and masson-trichrome stain. Ann. Anat. 2020;227:151431. doi: 10.1016/j.aanat.2019.151431. [DOI] [PubMed] [Google Scholar]
- 15.Wu J.J., Cao C.M., Meng T.T., Zhang Y., Xu S.L., Feng S Bin. Induction of immune responses and allergic reactions in piglets by injecting glycinin. Ital. J. Anim. Sci. 2016;15(1):166–173. doi: 10.1080/1828051X.2016.1144488. [DOI] [Google Scholar]
- 16.Zhu Y., Pan W.H., Wang X.R., Liu Y., Chen M., Xu X.G. Tryptase and protease-activated receptor-2 stimulate scratching behavior in a murine model of ovalbumin-induced atopic-like dermatitis. Int. Immunopharm. 2015;28(1):507–512. doi: 10.1016/j.intimp.2015.04.047. [DOI] [PubMed] [Google Scholar]
- 17.Berdún S., Bombuy E., Estrada O., Mans E., Rychter J., Clavé P. Peritoneal mast cell degranulation and gastrointestinal recovery in patients undergoing colorectal surgery. Neuro Gastroenterol. Motil. 2015;27(6):764–774. doi: 10.1111/nmo.12525. [DOI] [PubMed] [Google Scholar]
- 18.Stoikes N.F.N., Scott J.R., Badhwar A., Deeken C.R., Voeller G.R. Characterization of host response, resorption, and strength properties, and performance in the presence of bacteria for fully absorbable biomaterials for soft tissue repair. Hernia. 2017;21(5):771–782. doi: 10.1007/s10029-017-1726-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rueden C.T., Schindelin J., Hiner M.C., DeZonia B.E., Walter A.E., Arena E.T. ImageJ 2: ImageJ for the next generation of scientific image data. BMC Bioinf. 2017;18(1):1–36. doi: 10.1186/s12859-017-1934-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sammour T, Mittal A, Loveday BP, Kahokehr A, Phillips AR, Windsor JA, Hill AG Systematic review of oxidative stress associated with pneumoperitoneum. Br. J. Surg. 96(8):836–850. 10.1002/bjs.6651. [DOI] [PubMed]
- 21.Patel S., Yadav A. Prevention of adhesion in laparoscopic gynaecological surgery. Int J Reprod Contracept, Obstet Gynecol. 2016;5(12):4099–4105. doi: 10.18203/2320-1770.ijrcog20164311. [DOI] [Google Scholar]
- 22.Mueller C. Danger-associated molecular patterns and inflammatory bowel disease: is there a connection? Dig. Dis. 2013;30(SUPPL. 3):40–46. doi: 10.1159/000342600. [DOI] [PubMed] [Google Scholar]
- 23.Baldwin A.G., Brough D., Freeman S. Inhibiting the inflammasome: a chemical perspective. J. Med. Chem. 2016;59(5):1691–1710. doi: 10.1021/acs.jmedchem.5b01091. [DOI] [PubMed] [Google Scholar]
- 24.Kalesnikoff J., Galli S.J. New developments in mast cell biology. Nat. Immunol. 2008;9(11):1215–1223. doi: 10.1038/ni.f.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Souza D.A., Santana A.C., Da Silva E.Z.M., Oliver C., Jamur M.C. The role of mast cell specific chymases and tryptases in tumor angiogenesis. BioMed Res. Int. 2015;2015 doi: 10.1155/2015/14235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knight P.A., Wright S.H., Lawrence C.E., Paterson Y.Y.W., Miller H.R.P. Delayed Expulsion of the nematode Trichinella spiralis in mice lacking the mucosal mast cell–specific granule chymase, mouse mast cell protease-1. J. Exp. Med. 2000;192(12):1849–1856. doi: 10.1084/jem.192.12.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dawicki W., Marshall J.S. New and emerging roles for mast cells in host defence. Curr. Opin. Immunol. 2007;19(1):31–38. doi: 10.1016/j.coi.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Horny H.P., Sotlar K., Valent P., Hartmann K. Mastocytosis: a disease of the hematopoietic stem cell. Deutsches Ärzteblatt International. 2008 Oct;105(40):686. doi: 10.3238/arztebl.2008.0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poerwosusanta H, Utomo DH, Noor Z, Oktaviyanti IK, Mintaroem K, Pardjianto B, Widodo MA, Widjajanto E. Eleutherine americana Merr. extract regulates mitochondrial calcium homeostasis in intra-abdominal adhesion: a computational study. DIT 11(3):526–530.
- 30.Mungai P.T., Waypa G.B., Jairaman A., Prakriya M., Dokic D., Ball M.K. Hypoxia triggers AMPK activation through reactive oxygen species-mediated activation of calcium release-activated calcium channels. Mol. Cell Biol. 2011;31(17):3531–3545. doi: 10.1128/mcb.05124-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krystel-Whittemore M., Dileepan K.N., Wood J.G. Mast cell: a multi-functional master cell. Front. Immunol. 2016;6(JAN):620. doi: 10.3389/fimmu.2015.00620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chelombitko M.A., Fedorov A.V., Ilyinskaya O.P., Zinovkin R.A., Chernyak B.V. Role of reactive oxygen species in mast cell degranulation. Biochemistry (Mosc.) 2016 Dec 1;81(12):1564–1577. doi: 10.1134/S000629791612018X. [DOI] [PubMed] [Google Scholar]
- 33.Bischoff S.C. Role of mast cells in allergic and non-allergic immune responses: comparison of human and murine data. Nat. Rev. Immunol. 2007;7(2):93–104. doi: 10.1038/nri2018. [DOI] [PubMed] [Google Scholar]
- 34.Stokes L., MacKenzie A.B., Sluyter R. Editorial: roles of ion channels in immune cells. Front. Immunol. 2016;7(February):1–2. doi: 10.3389/fimmu.2016.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yao W., Yang H., Yin N., Ding G. Mast cell-nerve cell interaction at acupoint: modeling mechanotransduction pathway induced by acupuncture. Int. J. Biol. Sci. 2014;10(5):511–519. doi: 10.7150/ijbs.8631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi X.M., Zheng Y.F., Liu Z.R., Yang W.Z. A model of calcium signaling and degranulation dynamics induced by laser irradiation in mast cells. Chin. Sci. Bull. 2008;53(15):2315–2325. doi: 10.1007/s11434-008-0255-z. [DOI] [Google Scholar]
- 37.Maciver A.H., McCall M., James Shapiro A.M. Intra-abdominal adhesions: cellular mechanisms and strategies for prevention. Int. J. Surg. 2011;9(8):589–594. doi: 10.1016/j.ijsu.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 38.Bankova L.G., Lezcano C., Pejler G., Stevens R.L., Murphy G.F., Austen K.F. Mouse mast cell proteases 4 and 5 mediate epidermal injury through disruption of tight junctions. J Immunol [Internet] 2014;192(6):2812–2820. doi: 10.4049/jimmunol.1301794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bi J., Zhang S., Du Z., Zhang J., Deng Y., Liu C. Peripheral serotonin regulates postoperative intra-abdominal adhesion formation in mice. Sci. Rep. 2017;7(1):1–12. doi: 10.1038/s41598-017-10582-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yung S., Chan T.M. Pathophysiological changes to the peritoneal membrane during PD-related peritonitis: the role of mesothelial cells. Mediat. Inflamm. 2012;2012 doi: 10.1155/2012/484167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mao M., Alavi M.V., Labelle-Dumais C., Gould D.B. Type IV collagens and basement membrane diseases: cell biology and pathogenic mechanisms. Curr. Top. Membr. 2015 Jan 1;76:61–116. doi: 10.1016/bs.ctm.2015.09.002. Academic Press. [DOI] [PubMed] [Google Scholar]
- 42.Groschwitz K.R., Wu D., Osterfeld H., Ahrens R., Hogan S.P. Chymase-mediated intestinal epithelial permeability is regulated by a protease-activating receptor/matrix metalloproteinase-2-dependent mechanism. Am. J. Physiol. Gastrointest. Liver Physiol. 2013 Mar 1;304(5):G479–G489. doi: 10.1152/ajpgi.00186.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spencer V.A., Xu R., Bissell M.J. Gene expression in the third dimension: the ECM-nucleus connection. J. Mammary Gland Biol. Neoplasia. 2010;15(1):65–71. doi: 10.1007/s10911-010-9163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huleihel L., Hussey G.S., Naranjo J.D., Zhang L., Dziki J.L., Turner N.J. Matrix-bound nanovesicles within ECM bioscaffolds. Sci Adv. 2016;2(6) doi: 10.1126/sciadv.1600502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hermanowicz A., Debek W., Oksiuta M., Matuszczak E., Chyczewski L., Dzienis-Koronkiewicz E. Mast cells in peritoneal fluid in rats with experimentally induced peritoneal adhesions. Folia Histochem. Cytobiol. 2010;48(1):153–156. doi: 10.2478/v10042-010-0018-y. [DOI] [PubMed] [Google Scholar]
- 46.Arung W., Meurisse M., Detry O. Pathophysiology and prevention of postoperative peritoneal adhesions. World J. Gastroenterol. 2011;17(41):4545–4553. doi: 10.3748/wjg.v17.i41.4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.