Abstract
Background
Dual mobility (DM) constructs effectively reduce the risk of dislocation in revision and high risk primary total hip arthroplasty. However, modular DM designs require the use of a cobalt-chrome liner against a titanium cup which may induce corrosion, metal ions release, and associated biologic response. The purpose of this systematic review study was to collect all reported cases of serum metal ions after DM in the literature and ask the following questions: 1) what is the overall rate of significantly elevated Cobalt and Chromium metal ions and how do these levels change over time? 2) Does femoral head material composition influence serum metal ion levels? and 3) were there any atypical lymphocytic associated lesions after modular DM that required revision surgery?
Methods
A systematic review was performed according to PRISMA guidelines. In addition to patient demographics, information specific to the performance of the DM implant were recorded including: cobalt and chromium serum ion levels and all reported timepoints, the material composition of the femoral head, all revision and reoperations and any failure related to corrosion of the DM bearing. A significant elevation in cobalt or chromium was defined as >1.0 or >1.6 mcg/L.
Results
248 modular DM THAs were analyzed. The cumulative mean cobalt and chromium levels for all included studies was 0.47mcg/L and 0.53mcg/L, respectively. At final follow-up 13 patients (5.2%) had elevated cobalt ion levels and 4 patients (1.6%) had elevated chromium ion levels. Femoral head material composition trended towards but did not significantly increase serum ion levels. Ceramic heads had elevated cobalt and chromium ions in 4/135 (3%) of patients compared to metal heads which had elevated cobalt ions in 9/113 (8%) and elevated chromium ions in 0/113 (0%), (p = 0.09). There were no reoperations or revisions for metal related reactions at final follow-up (mean 27.4 months).
Conclusion
In this systematic review including 248 modular DM THAs, elevated serum cobalt ions were present in 5.2% of patients at a mean follow-up of 27.4 months. While a trend towards increased Cobalt serum ions with the use of cobalt chrome femoral heads, femoral head composition was not significantly associated with increased serum metal ion levels. At final follow-up, metal ion levels appear to decrease in the majority of patients between 1 and 2 years and no patient was revised for metal ion related complications. Continued serum metal ion surveillance is recommended to ensure the safety of DM constructs in THA with longer term follow-up.
Keywords: Total hip arthroplasty, Modular dual mobility, Serum metal ions, Cobalt, Chromium, Dislocation
1. Introduction
Total hip arthroplasty (THA) is a successful surgical treatment for end-stage arthritis in the hip and is associated with high rates of patient satisfaction and improvement in quality-of-life and overall physical function.1 However, complications do occur and prosthetic hip dislocation remains one of the most common complications requiring additional treatment such closed reduction under anesthesia and revision surgery.2,3 The dual mobility (DM) articulation was developed in Europe over 20 years ago and was introduced to the North America market in 2010. Several reports have found that DM articulations decrease dislocation rates in both primary and revision surgeries, and may be particularly effective in higher risk patients (spine fusion, abductor compromise, neurologic conditions).4, 5, 6, 7, 8 The DM design reduces dislocation risk through several design features: the large outer polyethylene bearing increased jump-distance secondary to the larger outer polyethylene bearing, and the increased the femoral head-neck ratio leading to increased range of motion prior to impingement.6,9
The two DM construct options currently available are either a monoblock acetabular component or a modular device with a hemispherical cup and modular cobalt chromium (CoCr) liner.10 The modular junction between the CoCr liner and Titanium shell has raised concerns over the corrosion potential at the dissimilar metal interface.11 Cobalt and chromium ions may be generated from bearing articulations or modular junctions in THA: the articular surface, modular neck-stem junction, and the modular head-neck junction (trunnionosis) have all been associated with adverse local tissue reactions (ALTR) requiring revision surgery.12, 13, 14, 15 In addition, as modifications continue to be made in both the design of the acetabular component and new DM systems are released to market, continued serum metal ion surveillance is warranted to ensure safety of this design. As DM construct usage continues to increase in the United States and around the world, it is imperative that we continue to evaluate the literature on reported serum metal ion levels after DM THA.
Several studies have reported on postoperative DM serum metal ion levels after DM THA. However, most of these reports contain small numbers of patients making it difficult to make any conclusions on overall DM performance. For this reason, we believe a systematic review collecting all DM cases with reported postoperative serum metal ion levels was warranted. The purpose of this study to perform a systematic review of all modular DM cases in the literature with reported serum metal ion levels and answer the following 3 questions: 1) to determine the overall rate of elevated Cobalt and Chromium serum metal ion levels; 2) to evaluate whether femoral head composition (cobalt-chromium and ceramic) influenced serum metal ion levels, and 3) to determine the number of revisions performed for adverse local tissue reactions to metal debris.
2. Methods
The present systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.16
2.1. Search criteria
The US National Library of Medicine (PubMed/MEDLINE) and the Cochrane Database of Systematic Reviews were queried for publications from January 2000 to February 2020 by utilizing the following keywords, relevant to modular dual mobility implants: Search terms included (“Dual Mobility”) AND (“Metal” OR “Ion”).
2.2. Inclusion and exclusion criteria
The inclusion criteria included: (1) studies describing human subjects of any age or gender; (2) use of a modular DM in either primary or revision THA; (3) studies reporting on metal ion levels in patients following implantation of a modular DM construct; (4) studies that also include postoperative clinical outcome measures; (5) studies with a minimum of 12 months follow-up. The exclusion criteria included: (1) review articles; (2) case studies; (3) studies examining the same patient group as another, previously published study; (4) non-English language publications. Articles meeting these criteria were thoroughly evaluated and the reference lists for the respective studies were examined to screen for additional articles that fit the aforementioned criteria for further analysis.
2.3. Data collection
Studies meeting inclusion criteria were retrieved and independently evaluated by two of the authors (A.K.S. and I.G.). Both reviewers discussed any disparities in study evaluation for those that were included or excluded. The methodological quality of each study and the different types of detected bias were assessed independently by each reviewer with the use of modified Coleman methodology score.17 The titles, authors, study design, year of publication, number of patients (and males/females), BMI, age, cases with modular DM implants, cases with fixed bearing implants, metal head usage, ceramic head usage, cobalt and chromium ion levels, follow-up time, and clinical outcome scores were all collected and summated during review of the data in the articles. Each paper utilized different cutoffs and reference ranges for what was considered an “elevated” metal ion level. According to current literature, metal ion levels ≥ 1mcg/L to 1.6 mcg/mL are considered clinically significantly elevated, depending on the study. Therefore, we used this threshold to define significantly elevated ion levels in our analysis.18, 19, 20
2.4. Statistics
Values are reported as means with ranges. Fischer exact square tests were utilized to analyze the difference in the rate of cobalt level elevation in patients with ceramic and CoCr femoral heads.
3. Results
3.1. Study selection
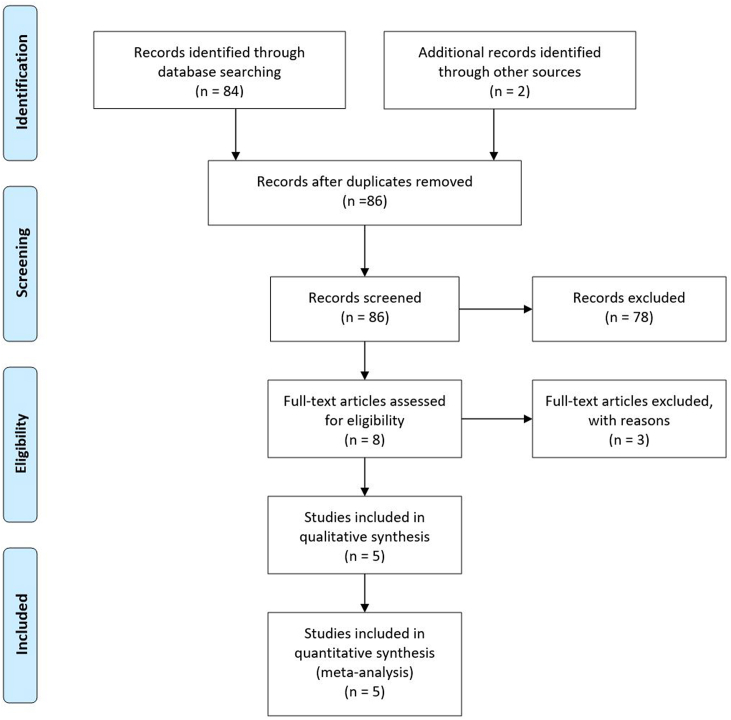
The initial search yielded 84 articles (Fig. 1). Inclusion and exclusion criteria were applied after the removal of duplicate articles resulting in 5 studies available for analysis (Table 1). Serum cobalt and chromium metal ion levels were reported in all included studies and, in addition, one study reported serum titanium levels and two reported serum cytokine levels (Table 2). Four of the studies reported on the use of modular DM in primary THA while one paper,21 included both primary and revision THA cases. Disagreements in evaluations between reviewers were discussed, and studies were included if consensus was achieved. The total mean modified Coleman score of the review was 47 (range: 43–54).
Fig. 1.
Flow chart diagram.
Table 1.
Studies that met inclusion criteria.
| Study ID | Journal | Year Published | Study Type | CEBM Evidence Level |
|---|---|---|---|---|
| Matsen Ko et al., 2016 | The Journal of Arthroplasty | 2016 | Retrospective | 3 |
| Nam et al., 2019 | The Journal of Arthroplasty | 2019 | Prospective | 2 |
| Chalmers et al., 2019 | The Bone & Joint Journal | 2019 | Prospective | 2 |
| Markel et al., 2019 | HIP International | 2019 | Retrospective | 3 |
| Markel et al., 2019 | The Bone & Joint Journal | 2019 | Prospective | 2 |
Table 2.
Implant specifications and recorded ion and outcome measurements.
| Study ID | Femoral Head Specifications | Serum Concentrations Recorded | Patient Recorded Outcomes |
|---|---|---|---|
| Matsen Ko et al., 2016 | Ceramic femoral head | Cobalt, chromium | Oxford Score |
| Cobalt chromium femoral head | |||
| Nam et al., 2019 | Cobalt alloy | Cobalt, chromium, titanium | UCLA Activity Score |
| Ceramic femoral head | Harris Hip Score | ||
| Chalmers et al., 2019 | Ceramic femoral head | Cobalt, chromium | Harris Hip Score |
| Markel et al., 2019 (HIP International) | Ceramic femoral head | Cobalt, chromium, CD14+ leukocyte, CD16+ leukocyte, CD3+ T lymphocytes, CD19+ B lymphocytes, CD4+ Helper T-cells, CD45 + RA memory vs. naïve T-cells | Harris Hip Score |
| 12-Item Short-Form Health Survey | |||
| Western Ontario and McMaster Universities Arthritis Index | |||
| Visual Analogue Scale for pain | |||
| Markel et al., 2019 (Bone and Joint Journal) | Ceramic femoral head | Cobalt, chromium, IL-2, IL-4, IL-5, IL-10, tumor necrosis factor, interferon gamma, CD3+ T lymphocytes expressing CD4 and/or CD45RA, CD19-expressing B lymphocytes, and monocytes expressing CD14 and/or CD16 | Harris Hip Score |
| 12-Item Short-Form Health Survey | |||
| Western Ontario and McMaster Universities Arthritis Index | |||
| Visual Analogue Scale for pain |
3.2. Patient demographics
Two-hundred and forty eight modular DM THA patients were collected among the 5 studies analyzed10,19,21, 22, 23 (Table 3). The mean sample size was 50 cases per study (range: 10–100 patients), a mean age of 60 years (range: 53–63 years), 67 female and 64 male patients (from two studies we cannot extract information for the gender), and mean body mass index (BMI) was 29.1 kg/m2 (range: 27.9–31.1 kg/m2). The average follow-up of the studies was 29.5 months (range: 24–48 months). There were 238 primary THAs (96.0%) and 10 revision THAs (4.0%). In one paper there were 7 patients who underwent revision operation to DM construct due to adverse local tissue reaction and they were excluded from the study.
Table 3.
Patient demographics.
| Study ID | Patients | Mean BMI (kg/m2) | Mean Age (years) | Male | Female | Primary THA | Revision THA | Mean Follow-up (Months) |
|---|---|---|---|---|---|---|---|---|
| Matsen Ko et al., 2016 | N = 100 | N/A | N/A | N/A | N/A | 100 | 0 | 27.6 |
| Nam et al., 2019 | N = 43 | 27.9 | 52.6 | 30 | 13 | 43 | 0 | 24 |
| Chalmers et al., 2019 | N = 17 | N/A | N/A | N/A | N/A | 7 | 10 | 48 |
| Markel et al., 2019 (HIP International) | N = 49 | 31.1 | 62.4 | 19 | 30 | 49 | 0 | 24 |
| Markel et al., 2019 (Bone and Joint Journal) | N = 39 | 28.4 | 61.7 | 15 | 24 | 39 | 0 | 24 |
3.3. Implants
In all patients a modular dual mobility (MDM) acetabular component was used (Stryker, Mahwah, New Jersey). The inner femoral head diameter size was 22 mm in 101 out of the 248 patients (41%) and 28 mm in 147 (59%).
3.4. Serum metal ions levels and changes in ion levels over time
248 primary and revision THAs utilizing a modular DM construct (Table 4) were analyzed. The mean cobalt ion levels ranged from 0.16 mcg/L to 0.70 mcg/L, with a cumulative mean of 0.47 mcg/L (Table 4). The individual cobalt ion levels ranged from as low as 0 to as high as 7 mcg/L, the highest of which was reported by Matsen Ko et al.10 The cumulative mean chromium level was 0.53 mcg/L, with a range of 0.14 mcg/L to 0.76 mcg/L (Table 4). The individual chromium ion levels ranged from as low as 0 to as high as 2.1 mcg/L.
Table 4.
Cases with modular dual mobility prostheses and distribution of metal and ceramic femoral heads as well as mean postoperative cobalt and chromium ion levels.
| Study ID | Cases with modular dual mobility prosthesis | Cobalt-chromium heads | Ceramic heads | Mean cobalt ion levels, mcg/L (range) | Mean chromium ion levels, mcg/L (range) |
|---|---|---|---|---|---|
| Matsen Ko et al., 2016 | 100 | 99 | 1 | 0.70 | 0.60 |
| Nam et al., 2019 | 43 | 14 | 29 | 0.16 | 0.14 |
| Chalmers et al., 2019 | 17 | N/A | 17 | 0.30 | 0.76 |
| Markel et al., 2019 (HIP International) | 49 | N/A | 49 | 0.57 | 0.50 |
| Markel et al., 2019 (Bone and Joint Journal) | 39 | N/A | 39 | 0.63 | 0.63 |
| Overall | 248 | 113 | 135 | 0.47 | 0.53 |
Of the 248 cases with a modular DM construct, 17 (6.9%) had significantly elevated ions (Co or Cr > 1mcg/mL). Matsen Ko et al.10 report the highest incidence, with 9 of 100 (9%) primary THA DM cases demonstrating significantly elevated metal ion levels. Chalmers et al.21 as well as Nam et al.,22 on the other hand, had no cases with significantly elevated ion levels following primary and revision THA. Markel et al.,23 report three cases out of 49 (6.1%) in one study whereas in the other one they report 5 cases out of 39 (12.8%)19 that are significantly elevated.
With continued follow-up there was a decrease in the number of cases with elevated serum metal ions. At 24 months follow-up, the number of cases with elevated ions had decreased to 8/88 (9.1%) (4 elevated cobalt ions and 4 elevated chromium ions) from 17% metal ion elevation the first year postoperatively. The follow-up time intervals varied in each study, with some studies reporting multiple follow-ups and corresponding ion levels. The values reflected in Table 4 represent the ion levels at the longest follow-up timepoint recorded for each study. Markel et al.19,23 and Nam et al.22 each had the shortest mean follow-up of 24 months. Chalmers et al.21 report a mean follow-up of 4 years (ranging from 2 to 7 years).
Three studies19,22,23 presented data on metal ion levels and different time intervals, Two studies19,23 which included 88 patients presented elevated ions at different time intervals and followed these patients over time (Table 5). Within the first year postoperatively, 15/88 cases (17%) had elevated cobalt and/or chromium ions (11 elevated cobalt ions and 4 elevated chromium ions).
Table 5.
Mean serum cobalt and chromium ion levels in patients with modular DM constructs at different time intervals.
| Study ID | ≤1 year |
24 months |
≤1 year |
24 months |
||
|---|---|---|---|---|---|---|
| Cobalt ion levels (mcg/L) |
Cobalt ion levels (mcg/L) |
Cobalt ion levels (mcg/L) | Chromium ion levels (mcg/L) |
Chromium ion levels (mcg/L) |
Chromium ion levels (mcg/L) | |
| 3 m | 12 m | 3 m | 12 m | |||
| Markel et al., 2019 (HIP International) | – | 0.63 (0.5–1.7) | 0.57 (0.5–1.2) | – | 0.53 (0.5–1.4) | 0.50 (0.5) |
| Markel et al., 2019 (Bone and Joint Journal) | 0.85 (SD 0.97) | 0.64 (SD 0.28) | 0.63 (SD 0.36) | 0.58 (SD 0.26) | 0.56 (SD 0.17) | 0.63 (SD 0.38) |
| Nam et al., 2019 | – | 0.30 (0.04–2.10)a | 0.16 (0.04–0.94)a | – | 0.17 (0.07–1.20) | 0.14 (0.07–0.26) |
| Mean Ion Levels | 0.85 | 0.52 | 0.45 | 0.58 | 0.42 | 0.42 |
Statistically significant.
3.5. Femoral head composition and serum metal ion levels
Femoral head composition was cobalt-chromium in 113 (46%) and ceramic in 135 (54%). There were 13 cases of elevated cobalt levels (5.2%) and 4 cases of significantly elevated chromium levels (1.6%) at the last follow-up. Three studies only had ceramic femoral heads while two reported serum metal ions for both CoCr and ceramic femoral heads (Table 4). For all the ceramic femoral heads reported, 8/135 patients (5.9%) had elevated cobalt and/or chromium metal ion (Table 6). In contrast, for the 113 cobalt-chrome femoral heads, 9 patients (8.0%) had elevated cobalt and/or chromium metal ion levels above the reference range (Table 7). More specifically, cobalt ions levels were elevated in 3.0% (4/135) of patients with ceramic heads elevated in 8.0% (9/113) of patients with metal heads but this was not statistically significant (p = 0.09).
Table 6.
Serum metal ion levels in patients with ceramic heads.
| Elevated Co ions | Elevated Cr ions | |
|---|---|---|
| Markel et al., 2019 (HIP International) | 2/49 | 1/49 |
| (4.1%) | (2.0%) | |
| Markel et al., 2019 (Bone and Joint Journal) | 2/39 | 3/39 |
| (5.1%) | (7.7%) | |
| Chalmers et al. | 0/17 | 0/17 (0%) |
| (0%) | ||
| Matsen Ko et al. | 0/1 | 0/1 |
| (0%) | (0%) | |
| Nam et al., 2019 | 0/29 | 0/29 |
| (0%) | (0%) | |
| Total | 4/135 | 4/135 |
| (3.0%) | (3.0%) |
Table 7.
Serum metal ion levels in patients with CoCr femoral heads.
| Elevated Co ions | Elevated Cr ions | |
|---|---|---|
| Markel et al., 2019 (HIP International) | – | – |
| Markel et al., 2019 (Bone and Joint Journal) | – | – |
| Chalmers et al. | – | – |
| Matsen Ko et al. | 9/99 | 0/99 |
| (9.1%) | ||
| Nam et al., 2019 | 0/14 | 0/14 |
| Total | 9/113 | 0/113 |
| (8.0%) | (0%) |
4. Discussion
The utilization of DM articulations is increasing in the United States as it has been shown to be effective in reducing the risk of dislocation in high risk primary and revision THA.6 Modular DM constructs, rather than monoblock DM constructs that are popular in Europe, were developed to allow continued use of titanium acetabular components and allow for supplementary screw fixation.24 However, due to recent experiences with large head metal on metal articulations and dual modular neck prostheses, there is concern the modular cobalt-chrome interface may lead to corrosion and subsequent ALTR. The studies that analyze serum metal ion levels in patients with modular DM constructs consist of small case series. As such, aggregate analysis of the data in a systematic review approach was the goal of this study in order to provide the most up to date information on serum ion levels after implantation of a modular DM articulation.
-
1)
What is the overall rate of significantly elevated Cobalt and Chromium metal ions and how do these levels change over time?
In this systematic review of 248 THAs with modular DM constructs, the mean Co serum level was 0.47 mcg/L and Cr was 0.53 mcg/L at a mean follow-up of 30 months. In total, 6.9% (17/248) patients had elevated cobalt, chromium, or both above the reference range. The total number of patients with elevated cobalt serum ions was 13/248 patients (5.2%). In multiple studies,19,22,23 the elevated serum ion levels were within the first few months postoperatively and decreased with longer term follow-up. The elevation in metal ions in the early postoperative period suggests fretting corrosion was unlikely.11,20 Alternative explanations for early ion elevation could be secondary to immediate metal ion release during impaction of the modular liner into the titanium shell,19 a sensitivity of component metals to the surrounding tissue environment (pH and temperature), or due to a “settling-in” period for the modular interface and poly-liner articulation.
Potential causes for the elevation in cobalt levels in 5% of patients is likely multifactorial. Corrosion chamber studies have analyzed if voltage changes are observed with mal-seating of the cobalt chrome liner occurs within the titanium shell.25 One such study reported an earlier fretting current onset at lower peak loads with liner mal-seating compared to well-seated liners in an experimental design. A retrospective clinical study 567 THAs found the rate of malseating to be 5.6% in this DM design.26 At last follow-up there was no correlation reported between liner malseating and risk of revision. Serum metal ion levels were not reported so no correlation between liner malseating and elevated metal ion levels can be made. Liner malseating was not reported in this systematic review so no further analysis on this relationship could be performed.
-
2)
Does femoral head material composition influence serum metal ion levels?
When categorized based on femoral head material composition, the cobalt ion levels were elevated in 3.0% (4/135) of patients with ceramic heads and 8.0% (9/113) of patients with metal heads but this was not statistically significant (p = 0.09). The safe level for serum metal ions in patients with DM bearing is unknown. Extrapolating metal ion data from other metal on polyethylene constructs that were concerning from trunnionosis at the head neck junction was a serum cobalt >1 mcg/L.27 Whether metal ion release from corrosion between the metal DM liner and titanium shell would produce metal ions of the same size and shape to trigger a similar ALTR type response with serum Co ions >1mcg/L is also unknown.
In studies focusing on head-neck taper corrosion, the reported pathognomonic Co to Cr ratio is usually greater than 2:1.28 This differential elevation is due to the preferential deposition of chromium at the head-neck junction in the form of chromium orthophosphate, while the released cobalt ions are systematically absorbed.29 In the present review, there were 113 cases with metal heads with an estimated mean Co:Cr ratio of 1.05 which suggests that metal ion elevation is not secondary to trunnion corrosion. Since we did not find a femoral head composition to have a significant effect on serum metal ion levels (p = 0.09) is it unlikely that the cobalt elevation is secondary to trunnion corrosion at the head-neck interface.
-
3)
Were there any atypical lymphocytic associated lesions after modular DM that required revision surgery?
At last reported follow-up in this systematic review of the literature, no patient out of the 248 DM THAs developed an ALTR that required revision surgery. However, even though no revisions were reported, cross-sectional imaging has demonstrated the presence of mild cytic ALTR lesions of unknown clinical significance but suggest continued surveillance of DM bearings is warranted. Matsen Ko et al.10 monitored underlying ALTR in the DM THAs who also underwent hip metal artifact reduction sequence (MARS) MRI for patients who presented with pain and elevated serum cobalt levels. Of the nine patients with an elevated serum cobalt level, four received MARS MRI. Two patients had serum cobalt ion levels greater than 4.5 mcg/L, but neither patient had findings consistent with ALTR. Of the other two imaged, one patient with a serum cobalt level of 2.3 mcg/L reported symptoms and was found to have low grade ALTR on imaging. The other patient had a serum cobalt level ranging from 2 to 3.1 mcg/L over a 6-month interval and, despite being asymptomatic, had MARS MRI findings indicating ALTR as well. These cases demonstrate the lack of direct correlation between cobalt ion levels and presence of ALTR on cross sectional imaging.
There are several limitations in this systematic review. First of all, three of the included studies19,21,22 were prospective whereas the other two studies included10,23 were retrospective. Additionally, three of the five studies included patients who received only ceramic femoral heads, excluding those who received metal heads19,21,23 and the other two studies10,22 included patients who received implants with either ceramic or metal femoral heads. Since there is no clear consensus regarding toxic levels of serum metal ions, the threshold above which the ion levels should be considered elevated was different in each study based on the reference range of the laboratory utilized. We defined an elevated level of 1 mcg/L or 1.6 mcg/L depending on the definition of each study in order to more uniformly analyze the metal ion levels across studies. There is also variability in the design of each study with three of them19,22,23 providing data in different time intervals while the other two10,21 did not. Moreover, the patients’ follow-up is approximately two years in 5 of the 6 included studies,10,19,22,23 with only one study21 having a follow up of more than two years.
5. Conclusion
Modular DM constructs have been shown to reduce the dislocation rate after both complex primary and revision THA. However, the additional cobalt-chrome liner modularity has raised concerns over corrosion at that junction and increased serum metal ion levels. Overall, in this cohort of 248 DM THA patients, the rate of serum Cobalt ion elevation was 5.2% for Cobalt and xx for Chromium. Reassuringly, femoral head composition was not associated with elevated with serum metal ions (p = 0.09) and serum metal ions levels appear to decrease with longer term follow-up. In addition, there were no revisions for adverse reactions to metal debris at final follow-up which supports the safety of these devices at mid-term follow-up.
References
- 1.Learmonth I.D., Young C., Rorabeck C. The operation of the century: total hip replacement. Lancet. 2007;370(9597):1508–1519. doi: 10.1016/S0140-6736(07)60457-7. [DOI] [PubMed] [Google Scholar]
- 2.Bozic K.J., Kurtz S.M., Lau E., Ong K., Vail T.P., Berry D.J. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91(1):128–133. doi: 10.2106/JBJS.H.00155. [DOI] [PubMed] [Google Scholar]
- 3.Fessy M.H., Putman S., Viste A. What are the risk factors for dislocation in primary total hip arthroplasty? A multicenter case-control study of 128 unstable and 438 stable hips. Orthop Traumatol Surg Res OTSR. 2017;103(5):663–668. doi: 10.1016/j.otsr.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 4.Hartzler M.A., Abdel M.P., Sculco P.K., Taunton M.J., Pagnano M.W., Hanssen A.D. Otto aufranc award: dual-mobility constructs in revision THA reduced dislocation, rerevision, and reoperation compared with large femoral heads. Clin Orthop. 2018;476(2):293–301. doi: 10.1007/s11999.0000000000000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdel M.P. Dual-mobility constructs in revision total hip arthroplasties. J Arthroplasty. 2018;33(5):1328–1330. doi: 10.1016/j.arth.2018.01.030. [DOI] [PubMed] [Google Scholar]
- 6.Jones C.W., De Martino I., D'Apolito R., Nocon A.A., Sculco P.K., Sculco T.P. The use of dual-mobility bearings in patients at high risk of dislocation. Bone Jt J. 2019;101-B(1_Supple_A):41–45. doi: 10.1302/0301-620X.101B1.BJJ-2018-0506.R1. [DOI] [PubMed] [Google Scholar]
- 7.Chalmers B.P., Pallante G.D., Taunton M.J., Sierra R.J., Trousdale R.T. Can dislocation of a constrained liner Be salvaged with dual-mobility constructs in revision THA? Clin Orthop. 2018;476(2):305–312. doi: 10.1007/s11999.0000000000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chalmers B.P., Perry K.I., Hanssen A.D., Pagnano M.W., Abdel M.P. Conversion of hip hemiarthroplasty to total hip arthroplasty utilizing a dual-mobility construct compared with large femoral heads. J Arthroplasty. 2017;32(10):3071–3075. doi: 10.1016/j.arth.2017.04.061. [DOI] [PubMed] [Google Scholar]
- 9.Plummer D.R., Christy J.M., Sporer S.M., Paprosky W.G., Della Valle C.J. Dual-mobility articulations for patients at high risk for dislocation. J Arthroplasty. 2016;31(9 Suppl):131–135. doi: 10.1016/j.arth.2016.03.021. [DOI] [PubMed] [Google Scholar]
- 10.Matsen Ko L.J., Pollag K.E., Yoo J.Y., Sharkey P.F. Serum metal ion levels following total hip arthroplasty with modular dual mobility components. J Arthroplasty. 2016;31(1):186–189. doi: 10.1016/j.arth.2015.07.035. [DOI] [PubMed] [Google Scholar]
- 11.Smith R.L., Schwarz E.M. Are biologic treatments a potential approach to wear- and corrosion-related problems? Clin Orthop. 2014;472(12):3740–3746. doi: 10.1007/s11999-014-3765-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steens W., von Foerster G., Katzer A. Severe cobalt poisoning with loss of sight after ceramic-metal pairing in a hip--a case report. Acta Orthop. 2006;77(5):830–832. doi: 10.1080/17453670610013079. [DOI] [PubMed] [Google Scholar]
- 13.Rizzetti M.C., Liberini P., Zarattini G. Loss of sight and sound. Could it be the hip? Lancet Lond Engl. 2009;373(9668):1052. doi: 10.1016/S0140-6736(09)60490-6. [DOI] [PubMed] [Google Scholar]
- 14.Tower S.S. Arthroprosthetic cobaltism: neurological and cardiac manifestations in two patients with metal-on-metal arthroplasty: a case report. J Bone Joint Surg Am. 2010;92(17):2847–2851. doi: 10.2106/JBJS.J.00125. [DOI] [PubMed] [Google Scholar]
- 15.Oldenburg M., Wegner R., Baur X. Severe cobalt intoxication due to prosthesis wear in repeated total hip arthroplasty. J Arthroplasty. 2009;24(5) doi: 10.1016/j.arth.2008.07.017. 825.e15-20. [DOI] [PubMed] [Google Scholar]
- 16.Moher D., Liberati A., Tetzlaff J., Altman D.G., The Prisma Group Preferred reporting Items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coleman B.D., Khan K.M., Maffulli N., Cook J.L., Wark J.D. Studies of surgical outcome after patellar tendinopathy: clinical significance of methodological deficiencies and guidelines for future studies. Victorian Institute of Sport Tendon Study Group. Scand J Med Sci Sports. 2000;10(1):2–11. doi: 10.1034/j.1600-0838.2000.010001002.x. [DOI] [PubMed] [Google Scholar]
- 18.Barlow B.T., Ortiz P.A., Boles J.W., Lee Y.-Y., Padgett D.E., Westrich G.H. What are normal metal ion levels after total hip arthroplasty? A serologic analysis of four bearing surfaces. J Arthroplasty. 2017;32(5):1535–1542. doi: 10.1016/j.arth.2016.11.024. [DOI] [PubMed] [Google Scholar]
- 19.Markel D.C., Bou-Akl T., Rossi M.D., Pizzimenti N., Wu B., Ren W. Blood metal levels, leucocyte profiles, and cytokine profiles in patients with a modular dual-mobility hip prosthesis: early results from a prospective cohort study. Bone Jt J. 2019;101-B(9):1035–1041. doi: 10.1302/0301-620X.101B9.BJJ-2019-0377.R2. [DOI] [PubMed] [Google Scholar]
- 20.Jacobs J.J., Skipor A.K., Patterson L.M. Metal release in patients who have had a primary total hip arthroplasty. A prospective, controlled, longitudinal study. J Bone Joint Surg Am. 1998;80(10):1447–1458. doi: 10.2106/00004623-199810000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Chalmers B.P., Mangold D.G., Hanssen A.D., Pagnano M.W., Trousdale R.T., Abdel M.P. Uniformly low serum cobalt levels after modular dual-mobility total hip arthroplasties with ceramic heads: a prospective study in high-risk patients. Bone Jt J. 2019;101-B(6_Supple_B):57–61. doi: 10.1302/0301-620X.101B6.BJJ-2018-1403.R1. [DOI] [PubMed] [Google Scholar]
- 22.Nam D., Salih R., Nahhas C.R., Barrack R.L., Nunley R.M. Is a modular dual mobility acetabulum a viable option for the young, active total hip arthroplasty patient? Bone Jt J. 2019;101-B(4):365–371. doi: 10.1302/0301-620X.101B4.BJJ-2018-0834.R1. [DOI] [PubMed] [Google Scholar]
- 23.Markel D., Bou-Akl T., Rossi M., Pizzimenti N.M., Wu B., Ren W.-P. Response profiles of circulating leukocytes and metal ions in patients with a modular dual-mobility hip implant. HIP Int. 2019;21 doi: 10.1177/1120700019865530. Published online July. 112070001986553. [DOI] [PubMed] [Google Scholar]
- 24.Huang R.C., Malkani A.L., Harwin S.F. Multicenter evaluation of a modular dual mobility construct for revision total hip arthroplasty. J Arthroplasty. 2019;34(7S):S287–S291. doi: 10.1016/j.arth.2019.03.027. [DOI] [PubMed] [Google Scholar]
- 25.Wright T.M., Wach A., Romero J.A., Padgett D.E. Malseated liners in modular dual mobility constructs demonstrate onset of fretting corrosion at physiologic loads: a simulated corrosion chamber study. Orthop Proc. 2019;101-B(suppl P_12) doi: 10.1302/1358-992X.2019.12.003. 3-3. [DOI] [Google Scholar]
- 26.Padgett D.E., Romero J., Wach A., Wright T.M. Incidence of liner malseating in dual mobility implants. Orthop Proc. 2019;101-B(suppl P_12):2. doi: 10.1302/1358-992X.2019.12.002. [DOI] [Google Scholar]
- 27.Cooper H.J., Della Valle C.J., Berger R.A. Corrosion at the head-neck taper as a cause for adverse local tissue reactions after total hip arthroplasty. J Bone Joint Surg Am. 2012;94(18):1655–1661. doi: 10.2106/jbjs.k.01352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plummer D.R., Berger R.A., Paprosky W.G., Sporer S.M., Jacobs J.J., Della Valle C.J. Diagnosis and management of adverse local tissue reactions secondary to corrosion at the head-neck junction in patients with metal on polyethylene bearings. J Arthroplasty. 2016;31(1):264–268. doi: 10.1016/j.arth.2015.07.039. [DOI] [PubMed] [Google Scholar]
- 29.Urban R.M., Jacobs J.J., Gilbert J.L., Galante J.O. Migration of corrosion products from modular hip prostheses. Particle microanalysis and histopathological findings. J Bone Joint Surg Am. 1994;76(9):1345–1359. doi: 10.2106/00004623-199409000-00009. [DOI] [PubMed] [Google Scholar]