Abstract
Purpose
to evaluate the results of Delta ceramic-on-ceramic (CoC) for total-hip-arthroplasty (THA).
Methods
261 THA using Delta-CoC, retrospectively analyzed. A 36 mm head was used in 189 cases and a 32/40 mm in the others. The series have been compared to a group of 89 THA with Forte-CoC.
Results
The Harris-Hip-Score improved from 49.1 ± 14.3 to 92.0 ± 8.9 (P < 0.001). In the Delta group there were one ceramic fracture and 2 dislocations. Two hips underwent revision. There were one revision in the Forte group for instability and one squeaking hip.
Conclusions
The new ceramic bearings provides a safe bearing for THA, with rare complications.
Keywords: Total hip arthroplasty, Ceramic on ceramic bearing, Delta ceramic
1. Introduction
Total hip arthroplasty (THA) is effective, reliable, and durable in relieving pain and improving function in patients with arthritis of the hip joint.1, 2, 3 However, despite an improvement in the wear properties of polyethylene,4, 5, 6, 7, 8 loosening remained a leading cause of failure in several large clinical series and worldwide registries.9, 10, 11 The generation of wear particles in active patients and the bio-reactivity of these particles after long term remained clinical concerns.11, 12, 13 With an increasing number of younger patients undergoing THA along with expected increases in longevity, there is a need for bearings used in THA to guarantee survivorship beyond the second decade of service.14 Ceramic bearings have been used in THA over the past 3 decades because of some positive characteristics including 1) low wear, 2) wettability, and 3) low bioreactivity.15, 16, 17, 18 Modern alumina on alumina (Forte) ceramic on ceramic (COC) THAs have demonstrated excellent survivorship with low rates of osteolysis and loosening even in the young and active patients.19, 20, 21 However, the risk for fracture and squeaking remained substantial barriers to wide adoption.22, 23, 24 Alumina matrix composite (Delta) ceramics are harder, more scratch and facture resistant compared to its Forte predecessor, but there is a lack of clinical data on whether these material improvements translate to improved clinical outcomes.
Therefore, the purpose of this study is to evaluate 1) the 5 year clinical outcomes and survivorship in a group of patients under COC THA using Delta ceramics, 2) complications including fractures and squeaking, and 3) compare these results to patients with Forte COC THA.
2. Materials and methods
The medical records of 261 consecutive delta on delta ceramic THAs performed between 2007 and 2011 implanted in 256 patients have been retrospectively reviewed. There were 108 men (109 hips) and 148 women (152 hips) with a mean age of 65.3 ± 11.9 years (range: 30–90). The preoperative diagnosis included primary hip osteoarthritis, hip dysplasia, osteonecrosis of the femoral head and other causes (Table 1). All hip replacements were performed using uncemented acetabular and femoral components. In 189 cases (72.4%) a 36 mm femoral head was used, while a smaller or larger head was used in the other hips as reported in Table 1. Patients characteristics and the types of implanted devices are outlined in Table 1. Clinical outcomes were evaluated using the Harris Hip Scores, while serial radiographs were assessed for component position, presence of osteolysis, development of progressive radiolucent lines and component loosening. Phone surveys were performed for patients who could not return for physical follow up. All patient data and complications were recorded including wound drainage, thromboembolic events, infection, dislocations, squeaking, fracture, and revision surgeries. Finally, the results have been compared to a contemporaneous group of 89 patients with Forte COC THA performed at another institution.
Table 1.
Demographic data Reported values are mean, Standard Deviation/SD. N = number.
| Baseline | Follow-up | |
|---|---|---|
| N° of patients | 256 | 210 |
| N° of hip | 261 | 213 |
| Age at surgery, mean ± SD | 65.3 ± 11.9 | 63.1 ± 11.3 |
| Sex (%): | ||
| Male | 108 (42.2%) | 93 (44.3%) |
| Female | 148 (57.8%) | 117 (55.7%) |
| Body Mass Index at surgery (BMI), mean ± SD | 26.5 ± 4.0 | 26.5 ± 3.6 |
| Side (%): | ||
| Right | 134 (51.3%) | 106 (49.8%) |
| Left | 127 (48.7%) | 107 (50.2%) |
| Diagnosis (%): | ||
| Primary coxarthrosis | 195 (74.4%) | |
| Congenital hip dysplasia | 37 (14.2%) | |
| Idiopathic osteonecrosis of the femoral head | 13 (5.0%) | |
| Post-traumatic coxarthrosis | 8 (3.1%) | |
| Post-traumatic necrosis of the femoral head | 2 (0.8%) | |
| Coxarthrosis in rheumatoid arthritis | 1 (0.4%) | |
| Pseudoarthrosis | 1 (0.4%) | |
| Coxa palna secondary to Perthes disease | 1 (0.4%) | |
| Coxarthosis secondary to poliomyelitis | 1 (0.4%) | |
| Coxarthosis secondary to arthrodesis | 1 (0.4%) | |
| Coxarthosis secondary to osteotomy | 1 (0.4%) | |
| Further surgery on the same leg | ||
| Yes | 36 (13.8%) | – |
| No | 225 (86.2%) | – |
| Contralateral THA | ||
| Yes | 60 (23.4%) | – |
| No | 196 (76.6%) | – |
| Hypometria | ||
| Yes | 116 (44.4%) | – |
| No | 145 (55.6%) | – |
2.1. Statistical analysis
Descriptive statistics was used for all demographic data. Continuous variables were presented with average and standard deviation (SD). Categorical variables are presented as frequency and percentages. The post-operative means HHS were compared and percentage improvement was measured. To analyze differences between pre-operative and post-operatively scores or data, T -test was applied to normally distribute continuous outcomes, while Chi-square test was used for categorical outcomes. For all tests, a P value less than 0.05 was considered statistically significant. All statistical analyses were performed using Microsoft Excel (Microsoft, Redmond WA).
3. Results
A total of 256 patients were involved in the study with an overall mean age of 65.3 ± 11.9 years (range: 30–90), for a total of 261 hips. Among these, 22 (8.6%) patients were untraceable while 24 patients (9.4%) died during the follow up. Consequently, 210 patients with an overall mean age of 63.1 ± 11.3 years (range: 30–85) were included in post-operative HHS evaluation, for a total of 213 hips (213 THAs). Of these, 108 patients (42.2%) were male and right hip was involved in 134 (51.3%) patients. Final follow-up was 95.5 months (range 80–128 months).
3.1. Clinical outcomes
Preoperatively, the mean Harris Hip score was 49.1 ± 14.3 points (range: 9–85). At last follow-up, the mean HHS was 92.0 ± 8.9 points (range: 36–100). There was significant improvement in every single component of HHS (P < 0.001). Most patients were satisfied and able to return to their pain-free daily activities. The detailed clinical results are reported in Table 2, while Table 3 reports the characteristics of the implants.
Table 2.
Harris Hip Score Results (HHS). Reported values are mean, Standard Deviation/SD.
| Harris Hip Score Results (HHS). | |||||
|---|---|---|---|---|---|
| PRE-OP. HHS | POST-OP. HHS | P VALUE | IMPROVEMENT (%) | ||
| Patients (n°) | 256 | 210 | – | – | |
| HHS (mean ± SD) | 49.1 ± 14.3 | 92.0 ± 8.9 | P < 0.001 | 87.37% | |
| Pain | 15.7 ± 8.3 | 41.5 ± 4.0 | P < 0.001 | ||
| Limp | 6.3 ± 2.3 | 10.3 ± 1.6 | P < 0.001 | ||
| Support | 8.0 ± 4.0 | 10.2 ± 2.2 | P < 0.001 | ||
| Distance walked | 5.3 ± 2.3 | 10.4 ± 1.3 | P < 0.001 | ||
| Sitting | 3.5 ± 1.3 | 5.0 ± 0.2 | P < 0.001 | ||
| Stairs | 1.3 ± 0.8 | 2.7 ± 1.2 | P < 0.001 | ||
| Put on shoes and socks | 1.8 ± 1.0 | 3.0 ± 1.0 | P < 0.001 | ||
| Enter public transportation | 0.4 ± 0.5 | 0.8 ± 0.4 | P < 0.001 | ||
| Absence of deformity | 4.0 ± 0.2 | 4.0 ± 0.0 | P = 0.204 | ||
| Flexion | 83.0 ± 20.4 | 102.9 ± 10.2 | P < 0.001 | ||
| Abduction | 20 ± 10.9 | 32.4 ± 7.8 | P < 0.001 | ||
| Adduction | 12.7 ± 8.2 | 18.7 ± 4.8 | P < 0.001 | ||
| Internal rotation | 5.1 ± 6.8 | 14.8 ± 6.9 | P < 0.001 | ||
| External rotation | 12.6 ± 10.2 | 19.2 ± 7.5 | P < 0.001 | ||
| Results (points) | P < 0.001 | ||||
| Poor (<70) | 248 (95.0%) | 7 (3.3%) | |||
| Fair (70–80) | 10 (3.8%) | 9 (4.2%) | |||
| Good (80–90) | 3 (1.2%) | 32 (15.0%) | |||
| Excellent (90–100) | 0 (0%) | 165 (77.5%) | |||
Table 3.
Delta PF and Delta TT acetabular cup, Limacorporate (Ita); Jump system acetabular cup, Permedica (Ita); Trilogy cup, Zimmer (USA), EP-Fit acetabular cup, Smith & Nephews. The table reports the number of the implants that completed the follow-up and in parenthesis the number of the hips implanted.
| Implant/Head size | 40 mm | 36 mm | 32 mm | Total |
|---|---|---|---|---|
| Delta PF | 19 (23) | 74 (89) | 2 (3) | 95 (115) |
| Delta TT | 11 (13) | 49 (53) | 1 (1) | 61 (67) |
| Jump system | – | 32 (44) | 17 (26) | 49 (70) |
| Trilogy | – | 1 (1) | 5 (6) | 6 (7) |
| EP-Fit | – | 2 (2) | – | 2 (2) |
| Total | 30 (36) | 158 (189) | 25 (36) | 213 (261) |
3.2. Radiological outcomes
Complete radiographic evaluation was performed on 170 of the 210 patients. The average acetabular tilt angle (ATA) was 44.5° (95% IC:43.6°–45.4°). There were no osteolytic lesions in any hips. Non-progressive radiolucent lines were present in 44 acetabular and 23 femoral components respectively (a case may have more than one line). At last follow up, two hips showed evidence of progressive acetabular radiolucency and required subsequent revision. There was no correlation between component position and the presence of radiolucent lines or implant loosening (p > 0.05).
3.3. Complications and reoperations
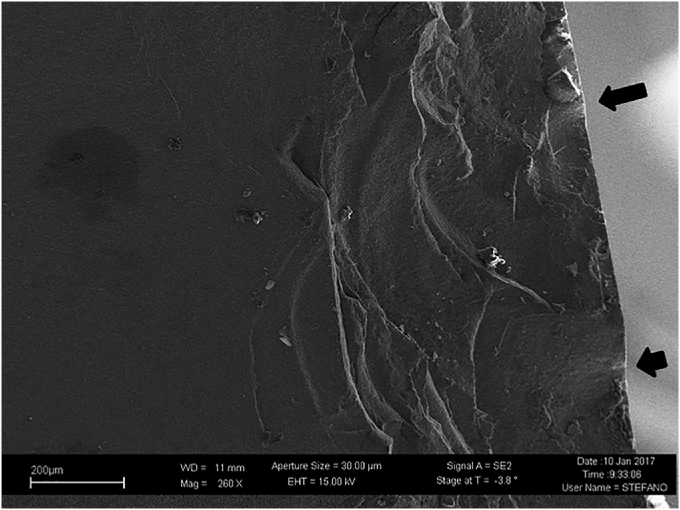
The complications are outlined in Table 4. Overall, 36 patients (36 hips) required reoperation or revision. The etiology for revision surgery included acetabular component loosening (n = 2), hip instability (n = 1), and a ceramic liner fracture which occurred 5 years after surgery. A morphological-compositional characterization of the fracture surfaces of the sample was performed by means of field emission scanning electron microscopy (Fig. 1 and Fig. 2). Another patient with instability did not require surgery after closed reduction. An additional patient required reoperation for severe heterotopic ossification, without implant removal. There were no cases of ball head fractures, squeaking, or femoral loosening. There were no infections requiring revision in this patient cohort.
Table 4.
Complications after surgery. Number and percentage.
| Complications | |
|---|---|
| Minor complications | |
| Skin blisters and disepithelization areas | 31 (14.6%) |
| Fever | 15 (7.0%) |
| Urinary infections | 9 (4.2%) |
| Hypotension and fall | 8 (3.8%) |
| Nausea/vomiting/heartburn | 7 (3.3%) |
| Hematoma | 6 (2.8%) |
| Oliguria | 4 (1.9%) |
| Oedema | 4 (1.9%) |
| Confusional state | 4 (1.9%) |
| Constipation | 4 (1.9%) |
| Hip pain | 3 (1.4%) |
| Abdominal pain | 2 (0.9%) |
| Paresthesia/dysesthesia | 2 (0.9%) |
| Diarrhea | 1 (0.5%) |
| Pharyngitis | 1 (0.5%) |
| Epididymitis | 1 (0.5%) |
| Angina pectoris | 1 (0.5%) |
| Atrial firillations |
1 (0.5%) |
|
Major complications | |
| Joint dislocation | 2(0.9%) |
| Aseptic loosening | 2(0.9%) |
| Liner fracture | 1 (0.5%) |
| Deep vein thrombosis | 1 (0.5%) |
| Partial deficit of common peroneal nerve | 1 (0.5%) |
| Supraventricular tachycardia | 1 (0.5%) |
| Fever and shivering after transfusion | 1 (0.5%) |
Fig. 1.
Morphological-compositional characterization of the fracture surfaces of the samples performed by means of field emission scanning electron microscopy (FESEM, SUPRATM 40, Zeiss) equipped with energy dispersive X-ray spectroscopy (EDS), at the Department of Applied Sciences and Technology (Politecnico di Torino, Italy). FESEM image from the bigger of the two fragments (magnification 235×). The clamping surfaces are evident. They developed due to the sliding of the material planes and appear as stratified beaches, witnessing the progress of the crack. There is no laminating trace (the dark lines are in fact caused by chrome plating).
Fig. 2.
FESEM image on the opposite side of the bigger of the two fragments (magnification 260×). The arrows indicate the fracture trigger. In ceramics the typical rupture mechanism is said to be brittle, as it is a fast, unstable and usually unstoppable process when triggered, unlike ductile rupture.
3.4. Delta COC versus forte COC THA
Compared to a control group of patients with Forte COC THA performed at another institution by one of the authors, there were no significant differences in 2 and 5 years survivorships, revision rate or fractures. There was one revision in the Forte group for instability; also one case of audible squeaking has been detected (the patient was doing well with no pain and decided not to have revision surgery). There were no fractures in the Forte group. Results of Forte group are reported in Table 5.
Table 5.
Patients characteristics and clinical results of the control group Forte on Forte. HHS: Harris Hip Score. Reported values are mean, Standard Deviation/SD and range. Implants from this group were: Depuy (Duraloc/Summit) (Duraloc/Corail); Stryker (Trident/Secure Fit); Smith and Nephew (R3 cup/Synergy) (Reflection/Synergy) (Reflection/Anthology).
| No of patients | 81(89 hips) | - | - |
|---|---|---|---|
| Age, mean ± SD | 61.2 ± 12.5 (range 25–70) |
- | - |
| Sex (%): | - | - | |
| Male | 51 (63%) | ||
| Female | 30 (37%) | ||
| Diameter of the head | - | - | |
| 28 mm | 2 | ||
| 32 mm | 80 | ||
| 36 mm | 7 | ||
| PRE-OP. | POST-OP. | P VALUE | |
| HHS | HHS | ||
| HHS (mean ± SD) | 44.5 ± 20.1 | 90.1 ± 15.0 | P < 0.001 |
| range (25–80) | range (77–100) |
4. Discussion
Improvements in ceramic materials aimed to reduce the historical complications such as ceramic component fractures and squeaking. Alumina matrix composite ceramics (Delta) are promising, nevertheless a lack of clinical data on whether the material improvements translate to improved clinical outcomes still remains. This study is one of the longest series about clinical performances of COC Delta in THA and focused on 1) the 7 years clinical outcomes and survivorship 2) the complications including fractures and squeaking, and 3) the comparison with a series of patients with the previous generation of COC (Forte).
This study has several limitations: first, the retrospective design makes it subject to the traditional limitations including recall selection, sampling and recall biases. However, the large sample size, relative long, and nearly complete clinical follow-up, provides a representative and accurate look at the clinical performance of these types of implants at medium term. Second, the comparative group was a group of Forte ceramic on ceramic patients performed at another institution in the United States (U.S). The decision to use this group to serve as a control can introduce selection bias to the study. However, during this time period, there was only one manufacturer with an FDA approved Delta on Delta hip implant in the U.S. As a result, most ceramic on ceramic THA were Forte on Forte articulations. In order to provide a relevant clinical comparator, we felt that it was important to select a contemporaneous group of patients performed with similar techniques and implants. Finally, there was a disparity in the distribution of head sizes between the Delta group compared to the Forte group. Most of the patients in the Delta group had 36 mm heads compared to 32 mm heads in the Forte group. This may have influenced fracture rates, as larger diameter ball heads are less likely to fracture21; nevertheless, although some authors sustain the contrary25 and in this study we had no head fractures. Thus, our results can simply show clinical equivalency but not superiority in terms of fractures of Delta on Delta articulations compared to Forte on Forte hips.
According to our result, Delta on Delta hip replacements are reliable and durable at mean follow up of more than 7 years, with a maximum of almost 12 years. These results are consistent with other published series of delta on delta THAs. Hamilton et al. reported a survivorship of 96.9% in a series of 345 delta C–O–C THAs at mean 5.3 year follow-up.26 Similarly, Lim and colleagues analyzed 749 delta on delta hips and reported a 98.6% survivorship at 6.5 years.27 Buttaro et al. also reported 99.3% implant survivorship at 2–10 years following surgery.28 Kim et al. reported a high rate of survivorship without evidence of osteolysis or fracture of ceramic material in 277 patients (334 hips) 50 years or younger for a mean follow-up of 7.8 years (range, 6–9).29 Aoude et al. reported good results at six years for 133 consecutive THA with Delta ceramic for patient sunder 65 years age.30 Lim et al. report excellent survivorship for a selected series of patients with osteonecrosis of femoral head (44 patients, 53 hips) at 5 years f-u, although with an audible hip noise identified in 2 (4%) of the 53 hips.31
However, the question remains: does the superior wear properties of ceramics translate into long term clinical superiority and longevity compared to ceramic on polyethylene articulations? Few randomized trials have evaluated this question.32, 33, 34 Atrey and colleagues reported comparable 15 year implant survivorship in a prospective randomized trial comparing these 2 articulations. However, osteolysis was more prevalent in the polyethylene group.32 Also Si et al. sustained that there are no clear evidence favoring the use of either a CoC or ceramic on polyethylene (CoP) bearing surfaces in primary THA.33 In a recent meta-analysis on five RCTs involving 897 patients with 974 hips Hu et al. aimed to compare ceramic-on-ceramic (COC) and metal-on-polyethylene (MOP) in terms of reliability and durability: despite more squeaking and intraoperative implant fracture, the findings supported the use of COC bearing surface which had lower rates of revision, osteolysis and radiolucent line, aseptic loosening, and dislocation compared with MOP.34
Consequently, the superiority of ceramic on ceramic hip implants in terms of wear and survivorship are unlikely to be demonstrated prior to the second decade. Longer term studies and registry level information will hopefully continue to provide additional information to the clinical benefits of ceramic on ceramic hip replacements.
Bearing complications were rare in this series of delta ceramic THAs. There was 1 case of ceramic liner fracture and no cases of squeaking or femoral ball head fractures. The fracture of this series occurred in a severe wrong positioning of the ceramic liner in the metal back, after 6 years in a 81 years active man, according to the literature which correlates orientation and fracture rate.35, 36, 37 While the rate of femoral head fractures have decreased with Delta ceramics compared to Forte,21 liner fractures continue to be rare but constant. Lee et al. reported one liner fracture (0.3%) in a series of 310 hips with no head fractures.38 Hamilton and colleagues reported a 0.9% rate of liner fractures in their series with no femoral head fractures.26 Similarly, Howard et al. analyzed the National Joint Registry and found comparable rates of fractures between Delta and Forte ceramic liners [0.126% vs. 0112%].24 Most of these fractures occurred relatively early suggesting either incomplete seating of the liner or chipping of the liner intraoperatively. Improvements in instrumentation and surgical technique may further decrease the rate of ceramic liner fractures: for example one of these implants is provided with a polar 2–3 mm thickness cylinder which drives the ceramic liner to exactly fit into the metal back (Delta PF and Delta TT, Limacorporate). Second, no patients in this series reported squeaking. The rates of squeaking reported in the literature range from 0 to 33% depending on the definition of squeaking; however, the incidence of revision for squeaking is less than 1%.39,40 Lim et al. reported a 6.4% of patients with audible noise from their hips with 2.5% reporting squeaking.27 Hamilton and colleagues had a 7.5% rate of patient reporting squeaking but only 1 (0.3%) could reproduce the acoustic phenomena in clinic.26 On the other side, a reduced squeaking with these new ceramic materials has been hypothesized41 and supported by our results. Therefore, bearing complications in terms of fractures and squeaking were not increased with a transition from pure alumina ceramics to alumina matrix composite THA.
These results also confirm that alumina matrix composite bearings do not affect performance or reliability in THA implant compared to pure alumina components. Due to the relatively small sample and the rarity of adverse events, we could not demonstrate superiority of Delta ceramic THA compared to Forte ceramic THAs. While zirconia-toughened Delta ceramics is stronger compared to its predecessors, concerns about alumina matrix composites surrounds the potential for phase transformation leading to increased surface roughness and changes in mechanical properties. Elpers et al. demonstrated in retrieved femoral heads that zirconia phase transformation can occur with increasing time in vivo but did not find a direct correlation between phase transformation and surface roughness.42 However, Parkes and colleagues used simulator testing to 5 million cycles and found that monoclinic content increased compared to virgin femoral heads and surface roughness in the test samples were increased only in the worn areas.43 Fortunately, has not been any reports of sudden increase of catastrophic failures in at the national joint registry levels.24 Consequently, Delta on Delta THA can be expected to function and perform similarly compared to Forte on Forte COC THA at short term.
In conclusion, Delta on Delta ceramic THA performed well with low complications at medium-term follow up. There were no increased adverse events and similar functional outcomes compared to a contemporaneous group of patients undergoing THA using Forte on Forte articulations.
Ethical standards
The work has been conducted according to ethical standards and complies with the current laws of the country in which it was performed.
Declaration of competing interest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article. The work described has not been published before and it is not under consideration for publication anywhere else; its publication has been approved by all co-authors.
Contributor Information
Alessandro Bistolfi, Email: abistolfi@cittadellasalute.to.it.
Riccardo Ferracini, Email: riccardoferraciniweb@gmail.com.
Alessandro Aprato, Email: ale_aprato@hotmail.com.
Alessandro Massè, Email: alessandro.masse@unito.it.
Walter Daghino, Email: walterdaghino@hotmail.com.
Sara Lea, Email: sara_lea@hotmail.it.
Stefano Artiaco, Email: sartiaco@cittadellasalute.to.it.
Gwo-Chin Lee, Email: gwo-chin.lee@uphs.upenn.edu.
References
- 1.Soderman P., Malchau H., Herberts P., Zugner R., Regner H., Garellick G. Outcome after total hip arthroplasty: part II. Disease-specific follow-up and the Swedish national total hip arthroplasty register. Acta Orthop Scand. 2001;72:113–119. doi: 10.1080/000164701317323345. [DOI] [PubMed] [Google Scholar]
- 2.Berry D.J., Harmsen W.S., Cabanela M.E., Morrey B.F. Twenty-five-year survivorship of two thousand consecutive primary Charnley total hip replacements: factors affecting survivorship of acetabular and femoral components. J Bone Joint Surg Am. 2002;84:171–177. doi: 10.2106/00004623-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Kurtz S., Ong K., Lau E., Mowat F., Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 4.Banche G., Bracco P., Allizond V. Do crosslinking and vitamin E stabilization influence microbial adhesions on UHMWPE-based biomaterials? Clin Orthop Relat Res. 2015;473(3):974–986. doi: 10.1007/s11999-014-4024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banche G., Allizond V., Bracco P. Interplay between surface properties of standard, vitamin E blended and oxidised ultra high molecular weight polyethylene used in total joint replacement and adhesion of Staphylococcus aureus and Escherichia coli. Bone Joint Lett J. 2014;96-B(4):497–501. doi: 10.1302/0301-620X.96B4.32895. [DOI] [PubMed] [Google Scholar]
- 6.Bistolfi A., Bellare A. The relative effects of radiation crosslinking and type of counterface on the wear resistance of ultrahigh-molecular-weight polyethylene. Acta Biomater. 2011;7(9):3398–3403. doi: 10.1016/j.actbio.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 7.Banche G., Bracco P., Bistolfi A. Vitamin E blended UHMWPE may have the potential to reduce bacterial adhesive ability. J Orthop Res. 2011;29(11):1662–1667. doi: 10.1002/jor.21432. [DOI] [PubMed] [Google Scholar]
- 8.Bistolfi A., Turell M.B., Lee Y.L., Bellare A. Tensile and tribological properties of high-crystallinity radiation crosslinked UHMWPE. J Biomed Mater Res B Appl Biomater. 2009;90(1):137–144. doi: 10.1002/jbm.b.31265. [DOI] [PubMed] [Google Scholar]
- 9.Korovessis P., Petsinis G., Repanti M., Repantis T. Metallosis after contemporary metal-on-metal total hip arthroplasty. Five to nine-year follow-up. J Bone Joint Surg Am. 2006;88-A:1183–1191. doi: 10.2106/JBJS.D.02916. [DOI] [PubMed] [Google Scholar]
- 10.Wang S., Zhang S., Zhao Y. A comparison of polyethylene wear between cobaltchrome ball heads and alumina ball heads after total hip arthroplasty: a 10-year follow-up. J Orthop Surg Res. 2013;8:20. doi: 10.1186/1749-799X-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmalzried T.P., Kwong L.M., Jasty M. The mechanism of loosening of cemented acetabular components in total hip arthroplasty. Analysis of specimens retrieved at autopsy. Clin Orthop Relat Res. 1992;274:60–78. [PubMed] [Google Scholar]
- 12.Willert HG, Bertram H, Buchhorn GH. Osteolysis in alloarthroplasty of the hip (1990) the role of ultra-high molecular weight polyethylene wear particles. Clin Orthop Relat Res 258:95-107. [PubMed]
- 13.Engh C.A., Jr., Hopper R.H., Jr., Huynh C., Ho H., Sritulanondha S., Engh C.A., Sr. A prospective, randomized study of crosslinked and non–cross-linked polyethylene for total hip arthroplasty at 10-year follow-up. J Arthroplasty. 2012;27(8):2–7. doi: 10.1016/j.arth.2012.03.048. [DOI] [PubMed] [Google Scholar]
- 14.Meftah M., Klingenstein G.G., Yun R.J., Ranawat A.S., Ranawat C.S. Long-term performance of ceramic and metal femoral heads on conventional polyethylene in young and active patients: a matched-pair analysis. J Bone Joint Surg Am. 2013;95(13):1193–1197. doi: 10.2106/JBJS.L.00432. [DOI] [PubMed] [Google Scholar]
- 15.Kurtz S.M., Lau E., Baykal D., Springer B.D. Outcomes of ceramic bearings after primary total hip arthroplasty in the medicare population. J Arthroplasty. 2017;32:743–749. doi: 10.1016/j.arth.2016.08.038. [DOI] [PubMed] [Google Scholar]
- 16.Lee G.C., Bistolfi A. Ceramic-on-ceramic total hip arthroplasty: a new standard. Semin Arthroplasty. 2015;26–1:11–15. [Google Scholar]
- 17.Kang B.J., Ha Y.C., Ham D.W., Hwang S.C., Lee Y.K., Koo K.H. Third-generation alumina-on-alumina total hip arthroplasty: 14 to 16-year follow-up study. J Arthroplasty. 2015;30(3):411–415. doi: 10.1016/j.arth.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 18.Kim Y.H., Park J.W., Kim J.S. Long-term results of third-generation ceramic-on-ceramic bearing cementless total hip arthroplasty in young patients. J Arthroplasty. 2016;31(11):2520–2524. doi: 10.1016/j.arth.2016.03.058. [DOI] [PubMed] [Google Scholar]
- 19.Zagra L., Giacometti Ceroni R. Ceramic-ceramic coupling with big heads: clinical outcome. Eur J Orthop Surg Traumatol. 2007;17(3):247–251. [Google Scholar]
- 20.Hernigou P., Homma Y., Pidet O., Guissou I., Hernigou J. Ceramic-on-ceramic bearing decreases the cumulative long-term risk of dislocation. Clin Orthop Relat Res. 2013;471(12):3875. doi: 10.1007/s11999-013-2857-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee G.C., Kim R.H. Incidence of modern alumina ceramic and alumina matrix composite femoral head failures in nearly 6 million hip implants. J Arthroplasty. 2017;32(2):546–551. doi: 10.1016/j.arth.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 22.Hannouche D., Nich C., Bizot P., Meunier A., Nizard R., Sedel L. Fractures of ceramic bearings: history and present status. Clin Orthop Relat Res. 2003;417:19–26. doi: 10.1097/01.blo.0000096806.78689.50. [DOI] [PubMed] [Google Scholar]
- 23.Salo P.P., Honkanen P.B., Ivanova I., Reito A., Pajamäki J., Eskelinen A. High prevalence of noise following Delta ceramic-on-ceramic total hip arthroplasty. Bone Joint Lett J. 2017;99–B:44–50. doi: 10.1302/0301-620X.99B1.37612. [DOI] [PubMed] [Google Scholar]
- 24.Howard D.P., Wall P.D.H., Fernandez M.A., Parsons H., Howard P.W. Ceramic-on-ceramic bearing fractures in total hip arthroplasty: an analysis of data from the National Joint Registry. Bone Joint Lett J. 2017;99-B(8):1012–1019. doi: 10.1302/0301-620X.99B8.BJJ-2017-0019.R1. [DOI] [PubMed] [Google Scholar]
- 25.Higuchi Y., Seki T., Hasegawa Y., Takegami Y., Morita D., Ishiguro N. 32-mm ceramic-on-ceramic total hip arthroplasty versus 28-mm ceramic bearings: 5- to 15-year follow-up study. Hip Int. 2018;1 doi: 10.1177/1120700018760971. 1120700018760971 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 26.Hamilton W.G., McAuley J.P., Blumenfeld T.J., Lesko J.P., Himden S.E., Dennis D.A. Midterm results of delta ceramic-on-ceramic total hip arthroplasty. J Arthroplasty. 2015;30:110–115. doi: 10.1016/j.arth.2015.02.047. [DOI] [PubMed] [Google Scholar]
- 27.Lim S.J., Ryu H.G., Eun H.J., Park C.W., Kwon K.B., Park Y.S. Clinical outcomes and bearing-specific complications following fourth-generation alumina ceramic-on-ceramic total hip arthroplasty: a single-surgeon series of 749 hips at a minimum of 5-year follow-up. J Arthroplasty. 2018;S0883–5403(18) doi: 10.1016/j.arth.2018.02.045. 30176-1. [DOI] [PubMed] [Google Scholar]
- 28.Buttaro M.A., Zanotti G., Comba F.M., Piccaluga F. Primary total hip arthroplasty with fourth-generation ceramic-on-ceramic: analysis of complications in 939 consecutive cases followed for 2-10 years. J Arthroplasty. 2017;32:480–486. doi: 10.1016/j.arth.2016.07.032. [DOI] [PubMed] [Google Scholar]
- 29.Kim Y.H., Park J.W., Kim J.S. Alumina delta-on-alumina delta bearing in cementless total hip arthroplasty in patients aged <50 years. J Arthroplasty. 2017;32(3):1048–1053. doi: 10.1016/j.arth.2016.10.035. [DOI] [PubMed] [Google Scholar]
- 30.Aoude A.A., Antoniou J., Epure L.M., Huk O.L., Zukor D.J., Tanzer M. Midterm outcomes of the recently FDA approved ceramic on ceramic bearing in total hip arthroplasty patients under 65 Years of age. J Arthroplasty. 2015;30(8):1388–1392. doi: 10.1016/j.arth.2015.03.028. [DOI] [PubMed] [Google Scholar]
- 31.Lim S.J., Kim S.M., Kim D.W., Moon Y.W., Park Y.S. Cementless total hip arthroplasty using Biolox®delta ceramic-on-ceramic bearing in patients with osteonecrosis of the femoral head. Hip Int. 2016;26(2):144–148. doi: 10.5301/hipint.5000311. [DOI] [PubMed] [Google Scholar]
- 32.Atrey A., Wolfstadt J.I., Hussain N. The ideal total hip replacement bearing surface in the young patient: a prospective randomized trial comparing alumina ceramic-on-ceramic with ceramic-on-conventional polyethylene: 15-year follow-up. J Arthroplasty. 2017;S0883–5403(17):31084–31087. doi: 10.1016/j.arth.2017.11.066. [DOI] [PubMed] [Google Scholar]
- 33.Si H.B., Zeng Y., Cao F., Pei F.X., Shen B. Is a ceramic-on-ceramic bearing really superior to ceramic-on-polyethylene for primary total hip arthroplasty? A systematic review and meta-analysis of randomised controlled trials. Hip Int. 2015;25(3):191–198. doi: 10.5301/hipint.5000223. [DOI] [PubMed] [Google Scholar]
- 34.Hu D., Tie K., Yang X., Tan Y., Alaidaros M., Chen L. Comparison of ceramic-on-ceramic to metal-on polyethylene bearing surfaces in total hip arthroplasty: a meta-analysis of randomized controlled trials. J Orthop Surg Res. 2015;10(1):22. doi: 10.1186/s13018-015-0163-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Al-Hajjar M., Leslie I.J., Tipper J., Williams S., Fisher J., Jennings L.M. Effect of cup inclination angle during microseparation and rim loading on the wear of BIOLOX® delta ceramic-on-ceramic total hip replacement. J Biomed Mater Res B Appl Biomater. 2010;95(2):263–268. doi: 10.1002/jbm.b.31708. [DOI] [PubMed] [Google Scholar]
- 36.Leslie I.J., Williams S., Isaac G., Ingham E., Fisher J. High cup angle and microseparation increase the wear of hip surface replacements. Clin Orthop Relat Res. 2009;467(9):2259–2265. doi: 10.1007/s11999-009-0830-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Angadji A., Royle M., Collins S.N., Shelton J.C. Influence of cup orientation on the wear performance of metal-on-metal hip replacements. Proc Inst Mech Eng H. 2009;223(4):449–457. doi: 10.1243/09544119JEIM518. [DOI] [PubMed] [Google Scholar]
- 38.Lee Y.K., Ha Y.C., Yoo J.I., Jo W.L., Kim K.C., Koo K.H. Mid-term results of the BIOLOX delta ceramic-on-ceramic total hip arthroplasty. Bone Joint Lett J. 2017;99-B(6):741–748. doi: 10.1302/0301-620X.99B6.BJJ-2016-0486.R3. [DOI] [PubMed] [Google Scholar]
- 39.Lee T.H., Moon Y.W., Lim S.J., Park Y.S. Meta-analysis of the incidence and risk factors for squeaking after primary ceramic-on-ceramic total hip arthroplasty in asian patients. Hip Pelvis. 2014;26(2):92–98. doi: 10.5371/hp.2014.26.2.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Owen D.H., Russell N.C., Smith P.N., Walter W.L. An estimation of the incidence of squeaking and revision surgery for squeaking in ceramic-on-ceramic total hip replacement: a meta-analysis and report from the Australian Orthopaedic Association National Joint Registry. Bone Joint Lett J. 2014;96-B(2):181–187. doi: 10.1302/0301-620X.96B2.32784. [DOI] [PubMed] [Google Scholar]
- 41.Hamilton W.G., McAuley J.P., Dennis D.A., Murphy J.A., Blumenfeld T.J., Politi J. THA with Delta ceramic on ceramic: results of a multicenter investigational device exemption trial. Clin Orthop Relat Res. 2010;468(2):358–366. doi: 10.1007/s11999-009-1091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elpers M., Nam D., Boydston-White S., Ast M.P., Wright T.M., Padgett D.E. Zirconia phase transformation, metal transfer, and surface roughness in retrieved ceramic composite femoral heads in total hip arthroplasty. J Arthroplasty. 2014;29(11):2219–2223. doi: 10.1016/j.arth.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 43.Parkes M., Sayer K., Goldhofer M., Cann P., Walter W.L., Jeffers J. Zirconia phase transformation in retrieved, wear simulated, and artificially aged ceramic femoral heads. J Orthop Res. 2017;35(12):2781–2789. doi: 10.1002/jor.23589. [DOI] [PMC free article] [PubMed] [Google Scholar]