Abstract
Background
Participation rates in colorectal cancer screening in Germany are low. We therefore investigated the effectiveness of different invitation models for immunological stool blood tests (fecal immunological tests, FITs).
Methods
A randomized controlled trial in 50- to 54-year-old clients of the health insurance provider AOK Baden-Wuerttemberg. A total of 17 532 insured persons were randomized to receive: (A) an invitation letter including a FIT (n = 5850); (B) an invitation letter including an option to request a FIT (n = 5844); or (C) an invitation letter only (n = 5838; control group, routine practice). Reminder letters were sent to half the members of groups A and B, selected at random, after 4 weeks. The primary endpoint was the use of a FIT within 1 year of the date of the invitation letter. IRRID: RR2–10.2196/16413. Registration: DRKS00011858.
Results
The invitation letter with a FIT enclosed (A) increased usage from 10% to 29.7% compared with the control group (+19.7% points, p < 0.0001; men: +19.4%, women: +18.8%). The invitation letter with a FIT request option (B) increased usage from 10% to 27.7% (+17.7% points, p < 0.0001; men: +17.7%, women: +17.4%). Reminders increased usage in group A by 7.5% points and in group B by 8.5% points. Participation among women was higher than among men in all groups. The FIT positivity rate was 6.9%. A subsequent colonoscopy was reported for 64.3% of FIT-positive participants, and advanced neoplasia was found in 21.3% of these cases.
Conclusion
Letters of invitation that include a FIT and those that offer low-threshold access to a FIT achieve strong, comparable increases in the usage of FIT in the context of colorectal cancer screening.
Colorectal cancer (CRC) is the second most commonly occurring cancer in Germany, accounting for approximately 60 000 new cases and 25 000 deaths per year (1). Randomized, observational and modeling studies have demonstrated that CRC incidence and mortality can be effectively reduced by screening with stool blood tests and endoscopy (2– 4). In Germany, fecal occult blood tests have been offered for early detection of CRC since 1977. In April 2017, guaiac-based fecal occult blood tests (gFOBTs) were replaced by more sensitive fecal immunological tests (FITs). Since then, FITs have been offered annually at ages 50–54 years and biennially from age 55 upwards. Moreover, colonoscopy was introduced as an alternative screening option from age 55 on in 2002; in April 2019 the starting age for men was lowered to 50 years. However, in the absence of an organized screening program (first introduced in July 2019), the uptake of both FIT-based and colonoscopy-based screening has remained low. We conducted a randomized trial to investigate the effects of different invitation models with low-threshold provision of FITs (FIT enclosed or provided via a user-friendly request option) on usage of the test by men and women aged 50–54 years.
Methods
Study design and population
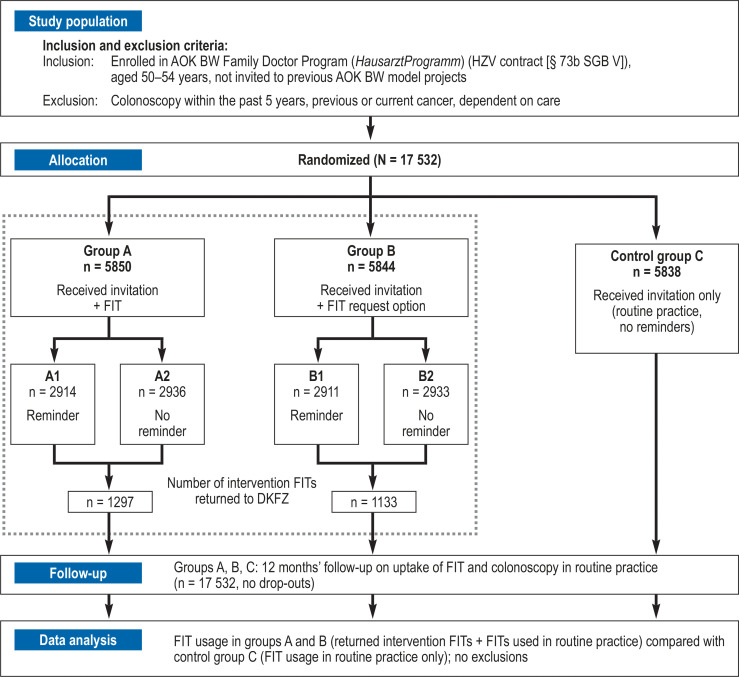
A three-armed, comparative, randomized controlled trial of personal invitation models for CRC screening was conducted in 2017–2019 by the German Cancer Research Center (DKFZ), Heidelberg in cooperation with a statutory health insurance provider in southwest Germany (AOK Baden-Wuerttemberg, AOK BW). Figure 1 shows an overview of the study design, population selection, interventions, and follow-up. The detailed research protocol has been published elsewhere (5). The ethics committee of Medical Faculty of the University of Heidelberg, Germany approved the study. The study was registered at the German Clinical Trials Register (DRKS), ID: DRKS00011858. We followed the CONSORT statement (6) and the FITTER guidelines (7); complementary information is provided in the protocol (5).
Figure 1.
Flow chart of the study.
AOK BW:
AOK Baden-Wuerttemberg;
DKFZ:
German Cancer Research Center;
FIT:
fecal immunological test;
HZV:
family doctor-centered care (Hausarztzentrierte Versorgung);
SGB:
Sozialgesetzbuch
Intervention
The eligible insurance clients of AOK BW (age 50–54 years; enrolled in the AOK BW Family Doctor Program) were randomized (by AOK BW: SQL command DBMS_RANDOM) to receive, between September 2017 and December 2017, personal invitation letters for CRC screening. Persons in intervention group A received an invitation letter with enclosed FIT (OC-Sensor FIT, Eiken Chemical, Tokyo, Japan). Persons in intervention group B received an invitation letter and were offered the opportunity to receive a FIT by mail from the DKFZ via a straightforward request (by online form, e-mail, fax, reply letter). In each intervention group, 50% of persons were selected (by renewed randomization) to receive a reminder letter after 4 weeks. Persons in control group C received only a general invitation letter (in accordance with AOK BW routine practice, with no reminder letters). Participants in the intervention groups could send the provided FITs to the DKFZ in a post-paid envelope for analysis in cooperation with a certified lab (Limbach Laboratory, Heidelberg, Germany; analyses conducted on OC-Sensor Pledia, analytical range 10 to 200 µg hemoglobin/g feces). Insurance clients in all study groups had routine access to FIT testing in the established way (FIT collected from and returned to a physician’s office, analysis in a certified lab, receipt of results via the physician’s office). Alternatively, they could enroll in the AOK Specialist Program for direct screening colonoscopy.
Intervention tests and subsequent colonoscopies
The median time from the usage of an intervention FIT to arrival at the DKFZ was 4 days, and 1 day (median) elapsed between arrival at the DKFZ (refrigeration at 2–8°C) and external lab analysis. The cut-off for test positivity was 10 µg hemoglobin/g feces. All participants received their FIT results by mail, and 81.8% agreed that their family physician could be informed. Physician contact was recommended after positive FIT results. After 6 months, the DKFZ followed up on subsequent colonoscopies. The data for primary outcome analyses were complete. Missing gender information was reported for a small number of participants in gender-specific analyses.
Fecal immunological tests and colonoscopies in routine practice
On the basis of billing data, AOK BW provided information on usage of FITs and colonoscopies conducted in routine practice within 1 year after initial invitation.
Statistical analyses
The primary outcome was overall usage of FIT (intervention FIT or FIT conducted in routine practice) within 1 year after sending the invitation letter in the respective groups (A, B, and C) of the randomized main study and in the respective subgroups of A and B (according to reminder status) in the randomized substudy. Overall usage was compared between groups by contingency table analysis using chi square tests. Statistical significance was defined by alpha levels of 0.05/3 for two-sided testing, adjusting for multiple pairwise testing according to the Bonferroni method. FIT usage was compared between intervention subgroups (with or without a reminder letter) by confirmatory two-sided testing at Bonferroni-adjusted alpha levels of 0.05/2. Furthermore, the relative uptake of FIT use was calculated with asymptotic 95% Wald confidence intervals, together with the proportions of positive FITs by 95% Clopper–Pearson confidence intervals. The secondary endpoints, addressed by descriptive analyses, included gender-specific FIT uptake and the performance of colonoscopy after positive FIT in the intervention groups. SAS version 9.4 was used for all analyses.
Results
In total, 17 532 persons aged 50–54 years who received an invitation letter were randomly allocated to the intervention groups A (invitation + FIT) and B (invitation + FIT request option) and to control group C (Figure 1). Reminders were sent to 50% of persons in the intervention groups. Intervention FITs were used by 22.2% of persons in group A and 19.4% in group B. An additional 7.5%, 8.3%, and 10.0% used a FIT in routine practice within 1 year after the invitation in groups A, B, and C, respectively.
Overall effect of interventions
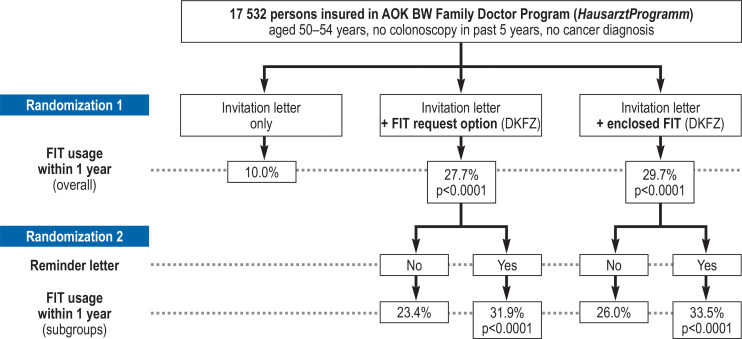
As shown in Table 1 and summarized in Figure 2, overall FIT use within 1 year was 29.7% in intervention group A (invitation + FIT), compared with 10.0% in the control group (difference 19.7% points, p < 0.0001). Comparable overall usage (27.7%) was observed in intervention group B (invitation + FIT request option), with an increase of 17.7% points compared with the control group (p < 0.0001). Usage of FIT in the control group was much lower in men (5.8%) than in women (14.9%). With almost identical absolute increases in usage among men and women, sex differences persisted for overall FIT usage in both intervention groups.
Table 1. Total FIT usage within 1 year after receiving a personal invitation with a FIT kit (A), FIT request options (B), or information only (C, control).
| Group C (control) | Group A (total usage rate and comparison with control group) | Group B (total usage rate and comparison with control group) | |||||||
| Information only (N=5838) | Invitation + FIT (N=5850) | Differencein % points | p-value*2 A vs. C | Relative FIT usage [95% CI] | Invitation + request option (N=5844) | Differencein % points | p-value*2 B vs. C | Relative FIT usage [95% CI] | |
| Total (%)*1 | 583 (10.0) | 1738 (29.7) | 19.7 | <0.0001 | 3.0 [2.7; 3.2] | 1616 (27.7) | 17.7 | < 0.0001 | 2.8 [2.5; 3.0] |
| Men (%)*3 | 182 (5.8) | 779 (25.2) | 19.4 | <0.0001 | 4.4 [3.8; 5.1] | 747 (23.5) | 17.7 | < 0.0001 | 4.1 [3.5; 4.8] |
| Women (%)*3 | 401 (14.9) | 932 (33.7) | 18.8 | <0.0001 | 2.3 [2.0; 2.5] | 861 (32.3) | 17.4 | < 0.0001 | 2.2 [1.9; 2.4] |
*1 Total FIT usage: FITs used in routine practice per group, plus returned intervention FITs in groups A and B
*2 Contingency table analysis using two-sided chi-squared test
*3 Number of men and women per group: 3086 men, 2764 women (A); 3176 men, 2668 women (B); 3153 men, 2685 women (C). Missing gender information in returned FITs: n=27 (A) and n=8 (B)
FIT: fecal immunological test; CI: confidence interval
Figure 2.
Main results of the study.
AOK BW:
AOK Baden-Wuerttemberg;
DKFZ: German Cancer Research Center;
FIT: fecal immunological test
Effect of reminder letters
Table 2 shows the effect of sending a reminder letter within each intervention group (summarized in Figure 2). The reminder letter increased overall FIT use by 7.5% points (p < 0.0001) in intervention group A and by 8.5% points (p < 0.0001) in intervention group B.
Table 2. Differences in FIT usage within 1 year in the intervention subgroups after receiving an invitation with or without a reminder.
| Group A (Comparison of FIT usage without/with reminder) | Group B (Comparison of FIT usage without/with reminder) | |||||||||
| A2 (N=2936) | A1 (N=2914) | Difference in % points | p-value*2A1 vs. A2 | Relative FIT use [95% CI] | B2 (N=2933) | B1 (N=2911) | Difference in % points | p-value*2B1 vs. B2 | Relative FIT use [95% CI] | |
| Total FIT usage (%)*1 | 763 (26.0) | 975 (33.5) | 7.5 | < 0.0001 | 1.3 [1.2; 1.4] | 687 (23.4) | 929 (31.9) | 8.5 | <0.0001 | 1.4 [1.3; 1.5] |
| Men (%)*3 | 334 (21.7) | 445 (28.7) | 7.0 | < 0.0001 | 1.3 [1.2; 1.5] | 312 (19.7) | 435 (27.3) | 7.6 | <0.0001 | 1.4 [1.2; 1.6] |
| Women (%)*3 | 414 (29.6) | 518 (37.9) | 8.3 | < 0.0001 | 1.3 [1.2; 1.4] | 370 (27.4) | 491 (37.2) | 9.8 | <0.0001 | 1.4 [1.2; 1.5] |
A2: Invitation + FIT without reminder; A1: invitation + FIT with reminder; B2: invitation + request option without reminder; B1: invitation + request option with reminder;
*1 Total FIT usage = FITs used in routine practice per subgroup plus returned “intervention” FITs per subgroup
*2 Contingency table analysis using two-sided chi-squared test
*3 Number of men and women per subgroup: 1548 men, 1366 women (A1); 1538 men, 1398 women (A2); 1592 men, 1319 women (B1); 1584 men, 1349 women (B2). Missing gender ‧information in returned FITs: n=27 (A) and n=8 (B)
FIT: Fecal immunological test; CI: confidence interval
Follow-up of results of fecal immunological tests and colonoscopies
Approximately 5% of all study participants had a colonoscopy within 1 year after the invitation: 5% in group A, 4.7% in group B, and 5.7% in group C (table 3). Altogether, 2284 of 2430 intervention FITs (94.0%), had valid results meeting all study quality criteria. Among the valid FITs, 157 (6.9%, 95% confidence interval [5.9; 8.0]) were positive—with comparable positivity rates between the intervention groups (table 3).
Table 3. Total colonoscopy usage within a year of invitation, and by outcome of FITs supplied as part of the intervention.
| Total | Group AInvitation + FIT | Group B Invitation + request option | Group CControl | |
| All invited insurance clients*1 | 17 532 | 5850 | 5844 | 5838 |
| Colonoscopy usage as recorded in billing data | ||||
| Colonoscopies, total (%) | 899 (5.1) | 292 (5.0) | 274 (4.7) | 333 (5.7) |
| Men (%) | 496 (5.3) | 163 (5.3) | 154 (4.8) | 179 (5.7) |
| Women (%) | 403 (5.0) | 129 (4.7) | 120 (4.5) | 154 (5.7) |
| Usage and outcome of FITs supplied as part of intervention*2 | ||||
| Correctly*3 used FITs supplied as part of intervention, total (%) | 2284 | 1205 | 1079 | – |
| Men (%) | 1171 (51.3) | 599 (49.7) | 572 (53.0) | |
| Women (%) | 1113 (48.7) | 606 (50.3) | 507 (47.0) | |
| Positive outcomes, total (%) | 157 (6.9) | 83 (6.9) | 74 (6.9) | |
| Men (%) | 99 (8.5) | 53 (8.8) | 46 (8.0) | |
| Women (%) | 58 (5.2) | 30 (5.0) | 28 (5.5) | |
| Colonoscopy usage*4 after FITs supplied as part of intervention*2 with positive outcome | ||||
| Colonoscopies, total (%) | 101 (64.3) | 56 (67.5) | 45 (60.8) | – |
| Men (%) | 63 (63.6) | 35 (66.0) | 28 (60.9) | |
| Women (%) | 38 (65.5) | 21 (70.0) | 17 (60.7) | |
| Colonoscopy usage*4 after FITs supplied as part of intervention*2 with negative outcome | ||||
| Colonoscopies, total (%) | 110 (5.2) | 71 (6.3) | 39 (3.9) | – |
| Men (%) | 60 (5.6) | 35 (6.4) | 25 (4.8) | |
| Women (%) | 50 (4.7) | 36 (6.3) | 14 (2.9) | |
FIT: fecal immunological test
*1 Number of men and women per group: 3086 men, 2764 women (A); 3176 men, 2668 women (B); 3153 men, 2685 women (C)
*2 Invitation with FIT enclosed (group A) or available upon straightforward request (group B).
*3 For 2284 of 2430 returned intervention FITs (94.0%), valid results were obtained (reasons for invalidity [number of cases]: > 7 days of uncooled transport [86]; missing informed consent for FIT analysis [29]; arrival of fecal sample after closing date of analysis [16]; failed laboratory testing [11]; faulty fecal sampling [4])
*4 Information available (90% group A; 89% group B) via informed consent for linking FIT results with routine billing data or by active follow-up of positive FITs
Information on uptake of colonoscopy after a positive or negative FIT result within 1 year of invitation was available for 1083 (90%) participants with valid FIT in group A and 956 (89%) in group B. To derive this information, on the one hand we actively followed up the positive FIT results, and on the other hand we linked the pseudonymized FIT results with the routine billing data of AOK BW on the basis of the informed consent obtained. Among these, colonoscopy was performed within 1 year after the invitation in 64.3% of the participants with a positive FIT result, compared with 5.2% of those with a negative FIT result.
Analysis of the available colonoscopy reports, obtained through active follow-up of participants with positive intervention FIT (n = 89), showed detection of CRC, advanced neoplasia (CRC or advanced adenoma), or neoplasia in 1 (1%), 19 (21%), and 37 (42%) of cases (etable).
eTable. Most advanced finding at diagnostic colonoscopy after positive results of FITs supplied in the context of the intervention, by gender.
| Most advanced finding*1 |
Total N = 89 |
Men n = 56 |
Women n = 33 |
pgender*2 | |||
| n | % | n | % | n | % | ||
| Carcinoma | 1 | 1.1 | 1 | 1.8 | 0 | 0 | 0.44 |
| Advanced adenoma | 18 | 20.2 | 15 | 26.8 | 3 | 9.1 | 0.05 |
| Non-advanced adenoma | 18 | 20.2 | 10 | 17.9 | 8 | 24.2 | 0.47 |
| Advanced neoplasia (carcinoma or advanced adenoma) | 19 | 21.3 | 16 | 28.6 | 3 | 9.1 | 0.03 |
| Neoplasia (carcinoma or adenoma) | 37 | 41.5 | 26 | 46.5 | 11 | 33.3 | 0.23 |
*1 Colonoscopy outcomes of both intervention groups, A and B, were combined for this analysis.
*2 p-value for gender difference in prevalence
FIT: fecal immunological test
Discussion
The results of this randomized controlled trial in 50- to 54-year-old clients of a statutory health insurance provider demonstrated a threefold increase in FIT usage by sending personal invitation letters with low-threshold access to FITs. Direct enclosure of a FIT increased overall usage within 1 year from 10% to 29.7%; however, this was not the only factor. The effect of a user-friendly FIT request option was almost as great, leading to overall usage of 27.7% within 1 year. Reminder letters were highly effective in both intervention groups, with response rates 7.5% points (group A) and 8.5% points (group B) higher than achieved by one-time invitation. Comparable increases in FIT usage were seen in both genders.
To our knowledge, this is the first study to investigate invitations with straightforward request options as an alternative to invitations with a FIT enclosed. At almost equal usage rates, the request option yields economic and environmental advantages. Sending FITs upon request achieves substantial savings (material, postage, ecological costs). In our study, almost 80% of directly mailed FITs in group A remained unused, compared with 35% of the FITs mailed on request in group B. Most requests in group B were received by reply letter, smaller numbers via internet, e-mail or fax. This preference may be due to the convenience of the addressed postpaid envelope, limited internet affinity, or concerns about data protection when placing orders online. Although we do not know how well the request options in group B would have been accepted if made individually, it seems plausible to assume that offering alternative options will increase overall response rates.
Stool test usage in both FIT intervention groups was higher than in a previous randomized trial from Germany, in which a personal invitation letter with enclosed gFOBT raised participation in 50- to 54-year-olds to 25% (8). A possible explanation is the generally higher acceptance of FITs than of gFOBTs (9, 10). Official data on FIT utilization in the organized screening program in Germany are not yet available, because it was only launched in July 2019 (11). However, after the introduction of FITs in 2017 (without personal invitations) stool test usage for early detection of CRC actually declined further (12). This highlights the need for invitation programs that help to increase use of this effective screening option.
Despite the substantial increase in FIT use by enclosing the FIT or by providing a straightforward request option, usage rates were lower in this study than those achieved in organized screening programs in other European countries (10). Additional measures to optimize participation, such as letters announcing the mailing of FITs 2 weeks in advance, achieved usage rates >60% in the Netherlands, for example (13– 16). This suggests that even higher uptake might be achieved by further modification of the invitation models. Another potential reason for the somewhat lower usage rate in our study is the relatively high prevalence of colonoscopy in Germany. For example, in a recent study from this country, 47.1% of 50- to 54-year-old participants reported a previous colonoscopy (17). Although we excluded persons for whom colonoscopy had been billed in the past 5 years, persons with colonoscopy 5–10 years ago or those with a recent screening not yet included in billing records could not be excluded. Performance of a FIT is generally not indicated in such persons. A further reason for the somewhat lower usage rate may have been the requirement for specific informed consent for access to personal information, fecal samples and FIT results in the context of this study. Another difference from the findings of organized screening programs in other countries is the major gender difference in usage rate. However, this was due to differences in FIT usage in routine practice in Germany, where FITs are commonly offered by gynecologists in conjunction with cervical cancer screening (18).
The FIT positivity rate of 6.9% in our study is within the range of 3.3%–9.8% observed—with varying tests, cut-offs, and age groups—in other European countries (10). The proportion of people undergoing colonoscopy after positive FIT was 12 times higher than after negative FIT (64.3% versus 5.2%). Although this large difference indicates that the FITs fulfilled their function of selecting people for colonoscopy, the utilization of colonoscopy after a positive FIT needs to be greatly improved and is distinctly lower than the average 80.9% uptake seen in other European countries (10). These results emphasize the necessity of systematic follow-up of positive FIT results in an organized screening system, which has not yet been implemented in the German cancer screening program. Better provision of information, including short explanatory videos, might help to increase colonoscopy usage by reducing anxiety about colonoscopy and related measures such as bowel preparation (19).
Overall, the colonoscopy rates in the intervention groups and the control group were similar. Therefore, the higher number of colonoscopies in FIT-positive persons and the lower number in FIT-negative persons more or less balanced each other out. Thus, despite similar colonoscopy uptake rates, the proportion of people who were more likely to benefit from colonoscopy was increased. The rate of detection of advanced neoplasia among persons with a positive FIT in this trial (21.3%) was over 3 times as high as in those undergoing colonoscopy with no preceding FIT in a comparable population of 50- to 54-year-old AOK BW clients (6.8%) (20) which underlines the suitability of FITs for effective preselection of candidates for colonoscopy.
The specific strengths of our study are its size and the collaboration with a statutory health insurance provider, which support transferability of the findings to routine practice. Although trial participation was restricted to persons enrolled in the AOK BW Family Doctor Program, the uptake of stool blood tests in routine practice was similar to that in a previous project that was not focused on a specific subgroup of insured persons (8). Owing to time-lags in the recording of billing information, some people may have been included who had completed a screening shortly beforehand. This could have led to underestimation of the true intervention effects, since it must be assumed that such persons would not undergo another screening test.
In conclusion, these results demonstrate the large potential of offering low-threshold access to FITs in personal invitation letters for CRC screening. Provision of FITs upon request might be a promising, economic, and easy-to-implement method whose adoption could help to substantially increase the effectiveness of CRC screening. Future studies should investigate further ways to improve the uptake of effective screening offers and to increase colonoscopy usage after positive FIT.
Key Messages.
Personal invitation letters were implemented in the screening program for colorectal cancer in Germany in July 2019, but they do not include direct or low-threshold provision of FITs.
In this randomized controlled trial in 17 532 men and women aged 50–54 years, enclosure of FITs in invitation letters significantly increased FIT usage within 1 year from 10% to almost 30%. A comparable effect was seen for invitations with multiple low-threshold options for requesting a free FIT (by internet, e-mail, fax, or reply letter).
The sending of a reminder letter significantly increased FIT usage in the intervention subgroups. Overall FIT usage came close to 40% among women who received a reminder letter following a written invitation with enclosed FIT or request options.
About one third of persons with positive FIT results declined colonoscopy to clarify the origin of the occult blood.
In more than 20% of persons who chose to undergo colonoscopy after a positive FIT result, advanced neoplasia (carcinoma or advanced adenoma) was found; the prevalence was considerably higher in men than in women.
Acknowledgments
Acknowledgements
We are grateful for the excellent cooperation between AOK BW and the German Cancer Research Center (DKFZ) and the respective collaborators who made this study possible. We would like to thank the documentation team and laboratory staff of the Division of Clinical Epidemiology and Aging Research, (DKFZ) for the excellent teamwork and support during the trial, with special thanks to Claudia El Idrissi-Lamghari, but also to Konstanze Kuhlmann, Ute Schneider, Marianne Marquard, Katarina Cuk, and Sabine Eichenherr. Furthermore, we thank Volker Knapp for creating and maintaining the website for online requests.
Funding
The study was funded by AOK Baden-Wuerttemberg.
Data sharing statement
A completely anonymized participant dataset on which the results presented in this paper are based may be shared upon reasonable request.
Footnotes
Conflict of interest statement
Stefan Meny is employed by AOK Baden-Wuerttemberg.
Prof. Brenner has received a lecture honorarium and reimbursement of travel costs from the Falk Foundation.
The remaining authors declare that no conflict of interest exists.
References
- 1.Robert Koch-Institut und die Gesellschaft der epidemiologischen Krebsregister in Deutschland e.V. (eds.) Robert Koch-Institut. Berlin: 2019. Krebs in Deutschland für 2015/2016. 12. Edition. [Google Scholar]
- 2.Shaukat A, Mongin SJ, Geisser MS, et al. Long-term mortality after screening for colorectal cancer. N Engl J Med. 2013;369:1106–1114. doi: 10.1056/NEJMoa1300720. [DOI] [PubMed] [Google Scholar]
- 3.Brenner H, Stock C, Hoffmeister M. Effect of screening sigmoidoscopy and screening colonoscopy on colorectal cancer incidence and mortality: systematic review and meta-analysis of randomised controlled trials and observational studies. BMJ. 2014;348 doi: 10.1136/bmj.g2467. g2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buskermolen M, Cenin DR, Helsingen LM, et al. Colorectal cancer screening with faecal immunochemical testing, sigmoidoscopy or colonoscopy: a microsimulation modelling study. BMJ. 2019;367 doi: 10.1136/bmj.l5383. l5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gruner LF, Hoffmeister M, Ludwig L, Brenner H. Effect of various invitation schemes on the use of fecal immunochemical tests for colorectal cancer screening: protocol for a randomized controlled trial. JMIR Res Protoc. 2020;9 doi: 10.2196/16413. e16413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340 doi: 10.1136/bmj.c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fraser CG, Allison JE, Young GP, Halloran SP, Seaman HE. Improving the reporting of evaluations of faecal immunochemical tests for haemoglobin: the FITTER standard and checklist. Eur J Cancer Prev. 2015;24:24–26. doi: 10.1097/CEJ.0000000000000016. [DOI] [PubMed] [Google Scholar]
- 8.Hoffmeister M, Holleczek B, Zwink N, Stock C, Stegmaier C, Brenner H. Screening for bowel cancer: increasing participation via personal invitation—a randomized intervention study. Dtsch Arztebl Int. 2017;114:87–93. doi: 10.3238/arztebl.2017.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akram A, Juang D, Bustamante R, et al. Replacing the guaiac fecal occult blood test with the fecal immunochemical test increases proportion of individuals screened in a large healthcare setting. Clin Gastroenterol Hepatol. 2017;15:1265–1270 e1. doi: 10.1016/j.cgh.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 10.Senore C, Basu P, Anttila A, et al. Performance of colorectal cancer screening in the European Union member states: data from the second European screening report. Gut. 2019;68:1232–1244. doi: 10.1136/gutjnl-2018-317293. [DOI] [PubMed] [Google Scholar]
- 11.Gemeinsamer Bundesausschuss (G-BA) Richtlinie des Gemeinsamen Bundesausschusses für organisierte Krebsfrüherkennungsprogramme. Geänderte Fassung vom 22.11.2018, in Kraft getreten am 01.07.2019. www.g-ba.de/downloads/62-492-1844/oKFE-RL-2018-11-22-iK-2019-07-01.pdf (last accessed on 5 July 2019) [Google Scholar]
- 12.Gemeinsamer Bundesausschuss (G-BA) Der iFOBT im Darmkrebs-Screening: Ergebnisse der medizinischen Laboratorien für das Jahr 2018. www.g-ba.de/downloads/17-98-4777/2019-03-25_G-BA_iFOBT_Quartalsbericht_2018_.pdf (last accessed on 17 June 2019) [Google Scholar]
- 13.Kapidzic A, Grobbee EJ, Hol L, et al. Attendance and yield over three rounds of population-based fecal immunochemical test screening. Am J Gastroenterol. 2014;109:1257–1264. doi: 10.1038/ajg.2014.168. [DOI] [PubMed] [Google Scholar]
- 14.Kapidzic A, van Roon AH, van Leerdam ME, et al. Attendance and diagnostic yield of repeated two-sample faecal immunochemical test screening for colorectal cancer. Gut. 2017;66:118–123. doi: 10.1136/gutjnl-2014-308957. [DOI] [PubMed] [Google Scholar]
- 15.van Roon AH, Hol L, Wilschut JA, et al. Advance notification letters increase adherence in colorectal cancer screening: a population-based randomized trial. Prev Med. 2011;52:448–451. doi: 10.1016/j.ypmed.2011.01.032. [DOI] [PubMed] [Google Scholar]
- 16.Erasmus MC - NKI / AvL. Monitor 2017, national monitoring of the colorectal cancer screening programme. www.rivm.nl/sites/default/files/2018-11/DarmMon2017-Engels5.pdf (last accessed on 20 January 2020) [Google Scholar]
- 17.Weigl K, Tikk K, Hoffmeister M, et al. Prevalence of a first-degree relative with colorectal cancer and uptake of screening among persons 40 to 54 years old. Clin Gastroenterol Hepatol. 2019 doi: 10.1016/j.cgh.2019.11.044. pii: S1542-3565(19)31384-9. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 18.Stock C, Ihle P, Schubert I, Brenner H. Colonoscopy and fecal occult blood test use in Germany: results from a large insurance-based cohort. Endoscopy. 2011;43:771–781. doi: 10.1055/s-0030-1256504. [DOI] [PubMed] [Google Scholar]
- 19.Yang C, Sriranjan V, Abou-Setta AM, Poluha W, Walker JR, Singh H. Anxiety associated with colonoscopy and flexible sigmoidoscopy: a systematic review. Am J Gastroenterol. 2018;113:1810–1818. doi: 10.1038/s41395-018-0398-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brenner H, Zwink N, Ludwig L, Hoffmeister M. Should screening colonoscopy be offered from age 50? Results from a statewide pilot project, and from a randomized intervention study. Dtsch Arztebl Int. 2017;114:94–100. doi: 10.3238/arztebl.2017.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]