Abstract
COVID-19 has rapidly become a major concern for the health systems worldwide. Its high contagiousness and associated mortality demand the discovery of helpful interventions with promising safety profile. Here, we report 3 severe COVID-19 cases, which achieved rapid and sustained improvement in outcome with the use of ruxolitinib, a JAK/STAT pathway inhibitor.
Keywords: COVID-19, Ruxolitinib, JAK/STAT, Inflammation
Introduction
COVID-19 has presented a serious challenge for most healthcare systems across the world, mainly due to the lack of effective therapies and incomplete understanding of its pathogenesis. Patients with severe COVID-19 have increased secretion of cytokines, such as IL-18, IFNγ-inducible protein (IP10), and macrophage inflammatory protein (MIP1A), as the first immune response. Later, lymphocytes, macrophages, and polymorphonuclear cells are recruited to secrete IL-15, IFNγ/IFNγ, IL-12, and IL-21, necessary for viral clearance, as well as producing an excess of IL-6, TNFα, IL-17a, GM-CSF, and G-CSF, leading to the consequent pulmonary and systemic hyperinflammatory state [1]. Such phenomena, when sustained, result in acute respiratory distress and multiple organ dysfunction. Furthermore, with the massive release of cytokines (cytokine storm), patients show high levels of macrophage activation markers, such as ferritin and sCD25 (IL-2RA).
In recent years, the JAK/STAT pathway has been described to play a key role in various conditions of excessive inflammation, for example in graft-versus-host disease, several autoimmune diseases, and in refractory hemophagocytic syndrome [2, 3, 4]. This signaling pathway is highly efficient and plays a crucial role in the inflammatory process, since it is necessary for both secretion and the activity of several interleukins.
The possibility of inhibiting the JAK/STAT pathway could promptly stop inflammatory lymphocyte activation and recruitment. Here, we report the cases of 3 patients with hyperinflammatory response linked to COVID-19, who had a rapid favorable outcome after the use of a JAK1/JAK2 kinase inhibitor ruxolitinib. The ruxolitinib schedule included 16 days of treatment: first week with 10 mg q12, second week with 5 mg q12, and the following 2 days with 5 mg a day.
Case Report/Case Presentation
Case 1
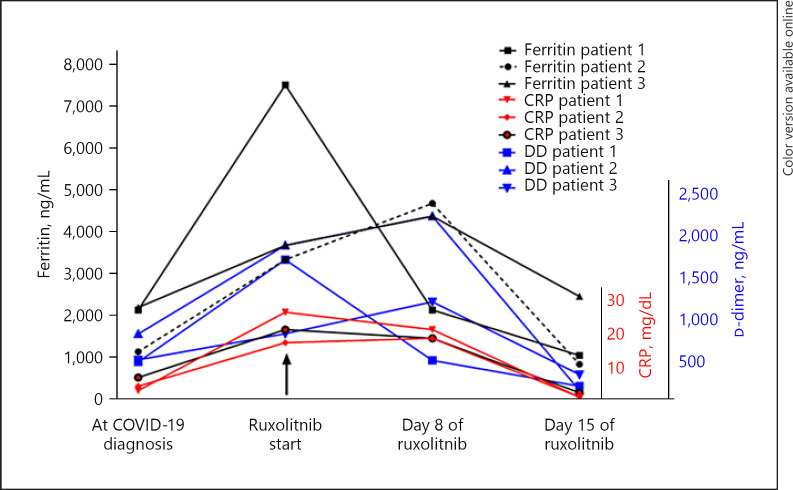
A 56-year-old male patient with arterial hypertension, being treated with losartan, was admitted due to a week of fatigue and fever. In the recent past, he had occupational exposure to a patient with COVID-19. Physical examination revealed no alteration, and O2 saturation was 97% with FiO2 21%. COVID-19 RT-PCR was positive, and CT scan showed the presence of diffuse interstitial pulmonary infiltrates, which were nonspecific, but attributable to acute viral infection. During the following 48 h, fever was persistent, and treatment with hydroxychloroquine/azithromycin showed no response. Monitoring of laboratory parameters showed intense elevation of D-dimer, CRP, and ferritin (Table 1; Fig. 1). Other treatments initiated included: cefepime for possible bacterial infection, prophylactic enoxaparin, and symptomatic relief medication with intravenous acetaminophen. After 72 h of admission, increase in heart rate and mild hypoxemia developed, along with duplication of the initial inflammatory parameters (Table 1). He was transferred to the intensive care unit and tocilizumab 8 mg/kg i.v. was administered, with a favorable response in clinical parameters. However, during the following 72 h, intense progression of fatigue and progressive elevation of ferritin D-dimer was noted. After 6 days of worsening symptoms, administration of 10 mg of ruxolitinib, every 12 h, was initiated. Clinical improvement was remarkable, leading to his discharge from hospital the day after initiating ruxolitinib treatment. After 16 days of ruxolitinib intake, inflammatory parameters were normalized. No control lung imaging has been performed yet. After 6 weeks of COVID-19 detection, viral RT-PCR was negative.
Table 1.
Evolution of laboratory findings in patients with hyperinflammatory state
| At diagnosis of COVID-19 |
At ruxolitinib start |
Day 8 of ruxolitinib |
Day 15 of ruxolitinib |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CRP, mg/dL | ferritin, ng/mL | D-dimer, ng/mL | CRP, mL mg/ferritin, ng/mL | D-dimer, ng/mL | CRP, mg/dL | ferritin, ng/mL | D-dimer, ng/mL | CRP, mg/dL | ferritin, ng/mL | D-dimer, ng/mL | COVID-19 PCR | ||
| Case 1 | 2 | 2,051 | 856 | 20 | 7,231 | 1,009 | 16 | 2,052 | 890 | 0.5 | 1,006 | 300 | negative |
| Case 2 | 3 | 1,090 | 1,500 | 13 | 3,209 | 658 | 4 | 4,506 | 1,185 | 5 | 802 | 201 | negative |
| Case 3 | 5 | 2,111 | 905 | 16 | 3,540 | 1,508 | 14 | 4,211 | 2,241 | 1.57 | 2,364 | 568 | negative |
Fig. 1.
Inflammatory markers before and after ruxolitinib treatment.
Case 2
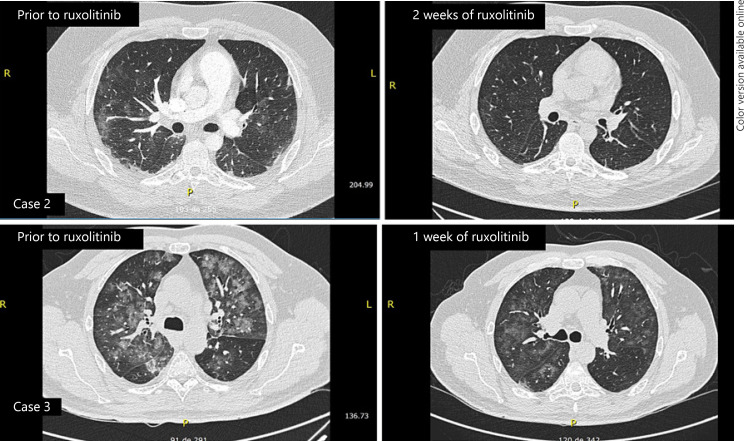
A 29-year-old male patient with Phi(−) acute lymphoblastic leukemia, currently in the 3rd week of methotrexate phase of augmented BFM chemotherapy regimen, with habitual mild cytopenia, developed headache, and mild nasal discharge. COVID-19 RT-PCR was positive. The initial evolution was satisfactory, with spontaneous regression of symptoms; however, fever and dry cough evolved after 1 week of infection. A chest CT scan was performed, showing mild pleural effusion and diffuse interstitial infiltrates, suggestive of viral infection. At the same time, intense systemic inflammation was noted, with elevated D-dimer and ferritin, along with further development of mild hypoxemia and supplemental oxygen requirements (Table 1; Fig. 1). Ruxolitinib treatment was started and prompt improvement in respiratory function, with cessation of supplemental oxygen needs, was noted in less than 24 h. After 2 weeks of treatment, a new chest CT was performed, which showed almost complete regression of the thoracic alterations (Fig. 2).
Fig. 2.
CT chest scan changes with ruxolitinib treatment. DD, D-dimer.
Case 3
A 58-year-old male patient was admitted to our hematology department with diffuse bone pain and spontaneous T11 vertebral fracture. Detailed investigation showed the presence of IgG multiple myeloma, R-ISS 2. Treatment with localized radiation therapy and cyclophosphamide-bortezomib-dexamethasone regimen was initiated, with good tolerance and pain improvement. During the second week of treatment, he developed fever and mild dyspnea. COVID-19 test result was positive. A chest CT scan was performed, and acute diffuse interstitial infiltrates seen, suggestive of viral infection. He was kept under observation for a week, under treatment with prophylactic antibiotics, enoxaparin, and intravenous acetaminophen. Unfortunately, he developed fever and showed progressive elevation of CRP, ferritin, and D-dimer (Table 1; Fig. 1). Promptly, ruxolitinib was initiated, and after a week of treatment, chest CT scan was performed again (Fig. 2); it showed almost complete regression of the pulmonary infiltrates, and after 2 more weeks, all inflammatory laboratory findings returned to normal values.
Discussion/Conclusion
Ruxolitinib is an oral inhibitor of JAK1/JAK2 kinases, with FDA approval for treating myeloproliferative neoplasm and graft-versus-host disease. It reduces IL-6, IL-13, MCP-1, and TNFα secretion in vitro [5]. This effect had also been demonstrated by CAR T-cell therapy, where the use of ruxolitinib prevented cytokine release syndrome without affecting the effectiveness of cell therapy [6], and in cases of refractory hemophagocytic lymphohistiocytosis [7].
Ruxolitinib has a very favorable safety profile, if used in low doses and for a limited number of days, and the occurrence of significant cytopenia and viral reactivations, like Herpesviridae and hepatitis B, is low [8]. It is also reported to inhibit HIV replication [9]. To date, there has been no publication related to its use in COVID-19, although its anti-inflammatory effects could have important implications in this disease [10].
Here, we report the first series of successful treatment of hyperinflammation, related to COVID-19, with ruxolitinib. Two of our patients had a hematologic neoplasm with ongoing chemotherapy treatment and were immunosuppressed owing to their cancer as well as the treatment; they cannot, therefore, be generalized with the non-cancer population.
Nevertheless, it would be worth highlighting that in the 2 patients with hematological diseases, treatment with ruxolitinib did not interfere with anti-leukemia or anti-myeloma treatment. In addition, the viral load turned negative during follow-up, hence suggesting that inhibition of the JAK/STAT pathway does not interfere with viral clearance.
Despite the short duration of treatment, we used a tapering schedule to avoid the theoretical risk of withdrawal syndrome and cytokine storm described with the abrupt discontinuation of ruxolitinib [11]. It may be argued that control of the hyperinflammatory state in the 3 patients could be due to the natural course of COVID-19; however, recent European data from patients with hematologic disease and COVID-19 have shown poor outcomes, with very high mortality (estimated as 40% in 1 month), which may be even higher with a longer follow-up [12]. We, therefore, believe the rapid improvement and discharge of the patient to be due to the active treatment of the hyperinflammatory state with ruxolitinib. For this reason, based on our small series, we concluded ruxolitinib treatment as the main therapeutic strategy that mitigated the cytokine storm and reversed the dismal outcome of COVID-19.
Recently, data from an RCT in China, by Cao et al. [13] have suggested the dose of 5 mg of ruxolitinib b.i.d. (20 patients) in patients with “severe COVID-19” (defined as having hypoxemia in room air or respiratory rate >30 breaths/min) to significatively result in early improvement of lungs (in CT scan) compared to placebo, and a reduction in the risk of intubation and death (though not significantly); however, no patient with hematologic neoplasm was included in this trial. Similar findings have been obtained by La Rosée et al. [14], in a case series of 14 patients; they reported treatment with ruxolitinib, in hyperinflammation due to COVID-19, to be safe with signs of efficacy to prevent or even overcome multiorgan failure.
In summary, COVID-19 induces a hyperinflammatory systemic state involving lungs, a phenomenon associated with cytokine storm and JAK/STAT hyperactivation. To the best of our knowledge, this is the first report of patients with severe hyperinflammatory COVID-19 and hematological disease being successfully treated with the JAK1/JAK2 kinase inhibitor ruxolitinib. Blocking the JAK/STAT pathway may be a promising therapy, especially in patients developing severe forms of COVID-19. Ongoing randomized studies will eventually report the best therapeutic strategy desperately required by all patients with COVID-19.
Statement of Ethics
All patients provided signed informed consent for ruxolitinib use and for publication of relevant clinical data with protection of identity. Clinical informed consent was regulated by the ethics board of our institution.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
The authors have no funding sources to declare.
Author Contributions
P.R. and M.S. collected the information and wrote the manuscript.
References
- 1.Schett G, Sticherling M, Neurath MF. COVID-19: risk for cytokine targeting in chronic inflammatory diseases? Nat Rev Immunol. 2020 May;20((5)):271–2. doi: 10.1038/s41577-020-0312-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birndt S, Schenk T, Heinevetter B, Brunkhorst FM, Maschmeyer G, Rothmann F, et al. Hemophagocytic lymphohistiocytosis in adults: collaborative analysis of 137 cases of a nationwide German registry. J Cancer Res Clin Oncol. 2020 Apr;146((4)):1065–77. doi: 10.1007/s00432-020-03139-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeiser R, von Bubnoff N, Butler J, Mohty M, Niederwieser D, Or R, et al. REACH2 Trial Group Ruxolitinib for Glucocorticoid-Refractory Acute Graft-versus-Host Disease. N Engl J Med. 2020 May;382((19)):1800–10. doi: 10.1056/NEJMoa1917635. [DOI] [PubMed] [Google Scholar]
- 4.Sarmiento Maldonado M, Ramírez Villanueva P, Bertín Cortes-Monroy P, Jara Arias V, Soto Donoso K, Uribe Gonzalez P, et al. Compassionate use of ruxolitinib in acute and chronic graft versus host disease refractory both to corticosteroids and extracorporeal photopheresis. Exp Hematol Oncol. 2017 Dec;6((1)):32. doi: 10.1186/s40164-017-0092-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hermans MA, Schrijver B, van Holten-Neelen CC, Gerth van Wijk R, van Hagen PM, van Daele PL, et al. The JAK1/JAK2- inhibitor ruxolitinib inhibits mast cell degranulation and cytokine release. Clin Exp Allergy. 2018 Nov;48((11)):1412–20. doi: 10.1111/cea.13217. [DOI] [PubMed] [Google Scholar]
- 6.Khadka RH, Sakemura R, Kenderian SS, Johnson AJ. Management of cytokine release syndrome: an update on emerging antigen-specific T cell engaging immunotherapies. Immunotherapy. 2019 Jul;11((10)):851–7. doi: 10.2217/imt-2019-0074. [DOI] [PubMed] [Google Scholar]
- 7.Wang J, Wang Y, Wu L, Wang X, Jin Z, Gao Z, et al. Ruxolitinib for refractory/relapsed hemophagocytic lymphohistiocytosis. Haematologica. 2020 May;105((5)):e210–2. doi: 10.3324/haematol.2019.222471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elli EM, Baratè C, Mendicino F, Palandri F, Palumbo GA. Mechanisms Underlying the Anti-inflammatory and Immunosuppressive Activity of Ruxolitinib. Front Oncol. 2019 Nov;9:1186. doi: 10.3389/fonc.2019.01186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gavegnano C, Detorio M, Montero C, Bosque A, Planelles V, Schinazi RF. Ruxolitinib and tofacitinib are potent and selective inhibitors of HIV-1 replication and virus reactivation in vitro. Antimicrob Agents Chemother. 2014;58((4)):1977–86. doi: 10.1128/AAC.02496-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kathryn D. JAK1 inhibition blocks lethal sterile immune responses: implications for COVID-19 therapy. bioRxiv. p. 2020.04.07.024455. https://doi.org/
- 11.Malard F, Genthon A, Brissot E, van de Wyngaert Z, Marjanovic Z, Ikhlef S, et al. COVID-19 outcomes in patients with hematologic disease. Bone Marrow Transplant. 2020 May; doi: 10.1038/s41409-020-0931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tefferi A, Pardanani A. Serious adverse events during ruxolitinib treatment discontinuation in patients with myelofibrosis. Mayo Clin Proc. 2011 Dec;86((12)):1188–91. doi: 10.4065/mcp.2011.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao Y, Wei J, Zou L, et al. Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): A multicenter, single-blind, randomized controlled trial [published online ahead of print, 2020 May 26] J Allergy Clin Immunol. 2020;S0091-6749((20)):30738–7. doi: 10.1016/j.jaci.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.La Rosée F, Bremer HC, Gehrke I, Kehr A, Hochhaus A, Birndt S, et al. The Janus kinase 1/2 inhibitor ruxolitinib in COVID-19 with severe systemic hyperinflammation [published online ahead of print, 2020 Jun 9] Leukemia. 2020 Jul;34((7)):1805–15. doi: 10.1038/s41375-020-0891-0. [DOI] [PMC free article] [PubMed] [Google Scholar]