Abstract
Background
The distribution of intestinal parasites among patients with tuberculosis in Ethiopia is not well understood.
Objective
This systematic review and meta-analysis was designed to determine the pooled national prevalence of intestinal parasites and its association with HIV among patients with tuberculosis in Ethiopia.
Methods
Original articles were searched in PubMed, Google Scholar, EMBASE, World Health Organization's HINARI portal, and supplemented by the hand searching of cross-references. Data were extracted using a standard data extraction checklist. Random-effects model was used to estimate the pooled prevalence of intestinal parasites and odds ratio of the association. The I2 statistic was utilized to quantify statistical heterogeneity across studies. Funnel plot asymmetry and Egger regression tests were used to check for publication bias. The analysis was done by STATA version 14 for Windows.
Results
Of 725 identified studies, 12 articles were eligible for inclusion in the final analysis. The pooled national prevalence of intestinal parasites among patients with tuberculosis in Ethiopia was 36.1% (95% CI, 22.1–50.1; I2 = 98.7%). Subgroup analysis based on study design indicated that the prevalence of intestinal parasite among case-control studies was 41.69% (95% CI, 28.6–54.8; I2 = 95.1%). The odds of intestinal parasites among patients with tuberculosis–HIV coinfection was not significantly different compared with patients with tuberculosis without HIV/AIDS (odds ratio = 0.99; 95% CI, 0.7—4.7; P = 0.96).
Conclusions
In Ethiopia, at least 1 out of 3 patients with tuberculosis have an intestinal parasite. These findings suggest a need of more attention on increasing screening tuberculosis patients for intestinal parasites and deworming interventions. (Curr Ther Res Clin Exp. 2020; 81:XXX–XXX)
Keywords: Ethiopia, HIV/AIDS, Intestinal parasite, Tuberculosis
Introduction
Parasitic and tuberculosis (TB) infections are among the most prevalent infections in humans in developing countries.1 The overlap of TB and parasitic disease morbidity presented with high and consistent figures all over the world.2, 3, 4, 5, 6 More than half of people with latent or active TB infections have intestinal parasite infection (IPI), which is common in high TB burden nations.7 In Africa, one-third of TB patients have an IPI8 that contributes to the high rate of therapeutic failure of pulmonary TB.9
It is evident that pulmonary TB and parasitic diseases were shown to be risk factors for each other and represented with high magnitude of comorbidity in developing countries.8,10 Coinfection may significantly inhibit the host's immune system, increase antibacterial therapy intolerance, and be detrimental to the prognosis of the disease. In addition, infection with parasitic diseases can alter the protective immune response to mycobacterium TB. This indicates that concomitant helminthic infections weaken immune resistance to mycobacterial infections.11,12 Furthermore, the presence of intestinal parasites affects the effectiveness of vaccine against TB and increases the chance of developing active TB diseases after vaccination.13
In addition, parasitic infection presented with more severe radiological pulmonary disease in the number of involved lung zones at the end of TB treatment. In particular, simultaneous intestinal helminth infection in patients with newly diagnosed TB alters a patient's immunity profile that could favor persistent mycobacterium TB infection and a more protracted clinical course of the disease.14,15
In developing countries, parasitic worm infections among TB patients are common and increases the chance for TB complications.16,17 Parasitic infection increases the chance of TB lung damage and induces the progression of a latent TB infection to active TB disease.16,17 The presence of high TB progression, delay in clinical response and sustained infectiousness leads to high transmission rate of TB to healthy individuals.18, 19, 20
There are factors that increase the risk of having parasites infection among TB patients. Personal hygiene, residence, eosinophils count, and habit of washing vegetables/fruits are some of the factors listed in previous studies.10,21,22 On 1 hand, the presence of immunocompromised diseases, such as HIV/AIDS, are associated with TB. On the other hand, HIV/AIDS is supposed to be a risk factor for IPIs.23 Currently, there is high prevalence of TB/HIV comorbidity that was estimated to be from 22% to 25.6% in Ethiopia.24, 25, 26 Parasitic infections represent a major public health problem in immunocompromised individuals.23 HIV infection accounts for the highest prevalence of IPIs among TB patients in comparison with TB patients without immunodeficiency,27 whereas another finding indicates significantly lower prevalence of IPIs among HIV-positive TB patients.2
In sum, high prevalence of TB and IPIs comorbidity can be explained by low level of socioeconomic and immunity status.28 Literature about the prevalence of and risk factors for intestinal parasites among these vulnerable populations exhorts policy makers and program planners to pay due attention and take appropriate measures. There is limited understanding of the extent of parasitosis comorbidities with TB in Ethiopia. The few available evidences are inconclusive and inconsistent. Hence, pointing out the overall level of comorbidity might alarm policy makers and ministries of health into developing interventional guidelines or bi-directional frameworks for effective treatment of TB and parasite infection comorbidity as well as successful implementation of a national TB control and prevention strategy. This systematic review and meta-analysis aimed to determine the pooled national prevalence of IPI and its association with HIV among patients with TB in Ethiopia.
Methods
Search approach and appraisal of studies
Articles were accessed through web-based electronic database searches, desk reviews of the grey literature, and cross-references of identified studies. The electronic databases searched were PubMed, Google Scholar, EMBASE, and the World Health Organization database portal for low- and middle-income countries that includes the Web of Science, SCOPUS, African Index Medicus, Cumulative Index to Nursing and Allied Health Literature, the World Health Organization Institutional Repository for Information Sharing, and African Journals Online databases. In addition, related articles were obtained through review of the grey literature available on institutional repository29 and from reviewing cross-references of identified articles.
Searching was done using the following key terms: intestinal diseases, parasitic, intestinal, diseases, parasitic, parasitic intestinal diseases, intestinal, parasites, intestinal parasites, intestinal helminthes, intestinal protozoa, soil-transmitted helminthes, tuberculosis, HIV, patients, and Ethiopia. These key terms were combined used AND and OR Boolean operators. To allow a comprehensive search strategy, these key terms were predefined that included all fields within records. Medical subject headings terms was also used to help expand the search in advanced PubMed search. The study period for searching of article was conducted from November 10, 2018, to February 1, 2019. Endnote citation manager software version X7 for Windows was utilized to collect and organize search outcomes and for removal duplicate articles.
Inclusion and exclusion criteria
All articles for the study were included if they met the following inclusion criteria: written in English-language, full-text articles on observational studies (case-control or cross-sectional), conducted in Ethiopia from 2004 to 2018, published in peer-reviewed journals or available at university repository, and used valid and reliable diagnostic criteria to diagnose intestinal parasite. Studies that did not report specific outcomes for intestinal parasites quantitatively were excluded from the final analysis.
Data abstraction and quality assessment
After preliminary assessment, 2 reviewers downloaded abstracts to assess for inclusion. The abstracts were assessed for agreement with the inclusion criteria. When it was unclear whether or not an abstract was relevant, it was included for retrieval. At this stage, articles deemed irrelevant or out of the scope of the study were excluded and the full-text of the remaining articles downloaded for a detailed review. Two reviewers then assessed the quality of potentially eligible articles using Newcastle-Ottawa Scale criteria30 that was validated previously.31 There are 3 main categories for Newcastle-Ottawa Scale criteria: selection, exposure, and comparability. A study can be awarded a maximum of 1 star for each numbered item within the selection and exposure categories. A maximum of 2 stars can be given for comparability categories. Then the number of stars that was given for each numbered item was computed to determine the quality score of each study. Discrepancies in quality assessment scores were resolved with a third reviewer whenever appropriate. The average of 2 independent reviewer's score was used to determine the quality of each article. The current systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocol guidelines.32
Data analysis
Using an Excel (Microsoft, Redmond, Washington) spreadsheet template based on Joanna Briggs Institute format, author name, publication year, region, dominant parasites, the age range of patients, and funding source were extracted from each study.33 These data were then imported to Stata version 14 software for Windows (Stata Corp, College Station, Texas) to compute the pooled prevalence of intestinal parasites and to examine the association with HIV. The statistical heterogeneity of study outcomes was assessed using the I2 statistic.34 Methodological heterogeneity was evaluated by comparing study design. Additionally, clinical heterogeneity that can arise from differences in participant characteristics (eg, sex, age, baseline disease severity, ethnicity, and comorbidities) and types or timing of outcome measurements was assessed with face-to-face discussion by the authors. First, we set and agreed on definitions of variations in sex, age, baseline disease severity, ethnicity, comorbidities, and types or timing of outcome measurements. Next, we discussed and reached consensus about how much this heterogeneity influenced our pooled analysis. A random-effects model was used to estimate the pooled prevalence of IPIs at a 95% CI. Because theoretically we expected that the study settings and socioeconomic contexts might differ radically across these studies, subgroup analysis was based on study design and group of parasites. We used visual examination of funnel plot asymmetry and Egger regression tests to check for publication bias.35 Meta-regression analysis was employed to identify the source of heterogeneity using publication years, sample size, and region as covariates. Sensitivity analysis was also performed to examine influence of each study on the overall effect size. We appraised the risk of bias using the 10-item rating scale developed by Hoy et al36 for prevalence studies. The tool assesses studies on 10 domains, including sampling, data collection, reliability and validity of study tools, case definition, and prevalence periods. Each study was assigned a score of 1 (yes answers to domain questions) or 0 (no answers to domain questions) for each domain, and these scores were summed to provide an overall study quality score. Scores of 8 to 10 were considered as having a low risk of bias, 6 to 7 a moderate risk of bias, and 0 to 5 a high risk of bias. For the final risk of bias classification, disagreements between the reviewers were resolved via consensus (see Supplemental Figure 1 in the online version).
Results
Identification and description of studies
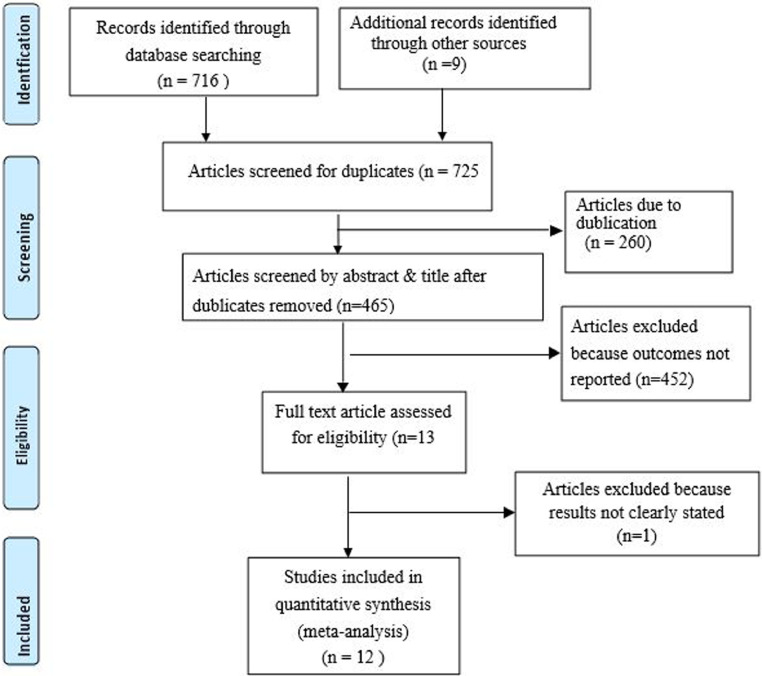
Of 725 identified studies, 260 duplicate articles were excluded after reviewing the titles and abstracts. Next, 452 articles were excluded with a reason of irrelevance. Finally, the full-text of the remaining 13 articles were downloaded and assessed for quality and for the presence of all required information. One article was excluded because the outcome was not clearly stated.17 The remaining 12 studies were included in the final meta-analysis (Fig. 1).
Figure 1.
Flow diagram showing the procedure of selecting of studies.
Characteristics of included studies
Twelve studies with a total sample of 1927 TB patients were included. Most of the study participants belonged to the age range 2 to 100 years. Eight studies were conducted in Amhara region,37, 38, 39, 40, 41, 42, 43, 44 2 in Oromia region,45,46 and 2 studies in Addis Ababa and SNNPR.21,22 All studies used direct microscope examination of stool to identify intestinal parasites. Half of the studies were case-control study. The quality score ranges from 6 to 7 with mean (SD) score of 6.8 (0.25) (Table 1).
Table 1.
Characteristics of included studies for the present systematic review and meta-analysis, 2004–2018, in Ethiopia.
| Reference | Publication year | Region | Diagnostic method for TB | Type of TB | Dominant parasite | Study design | Age of patients, y | Response rate, % | Funding | Prevalence, % | Quality score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Kassu et al43 | 2004 | Amhara | Histopathological evidence, sputum smear, radiographic examination consistent with TB, clinical response to anti-TB chemotherapy | Pulmonary and exteral pulmonary TB | Ascaris lumbricoides | Cross-sectional | Not stated | 100 | Ministry of Education, Culture, Sports, Science, and Technology of Japan; Yakult Ltd, Japan; Gondar University | 37.3 | 7 |
| Elias et al40 | 2006 | Amhara | Sputum smear microscopy | Pulmonary TB | A lumbricoides | Case control | ≥10 | 73.7 | Gondar University and SIDA/SAREC | 71 | 6.5 |
| Ramos et al45 | 2006 | Oromia | Sputum smear microscopy | Not specified | A lumbricoides | Cross-sectional | >12 | 100 | Not stated | 44 | 6.5 |
| Afework, et al42 | 2007 | Amhara | Clinical, radiological, histopathological, and laboratory features of the patients following standard procedures | Not specified | A lumbricoides | Cross-sectional | 15–80 | 100 | University of Gondar | 40.5 | 7 |
| Mohammed et al46 | 2011 | Oromia | Sputum smear microscopy | Pulmonary TB | Not stated | Case control | ≥15 | 100 | Jimma University | 40.1 | 7 |
| Abate et al37 | 2012 | Amhara | Sputum smear, radiographic examination | Pulmonary TB | A lumbricoides | Case control | 15–65 years | 100 | Not stated | 29 | 6.5 |
| Alemayehu et al39 | 2014 | Amhara | Sputum smear, Xpert MTB/RIF assay, and culture | Pulmonary TB | Hookworm | Cross-sectional | 2–80 | 100 | Not stated | 33.3 | 7 |
| Abate et al38 | 2015 | Amhara | Sputum smear, radiographic examination consistent with TB, clinical response to anti-TB chemotherapy | Not specified | A lumbricoides | Case control | 15–60 | 100 | Not stated | 40 | 7 |
| Hailu et al41 | 2015 | Amhara | AFB smear microscopy | Pulmonary TB | Not stated | Case control | 18–65 | 100 | Hawassa University | 49 | 7 |
| Alemu and Mama21 | 2017 | SNNPR | Sputum smear, radiographic examination | Pulmonary TB | A lumbricoides | Cross-sectional | 15–65 | 100 | Arba Minch University | 26.3 | 7 |
| Alemu et al20 | 2018 | Addis Ababa | Sputum smear, Xpert MTB/RIF assay, and culture | Pulmonary TB | Giardia lamblia | Case control | 19–34 | 100 | Addis Abeba University | 22 | 7 |
| Tegegne et al44 | 2018 | Amhara | Sputum smear | Pulmonary TB | A lumbricoides | Cross-sectional | ≥5 | 100 | University of Gondar | 2 | 6.5 |
AFB = acid-fast bacilli; MTB/RIF = mycobacterium tuberculosis/rifampin; SAREC = Sustainable Agriculture Research and Extension Center; SIDA = Swedish International Development Cooperation Agency; SNNPR = Southern Nations, Nationalities, and Peoples' Region; TB = tuberculosis.
Prevalence of intestinal parasite
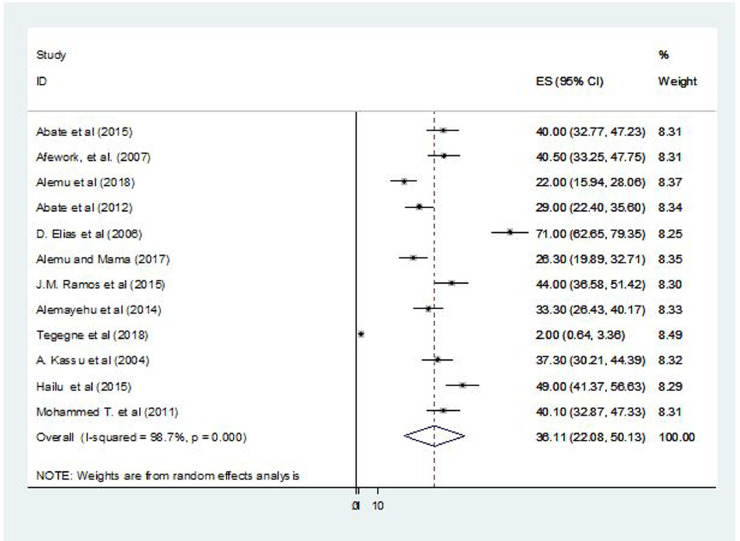
Both the lowest (2%)44 and highest (40.5%)42 prevalence of intestinal parasites were reported in Amhara region. The pooled national prevalence of intestinal parasites among TB patients in Ethiopia was 36.11% (95% CI, 22.08–50.13; I2 = 98.7%; P = 0.01) (Fig. 2). Visual examination of the funnel plot revealed deviation (see Supplemental Figure 2 in the online version). However, both Begg (P = 0.27) and Egger tests (P = 0.57) of the intercept indicated the absence of significant publication bias.
Figure 2.
Forest plot presentation showing the pooled prevalence of intestinal parasites among patients with tuberculosis in Ethiopia, 2004–2018.
Subgroup and sensitivity analysis
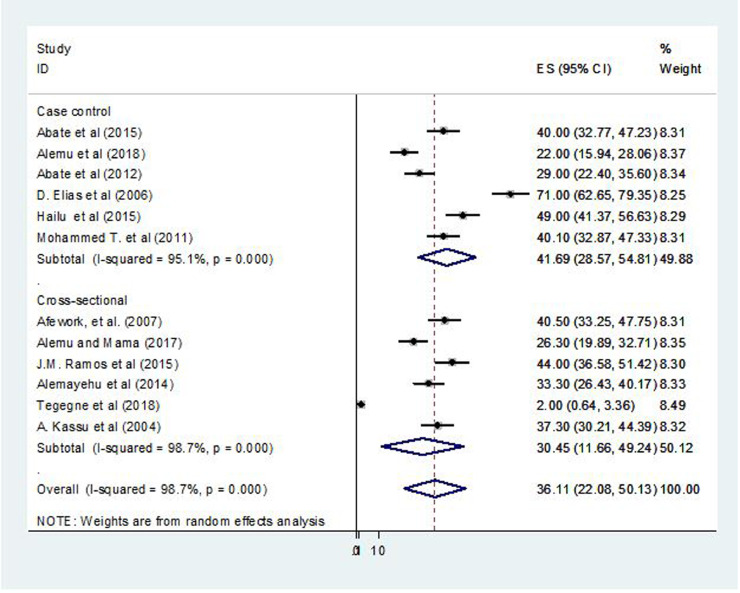
Based on the subgroup analysis, the pooled prevalence of intestinal parasites among case-control studies was 41.69% (95% CI, 28.57–54.81; P = 0.01; I2 = 95.1%) and 30.45% (95% CI, 11.66–49.24; P = 0.01; I2 = 98.7%) in cross-sectional studies (Fig. 3).
Figure 3.
Forest plot presentation showing the pooled prevalence of intestinal parasites by study design among patients with tuberculosis in Ethiopia, 2004–2018.
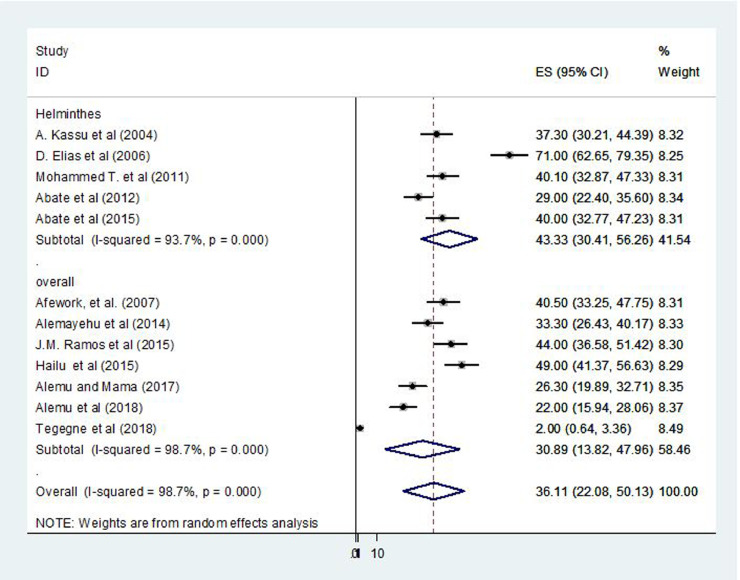
Additionally, subgroup analysis based on types of intestinal parasites showed that the highest percentage was reported for helminths species with a prevalence of 43.3% (95% CI, 30.4%–56.3%; I2 = 93.7%) (Fig. 4). The sensitivity analysis showed that none of the studies significantly influenced the overall pooled estimate of IPI.
Figure 4.
Forest plot presentation showing the pooled prevalence of intestinal parasites by category of intestinal parasite among patients with tuberculosis in Ethiopia, 2004–2018.
Meta-regression analysis
The univariate meta-regression analysis showed that a 1-person increases of sample size brought a variation on prevalence of each study by 0.2 (P = 0.03) (Table 2).
Table 2.
Meta regression results on selected variables.
HIV status and IPI
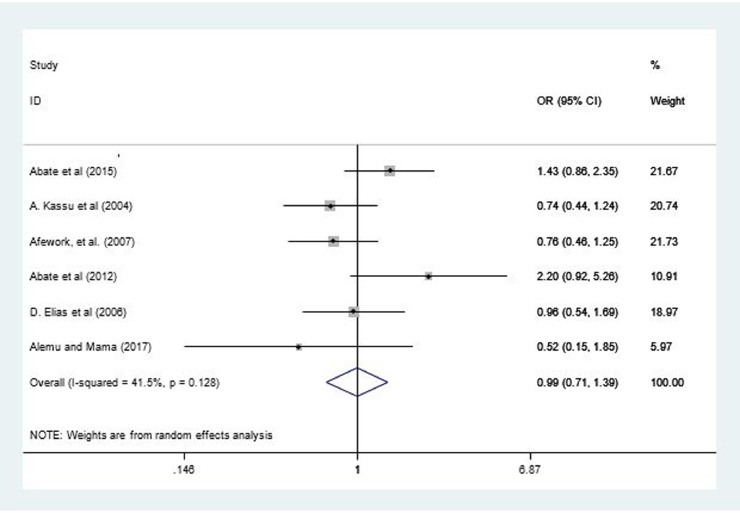
Six studies22,37,38,40,42,43 reported the association between HIV status and IPI. The odds of intestinal parasites among TB patients with HIV/AIDS was not significantly different compared with TB patients without HIV/AIDS (odds ratio = 0.99; 95% CI, 0.71–4.71; I2 = 41.5%; P = 0.13) (Fig. 5). Although the funnel plot showed slight deviation (see Supplemental Figure 3 in the online version), Begg (P = 0.57) and Egger (P = 0.91) tests showed the absence of significant publication bias.
Figure 5.
Forest plot of 6 studies examining the effect of HIV sero status on intestinal parasite among patients with tuberculosis in Ethiopia, 2004–2018.
Discussion
TB and intestinal parasites overlap geographically, and can represented with high prevalence in the area where there is poor socioeconomic status.47 Additionally, it is evident that due to complicated immune status of TB patients with immunodeficiency disorders, this group of patients are at higher risk of developing IPI.27 To our knowledge, this is the first meta-analysis in Ethiopia to provide the pooled national prevalence of intestinal parasites and its association with HIV among TB patients. The national prevalence of intestinal parasites among patients with TB was 36.11%. This finding is consistent with a report in Tanzania with prevalence of 31.8%.8
Our finding was high compared to a report in Egypt (16%)9 and China (21.7%).48 The Egyptian study9 was only assessed the burden of a single parasite, which was not a dominant parasite in our finding. In our study, Ascaris lumbricoides was the dominant parasite. Thus, high prevalence of A lumbricoides in our study might increase the overall IPIs prevalence and can be accounted for this variation. Ascariasis is the most common parasite that found in warm tropical and subtropical regions of the world, including Ethiopia.49 Due to the presence of high utilization of unsafe water, unhygienic living conditions, and unsanitary waste management,50 Ethiopia stands out for having the highest burden of ascariasis cases following Nigeria and the Democratic Republic of Congo. Almost one-third of Ethiopians have been infected with ascariasis.51 In A lumbricoides endemic areas, it is expected to have high rate of A lumbricoides and TB/HIV coinfection.52 Socioeconomic difference between Ethiopia and China explain this discrepancy. Ethiopia is low-income country whose peoples live under poverty whereby risk factors, such as poor sanitation, low health awareness, low urbanization, low health care service, and high burden of parasitosis are evident compared with China.53 In China, the Chinese Center for Disease Control and Prevention provide specialized hospital care for patients with IPIs. The presence of this care unit attributed to the nationwide scale-up of TB management and a national TB control program. Patients in a specialized care unit have better follow-up, receive appropriate examinations, treatment regimens, and rigorous supervision when compared with patients who take treatment at the hospital level.54 Consequently, the burden of IPIs in China might decrease.55
On the other hand, our finding was lower than the study report in Brazil (65%).7 This difference can be explained by the variation in the study area. Our study estimated the prevalence in studies conducted without consideration of socioeconomic class. However, the Brazilian study7 was conducted among a low-income community, in particular in the area where there is inadequate sanitary structure, latrines being shared with other rooms, well water as the main source of drinking water, and near to half of families had low income.
Our subgroup analysis result showed that estimate from case-control studies was higher than cross-sectional studies. This difference might happen related to the nature of sample selection. The unique advantage of cross-sectional surveys is that it is possible to determine the prevalence of an outcome because the study samples are selected randomly.56 Conversely, in case-control studies, cases and/or controls are selected on criteria related to the exposure of interest that can influence the overall prevalence.57 Based on the category of parasite subgroup analysis, the highest percentage was reported for helminths group with a prevalence of 43.3%. This finding was consistent with previous studies conducted among people with TB.2,10 However, our finding was totally different from large surveys report on non-TB people in Ethiopia,58 Ghana,59and Brazil60 with higher proportion of protozoal infection reported than helminths. This evidence suggests the presence of strong immunological and geographical connection between helminths and TB that contributes to high burden of helminthiases among TB patients.61, 62, 63
The present meta-analysis showed that the odds of intestinal parasites among TB patients was not significantly different in patients with or without HIV. Similarly, previous studies concluded that the presence of HIV could not contribute to high parasitosis among TB patients.8,10 This might be due to the presence of better follow-up for patients with HIV. Currently, there is better awareness of professionals in adopting prevention and treatment measures against opportunistic infection, such as intestinal parasites in HIV patients. In addition, the current Ethiopian antiretroviral therapy guideline recommend anthelminthic for antiretroviral therapy patients for deworming purpose. During the past few years, the presence of appropriate follow-up and treatment for IPIs at antiretroviral therapy clinics dramatically decreased the prevalence among HIV patients.64 Moreover, patients might regain their immunity after initiation of antiretroviral therapy that can minimize IPI.65
The presence high helminths infection among patients with TB in our finding implies that these patients are risk for sustained infectiousness that can lead to high transmission rate of TB to healthy individual. This issue directly interfere with the national TB control and prevention strategy.18 Antigens from helminths and mycobacteria have immunomodulatory activities that can affect immune responses for microbes. There is immunological evidence demonstrating that helminths clearly alter the magnitude of the mycobacteria-specific cytokine responses and the control of the mycobacteria growth. This delays the clinical response of TB patients.19,20
Ethiopia is a country that has a high burden of multidrug-resistant TB.66,67 Timely management of risk factors like intestinal parasitosis can improve the effectiveness of anti-TB treatment and reduce the magnitude of multidrug-resistant TB. It is known that the effectiveness of anti-TB treatment is greatly influenced by duration and patient adherence with anti-TB drugs.68 Patients with parasitosis comorbidities with TB presented with difficulty of adherence with anti-TB treatment and low effectiveness of overall TB therapy. Consequently, it increases the burden of health institution workload, the occurrence of new TB infection, and the national burden of multidrug-resistant TB.11
In most developing countries where TB infection is prevalent, parasitic infections are also predominant medical problems.11 Reducing the burden of these parasites is important to reduce the mortality and morbidity associated with TB infection. Parasitic infections have profoundly debilitating effects, particularly on the immune system of the host, potently compromising the host capacity to cope with TB infection and to mount efficacious immune responses. In addition, without the eradication of helminthic parasites, TB treatment would fail to confer protection for TB infection in TB endemic areas. This implies that eradication of helminthic infections or modulation of the immune change that they cause should be instituted before plans to reach TB zero prevalence in the country. For example, de-worming for eradication of helminthic infections throughout the country in the context of TB epidemics should be seriously considered.14,69
Study Strengths and Limitations
The present meta-analysis is the first in Ethiopia that can give nationally pooled evidence about the overall national burden of IPIs among patients with TB.
This meta-analysis has also limitations. First, most of the studies reviewed were published in small regional journals, making it difficult to gauge the extent of peer review and quality of studies. However, all included studies passed the quality screening criteria. Second, due to the absence of data, unadjusted odds ratios were used to estimate the effect size of HIV, which prevented us from excluding the confounding effect of other factors. The findings of this meta-analysis would be best interpreted while keeping these analytical limitations and the limitations of the original studies in mind.
Conclusions
In Ethiopia, at least 1 out of 3 patients with TB have intestinal parasites. These findings suggest a need for more attention on increasing screening of TB patients for intestinal parasites and deworming to reduce its influence on patients’ overall health status.
Declaration of Competing Interest
The authors have indicated that they have no conflicts of interest regarding the content of this article.
Acknowledgments
G. Dessie conceived and designed the research protocol. G. Dessie, B. Zeleke, H. Mulugeta, D. Amare, A. Negesse, D. Haile, T. D. Habetwold, B. Abebaw, and F. Wagnew conducted the literature review, data extraction, data analysis, interpreted the results and drafted the manuscript. G. Dessie, H. Mulugeta, and F. Wagnew assisted with assessment of quality of included studies. H. Mulugeta, T. D. Habetwold, F. Wagnew, and G. Dessie assisted in interpretation of results, manuscript revision and language copyediting. All authors were involved in revising and editing the manuscript.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.curtheres.2020.100603.
Appendix. Supplementary materials
Supplemental Figure 2 and 3. Meta funnel presentation for the studies include to determine prevalence of intestinal parasites among tuberculosis patients, 2004–2017, Ethiopia.
References
- 1.Haque R. Human intestinal parasites. Journal of health, population, and nutrition. 2007;25:387. [PMC free article] [PubMed] [Google Scholar]
- 2.Range N, Magnussen P, Mugomela A, Malenganisho W, Changalucha J. HIV and parasitic co-infections in tuberculosis patients: a cross-sectional study in Mwanza, Tanzania. Annals of Tropical Medicine & Parasitology. 2007;101:343–351. doi: 10.1179/136485907X176373. [DOI] [PubMed] [Google Scholar]
- 3.Escobar MA, Saravia NG, Weigle KA. Concurrent mucosal leishmaniasis and pulmonary tuberculosis. Clinical infectious diseases. 1996;23:836–837. doi: 10.1093/clinids/23.4.836. [DOI] [PubMed] [Google Scholar]
- 4.Delobel P, Launois P, Djossou F, Sainte-Marie D, Pradinaud R. American cutaneous leishmaniasis, lepromatous leprosy, and pulmonary tuberculosis coinfection with downregulation of the T-helper 1 cell response. Clinical infectious diseases. 2003;37:628–633. doi: 10.1086/376632. [DOI] [PubMed] [Google Scholar]
- 5.Duboucher C, Farto-Bensasson F, Chéron M, Peltier J-Y, Beaufils F. Lymph node infection by Trichomonas tenax: report of a case with co-infection by Mycobacterium tuberculosis. Human pathology. 2000;31:1317–1321. doi: 10.1053/hupa.2000.18502. [DOI] [PubMed] [Google Scholar]
- 6.Torresi J, Sievert W. Hepatosplenic schistosomiasis presenting as granulomatous hepatitis in an immigrant from the Philippines with pulmonary tuberculosis, tuberculous lymphadenitis, and a history of alcohol abuse. Journal of travel medicine. 2001;8:216–218. doi: 10.2310/7060.2001.22142. [DOI] [PubMed] [Google Scholar]
- 7.Cardoso BA, FdO Fonseca, AHAd Moraes Neto, Martins ACGS, NVdS Oliveira. Revista do Instituto de Medicina Tropical de São Paulo; 2017. Environmental aspects related to tuberculosis and intestinal parasites in a low-income community of the Brazilian Amazon; p. 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mhimbira F, Hella J, Said K, Kamwela L, Sasamalo M. Prevalence and clinical relevance of helminth co-infections among tuberculosis patients in urban Tanzania. PLoS neglected tropical diseases. 2017;11 doi: 10.1371/journal.pntd.0005342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hasanain AFA, Zayed AA-AH, Mahdy RE, Nafee AMA, Attia RA-MH. Hookworm infection among patients with pulmonary tuberculosis: Impact of co-infection on the therapeutic failure of pulmonary tuberculosis. International journal of mycobacteriology. 2015;4:318–322. doi: 10.1016/j.ijmyco.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Neto L, RdVCd Oliveira, Totino PR, Sant'Anna FM, VdO Coelho. Enteroparasitosis prevalence and parasitism influence in clinical outcomes of tuberculosis patients with or without HIV co-infection in a reference hospital in Rio de Janeiro (2000-2006) Brazilian Journal of Infectious Diseases. 2009;13:427–432. doi: 10.1590/s1413-86702009000600008. [DOI] [PubMed] [Google Scholar]
- 11.Li X-X, Zhou X-N. Co-infection of tuberculosis and parasitic diseases in humans: a systematic review. Parasites & vectors. 2013;6:79. doi: 10.1186/1756-3305-6-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elias D, Wolday D, Akuffo H, Petros B, Bronner U. Effect of deworming on human T cell responses to mycobacterial antigens in helminth‐exposed individuals before and after bacille calmette–guérin (BCG) vaccination. Clinical & Experimental Immunology. 2001;123:219–225. doi: 10.1046/j.1365-2249.2001.01446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elias D, Britton S, Kassu A, Akuffo H. Chronic helminth infections may negatively influence immunity against tuberculosis and other diseases of public health importance. Expert review of anti-infective therapy. 2007;5:475–484. doi: 10.1586/14787210.5.3.475. [DOI] [PubMed] [Google Scholar]
- 14.Resende Co T, Hirsch CS, Toossi Z, Dietze R, Ribeiro‐Rodrigues R. Intestinal helminth co‐infection has a negative impact on both anti‐Mycobacterium tuberculosis immunity and clinical response to tuberculosis therapy. Clinical & Experimental Immunology. 2007;147:45–52. doi: 10.1111/j.1365-2249.2006.03247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mkhize-Kwitshana ZL, Tadokera R, Mabaso MH. Helminthiasis: a systematic review of the immune interactions present in individuals coinfected with HIV and/or tuberculosis. Human Helminthiasis: InTech. 2017 [Google Scholar]
- 16.Monin L, Griffiths KL, Lam WY, Gopal R, Kang DD, Ahmed M. Helminth-induced arginase-1 exacerbates lung inflammation and disease severity in tuberculosis. The Journal of clinical investigation. 2015;125(12):4699–4713. doi: 10.1172/JCI77378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abate E. Linköping University Electronic Press; 2013. The impact of helminth infection in patients with active tuberculosis. [Google Scholar]
- 18.DiNardo AR, Nishiguchi T, Mace EM, Rajapakshe K, Mtetwa G. Schistosomiasis Induces Persistent DNA Methylation and Tuberculosis-Specific Immune Changes. The Journal of Immunology. 2018;201:124–133. doi: 10.4049/jimmunol.1800101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feleke BE, Alene GD, Feleke TE, Motebaynore Y, Biadglegne F. Clinical response of tuberculosis patients, a prospective cohort study. PloS one. 2018;13 doi: 10.1371/journal.pone.0190207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Méndez-Samperio P. Immunological mechanisms by which concomitant helminth infections predispose to the development of human tuberculosis. The Korean journal of parasitology. 2012;50:281. doi: 10.3347/kjp.2012.50.4.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alemu A. Addis Ababa Addis Ababa University; 2018. Intestinal Parasites Co-infection and Associated factors Among Active Pulmonary Tuberculosis Patients in Selected Health Centers, Addis Ababa, Ethiopia: A Case control stud [Research thesis] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alemu G, Mama M. Intestinal helminth co-infection and associated factors among tuberculosis patients in Arba Minch, Ethiopia. BMC infectious diseases. 2017;17:68. doi: 10.1186/s12879-017-2195-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.AK D, Gaber M, Hassan T, El Kady A, Badary D, Mahmoud H. Parasites Associated With Human Immune-Deficiency Virus (Hiv) Infection in Assiut University Hospitals, Egypt. Madridge J Vacc. 2018;2(1):49–54. [Google Scholar]
- 24.Teweldemedhin M, Asres N, Gebreyesus H, Asgedom SW. Tuberculosis-Human Immunodeficiency Virus (HIV) co-infection in Ethiopia: a systematic review and meta-analysis. BMC infectious diseases. 2018;18:676. doi: 10.1186/s12879-018-3604-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tesfaye B, Alebel A, Gebrie A, Zegeye A, Tesema C. The twin epidemics: Prevalence of TB/HIV co-infection and its associated factors in Ethiopia; A systematic review and meta-analysis. PloS one. 2018;13 doi: 10.1371/journal.pone.0203986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Endalamaw A, Ambachew S, Geremew D, Habtewold T. HIV infection and unknown HIV status among tuberculosis patients in Ethiopia: a systematic review and meta-analysis. The International Journal of Tuberculosis and Lung Disease. 2019;23:187–194. doi: 10.5588/ijtld.18.0363. [DOI] [PubMed] [Google Scholar]
- 27.Taghipour A, Azimi T, Javanmard E, Pormohammad A, Olfatifar M. Immunocompromised patients with Pulmonary Tuberculosis; a susceptible group to Intestinal parasites. Gastroenterology and Hepatology from Bed to Bench. 2018 [PMC free article] [PubMed] [Google Scholar]
- 28.Gabrie JA, Rueda MM, Rodriguez CA, Canales M, Sanchez AL. Vol. 2016. 2016. (Immune Profile of Honduran Schoolchildren with Intestinal Parasites: The Skewed Response against Geohelminths). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.AAU Institutional repository/Electronic Thesis and Dissertation at "http://www.aau.edu.et/library/resources/aau-institutional-repositoryelectronic-thesis-and-dissertation/, june 2018.
- 30.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. European journal of epidemiology. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 31.Hartling L, Hamm M, Milne A, Vandermeer B, Santaguida PL. 2012. Validity and inter-rater reliability testing of quality assessment instruments. [PubMed] [Google Scholar]
- 32.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of internal medicine. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 33.Munn Z, Tufanaru C, Aromataris E. JBI's systematic reviews: data extraction and synthesis. AJN The American Journal of Nursing. 2014;114:49–54. doi: 10.1097/01.NAJ.0000451683.66447.89. [DOI] [PubMed] [Google Scholar]
- 34.Institute JB Joanna Briggs Institute Reviewers’ Manual: 2017 edition. Australia: The Joanna Briggs Institute; 2017. Gaston et al Venous Thromboembolism (VTE) Risk Assessment and Prophylaxis: A Comprehensive Systematic.
- 35.Huedo-Medina TB, Sánchez-Meca J, Marín-Martínez F, Botella J. Assessing heterogeneity in meta-analysis: Q statistic or I² index? Psychological methods. 2006;11:193. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- 36.Hoy D, Brooks P, Woolf A, Blyth F, March L. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. Journal of clinical epidemiology. 2012;65:934–939. doi: 10.1016/j.jclinepi.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 37.Abate E, Belayneh M, Gelaw A, Idh J, Getachew A. The impact of asymptomatic helminth co-infection in patients with newly diagnosed tuberculosis in north-west Ethiopia. PLoS One. 2012;7:e42901. doi: 10.1371/journal.pone.0042901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abate E, Belayneh M, Idh J, Diro E, Elias D. Asymptomatic helminth infection in active tuberculosis is associated with increased regulatory and Th-2 responses and a lower sputum smear positivity. PLoS neglected tropical diseases. 2015;9 doi: 10.1371/journal.pntd.0003994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alemayehu M, Birhan W, Belyhun Y, Sahle M, Tessema B. Prevalence of smear positive tuberculosis, intestinal parasites and their co-infection among tuberculosis suspects in Gondar University Hospital and Gondar Poly Clinic, North West Ethiopia. J Microb Biochem Technol. 2014;6:179–184. [Google Scholar]
- 40.Elias D, Mengistu G, Akuffo H, Britton S. Are intestinal helminths risk factors for developing active tuberculosis? Tropical Medicine & International Health. 2006;11:551–558. doi: 10.1111/j.1365-3156.2006.01578.x. [DOI] [PubMed] [Google Scholar]
- 41.Hailu AW. The case control studies of HIV and Intestinal parasitic infections rate in active pulmonary tuberculosis patients in Woldia General Hospital and Health Center in North Wollo, Amhara Region, Ethiopia. International journal of pharma sciences. 2015;5:1092. [PMC free article] [PubMed] [Google Scholar]
- 42.Kassu A, Mengistu G, Ayele B, Diro E, Mekonnen F. HIV and intestinal parasites in adult TB patients in a teaching hospital in Northwest Ethiopia. Tropical doctor. 2007;37:222–224. doi: 10.1258/004947507782333026. [DOI] [PubMed] [Google Scholar]
- 43.Kassu A, Mohammad A, Fujimaki Y, Moges F, Elias D. Serum IgE levels of tuberculosis patients in a tropical setup with high prevalence of HIV and intestinal parasitoses. Clinical & Experimental Immunology. 2004;138:122–127. doi: 10.1111/j.1365-2249.2004.02597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tegegne Y, Wondmagegn T, Worku L, Jejaw Zeleke A. Prevalence of Intestinal Parasites and Associated Factors among Pulmonary Tuberculosis Suspected Patients Attending University of Gondar Hospital, Gondar, Northwest Ethiopia. Journal of parasitology research 2018. 2018 doi: 10.1155/2018/9372145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manuel Ramos J, Reyes F, Tesfamariam A. Intestinal parasites in adults admitted to a rural Ethiopian hospital: Relationship to tuberculosis and malaria. Scandinavian journal of infectious diseases. 2006;38:460–462. doi: 10.1080/00365540500525187. [DOI] [PubMed] [Google Scholar]
- 46.Taha M, Deribew A, Tessema F, Assegid S, Duchateau L. Risk factors of active tuberculosis in people living with HIV/AIDS in southwest Ethiopia: a case control study. Ethiopian journal of health sciences. 2011;21:131–140. doi: 10.4314/ejhs.v21i2.69053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simon GG. Impacts of neglected tropical disease on incidence and progression of HIV/AIDS, tuberculosis, and malaria: scientific links. International Journal of Infectious Diseases. 2016;42:54–57. doi: 10.1016/j.ijid.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 48.Disease Control Bureau of the Ministry of Health . People's Medical Publishing House; Beijing, China: 2008. National Institute of Parasitic Diseases of the Chinese Center for Disease Control and Prevention .Report on the national survey of current status of major human parasitic diseases in China. [Google Scholar]
- 49.Hadush A, Pal M. Ascariasis: Public Health Importance and its Status in Ethiopia. Air Water Borne Diseases. 2016;5:126. [Google Scholar]
- 50.King JD, Endeshaw T, Escher E, Alemtaye G, Melaku S. Intestinal parasite prevalence in an area of Ethiopia after implementing the SAFE strategy, enhanced outreach services, and health extension program. PLoS Negl Trop Dis. 2013;7:e2223. doi: 10.1371/journal.pntd.0002223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deribe K, Meribo K, Gebre T, Hailu A, Ali A. The burden of neglected tropical diseases in Ethiopia, and opportunities for integrated control and elimination. Parasites & vectors. 2012;5:240. doi: 10.1186/1756-3305-5-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alemu A, Kebede A, Dagne B, Amare M, Diriba G. Intestinal parasites co-infection and associated factors among active pulmonary tuberculosis patients in selected health centers, Addis Ababa, Ethiopia: unmatched case control study. BMC Infectious Diseases. 2019;19:407. doi: 10.1186/s12879-019-4009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hesham M, Edariah A, Norhayati M. Intestinal parasites infections and micronutrient deficiency. Med J Malaysia. 2003;58:2. [PubMed] [Google Scholar]
- 54.Wang L, Zhang H, Ruan Y, Chin DP, Xia Y. Tuberculosis prevalence in China, 1990–2010; a longitudinal analysis of national survey data. The Lancet. 2014;383:2057–2064. doi: 10.1016/S0140-6736(13)62639-2. [DOI] [PubMed] [Google Scholar]
- 55.Yuan L, Zhang H, Zhou C, Jiang W, Zhao Q. Better care provided to patients with tuberculosis at county designated TB hospitals (CTD) compared to non-CTDs in rural China. BMC infectious diseases. 2017;17:71. doi: 10.1186/s12879-016-2108-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Omair A. Selecting the appropriate study design for your research: Descriptive study designs. Journal of Health Specialties. 2015;3:153. [Google Scholar]
- 57.Bailey L, Vardulaki K, Langham J, Chandramohan D. In: Black N, Raine R, editors. Open University Press in collaboration with LSHTM; London: 2006. [Google Scholar]
- 58.Hailegebriel T. Prevalence of intestinal parasitic infections and associated risk factors among students at Dona Berber primary school, Bahir Dar, Ethiopia. BMC infectious diseases. 2017;17:362. doi: 10.1186/s12879-017-2466-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Forson AO, Arthur I, Olu-Taiwo M, Glover KK, Pappoe-Ashong PJ. Intestinal parasitic infections and risk factors: a cross-sectional survey of some school children in a suburb in Accra, Ghana. BMC research notes. 2017;10:485. doi: 10.1186/s13104-017-2802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Seguí R, Muñoz-Antoli C, Klisiowicz DR, Oishi CY, Köster PC. Prevalence of intestinal parasites, with emphasis on the molecular epidemiology of Giardia duodenalis and Blastocystis sp., in the Paranaguá Bay, Brazil: a community survey. Parasites & vectors. 2018;11:490. doi: 10.1186/s13071-018-3054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Babu S, Nutman TB. Helminth-tuberculosis co-infection: an immunologic perspective. Trends in immunology. 2016;37:597–607. doi: 10.1016/j.it.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Potian JA, Bhatt K, Liu Z, Gause W, Salgame P. Helminthic infection enhances susceptibility to tuberculosis in a murine coinfection model (43.31) Am Assoc Immnol. 2007 [Google Scholar]
- 63.Sikalengo G, Hella J, Mhimbira F, Rutaihwa LK, Bani F. Distinct clinical characteristics and helminth co-infections in adult tuberculosis patients from urban compared to rural Tanzania. Infectious diseases of poverty. 2018;7:24. doi: 10.1186/s40249-018-0404-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Missaye A, Dagnew M, Alemu A, Alemu A. Prevalence of intestinal parasites and associated risk factors among HIV/AIDS patients with pre-ART and on-ART attending dessie hospital ART clinic, Northeast Ethiopia. AIDS research and therapy. 2013;10:7. doi: 10.1186/1742-6405-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cerveja BZ, Tucuzo RM, Madureira AC, Nhacupe N, Langa IA. Prevalence of Intestinal Parasites Among HIV Infected and HIV Uninfected Patients Treated at the 1 De Maio Health Centre in Maputo, Mozambique. EC microbiology. 2017;9:231. [PMC free article] [PubMed] [Google Scholar]
- 66.Asgedom SW, Teweldemedhin M, Gebreyesus H. Prevalence of Multidrug-Resistant Tuberculosis and Associated Factors in Ethiopia: A Systematic Review. Journal of pathogens. 2018 doi: 10.1155/2018/7104921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Girum T, Muktar E, Lentiro K, Wondiye H, Shewangizaw M. Epidemiology of multidrug-resistant tuberculosis (MDR-TB) in Ethiopia: a systematic review and meta-analysis of the prevalence, determinants and treatment outcome. Tropical Diseases, Travel Medicine and Vaccines. 2018;4:5. doi: 10.1186/s40794-018-0065-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alipanah N, Jarlsberg L, Miller C, Linh NN, Falzon D. Adherence interventions and outcomes of tuberculosis treatment: A systematic review and meta-analysis of trials and observational studies. PLoS medicine. 2018;15 doi: 10.1371/journal.pmed.1002595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bentwich Z, Horner R, Borkow G. De-worming in developing countries as a feasible and affordable means to fight co-endemic infectious diseases. Open Biology Journal. 2010;3:97–103. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 2 and 3. Meta funnel presentation for the studies include to determine prevalence of intestinal parasites among tuberculosis patients, 2004–2017, Ethiopia.