Abstract
Background
Lifestyle involving uncontrolled alcohol consumption coupled regularly with red meat and other iron sources has detrimental effects on the liver, which in the long term, results in Alcoholic Liver Disease (ALD). Procyanidin has lately garnered increasing attention and has become the focus of research owing to its antioxidant properties. This study explores the anti-inflammatory effects of procyanidins, in preventing ALD, by analyzing the biological activities of the compound on liver injury caused by excessive alcohol and iron.
Method
Male SPF Wistar rats were placed in 4 groups; the control Group A (basic diet); the model Group B (excess alcohol 8–12 mL/kg/d and iron 1000 mg/kg diet); the low dose procyanidin Group C (model group diet plus 60 mg/kg/d of procyanidin); and the high dose procyanidin Group D (model group diet plus 120 mg/kg/d of procyanidin). Serum biochemical markers for liver damage were measured spectrophotometrically. The NFκB and IκB mRNA expression levels were determined using RT-PCR; the NFκB p65 and IκB protein expression levels were assessed via western blotting, while ELISA was used to detect serum inflammatory factors.
Results
The pathological score of the model Group B, low and high dose procyanidin Groups C and D were 6.58 ± 0.90,4.69 ± 0.70 and 2.00 ± 0.73, respectively (P < 0.05). The results showed that high alcohol and iron contents in the model group led to significant damage of liver structure, increased low-density lipoproteins (LDLs), steatosis, and increased levels of inflammatory cytokines. High amounts of procyanidins led to the preservation of the liver structure, production of high-density lipoproteins, and reduction in serum inflammatory cytokines while also significantly decreasing the expression levels of NFκB p65.
Conclusion
The results prove that procyanidins have hepatoprotective potential and could be effective in reversing histopathology, possibly by alleviating inflammation and improving lipid metabolism.
Keywords: Cell biology, Adaptation, Inflammation, Immune response, Microorganism, Enzymology, Immunology, ALD, Hepatic injury, Oxidative stress, Hepatoprotective
Cell biology; Adaptation; Inflammation; Immune response; Microorganism; Enzymology; Immunology; ALD; Hepatic injury; Oxidative stress; Hepatoprotective.
1. Introduction
Jacques Masquelier was the first person who began intensive research on procyanidins in the 1940s when he investigated the pine bark brew used by Native Americans to treat Scurvy. The researcher determined that monomeric proanthocyanidins were the main components that gave the brew its healing properties, while also being safe for consumption [1, 2]. Procyanidins, molecular formula C30H26O13, are polyphenols that are homo-oligomeric (epi)catechin having two B-ring hydroxyl groups and are built from (−)-epicatechin and flavan-3-ols (+)-catechin [1]. The compound can be categorized into A-type, which has an interflavan bond and an ether linkage between the carbon-2 and the hydroxyl group of the A-ring, while B-type only has a single interflavan bond connecting the carbon-4 of the B-ring to the carbon-6 or carbon-8 of the C-ring [3].
Procyanidin, a natural health food, is commonly found in plants such as vegetables, fruits, legumes, grains, and nuts. Foods such as red wine, grapes, berries, chocolate, cranberry juice, and certain varieties of apples are known to contain large amounts of procyanidins [4, 5, 6]. Procyanidins, also known as condensed tannins, have drawn much attention for their hepatoprotective effect at the microscopic level [7] and potent activities like inhibiting levels of NO, PGE2, TNF-α, and ROS, and altering NFκB and activating IκB [8, 9]. They have been under intense investigation by researchers as they exhibit cardioprotective, anti-oxidant, anti-cancer, anti-inflammatory, and anti-diabetic properties [6, 10, 11]. Their medicinal properties have seen their increased use in dietary supplements and alternative medicines [12].
The harmful effect of alcoholism counts for annually three million global causalities representing 5.3% of all deaths [13]. As the liver is the primary location for alcohol metabolism, it sustains the highest degree of damage [14]. Chronic alcoholism leads to alcohol-related liver disease, which is characterized by the development of hepatic steatosis, alcoholic hepatitis, fibrosis, cirrhosis, and may eventually lead to end-stage liver disease [15]. The early sign of heavy drinking is the build-up of fat in the liver, known as steatosis [15]. This simple steatosis leads to inflammation followed by fibrosis, which may eventually result in further damage as cirrhosis [16]. Alcoholic fatty liver is a reversible condition, fibrosis is variable, but cirrhosis in most cases is a sign of permanent damage [17].
The liver also plays a noteworthy role in iron homeostasis, a process that maintains plasma iron levels within a specific range [18]. High iron intake causes the activation of hepatocytes, which produce hepcidin that ensures homeostasis [19]. More than one-half of the cases with advanced ALD and one-third of the alcohol-dependent subjects exhibit high liver iron content [20], as alcohol-induced oxidative stress down-regulates hepcidin production [21, 22]. This increased iron buildup in the liver is associated with greater mortality from alcoholic cirrhosis, indicating a pathogenic role for iron in alcohol-related liver disease [22].
In the liver tissue, alcohol metabolism and high iron deposits are involved in the generation of reactive oxygen species (ROS) and oxidative stress development [23, 24], causing changes in lipid metabolism and inflammatory factors [25, 26]. Thus, drinking alcohol while eating iron-rich foods can have detrimental effects on one's health status as the two substances mediate and promote oxidative stress and hepatic fibrosis, revealing the close relation between hemochromatosis and excessive alcohol consumption [27], this means that these two substances, help in facilitating ALD development.
For determining whether procyanidin, a powerful antioxidant, can be used to rectify liver damage in patients with ALD and its effectiveness in preventing ALD, our study aimed to explore their positive effect on lipid metabolism and inflammation in rats with alcohol and iron-induced hepatic injury.
2. Materials and methods
2.1. Reagents
All reagents used in our research were of analytical purity. Grape seed procyanidin extract (GSPE), was obtained from Xi'an Tianxingjian Natural Biological Products Co., Ltd. China. At the same time, Iron(II) sulfate heptahydrate (FeSO4.7H2O) was purchased from Shanghai Maclean Biochemical Technology Co., Ltd. China, and ethanol was obtained from Sinopharm Chemical Reagent Beijing Co., Ltd. China.
2.2. Animal treatment and experimental design
Male SPF Wistar rats (n = 51, weighing 150–160 g) were bought from Shandong Lukang Pharmaceutical Co., Ltd. The animals (Certificate of Quality No. SCXK (Lu) 20140007) were provided humane care in compliance with international standards for the care and management of Laboratory Animals (Laboratory Animal Resources Institute, Life Science Commission, National Research Council, 1996). The research was approved by the Animal Ethics Committee of Qingdao Medical University. The animals (6 weeks old) were housed in a standard environment (25 ± 1 °C temperature, 55 ± 5% humidity, and 12/12 h natural light/dark cycle) with unlimited rodent chow and water access.
After acclimatizing the rats for three weeks, they were assigned randomly to four groups: A, Control Group (n = 11) was provided basic diet (iron content of 50 mg/kg) and normal saline; B, Model Group (n = 12) was provided with iron-rich food (1000 mg/kg) while administering 50% v/v ethanol (8 ml kg−1 d−1 for 2 weeks + 12 ml kg−1 d−1 gavage for 10 weeks); C, Low-dose Procyanidin Treatment Group (n = 14) and D, High-dose Procyanidin Treatment Group (n = 14) were given the same model group diet rich in alcohol and iron content, while also being administered procyanidin aqueous solution of 60 mg kg−1 d−1 and 120 mg kg−1 d−1, respectively. They were kept under strict observation.
After 12 weeks under sterile conditions, the animals were anesthetized with 30 mg/kg sodium pentobarbital. For serum lipid analysis, blood samples were drawn from the abdominal aorta. Liver biopsies were taken, and the tissue samples were divided into two portions; one treated for pathological and the other for biochemical analysis. The remaining samples were frozen in liquid nitrogen and stored at -80 °C.
2.3. Liver index calculation
| Formula for liver index calculation: Liver index (%) = liver mass/body mass × 100. |
2.4. Preparation of liver tissue for pathological examination
Formalin-fixed liver tissues (0.9 × 0.9 × 0.5 cm) were paraffin-embedded and stained with hematoxylin-eosin (HE) to assess inflammation and steatosis, as previously described [28, 29]. Pathological observation and photography were performed using an Olympus BX60 multi-function microscope. Liver pathology scoring was done as previously described by Yin et al [30].
2.5. Preparation of liver tissue for ultrastructural observation with transmission electron microscopy (TEM)
For ultrastructural observation, as previously described [28], a portion of tissue was fixed with 2.5% glutaraldehyde for 24 hrs at 4 °C for pathological analysis (1 × 1 × 3 mm) and then washed with 0.1M cacodylate buffer, dehydrated with ethanol, fixed with 1% citric acid, dehydrated with acetone, embedded with epoxy resin (EPON812). Then the ultra-thin sections (50–70 nm) were placed on grids, stained with uranyl acetate and lead citrate, and then observed in a TEM.
2.6. Preparation of serum and liver tissue for detection of serum enzyme indices and liver metabolic indicators
Blood samples were collected into sodium citrate monovette for measuring biochemical markers such as serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and gamma-glutamyl transpeptidase (GGT). These markers, along with serum and liver tissue TG, TC, LDL, and HDL were measured spectrophotometrically by the automatic biochemical analyzer (AU5400, Beckman, USA) according to the kit instructions, as described previously [31].
2.7. Preparation of liver tissue for detection of NFκB and IκB expression levels
2.7.1. Detection of NFκB and IκB mRNA expression levels in liver tissue
RNA extraction, cDNA synthesis, and RT-PCR were done as previously published methods [29, 32].
2.7.1.1. Primer synthesis
Specific primers of NFκB and IκB mRNA were designed by the literature search. The gene sequences and the specificity of each primer sequence were compared by the NCBI BLAST gene pool. Shanghai Shenggong Bioengineering Co. Ltd. synthesized the primers with β-actin as the internal reference. Listed in Table 1 are the primer sequences.
Table 1.
Primer sequences.
| Gene | Sequence | Product length (bp) |
|---|---|---|
| β-actin | Upstream: CCTAGACTTCGAGCAAGAGA | 140 |
| Downstream: GGAAGGAAGGCTGGAAGA | ||
| NFκB p65 | Upstream: TCTGTTTCCCCTCATCTTT | 155 |
| Downstream: TGGTATCTGTGCTTCTCTC | ||
| IκB-α | Upstream: ATGGAAGTGATTGGTCAGGTGA | 184 |
| Downstream: AGGCAAGATGGAGAGGGGTATT |
2.7.1.2. Extraction of sample total RNA
Tissue was homogenized with liquid nitrogen, then incubated with Trizol, and allowed to stand for 5–10 min at room temperature. Again 0.2 ml chloroform was added and mixed vigorously for 15–30s. It was then allowed to stand for 3 min and centrifuged at 12,000 rpm for 15 min at 4 °C. The aqueous phase containing the RNA was transferred to a new EP tube, 0.5 ml isopropanol was added, mixed, kept in an ice bath for 10 min, and centrifuged at 4 °C, 12,000 rpm for 10 min. The aqueous phase was transferred to a fresh EP tube again, precipitated with 75% ethanol in ice, centrifuged for 5 min at 12,000 rpm at 4 °C. It was then dried and treated with DEPC to dissolve RNA, then RNA concentration was detected and stored at -80 °C. Electrophoresis of 5 μL of RNA was done on 1% agarose gel to detect RNA integrity.
2.7.1.3. Reverse transcription synthesis of cDNA
TIANScript RT kit instructions were followed strictly. Preparation of RT reaction was done by combining the following components in a sterile microcentrifuge tube: 1μg total RNA, 2 μL Oligo (dT)15, 2 μL Super Pure dNTP and RNase-Free ddH2O to make a volume of 14.5 μL. The mixture was heated at 70 °C for 5 min and cooled rapidly on ice for 2 min. Briefly centrifuged with the addition of the following components: 0.5 μL 5X First-Strand Buffer (including DTT), 0.5 μL RNasin and 1 μL TIANScript M-MLV; and mixed gently, with incubation at 25 °C for 10 min, 42 °C for 50 min, followed by heating at 95 °C for 5 min. The reaction system was diluted to 50 μL with RNase-Free ddH2O and stored at -20 °C.
2.7.1.4. Real-time PCR reaction
The NFκB and IκB mRNA were amplified by the target gene primer and the reference gene primer by fluorescence quantitative PCR, and the dissolution curve was analyzed at 60–95 °C. The experiment was carried out in strict accordance with the product specifications. The reaction system was prepared by mixing 10μL of SuperReal PreMix Plus, 0.6μL of upstream primer (10 μM), 0.6μL of downstream primer (10 μM), 100ng of cDNA, 0.4μL of ROX Reference Dye and RNase-Free ddH2O to make a volume of 20μL. The PCR amplification procedures included initial denaturation at 95 °C for 15 min, followed by 40–50 cycles at 95 °C for 10 s, 58 °C for 30 s, and 72 °C for 30 s. The samples were heated gradually from 72 °C to 95 °C to obtain melting curves and the fusion temperature of amplicons. See Supplementary Figure 1 for amplification and dissolution curves.
Relative quantitative analysis of the data was performed using the 2–∆∆Ct method. The formula is as follows: the ratio of the target gene of the intervention group to the target gene expression of the control group = 2–∆∆Ct, △△Ct=(Ct1-Ct2) -(Ct3-Ct4),
Ct1: Ct value of the gene of the intervention group sample;
Ct2: Ct value of the internal reference gene of the intervention group sample;
Ct3: Ct value of the target gene of the control group;
Ct4: Ct value of the internal reference gene of the control group.
2.7.2. Western blot analysis
The western blot analysis was done according to a published method [33]. Proteins were extracted from liver homogenates by SDS-polyacrylamide gel electrophoresis and then transferred onto a PVDF membrane (Millipore, Bedford, MA) for 1 h at room temperature. The membranes were blocked with TBST containing 10% skimmed milk for 2 h at room temperature and then probed with the primary antibody against NFκB p65 (1:1000) (Santa Cruz Biotechnology, California, US), IκB (1:1000) (Santa Cruz Biotechnology, California, US), β-actin (1:5000) (Easybio, Beijing, China), and Histone H3 (1:5000) (Easybio, Beijing, China) overnight at 4 °C. After three 10-minute washes in TBST, the membranes were incubated with the horseradish peroxidase-conjugated secondary antibody (1:10000) at room temperature for 60 min. The membranes were washed with TBST three times. The protein bands were then visualized with an enhanced chemiluminescence detection kit (Beyotime, Shanghai, China). The blots were analyzed by scanning densitometry with a GS-700 imaging densitometer and Quantity One software. The optical density ratio of the target proteins to their internal reference proteins was used as the relative expression of the target protein.
2.8. ELISA method for detection of serum inflammatory factors
Serum TNF-α, IL-6, IL-10, and IL-4 were detected strictly following the kit (USCN KIT Inc., Hubei, China) instructions, as previously reported [34].
2.9. Statistical analysis
Statistical analysis was performed on SPSS 17.0 software. ANOVA was used to compare multiple groups, expressed as (±S), the test level α = 0.05. P < 0.05 was considered statistically significant.
3. Results
3.1. Liver index of rats in each group
There was a statistically significant difference in the liver index when comparing the high dose procyanidin Group D to the model Group B and low dose procyanidin Group C (P < 0.05) (Supplementary Table 1).
3.2. Effect of procyanidins on pathology (H&E) and ultrastructure (TEM) of rat liver tissue
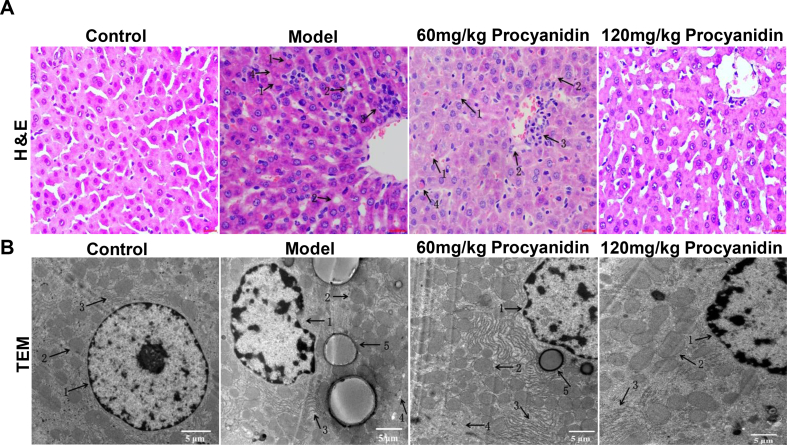
H&E staining of the control Group A showed normal lobular structure, orderly hepatic cord, absence of steatosis. In the model Group B, alcohol and iron exhibited liver damage such as Mallory body, fat vacuoles, inflammatory infiltration, hepatocyte hyaline degeneration, and microvesicular steatosis. Alcohol and iron significantly increased liver pathology score in the model Group B as compared to the control Group A. In the different procyanidin Groups C and D, the liver pathology score was improved, the structure of the hepatic lobules was also damaged, but the degree of hepatocyte necrosis was significantly reduced in comparison with the model Group B. In the low dose procyanidin Group C, fat droplets were found in the cytoplasm of the hepatocytes, the hepatic cord was irregularly arranged and showed inflammatory cell infiltration. In high dose procyanidin Group D, steatosis was reduced, and hepatic histopathological changes improved significantly, hepatic cord arrangement and tissue structure were normal. High dose procyanidin improved liver pathology score and steatosis markedly as compared to low dose procyanidin. The pathological score of the model Group B, low and high dose procyanidin Groups C and D are 6.58 ± 0.90, 4.69 ± 0.70, and 2.00 ± 0.73, respectively (P < 0.05) (Figure 1A) (Supplementary Table 2).
Figure 1.
Effect of procyanidins on pathology and ultrastructure of rat liver tissue. (A) Representative histological sections of H&E stained rat liver tissue at 12 weeks in each group. 1: Mallory body; 2: Fat Vacuoles; 3: Inflammatory Infiltration; 4: Hepatocyte Hyaline Degeneration. (B) Ultrastructure of rat liver tissue at 12 weeks in each group. 1: Nucleus; 2: Mitochondria; 3: Rough Endoplasmic Reticulum; 4: Capillary Bile Duct; 5: Lipid Droplets. Scale bar = 50 μm for A, 5 μm for B.
Hepatic ultrastructure of the control Group A showed hepatocytes without droplet fat, intact nuclear membrane, oval or round nuclei, clear nucleolus, clear ridge structure, normal mitochondrial morphology and rough endoplasmic reticulum, abundant ribosomes, capillary bile ducts, and tight junctions were in the cell boundary when compared to the model Group B. The model Group B showed the hepatic cells with irregular shape and increased droplet fat, mitochondria showed deformity with swelling, the nuclear membrane was fuzzy or irregular, ridge structure was fuzzy, lysosomes increased, and the rough endoplasmic reticulum showed disorganization, swelling, and fracture. In the low dose procyanidin Group C, the mitochondrial lesion decreased, and the number of mitochondria increased, the degree of rough endoplasmic reticulum breakage and disorder was improved, and the number of lysosomes and lipid droplets decreased. In high dose procyanidin Group D as compared to the model Group B the morphology of liver cells was normal, with the absence of fat droplets in the cytoplasm, the nuclear membrane remained intact, the mitochondria were nearly normal, with reduced pathological changes, while the disordered and degraded rough ER improved (Figure 1B).
3.3. Effect of procyanidins on liver function and lipid metabolism in rats
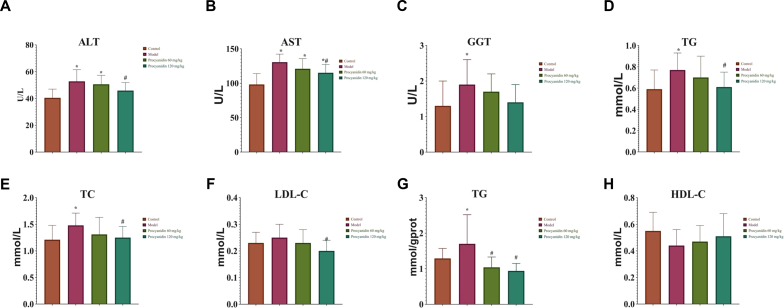
Serum marker activity of liver functions showing levels of ALT, AST, and GGT in the model Group B were increased significantly in comparison with the control Group A (P < 0.05). The procyanidin Groups C and D showed a dose-dependent decrease in ALT, AST, and GGT levels. Model Group B showed an escalation in the plasma level concentrations of TG, TC, LDL, and a decrease in HDL. Plasma lipid levels were not improved in low dose procyanidin treatment. High dose procyanidin reduced TG, TC, LDL, and increased HDL, suggestive of improved hepatic and blood lipid levels (P < 0.05) (Figure 2) (Supplementary Table 3 and 4).
Figure 2.
Effect of procyanidins on liver function and lipid metabolism in rats. The serum lipid levels of (A) ALT, (B) AST, (C) GGT showed a significant reduction with the increased dosage of procyanidins as compared to the model Group B. The lipid metabolic indicators (D) TG mmol/L, (E) TC, (F) LDL-C, (G) TG mmol/gprot showed reduction after treatment with procyanidin, with the exception of (H) HDL-C which showed an increase in high dose procyanidin Group D (∗P < 0.05 vs Control; #P < 0.05 vs Model).
3.4. Effect of procyanidins on NFκB, IκB protein and mRNA expression levels in rat liver tissue
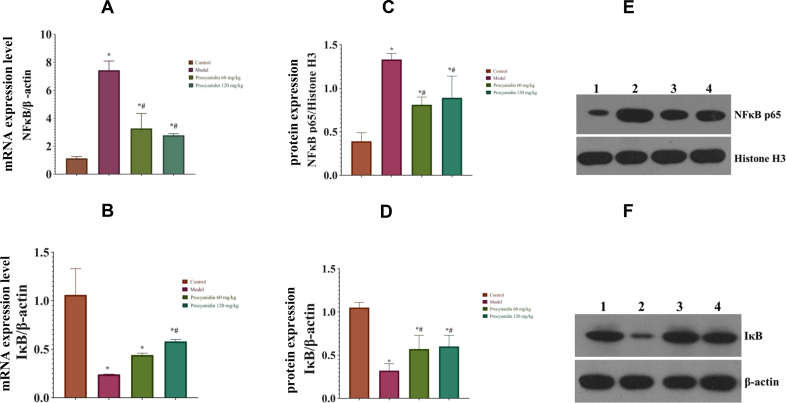
Compared to model Group B, procyanidin treated Groups C and D had significantly reduced mRNA expression levels of NFκB (P < 0.05). The expression level of IκB mRNA in the procyanidin Groups C and D significantly increased (P < 0.05), as compared to the model Group B. The western blotting results revealed, NFκB p65 protein expression level was decreased, and IκB protein expression level was increased in procyanidin Groups C and D as compared to the model Group B (P < 0.05). Procyanidins in a dosage-dependent manner affect protein, and their expression levels (Figure 3) (Supplementary Table 5 and 6).
Figure 3.
Effect of procyanidins on NFκB, IκB protein, and mRNA expression levels in rat liver tissue. (A) The above graphical representations show that NFκB mRNA expression is increased in the model Group B but lowered after procyanidin treatment. (B) The IκB mRNA expression levels are increased in the procyanidin treated groups. (C) NFκB protein levels increased in model Group B as compared to the procyanidin groups (D) IκB levels are increased in procyanidin treatment groups. (E) and (F) Western blot analysis was used to detect the expression levels of NFκB p65 and IκB protein in liver tissue. 1: Control Group A; 2: Model Group B; 3: Low dose procyanidin Group C; 4: High dose procyanidin Group D (∗P < 0.05 vs Control; #P < 0.05 vs Model). The full, uncropped images of the western blots are presented in Supplementary Figure 2.
3.5. Effect of procyanidins on serum inflammatory factor levels in rats
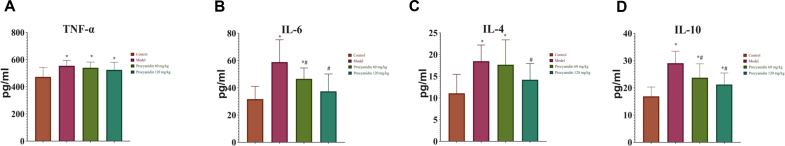
The concentration of cytokines TNF-α, IL-6, IL-4, and IL-10 were measured and found to be elevated in the model Group B. TNF-α and IL-4 concentrations increased in the low dose procyanidin Group C compared to the control Group A, but no significant difference compared to the model Group B. The concentrations of IL-6 and IL-10 in low dose Group C significantly increased as compared to the control Group A and decreased comparatively with the model Group B. High dose procyanidin treated Group D showed significant improvement in cytokine levels as compared to the model Group B, signifying the anti-inflammatory effect of procyanidins (P < 0.05) (Figure 4) (Supplementary Table 7).
Figure 4.
Effect of procyanidins on serum inflammatory factor levels in rats. (A) TNF-α, (B) IL-6, (C) IL-4, (D) IL-10 levels are reduced with the increasing dose of procyanidins as compared with the model Group B (∗P < 0.05 vs Control; #P < 0.05 vs Model).
4. Discussion
The outcome of the study highlights that procyanidins, members of the procyanidin class of flavonoids, are effective in reversing the adverse effects of high alcohol consumption and dangerous levels of iron in the liver, which are responsible for causing ALD. These beneficial effects of procyanidin are obvious from the following improvements: decreased histopathological damage, suppressed serum biochemical indices, improved serum lipid profiles, attenuated serum inflammatory cytokines, down-regulated NFκB mRNA, and NFκB p65 expressions, and up-regulated IκB mRNA expression. Procyanidin's hepatoprotective effect could be the result of its ROS scavenging nature, whose overproduction and buildup are sparked by oxidative stress, thereby alleviating hepatic injury. These findings strongly suggest that procyanidin can effectively prevent the progression of hepatic damage caused by high alcohol and iron. The result of the study correlates with previous findings on similar subjects that evaluated the effect of procyanidins on liver functions. A study by Blumberg et al. also indicated that procyanidins in cranberry juice resulted in better serum lipid profiles, glucoregulation, and improvement in the activities of serum biomarkers for inflammation control [35]. In another study, researchers confirmed the protective ability of procyanidin against hepatotoxicity in rats at the mitochondria level, owing to its antioxidant, metal chelating, and free radical scavenging properties [7].
Previous studies have shown that alcohol-iron exposure can cause histopathological changes in the liver, like hepatocyte swelling, the appearance of fat droplets, inflammatory cell infiltration, and hepatic cord derangement, reflecting the functional and morphological damage in alcohol-related liver injury [20]. Typical ALD characteristics demonstrated in Group B included the existence of Mallory bodies, fat vacuoles, inflammatory cell infiltration, hepatocyte hyaline degeneration, and microvesicular steatosis. Although there was evidence of liver damage in the procyanidin groups C and D, the degree of hepatocyte necrosis was significantly lower compared to the model Group B, as indicated by HE. However, a better recovery was observed in the high-dose procyanidin Group D, with animals exhibiting less steatosis, inflammation, and necrosis, showing that the effect of procyanidin in addressing liver damage is dose-dependent. Our analysis revealed that procyanidin intervention alleviated inflammation and fat accumulation in the liver and improved pathological injuries, the result being consistent with a previous report [36].
The high presence of iron in the liver is a major cause of oxidative stress, as research shows that it induces and increases the Fenton reaction, resulting in high production of free radicals in large amounts [37, 38]. The free radicals-antioxidant interaction is a normal process that occurs in hepatocyte cells, where the liver tries to limit the free radicals production and accumulation in the body through antioxidants [39, 40]. When the ROS production surpasses the ability of the antioxidants to neutralize them, it is referred to as oxidative stress [41]. ROS such as hydroxyl radicals, singlet oxygen, superoxide radicals, and hydrogen peroxide are produced usually by the mitochondria of inflammatory and immune cells such as the Kupffer cells and in other processes such as arachidonic acid metabolism [41]. Cells use superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) as antioxidants to defend themselves against the increased ROS in the body, they are not effective against high amounts of ROS produced when the presence of iron in the liver is high [38, 41]. The high production of ROS affects the other cell organelles, which was noted in the model Group B where the liver cells presented irregular shapes with increasing droplet fat characterized by a fuzzy or irregular nuclear membrane, swollen and deformed mitochondria, larger lysosomes, fuzzy ridge structure, with a swollen, fractured and disorganized rough endoplasmic reticulum, as indicated by TEM.
Enzyme markers, such as ALT, AST, and GGT, are widely used in animal research as indices of liver function and injury [42, 43]. The liver TG level is known to be an indicator of hepatic lipid accumulation [44]. In the current study, elevated serum ALT, AST, GGT, and liver TG levels verified the hepatic injury in rats treated with alcohol and iron. The results demonstrated that procyanidin intervention could decrease the activity levels of serum ALT, AST, GGT, and liver TG. In conclusion, procyanidin induced hepatoprotective effects in alcohol-iron induced liver injury, as indicated by liver histopathological examinations and liver function-enzyme assays.
Ioannou et al. had previously stated that chronic alcohol consumption causes increased ferritin concentration and serum transferrin saturation, and also elevated hepatic iron stores [45]. Alcohol also increases cholesterol metabolism in the liver by elevating its synthesis, absorption, and excretion while limiting the absorption of cholesterol in the intestines [31, 46]. Silva et al. found that high iron intake could cause lipid metabolism disorder in rats [47]. Alcohol and iron also cause high serum LDL concentration and proliferation of serum markers, which result in the interference of hepatocyte functions, thus making alcohol and iron, the immediate culprits of causing hepatosteatosis [48, 49]. In contrast, high levels of procyanidins are associated with the prevention of hepatosteatosis due to the downregulation of production of TG, TC, and LDL, which resulted in the preservation of liver structure and functions as found in the study.
The result of the experiment also showed that there was a marked increase in serum inflammatory cytokines TNF-α, IL-6, IL-4, and IL-10 in the model Group B. The high dose procyanidin Group D showed significant improvement in cytokine levels as compared to the model Group B signifying the anti-inflammatory effect of procyanidins [1, 8]. Research shows that ethanol-mediated microbial colonization and increase in the gut opens up the tight junctions in the intestines, facilitating the release of large amounts of endotoxins into the intestinal lumen and subsequently transported to the liver [50]. The inability of the liver to clear these high amounts of endotoxins in the blood causes them to accumulate, resulting in the activation of the immune system [51, 52]. This activation is mainly by Kupffer cells, which release large amounts of chemokines and pro-inflammatory cytokines in response to the metabolic and functional deficiencies brought about by the high presence of endotoxins [53]. Lipopolysaccharide (LPS) and other endotoxins affect the biological functions of the non-immune, immune, and parenchymal cells, and the observed inflammatory response in the experiment is usually a primary occurrence in the development of ALD.
Another observation in the experiment that can be explained using research findings is the increased level of protein expression of NFκB p65 and subsequent inhibition of IκB in the model Group B. The occurrence of this phenomenon is related to the ethanol-mediated proliferation of gram-negative bacteria in the gut, which results in the production of endotoxins. The IκB helps in the cellular reaction to inflammation and is usually phosphorylated by LPS, antigen receptors, growth factors, and cytokines through activation of the IKK complex [54]. The activity of the IκB is affected by the upregulation of NFκB p65 in the presence of high amounts of intravenous endotoxins because the latter participates in the production of cytokines, which explains why the serum inflammatory cytokines TNF-α, IL-6, IL-4, and IL-10 were observed in high amounts in the model Group B [54]. The NFκB dimer is usually kept inactive by IκB in the cytoplasm of normal cells. But when the body is invaded by pathogens, the NFκB p65 signaling pathway is activated, causing an immune response [54]. The activation of the NFκB helps in the production of antibodies that try to limit the number of endotoxins in the bloodstream. It is usually a crucial stage in ALD development as it signifies the inability of the liver to eliminate numerous endotoxins in the blood, requiring the innate immune response to help in ameliorating the conditions. In this study, high dose procyanidin Group D showed a lower NFκB activity in the liver, which is a sign of a reduction in the expression of hepatic inflammatory markers, underlining the ability of procyanidins to control liver inflammation by inhibiting the production of proinflammatory cytokines.
A study conducted by Terra et al. compared rats fed with a high-fat diet with those on a high-fat diet together with procyanidins from grape seeds. The researchers found that rats fed with only a high-fat diet had high production of C-reactive protein (CRP), while those that consumed procyanidins had lower plasma CRP levels, which is because of the downregulation of CRP mRNA expression especially in the mesenteric white adipose tissue and the liver [55]. The researchers also found out that procyanidin downregulated the expression of TNF-α and IL-6 pro-inflammatory cytokines, while increased adiponectin mRNA levels in the mesenteric white adipose tissue [55]. The study shows that procyanidin controls CRP when it is being synthesized, and in addition to the inhibition of the expression of proinflammatory cytokines TNF-α and IL-6 and the upregulation of anti-inflammatory cytokine adiponectin the compound, can be useful in treating inflammatory illnesses [55]. The findings from this study and that from the literature underline the potential of procyanidins in lowering obesity-related adipokine dysregulation, which can be useful in managing metabolic and cardiovascular risk factors.
Researchers have also investigated the effectiveness of procyanidins in preventing oxidative stress and preserving liver integrity. In a study by Decorde et al., the researchers examined the effectiveness of polyphenolic grape seed extract (GSE) in overcoming obesity by reducing oxidative stress and addressing adipokine imbalance [56]. The study showed that procyanidins in the GSE reduced abdominal fat, insulinemia, higher plasma glucose, and leptinemia by more than 16.5% and increased the levels of adiponectin by 61% [56]. Rodríguez-Ramiro et al. determined that cocoa polyphenolic extract together with procyanidin, are effective in preventing oxidative stress caused by dietary acrylamide, by enhancing the redox status of cells and by obstructing the apoptotic pathways created by acrylamide [57]. Polyphenols such as procyanidin and epicatechin can greatly help to improve personal health.
The effectiveness of polyphenols such as procyanidins, epicatechin, phenolic acids, carotenoids, and flavonoids in preventing a myriad of illnesses such as cancers, tumors, diabetes, heart problems, liver issues, and neurogenerative disorders is due to their activities as antioxidants [56]. Antioxidants are free radical scavengers, helping to control the production of these reactive oxygen species (ROS) that are responsible for damaging small blood vessels and releasing inflammatory cytokines that result in organ damages [58]. When ROS production overwhelms the body's ability to regulate them, oxidative stress ensues a condition that adversely alters proteins, lipids, and DNA [59]. Therefore, the ability of antioxidants such as procyanidins, to control the production of ROS and reduce oxidative stress is significant in preventing diseases.
Patients with chronic ALD are usually at risk of liver failure, which can put their lives in danger when the situation is not addressed. The present study highlighted the potential of procyanidins to attenuate the debilitating effects of excess alcohol consumption and iron overload, which are the major risk factors of acquiring ALD [60, 61]. The compound can be used by individuals who are at risk of getting ALD, and in alleviating situations where prevention of liver inflammation is required to ensure the liver disease does not worsen. Procyanidins are highly abundant in plants and can be a cheaper alternative to enhancing personal health as supplements instead of relying on expensive medications. This study shows that there is great potential in using the compound to treat and prevent other ailments emanating from oxidative stress in the liver, such as diabetes and associated illnesses.
Declarations
Author contribution statement
A. Lobo: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Y. Liu: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data.
Y. Song: Performed the experiments.
S. Liu and R. Zhang: Analyzed and interpreted the data.
H. Xin and H. Liang: Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by National Natural Science Foundation of China (Grant No. 81573137, Grant No. 31171671) and Key Research and Development Program in Shandong Province of China (Grant No. 2017GSF18167).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Thanks to Feixiang Zhan from the Realbio Genomics Institute for his valuable assistance with regards to this research paper.
Contributor Information
Hui Liang, Email: qdlianghui@126.com.
Hui Xin, Email: xinhuiqy@163.com.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Rue E.A., Rush M.D., van Breemen R.B. Procyanidins: a comprehensive review encompassing structure elucidation via mass spectrometry. Phytochemistry Rev. 2018;17(1):1–16. doi: 10.1007/s11101-017-9507-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rastogi S., Arora V.S., Bhalla V. 2015. Pycnogenol: the hercules of Antioxidants. [Google Scholar]
- 3.Bittner K., Rzeppa S., Humpf H.U. Distribution and quantification of flavan-3-ols and procyanidins with low degree of polymerization in nuts, cereals, and legumes. J. Agric. Food Chem. 2013;61(38):9148–9154. doi: 10.1021/jf4024728. [DOI] [PubMed] [Google Scholar]
- 4.Gu L. Concentrations of proanthocyanidins in common foods and estimations of normal consumption. J. Nutr. 2004;134(3):613–617. doi: 10.1093/jn/134.3.613. [DOI] [PubMed] [Google Scholar]
- 5.Hammerstone J., Lazarus S., Schmitz H. Procyanidin content and variation in some commonly consumed foods. J. Nutr. 2000;130 doi: 10.1093/jn/130.8.2086S. 2086S-92S. [DOI] [PubMed] [Google Scholar]
- 6.Rauf A. Proanthocyanidins: a comprehensive review. Biomed. Pharmacother. 2019;116:108999. doi: 10.1016/j.biopha.2019.108999. [DOI] [PubMed] [Google Scholar]
- 7.Miltonprabu S., Nazimabashir, Manoharan V. Hepatoprotective effect of grape seed proanthocyanidins on Cadmium-induced hepatic injury in rats: possible involvement of mitochondrial dysfunction, inflammation and apoptosis. Toxicol. Rep. 2016;3:63–77. doi: 10.1016/j.toxrep.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gentile C. Polymeric proanthocyanidins from Sicilian pistachio (Pistacia vera L.) Nut extract inhibit lipopolysaccharide-induced inflammatory response in RAW 264.7 cells. Eur. J. Nutr. 2012;51(3):353–363. doi: 10.1007/s00394-011-0220-5. [DOI] [PubMed] [Google Scholar]
- 9.Vázquez-Agell M. Cocoa consumption reduces NF-κb activation in peripheral blood mononuclear cells in humans. Nutr. Metabol. Cardiovasc. Dis. 2013;23(3):257–263. doi: 10.1016/j.numecd.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 10.Arranz S. Cardioprotective effects of cocoa: clinical evidence from randomized clinical intervention trials in humans. Mol. Nutr. Food Res. 2013;57(6):936–947. doi: 10.1002/mnfr.201200595. [DOI] [PubMed] [Google Scholar]
- 11.Gossé F. Chemopreventive properties of apple procyanidins on human colon cancer-derived metastatic SW620 cells and in a rat model of colon carcinogenesis. Carcinogenesis. 2005;26(7):1291–1295. doi: 10.1093/carcin/bgi074. [DOI] [PubMed] [Google Scholar]
- 12.Oligomeric proanthocyanidins (opcs) Monograph. Altern Med Rev. 2003;8(4):442–450. [PubMed] [Google Scholar]
- 13.Global Status Report on Alcohol and Health. 2018. [Google Scholar]
- 14.Lieber C.S. Alcoholic liver disease: new insights in pathogenesis lead to new treatments. J. Hepatol. 2000;32(1 Suppl):113–128. doi: 10.1016/s0168-8278(00)80420-1. [DOI] [PubMed] [Google Scholar]
- 15.Osna N.A., Donohue T.M., Jr., Kharbanda K.K. Alcoholic liver disease: pathogenesis and current management. Alcohol Res. Curr. Rev. 2017;38(2):147–161. [PMC free article] [PubMed] [Google Scholar]
- 16.Tanwar S. Inflammation and fibrosis in chronic liver diseases including non-alcoholic fatty liver disease and hepatitis C. World J. Gastroenterol. 2020;26(2):109–133. doi: 10.3748/wjg.v26.i2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szabo G., Mandrekar P. Focus on: alcohol and the liver. Alcohol Res. Health : J. National Institute Alcohol Abuse Alcohol. 2010;33(1-2):87–96. [PMC free article] [PubMed] [Google Scholar]
- 18.Milic S. The role of iron and iron overload in chronic liver disease. Med. Sci. Mon. 2016;22:2144–2151. doi: 10.12659/MSM.896494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sangkhae V., Nemeth E. Regulation of the iron homeostatic hormone hepcidin. Adv. Nutr. (Bethesda, Md) 2017;8(1):126–136. doi: 10.3945/an.116.013961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma Y. Protective effect of aplysin supplementation on intestinal permeability and microbiota in rats treated with ethanol and iron. Nutrients. 2018;10(6):681. doi: 10.3390/nu10060681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harrison-Findik D.D. Alcohol metabolism-mediated oxidative stress down-regulates hepcidin transcription and leads to increased duodenal iron transporter expression. J. Biol. Chem. 2006;281(32):22974–22982. doi: 10.1074/jbc.M602098200. [DOI] [PubMed] [Google Scholar]
- 22.Harrison-Findik D.D. Role of alcohol in the regulation of iron metabolism. World J. Gastroenterol. 2007;13(37):4925–4930. doi: 10.3748/wjg.v13.i37.4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haorah J. Mechanism of alcohol-induced oxidative stress and neuronal injury. Free Radic. Biol. Med. 2008;45(11):1542–1550. doi: 10.1016/j.freeradbiomed.2008.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galaris D., Pantopoulos K. Oxidative stress and iron homeostasis: mechanistic and health aspects. Crit. Rev. Clin. Lab Sci. 2008;45(1):1–23. doi: 10.1080/10408360701713104. [DOI] [PubMed] [Google Scholar]
- 25.Borza C. 2013. Oxidative Stress and Lipid Peroxidation – A Lipid Metabolism Dysfunction. [Google Scholar]
- 26.Hussain T. Oxidative stress and inflammation: what polyphenols can do for us? Oxidative Med. Cellular Longevity. 2016;2016:7432797. doi: 10.1155/2016/7432797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma J. JCI Insight; 2020. Chronic-plus-binge Alcohol Intake Induces Production of Proinflammatory Mtdna-Enriched Extracellular Vesicles and Steatohepatitis via ASK1-p38mapkα-dependent Mechanisms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xue M. Protective effect of aplysin on liver tissue and the gut microbiota in alcohol-fed rats. PloS One. 2017;12(6):E0178684–e0178684. doi: 10.1371/journal.pone.0178684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bak J. Oligonol ameliorates ccl4-induced liver injury in rats via the NF-Kappa B and MAPK signaling pathways. Oxidative Med. Cellular Longevity. 2016;2016:3935841. doi: 10.1155/2016/3935841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yin M. Glycine accelerates recovery from alcohol-induced liver injury. J. Pharmacol. Exp. Therapeut. 1998;286(2):1014–1019. [PubMed] [Google Scholar]
- 31.Li B. Alcohol induces more severe fatty liver disease by influencing cholesterol metabolism. Evid. base Compl. Alternative Med. 2019;2019:7095684. doi: 10.1155/2019/7095684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu X. The mediation of interleukin-17 and chemokine ligand 2 in pelvic pain of experimental autoimmune prostatitis. Experimental Therapeutic Med. 2017;14(1):51–58. doi: 10.3892/etm.2017.4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang Zhishang L.Y., Su Ai, Wang Wencheng, Xu Hongwei, Jiang Yushan, Liang Hui. Protective effect and underlying mechanism of aplysin on ethanol-induced liver injury in rats. Food Sci. 2020;(7):131–139. [Google Scholar]
- 34.Jiang Y. Nicotinamide riboside alleviates alcohol-induced depression-like behaviours in C57BL/6J mice by altering the intestinal microbiota associated with microglial activation and BDNF expression. Food Funct. 2020;11(1):378–391. doi: 10.1039/c9fo01780a. [DOI] [PubMed] [Google Scholar]
- 35.Blumberg J.B. Impact of cranberries on gut microbiota and cardiometabolic health: proceedings of the cranberry health research conference 2015. Adv Nutr. 2016;7(4) doi: 10.3945/an.116.012583. 759s-70s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dai N. Antioxidant properties of proanthocyanidins attenuate carbon tetrachloride (ccl4)-induced steatosis and liver injury in rats via CYP2E1 regulation. J. Med. Food. 2014;17(6):663–669. doi: 10.1089/jmf.2013.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walter M. Identifying the reactive sites of hydrogen peroxide decomposition and hydroxyl radical formation on chrysotile asbestos surfaces. Part. Fibre Toxicol. 2020;17(1):3. doi: 10.1186/s12989-019-0333-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Preziosi M.E. Mice lacking liver-specific β-catenin develop steatohepatitis and fibrosis after iron overload. J. Hepatol. 2017;67(2):360–369. doi: 10.1016/j.jhep.2017.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mehta K.J., Farnaud S.J., Sharp P.A. Iron and liver fibrosis: mechanistic and clinical aspects. World J. Gastroenterol. 2019;25(5):521–538. doi: 10.3748/wjg.v25.i5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pham-Huy L.A., He H., Pham-Huy C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. : IJBS. 2008;4(2):89–96. [PMC free article] [PubMed] [Google Scholar]
- 41.Pizzino G. Oxidative stress: harms and benefits for human health. Oxid. Med. Cell Longev. 2017;2017:8416763. doi: 10.1155/2017/8416763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chiu W.C. Synbiotics reduce ethanol-induced hepatic steatosis and inflammation by improving intestinal permeability and microbiota in rats. Food Funct. 2015;6(5):1692–1700. doi: 10.1039/c5fo00104h. [DOI] [PubMed] [Google Scholar]
- 43.Qu B.-G. Association between circulating inflammatory molecules and alcoholic liver disease in men. Cell Stress Chaperones. 2016;21(5):865–872. doi: 10.1007/s12192-016-0711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao J., Chen H., Li Y. Protective effect of bicyclol on acute alcohol-induced liver injury in mice. Eur. J. Pharmacol. 2008;586:322–331. doi: 10.1016/j.ejphar.2008.02.059. [DOI] [PubMed] [Google Scholar]
- 45.Ioannou G. The effect of alcohol consumption on the prevalence of iron overload, iron deficiency, and iron deficiency anemia. Gastroenterology. 2004;126:1293–1301. doi: 10.1053/j.gastro.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 46.You M., Arteel G.E. Effect of ethanol on lipid metabolism. J. Hepatol. 2019;70(2):237–248. doi: 10.1016/j.jhep.2018.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Silva M. Iron overload alters glucose homeostasis, causes liver steatosis, and increases serum triacylglycerols in rats. Nutr. Res. 2008;28(6):391–398. doi: 10.1016/j.nutres.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 48.Wang M. Iron overload correlates with serum liver fibrotic markers and liver dysfunction: potential new methods to predict iron overload-related liver fibrosis in thalassemia patients. United European Gastroenterol. J. 2017;5(1):94–103. doi: 10.1177/2050640616646525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bala S. The pro-inflammatory effects of mir-155 promote liver fibrosis and alcohol-induced steatohepatitis. J. Hepatol. 2016;64(6):1378–1387. doi: 10.1016/j.jhep.2016.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Szabo G. Gut-liver axis in alcoholic liver disease. Gastroenterology. 2015;148(1):30–36. doi: 10.1053/j.gastro.2014.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Szabo G., Bala S. Alcoholic liver disease and the gut-liver axis. World J. Gastroenterol. 2010;16(11):1321–1329. doi: 10.3748/wjg.v16.i11.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wheeler M.D. The role of Kupffer cell oxidant production in early ethanol-induced liver disease. Free Radic. Biol. Med. 2001;31(12):1544–1549. doi: 10.1016/s0891-5849(01)00748-1. [DOI] [PubMed] [Google Scholar]
- 53.Thakur V. Regulation of macrophage activation in alcoholic liver disease. J. Gastroenterol. Hepatol. 2007;22(Suppl 1):S53–S56. doi: 10.1111/j.1440-1746.2006.04650.x. [DOI] [PubMed] [Google Scholar]
- 54.Oeckinghaus A., Ghosh S. The NF-kappab family of transcription factors and its regulation. Cold Spring Harb Perspect. Biol. 2009;1(4):A000034. doi: 10.1101/cshperspect.a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Terra X. Grape-seed procyanidins prevent low-grade inflammation by modulating cytokine expression in rats fed a high-fat diet. J. Nutr. Biochem. 2009;20(3):210–218. doi: 10.1016/j.jnutbio.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 56.Décordé K. Chardonnay grape seed procyanidin extract supplementation prevents high-fat diet-induced obesity in hamsters by improving adipokine imbalance and oxidative stress markers. Mol. Nutr. Food Res. 2009;53(5):659–666. doi: 10.1002/mnfr.200800165. [DOI] [PubMed] [Google Scholar]
- 57.Rodríguez-Ramiro I. Procyanidin B2 and a cocoa polyphenolic extract inhibit acrylamide-induced apoptosis in human Caco-2 cells by preventing oxidative stress and activation of JNK pathway. J. Nutr. Biochem. 2011;22(12):1186–1194. doi: 10.1016/j.jnutbio.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 58.Lawson M. Free radicals and antioxidants in human disease. In: Al-Gubory K.H., Laher I., editors. Nutritional Antioxidant Therapies: Treatments and Perspectives. Springer International Publishing; Cham: 2017. pp. 283–305. [Google Scholar]
- 59.Lobo V. Free radicals, antioxidants and functional foods: impact on human health. Phcog. Rev. 2010;4(8):118–126. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kowdley K.V. Iron overload in patients with chronic liver disease. Gastroenterol. Hepatol. 2016;12(11):695–698. [PMC free article] [PubMed] [Google Scholar]
- 61.Shepard B.D., Tuma P.L. Alcohol-induced protein hyperacetylation: mechanisms and consequences. World J. Gastroenterol. 2009;15(10):1219–1230. doi: 10.3748/wjg.15.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.