Abstract
Chronic granulomatous disease (CGD) is caused by gene mutations that affect the phagocyte NADPH oxidase. This results in recurrent infections by catalase-positive bacteria or fungi. Here, we report a case of X-linked CGD presenting a mixed infection with Burkholderia cepacia and Aspergillus. A novel mutation was found by bioinformatics analyses of his genealogy (c.1234delG), which perhaps changed the structure and function of the related proteins.
Keywords: Chronic granulomatous disease, Mutation, CYBB gene, Burkholderia cepacia, Aspergillus
1. Introduction
Chronic granulomatous disease (CGD) is a rare primary immunodeficiency disease (PID), with an incidence of 1/250000 to 1/200000 [1]. It is caused by defects in any of the five subunits of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase complex, which is responsible for the respiratory burst in phagocytic leukocytes. Mutations in the CYBB gene that encodes gp91phox (NOX2) are X-linked and are detected in two-thirds of all CGD cases [2]. Patients with CGD are prone to life-threatening infections with catalase-positive bacteria and fungi, such as Staphylococcus aureus, Burkholderia onionensis, Serratia mucilage, Nocardia, and Aspergillus [3]. Herein, we report the case of a child presenting infections with Burkholderia cepacia and Aspergillus, who was diagnosed with X-linked CGD after death, based on the detection of a novel mutation in CYBB.
2. Case presentation
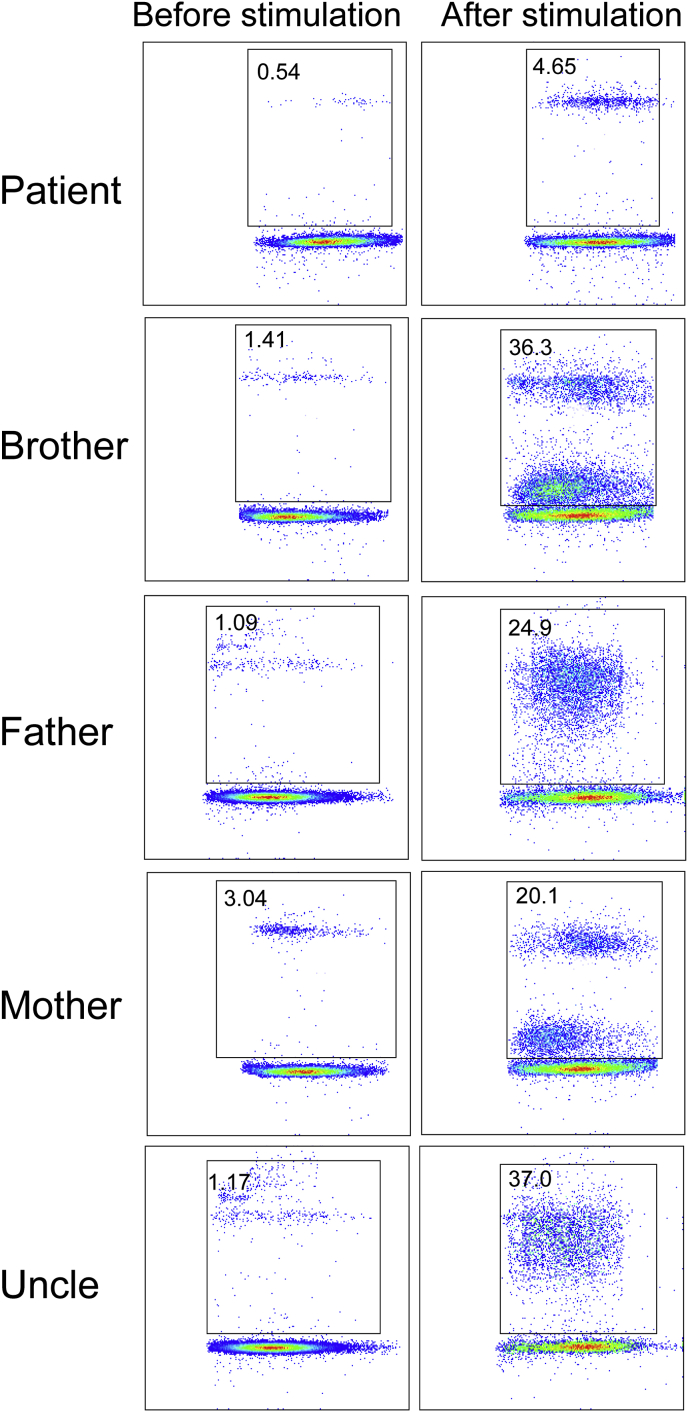
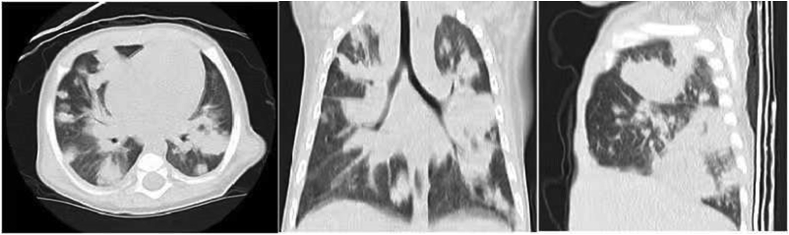
In November 2018, an 8-month-old boy was hospitalized due to fever and cough for 10 days. Even though the patient was susceptible to upper respiratory tract infections, he had no history of severe infectious diseases nor was he allergic to food or drugs. The patient was born at term with no complications. His family history revealed that his maternal uncle died early in infancy due to pneumonia; the patient's brother was 4 years old and healthy. On admission, the patient underwent physical examination; a few moist rales were observed in both lungs. Laboratory findings showed high IgE and IgA levels (Table 1). Test results for complement levels, complete blood count, and mitogen-induced T-cells were normal. Neutrophil phagocytosis assays revealed that the patient's peripheral blood neutrophils presented impaired phagocytotic functions (Fig. 1). After PMA stimulation, only 4.65% of the patient neutrophils could devour APC beads while in the other samples, normal phagocytosis was observed in >20% neutrophils. Serological tests did not reveal infection with human immunodeficiency virus and he was antibody-negative for some common viruses, including cytomegalovirus (CMV), Epstein-Barr virus (EBV), and other herpes viruses. Additionally, the patient tested negative for Mycobacterium tuberculosis and Mycoplasmal pneumonia by pharyngeal swab antigen test and cultures. High resolution computed tomography (HRCT) revealed multiple patchy infiltrating shadows and a diffuse consolidation with air bronchograms on admission (Fig. 2). His condition deteriorated rapidly after admission to the hospital, quickly progressing to respiratory failure, shock, and organ dysfunction and failure. He died three days after admission despite being administered anti-infective therapy (vancomycin and meropenem) and all intensive care (transfusion, mechanical ventilation, and continuous blood purification). After his death, sputum and blood cultures yielded multiple drug-resistant Burkholderia cepacia, whereas microscopic examination of the transbronchial lung biopsy (TBLB) revealed Aspergillus hyphae.
Table 1.
Laboratory Findings of the patient.
| Laboratory index | Results | Reference range |
|---|---|---|
| WBC( × 109/L) | 13.50 | 5–12 |
| N ( × 109/L) | 7.87 | 5–12 |
| Hb (g/dL) | 96.00 | 120–160 |
| PLT ( × 109/L) | 224.00 | 100–160 |
| CRP (mg/L) | 166.10 | <8.2 |
| IgG (g/L) | 8.63 | 3.6–9.2 |
| IgA(g/L) | 1.00 | 0.08–0.56 |
| IgM(g/L) | 1.57 | 0.38–1.26 |
| IgE(IU/ML) | 191.00 | 0–15 |
| CD3+4+ (cells/ul) | 787.00 | 410–1590 |
| CD3+8+ (cells/ul) | 330.00 | 190–1140 |
| CD19+ (cells/ul) | 1055.00 | 90–660 |
| Th/Ts (%) | 2.39 | 0.68–2.47 |
| NK (cells/ul) | 143.00 | 90–590 |
WBC, white blood count; N, neutrophils; Hb, hemoglobin; PLT, platelet count; CRP, C-reactive protein; Th, T helper T cells; Ts, inhibited T cells; NK, Natural Killer.
Fig. 1.
Functional assays of the family members. After PMA stimulation, only 4.65% of the patient neutrophils could devour APC beads. Other members presented a number of neutrophils with normal phagocytotic functions of >20%.
Fig. 2.
HRCT showed multiple patchy infiltrating shadows and ill-defined nodular areas of consolidation with air bronchograms in the bilateral regions.
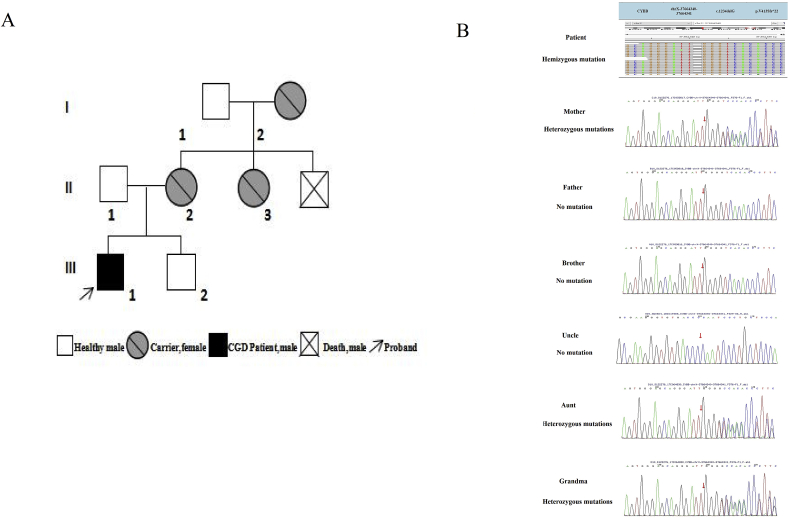
The family tree of the patient (Fig. 3A) includes three generations with a total of eight people. The proband has been diagnosed with X-linked CGD. Apart from his uncle's death in infancy because of pneumonia, others have no history of recurrent or severe respiratory infection. The proband detected a novel mutation on the CYBB gene in the patient, a G deletion in locus c.1234 on the CYBB gene of X chromosome (c.1234delG) (Fig. 3B). This mutation shifts valine to serine at residue 413 creating a premature stop codon (p. V413Sfs*22). Besides, the same mutation was detected on one of the X chromosomes of his mother, aunt, and grandmother, who we called carriers. However, no CYBB gene mutation was detected in the infant's father, his other uncle or his brother. Thus, it was clear that the mutated gene came from his mother who inherited it from his grandmother.
Fig. 3.
(A) Family tree; (B) Analysis of CYBB gene expression of the patient, showed G deletion at locus c.1234 on the CYBB gene of X chromosome, the same mutation was detected on one of the X chromosomes of his mother, aunt, and grandmother.
3. Discussion
Chronic granulomatous disease (CGD) is caused by a defective generation of reactive oxygen species by the phagocyte nicotinamide adenine dinucleotide phosphate (NADPH) [4]. We presented the case of a patient who was hospitalized with symptoms of acute respiratory infection, without a history of serious infections. He suffered a fulminating clinical course during his hospitalization, characterized by a mixed infection with Burkholderia cepacia and Aspergillus. At least 1 episode of pulmonary infection occurs in 87% of CGD patients [5]. Aspergillus is the most common pathogen in X-linked CGD patients whereas Burkholderia cepacia infections are rarely observed in patients with X-linked CGD [6]. Mixed infections in CGD patients have been often reported, especially in patients with X-linked gp91phox [5], which was the precise mutation site in this patient. However, mixed infections with Aspergillus and Burkholderia cepacia have been rarely reported in X-linked CGD patients. Although this patient received broad-spectrum antibiotics early during treatment, his condition continuously deteriorated probably due to a delayed pathogen evidence. Therefore, the early identification and diagnosis of CGD patients plays a significant role in making an effective treatment strategy and improving prognosis.
This patient was diagnosed with X-linked CGD with a novel mutation in CYBB. His family members, including his mother, aunt, and grandmother, were verified carriers of this mutation. Functional assay results supported the diagnosis, and showed that the patient's neutrophils could not move after PMA stimulation, indicating that CYBB mutations may affect neutrophils functions. Genetic analysis showed a guanine deletion at the locus c.1234 in CYBB on the X chromosome, leading to a change from valine to serine at residue 413 and creating a premature stop codon. The mutation affected the NADPH-binding region of the protein (gp91phox), which had a low mutation rate (17%) [7]. A pediatric patient with a missense mutation (c.1234 G > C) in CYBB was reported to be alive at 12 years of age [8]. Nevertheless, our patient was found to have a new deletion at the same site, experiencing a bad prognosis with a rapid course.
4. Conclusion
In conclusion, we identified a novel mutation in a X-linked CGD patient and his family members. This case highlights the importance of mixed infection for patients with X-linked CGD, and also the importance of functional assays and genetic testing of the family in early diagnosis.
Funding
No external or intramural funding was received.
Declaration of competing interest
The authors report no actual or potential conflicts of interests.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.rmcr.2020.101213.
Contributor Information
Xuehua Xu, Email: 208854408@qq.com.
Bingtai Lu, Email: lubingtaip@163.com.
Yaping Xie, Email: xieyp520@163.com.
Diyuan Yang, Email: diyuanyang@126.com.
Gen Lu, Email: lugen5663330@sina.com.
Huifeng Fan, Email: sonny-000@163.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Mahdaviani S.A., Mohajerani S.A., Rezaei N., Casanova J.L., Mansouri S.D., Velayati A.A. Pulmonary manifestations of chronic granulomatous disease. Expet Rev. Clin. Immunol. 2013;9(2):153–160. doi: 10.1586/eci.12.98. [DOI] [PubMed] [Google Scholar]
- 2.Warren M., Shimada H. Cytologic and ultrastructural findings of bronchoalveolar lavage in patients with chronic granulomatous disease. Pediatric and Developmental Pathology. 2018;21(4):347–354. doi: 10.1177/1093526617736188. [DOI] [PubMed] [Google Scholar]
- 3.Roos D. Chronic granulomatous disease. Br. Med. Bull. 2016;118(1):50–63. doi: 10.1093/bmb/ldw009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rider N.L., Jameson M.B., Creech C.B. Chronic granulomatous disease: epidemiology, pathophysiology, and genetic basis of disease. Journal of the Pediatric Infectious Diseases Society. 2018;7(suppl_1):S2–S5. doi: 10.1093/jpids/piy008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marciano B.E., Spalding C., Fitzgerald A., Mann D., Brown T., Osgood S., Yockey L., Darnell D.N., Barnhart L., Daub J., Boris L., Rump A.P., Anderson V.L., Haney C., Kuhns D.B., Rosenzweig S.D., Kelly C., Zelazny A., Mason T., DeRavin S.S., Kang E., Gallin J.I., Malech H.L., Olivier K.N., Uzel G., Freeman A.F., Heller T., Zerbe C.S., Holland S.M. Common severe infections in chronic granulomatous disease. Clin. Infect. Dis. 2015;60(8):1176–1183. doi: 10.1093/cid/ciu1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winkelstein J.A., Marino M.C., Johnston R.B., Jr., Boyle J., Curnutte J., Gallin J.I., Malech H.L., Holland S.M., Ochs H., Quie P., Buckley R.H., Foster C.B., Chanock S.J., Dickler H. Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine. 2000;79(3):155–169. doi: 10.1097/00005792-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Jirapongsananuruk O., Niemela J.E., Malech H.L., Fleisher T.A. CYBB mutation analysis in X-linked chronic granulomatous disease. Clin. Immunol. 2002;104(1):73–76. doi: 10.1006/clim.2002.5230. [DOI] [PubMed] [Google Scholar]
- 8.Bakri F.G., Martel C., Khuri-Bulos N., Mahafzah A., El-Khateeb M.S., Al-Wahadneh A.M., Hayajneh W.A., Hamamy H.A., Maquet E., Molin M., Stasia M.J. First report of clinical, functional, and molecular investigation of chronic granulomatous disease in nine Jordanian families. J. Clin. Immunol. 2009;29(2):215–230. doi: 10.1007/s10875-008-9243-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.