Abstract
Background
Accelerometer-based computer-assisted navigation systems (ABCANSs) have been shown to improve alignment accuracy in total knee arthroplasty (TKA) and are effective in treating complex extra-articular deformity. We present an ABCANS-assisted TKA performed in a 68-year-old male with end-stage arthritis of the right knee, in the setting of a severe valgus deformity secondary to multiple hereditary exostoses.
Methods
The KneeAlign 2 system (OrthAlign, Inc.; Aliso Viejo, CA) was used to perform the TKA in this clinical scenario, given its functionality, which allows angular correction to be tailored to a given deformity, and its reported accuracy in performance of bony resection in TKA. The patient was prospectively followed up for one year postoperatively. Radiographs, PROMs, and patient satisfaction were reported.
Results
After the ABCANS-assisted TKA, the patient's alignment was improved from 25° to 4° of valgus. His final range of motion was 0-135° without an instability. In addition, the patient reported excellent scores on multiple joint-specific outcome measures, including the Knee Injury and Osteoarthritis Outcome Score for Joint Replacement, the Forgotten Joint Score, and the Oxford Knee Score.
Conclusion
This case report illustrates the rationale, technique, and the excellent clinical outcomes achieved in a complex patient with extra-articular deformity using an ABCANS-assisted TKA.
Keywords: Computer navigation, Complex primary total knee arthroplasty, Multiple hereditary exostosis, Osteochondromas, Valgus knee deformity
Introduction
Accelerometer-based computer-assisted navigation systems (ABCANSs) have previously been shown to improve alignment accuracy in total knee arthroplasty (TKA) [[1], [2], [3]] and are safe, effective surgical tools in complex cases such as in patients with retained hardware or extra-articular deformity [[4], [5], [6]]. When compared with traditional extramedullary instrumentation for performance of tibial resection, OrthAlign has been noted to be within 2° of neutral coronal alignment in 95.7% of cases, whereas traditional instrumentation hits this target in only 68.2% of cases [7]. With respect to the tibial slope, OrthAlign is able to remain within 2° of target position in 95% of cases compared with 72.1% using traditional instrumentation [7]. Distal femoral resection using OrthAlign has also been shown to be accurate within 2° of 90° relative to the femoral mechanical axis in 95.8% of cases [3]. The use of OrthAlign in TKA for cases of multiple hereditary exostoses (MHE), however, has not been described.
MHE, or hereditary multiple osteochondromas, describes a genetic condition in which patients develop several benign osseocartilaginous lesions, typically characterized as a bony stalk with a cartilaginous cap, most often located near the metaphyseal regions of long bones [8]. Although the true incidence of the disease is unknown because of the number of subclinical cases, MHE has been estimated to affect up to 1 in 50,000 individuals in Caucasian populations [[8], [9], [10]]. MHE is inherited in autosomal dominant fashion, typically with complete penetrance, and sequencing analysis has implicated the EXT1 or EXT2 genes in 70%-95% of affected individuals [11]. Mutations in these genes result in altered regulation of chondrocyte differentiation and maturation, which subsequently cause abnormal endochondral ossification in the growing bone [8]. As such, MHE is associated with highly variable presentations of skeletal abnormalities, including alterations of the limbs, chest, and spine; limb-length discrepancy; spinal stenosis; scoliosis; and short stature [12].
The knee is the most common site of lesions in MHE, and valgus deformity is a well-known phenotypical manifestation [8]. Although data remain sparse, a few recent case reports have examined the role of TKA as a treatment option in instances of MHE affecting the knee. Although results have been acceptable, these cases continue to present significant technical challenges to surgeons, and the importance of preoperative templating has been highlighted [[13], [14], [15]].
Case history
Preoperative evaluation
A 68-year-old male with a known history of MHE and hypertension presented for evaluation of worsening right knee pain for a 6-month duration. He noted a painful grinding sensation in his right knee that restricted his walking distance to 2 blocks, and he had difficulty going up and down stairs. His pain was not well controlled despite daily use of naproxen, aspirin, and hydrocodone-acetaminophen of 10-325 mg 4 times daily. The patient had previously undergone multiple surgeries of the right knee for excision of exostoses, starting at the age of 2 years and most recently at the age of 19 years.
On physical examination, inspection of the right lower extremity revealed multiple well-healed surgical scars on the medial and lateral aspects of the knee, as well as a pronounced valgus deformity. The patient was tender to palpation over the medial and lateral joint lines and was found to have mild medial-sided opening with valgus stress. Evaluation of the patellofemoral compartment was remarkable for positive patellar grind and inhibition examinations. The range of motion of the right knee was 10°-110° with crepitus. Strength of the bilateral lower extremities was 5 of 5 in all muscle groups, as evaluated by the Oxford muscle grading scale. The patient was able to ambulate without an assistive device but with an antalgic gait of the right leg. There were no abnormalities noted on the remainder of the neurovascular assessment. Provocative examinations of the right hip and ankle were devoid of pain.
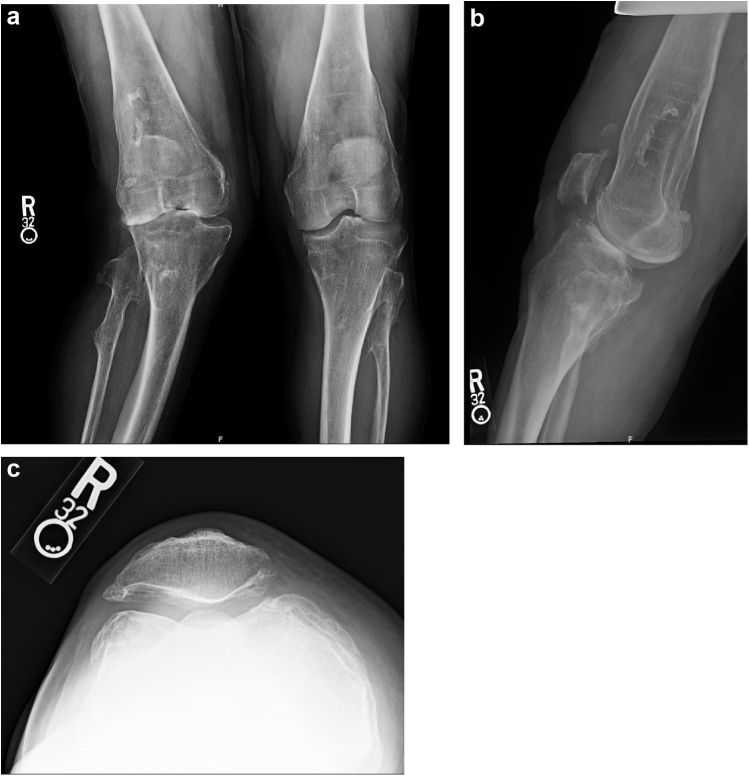
Radiographic evaluation using three views of the right knee revealed chronic deformative changes of the distal femur and proximal tibia and fibula, characteristic of MHE, and status after multiple prior osteotomies. End-stage osteoarthritis most severe in the lateral and patellofemoral compartments was also identified. This was classified as Kellgren-Lawrence grade 4, with severe joint space narrowing, peripheral osteophyte formation, and subchondral sclerosis [16] (Fig. 1). Full-length views of the lower extremities were also obtained to better characterize the valgus deformity, which was measured as 25 degrees (Fig. 2).
Figure 1.
Preoperative AP (a), lateral (b), and sunrise (c) radiographs of the right knee demonstrating KL grade 4 osteoarthritis with severe joint space narrowing, subchondral sclerosis, and osteophyte formation. AP, anteroposterior; KL, Kellgren-Lawrence.
Figure 2.
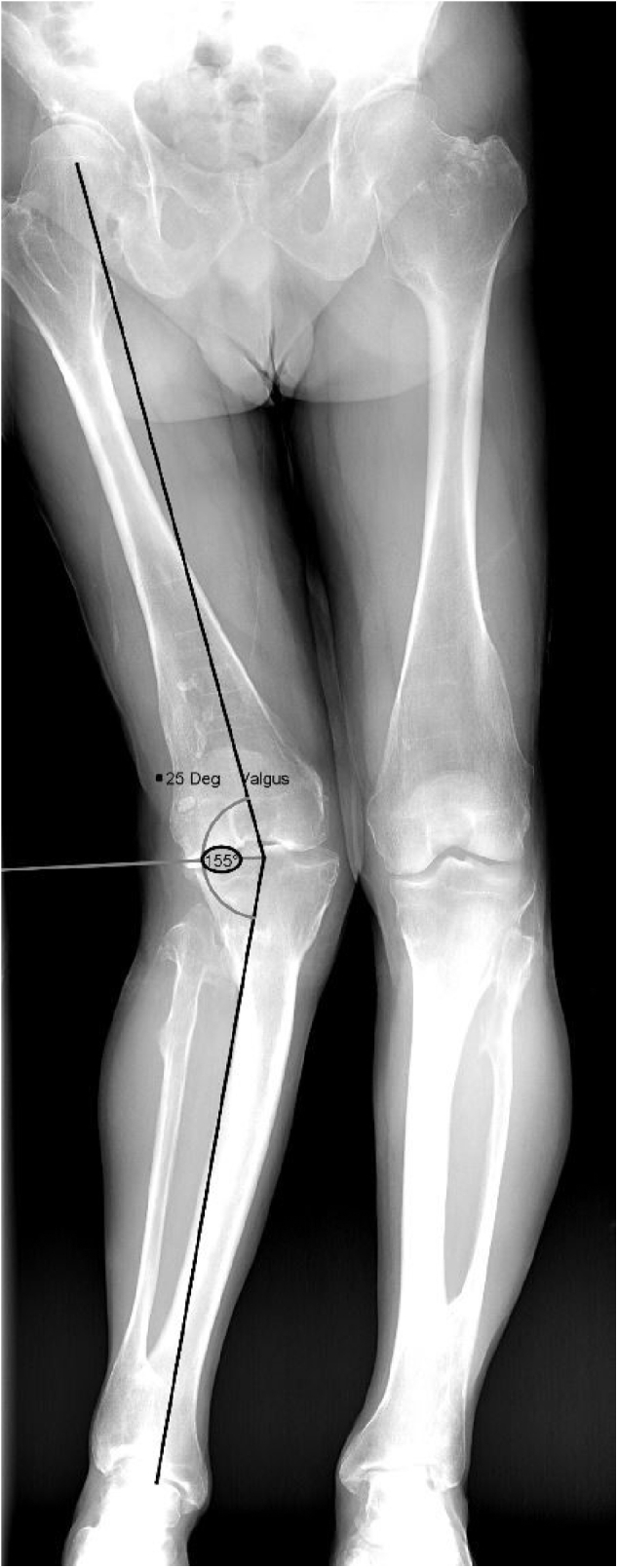
Preoperative full-length standing radiographs demonstrating the 25-degree valgus deformity of the right knee.
Surgical technique
The surgery was performed by the senior authors with use of a tourniquet. An anterior midline incision and medial parapatellar arthrotomy were used for exposure. Severe tricompartmental osteoarthritic changes were noted on entrance of the joint space. A limited medial release of the soft tissues from the proximal tibia was performed at the joint line to the midcoronal plane, and the anterior cruciate ligament (ACL), posterior cruciate ligament (PCL), and meniscal remnants were excised.
Attention was then directed to the distal femur. The KneeAlign 2 distal femoral microblock was placed using a guide pin directed at the center of the femoral head, secured with 2 additional headed pins, and the hip center of rotation was registered. Our preoperative plan dictated the need to make our tibial cut in slight valgus orientation to accommodate the tibial component, given the severity of the valgus tibial bow. To counteract this, and in an effort to generate an overall orthogonal alignment to the limb, we set our distal femoral cut in 2° of varus alignment. We also set our distal femoral resection in 3° of flexion to match the femoral component selected. For most TKA procedures using this particular implant, it is the senior author’s standard practice to resect 9 mm of the distal femur. Given this patient’s flexion contracture, the distal resection depth was increased to 11 mm.
The tibial jig for the KneeAlign 2 system was then pinned into position, referencing the medial third of the tibial tubercle and the ACL footprint. A significant rotational deformity of the tibia was noted, in addition to the angular deformity, excessive native posterior slope, and a large posterolateral defect. We registered the offset of the tibial jig relative to the ACL footprint as well as the medial and lateral malleoli of the ankle to establish the mechanical axis of the tibia. We set our tibial resection in 3° of valgus relative to the mechanical axis of the tibia to avoid over-resection of the medial plateau, which could have otherwise compromised the medial collateral ligament (MCL) insertion. In addition, based on preoperative templating, we aimed to ensure a resection angle such that the orthogonal trajectory of the tibial stem would not perforate the lateral tibial cortex. Subsequently, the posterior slope was set to 4° with a resection depth of 8 mm off the medial side, and the resection was performed.
A complete lateral release of soft tissues from the lateral epicondyle of the femur and release of the popliteus tendon were necessary to equalize the medial and lateral extension gaps. Femoral rotation was then verified using the transepicondylar axis. The femur was sized, and the femoral cuts were performed. The flexion gap was checked with the knee in 90° of flexion and was found to be well balanced relative to the extension gap.
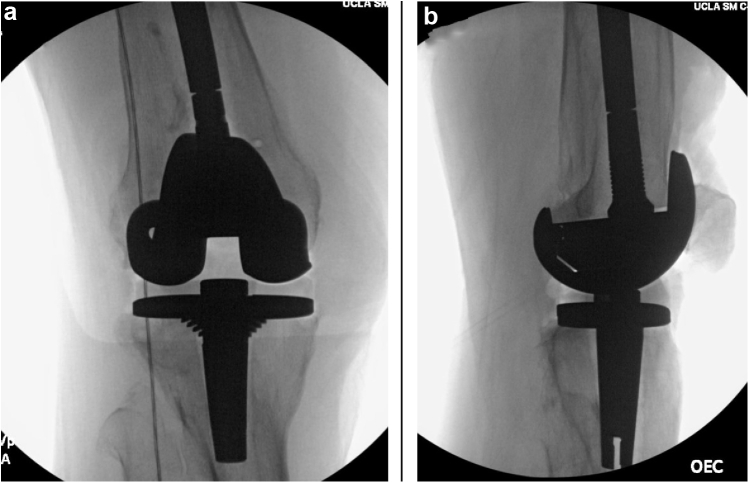
The femoral box cut was performed, and the tibia was drilled and punched in proper rotation. The patella was resurfaced using measured resection. Trial components (PFC TC3 size 5, posterior-stabilized femoral component with a 13-mm stem, size 4 MBT tibial baseplate, 10-mm polyethylene insert, size 38 patellar button; DePuy Synthes, Raynham, MA) were then placed. The range of motion and stability were assessed: the knee achieved full extension and flexion to 120° with excellent patellar tracking, and the construct was stable to varus and valgus stress in full extension and at 30° and 90° of flexion. Intraoperative fluoroscopic images demonstrated an overall mild valgus alignment, although the result was a significant improvement when compared with the preoperative state (Fig. 3). Owing to the abutment of the tip of the tibial keel with the lateral cortex and the associated risk of cortical perforation, the implantation of a longer stemmed tibial component was precluded. However, given the overall improvement in alignment, mechanical stability, and intact nature of the femoral and tibial cortices, we accepted the construct and proceeded with cementation and closure. Given the patient’s history of multiple prior surgeries, 10 mL of absorbable calcium sulfate beads (STIMULAN, Biocomposites Ltd, Keele, United Kingdom) impregnated with 1 g of powdered vancomycin and 1.2 g of powdered tobramycin were placed in the joint space before closure. The use of absorbable calcium sulfate beads has been shown to be effective as a prophylactic measure for decreasing the risk of periprosthetic joint infection (PJI) in primary hip and knee replacement surgery in high-risk patients [17,18].
Figure 3.
AP (a) and lateral (b) intraoperative fluoroscopic images of the right knee with the implants placed, demonstrating significant improvement in alignment compared with the preoperative state.
Postoperative neurovascular check demonstrated no abnormalities. The patient was able to ambulate 350 feet on postoperative day 1 and was discharged home on postoperative day 2 with no activity restrictions.
Postoperative evaluation
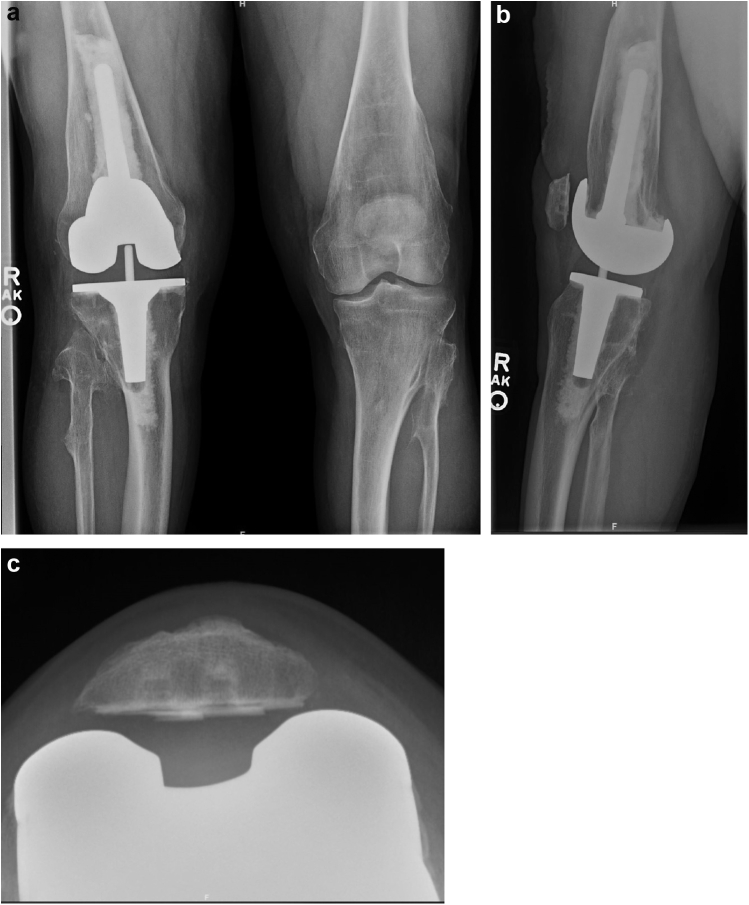
At 1 week postoperatively, the patient was progressing well and was ambulating without any assistive device. By 6 weeks postoperatively, he was not requiring any medications for pain, and range of motion of the right knee was 0°-135° with no varus or valgus instability. At the most recent follow-up of 14 months postoperatively, radiographs demonstrated acceptable alignment of the right lower extremity and no evidence of implant loosening (Fig. 4). Standing, full-length, limb alignment views were obtained at the 14-month follow-up visit which demonstrated the mechanical limb alignment to be 4° of valgus; this represented a 21° correction from preoperative alignment (Fig. 5). In addition, the patient reported excellent scores on multiple joint-specific outcome measures, including the Knee Injury and Osteoarthritis Outcome Score for Joint Replacement, the Forgotten Joint Score, and the Oxford Knee Score (Table 1). His University of California activity score indicated that he “sometimes participated in moderate activities or could do unlimited housework or shopping.” On assessment of his overall status via the Patient-Reported Outcome Measurement Information System Global Health form, he indicated his physical health as “good” and his quality of life as “very good” and described his average level of pain as “no pain.” He did not experience any postoperative complications. The patient provided our team with informed consent to publish his case in the orthopaedic literature.
Figure 4.
Postoperative AP (a), lateral (b), and sunrise (c) radiographs of the right knee at the final follow-up of 14 months, demonstrating acceptable limb alignment and no evidence of implant loosening.
Figure 5.
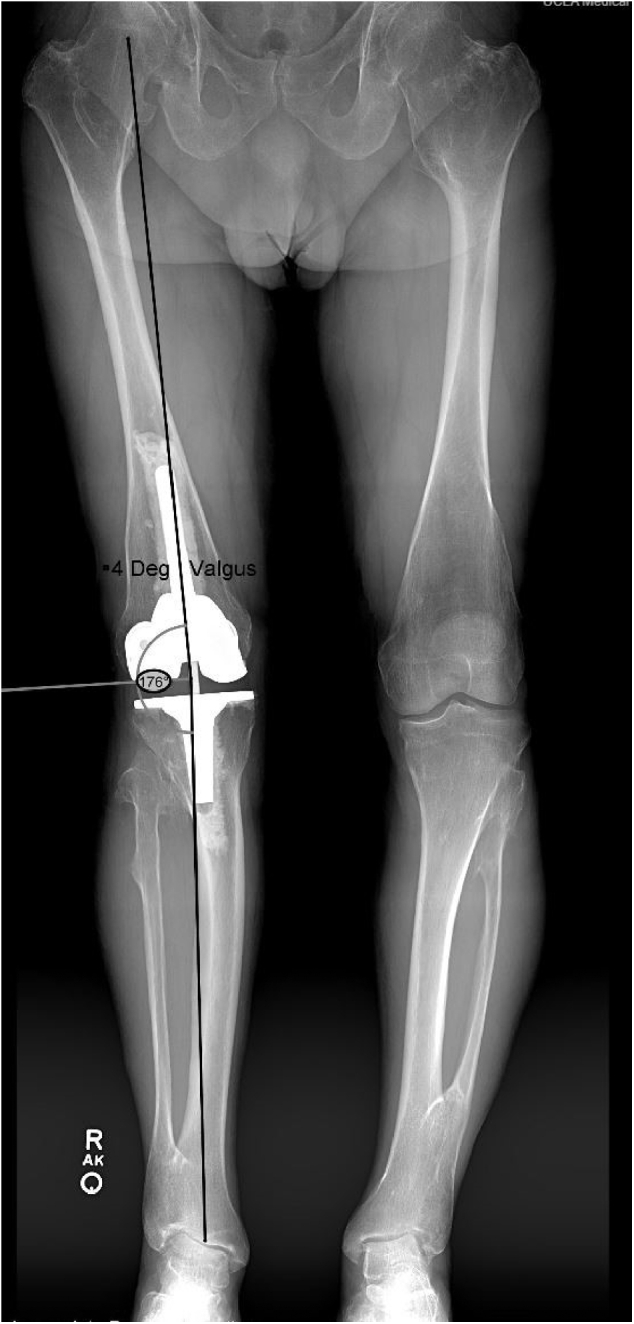
Full-length standing radiographs at 14-month follow-up demonstrated 4° of valgus alignment, representing a 21° correction when compared with the preoperative state.
Table 1.
Patient-reported outcome measures at the final follow-up.
| Outcome measure | Raw score (% of max.) |
|---|---|
| KOOS, JRa | 100 (100) |
| FJS-12a | 98 (98) |
| OKSa | 48 (100) |
| UCLA | 5 (50) |
| PROMIS GH | |
| Physical | 17 (85) |
| Mental | 15 (75) |
KOOS, JR, Knee Injury and Osteoarthritis Outcome Score for Joint Replacement; FJS-12, Forgotten Joint Score, 12 items; OKS, Oxford Knee Score; UCLA, University of California, Los Angeles Activity Scale; PROMIS GH, Patient-Reported Outcome Measurement Information System Global Health Assessment.
Joint-specific outcome measures.
Discussion
MHE, although a rare condition, can present in a variety of phenotypic manifestations. Among these, valgus knee deformity is one well-known presentation. This deformity is usually secondary to lesions of the distal femur or proximal tibia, which are present in 70% to 98% of MHE cases [8,14] or the result of a shortened fibula causing valgus tibial angulation [19]. Several surgical treatment options have been used for correction of the valgus knee deformity in MHE, depending on the cause. In cases of valgus-causing femoral lesions, opening-wedge osteotomy and blade-plate fixation have shown acceptable results, while hemiepiphysiodesis or high tibial osteotomy has been successfully used for lesions involving the proximal tibia [20]. If present intra-articularly, osteochondromas can also be effectively removed via arthroscopic resection [21]. Ultimately, these treatment strategies aim to anatomically realign the limb, correct limb-length discrepancies, and restore patients’ function while minimizing pain. At the time of our patient’s presentation, he had undergone several attempts at surgical resection of exostoses but continued to struggle with malalignment, pain, and severely impaired functionality secondary to end-stage arthritis.
TKA for treatment of valgus knee deformity in MHE has been described, although the literature on this topic remains sparse. Kim et al. [22] used TKA in 12 patients with various types of skeletal dysplasia, including 5 knees in patients with MHE and a preoperative valgus deformity ranging from 11° to 45°. At a minimum follow-up of 3 years among the patients with MHE, the postoperative limb alignment was only 4° to 5° of valgus, postoperative Knee Society Score was 87/100 or greater, and all patients were pain free. Mesfin et al. [14] have also described a case of posterior-stabilized TKA for valgus knee deformity secondary to MHE, demonstrating excellent limb alignment and complete resolution of pain at 39-month follow-up. Finally, Fernandez-Perez et al. [13] recently described their use of TKA with metaphyseal sleeves for this indication at 6-month postoperative evaluation, the limb was anatomically aligned, range of motion was 10° to 105°, Knee Society Score was 82, and the Western Ontario McMaster score was 90.2. In the majority of previous cases, at least some degree of lateral-sided release (lateral epicondylar osteotomy, release of the iliotibial band, release of the popliteus tendon, etc.) was necessary to achieve adequate gap balancing. In our case, we used a complete release of all structures from the lateral femoral epicondyle and release of the popliteus tendon with excellent results. Given the complexity of these cases, it is advised that surgeons be intimately familiar with multiple techniques to achieve adequate gap balancing.
Notably, the only literature support of accelerometry-based navigation to treat arthritis in patients with skeletal dysplasia comes from Kim et al. [22], who used these systems in 2 patients, both of whom had a varus deformity and considerable femoral bowing secondary to achondroplasia. As such, to our knowledge, this is the first report detailing the use of ABCANSs in the setting of TKA for valgus knee deformity secondary to MHE. ABCANSs in TKA have been the subject of several investigations in recent years, and results have been overwhelmingly positive with respect to their effectiveness. In a series of 42 knee replacements using such a system, Nam et al. demonstrated that 97.6% of tibial components were placed within 2° perpendicular to the mechanical axis in the coronal plane, and 96.2% of tibial components were placed within 3° ± 2° to the mechanical axis in the sagittal plane [2]. In a series of 48 TKAs using the same system, the same authors demonstrated similarly impressive accuracy with regard to placement of the femoral components [3]. In addition, ABCANSs have been shown to be as accurate as large-console, imageless computer-assisted surgery systems in producing anatomic limb alignment [1]. Still, perhaps the most compelling evidence in support of ABCANSs comes from a 2014 randomized controlled trial, also conducted by Nam et al [7]. The authors compared postoperative tibial alignment in knees replaced with ABCANSs with those replaced with extramedullary tibial guides and found that ABCANSs were significantly superior with regard to the percentage of tibial components fixed within 2° perpendicular to the tibial mechanical axis (95.7% vs 68.1%, P < .001) and with regard to the percentage of tibial components within 2° of a 3° posterior slope (95.0% vs 72.1%, P = .007).
In the present report, our use of ABCANSs allowed us to execute a preoperatively templated plan of a femoral cut in 2° of varus and a tibial cut in 3° of valgus, each cut relative to the mechanical axis of each bone. This ultimately allowed us to achieve overall near-anatomic alignment of the lower extremity, while concurrently preserving the integrity of the femoral and tibial cortices. Without the use of ABCANSs, it is plausible that less accurate distal femoral and proximal tibial cuts could have resulted in malalignment or perforation of the lateral tibial cortex. Although such cortical perforations are rare, they may occur more often in patients who have previously undergone a high tibial osteotomy, a documented treatment for valgus knee deformity in MHE [20,23]. In addition, despite our effective use of ABCANSs, the positioning of our implants resulted in abutment of the tibial component on the lateral tibial cortex. This finding underscores the extremely narrow window for proper implant placement and highlights the necessity for precise resection in these difficult cases. For these reasons, we recommend that TKA in cases of valgus knee deformity secondary to MHE be performed by surgeons with considerable experience in surgical correction of knee deformity.
Current controversies and future considerations
The management of valgus knee deformity with end-stage arthritis in MHE represents a complicated surgical problem. TKA is an effective treatment, particularly for those with profound malalignment in the setting of arthritis. These surgeries, although complex and difficult, can be successfully performed with use of meticulous preoperative planning including radiographic templating. As demonstrated in the current report, the use of handheld ABCANSs can be an extremely useful tool to ensure accuracy of distal femoral and proximal tibial resections. The use of this navigation tool can help prevent inadvertent cortical perforation or malalignment, either of which is potentially more likely in the presence of bony deformity such as that seen in patients with skeletal dysplasias such as MHE. Currently, the literature on the use ABCANSs in cases of knee deformity is quite limited—larger-scale, long-term investigations are necessary to validate the use of these navigation systems in cases of knee deformity secondary to skeletal dysplasias such as MHE.
Summary
MHE is a genetic disorder that typically affects the metaphyseal regions of long bones. Although it is a rare condition, it can manifest in several phenotypes, one of which is valgus knee deformity. These cases have previously been managed with surgical excision of exostoses, but TKA for this indication can be a viable option. ABCANSs can be particularly useful in these difficult cases, as they allow for precise, patient-specific measurements to determine appropriate femoral and tibial cuts during surgery. This approach can aid the surgeon in anatomic realignment and appropriate implant positioning to help ensure proper function and longevity of the arthroplasty.
Key points
-
•
Multiple hereditary exostoses is a rare, highly variable genetic disease that commonly presents with lesions of the knee, often causing valgus deformity.
-
•
Treatment has historically comprised of removal of exostoses, but, in the setting of arthritis, total knee arthroplasty (TKA) is also an excellent option. TKA should only be undertaken with meticulous preoperative planning and an experienced surgical team.
-
•
The use of accelerometer-based computer-assisted navigation systems has been shown to be effective in increasing the accuracy of placement of tibial and femoral-sided components in TKA to improve limb alignment.
-
•
The use of accelerometer-based computer-assisted navigation systems should be a prominent consideration when performing TKA for valgus knee deformity in multiple hereditary exostoses, especially given the narrow window for accurate tibial and femoral resection to avoid complications.
Conflict of interests
E. Zeegen holds stock or stock options in RadLink Inc. and is a member of the editorial or governing board of the Journal of Arthroplasty and Arthroplasty Today; A. Sassoon is a paid consultant for Biocomposites Inc, OrthAlign, and Smith and Nephew and is a board or committee member of the American Association of Hip and Knee Surgeons; B.H. Patel declares no potential conflicts of interest.
Supplementary data
References
- 1.Nam D., Weeks K.D., Reinhardt K.R., Nawabi D.H., Cross M.B., Mayman D.J. Accelerometer-based, portable navigation vs imageless, large-console computer-assisted navigation in total knee arthroplasty: a comparison of radiographic results. J Arthroplasty. 2013;28(2):255. doi: 10.1016/j.arth.2012.04.023. [DOI] [PubMed] [Google Scholar]
- 2.Nam D., Jerabek S.A., Haughom B., Cross M.B., Reinhardt K.R., Mayman D.J. Radiographic analysis of a hand-held surgical navigation system for tibial resection in total knee arthroplasty. J Arthroplasty. 2011;26(8):1527. doi: 10.1016/j.arth.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 3.Nam D., Nawabi D.H., Cross M.B., Heyse T.J., Mayman D.J. Accelerometer-based computer navigation for performing the distal femoral resection in total knee arthroplasty. J Arthroplasty. 2012;27(9):1717. doi: 10.1016/j.arth.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Tigani D., Masetti G., Sabbioni G., Ben Ayad R., Filanti M., Fosco M. Computer-assisted surgery as indication of choice: total knee arthroplasty in case of retained hardware or extra-articular deformity. Int Orthop. 2012;36(7):1379. doi: 10.1007/s00264-011-1476-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manzotti A., Chemello C., Pullen C., Cerveri P., Confalonieri N. Computer-assisted total knee arthroplasty after prior femoral fracture without hardware removal. Orthopedics. 2012;35(10 Suppl):34. doi: 10.3928/01477447-20120919-55. [DOI] [PubMed] [Google Scholar]
- 6.Hendricks T.J., Chong A.C., Bhargava T. The use of precision alignment technology to Circumvent patient-specific roadblocks in performing total knee arthroplasty: a case series. Kans J Med. 2017;10(3):1. [PMC free article] [PubMed] [Google Scholar]
- 7.Nam D., Cody E.A., Nguyen J.T., Figgie M.P., Mayman D.J. Extramedullary guides versus portable, accelerometer-based navigation for tibial alignment in total knee arthroplasty: a randomized, controlled trial: winner of the 2013 HAP PAUL award. J Arthroplasty. 2014;29(2):288. doi: 10.1016/j.arth.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Stieber J.R., Dormans J.P. Manifestations of hereditary multiple exostoses. J Am Acad Orthop Surg. 2005;13(2):110. doi: 10.5435/00124635-200503000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Wicklund C.L., Pauli R.M., Johnston D., Hecht J.T. Natural history study of hereditary multiple exostoses. Am J Med Genet. 1995;55(1):43. doi: 10.1002/ajmg.1320550113. [DOI] [PubMed] [Google Scholar]
- 10.Schmale G.A., Conrad E.U., 3rd, Raskind W.H. The natural history of hereditary multiple exostoses. J Bone Joint Surg Am. 1994;76(7):986. doi: 10.2106/00004623-199407000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Wuyts W., chmale G.A., Chansky H.A., Raskind W.H. Hereditary multiple osteochondromas. In: Adam M.P., Ardinger H.H., Pagon R.A., editors. GeneReviews. University of Washington, Seattle. 1993; Seattle, WA: 2013. [PubMed] [Google Scholar]
- 12.Pacifici M. Hereditary multiple exostoses: new insights into pathogenesis, clinical complications, and potential treatments. Curr Osteoporos Rep. 2017;15(3):142. doi: 10.1007/s11914-017-0355-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandez-Perez S.A., Rodriguez J.A., Jr., Beaton-Comulada D., Colon-Miranda R.G., Soler-Salas A.H., Otero-Lopez A. Total knee arthroplasty in patients with multiple hereditary exostoses. Arthroplasty Today. 2018;4(3):325. doi: 10.1016/j.artd.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mesfin A., Goddard M.S., Tuakli-Wosornu Y.A., Khanuja H.S. Total hip and knee arthroplasty in patients with hereditary multiple exostoses. Orthopedics. 2012;35(12):e1807. doi: 10.3928/01477447-20121120-29. [DOI] [PubMed] [Google Scholar]
- 15.Wells M., Birchard Z. A 40-year-old male presenting with hereditary multiple exostosis: management and considerations. Case Rep Orthop. 2019;4:2019. doi: 10.1155/2019/4793043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kohn M.D., Sassoon A.A., Fernando N.D. Classifications in brief: Kellgren-Lawrence Classification of osteoarthritis. Clin Orthop Relat Res. 2016;474(8):1886. doi: 10.1007/s11999-016-4732-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lum Z.C., Pereira G.C. Local bio-absorbable antibiotic delivery in calcium sulfate beads in hip and knee arthroplasty. J Orthop. 2018;15(2):676. doi: 10.1016/j.jor.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stancil R., Summers N., Fernando N., Chansky H., Sassoon A. Western Orthopaedic Association; 2016. Prophylactic antibiotic impregnated calcium sulfate beads in high-risk hip and knee arthroplasty. Podium Presentation. [Google Scholar]
- 19.Nawata K., Teshima R., Minamizaki T., Yamamoto K. Knee deformities in multiple hereditary exostoses. A longitudinal radiographic study. Clin Orthop Relat Res. 1995;(313):194. [PubMed] [Google Scholar]
- 20.Pierz K.A., Stieber J.R., Kusumi K., Dormans J.P. Hereditary multiple exostoses: one center's experience and review of etiology. Clin Orthop Relat Res. 2002;(401):49. doi: 10.1097/00003086-200208000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi M., Nishihara A., Ohishi T., Shiga K., Yamamoto K., Nagano A. Arthroscopic resection of an intra-articular osteochondroma of the knee in the patient with multiple osteochondromatosis. Arthroscopy. 2004;20(Suppl 2):28. doi: 10.1016/j.arthro.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 22.Kim R.H., Scuderi G.R., Dennis D.A., Nakano S.W. Technical challenges of total knee arthroplasty in skeletal dysplasia. Clin Orthop Relat Res. 2011;469(1):69. doi: 10.1007/s11999-010-1516-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoo J.D., Kim N.K. Periprosthetic fractures following total knee arthroplasty. Knee Surg Relat Res. 2015;27(1):1. doi: 10.5792/ksrr.2015.27.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.