Abstract
Regulatory science for medical devices aims to develop new tools, standards and approaches to assess the safety, effectiveness, quality and performance of medical devices. In the field of biomaterials, hernia mesh is a class of implants that have been successfully translated to clinical applications. With a focus on hernia mesh and its regulatory science system, this paper collected and reviewed information on hernia mesh products and biomaterials in both Chinese and American markets. The current development of regulatory science for hernia mesh, including its regulations, standards, guidance documents and classification, and the scientific evaluation of its safety and effectiveness was first reported. Then the research prospect of regulatory science for hernia mesh was discussed. New methods for the preclinical animal study and new tools for the evaluation of the safety and effectiveness of hernia mesh, such as computational modeling, big data platform and evidence-based research, were assessed. By taking the regulatory science of hernia mesh as a case study, this review provided a research basis for developing a regulatory science system of implantable medical devices, furthering the systematic evaluation of the safety and effectiveness of medical devices for better regulatory decision-making. This was the first article reviewing the regulatory science of hernia mesh and biomaterial-based implants. It also proposed and explained the concepts of evidence-based regulatory science and technical review for the first time.
Keywords: Regulatory science, Safety and effectiveness, Hernia mesh, Regeneration, Remolding
Graphical abstract
Highlights
-
•
Hernia mesh is a class of biomaterials-based implants that have been successfully translated to clinical applications.
-
•
This is the first article reviewing the current status and future perspectives of regulatory science for hernia mesh.
-
•
Research on regenerative medicine, computational modeling, big data and evidence-based research could promote the development.
-
•
Evidence-based regulatory science and technical review were also proposed and defined for the first time.
1. Introduction
Breakthroughs in science and technology and the huge clinical demand brought about by aging population are accelerating the growth of the health care market, including medical devices. The global market of medical products is expected to reach $400 billion in 2020 [1]. As the second largest medical device market in the world, China saw the revenues of its medical device manufacturers in 2018 standing at around 638 billion yuan, and the number is expected to exceed one trillion yuan in 2021–2022 [2]. The burgeoning medical device industry has brought unprecedented challenges to the regulators, boosting the development of regulatory science for medical devices.
Regulatory science for medical devices is “the science of developing new tools, standards, and approaches to assess the safety, effectiveness, quality, and performance of regulated medical device products.” [3].
In 2010, the FDA initiated the framework of regulatory science [4] and the drafting of its strategic plan [5]. The FDA Center for Devices and Radiological Health (CDRH) proposed top ten regulatory science priorities for medical devices in 2017, including leveraging “big data” for regulatory decision-making, modernized biocompatibility evaluation, computational modeling technologies, precision medicine and biomarkers [6].
In April 2019, China National Medical Products Administration launched the first round of regulatory science action plans for medical products. Projects related to regulatory science for medical devices included safety and effectiveness evaluation of artificial intelligence medical devices, research on regulatory science for novel materials-based medical devices, the technical evaluation of combination products and the methodological research on clinical evaluation of medical devices based on real-world data [7].
Regulatory science provides crucial basis and support for the scientific regulation of medical devices. Medical device regulation involves many aspects, including but not limited to the formulation of laws and regulations, registration review and approval, inspection of manufacturing quality system and evaluation of adverse events. How to evaluate the safety and effectiveness of medical devices in a scientific and systematic way is one of the main contents of research on regulatory science for medical devices.
Hernia mesh is a typical biomaterial-based implant. The current paper focuses on the safety and effectiveness evaluation of hernia mesh. This paper starts with a description of the mechanism, classification, marketed products and materials of hernia mesh. Next, the general information of regulatory science for medical devices is introduced, with a focus on the current development of regulatory science for hernia mesh, including relevant laws and regulations, standards and guidance documents. The evidence for safety and effectiveness evaluation at all stages, including preclinical testing on physical and chemical characterization, biocompatibility and biosafety research, animal study and clinical evaluation, is analyzed. Finally, future research directions of regulatory science for hernia mesh are proposed.
2. Hernia mesh
Hernia refers to a raised structure that organs or tissues in the abdominal cavity protrude from defects in abdominal wall, groin and other areas. Abdominal wall defects can be congenital or postnatal, with higher incidence among the elderly [8,9]. According to two large epidemiological surveys, the incidence rate of inguinal hernia in China is 5.5–7 per 1000 [10,11]. With the world's aging population, hernia will likely become a major disease from which tens of millions will suffer.
Hernia repair is an effective method to cure hernia and abdominal wall defect. It can be divided into tension hernia repair and tension-free hernioplasty. In 1986, Irving Lichtenstein first proposed to use synthetic materials for inguinal hernia repair to achieve tension-free hernia repair [12]. Now, tension-free hernioplasty has become a regular choice for clinicians around the world.
This section puts forward and discusses the classification of hernia mesh based on four criteria from the perspective of safety and effectiveness evaluation. Hernia mesh products on the Chinese and the U.S. markets are introduced, as well as hernia mesh products that have passed the special procedure for review and approval of innovative medical devices in China.
2.1. Scope and classification
There are different classification methods for hernia mesh. In guidelines issued by European, U.S. and Asia-Pacific hernia societies, hernia mesh is generally divided into two categories. One is synthetic mesh made of synthetic materials such as polypropylene, polyethylene terephthalate and polytetrafluoroethylene. The other is biologic/biological mesh made of animal-derived or allogeneic materials [13,14]. In addition, it can also be classified into large porous mesh and lightweight mesh [8]. According to the type of composed materials, some reviewers classified it into single material mesh, composite mesh with two or more layers, woven or machine-knit mesh with two kinds of fibers, and biological mesh [15].
Based on the safety and effectiveness evaluation of hernia mesh, this paper classifies hernia mesh by the following four different categories, mechanism of repair, anatomical site of implanted mesh, indications and location of use, and type of material.
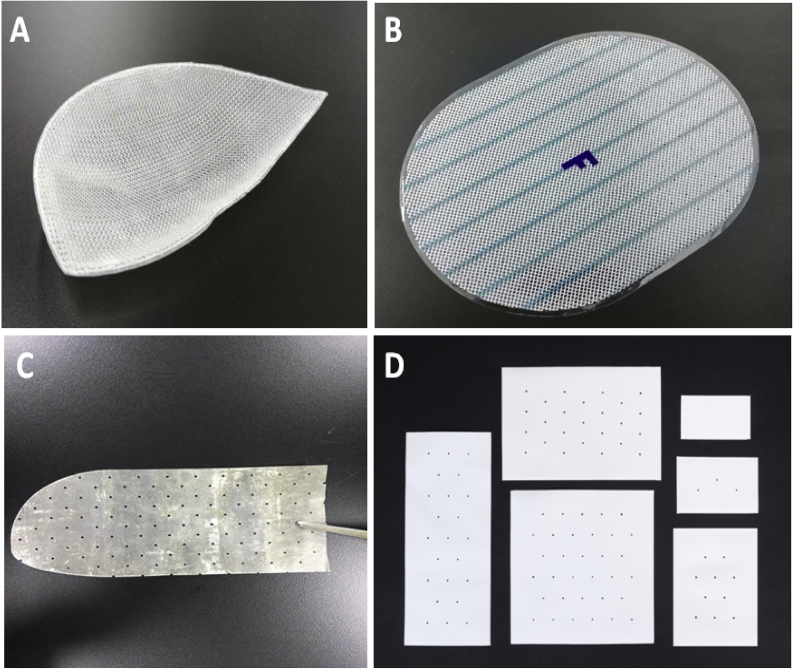
First, according to different repair mechanisms, hernia mesh can be divided into non-remodeling hernia mesh (NRHM) (see Fig. 1A and B) and tissue remodeling hernia mesh (TRHM) (see Fig. 1C and D), respectively corresponding to the two mechanisms of biological tissue defect healing, i.e. tissue scarring and remodeling/regeneration [16,17]. The former can repair the defect by stimulating the scar tissue formation to fill the pores of the mesh through the reaction to foreign body, and the foreign body exists in the human body for a long time [18]. The latter has a long-lasting effect on the repair of hernia and abdominal wall defect by using endogenous tissue regeneration and remodeling, resulting in fewer scar tissues in the repaired defect [16].
Fig. 1.
Representative Images of Hernia Mesh. (A) Inguinal hernia mesh with anatomical shape; (B) Intraperitoneal mesh with coating; (C) Biological mesh; (D) Fiber-based absorbable mesh (Images were provided by different clinical sources).
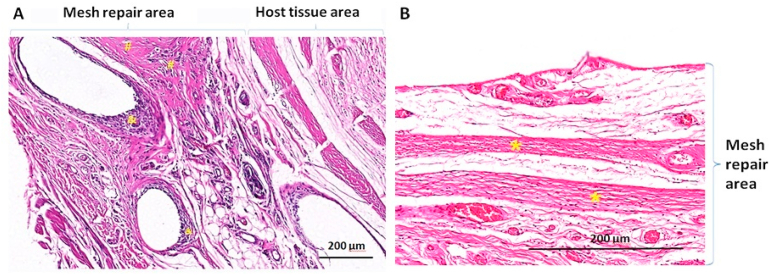
NRHM is often made of non-absorbable materials, while TRHM is composed of absorbable materials [19]. Since the degradation of implant materials varies and tissue reactions differ, tissue repair is achieving toward different directions. The representational histological staining images are shown in Fig. 2:
Fig. 2.
Histologic appearance of Scar Repair and Remodeling & Regenerative Repair. (A) Scar repair with non-absorbable Polyvinylidene fluoride (PVDF) synthetic mesh (hematoxylin & eosin (H&E) stained). A large number of inflammatory cells are around the mesh fibers. The disordered deposition of scar tissue collagen fibers can be seen at the interface between the mesh and the host tissue; and (B) Remodeling & regenerative repair with absorbable porcine urinary bladder matrix biological mesh (H&E stained). The ordered deposition of regenerated collagen fibers appeared in mesh repair area.
#: Scar tissue; &: Inflammatory response area; *: The regenerated collagen fibers appeared in an orderly arrangement.
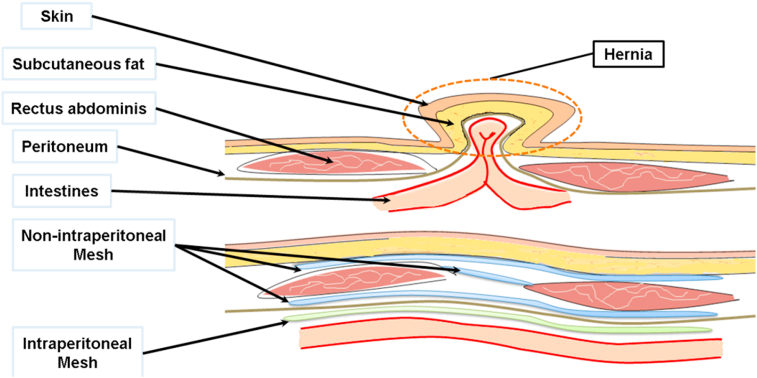
Second, according to the anatomical site of implanted mesh, hernia mesh can be divided into two types: intraperitoneal hernia mesh and non-intraperitoneal hernia mesh [20]. As shown in Fig. 3, for abdominal wall hernia, intraperitoneal hernia mesh is implanted under the peritoneum. Due to the possibility of adhesion with the tissues and organs in abdomen, the product is specially designed to reduce adhesion. As shown in Fig. 1 (B), the mesh has a layer of film to reduce adhesion. In Fig. 3, the meshes implanted in other anatomical sites are all non-intraperitoneal hernia mesh.
Fig. 3.
Implanted Anatomical Position of Hernia Mesh (Image modified from Refs. [20]).
Third, according to the clinical indications and types of hernia, it can be divided into inguinal hernia, femoral hernia and abdominal wall hernia (incisional ventral hernia, parastomal hernia, umbilical hernia, epigastic hernia and semilunar hernia), diaphragmatic hernia, hiatal hernia and pelvic hernia without organ prolapse [20,21]. It should be noted that while some mesh can be used for various types of hernia repair, there are special-purpose mesh products designed only for certain types of hernia. As the mesh shown in Fig. 1 (A), it can only be used for inguinal hernia.
Fourth, according to materials, hernia mesh can be made of synthetic polymer materials such as polypropylene, biological tissue membrane materials such as porcine small intestinal submucosa, and composite made of various materials [9]. Table 1, Table 2, Table 3 show the materials used in approved/cleared hernia mesh published by the official websites of the China NMPA and the U.S. FDA. For the convenience of description, this paper reviews materials of hernia mesh as following: the key materials of hernia mesh (Table 1), the materials of the layer that minimizes tissue adhesions or attachment for intraperitoneal hernia mesh (Table 2), and the materials of functional components (Table 3).
Table 1.
Key Materials of marketed hernia mesh in China and the U.S.
| No. | Type of material | Chemical name of material | Ref. |
|---|---|---|---|
| 1 | A: Non-absorbable synthetic materials | Polypropylene (PP) | [22] |
| 2 | Polytetrafluoroethylene (PTFE) | [23] | |
| 3 | Expanded polytetrafluoroethylene (ePTFE) | [24] | |
| 4 | Polyvinylidene fluoride (PVDF) | [24] | |
| 5 | Polyethylene terephthalate (PET) | [23] | |
| 6 | nylon 6 | [25] | |
| 7 | Polycarbonate polyurethane urea | [26] | |
| 8 | Cross-linked acrylic polymer | [27] | |
| 9 | Polycaprolactone (PCL) based poly (urethane urea) (PUU) | [28] | |
| 10 | expanded polytetrafluoroethylene (ePTFE) reinforced with fluorinated ethylene propylene (FEP) | [29] | |
| 11 | Co-knitted using PP and PVDF | [30] | |
| 12 | Co-knitted using PP and Polyurethane (PU) | [31] | |
| 13 | Co-knitted using PP and Polycarbonate polyurethane urea | [32] | |
| 14 | PP with titanium coating (plasma activated chemical vapor deposition of atomic Titanium) | [19] | |
| 15 | PP with Titanium Dioxide coating (Wet-chemical coating procedure) | [19] | |
| 16 | B: Fiber-based absorbable synthetic materials scaffold | Polyglycolide (PGA) | [33] |
| 17 | Polylactide (PLA) | [34] | |
| 18 | Polydioxanone (PDO) | [35] | |
| 19 | Poly (lactide-co-glycolide) (PLGA) | [33] | |
| 20 | Poly (glycolide-co-trimethylene carbonate) (P (GA-TMC)) | [36] | |
| 21 | Co-knitted using Poly (glycolide-co-trimethylene carbonate) (P (GA-TMC)) and Poly (lactide-co-trimethylene carbonate) copolymer (P (LA-TMC)) | [37,38] | |
| 22 | Co-knitted using Poly (glycolide-co-lactide-co-trimethylene carbonate) copolymer (P (GA-LA-TMC)) and Poly (lactide-co-trimethylene carbonate) copolymer (P (LA-TMC)) | [38] | |
| 23 | Bioresorbable l-tyrosine succinate polymer | [24] | |
| 24 | C: Fiber-based absorbable natural materials scaffold | Silk fibroin protein | [39] |
| 25 | Poly-4-hydroxybutyrate (P4HB) | [40,41] | |
| 26 | D: Absorbable or part-absorbable allogenic acellular tissue matrix | Allogenic acellular dermal matrix (A-ADM) | [42] |
| 27 | E: Absorbable or part-absorbable xenogenic acellular tissue matrix | Porcine intestinal submucosa (SIS) | [43] |
| 28 | Porcine urinary bladder matrix (P-UBM) | [44,45] | |
| 29 | Porcine liver matrix | [46] | |
| Porcine acellular dermal matrix (P-ADM) | [47] | ||
| Bovine acellular dermal matrix (B-ADM) | [48] | ||
| 30 | Collagen derived from bovine pericardium | [49] | |
| 31 | Collagen derived from porcine pleura | [50] | |
| 32 | Cross-linked porcine dermal collagen | [51] | |
| 33 | Cross-linked fibrous collagen derived from bovine hides | [52] | |
| 34 | F: Non-absorbable or part-absorbable biological tissue | Porcine pericardium (P-PC) | [53] |
| 35 | Bovine pericardium (P-PC) | [49] | |
| 36 | Equine pericardium (E-PC) | [54] | |
| 37 | G: Non-absorbable natural materials | microbial-derived cellulose | [55] |
| 38 | Composite material: A + B | Co-knitted using PP and Polyglycolide (PGA) | [56] |
| 39 | Co-knitted using PP and PDO | [57] | |
| 40 | Co-knitted using PP and poly (glycolide-co-caprolactone) (P (GA-CL)) | [8] | |
| 41 | Co-knitted using PP and PLGA | [19] | |
| 42 | Composite of PVDF and poly (1,4-butylene adipate)PBA | [58] | |
| 43 | Hybrid mesh: Multi-layer using PTFE and porous PGA/TMC | [40] | |
| 44 | Composite material: A + E | Hybrid mesh: Multi-layer using PP and SIS | [40,59] |
| 45 | Hybrid mesh: Ovine forestomach or rumen derived extra cellular matrix (ECM) with PP embroidery | [40,60,61] | |
| 46 | Composite material: B + C | Electrospinning technology: Poly (l-lactide-co-caprolactone) (P (LLA-CL)) and Porcine fibrinogen (P-FIB) | [62] |
| 47 | Co-knitted using P4HB and PGA | [63] | |
| 48 | Multi-layers using lyophilized porcine collagen and PLA | [64] | |
| 49 | Composite material: B + E | Ovine forestomach or rumen derived extra cellular matrix (ECM) with PGA embroidery | [40,60,61] |
* Table 1 only lists the key mesh materials for hernia repair. The information of the composite mesh materials with the adhesion-reducing layers is listed in Table 2, such as the ePTFE of Composix product and the P (GA-CL) of PHYSIOMESH product. The material information of functional components in multi-component hernia mesh is listed in Table 3, such as the self-gripping system of the PLA of ProGrip product.
Table 2.
Materials of the layer that could minimize tissue adhesions or attachment in the marketed intraperitoneal hernia mesh in China and the U.S.
| No. | Type of material | Chemical name of material | Ref. |
|---|---|---|---|
| 1 | Non-absorbable synthetic materials | ePTFE | [65] |
| 2 | PVDF | [30] | |
| 3 | Cross-linked acrylic polymer with polyamide | [27] | |
| 4 | Polyurethane | [31] | |
| 5 | silicone | [66] | |
| 6 | Absorbable synthetic materials | P (LA-CL) | [32,67] |
| 7 | P (GA-CL) | [31] | |
| 8 | P (GA-TMC) | [40] | |
| 9 | P (GA-CL-TMC-LA) | [68] | |
| 10 | Oxidized regenerated cellulose (ORC) | [56,69] | |
| 11 | Natural materials | Bioabsorbable Oil Fatty Acid (O3FA): fatty acids, lipids and glycerides | [31] |
| 12 | Cross-linked resorbable collagen film of porcine origin | [53] | |
| 13 | Composite materials | Porcine collagen, polyethylene glycol (PEG)and glycerol | [64,70] |
| 14 | Porcine collagen and glycerol | [71] | |
| 15 | Sodium hyaluronate (HA) and carboxymethyl cellulose (CMC) | [70] | |
| 16 | Sodium hyaluronate, carboxymethyl cellulose and polyethylene glycol (HA-CMC-PEG) | [56,63] |
Table 3.
Materials of functional components in the marketed hernia mesh in China and the U.S.
| No. | Type of functional component | Chemical name of material | Ref. |
|---|---|---|---|
| 1 | Monofilament resorbable pins or grips for Self-Gripping | PLA | [72] |
| 2 | Self-expanding rings | PET | [73] |
| 3 | Expanding rings | PLGA | [56] |
| 4 | Recoil ring | PDO | [56] |
| 56 | Antibiotic drugs coating | Silver carbonate and chlorhexidine diacetate | [24] |
| Bioresorbable l-tyrosine succinate polymer and antimicrobial agents Rifampin and Minocycline | [24] | ||
| 6 | Self-expanding Nitinol framed | Ni–Ti shape-memory alloy | [74] |
| 7 | Coating provide additional stiffness | bioresorbable polyarylate (PAR) | [24] |
The U.S.: https://www.fda.gov/medical-devices.
*Hernia mesh products official database websites.
2.2. Marketed hernia mesh products in China and the U.S
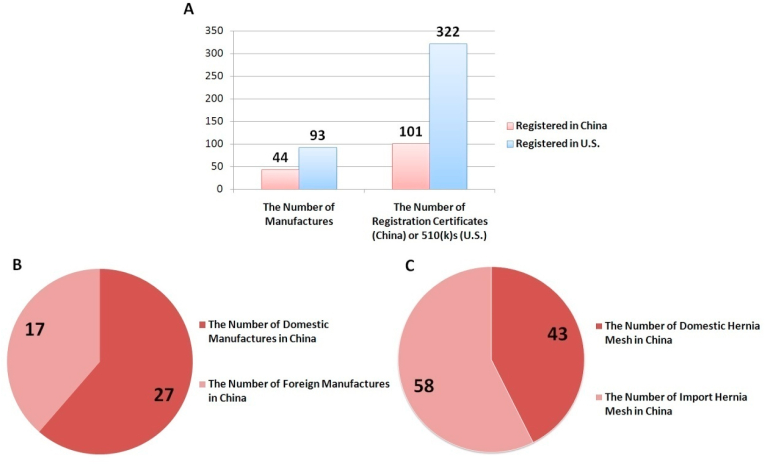
According to the official websites of the NMPA and the FDA, the information of hernia mesh approved or cleared for market entry in China and the U.S. up to April 15, 2020 is summarized in Fig. 4.
Fig. 4.
Statistics of Hernia Mesh Approved in China and the U.S. (A)The number of approved hernia mesh and manufacturers in China and the U.S.(B) The number of domestic and foreign hernia mesh manufacturers in China. (C) The number of domestic and imported hernia mesh in China. As of April 15, 2020, forty-four hernia mesh manufacturers obtained market approval in China, including twenty-seven domestic manufacturers and seventeen foreign manufacturers. Currently, there are 101 registration certificates issued for various kinds of hernia mesh, forty-three of which are Chinese products and fifty-eight are imported. Ninety-two manufacturers in the United States obtained clearance, with 322 currently valid 510(k) Premarket Notifications.
* Hernia mesh products official database websites: China: http://qy1.sfda.gov.ex2.ipv6.nmpa.gov.cn/datasearchcnda/face3/dir.html; The U.S.: https://www.fda.gov/medical-devices.
To sum up, among the approved devices in China, there are eight TRHMs and nineteen intraperitoneal hernia mesh devices designed to reduce adhesions. In the United States, there are seventy-eight TRHMs on the 510 (k) premarket notification and fifty-three intraperitoneal mesh designed to reduce adhesions. The hybrid hernia mesh made of absorbable tissue-remodeling mesh and non-absorbable synthetic materials has not yet been approved in China, whereas there are three hybrid hernia mesh products in the United States.
2.3. Innovative hernia mesh devices
Since 2014, the former China Food and Drug Administration (CFDA) had launched the special procedure for review and approval of innovative medical devices to promote innovation [75]. At present, the hernia mesh products which have applied for innovative medical device status are all TRHMs. Compared with scarring repair, this kind of mesh is considered as an ideal medical device for soft tissue defect repair [8,76].
As of the submission of this article, two hernia mesh products have passed the special procedure for innovative medical devices in China. Among them, the composite hernia mesh has been approved for market entry in China. The product is made of the blend of l-lactide, caprolactone and porcine fibrinogen by electrostatic spinning technology [62,77]. The other innovative medical device is the biological hernia mesh, which is a “sandwich” structure made of porcine urinary bladder basement membrane and small intestinal submucosa extracellular matrix by vacuum lamination [78].
The evaluation of the safety and effectiveness of innovative medical devices poses a challenge to regulators, in terms of how to evaluate the induced tissue regeneration and how to distinguish induced tissue regeneration from tissue remodeling. Related information will be discussed in detail in the later sections.
3. Current status
Regulatory science for hernia mesh should cover the total product life cycle of hernia mesh, including the premarket and post-marketing phases. The premarket safety and effectiveness evaluation is a good representative in the research of regulatory science. The constructing of premarket regulatory science system involves laws, regulations, standards, guidance documents and other documents issued by the regulators of medical products, the standardization technical committee and other institutions.
This section focuses on the safety and effectiveness evaluation of hernia mesh devices. It starts with the introduction of key regulatory documents including Rules, Regulations, Standards and Technical Guidance Documents. Next, scientific evidence on safety and effectiveness evaluation for hernia mesh is summarized. The quality of evidence is analyzed and discussed from the three areas of preclinical chemical properties testing, preclinical animal study and premarket clinical evaluation. Finally, the regulatory pathways for hernia mesh devices in both the U.S. and China are described.
3.1. Key regulatory documents
In August 2015, the State Council of China issued the Opinions on the Reform of the Review and Approval System for Drugs and Medical Devices [79](GF [2015] No. 44), and launched the reform of the review and approval system of medical devices in China. Under the guidance of the document, the NMPA formulated and issued a series of regulatory and normative documents related to the reform of medical device review and approval (refer to article [80] for details). These regulations and normative documents are commonly applicable, not limited to specific medical devices such as hernia mesh.
Standard serves as an important reference for the safety and effectiveness evaluation of medical devices. Standards that referred by the NMPA for medical devices include international standards from both ISO and ASTM, Chinese national standards (GB) and Chinese medical device industrial standards (YY). Chinese standards are also divided into mandatory standards and recommended standards [81].
Guidance documents are important technical documents to navigate the safety and effectiveness evaluation of medical devices. The general guidance documents of medical devices cover the safety and effectiveness evidence of preclinical performance research [82], biological safety evaluation of animal-derived medical devices [83,84], general principles of the design of animal studies [85], and clinical evaluation [[86], [87], [88]], among other contents [89,90]. The technical review of hernia mesh can refer to the applicable general guidance or the applicable contents in the guidance documents.
Since 2013, the NMPA has issued a series of specialized guidance documents for the technical review of hernia mesh. A system of technical review science for hernia mesh in line with the general guidance documents has been established (see Table 4 for more specifics). In 1999, the United States also issued a guidance document to guide the 510(k) application of surgical mesh [91].
Table 4.
Guidance documents of technical review for hernia mesh in China.
| Year and document No. | Titles | Date of Implementation |
|---|---|---|
| 2013 SFDA Announcement No.7 | Guidance of Technical Review for Product Registration of Hernia Mesh [92] | Oct. 12st, 2013 |
| 2019 NMPA Announcement No.18 | Guidance of Technical Review for Animal Experiment of Intraperitoneal Hernia Mesh [93] | April 18th, 2019 |
| / | Guidance for Clinical Trail of Hernia Mesh [94] | To be released |
*SFDA is the abbreviation of State Food and Drug Administration of China before 2013.
NMPA is the abbreviation of National Medical Product Administration of China since 2018.
The Guidance of Technical Review for Product Registration of Hernia Mesh mainly regulates the submission of non-clinical documents and data of hernia mesh [92]. The guidance covers areas including physical and chemical bench performance tests, biocompatibility assessment, sterilization validation, shelf life tests, and technical specifications and tests.
3.2. Evaluation of safety and effectiveness in regulatory science
To ensure the safety and effectiveness of medical devices is the responsibility and mission of medical products regulators worldwide. Technical review is an important link in the regulation of medical devices. Regulatory science on the technical review of medical devices can be defined as technical review science, which focuses on the scientific and efficient evaluation on the safety and effectiveness of medical devices before market entry.
Safety and effectiveness are the prerequisites for the approval or clearance of medical devices. The International Medical Device Regulators Forum (IMDRF), initiated by medical device regulators from many countries, has issued the Essential Principles of Safety and Performance of Medical Devices and IVD Medical Devices which defines “safety” as “the freedom from unacceptable risk” and “effectiveness” as “the ability of a medical device or IVD medical device to provide clinically significant results in a significant portion of the target population” [95].
The top three clinical risks of hernia mesh are reoccurrence, pain and infection [19,24,96]. Regulatory science focuses on how to scientifically evaluate hernia mesh devices in terms of acceptance of their potential risks as well as sufficient evidence to prove safety and effectiveness. For example, potential reoccurrence can be evaluated via preclinical bench top mechanical testing, animal study and clinical evaluation. Potential infection risk can be evaluated through biocompatibility evaluation and sterilization validation. The following will discuss these information in detail.
3.3. Summary of scientific evidence
For hernia mesh, the evidence required for safety and effectiveness evaluation includes preclinical in vitro performance test, animal study, clinical evaluation and other aspects. For the convenience of discussion, the types of evidence and specific items are presented in Table 5. Please note that Table 5 is not an exhausted but a representative list of relevant evidence to be provided.
Table 5.
Summary of evidence for the safety and effectiveness of hernia mesh.
| Type of evidence | Corresponding risks | List of specific items |
|---|---|---|
| quality control of raw materials | Affecting the safety and effectiveness of final products. | Applicable items such as chemical composition analysis, qualitative and quantitative analysis of impurity, fiber size and physical strength, biocompatibility evaluation. |
| Physical properties [97,98] | Risk of hernia recurrence due to insufficient mechanical properties. | Appearance, physical dimensions (thickness) [23], monofilament diameter [22], pore properties [23,99] (pore size, pore density, porosity), weight per unit area/Density [23,[100], [101], [102]], uniaxial tensile strength [23,100,[103], [104], [105], [106]], tensile elongation [106,107], burst strength [23,100,104], suture retention [23,100,104], suture pullout strength [108], connection strength between components and layers (when applicable), tear resistance [23,100,104] (when applicable), fading test (when applicable), stiffness [109]. |
| Chemical properties | Toxic reaction due to chemical residues. | Qualitative analysis of chemical composition of the mesh [69] (also consider its degradation products).a |
| Impurity residues (including qualitative and quantitative analysis, items reflecting the total amount of impurities indirectly and specific impurity residues).a | ||
| Research on bench performance tests | Functional failure. | Applicable items such as in vitro tests of fixation strength of self-fixed mesh without suture, research on mesh shape with human groin anatomical structure, etc. |
| Degradation cycle [38] and degradation rate study (when applicable) | ||
| Process validation | Instability of product quality or safety risks caused by process variations. | Applicable items including but not limited to cleaning process and impurity removal process, and weaving process. |
| Biocompatibility [110] | Toxicological response caused by poor biocompatibility. | Evaluation without performing biological tests: toxicological equivalence analysis with similar products on the Chinese market, toxicological analysis of leachable substances. |
| Biological tests: cytotoxicity, sensitization, irritation or intracutaneous reactivity, systemic toxicity (acute/subchronic/pyrogenicity), genotoxicity, implantation (with histology of the surrounding tissue). If the materials have never been used in marketed long-term implantable medical devices in China, long-term implantation responses, chronic toxicity and carcinogenicity should be evaluated. | ||
| Research data on the metabolism of degradation products (when applicable) and bacterial endotoxin. | ||
| Biosafety studyb | Risk of virus and infectious pathogen, immunogenicity risks. | Safety data of animal or allogeneic source control, such as quarantine and epidemic prevention documents, donor donation letters, virus test reports. |
| Risk analysis of viruses and/or infectious pathogens, description and verification of corresponding control measures. | ||
| Risk analysis of product immunogenicity (immunoreaction), description and verification of control processes, test of items related to quality control of immunogen (applicable items such as hetero protein, DNA, antigen composition) and immunotoxicity test (if necessary). | ||
| Sterilization validation | Infections associated with the non-sterile products. | Studies on the tolerance of hernia mesh to sterilization process, sterility assurance level, sterility test, irradiation dose (when applicable), residues of ethylene oxide and other disinfectants and their derivative (when applicable). |
| Shelf life Study | Risk of Product deterioration within the expiration date, damaged sterile packaging. | Mesh stability study. |
| Packaging study. | ||
| Simulated transportation study. | ||
| Animal studies | Tissue adhesion with intraperitoneal mesh, hernia recurrence caused by rapid degradation of remodeling mesh. | Studies on adhesions of intraperitoneal hernia mesh (when applicable). |
| Studies on tissue regeneration and remodeling effectiveness of remodeling mesh [38] (when applicable). | ||
| Studies on the shrinkage of mesh after long-term implantation (when applicable). | ||
| Other performances of product (when applicable) [111]. | ||
| Premarket clinical evaluation | Defect recurrence, adverse events and complications after clinical application [112,113]. | Prospective clinical trials, clinical literature, clinical cases studies and other evidence. |
| The comparison with registered product and predicate medical devices to demonstrate that they are substantial equivalent (SE)in terms of safety and effectiveness. | ||
| Post-marketing clinical evaluation | Post-market clinical trials, registered studies (when applicable), clinical follow-up and quality tracking [67]. |
There are differences in the chemical properties of hernia mesh made of different materials (see Table 7 for details).
Biosafety is applicable to mesh made from animal-derived and allogeneic materials.
Considering the wide varieties of hernia mesh, it can be well classified according to four categories which are in line with the product characteristics, as described in Section One, for better regulation of such products. Depending on types of hernia mesh in each category, the safety and effectiveness may be evaluated differently.
Some types of evidence are not applicable to certain hernia mesh. Table 6 gives the information on the applicability of evidence to different types of hernia mesh based on Table 5. For mesh containing materials derived from animal sources, allogeneic material and bioactive components, research proof on biosafety is required. At present, in line with the Guidance for Technical Review of Animal Study of Medical Devices Part 1: Decision-making Principles [85], if the NRHM implanted extra peritoneal does not apply a brand new material nor come up with a new structural design, it may not be necessary to carry out animal study during the phase of design control for the product. For NRHM that does not have the tissue remodeling properties, clinical trial may not be necessary if substantial equivalence exists in terms of the safety and effectiveness between the mesh and those of the marketed predicate products in China. The specific contents of animal studies and clinical evaluation will be discussed in the following sections.
Table 6.
Supplementary list of the evidence of safety and effectiveness for different types of hernia mesh.
| Type of evidence | Type of mesh |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Repair mechanism |
Non- remodeling |
Tissue remodeling |
|||||||
| Anatomical site |
Non-intraperitoneal |
Intraperitoneal |
Non-intraperitoneal |
Intraperitoneal |
|||||
| Materials sources | Animal or allogeneic human | Synthetic and other natural sources | Animal or allogeneic human | Synthetic and other natural sources | Animal or allogeneic human | Synthetic and other natural sources | Animal or allogeneic human | Synthetic and other natural sources | |
| Biosafety | ✓ | / | ✓ | / | ✓ | / | ✓ | / | |
| Animal study | / | ✓ | |||||||
| Premarket clinical investigation | * | ✓ | |||||||
Note: Other evidence types not listed are applicable to all types of hernia mesh.
*: Premarket clinical investigation is unnecessary if the subject product is substantial equivalent to marketed predicate products in China.
3.4. Preclinical chemical properties testing
For hernia mesh made of different materials, their chemical properties are also different. This paper summarizes the chemical properties of some of the typical marketed hernia mesh products (see Table 7). Synthetic materials usually need to be characterized and tested for chemical residues. Materials derived from animal and allogeneic sources need to establish quality control standards for residues that may generate immunogenic reactions. In addition, the residual amount of specific hazardous small molecules and macromolecules should be analyzed according to the condition of use for raw materials and additives during the manufacturing process.
Table 7.
Chemical properties of hernia mesh [92].
| Type of Material | Chemical composition of material | Chemical properties |
|||
|---|---|---|---|---|---|
| Material characterization | Safety items | ||||
| Synthetic polymer materials | Non-absorbable materials | PP, PET, PVDF, PTFE, etc. | Infrared identification [114] | Potential of hydrogen (PH) | Residues of heavy metals, manufacturing reagents, harmful small molecular and macromolecular substances in final products, such as ethylene oxide (when applicable) |
| Evaporation residue | |||||
| Reducing substance | |||||
| Ultraviolet Absorbance (UVA) | |||||
| Absorbable materials | PGA,PLA,PCL,PDO, etc. | Infrared or nuclear magnetic resonance identification [114] | Monomer residue | ||
| Molecular weight and molecular weight distribution | Catalyst residue (Tin) | ||||
| Monomer unit ratio (if applicable) | Organic solvents residue | ||||
| Optical rotation (if applicable) | Water residue | ||||
| Natural materials | Non-absorbable materials | Bovine pericardium (B-PC), Porcine pericardium (P-PC) | Cross-linking reagents (Glutaraldehyde, epoxide) | ||
| DNA, Antigen and other immunogenic substances | |||||
| Absorbable materials | Absorbable biological membrane materials such as Porcine small intestine submucosa | ||||
| Purified collagen | Identification | PH | |||
| Collagen purity | Residue on ignition | ||||
| Hydroxyproline content | Impurity protein analysis | ||||
3.5. Preclinical animal study
Animal study is an important stage during the design and development processes of medical devices. For the medical devices that need to conduct premarket clinical trials, animal study could reduce the potential risks that trial subjects and investigators may face. It also serves as a reference for the design of the clinical trial of the device. Although animal study is of great significance, it is not necessary for all medical devices. In 2019, the NMPA issued the Guidance for Technical Review of Animal Study of Medical Devices Part 1: Decision-making Principles [85]. The guidance proposes principles related to animal welfare and ethics, as well as risk management. A decision-making flow chart of animal study has been developed, which provides reference for medical device sponsors to decide whether to carry out animal study or not.
For different types of hernia mesh, different decision outcomes may be drawn when referring to the decision-making principles due to the distinctive characteristics of each product [85]. In general, for intraperitoneal hernia mesh, adhesion conditions should be evaluated through animal study, while for TRHM, effectiveness of tissue remodeling should be evaluated via animal study. Other hernia mesh which are made of new materials, or adopt new designs and designed with special functions may also need to conduct animal study.
In 2019, the NMPA issued the Guidance of Technical Review for Animal Study of Intraperitoneal Hernia Mesh [93], tackling the controversy over how to evaluate adhesion between mesh and intra-abdominal tissues. The guidance was expected to change the current confounding situation for the design of animal study [115]. The fundamental elements in the design of animal studies for hernia mesh were elucidated in the guidance (see Table 8 for specifics). The guidance established a brand-new composite index of both adhesion strength and adhesion area. As a result, an evaluation system of tissue adhesion for hernia mesh was developed for the first time.
Table 8.
Fundamental elements in the design of animal study for intraperitoneal hernia mesh [93].
| Fundamental elements | Relevant content in the guidance |
|---|---|
| Animal species | Large mature animals (such as minipigs and beagles) |
| Selection of control | The most similar marketed intraperitoneal mesh in terms of material and structural design in China. |
| Selection of evaluation criteria | Adhesion strength, adhesion area, acceptable rate of adhesion, condition of newly-grown peritoneum, adverse reactions, and histological reactions. |
| Selection of observation time | For non-absorbable adhesion reducing materials, at least observe for 28 days after implantation. For absorbable adhesion reducing materials, the observation period should be determined according to the expected time of complete degradation of the product. |
For other aspects such as the evaluation of the effectiveness of tissue remodeling, the NMPA will continue to carry out research on the evaluation of hernia mesh animal study and formulate new guidance documents. Detailed discussion can be found in Section Four.
3.6. Premarket clinical evaluation
According to China's current laws and regulations for medical device registration, such as the Provisions for Medical Device Registration [116] and the Guidance for Clinical Evaluation of Medical Devices, there are three main approaches for clinical evaluation. First, for medical devices listed in the Catalogue of Medical Devices Exempted from Clinical Trial [[117], [118], [119], [120], [121]], premarket clinical investigation is exempted. Second, for the medical devices that are not listed in the catalogue, clinical evaluation can be conducted through equivalent comparison of safety and effectiveness with the marketed medical devices. Third, for devices that carry out premarket clinical investigation, clinical data from overseas may also applicable for imported medical devices to apply for registration in China.
Table 9 lists hernia mesh in the Catalogue of Medical Devices Exempted from Clinical Trial since 2014 [[117], [118], [119], [120], [121]].
Table 9.
Hernia mesh in the Catalogue of Medical Devices Exempted from Clinical Trial.
| Year and document No. | Revision of the catalogue and types of hernia mesh |
|---|---|
| 2014CFDA Announcement No.13 [117]2016CFDA Announcement No.133 [118]2017CFDA Announcement No.170 [119] | The non-tissue remodeling non-intraperitoneal hernia mesh was listed in the Catalogue of Medical Devices Exempted from Clinical Trial as first batch of implantable medical devices. The key material of the mesh can only be polypropylene or polyethylene terephthalate, with partial absorbable materials. |
| 2018 NPMA Announcement No.94 [120] | Non-intraperitoneal hernia mesh of PVDF material was added in the catalogue. |
| 2019 NPMA Announcement No.91 [121] | After the release of the Guidance of Technical Review for Animal Study of Intraperitoneal Hernia Mesh, intraperitoneal NRHM was added to the exemption catalogue considering that the animal study for intraperitoneal hernia mesh could be consistent with the guidance document on animal study. |
*CFDA is the abbreviation of China Food and Drug Administration from 2013 to 2018.
NMPA is the abbreviation of National Medical Product Administration of China since 2018.
In 2019, the NMPA formulated the Guidance of Technical Review for Animal Study of Intraperitoneal Hernia Mesh [93] on the basis of technical review of hernia mesh clinical trial data and a comprehensive review of relevant literature. The guidance document reviewed related design elements of clinical trial and offered corresponding suggestions, such as the selection/exclusion criteria, endpoint, follow-up time, control devices, sample size (including the proportion of different types of hernia being studied together), statistical methods and results evaluation. In addition, the impact of test bias in terms of laparoscopic surgery or open surgery, and doctors' operation quality on clinical trial design were also analyzed and discussed.
3.7. Regulatory pathways for hernia mesh: U.S. FDA vs. NMPA
U.S. FDA has been conducting research on regulatory science for hernia mesh devices. In terms of safety and effectiveness evaluation, most of the products were cleared via premarket notification process, i.e., 510(k). Submitted products were evaluated via comparing with predicate devices to demonstrate substantially equivalency. Clinical trials are not required [91].
NMPA has similar regulatory pathways for hernia mesh devices, which are listing in the Catalogue of Medical Devices Exempted from Clinical Trial [[117], [118], [119], [120], [121]] and clinical evaluation through equivalency comparison of safety and effectiveness with the marketed medical device [86]. However, clinical trials are typically required for mesh with tissue remodeling claims, which often cannot be demonstrated via equivalency comparison.
4. Future perspectives
This section looks into the future prospects of regulatory science for hernia mesh from the following aspects: evaluation methods of tissue-remodeling and regeneration, application of computational modeling technology, smart regulation based on big data platforms and real-world data, and evidence analysis though evidence-based research methods. The aspects mentioned above in the regulatory science for hernia mesh are also cutting-edge research areas in biomaterial science, regenerative medicine, and big data application.
4.1. Evaluation of tissue remodeling and regeneration
Tissue remodeling and regeneration performance are critical to the safety and effectiveness of TRHM. One of the research areas for regulatory science of hernia mesh is to identify the tools and methods for standardized evaluation of tissue regeneration and remodeling in the dynamics of changing local tissue microenvironment, material degradation and host tissue response. Since the in vivo immune response to the mesh directly affects the effectiveness of tissue remodeling and regeneration, the evaluation method should be established based on animal study [122].
At present, the following aspects should be considered for evaluating remodeling and regeneration in the animal study. First, animal models with abdominal wall defect should be selected. Material samples are removed regularly after implantation. On one hand, histological analysis should be carried out to examine the degree of neovascularization and other factors [123]. On the other hand, matching between the degradation rate of mesh and tissue remodeling rate should be studied through analysis of retrieved implants at different in vivo periods [124].
At present, there are still a series of challenge facing the evaluation of tissue remodeling and regeneration of hernia mesh. For example, how to quantify the evaluation criteria of remodeling and regeneration? Reports reveal that the polarization typing of macrophages M1 and M2 has a strong correlation with tissue remodeling. In situ polarization of macrophages such as M1/M2 ratio can be used to accurately and quantitatively characterize the remodeling progress [122,125,126]. These studies shed light upon the quantitative criteria to evaluate the tissue remodeling or regeneration in different hernia mesh. The interaction between different hernia mesh and the host tissue in situ causes different polarization results of macrophages. That is due to the differences in microporous topological structure, degradation cycle and degradation products among different hernia mesh. Scientific research on tissue remodeling and regeneration also includes topics such as collagen structure proportion [127], biomarkers related to host cell proliferation, differentiation and regulation [127,128], characterization parameters and test methods of tissue-remodeling microenvironment [129], and advanced manufacturing technology of biomimetic ECM [126,130].
4.2. Computational modeling
In the R & D and manufacturing of medical devices, device failure models can be established utilizing finite element analysis (FEA) [131,132] to predict the durability, fatigue and other performances after long-term implantation. By identifying the worst-case scenario via computational modeling, the manufacturer could save costs, shorten the R & D cycle, and accelerate market entry of products. As for hernia mesh, research is focused on evaluating the mechanical compatibility between the mesh and abdominal wall [106,133,134]. The mechanical response of the mesh implanted in various anatomical layers of the abdominal wall can also be simulated and analyzed through FEA [106,135,136].
Computational modeling technologies use mathematical methods to simulate the real-world situation. However, the study of biodegradation and tissue-remodeling of hernia mesh involves the complex mechanisms of cell proliferation and metabolism in vivo. Therefore, computational modeling results must be combined with animal study results to provide persuasive evidence.
4.3. Big data platform
Big data platforms bring new tools for regulatory science of medical devices. For instance, there are 129 marketed hernia mesh products in China, which provide massive data on safety and effectiveness evaluation from in vitro bench performance tests, animal study and clinical investigation. Hernia mesh manufacturers, R&D institutes and regulatory agencies may upload their data into different database. Regulatory agencies manage big data and conduct intelligent analysis on different types of research. It is helpful to evaluate the safety and effectiveness of future hernia mesh products.
Establishing the big data platforms is also beneficial to the post-marketing surveillance of hernia mesh. Clinical registration database provides the most concrete evidence for tracking adverse events, re-evaluation of medical devices and revocation of registration certificate. Since 1992, at least nine countries in Europe and the United States have established domestic or international registration data platforms for hernia operations. The data should include the following information: information of patient, surgery, types of hernia mesh used, intra-operative and postoperative complications and follow-up time [20,137]. The real-world data obtained from the above data platforms help researchers evaluate both the long-term safety and effectiveness of hernia mesh after implantation [67]and the efficacy of a certain surgical approach [138].
On December 29, 2017, the Chinese hernia registry and follow-up system was officially launched, initiated by Beijing Chaoyang Hospital of Capital Medical University of China. By the end of August 2019, a total of 354 hospitals in China had joined the follow-up system, with a total of 96,705 patients [139]. This big data platform collects and records the clinical performance and adverse events of hernia surgery involving devices such as hernia mesh and mesh fixing devices. It also provides systematic and traceable real-world data for the regulatory decision-making of hernia mesh after market entry.
4.4. Evidence-based research
Evidence-based research is one of the key developments of regulatory science for medical devices, because it generates powerful and substantial evidence for the safety and effectiveness evaluation of medical devices. This paper advocates “evidence-based regulatory science” and “evidence-based technical review” for medical devices for the first time by applying the evidence-based theory. By definition, “evidence-based regulatory science” is “evaluating the safety, effectiveness, quality and performance of medical devices in the total product life cycle on the basis of the actual needs of regulatory science and in accordance with the theory of evidence-based research and its relevant methods, standards and tools”. “Evidence-based technical review” is the specific practice of technical review in evidence-based regulatory science. It can be defined as “making scientific review decisions on the safety, effectiveness and risk/benefit evaluation of medical devices on the basis of the scientific evidence obtained from evidence-based research, together with the expertise and experience of reviewers and with full consideration over the clinical urgency for the devices by people of the country due to public health contingencies and other reasons".
The application of evidence-based research in the regulatory science for hernia mesh and other medical devices has the following two advantages. First, the registrant can obtain the supporting evidence of the safety and effectiveness to the greatest extent through evidence-based research method, so as to eliminate the unnecessary burden caused by repeated research. Second, evidence-based research methods such as systematic review and meta-analysis enable reviewers to have full access to the accumulated evidence on the safety and effectiveness of medical devices. Such evidence also helps identify the key risks of the devices for future review decisions.
There have been evidence-based research reports on hernia mesh [[140], [141], [142], [143], [144]]. Systematic reviews provide surgeons with reliable evidence-based medical reference to choose the ideal hernia and abdominal wall repair surgical technique [145]. They also present real-world clinical data of permanent implanted medical devices such as hernia mesh [146,147]. The post-market clinical research, long-term clinical follow-up and related systematic reviews supply valuable evidence for the long-term clinical safety and effectiveness of hernia mesh [148].
Evidence-based research can also be used to extract high-quality evidence of safety and effectiveness from preclinical research data such as hernia mesh bench performance tests, biocompatibility evaluation, and preclinical animal study. For instance, the systematic review of preclinical research can help determine whether a certain kind of material is suitable to be used for hernia mesh. H. Liu systematically reviewed previous intraperitoneal hernia mesh animal study literatures on the evaluation of adhesions, and conducted a meta-analysis on adhesions of intraperitoneal hernia mesh coated with hyaluronic acid and carboxymethylcellulose (HA/CMC) in animals. The results showed that the HA/CMC coating effectively reduced adhesions of the hernia mesh at the 4th week [70].
5. Conclusion
Regulatory science for medical devices provides new tools, standards and approaches for evaluating the safety, effectiveness, quality and performance of medical devices in their total product life cycles. It is the multidisciplinary science in the service of medical device regulation.
Based on the evaluation of the safety and effectiveness of hernia mesh, a typical biomaterial-based implantable medical device, this paper categorizes the hernia mesh products based on four criteria, including the repair mechanism, anatomical site, clinical indications and hernia types, and manufacturing materials. Given the great varieties of hernia mesh materials, the complexity of materials and designs poses both challenges and opportunities to the regulators, stimulating development of regulatory science for such products.
The premarket technical review on safety and effectiveness is one of the core research topics in regulatory science for hernia mesh. At present, China NMPA has established an initial frame work of regulatory science for the safety and effectiveness evaluation of hernia mesh, which consists of relevant laws, regulations, standards and technical guidance documents. The framework also embodies safety and effectiveness evaluation evidence covering bench performance tests, biocompatibility evaluation, biosafety assessment, animal study and clinical evaluation. In the future, there will be several promising directions in the research of regulatory science for hernia mesh, including the application of computational modeling technologies, tissue remodeling and regeneration evaluation in animal study, big data platform, real-world data and evidence-based research methods. As a result of regulatory science research, new tools, methods and standards are expected to be applied to the regulation of hernia mesh and other medical devices.
CRediT authorship contribution statement
Wenbo Liu: Conceptualization, Investigation, Writing - original draft. Yajie Xie: Writing - review & editing. Yudong Zheng: Supervision, Writing - review & editing. Wei He: Investigation. Kun Qiao: Investigation. Haoye Meng: Investigation.
Declaration of competing interest
There are no conflicts to declare.
Acknowledgement
The authors want to thank Xingdong Zhang from Sichuan University for contribution to the Regulatory Science for medical device in China, and China Hernia Society. CSS. CMA. for supporting the hernia mesh guidance documents drafting, and all the NMPA employees for their hard work in safety and effectiveness evaluation of hernia mesh. The authors thank the financial support from National Natural Science Foundation of China (51973018), National Natural Science Foundation of China (51773018), and Beijing Municipal Science and Technology Commission Projects (No. Z191100002019017).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Wenbo Liu, Email: bobole@live.cn.
Yajie Xie, Email: ch0602120126@126.com.
Yudong Zheng, Email: zhengyudong@mater.ustb.edu.cn.
Wei He, Email: hewei2016@ustb.edu.cn.
Kun Qiao, Email: jorkun@vip.qq.com.
Haoye Meng, Email: menghaoye@126.com.
References
- 1.Yadwadkar A. Analysis of the thermoplastic copolyester elastomers market influenced by widening scope of end-use applications. Reinforc Plast. 2019;63(6):314–316. doi: 10.1016/j.repl.2019.02.021. [DOI] [Google Scholar]
- 2.China Society for Drug Regulation, Blue Book of Medical Device Industry. Social sciences academic press; China: 2019. [Google Scholar]
- 3.Us Food and Drug Administration Regulatory science priorities (FY2017) 2017. https://www.fda.gov/media/100299/download [accessed 2020 May 6]. Available at:
- 4.Us Food and Drug Administration Advancing regulatory science for public health. 2010. https://www.fda.gov/downloads/ScienceResearch/SpecialTopics/RegulatoryScience/UCM228444.pdf [accessed 2020 May 6]. Available at:
- 5.Us Food and Drug Administration FDA advancing regulatory science—a strategic plan august 2011. 2011. https://www.fda.gov/downloads/ScienceResearch/SpecialTopics/RegulatoryScience/UCM268225.pdf [accessed 2020 May 6]. Available at:
- 6.Us Food and Drug Administration CDRH's regulatory science priorities. 2019. https://www.fda.gov/medical-devices/science-and-research-medical-devices/cdrh-regulatory-science-priorities [accessed 2020 May 6]. Available at:
- 7.China National Medical Product Administration NMPA launched the regulatory science action plan for medical products in China. http://www.nmpa.gov.cn/WS04/CL2056/337150.html [accessed 2020 May 6]. Available at:
- 8.HerniaSurge Group International guidelines for groin hernia management, Hernia. 2018. 22, 1, 1-165. [DOI] [PMC free article] [PubMed]
- 9.China Hernia SocietyCss.Cma Chinese College of Surgeons, Guidelines on the treatment of inguinal hernia in adult patients (The 2018 edition) Chin. J. Pract. Surg. 2018;38:704–706. doi: 10.19538/j.cjps.issn1005-2208.2018.07.02. [DOI] [Google Scholar]
- 10.Chen Jianmin, Jiang Yanan, Wang Dianchen, Chen Yake, Nan Hong, Li Weijie. An epidemiological study of groin hernia in Taikang County of Henan Province. Chin. J. Hernia Abdom. Wall Surg.(Electron. Ed.) 2014;8(5):421–423. doi: 10.3877/cma.j.issn.1674-392X.2014.05.006. [DOI] [Google Scholar]
- 11.Zhou Yisheng, Ding yan, Zhu Chengxin, Chen Ruixiang, Xiao Wenfa, Lan Zhijun, Shen Jianqing, Ding Qingguo, Zhang Qi, Xu Baijun. An epidemiological study of groin hernia in Xiaoshan County of Hangzhou city. Chin. J. Hernia Abdom. Wall Surg.(Electron. Ed.) 2016;10(5):385–386. doi: 10.3877/cma.j.issn.1674-392X.2016.05.021. [DOI] [Google Scholar]
- 12.Il L. 1986. Hernia Repair without Disability. [Google Scholar]
- 13.Bittner R., Bingener-Casey J., Dietz U., Fabian M., Ferzli G.S., Fortelny R.H., Köckerling F., Kukleta J., LeBlanc K., Lomanto D., Misra M.C., Bansal V.K., Morales-Conde S., Ramshaw B., Reinpold W., Rim S., Rohr M., Schrittwieser R., Simon T., Smietanski M., Stechemesser B., Timoney M., Chowbey P. Guidelines for laparoscopic treatment of ventral and incisional abdominal wall hernias (International Endohernia Society (IEHS)—Part 1. Surg. Endosc. 2014;28(1):2–29. doi: 10.1007/s00464-013-3170-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Menzo E.L., Hinojosa M., Carbonell A., Krpata D., Carter J., Rogers A.M. American Society for Metabolic and Bariatric Surgery and American Hernia Society consensus guideline on bariatric surgery and hernia surgery. Surg. Obes. Relat. Dis. 2018;14(9):1221–1232. doi: 10.1016/j.soard.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Coda A., Lamberti R., Martorana S. Classification of prosthetics used in hernia repair based on weight and biomaterial. Hernia. 2012;16(1):9–20. doi: 10.1007/s10029-011-0868-z. [DOI] [PubMed] [Google Scholar]
- 16.Keane T.J., Horejs C.-M., Stevens M.M. Scarring vs. functional healing: matrix-based strategies to regulate tissue repair. Adv. Drug Deliv. Rev. 2018;129:407–419. doi: 10.1016/j.addr.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cramer M.C., Badylak S.F. Extracellular matrix-based biomaterials and their influence upon cell behavior. Ann. Biomed. Eng. 2019 doi: 10.1007/s10439-019-02408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nie X., Xiao D., Wang W., Song Z., Yang Z., Chen Y., Gu Y. Comparison of porcine small intestinal submucosa versus polypropylene in open inguinal hernia repair: a systematic review and meta-analysis. PloS One. 2015;10(8) doi: 10.1371/journal.pone.0135073. e0135073-e0135073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalaba S., Gerhard E., Winder J.S., Pauli E.M., Haluck R.S., Yang J. Design strategies and applications of biomaterials and devices for hernia repair. Bioact Mater. 2016;1(1):2–17. doi: 10.1016/j.bioactmat.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muysoms F., Campanelli G., Champault G.G., DeBeaux A.C., Dietz U.A., Jeekel J., Klinge U., Köckerling F., Mandala V., Montgomery A., Morales Conde S., Puppe F., Simmermacher R.K.J., Śmietański M., Miserez M. EuraHS: the development of an international online platform for registration and outcome measurement of ventral abdominal wall hernia repair. Hernia. 2012;16(3):239–250. doi: 10.1007/s10029-012-0912-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muysoms F.E., Miserez M., Berrevoet F., Campanelli G., Champault G.G., Chelala E., Dietz U.A., Eker H.H., El Nakadi I., Hauters P., Hidalgo Pascual M., Hoeferlin A., Klinge U., Montgomery A., Simmermacher R.K.J., Simons M.P., Smietański M., Sommeling C., Tollens T., Vierendeels T., Kingsnorth A. Classification of primary and incisional abdominal wall hernias. Hernia. 2009;13(4):407–414. doi: 10.1007/s10029-009-0518-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haigh J.N., Dargaville T.R., Dalton P.D. Additive manufacturing with polypropylene microfibers. Mater. Sci. Eng. C. 2017;77:883–887. doi: 10.1016/j.msec.2017.03.286. [DOI] [PubMed] [Google Scholar]
- 23.Deeken C.R., Abdo M.S., Frisella M.M., Matthews B.D. Physicomechanical evaluation of polypropylene, polyester, and polytetrafluoroethylene meshes for inguinal hernia repair. J. Am. Coll. Surg. 2011;212(1):68–79. doi: 10.1016/j.jamcollsurg.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 24.Guillaume O., Pérez-Tanoira R., Fortelny R., Redl H., Moriarty T.F., Richards R.G., Eglin D., Petter Puchner A. Infections associated with mesh repairs of abdominal wall hernias: are antimicrobial biomaterials the longed-for solution? Biomaterials. 2018;167:15–31. doi: 10.1016/j.biomaterials.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 25.Yenli E.M.T., Abanga J., Tabiri S., Kpangkpari S., Tigwii A., Nsor A., Amesiya R., Ekremet K., Abantanga F.A. Our experience with the use of low cost mesh in tension-free inguinal hernioplasty in northern Ghana. Ghana Med. J. 2017;51(2):78–82. [PMC free article] [PubMed] [Google Scholar]
- 26.Fine A. Laparoscopic repair of inguinal hernia with biomimetic matrix. J. Soc. Laparoendosc. Surg. 2012;16(4):564–568. doi: 10.4293/108680812X13462882737050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tension-free repair of abdominal wall hernias using glue in experiment. 2013. http://www.stm-journal.ru/en/numbers/2013/2/980/pdf [accessed 2020 July 11]. Available at:
- 28.Singhal P., Small W., Cosgriff-Hernandez E., Maitland D.J., Wilson T.S. Low density biodegradable shape memory polyurethane foams for embolic biomedical applications. Acta Biomater. 2014;10(1):67–76. doi: 10.1016/j.actbio.2013.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patrick C.W., Jr. Tissue engineering strategies for adipose tissue repair. 2001. 263(4) 361-366. [DOI] [PubMed]
- 30.Tandon A., Shahzad K., Pathak S., Oommen C.M., Nunes Q.M., Smart N. Parietex™ Composite mesh versus DynaMesh(®)-IPOM for laparoscopic incisional and ventral hernia repair: a retrospective cohort study. Ann. R. Coll. Surg. Engl. 2016;98(8):568–573. doi: 10.1308/rcsann.2016.0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gómez-Gil V., Pascual G., Bellón J.M. Biomaterial implants in abdominal wall hernia repair: a review on the importance of the peritoneal. Interface. 2019;7(2):105. doi: 10.3390/pr7020105. [DOI] [Google Scholar]
- 32.Huber A., Boruch A.V., Nieponice A., Jiang H., Medberry C., Badylak S.F. Histopathologic host response to polypropylene-based surgical mesh materials in a rat abdominal wall defect model. 2012. 100B(3) 709-717. [DOI] [PubMed]
- 33.Rastegarpour A., Cheung M., Vardhan M., Ibrahim M.M., Butler C.E., Levinson H. Surgical mesh for ventral incisional hernia repairs: understanding mesh design. Plast Surg (Oakv) 2016;24(1):41–50. doi: 10.4172/plastic-surgery.1000955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee C.H., Kim H., Han I.W., Kim S.M., Kwak B.S., Baik Y.H., Park Y.J., Oh M.G. Effect of polylactic film (Surgi-Wrap) on preventing postoperative ileus after major hepato-pancreato-biliary surgery. Ann Hepatobiliary Pancreat Surg. 2016;20(4):191–196. doi: 10.14701/ahbps.2016.20.4.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang K., Ding X., Lv B., Wei L., Sun J., Xu Z., Qin X., Tang H. Reconstruction of large-size abdominal wall defect using biodegradable poly-p-dioxanone mesh: an experimental canine study. World J. Surg. Oncol. 2014;12(1):57. doi: 10.1186/1477-7819-12-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stoikes N.F.N., Scott J.R., Badhwar A., Deeken C.R., Voeller G.R. Characterization of host response, resorption, and strength properties, and performance in the presence of bacteria for fully absorbable biomaterials for soft tissue repair. Hernia. 2017;21(5):771–782. doi: 10.1007/s10029-017-1638-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruiz-Jasbon F., Norrby J., Ivarsson M.L., Björck S. Inguinal hernia repair using a synthetic long-term resorbable mesh: results from a 3-year prospective safety and performance study. Hernia. 2014;18(5):723–730. doi: 10.1007/s10029-014-1249-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hjort H., Mathisen T., Alves A., Clermont G., Boutrand J.P. Three-year results from a preclinical implantation study of a long-term resorbable surgical mesh with time-dependent mechanical characteristics. Hernia. 2012;16(2):191–197. doi: 10.1007/s10029-011-0885-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clemens M.W., Downey S., Agullo F., Lehfeldt M.R., Kind G.M., Palladino H., Marshall D., Jewell M.L., Mathur A.B., Bengtson B.P. Clinical application of a silk fibroin protein biologic scaffold for abdominal wall fascial reinforcement. Plast. Reconstr. Surg. Glob. Open. 2014;2(11) doi: 10.1097/GOX.0000000000000217. e246-e246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lake S.P., Stoikes N.F.N., Badhwar A., Deeken C.R. Contamination of hybrid hernia meshes compared to bioresorbable Phasix™ Mesh in a rabbit subcutaneous implant inoculation model. Ann Med Surg (Lond) 2019;46:12–16. doi: 10.1016/j.amsu.2019.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Messa C.A.t., Kozak G., Broach R.B., Fischer J.P. When the mesh goes away: an analysis of poly-4-hydroxybutyrate mesh for complex hernia repair. Plast. Reconstr. Surg Glob Open. 2019;7(11) doi: 10.1097/GOX.0000000000002576. e2576-e2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ward K.C., Costello K.P., Baalman S., Pierce R.A., Deeken C.R., Frisella M.M., Michael Brunt L., Matthews B.D. Effect of acellular human dermis buttress on laparoscopic hiatal hernia repair. Surg. Endosc. 2015;29(8):2291–2297. doi: 10.1007/s00464-014-3946-3. [DOI] [PubMed] [Google Scholar]
- 43.Sun L., Chen J., Shen Y. Randomized controlled trial of Lichtenstein repair of indirect inguinal hernias with two biologic meshes from porcine small intestine submucosa. Therapeut. Clin. Risk Manag. 2019;15:1277–1282. doi: 10.2147/TCRM.S208185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Young D.A., McGilvray K.C., Ehrhart N., Gilbert T.W. Comparison of in vivo remodeling of urinary bladder matrix and acellular dermal matrix in an ovine model. 2018. 13(7) 759-773. [DOI] [PubMed]
- 45.Young D.A., Jackson N., Ronaghan C.A., Brathwaite C.E., Gilbert T.W. Retrorectus repair of incisional ventral hernia with urinary bladder matrix reinforcement in a long-term porcine model. 2018. 13(4) 395-408. [DOI] [PubMed]
- 46.Petro C.C., Prabhu A.S., Liu L., Majumder A., Anderson J.M., Rosen M.J. An in vivo analysis of Miromesh—a novel porcine liver prosthetic created by perfusion decellularization. J. Surg. Res. 2016;201(1):29–37. doi: 10.1016/j.jss.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 47.Kamarajah S.K., Chapman S.J., Glasbey J., Morton D., Smart N., Pinkney T., Bhangu A. Systematic review of the stage of innovation of biological mesh for complex or contaminated abdominal wall closure. BJS Open. 2018;2(6):371–380. doi: 10.1002/bjs5.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adelman D.M., Cornwell K.G. Bioprosthetic versus synthetic mesh: analysis of tissue adherence and revascularization in an experimental animal model. Plast. Reconstr. Surg. Glob. Open. 2018;6(5) doi: 10.1097/GOX.0000000000001713. e1713-e1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gurrado A., Franco I.F., Lissidini G., Greco G., De Fazio M., Pasculli A., Girardi A., Piccinni G., Memeo V., Testini M. Impact of pericardium bovine patch (Tutomesh®) on incisional hernia treatment in contaminated or potentially contaminated fields: retrospective comparative study. Hernia. 2015;19(2):259–266. doi: 10.1007/s10029-014-1228-6. [DOI] [PubMed] [Google Scholar]
- 50.Ren Tongli W.W. Utility of different grafting materials in endoscopic myringoplasty. Chin. J. Otol. 2017;15(4):412–415. doi: 10.3969/j.issn.1672-2922.2017.04.005. [DOI] [Google Scholar]
- 51.Giordano P., Pullan R.D., Ystgaard B., Gossetti F., Bradburn M., McKinley A.J., Smart N.J., Daniels I.R. The use of an acellular porcine dermal collagen implant in the repair of complex abdominal wall defects: a European multicentre retrospective study. Tech. Coloproctol. 2015;19(7):411–417. doi: 10.1007/s10151-015-1307-4. [DOI] [PubMed] [Google Scholar]
- 52.Sullivan E.K., Kamstock D.A., Turner A.S., Goldman S.M., Kronengold R.T. Evaluation of a flexible collagen surgical patch for reinforcement of a fascial defect: experimental study in a sheep model. J. Biomed. Mater. Res. B Appl. Biomater. 2008;87(1):88–94. doi: 10.1002/jbm.b.31073. [DOI] [PubMed] [Google Scholar]
- 53.Zouhair S., Dal Sasso E., Tuladhar S.R., Fidalgo C., Vedovelli L., Filippi A., Borile G., Bagno A., Marchesan M., De Rossi G., Gregori D., Wolkers W.F., Romanato F., Korossis S., Gerosa G., Iop L. A comprehensive comparison of bovine and porcine decellularized pericardia. New Insights for Surgical Applications. 2020;10(3):371. doi: 10.3390/biom10030371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Petter-Puchner A.H., Fortelny R.H., Silic K., Brand J., Gruber-Blum S., Redl H. Biologic hernia implants in experimental intraperitoneal onlay mesh plasty repair: the impact of proprietary collagen processing methods and fibrin sealant application on tissue integration. Surg. Endosc. 2011;25(10):3245–3252. doi: 10.1007/s00464-011-1700-7. [DOI] [PubMed] [Google Scholar]
- 55.Ludwicka K., Kolodziejczyk M., Gendaszewska-Darmach E., Chrzanowski M., Jedrzejczak-Krzepkowska M., Rytczak P., Bielecki S. Stable composite of bacterial nanocellulose and perforated polypropylene mesh for biomedical applications. 2019. 107(4) 978-987. [DOI] [PubMed]
- 56.García-Moreno F., Pérez-López P., Sotomayor S., Pérez-Köhler B., Bayon Y., Pascual G., Bellón J.M. Comparing the host tissue response and peritoneal behavior of composite meshes used for ventral hernia repair. J. Surg. Res. 2015;193(1):470–482. doi: 10.1016/j.jss.2014.07.049. [DOI] [PubMed] [Google Scholar]
- 57.Sutton N., MacDonald M.H., Lombard J., Ilie B., Hinoul P., Granger D.A. Preclinical evaluation of the effect of the combined use of the ethicon Securestrap(®) open absorbable strap fixation device and ethicon Physiomesh™ open flexible composite mesh device on surgeon stress during ventral hernia repair. Med. Devices (Auckl) 2017;11:1–9. doi: 10.2147/MDER.S146761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sharma A., Faubion W.A., Dietz A.B. Regenerative materials for surgical reconstruction: current spectrum of materials and a proposed method for classification. Mayo Clin. Proc. 2019;94(10):2099–2116. doi: 10.1016/j.mayocp.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 59.FitzGerald J.F., Kumar A.S. Biologic versus synthetic mesh reinforcement: what are the pros and cons? Clin. Colon Rectal Surg. 2014;27(4):140–148. doi: 10.1055/s-0034-1394155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sawyer M.A.J. New ovine polymer-reinforced bioscaffold in hiatal hernia repair. J. Soc. Laparoendosc. Surg. 2018;22(4) doi: 10.4293/JSLS.2018.00057. e2018.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ferzoco S.J. Early experience outcome of a reinforced Bioscaffold in inguinal hernia repair: a case series. Int. J. Surg. Open. 2018;12:9–11. doi: 10.1016/j.ijso.2018.06.001. [DOI] [Google Scholar]
- 62.Li S., Xiao H., Yang L., Hua L., Qiu Z., Hu X., Ping D., Zheng K., He H., Tang J. Electrospun P(LLA-CL) nanoscale fibrinogen patch vs porcine small intestine submucosa graft repair of inguinal hernia in adults: a randomized, single-blind, controlled, multicenter, noninferiority trial. J. Am. Coll. Surg. 2019;229(6):541–551. doi: 10.1016/j.jamcollsurg.2019.08.1446. e1. [DOI] [PubMed] [Google Scholar]
- 63.Scott J.R., Deeken C.R., Martindale R.G., Rosen M.J. Evaluation of a fully absorbable poly-4-hydroxybutyrate/absorbable barrier composite mesh in a porcine model of ventral hernia repair. Surg. Endosc. 2016;30(9):3691–3701. doi: 10.1007/s00464-016-5057-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gruber-Blum S., Petter-Puchner A.H., Brand J., Fortelny R.H., Walder N., Oehlinger W., Koenig F., Redl H. Comparison of three separate antiadhesive barriers for intraperitoneal onlay mesh hernia repair in an experimental model. 2011. 98(3) 442-449. [DOI] [PubMed]
- 65.Costa A., Adamo S., Gossetti F., D'Amore L., Ceci F., Negro P., Bruzzone P. Biological scaffolds for abdominal wall repair: future in clinical application? Materials. 2019;12(15):2375. doi: 10.3390/ma12152375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Panaro F., Matos-Azevedo A.M., Fatas J.A., Marin J., Navarro F., Zaragoza-Fernandez C. Endoscopic and histological evaluations of a newly designed inguinal hernia mesh implant: experimental studies on porcine animal model and human cadaver. Ann. Med. Surg. 2015;4(2):172–178. doi: 10.1016/j.amsu.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Köckerling F., Simon T., Hukauf M., Hellinger A., Fortelny R., Reinpold W., Bittner R. The importance of registries in the postmarketing surveillance of surgical meshes. Ann. Surg. 2018;268(6):1097–1104. doi: 10.1097/SLA.0000000000002326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.ClinicalTrailgov Post market study of Parietene™ DS composite mesh in ventral hernia repair (PPDS) https://clinicaltrials.gov/ct2/show/NCT03495154 [accessed 2020 May 17]. Available at:
- 69.Aydemir Sezer U., Sanko V., Gulmez M., Aru B., Sayman E., Aktekin A., Vardar Aker F., Yanıkkaya Demirel G., Sezer S. Polypropylene composite hernia mesh with anti-adhesion layer composed of polycaprolactone and oxidized regenerated cellulose. Mater. Sci. Eng. C. 2019;99:1141–1152. doi: 10.1016/j.msec.2019.02.064. [DOI] [PubMed] [Google Scholar]
- 70.Liu H., van Steensel S., Gielen M., Vercoulen T., Melenhorst J., Winkens B., Bouvy N.D. Comparison of coated meshes for intraperitoneal placement in animal studies: a systematic review and meta-analysis. Hernia. 2019 doi: 10.1007/s10029-019-02071-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lacour M., Ridereau Zins C., Casa C., Venara A., Cartier V., Yahya S., Barbieux J., Aubé C. CT findings of complications after abdominal wall repair with prosthetic mesh. Diagn. Intervent. Imag. 2017;98(7):517–528. doi: 10.1016/j.diii.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 72.Percalli L., Pricolo R., Passalia L., Riccò M. Comparison between self-gripping, semi re-absorbable meshes with polyethylene meshes in Lichtenstein, tension-free hernia repair: preliminary results from a single center. Acta Biomed. Atenei Parmensis. 2018;89(1):72–78. doi: 10.23750/abm.v89i1.6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zimkowski M.M., Rentschler M.E., Schoen J., Rech B.A., Mandava N., Shandas R. Integrating a novel shape memory polymer into surgical meshes decreases placement time in laparoscopic surgery: an in vitro and acute in vivo study. J. Biomed. Mater. Res. 2013;101(9):2613–2620. doi: 10.1002/jbm.a.34556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.D'Hondt M., Nuytens F., Yoshihara E., Adriaens E., Vansteenkiste F., Pottel H. Totally extraperitoneal laparoscopic inguinal hernia repair using a self-expanding nitinol framed hernia repair device: a prospective case series. Int. J. Surg. 2017;40:139–144. doi: 10.1016/j.ijsu.2017.02.091. [DOI] [PubMed] [Google Scholar]
- 75.CFDA Special procedure of review and approval for innovative medical devices (draft, CFDA 2014 [SYJXG] No. 13) 2014. https://www.nmpa.gov.cn/xxgk/fgwj/gzwj/gzwjylqx/20140207154501788.html [accessed 2020 Aug 7]. Available at:
- 76.Deepthi S., Jayakumar R. Alginate nanobeads interspersed fibrin network as in situ forming hydrogel for soft tissue engineering. Bioact. Mater. 2018;3(2):194–200. doi: 10.1016/j.bioactmat.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.NMPA Composite hernia mesh approved for market. 2018. http://www.nmpa.gov.cn/WS04/CL2056/329889.html [accessed 2020 May 6]. Available at:
- 78.CMDE Public summons of innovative medical devices conform to special procedure for review and approval (No.8, 2018) 2018. https://www.cmde.org.cn/CL0050/7930.html [accessed 2020 May 6]. Available at:
- 79.State Council of the People’s Republic of China Opinions on the reform of the review and approval system for Drugs and medical devices (state Council No. 44) 2015. http://www.gov.cn/zhengce/content/2015-08/18/content_10101.htm [accessed 2020 May 6]. Available at:
- 80.Liu W., Shi X., Lu Z., Wang L., Zhang K., Zhang X. Review and approval of medical devices in China: changes and reform. J. Biomed. Mater. Res. Part B. 2018;106(6):2093–2100. doi: 10.1002/jbm.b.34031. [DOI] [PubMed] [Google Scholar]
- 81.Zheng Jia, Yu Xinhua. Analysis of the basic attributes and positioning of medical device standards in the new era. China Food Durg Admin. Mag. 2019;10(189):28–33. doi: 10.3969/j.issn.1673-5390.2019.10.03. [DOI] [Google Scholar]
- 82.CFDA Guidance for the shelf life registration Application Materials of non-active implanted medical devices (CFDA No. 75) 2017. http://www.nmpa.gov.cn/WS04/CL2138/300350.html [accessed 2020 May 6]. Available at:
- 83.CFDA Guidance for the registration of Medical devices utilizing animal tissues and their derivatives (revised in 2017) (CFDA No. 224) 2017. https://www.cmde.org.cn/CL0056/6883.html [accessed 2020 May 6]. Available at:
- 84.SFDA Guidance for Technical Review of virus inactivation process validation of Allograft medical devices([SYJBXH] No. 116) 2011. https://www.cmde.org.cn/CL0055/1388.html [accessed 2020 May 6]. Available at:
- 85.NMPA Guidance for technical review of animal study of medical devices Part 1: decision-making principles (CFDA No. 13) 2018. http://www.nmpa.gov.cn/WS04/CL2138/336317.html [accessed 2020 May 6]. Available at:
- 86.CFDA Technical guidance for medical device clinical evaluation (CFDA No. 14) 2015. https://www.cmde.org.cn/CL0058/3631.html [accessed 2020 May 6]. Available at:
- 87.CFDA Guidance for principles of clinical investigation design (CFDA No. 6) 2018. http://www.nmpa.gov.cn/WS04/CL2138/300479.html [accessed 2020 May 6]. Available at:
- 88.CFDA Technical guidance for acceptance of overseas clinical trial data of medical devices (CFDA No. 13) 2018. http://www.nmpa.gov.cn/WS04/CL2093/229311.html [accessed 2020 May 6]. Available at:
- 89.SFDA Guidance for the registration of non-active implanted medical devices([SYJXH] No. 519) 2009. http://www.nmpa.gov.cn/WS04/CL2197/324634.html [accessed 2020 May 6]. Available at:
- 90.NMPA Essential principles of safety and performance of medical devices (NMPA No. 18) 2020. http://www.nmpa.gov.cn/WS04/CL2138/375521.html [accessed 2020 May 6]. Available at:
- 91.US Food and Drug Administration Guidance for the preparation of a premarket notification application for a surgical mesh. 1999. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-preparation-premarket-notification-application-surgical-mesh-guidance-industry-andor-fda [accessed 2020 May 6]. Available at:
- 92.CFDA Guidance of technical review for product registration of hernia mesh (CFDA No. 7) 2013. http://www.nmpa.gov.cn/WS04/CL2138/299954.html [accessed 2020 May 6]. Available at:
- 93.NMPA Guidance of technical review for animal experiment of intraperitoneal hernia mesh (NMPA No. 18) 2019. http://www.nmpa.gov.cn/WS04/CL2138/336317.html [accessed 2020 May 6]. Available at:
- 94.NMPA Guidance for clinical trail of hernia mesh (draft) 2019. https://www.cmde.org.cn/CL0063/19564.html [accessed 2020 May 6]. Available at:
- 95.IMDRF Essential principles of safety and performance of medical devices and IVD medical devices. 2018. http://www.imdrf.org/docs/imdrf/final/technical/imdrf-tech-181031-grrp-essential-principles-n47.pdf [accessed 2020 July 12]. Available at: [DOI] [PubMed]
- 96.Axman E., Holmberg H., Nordin P., Nilsson H. Chronic pain and risk for reoperation for recurrence after inguinal hernia repair using self-gripping mesh. Surgery. 2020;167(3):609–613. doi: 10.1016/j.surg.2019.11.011. [DOI] [PubMed] [Google Scholar]
- 97.Deeken C.R., Lake S.P. Mechanical properties of the abdominal wall and biomaterials utilized for hernia repair. Journal of the Mechanical Behavior of Biomedical Materials. 2017;74:411–427. doi: 10.1016/j.jmbbm.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 98.Todros S., Pavan P.G., Pachera P., Natali A.N. Synthetic surgical meshes used in abdominal wall surgery: Part II—biomechanical aspects. 2017. 105(4) 892-903. [DOI] [PubMed]
- 99.Lake S.P., Ray S., Zihni A.M., Thompson D.M., Gluckstein J., Deeken C.R. Pore size and pore shape – but not mesh density – alter the mechanical strength of tissue ingrowth and host tissue response to synthetic mesh materials in a porcine model of ventral hernia repair. J. Mech. Behav. Biomed. Mater. 2015;42:186–197. doi: 10.1016/j.jmbbm.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 100.Deeken C.R., Abdo M.S., Frisella M.M., Matthews B.D. Physicomechanical evaluation of absorbable and nonabsorbable barrier composite meshes for laparoscopic ventral hernia repair. Surg. Endosc. 2011;25(5):1541–1552. doi: 10.1007/s00464-010-1432-0. [DOI] [PubMed] [Google Scholar]
- 101.Earle D.B., Mark L.A. Prosthetic material in inguinal hernia repair: how do I choose? Surg. Clin. 2008;88(1):179–201. doi: 10.1016/j.suc.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 102.Klosterhalfen B., Junge K., Klinge U. The lightweight and large porous mesh concept for hernia repair. Expet Rev. Med. Dev. 2005;2(1):103–117. doi: 10.1586/17434440.2.1.103. [DOI] [PubMed] [Google Scholar]
- 103.Pui C.L., Tang M.E., Annor A.H., Ebersole G.C., Frisella M.M., Matthews B.D., Deeken C.R. Effect of repetitive loading on the mechanical properties of biological scaffold materials. J. Am. Coll. Surg. 2012;215(2):216–228. doi: 10.1016/j.jamcollsurg.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 104.Deeken C.R., Eliason B.J., Pichert M.D., Grant S.A., Frisella M.M., Matthews B.D. Differentiation of biologic scaffold materials through physicomechanical, thermal, and enzymatic degradation techniques. Ann. Surg. 2012;255(3):595–604. doi: 10.1097/SLA.0b013e3182445341. [DOI] [PubMed] [Google Scholar]
- 105.Ibrahim M.M., Poveromo L.P., Glisson R.R., Cornejo A., Farjat A.E., Gall K., Levinson H. Modifying hernia mesh design to improve device mechanical performance and promote tension-free repair. J. Biomech. 2018;71:43–51. doi: 10.1016/j.jbiomech.2018.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pavan P.G., Pachera P., Todros S., Tiengo C., Natali A.N. Mechanical characerization of animal derived grafts for surgical implantation. 2016. 16(03) 1650023. [DOI]
- 107.Junge K., Klinge U., Prescher A., Giboni P., Niewiera M., Schumpelick V. Elasticity of the anterior abdominal wall and impact for reparation of incisional hernias using mesh implants. Hernia. 2001;5(3):113–118. doi: 10.1007/s100290100019. [DOI] [PubMed] [Google Scholar]
- 108.ARNOLD G.A., MATHEWS K.G., ROE S., Mente P., Seaboch T. Biomechanical comparison of four soft tissue replacement materials: an in vitro evaluation of single and multilaminate porcine small intestinal submucosa. Canine Fascia Lata Polypropylene Mesh. 2009;38(7):834–844. doi: 10.1111/j.1532-950X.2009.00577.x. [DOI] [PubMed] [Google Scholar]
- 109.Pott P.P., Schwarz M.L.R., Gundling R., Nowak K., Hohenberger P., Roessner E.D. Mechanical properties of mesh materials used for hernia repair and soft tissue augmentation. PloS One. 2012;7(10) doi: 10.1371/journal.pone.0046978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Piasecka-Zelga J., Zelga P., Szulc J., Wietecha J., Ciechańska D. An in vivo biocompatibility study of surgical meshes made from bacterial cellulose modified with chitosan. Int. J. Biol. Macromol. 2018;116:1119–1127. doi: 10.1016/j.ijbiomac.2018.05.123. [DOI] [PubMed] [Google Scholar]
- 111.Majumder A., Scott J.R., Novitsky Y.W. Evaluation of the antimicrobial efficacy of a novel Rifampin/minocycline-coated, noncrosslinked porcine acellular dermal matrix compared with uncoated scaffolds for soft tissue repair. Surg. Innovat. 2016;23(5):442–455. doi: 10.1177/1553350616656280. [DOI] [PubMed] [Google Scholar]
- 112.Robinson T.N., Clarke J.H., Schoen J., Walsh M.D. Major mesh-related complications following hernia repair. Surg. Endosc. Other Intervent. Tech. 2005;19(12):1556–1560. doi: 10.1007/s00464-005-0120-y. [DOI] [PubMed] [Google Scholar]
- 113.Harth K.C., Rosen M.J. Major complications associated with xenograft biologic mesh implantation in abdominal wall reconstruction. Surg. Innovat. 2009;16(4):324–329. doi: 10.1177/1553350609353609. [DOI] [PubMed] [Google Scholar]
- 114.Liu P., Fu K., Zeng X., Chen N., Wen X. Fabrication and characterization of composite meshes loaded with antimicrobial peptides. ACS Appl. Mater. Interfaces. 2019;11(27):24609–24617. doi: 10.1021/acsami.9b07246. [DOI] [PubMed] [Google Scholar]
- 115.Vogels R.R.M., Kaufmann R., van den Hil L.C.L., van Steensel S., Schreinemacher M.H.F., Lange J.F., Bouvy N.D. Critical overview of all available animal models for abdominal wall hernia research. Hernia. 2017;21(5):667–675. doi: 10.1007/s10029-017-1605-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.CFDA Provisions for medical device registration (CFDA order No. 4) 2017. http://www.nmpa.gov.cn/WS04/CL2197/324829.html [accessed 2020 May 6]. Available from:
- 117.CFDA Catalogue of class III medical devices exempted from clinical trial (CFDA No. 13) 2014. http://www.nmpa.gov.cn/WS04/CL2138/299982.html [accessed 2020 May 6]. Available at:
- 118.CFDA Catalogue of class III medical devices exempted from clinical trial (CFDA No. 133) 2016. http://www.nmpa.gov.cn/WS04/CL2138/300213.html [accessed 2020 May 6]. Available.
- 119.CFDA Catalogue of class III medical devices exempted from clinical trial (CFDA No. 170) 2017. http://www.nmpa.gov.cn/WS04/CL2138/300422.html [accessed 2020 May 6]. Available.
- 120.NMPA Catalogue of class III medical devices exempted from clinical trial (NMPA No. 94) 2018. http://www.nmpa.gov.cn/WS04/CL2093/331201.html [accessed 2020 May 6]. Available at:
- 121.NMPA Catalogue of class III medical devices exempted from clinical trial (NMPA No. 91) 2019. http://www.nmpa.gov.cn/WS04/CL2093/372288.html [accessed 2020 May 6]. Available at:
- 122.Brown B.N., Londono R., Tottey S., Zhang L., Kukla K.A., Wolf M.T., Daly K.A., Reing J.E., Badylak S.F. Macrophage phenotype as a predictor of constructive remodeling following the implantation of biologically derived surgical mesh materials. Acta Biomater. 2012;8(3):978–987. doi: 10.1016/j.actbio.2011.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cavallo J.A., Greco S.C., Liu J., Frisella M.M., Deeken C.R., Matthews B.D. Remodeling characteristics and biomechanical properties of a crosslinked versus a non-crosslinked porcine dermis scaffolds in a porcine model of ventral hernia repair. Hernia. 2015;19(2):207–218. doi: 10.1007/s10029-013-1070-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Badylak S., Kokini K., Tullius B., Whitson B. Strength over time of a resorbable bioscaffold for body wall repair in a dog model. J. Surg. Res. 2001;99(2):282–287. doi: 10.1006/jsre.2001.6176. [DOI] [PubMed] [Google Scholar]
- 125.Wynn T.A., Vannella K.M. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016;44(3):450–462. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Guo R., Merkel A.R., Sterling J.A., Davidson J.M., Guelcher S.A. Substrate modulus of 3D-printed scaffolds regulates the regenerative response in subcutaneous implants through the macrophage phenotype and Wnt signaling. Biomaterials. 2015;73:85–95. doi: 10.1016/j.biomaterials.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lundy J.B. A primer on wound healing in colorectal surgery in the age of bioprosthetic materials. Clin. Colon Rectal Surg. 2014;27(4):125–133. doi: 10.1055/s-0034-1394086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Baker E.A., Leaper D.J. The plasminogen activator and matrix metalloproteinase systems in colorectal cancer: relationship to tumour pathology. Eur. J. Canc. 2003;39(7):981–988. doi: 10.1016/S0959-8049(03)00065-0. [DOI] [PubMed] [Google Scholar]
- 129.Sadtler K., Elisseeff J.H. Analyzing the scaffold immune microenvironment using flow cytometry: practices, methods and considerations for immune analysis of biomaterials. Biomater. Sci. 2019;7(11):4472–4481. doi: 10.1039/c9bm00349e. [DOI] [PubMed] [Google Scholar]
- 130.Stratton S., Shelke N.B., Hoshino K., Rudraiah S., Kumbar S.G. Bioactive polymeric scaffolds for tissue engineering. Bioact Mater. 2016;1(2):93–108. doi: 10.1016/j.bioactmat.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tomaszewska A., Lubowiecka I., Szymczak C. Mechanics of mesh implanted into abdominal wall under repetitive load. Exp. Numer. Study. 2019;107(5):1400–1409. doi: 10.1002/jbm.b.34232. [DOI] [PubMed] [Google Scholar]
- 132.Wu H., Zhao C., Ni J., Zhang S., Liu J., Yan J., Chen Y., Zhang X. Research of a novel biodegradable surgical staple made of high purity magnesium. Bioact. Mater. 2016;1(2):122–126. doi: 10.1016/j.bioactmat.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pachera P., Pavan P.G., Todros S., Cavinato C., Fontanella C.G., Natali A.N. A numerical investigation of the healthy abdominal wall structures. J. Biomech. 2016;49(9):1818–1823. doi: 10.1016/j.jbiomech.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 134.Hernández-Gascón B., Mena A., Peña E., Pascual G., Bellón J.M., Calvo B. Understanding the passive mechanical behavior of the human abdominal wall. Ann. Biomed. Eng. 2013;41(2):433–444. doi: 10.1007/s10439-012-0672-7. [DOI] [PubMed] [Google Scholar]
- 135.Hernandez-Gascon B., Pena E., Melero H., Pascual G., Doblare M., Ginebra M.P., Bellon J.M., Calvo B. Mechanical behaviour of synthetic surgical meshes: finite element simulation of the herniated abdominal wall. Acta Biomater. 2011;7(11):3905–3913. doi: 10.1016/j.actbio.2011.06.033. [DOI] [PubMed] [Google Scholar]
- 136.Hernández-Gascón B., Espés N., Peña E., Pascual G., Bellón J.M., Calvo B. Computational framework to model and design surgical meshes for hernia repair. Comput. Methods Biomech. Biomed. Eng. 2014;17(10):1071–1085. doi: 10.1080/10255842.2012.736967. [DOI] [PubMed] [Google Scholar]
- 137.Kyle-Leinhase I., Köckerling F., Jørgensen L.N., Montgomery A., Gillion J.F., Rodriguez J.A.P., Hope W., Muysoms F. Comparison of hernia registries: the CORE project. Hernia. 2018;22(4):561–575. doi: 10.1007/s10029-017-1724-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Köckerling F., Simon T., Adolf D., Köckerling D., Mayer F., Reinpold W., Weyhe D., Bittner R. Laparoscopic IPOM versus open sublay technique for elective incisional hernia repair: a registry-based, propensity score-matched comparison of 9907 patients. Surg. Endosc. 2019;33(10):3361–3369. doi: 10.1007/s00464-018-06629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Qin Changfu, Chen Jie, Shen Yingmo, Cheng Lili, Liu Jing. The promoting effect of Chinese hernia registration and follow-up system in hernia and abdominal wall surgery. J. Clin. Surg. 2019;27:734–736. doi: 10.3969/j.issn.1005-6483.2019.09.002. [DOI] [Google Scholar]
- 140.Andresen K., Rosenberg J. Open preperitoneal groin hernia repair with mesh: a qualitative systematic review. Am. J. Surg. 2017;213(6):1153–1159. doi: 10.1016/j.amjsurg.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 141.Barnes T.G., McWhinnie D.L. Laparoscopic spigelian hernia repair: a systematic review, surgical laparoscopy, endoscopy & percutaneous techniques. 2016. 26(4) 265-270. [DOI] [PubMed]
- 142.Cross A.J., Buchwald P.L., Frizelle F.A., Eglinton T.W. Meta-analysis of prophylactic mesh to prevent parastomal hernia. Br. J. Surg. 2017;104(3):179–186. doi: 10.1002/bjs.10402. [DOI] [PubMed] [Google Scholar]
- 143.Shubinets V., Carney M.J., Colen D.L., Mirzabeigi M.N., Weissler J.M., Lanni M.A., Braslow B.M., Fischer J.P., Kovach S.J. Management of infected mesh after abdominal hernia repair: systematic review and single-institution experience. Ann. Plast. Surg. 2018;80(2):145–153. doi: 10.1097/sap.0000000000001189. [DOI] [PubMed] [Google Scholar]
- 144.Zhang C., Liu D., Li F., Watson D.I., Gao X., Koetje J.H., Luo T., Yan C., Du X., Wang Z. Systematic review and meta-analysis of laparoscopic mesh versus suture repair of hiatus hernia: objective and subjective outcomes. Surg. Endosc. 2017;31(12):4913–4922. doi: 10.1007/s00464-017-5586-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lockhart K., Dunn D., Teo S., Ng J.Y., Dhillon M., Teo E., van Driel M.L. Mesh versus non-mesh for inguinal and femoral hernia repair. Cochrane Database Syst. Rev. 2018;9(9) doi: 10.1002/14651858.CD011517.pub2. CD011517-CD011517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhong C., Wu B., Yang Z., Deng X., Kang J., Guo B., Fan Y. A meta-analysis comparing lightweight meshes with heavyweight meshes in Lichtenstein inguinal hernia repair. Surg. Innovat. 2013;20(1):24–31. doi: 10.1177/1553350612463444. [DOI] [PubMed] [Google Scholar]
- 147.Antoniou S.A., Pointner R., Granderath F.-A., Köckerling F. The use of biological meshes in diaphragmatic defects - an evidence-based review of the literature. Front. Surg. 2015;2 doi: 10.3389/fsurg.2015.00056. 56-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kokotovic D., Bisgaard T., Helgstrand F. Long-term recurrence and complications associated with elective incisional hernia repair. JAMA. 2016;316(15):1575–1582. doi: 10.1001/jama.2016.15217. [DOI] [PubMed] [Google Scholar]