Abstract
Over the last 60 years, many reports have investigated carotid endarterectomy (CEA) and techniques have thus changed and improved. In this paper, we review the recent literature regarding operational maneuvers for CEA and discuss future problems for CEA. Longitudinal skin incision is common, but the transverse incision has been reported to offer minimal invasiveness and better cosmetic effects for CEA. Most surgeons currently use microscopy for dissection of the artery and plaque. Although no monitoring technique during CEA has been proven superior, multiple monitors offer better sensitivity for predicting postoperative neurological deficit. To date, data are lacking regarding whether routine shunt or selective shunt is better. Individual surgeons thus need to select the method with which they are more comfortable. Many surgical techniques have been reported to obtain distal control of the internal carotid artery in patients with high cervical carotid bifurcation or high plaque, and minimally invasive techniques should be considered. Multiple studies have shown that patch angioplasty reduces the risks of stroke and restenosis compared with primary closure, but few surgeons in Japan have been performing patch angioplasty. Most surgeons thus experience only a small volume of CEAs in Japan, so training programs and development of in vivo training models are important.
Keywords: carotid endarterectomy surgical technique, shunt, high plaque, monitoring
Introduction
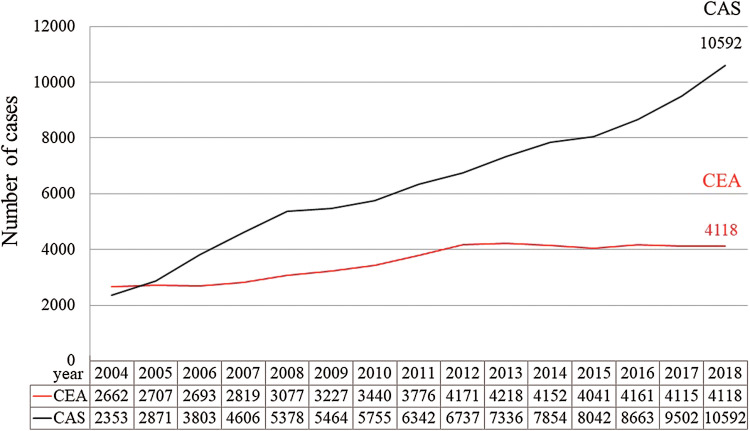
Carotid endarterectomy (CEA) was first reported by Eascott et al. in 1954.1) Over the last 60 years, many reports about CEA have been published and techniques have changed and improved.2–4) Recently, despite the increasing use of carotid artery stenting (CAS), CEA remains an effective treatment for carotid artery stenosis both around the world and in Japan (Fig. 1).5,6) Particularly in patients with symptomatic carotid artery stenosis, CEA has been shown to be superior to CAS in several randomized studies and a meta-analysis.7–9)
Fig. 1.
Annual changes in CEA and CAS. These data are prepared with permission from The Japan Neurosurgical Society. CAS: carotid artery stenting, CEA: carotid endarterectomy.
Some controversies remain regarding surgical strategies for CEA. In this paper, we review the recent literature about the operational maneuvers and techniques for CEA and discuss future problems.
Positioning for CEA
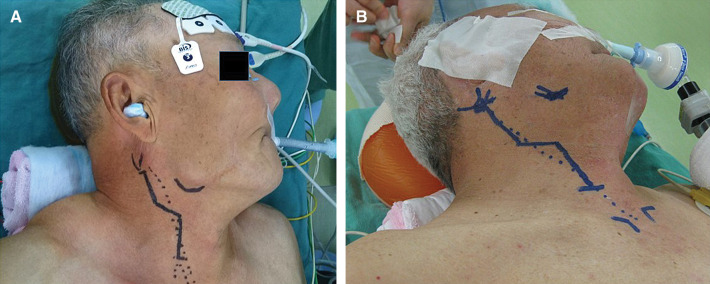
The most important point of discussion regarding positioning for CEA is how the operators extend the neck. All patients are placed in the supine position, then the head is placed on a firm holder and the neck is extended and turned away from the side of operation. Surgeons use various methods to extend the neck of the patient,4) and place a pillow under the neck and shoulder. One advantage of head rotation ≥45° is the possibility of dissecting the internal carotid artery (ICA) more distally or accessing a twisted carotid bifurcation.10,11) For expansion of the distal ICA, Takigawa et al. used a radiolucent head frame to extend the patient’s neck.12) In any case, the surgeon takes the head and neck position to expose the distal ICA for safe CEA (Fig. 2).
Fig. 2.
Neck extension and head rotation. (A) Routine neck extension and head rotation (about 45°) to the opposite side. (B) Nasotracheal intubation and extensive rotation (≥45°) and extension for exposure of the distal internal carotid artery.
Skin Incision
The skin incision is decided based on two considerations: good exposure of the carotid artery and cosmetic effects. Traditionally, a longitudinal incision has been made along the anterior margin of the sternocleidomastoid muscle (SCM) (Fig. 3A). The transverse incision, however, has been reported as a minimally invasive and cosmetic effect for CEA (Fig. 3B).11,13) Kazimierczak et al.14) reported a randomized study of camouflage (transverse) incision versus longitudinal incision. Although no significant difference in mortality or morbidity was seen between groups, scar assessment scale score was significantly better in patients with camouflage incision than in patients with longitudinal incision.14) However, if stenosis of the ICA extended to a high position, the transverse incision was limited to exposing the distal side of the ICA, and most surgeons use an extended longitudinal skin incision to improve distal ICA exposure (Fig. 3C). When we expose the upper edge of the platysma and SCM, attention must be paid to the great auricular nerve. This nerve exits from the lateral border of the SCM, crosses over the surface of the SCM and courses to the parotid gland and inferior part of the auricle. We should preserve this nerve as much as possible.15)
Fig. 3.
Skin incision for CEA. (A) Longitudinal incision along the anterior margin of SCM. (B) Transverse incision across the anterior margin of SCM. C: Combined incision, CEA: carotid endarterectomy, SCM: sternocleidomastoid muscle (#).
Microsurgical Dissection of the Carotid Artery Using Mini-Hooks
In the past, many surgeons have opened the surgical filed using various retractors. Mini-hooks with rubber bands are used for lifting and developing the surgical field.11,16) The carotid artery is moved more superficially. Moreover, this system helps prevents nerve palsy and crush damage to surrounding tissues (Fig. 4).
Fig. 4.
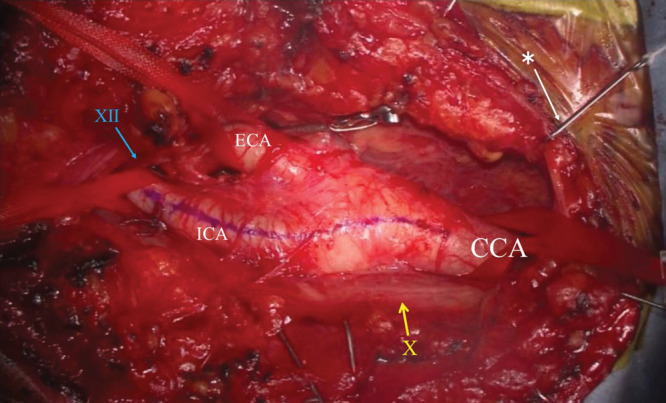
Photograph of the operative field. Using mini-hooks with rubber bands, the carotid artery is lifted to the surface. The surgical field is also widely developed using mini-hooks. CCA: common carotid artery, ICA: internal carotid artery, ECA: external carotid artery, yellow bar: cranial nerve X (vagus nerve), blue bar: cranial nerve XII (hypoglossal nerve), White bar: * mini- hooks with rubber bands.
Before the 1990s, most surgeons performed CEA without using a microscope. Since Spetzler et al.17) and Findlay18) reported on CEA using microsurgical techniques, most surgeons have adopted the use of a microscope for dissection of the artery and plaque. More recently, the high-definition exoscope has been reported as a novel instrumentation.19) While few reports have described the use of such devices in CEA, their use seems likely to increase.
Block of the Carotid Body
Post-CEA hemodynamic instability is associated with postoperative morbidity and mortality.20,21) Most surgeons perform local anesthetic blockade of the carotid sinus at dissection of the carotid artery to protect against hypotension during CEA.22) However, this effect remains controversial. Many studies have demonstrated that lidocaine blockade of the carotid sinus did not influence hypotension or bradycardia during CEA under general or local anesthesia.23,24) Tang et al.25) reported that no evidence currently supports routine use of local anesthetic to block the carotid sinus to reduce postoperative fluctuations in blood pressure. We may therefore use anesthetic blockade of the carotid sinus if we observe bradycardia or decreased blood pressure during dissection of or compression on the ICA.
Monitoring Technique in CEA
Several studies have discussed the best monitoring systems for use during CEA.26–30) Some of those studies considered the need for shunt,29) but some used monitoring to predict neurological deficit.28,30) To date, no monitoring technique during CEA has proven clearly superior to any other.
For many years, electroencephalograms (EEGs) and stump pressure have been used to monitor cerebral function and collateral cerebral flow during CEA.31) Stump pressure <40 mmHg has been used as the threshold for defining cerebral ischemia.32) Findlay et al.31) demonstrated CEA stump pressure <40 mmHg in 21% of 300 cases.
Recently, somatosensory-evoked potential (SSEP) has seen use as a standard method for monitoring CEA. Nwachuku et al.27) demonstrated that patients with perioperative neurological deficit were 14 times more likely to have shown changes in SSEP during CEA. Thiagarajan et al.26) reported that a change in either EEG or SSEP was 17 times more likely in patients with perioperative stroke, and dual modality monitoring was found to be more sensitive for predicting perioperative neurological deficit.
Recently, transcranial Doppler (TCD),33,34) near-infrared spectroscopy (NIRS),35,36) and transcranial motor-evoked potential (MEP)37,38) have also been used for monitoring. Patients with perioperative stroke were four times more likely to have shown TCD changes during CEA compared to patients without stroke.34) Using TCD and NIRS during and after CEA, hyperperfusion can be predicted.33,35) Although cerebral ischemia of the middle cerebral artery area is detected by SSEP and/or MEP, that of the anterior cerebral artery (ACA) area remains undetected by these modalities. Therefore, a combination of SSEP, MEP, and NIRS to allow detection of cerebral ischemia in the ACA territory is very useful (Fig. 5).
Fig. 5.
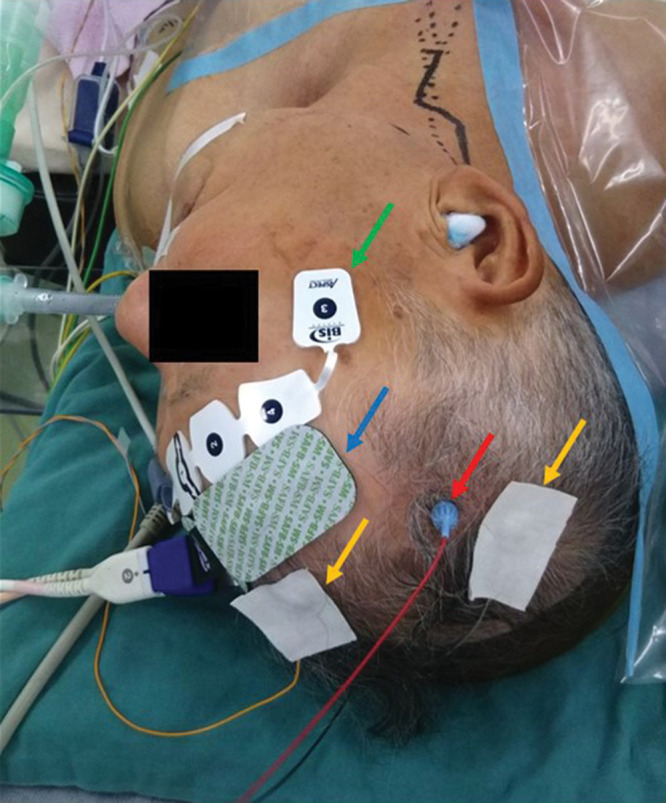
Intraoperative monitoring for CEA. MEP, somatosensory evoked potential (SEP) and NIRS as intraoperative monitoring for CEA. BIS is used to monitor anesthesia. Red arrow, MEP electrode; yellow arrows, electrodes for SEP; blue arrow, electrode for NIRS; green arrow, electrode for BIS. BIS: bispectral index, CEA: carotid endarterectomy, MEP: motor-evoked potential, NIRS: near-infrared spectroscopic topography, SEP: sensory-evoked potential.
We should choose the method of monitoring according to the purpose. Dual or multiple monitors offer better sensitivity for predicting postoperative neurological deficit.37,39,40)
Selective Shunt or Routine Shunt
Shunt usage during CEA has been the subject of considerable debate, with most surgeons favoring routine use or selective use based on the results of intraoperative monitoring.41–43) Cooley et al. reported shunt use during surgery on the carotid artery in 1956.2) Authors reported on external shunt as a new device and discussed the indications in 2001, 45 years after the initial report.44) We also demonstrated that in 30 of the 131 procedures (22.9%), intraoperative monitoring disclosed abnormalities after cross-clamping of the ICA using selective shunt during CEA.29) On the other hands, several arguments have been made against the use of routine shunts, including unnecessary use in approximately 80–85% of patients.45–47) Aburahma et al.45) reported a prospective randomized study comparing routine and selective shunting. They showed that both groups were associated with low stroke rates (routine shunt vs. selective shunt: 0% vs. 2%, p = 0.498), with no significant differences in outcome. At present, the individual surgeon should select the method with which they are comfortable.42,48)
Dissecting the Distal ICA Plaque
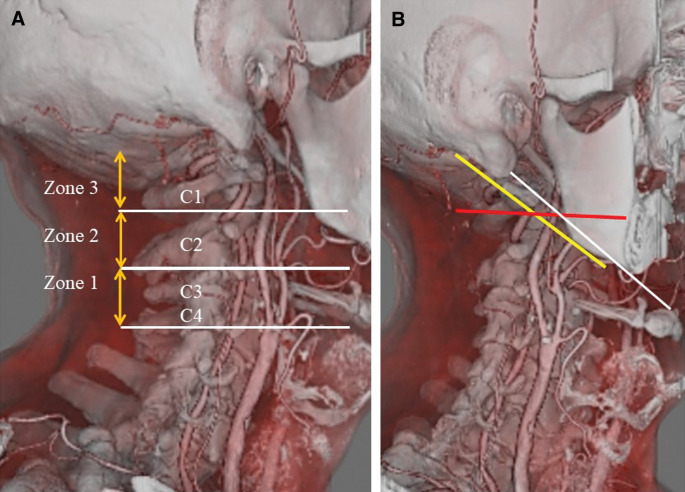
Distal ICA exposure is necessary to obtain distal control in patients with high cervical carotid bifurcation or high plaque. However, the definition of high cervical bifurcation is still ambiguous (Fig. 6).42,49–54) Hans et al. divided the ICA into three zones and defined a high plaque as located above Zone 2 (Fig. 6A).50,51) The mastoid-mandibular line has been proposed as a border for high position of the stenosis.52) Uno et al.53) reported the intersection of the occipital artery and ICA on lateral carotid angiography as another borderline. Kubota et al.54) reported the C1 transverse process-hyoid bone line as an useful indicator for accessible ICA (Fig. 6B).
Fig. 6.
Definition of high plaque. (A) Definition of high plaque by Hans et al. The ICA is divided into three zones, with high plaque defined as that located above Zone 2. (B) Various definition of high plaque. Yellow line is the mastoid-mandibular line. Red line is the intersection of the occipital artery and internal carotid artery. White line is the C1 transverse process-hyoid bone line. ICA: internal carotid artery.
The limited accessibility of the distal ICA has resulted in the development of various operative techniques for exposing this region. Nasotracheal intubation and a chin-up position can be helpful to expose the distal ICA (Fig. 2B). Weiss et al. described this method as obtaining as much as 2.5 cm of more distal exposure.55) Takigawa et al. also mentioned chin-up and extension of the neck as useful.12) However, CAS is currently selected in most of patients with high plaque, so nasotracheal intubation is rarely adopted. Moreover, we should consider the side effects such as nasal bleeding and infection in the respiratory tract.
Temporary mandibular subluxation49,56,57) or mandibular osteotomy58) have been used for exposure of high distal ICA sites. These techniques are useful to expose more distal sites of the ICA, but are more invasive for the patient, and use of these methods has decreased.
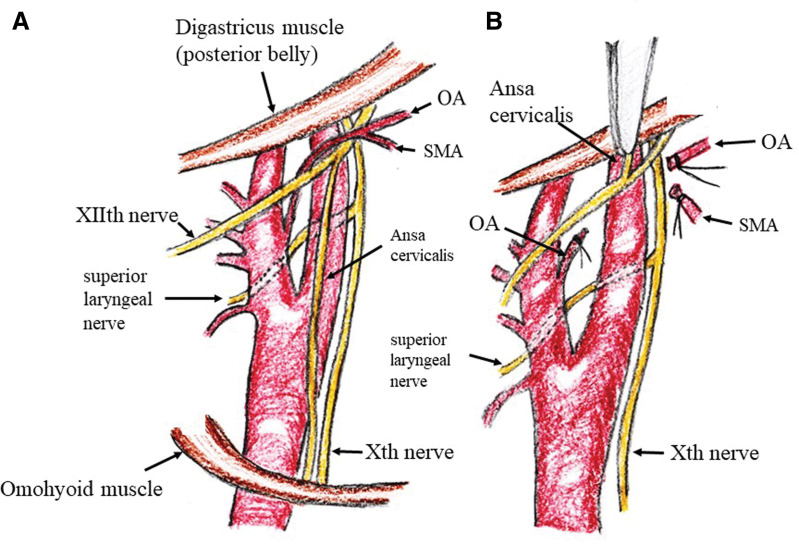
On the other hand, as a minimally invasive method, several surgeons cut the ansa cervicalis at the origin to lift the hypoglossal nerve.51,53,59) If the occipital artery gets in the way of securing the distal site of the ICA, it can be cut (Fig. 7).51,53,59) A high plaque can be managed in almost all cases using these methods.
Fig. 7.
Dissection of the distal ICA. (A) Correlation of carotid arteries and lower cranial nerves. (B) Dissection of the distal ICA. Ansa cervicalis is cut and pulled up alongside the hypoglossal nerve. If the occipital artery (OA) and/or sternocleidomastoid muscle artery (SMA) prevent securing of the distal ICA, they should be cut. ICA: internal carotid artery.
Protection Against Embolic Complications and Lower Cranial Nerve Palsy
The major cause of ischemic complications associated with CEA is embolism.60,61) To protect against embolic complication, several reports have examined different methods during exposure and dissection of the carotid artery. First, we administer heparin intravenously to reduce thrombus formation during ICA clamping. Hannan et al. reported that all surgical specialists used heparin extensively, with 97.5% of all patients having received heparin.62) Other strategies to avoid or minimize intraoperative cerebral plaque embolism have been reported. Pratesi et al. and Bourke et al. reported early control of the distal ICA during CEA.38,63) They demonstrated that use of this modified surgical strategy was independently associated with a lower risk of developing intraoperative neurological deficit.38,63) Yoshida et al. showed another method, named the “flow-control” CEA technique.61) They clamped the proximal common carotid artery (CCA), external carotid artery and superior thyroid artery, then dissected the bifurcation of the CCA and ICA before clamping the distal ICA. Under this method, new embolic lesions as detected by diffusion-weighted MRI were significantly decreased compared with the conventional method of CEA.61)
Lower cranial nerve injuries during CEA represent a considerable complication.64) The reported frequency of cranial nerve injuries in patients who underwent CEA ranges from 2% to over 50%.64,65) Most of these nerve injuries are transient, and vagus nerve and/or hypoglossal nerve palsies are the most common.64–66) Most of these injuries are due to excessive retraction, and we therefore have to pay attention to protecting against lower nerve injuries. Several strategies have been reported.62,66,67) During carotid artery dissection, trauma may occur if the dissection is not kept close to the wall of the artery.62) The vagus and/or hypoglossal nerves are sometimes retracted excessively, and we therefore have to perform sharp dissection of the nerve and vessels, and remove excessive tension on nerves by detaching them from the surrounding tissues. Aldoori et al.67) reported that the pharyngeal veins, which drain into the internal jugular vein, should be cut if they cross over the hypoglossal or vagus nerve.
Patch Angioplasty vs. Primary Suture
The ideal surgical technique for vessel closure during CEA remains controversial. In Japan, primary closure of the carotid artery is common. However, multiple studies have shown that patch angioplasty after CEA reduces the risk of stroke and restenosis compared with primary closure.68,69) Some studies have shown a high risk of restenosis after 5 years in patients with primary closure.70,71) A recent meta-analysis by Rerkasem et al.72) demonstrated that carotid patch angioplasty reduced the combined perioperative and long-term risks of stroke and restenosis. In contrast, some retrospective studies have shown no significant difference between primary closure and patch angioplasty for postoperative outcomes and restenosis.73–75)
Considerable debate remains over the choice of patch material. Biological, synthetic (e.g., Dacron or polytetrafluoroethylene), or vein patches have traditionally been used in CEA.76) Hemostasis time is longer in CEA with PTFE patch than in that with venous patch or Dacron patch. The overall perioperative and long-term mortality rates, stroke rates, frequency of restenosis, and operative time appear similar between these patch materials.76) Saphenous vein patch angioplasty has been shown to be prone to aneurysmal dilatation77) and suture rupture with a rate of 0.1–4%,78) and many surgeons have thus changed to using synthetic patch recently.
Skin Closure
Before skin closure, some surgeons administer protamine to reverse the heparin. No consensus has yet been reached about reversing the effects of heparin at the end of CEA. However, several reports have demonstrated that use of protamine is associated with a reduction in bleeding complications, without increasing major thrombotic outcomes, including stroke, myocardial infarction, or death.62,79,80)
Another point is whether to use a subcutaneous drain insert at the skin closure. Smolock et al.81) demonstrated that drain placement shows no benefit. However, many surgeons in Japan still place a subcutaneous drain.
Effect of Surgeon Experience on Perioperative Outcomes
Several studies have demonstrated better outcome of CEA at high-volume centers. AbuRuhma et al.82) reported that high-volume surgeons (≥30 CEA/year) achieved significantly better outcomes than low-volume surgeons (stroke/death: 1.3% vs. 4.2%, p = 0.005).
Another point is the difference in outcome of CEA between experienced and younger surgeons. Several reports have demonstrated similar complication rates compared with experienced and younger surgeons.83,84) Cacioppa et al. reported a teaching program for trainees learning CEA. With this program (5 years of training), trainees can obtain results similar to those of experienced surgeons in terms of outcomes of CEA.83)
In Japan, most surgeons encounter only a small volume of CEAs, and gaining experience with a sufficient number of cases is difficult. We have already reported on training models as an “in vivo” CEA procedure,85) but the extent to which such models have gained acceptance is hard to say.
Conclusion
CEA had been performed as a relatively constant maneuver, but new maneuvers and equipment have been introduced over the course of 60 years. The technical aspects of CEA have changed little by little, but room for discussion remains in various aspects. To maintain the CEA procedure, improvements and the establishment of safer techniques are necessary.
Footnotes
Conflicts of Interest Disclosure
The authors have no conflicts of interest to declare regarding this study or its findings.
References
- 1).Eastcott HH, Pickering GW, Rob CG: Reconstruction of internal carotid artery in a patient with intermittent attacks of hemiplegia. Lancet 264: 994–996, 1954 [DOI] [PubMed] [Google Scholar]
- 2).Cooley DA, Al-naaman YD, Carton CA: Surgical treatment of arteriosclerotic occlusion of common carotid artery. J Neurosurg 13: 500–506, 1956 [DOI] [PubMed] [Google Scholar]
- 3).Murphey F, Miller JH: Carotid insufficiency: diagnosis and surgical treatment; a report of twenty-one cases. J Neurosurg 16: 1–23, 1959 [DOI] [PubMed] [Google Scholar]
- 4).Loftus CM: Technical aspects of carotid endarterectomy with Hemashield patch graft. Neurol Med Chir (Tokyo) 37: 805–818, 1997 [DOI] [PubMed] [Google Scholar]
- 5).North American Symptomatic Carotid Endarterectomy Trial Collaborators. Barnett HJM, Taylor DW, et al. : Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med 325: 445–453, 1991 [DOI] [PubMed] [Google Scholar]
- 6).Endarterectomy for asymptomatic carotid artery stenosis Executive Committee for the asymptomatic carotid atherosclerosis study. JAMA 273: 1421–1428, 1995 [PubMed] [Google Scholar]
- 7).Mas JL, Chatellier G, Beyssen B, et al. : Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med 355: 1660–1671, 2006 [DOI] [PubMed] [Google Scholar]
- 8).Columbo JA, Martinez-Camblor P, MacKenzie TA, et al. : Comparing long-term mortality after carotid endarterectomy vs carotid stenting using a novel instrumental variable method for risk adjustment in observational time-to-event data. JAMA Netw Open 1: e181676, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Batchelder AJ, Saratzis A, Ross Naylor A: Editor's choice - overview of primary and secondary analyses from 20 randomised controlled trials comparing carotid artery stenting with carotid endarterectomy. Eur J Vasc Endovasc Surg 58: 479–493, 2019 [DOI] [PubMed] [Google Scholar]
- 10).Loftus CM: Side by side Carotid endarterctomy Principles and technique Quality Medical Publishing, St Louis: pp. 48–49, 1995 [Google Scholar]
- 11).Uno M, Yagi K, Takai H, et al. : Diagnosis and operative management of carotid endarterectomy in patients with twisted carotid bifurcation. Neurol Med Chir (Tokyo) 60: 383–389, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Takigawa T, Yanaka K, Yasuda M, Asakawa H, Matsumaru Y, Nose T: Head and neck extension-fixation with a head frame for exposure of the distal internal carotid artery in carotid endarterectomy–technical note. Neurol Med Chir (Tokyo) 43: 271–273; discussion 273, 2003 [DOI] [PubMed] [Google Scholar]
- 13).Deck M, Kopriva D: Patient and observer scar assessment scores favour the late appearance of a transverse cervical incision over a vertical incision in patients undergoing carotid endarterectomy for stroke risk reduction. Can J Surg 58: 245–249, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Kazimierczak A, Rybicka A, Rynio P, Gutowski P, Wiernicki I: Cosmetic effects of skin-crease camouflage incision versus longitudinal incision following carotid endarterectomy. Wideochir Inne Tech Maloinwazyjne 13: 102–110, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Kondo T, Ota N, Göhre F, et al. : High cervical carotid endarterectomy-outcome analysis. World Neurosurg 136: e108–e118, 2020 [DOI] [PubMed] [Google Scholar]
- 16).Yoneyama T, Kawamata T, Tanaka M, Yamaguchi K, Okada Y: Omnidirectional retractor-supporting ring as a new device for carotid endarterectomy. J Neurosurg 122: 148–151, 2015 [DOI] [PubMed] [Google Scholar]
- 17).Spetzler RF, Martin N, Hadley MN, Thompson RA, Wilkinson E, Raudzens PA: Microsurgical endarterectomy under barbiturate protection: a prospective study. J Neurosurg 65: 63–73, 1986 [DOI] [PubMed] [Google Scholar]
- 18).Findlay JM, Lougheed WM: Carotid microendarterectomy. Neurosurgery 32: 792–797; discussion 798, 1993 [DOI] [PubMed] [Google Scholar]
- 19).Murai Y, Sato S, Yui K, et al. : Preliminary clinical microneurosurgical experience with the 4K3- dimensional microvideoscope (ORBEYE) system for microneurological surgery: observation study. Oper Neurosurg (Hagerstown) 16: 707–716, 2019 [DOI] [PubMed] [Google Scholar]
- 20).Englund R, Dean RH: Blood pressure aberrations associated with carotid endarterectomy. Ann Vasc Surg 1: 304–309, 1986 [DOI] [PubMed] [Google Scholar]
- 21).Tan TW, Eslami MH, Kalish JA, et al. : The need for treatment of hemodynamic instability following carotid endarterectomy is associated with increased perioperative and 1-year morbidity and mortality. J Vasc Surg 59: 16–24.e1-2, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Cafferata HT, Merchant RF, DePalma RG: Avoidance of postcarotid endarterectomy hypertension. Ann Surg 196: 465–472, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Ajduk M, Tudorić I, Sarlija M, et al. : Effect of carotid sinus nerve blockade on hemodynamic stability during carotid endarterectomy under local anesthesia. J Vasc Surg 54: 386–393, 2011 [DOI] [PubMed] [Google Scholar]
- 24).Al-Rawi PG, Sigaudo-Roussel D, Gaunt ME: Effect of lignocaine injection in carotid sinus on baroreceptor sensitivity during carotid endarterectomy. J Vasc Surg 39: 1288–1294, 2004 [DOI] [PubMed] [Google Scholar]
- 25).Tang TY, Walsh SR, Gillard JH, Varty K, Boyle JR, Gaunt ME: Carotid sinus nerve blockade to reduce blood pressure instability following carotid endarterectomy: a systematic review and meta-analysis. Eur J Vasc Endovasc Surg 34: 304–311, 2007 [DOI] [PubMed] [Google Scholar]
- 26).Thiagarajan K, Cheng HL, Huang JE, et al. : Is two really better than one? Examining the superiority of dual modality neurophysiological monitoring during carotid endarterectomy: a meta-analysis. World Neurosurg 84: 1941–1949.e1, 2015 [DOI] [PubMed] [Google Scholar]
- 27).Nwachuku EL, Balzer JR, Yabes JG, Habeych ME, Crammond DJ, Thirumala PD: Diagnostic value of somatosensory evoked potential changes during carotid endarterectomy: a systematic review and meta- analysis. JAMA Neurol 72: 73–80, 2015 [DOI] [PubMed] [Google Scholar]
- 28).Domenick Sridharan N, Chaer RA, Thirumala PD, et al. : Somatosensory evoked potentials and electroencephalography during carotid endarterectomy predict late stroke but not death. Ann Vasc Surg 38: 105–112, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Uno M, Suzue A, Nishi K, Nagahiro S: Hemodynamic cerebral ischemia during carotid endarterectomy evaluated by intraoperative monitoring and post- operative diffusion-weighted imaging. Neurol Res 29: 70–77, 2007 [DOI] [PubMed] [Google Scholar]
- 30).Okuyama S, Nishimura S, Takahashi Y, et al. : Limitations of median nerve somatosensory evoked potential monitoring during carotid endarterectomy. J Neurosurg 131: 750–756, 2018 [DOI] [PubMed] [Google Scholar]
- 31).Findlay JM, Kesarwani R, Jacka M, Marchak BE: Combined stump pressure and oximetry for shunt use during carotid endarterectomy. Can J Neurol Sci 44: 692–696, 2017 [DOI] [PubMed] [Google Scholar]
- 32).Hans SS, Jareunpoon O: Prospective evaluation of electroencephalography, carotid artery stump pressure, and neurologic changes during 314 consecutive carotid endarterectomies performed in awake patients. J Vasc Surg 45: 511–515, 2007 [DOI] [PubMed] [Google Scholar]
- 33).Pennekamp CW, Tromp SC, Ackerstaff RG, et al. : Prediction of cerebral hyperperfusion after carotid endarterectomy with transcranial Doppler. Eur J Vasc Endovasc Surg 43: 371–376, 2012 [DOI] [PubMed] [Google Scholar]
- 34).Udesh R, Natarajan P, Thiagarajan K, et al. : Transcranial doppler monitoring in carotid endarterectomy: a systematic review and meta-analysis. J Ultrasound Med 36: 621–630, 2017 [DOI] [PubMed] [Google Scholar]
- 35).Pennekamp CW, Immink RV, den Ruijter HM, et al. : Near-infrared spectroscopy to indicate selective shunt use during carotid endarterectomy. Eur J Vasc Endovasc Surg 46: 397–403, 2013 [DOI] [PubMed] [Google Scholar]
- 36).Pennekamp CW, Immink RV, den Ruijter HM, et al. : Near-infrared spectroscopy can predict the onset of cerebral hyperperfusion syndrome after carotid endarterectomy. Cerebrovasc Dis 34: 314–321, 2012 [DOI] [PubMed] [Google Scholar]
- 37).Malcharek MJ, Kulpok A, Deletis V, et al. : Intraoperative multimodal evoked potential monitoring during carotid endarterectomy: a retrospective study of 264 patients. Anesth Analg 120: 1352–1360, 2015 [DOI] [PubMed] [Google Scholar]
- 38).Pratesi C, Dorigo W, Innocenti AA, et al. : Reducing the risk of intraoperative neurological complications during carotid endarterectomy with early distal control of the internal carotid artery. Eur J Vasc Endovasc Surg 28: 670–673, 2004 [DOI] [PubMed] [Google Scholar]
- 39).Malcharek MJ, Hesse J, Hesselbarth K, et al. : Warning criteria for MEP monitoring during carotid endarterectomy: a retrospective study of 571 patients. J Clin Monit Comput 37: 589–595, 2019 [DOI] [PubMed] [Google Scholar]
- 40).Alcantara SD, Wuamett JC, Lantis JC, et al. : Outcomes of combined somatosensory evoked potential, motor evoked potential, and electroencephalography monitoring during carotid endarterectomy. Ann Vasc Surg 28: 665–672, 2014 [DOI] [PubMed] [Google Scholar]
- 41).Kong J, Li J, Ye Z, et al. : Carotid endarterectomy with routine shunt for patients with contralateral carotid occlusion. Ann Thorac Cardiovasc Surg 23: 227–232, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42).Chongruksut W, Vaniyapong T, Rerkasem K: Routine or selective carotid artery shunting for carotid endarterectomy (and different methods of monitoring in selective shunting). Cochrane Database Syst Rev 6: CD000190, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43).Thompson JE, Austin DJ, Patman RD: Carotid endarterectomy for cerebrovascular insufficiency: long-term results in 592 patients followed up to thirteen years. Surg Clin North Am 66: 233–253, 1986 [DOI] [PubMed] [Google Scholar]
- 44).Uno M, Nishi K, Shinno K, Nagahiro S: Carotid endarterectomy with external shunt: a new device and indication for use: technical note. Neurosurgery 48: 1174–1177, 2001 [DOI] [PubMed] [Google Scholar]
- 45).Aburahma AF, Stone PA, Hass SM, et al. : Prospective randomized trial of routine versus selective shunting in carotid endarterectomy based on stump pressure. J Vasc Surg 51: 1133–1138, 2010 [DOI] [PubMed] [Google Scholar]
- 46).Calligaro KD, Dougherty MJ: Correlation of carotid artery stump pressure and neurologic changes during 474 carotid endarterectomies performed in awake patients. J Vasc Surg 42: 684–689, 2005 [DOI] [PubMed] [Google Scholar]
- 47).Hans SS, Catanescu I: Selective shunting for carotid endarterectomy in patients with recent stroke. J Vasc Surg 61: 915–919, 2015 [DOI] [PubMed] [Google Scholar]
- 48).Aburahma AF, Mousa AY, Stone PA: Shunting during carotid endarterectomy. J Vasc Surg 54: 1502–1510, 2011 [DOI] [PubMed] [Google Scholar]
- 49).Frim DM, Padwa B, Buckley D, Crowell RM, Ogilvy CS: Mandibular subluxation as an adjunct to exposure of the distal internal carotid artery in endarterectomy surgery. Technical note. J Neurosurg 83: 926–928, 1995 [DOI] [PubMed] [Google Scholar]
- 50).Hans SS, Shah S, Hans B: Carotid endarterectomy for high plaques. Am J Surg 157: 431–434; discussion 434–435, 1989 [DOI] [PubMed] [Google Scholar]
- 51).Vang S, Hans SS: Carotid endarterectomy in patients with high plaque. Surgery 166: 601–606, 2019 [DOI] [PubMed] [Google Scholar]
- 52).Blaisdell WF, Clauss RH, Galbraith JG, Imparato AM, Wylie EJ: Joint study of extracranial arterial occlusion. IV. A review of surgical considerations. JAMA 209: 1889–1895, 1969 [PubMed] [Google Scholar]
- 53).Uno M, Suzue A, Nishi K, Satoh K, Shinno K, Nagahiro S: Carotid endarterectomy for patients with high cervical carotid artery stenosis. Surg Cereb Stroke 31: 61–66, 2003. (Japanese) [Google Scholar]
- 54).Kubota H, Sanada Y, Yoshioka H, et al. : C1 transverse process-hyoid bone line for preoperative evaluation of the accessible internal carotid artery on carotid endarterectomy: technical note. Acta Neurochir (Wien) 157: 43–48, 2015 [DOI] [PubMed] [Google Scholar]
- 55).Weiss MR, Smith HP, Patterson AK, Weiss RM: Patient positioning and nasal intubation for carotid endarterectomy. Neurosurgery 19: 256–257, 1986 [DOI] [PubMed] [Google Scholar]
- 56).Dossa C, Shepard AD, Wolford DG, Reddy DJ, Ernst CB: Distal internal carotid exposure: a simplified technique for temporary mandibular subluxation. J Vasc Surg 12: 319–325, 1990 [PubMed] [Google Scholar]
- 57).Yoshino M, Fukumoto H, Mizutani T, Yuyama R, Hara T: Mandibular subluxation stabilized by mouthpiece for distal internal carotid artery exposure in carotid endarterectomy. J Vasc Surg 52: 1401–1404, 2010 [DOI] [PubMed] [Google Scholar]
- 58).Balagura S, Carter JB, Gossett DL: Surgical approach to the high subcranial internal carotid artery. Neurosurgery 16: 402–405, 1985 [DOI] [PubMed] [Google Scholar]
- 59).Kondo T, Ota N, Göhre F, et al. : High Cervical carotid endarterectomy-outcome analysis. World Neurosurg 136: e108–118, 2019 [DOI] [PubMed] [Google Scholar]
- 60).Riles TS, Imparato AM, Jacobowitz GR, et al. : The cause of perioperative stroke after carotid endarterectomy. J Vasc Surg 19: 206–214; discussion 215–216, 1994 [DOI] [PubMed] [Google Scholar]
- 61).Yoshida K, Kurosaki Y, Funaki T, et al. : Surgical dissection of the internal carotid artery under flow control by proximal vessel clamping reduces embolic infarcts during carotid endarterectomy. World Neurosurg 82: e229–234, 2014 [DOI] [PubMed] [Google Scholar]
- 62).Hannan EL, Popp AJ, Feustel P, et al. : Association of surgical specialty and processes of care with patient outcomes for carotid endarterectomy. Stroke 32: 2890–2897, 2001 [DOI] [PubMed] [Google Scholar]
- 63).Bourke BM, Crimmins DS: Early control of the distal internal carotid artery during endarterectomy: achievability and results. J Vasc Surg 36: 70–74, 2002 [DOI] [PubMed] [Google Scholar]
- 64).Cunningham EJ, Bond R, Mayberg MR, Warlow CP, Rothwell PM: Risk of persistent cranial nerve injury after carotid endarterectomy. J Neurosurg 101: 445–448, 2004 [DOI] [PubMed] [Google Scholar]
- 65).Kakisis JD, Antonopoulos CN, Mantas G, Moulakakis KG, Sfyroeras G, Geroulakos G: Cranial nerve injury after carotid endarterectomy: incidence, risk factors, and time trends. Eur J Vasc Endovasc Surg 53: 320–335, 2017 [DOI] [PubMed] [Google Scholar]
- 66).Massey EW, Heyman A, Utley C, Haynes C, Fuchs J: Cranial nerve paralysis following carotid endarterectomy. Stroke 15: 157–159, 1984 [DOI] [PubMed] [Google Scholar]
- 67).Aldoori J, Mahadevan V, Aldoori M: The significance of the pharyngeal veins during carotid endarterectomy: description of an anatomical triangle. Ann R Coll Surg Engl 100: 125–128, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68).Okazaki T, Kanematsu Y, Shimada K, et al. : A single- center retrospective study with 5- and 10-year follow- up of carotid endarterectomy with patch graft. Neurol Med Chir (Tokyo) 59: 231–237, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69).Bond R, Rerkasem K, Naylor AR, Aburahma AF, Rothwell PM: Systematic review of randomized controlled trials of patch angioplasty versus primary closure and different types of patch materials during carotid endarterectomy. J Vasc Surg 40: 1126–1135, 2004 [DOI] [PubMed] [Google Scholar]
- 70).Rerkasem K, Gallagher PJ, Grimble RF, Calder PC, Shearman CP: Sex difference in composition of plaques of patients undergoing carotid endarterectomy. Vascular 18: 77–81, 2010 [DOI] [PubMed] [Google Scholar]
- 71).De Letter JA, Moll FL, Welten RJ, et al. : Benefits of carotid patching: a prospective randomized study with long-term follow-up. Ann Vasc Surg 8: 54–58, 1994 [DOI] [PubMed] [Google Scholar]
- 72).Rerkasem K, Rothwell PM: Systematic review of randomized controlled trials of patch angioplasty versus primary closure and different types of patch materials during carotid endarterectomy. Asian J Surg 34: 32–40, 2011 [DOI] [PubMed] [Google Scholar]
- 73).Maertens V, Maertens H, Kint M, Coucke C, Blomme Y: Complication rate after carotid endarterectomy comparing patch angioplasty and primary closure. Ann Vasc Surg 30: 248–252, 2016 [DOI] [PubMed] [Google Scholar]
- 74).Ben Ahmed S, Daniel G, Benezit M, et al. : Does the technique of carotid endarterectomy determine postoperative hypertension? Ann Vasc Surg 29: 1272–1280, 2015 [DOI] [PubMed] [Google Scholar]
- 75).Clagett GP, Patterson CB, Fisher DF, et al. : Vein patch versus primary closure for carotid endarterectomy. a randomized prospective study in a selected group of patients. J Vasc Surg 9: 213–223, 1989 [DOI] [PubMed] [Google Scholar]
- 76).Ren S, Li X, Wen J, Zhang W, Liu P: Systematic review of randomized controlled trials of different types of patch materials during carotid endarterectomy. PLoS ONE 8: e55050, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77).Lord RS, Raj TB, Stary DL, Nash PA, Graham AR, Goh KH: Comparison of saphenous vein patch, polytetrafluoroethylene patch, and direct arteriotomy closure after carotid endarterectom. Part I. Perioperative results. J Vasc Surg 9: 521–529, 1989 [PubMed] [Google Scholar]
- 78).Berner M, Lattmann T, Stalder P, Wigger P: Vein patch closure using below the knee greater saphenous vein for femoral endarterectomy procedures is not always a safe choice. EJVES Short Rep 37: 22–24, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79).Newhall KA, Saunders EC, Larson RJ, Stone DH, Goodney PP: Use of protamine for anticoagulation during carotid endarterectomy: a meta-analysis. JAMA Surg 151: 247–255, 2016 [DOI] [PubMed] [Google Scholar]
- 80).Kakisis JD, Antonopoulos CN, Moulakakis KG, Schneider F, Geroulakos G, Ricco JB: Protamine reduces bleeding complications without increasing the risk of stroke after carotid endarterectomy: a meta- analysis. Eur J Vasc Endovasc Surg 52: 296–307, 2016 [DOI] [PubMed] [Google Scholar]
- 81).Smolock CJ, Morrow KL, Kang J, Kelso RL, Bena JF, Clair DG: Drain placement confers no benefit after carotid endarterectomy in the Vascular Quality Initiative. J Vasc Surg 72: 204–208, 2020 [DOI] [PubMed] [Google Scholar]
- 82).AbuRahma AF, Stone PA, Srivastava M, et al. : The effect of surgeon's specialty and volume on the perioperative outcome of carotid endarterectomy. J Vasc Surg 58: 666–672, 2013 [DOI] [PubMed] [Google Scholar]
- 83).Cacioppa LM, Pini R, Longhi M, et al. : The value of carotid endarterectomy as a learning tool for trainees. Ann Vasc Surg 47: 195–199, 2018 [DOI] [PubMed] [Google Scholar]
- 84).Rijbroek A, Wisselink W, Rauwerda JA: The impact of training in unselected patients on mortality and morbidity in carotid endarterectomy in a vascular training center and the recommendations of the European Board of Surgery Qualification in Vascular Surgery. Eur J Vasc Endovasc Surg 26: 256–261, 2003 [DOI] [PubMed] [Google Scholar]
- 85).Uno M: Development of a model simulating carotid endarterectomy. Surg Cereb Stroke 41: 143–147, 2013. (Japanese) [Google Scholar]