Abstract
Background
Several risk factors have been associated with the development of postoperative atrial fibrillation (AF). However, some important factors that may play substantial roles have been neglected in the final suggested risk models. In this study, we aimed to derive a new clinical risk index to predict AF in coronary artery bypass graft (CABG) patients.
Methods
In this retrospective cohort study we enrolled 3047 isolated CABG patients. A random sample of 2032 patients was used to derive a risk index for the prediction of post-CABG AF. A multivariate logistic regression model identified the independent preoperative predictors of post-CABG AF, and a simple risk index to predict AF was constructed. This risk index was cross-validated in a validation set of 1015 patients with isolated CABG.
Results
Post-CABG AF occurred in 15.9% and 15.7% of the patients in the prediction and validation sets, respectively. Using multivariate stepwise analysis, four preoperative variables including advanced age, left atrial (LA) enlargement, hypertension and cerebrovascular accident contributed to the prediction model (area under the receiver operating characteristic curve curve = 0.66). The effect of advanced age appeared to be dominant [age ≥ 75 years; odds ratio: 4.134, 95% confidence interval (CI): 2.791-6.121, p < 0.001]. Moderate to severe LA enlargement had an odds ratio of 2.176 (95% CI: 1.240-3.820, p = 0.013) for developing AF in our risk index.
Conclusions
LA size was an important factor in risk stratification of post-CABG AF, which remained significant in the final model. Future scoring system studies might benefit from the use of this variable to obtain a more robust predictive value.
Keywords: Atrial fibrillation, Coronary artery bypass graft, Risk prediction
INTRODUCTION
Atrial fibrillation (AF) is the most frequent arrhythmia after cardiac surgery, and occurs in approximately 10-60% of patients.1-4 Several factors related to the clinical course of patients and heart anatomy have been associated with AF, of which left atrial (LA) size is one of the most important.5,6 Accordingly, previous large sample size studies have identified an association between M-mode anteroposterior LA diameter and the development of AF, especially in those with cardiomyopathy.7-9 Nevertheless, the clinical impact and utility of LA size in the development of post-coronary artery bypass graft (CABG) AF were rejected in the findings of a small population-based study.10
Although several intraoperative and postoperative factors have been reported to have adequate discriminative power with regards to the risk estimation of postoperative AF,11-14 the lack of LA size in the final model, given its inherent importance as noted above in predicting post-CABG AF, was surprising. To the best of our knowledge, all of the previous prediction models for post-CABG AF have failed to include the variable "LA size".15
Therefore, the aim of this study was to derive a simple clinical index for discriminating patients at high risk of AF using available preoperative independent predictors of AF, including LA diameter. We assumed that this could be considered as an acknowledgment of the importance of LA size in further AF risk index models.
METHODS
Study population
In this observational cohort study, we retrieved the data from the Adult Cardiac Surgery Databank16 related to patients who underwent isolated CABG at Tehran Heart Center between April 2010 and February 2012. The study was approved by the hospital’s local review board. Overall, 3047 isolated CABG patients were identified. The exclusion criteria were concomitant cardiac procedures, or patients with a history of AF, previous cardiac surgery, pacemaker implantation, or the use of preoperative anti-arrhythmic drugs except for beta-blockers.
The occurrence of AF in the first 72 hours following the cardiac surgery was documented by intensive care unit (ICU) and post-ICU nurses through continuous cardiac monitoring systems, and then confirmed by a cardiologist. Our post-ICU nurses are ordinary ward nurses who are trained for ICU care, and they are expert in arrhythmia and necessary nursing care. After 72 hours, an electrocardiogram was recorded in symptomatic patients. The new onset of post-CABG AF was defined as the occurrence of AF (episodes lasting > 30 seconds) following isolated CABG which resolved spontaneously or required pharmacologic or electrical cardioversion. In the present study, none of the patients were discharged with AF and only a few needed electrical cardioversion. All entries were based on definitions of the Society of Thoracic Surgeons. Echocardiographic parameters were classified using the American Society of Echocardiography recommendations.17
The baseline demographic and clinical characteristics of the patients were recorded, including the past medical and drug history, intraoperative and angiographic information, and preoperative echocardiographic data on LA size.
LA diameter was measured at the level of the aortic valve leaflets in the parasternal long-axis view and was classified as a gender-specific variable, such that LA enlargement was defined as an LA diameter of ≥ 41 mm in men or ≥ 39 mm in women; an LA diameter below these values was defined as normal LA size. LA enlargement was further categorized as mild (LA diameter 41 to 46 mm in men or 39 to 42 mm in women), moderate (47 to 51 mm in men or 43 to 46 mm in women) or severe (≥ 52 mm in men or ≥ 47 mm in women) according to the recommendations of the American Society of Echocardiography.17
Statistical analysis
Continuous variables were presented as mean ± standard deviation (SD) and were compared across the two groups (with and without AF) using the Student’s t test or Mann-Whitney U test. Categorical variables were summarized using frequencies and percentages and were compared between the two groups using the chi-square test or Fisher’s exact test.
We used the one-time data splitting method, a simple method for internal validation, in which the study samples were randomly split into two development and validation sets.18 Of the 3047 eligible participants, almost two-thirds (n = 2032) were randomly selected for the prediction (derivation) set, and the remaining one-third (n = 1015) were reserved as a validation set. The prediction set was used to develop the prediction model through the following steps; variables with p-values less than or equal to 0.2 in the univariate analysis were candidates to enter into the multivariate model. A multivariate backwards logistic regression model with a removal probability of 0.1 was applied to determine AF predictors after CABG. The effects of independent variables on AF were reported using odds ratios with 95% confidence intervals (CIs).
To provide clinicians with a convenient way to categorize post-CABG AF risk, we developed a scoring system the simple model. Based on the magnitude of corresponding regression coefficients (log odds ratios) estimated for each predictive variable, a simple point value, i.e. a whole number proportional to the individual estimated coefficient, was assigned to each of the risk factors.19 In the next step, the total score was calculated for each patient by summing the points with maximum 2 points awarded for advanced age and LA enlargement variables, and 1 point for each of the three other predictors; the maximum score attainable was therefore 6. Only the total score was included as an explanatory variable in the final logistic regression model to assess the discriminatory power of this risk index in the prediction set. Finally, in order to cross-validate the prediction model, it was tested in the validation set.
For all of the models, the discriminatory power of the model was measured using the area under the receiver operating characteristic curve (AUC), and model calibration was assessed using the Hosmer-Lemeshow goodness-of-fit test. All analyses were done using IBM SPSS statistics software for Windows, version 20.0 (Armonk, NY: IBM Corp.).
RESULTS
As mentioned above, the patients were randomly stratified into two groups of derivation (prediction) and validation samples including 2032 and 1015 patients, respectively. The baseline and clinical characteristics of the patients in both sets are summarized in Table 1. The incidence of post-CABG AF in the early postoperative period was 15.8% in the entire study population (n = 3047). This incidence was similar among the patients in the prediction (15.9%) and validation (15.7%) sets (p = 0.88). Dyslipidemia (50.1% vs. 45.2%, p = 0.012) and cigarette smoking (16.5% vs. 13%, p = 0.012) were more common among the patients in the prediction set, whereas chronic obstructive pulmonary disease (COPD) was more frequent in the validation set. Other baseline characteristics were not significantly different between the two groups.
Table 1. Baseline and clinical characteristics of the patients in the derivation and validation cohorts.
| Prediction set (n = 2032) | Validation set (n = 1015) | p value | |
| Atrial fibrillation | 319 (15.9) | 159 (15.7) | 0.883 |
| Age (year) | 0.107 | ||
| < 65 | 1273 (62.6) | 667 (65.7) | |
| 65-74 | 613 (30.2) | 269 (26.5) | |
| ≥ 75 | 146 (7.2)0 | 79 (7.8) | |
| Male gender | 1478 (73.7) | 770 (75.9) | 0.199 |
| Body mass index (kg/m2) | 27.21 ± 4.30 | 26.96 ± 4.00 | 0.122 |
| Diabetes mellitus | 794 (39.6) | 412 (40.6) | 0.633 |
| Dyslipidemia | 1005 (50.1) | 459 (45.2) | 0.012 |
| Hypertension | 975 (48.6) | 472 (46.5) | 0.310 |
| Cigarette smoker | 331 (16.5) | 132 (13.0) | 0.012 |
| History of CVA | 80 (4.0) | 45 (4.4) | 0.513 |
| History of COPD | 15 (0.7) | 22 (2.2) | 0.001 |
| CHF | 365 (18) | 178 (17.5) | 0.772 |
| Preoperative MI | 722 (36.0) | 367 (36.2) | 0.849 |
| LA enlargement | 0.654 | ||
| Normal | 1579 (78.7) | 818 (80.6) | |
| Mild | 361 (18.0) | 164 (16.2) | |
| Moderate to severe | 66 (3.3) | 33 (3.3) | |
| Coronary vessel involvement | |||
| LMCA | 134 (6.7) | 71 (7.0) | 0.740 |
| RCA | 730 (36.4) | 381 (37.5) | 0.542 |
| LCX | 661 (33.0) | 315 (31.0) | 0.283 |
| LAD | 1062 (52.9) | 521 (51.3) | 0.403 |
| Statin use | 1848 (92.0) | 938 (92.4) | 0.712 |
| Β-blocker use | 1667 (83.1) | 840 (82.8) | 0.777 |
| Creatinine (mg/dl) | 1.01 ± 0.67 | 0.97 ± 0.42 | 0.300 |
| LVEF (%) | 46.71 ± 9.17 | 47.10 ± 8.85 | 0.427 |
| Aortic cross-clamp time (minute) | 42.30 ± 21.67 | 41.72 ± 14.39 | 0.629 |
| Pump time (minute) | 74.89 ± 29.04 | 74.46 ± 26.61 | 0.832 |
Continuous variables are presented as mean ± standard deviation, and categorical variables as number and percentages within parentheses.
CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; LA, left atrium; LAD, left anterior descending artery; LCX, left circumflex artery; LMCA, left main coronary artery; LVEF, left ventricular ejection fraction; MI, myocardial infarction; RCA, right coronary artery.
The clinical and angiographic characteristics of the patients with and without AF in the prediction set are presented in Table 2. The patients with AF were generally older (p < 0.001) and were more likely to have an enlarged LA (p < 0.001), hypertension (p = 0.001) and a history of cerebrovascular accident (CVA) (p < 0.001). They also more frequently showed the involvement of the left main coronary artery and right coronary artery in the coronary artery tree (p = 0.002 and 0.046, respectively). The surgical data are shown in Table 2. The patients with AF had a longer aortic cross-clamp time (45.6 vs. 41.6 minutes, p = 0.03) and pump time (79.6 vs. 73.9 minutes, p = 0.002) compared to those who did not have AF. There was no statistically significant difference in body mass index between the two groups. There was also no significant difference in the frequency of dyslipidemia between the patients with and without AF, however the patients who did not develop post-CABG AF had a significantly higher usage of statins compared to the patients with AF (92.8% vs. 88.1%, p = 0.005).
Table 2. Baseline characteristics of the patients in derivation set stratified by atrial fibrillation status.
| No atrial fibrillation (n = 1687) | Atrial fibrillation (n = 319) | p value | |
| Age (year) | < 0.001 | ||
| < 65 | 1125 (66.7) | 135 (42.3) | |
| 65-74 | 468 (27.7) | 134 (42) | |
| ≥ 75 | 94 (5.6) | 50 (15.7) | |
| Male gender | 1249 (74.0) | 229 (71.8) | 0.403 |
| BMI (kg/m2) | 27.23 ± 4.29 | 27.13 ± 4.28 | 0.497 |
| Diabetes mellitus | 665 (39.4) | 130 (40.8) | 0.665 |
| Dyslipidemia | 854 (50.6) | 149 (46.7) | 0.200 |
| Hypertension | 789 (46.8) | 181 (56.7) | 0.001 |
| Cigarette smoker | 290 (17.3) | 40 (12.5) | 0.037 |
| History of CVA | 55 (3.3) | 24 (7.5) | < 0.001 |
| History of COPD | 11 (0.7) | 4 (1.3) | 0.252 |
| CHF | 297 (17.6) | 63 (19.7) | 0.360 |
| Preoperative MI | 615 (36.5) | 104 (32.6) | 0.188 |
| LA enlargement | < 0.001 | ||
| Normal | 1357 (80.4) | 223 (69.9) | |
| Mild | 284 (16.8) | 76 (23.8) | |
| Moderate to severe | 46 (2.7) | 20 (6.3) | |
| Coronary vessel involvement | |||
| LMCA | 100 (5.9) | 34 (10.7) | 0.002 |
| RCA | 599 (35.5) | 132 (41.4) | 0.046 |
| LCX | 546 (32.4) | 116 (36.4) | 0.164 |
| LAD | 894 (53.0) | 168 (52.7) | 0.914 |
| Statin use | 1565 (92.8) | 281 (88.1) | 0.005 |
| Β-blocker use | 1410 (83.6) | 260 (81.5) | 0.363 |
| Creatinine (mg/dl) | 1.00 ± 0.66 | 1.07 ± 0.75 | 0.067 |
| LVEF (%) | 46.82 ± 9.18 | 46.26 ± 9.06 | 0.317 |
| Aortic cross-clamp time (minute) | 41.61 ± 14.58 | 45.61 ± 43.04 | 0.031 |
| Pump time (minute) | 73.91 ± 28.45 | 79.60 ± 31.79 | 0.002 |
Continuous variables are presented as mean ± standard deviation, and categorical variables as number and percentages within parentheses.
CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; LA, left atrium; LAD, left anterior descending artery; LCX, left circumflex artery; LMCA, left main coronary artery; LVEF, left ventricular ejection fraction; MI, myocardial infarction; RCA, right coronary artery.
Table 3 presents the predictors of post-CABG AF among the patients in the prediction set. In order to derive a simple scoring model, only the preoperative clinical data and echocardiographic information were entered into the final model. Using multivariate stepwise analysis, four preoperative variables were identified to be independently associated with the risk of AF: advanced age, LA enlargement, hypertension (HTN) and CVA. The model including these four variables had an AUC of 0.66 (95% CI: 0.61-0.698), and the addition of other variables did not significantly improve the AUC of this 4-variable model. The AF risk index (computed from the prediction set) predicted incidence cases of AF in the validation set with an AUC of 0.665 (95% CI: 0.61 to 0.698).
Table 3. Predictors and clinical risk index for postoperative atrial fibrillation following coronary artery bypass graft.
| Predictors | OR (95% CI) | Post-CABG AF incidence [no/total (%)] | p value | Regression coefficients | Risk points |
| Age | |||||
| < 65 | 1 | 135/1260 (10.7) | < 0.001 | Ref. | 0 |
| 65-74 | 2.234 (1.713-2.913) | 134/602 (22.3) | < 0.001 | 0.804 | 1 |
| ≥ 75 | 4.134 (2.791-6.121) | 50/144 (34.7) | < 0.001 | 1.419 | 2 |
| LA enlargement | |||||
| Normal | 1 | 223/1580 (14.1) | 0.003 | Ref. | 0 |
| Mild | 1.497 (1.110-2.021) | 76/360 (21.1) | 0.009 | 0.404 | 1 |
| Moderate to severe | 2.176 (1.240-3.820) | 20/66 (30.3) | 0.013 | 0.778 | 2 |
| HTN | 1.312 (1.022-1.685) | 181/970 (18.7) | 0.015 | 0.272 | 1 |
| CVA | 2.025 (1.211-3.385) | 24/79 (30.4) | 0.009 | 0.705 | 1 |
| AUC (95% CI) for risk index | |||||
| Prediction set | 0.665 (0.61-0.698) | ||||
| Validation set | 0.627 (0.576-0.677) |
AF, atrial fibrillation; AUC, area under the ROC curve; CABG, coronary artery bypass graft surgery; CI, confidence interval; CVA, cerebrovascular accident; HTN, hypertension; LA, left atrium; OR, odds ratio; Ref., reference category.
Although the maximum score attainable was 6, none of the patient in either group had a score of 6. Figure 1 shows the distribution of the AF risk score in the two groups. These were relatively similar to the highest proportion of individuals with a score of 1 (36.8% in the derivation set vs. 38% in the validation set). The incidence of post-CABG AF increased with increasing score in both groups (Figure 2).
Figure 1.
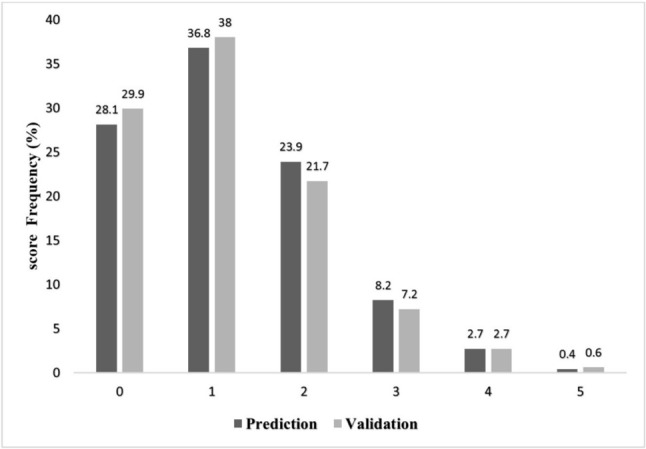
Frequency of score points in prediction and validation sets.
Figure 2.
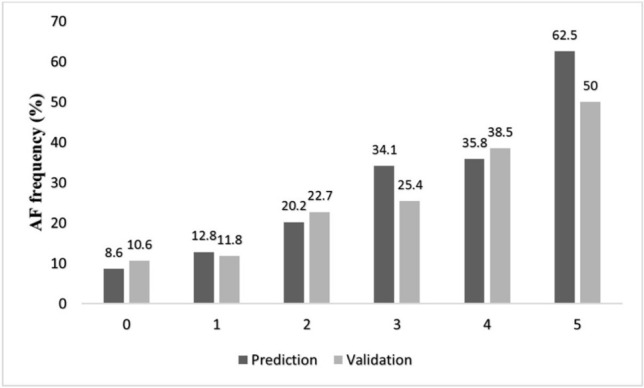
Frequency of AF in different score points in prediction and validation sets.
DISCUSSION
In the present investigation, we used a relatively large single center database of patients undergoing cardiac surgery to create a simple scoring system for predicting the incidence of post-CABG AF. We excluded patients who underwent concomitant valve surgery and those who had previous episodes of AF, because these patients are at an especially high risk of developing AF postoperatively and may benefit from prophylactic treatment.20 Since most preventive measures should be started preoperatively,21 we also did not include intraoperative and postoperative factors in the model to make our risk index clinically more useful.
Some intraoperative-related factors have been reported to have strong predictive role in the occurrence of postoperative AF. However, the determinative role of some variables such as LA size in the development of AF has been ignored in the final risk indexing system. Our scoring system requires that practitioners determine only 4 risk factors, namely advanced age, LA enlargement, HTN and CVA, to predict post-CABG AF for isolated CABG patients with an acceptable degree of accuracy. To the best of our knowledge, unlike other similar previous studies,11,12,15 this is the first study to include LA size in the risk score model. Moreover, the robustness of our model was preserved in the validation set, since the logistic regression model showed only a slight decrease in discrimination (from 0.66 to 0.62). We therefore assume that this risk index is simple to use and well validated, and might be used to help in clinical decision making.
To the best of our knowledge, a few risk indices for predicting AF after cardiac surgery have been developed on the basis of large studies, however none of them has been widely applied in clinical practice.11,12,15 Recent studies have focused on the utility of the CHA2DS2-VASc scoring system in the prediction of post cardiac surgery AF.22-24 However, this risk index was initially developed to estimate the risk of ischemic stroke in patients with AF. Of note, the sample sizes of the aforementioned investigations were not sufficient enough to provide definite findings with sufficient power, and one study could not find a significant independent predictive role for the CHA2DS2-VASc score in developing AF.25 Among the other suggested risk score models, the first index published in 2004 was proposed by Mathew et al.12 using a cohort of 4657 patients who underwent CABG with or without concomitant valve surgery, with 3093 in a derivation data set and 1564 in a validation data set. This risk index was based on patient age, history of AF, COPD, concomitant valve surgery, and withdrawal of postoperative β-blockers or angiotensin-converting enzyme inhibitors, while in the current study we initially excluded patients with a previous history of AF and cases with concomitant cardiac surgery.
The second suggested index was developed by Amar et al. using a cohort of 1553 patients who underwent pure on-pump CABG.11 This risk index was based on age, history of AF, P wave duration > 110 ms on electrocardiography, and low cardiac output postoperatively. In the third index proposed by El-Chami et al.,15 age, height, weight, and peripheral vascular disease were the four clinical factors included in the prediction model. The authors used a large sample size of 18517 patients in a derivation cohort, that was then validated in a group of 1378 consecutive patients.
The first risk index seems to be comprehensive with a robust discriminative power (AUC, 0.78), but its major limitation is that it is too cumbersome to be readily applied in clinical practice.12 In that index, risk is determined by assigning points from scales (from -11 to +42) for each of the predictive factors in that model, and then the total scores attained are correlated to the risk of AF through a nomogram. The proposed scoring model by Amar et al.11 assigns 1 point per year of age, 12 points for a history of AF, 10 points for low cardiac output, and 3 points for a P wave duration of more than 110 ms. A nomogram is then used to correlate the total score with the risk of AF. This makes it cumbersome and less clinically practical. In addition, the model did not undergo outside validation, the discriminative power is moderate (AUC, 0.69), and most importantly, including the development of a low cardiac output following surgery, which is a postoperative event, in the model restricts its value as a preoperative evaluation tool. The suggested scoring model by El-Chami et al.15 provides a simple tool to predict the risk of AF in patients undergoing isolated CABG, and it is the largest analysis to date. The major limitation of that study, as the authors noted, is that they failed to include ‘left atrial size’ in their prediction models.15
Each of these models uses different variables, and the results of the different studies are inconsistent. This discrepancy might be explained by the fact that the exact mechanism of AF has yet to be defined, and it is considered to be a multi-factorial event. Another possible explanation is that most of the studies were observational and retrospective with different inclusion criteria. Advanced age is the exception that appeared in all three models as the most potent predictor of post-CABG AF,11,12,15 as it did in our model. Aging has been shown to result in various electrophysiological and structural changes including atrial dilatation, and each 10-year increase in age has been associated with a 75% higher rate of post-CABG AF.10,26 On the other hand, the frequency of HTN is higher among old patients, which could make the atrium prone to the development of AF.26 Moreover, HTN may be associated with fibrosis and dispersion of atrial refractoriness, and it has been reported to be the most common cardiovascular risk factor for AF.12,27 In the current study, we found an odds ratio of 1.3 for the development of post-CABG AF in patients who suffered from HTN.
Several previous studies have suggested that LA enlargementis a predictor of postoperative AF either in CABG patients or in patients following heart valvular surgery.2,22,28 However, LA size was not found to be different between the patients with AF and those with sinus rhythm in a study by Zaman et al.,10 although it should be noted that the investigators included only 64 patients as a subgroup of a larger study population.10 The large sample size in the present study may mean that our results are superior in determining the aforementioned association.
One of the limitations of our study is that the discriminative power of our model was only moderate (AUC, 0.66). However, previous investigators have reported similar results for prediction models of postoperative AF after isolated CABG with AUC values of 0.62,29 0.65,4 0.68,15 and 0.69.11 However the easily obtainable preoperative variables used in the final risk stratification model in our study were the most superior.
CONCLUSION
LA size was found to be an important factor in risk stratification of post-CABG AF, which remained significant in the final model. Other scoring systems might benefit from the use of this variable to obtain a more robust predictive value. Meanwhile, the simple and validated AF risk score developed here can be applied in patients undergoing CABG as a tool to determine those at high risk of postoperative AF. Future studies might focus on testing the validity and generalizability of this risk index across different countries.
Limitation
Although increased LA diameter has been found to be associated with the development of AF, it has been reported that the value of LA volume in predicting post-CABG AF is superior to LA diameter or area.7,8,30 However, in this study, we did not have access to data of LA volume which may have provided more robust results.
FUNDING
This work was supported by Tehran Heart Center, Tehran University of Medical Sciences.
CONFLICT OF INTERESTS
All authors declare no conflicts of interest.
REFERENCES
- 1.Yamashita K, Hu N, Ranjan R, et al. Clinical risk factors for postoperative atrial fibrillation among patients after cardiac surgery. Thorac Cardiovasc Surg. 2019;67:107–116. doi: 10.1055/s-0038-1667065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maesen B NJ, Maessen J, Allessie M, Schotten U. Postoperative atrial fibrillation: a maze of mechanisms. Eurospace. 2012;14:159–174. doi: 10.1093/europace/eur208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akintoye E, Sellke F, Marchioli R, et al. Factors associated with postoperative atrial fibrillation and other adverse events after cardiac surgery. J Thorac Cardiovasc Surg. 2018;155:242–251. doi: 10.1016/j.jtcvs.2017.07.063. [DOI] [PubMed] [Google Scholar]
- 4.Shen J, Lall S, Zheng V, et al. The persistent problem of new-onset postoperative atrial fibrillation: a single-institution experience over two decades. J Thorac Cardiovasc Surg. 2011;141:559–570. doi: 10.1016/j.jtcvs.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mulukutla S, Althouse AD, Jain SK, et al. Increased left atrial size is associated with higher atrial fibrillation recurrence in patients treated with antiarrhythmic medications. Clin Cardiol. 2018;41:825–829. doi: 10.1002/clc.22964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsao HM, Hu WC, Tsai PH, et al. Functional remodeling of both atria is associated with occurrence of stroke in patients with paroxysmal and persistent atrial fibrillation. Acta Cardiol Sin. 2017;33:50. doi: 10.6515/ACS20160411A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang MK, Joung B, Shim CY, et al. Post-operative left atrial volume index is a predictor of the occurrence of permanent atrial fibrillation after mitral valve surgery in patients who undergo mitral valve surgery. Cardiovasc Ultrasound. 2018;16:5. doi: 10.1186/s12947-018-0123-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klopotowski M, Kwapiszewska A, Kukula K, et al. Clinical and echocardiographic parameters as risk factors for atrial fibrillation in patients with hypertrophic cardiomyopathy. Clin Cardiol. 2018;41:1336–1340. doi: 10.1002/clc.23050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Losi MA, Betocchi S, Aversa M, et al. Determinants of atrial fibrillation development in patients with hypertrophic cardiomyopathy. Am J Cardiol. 2004;94:895–900. doi: 10.1016/j.amjcard.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 10.Zaman AG, Archbold RA, Helft G, et al. Atrial fibrillation after coronary artery bypass surgery: a model for preoperative risk stratification. Circulation. 2000;101:1403–1408. doi: 10.1161/01.cir.101.12.1403. [DOI] [PubMed] [Google Scholar]
- 11.Amar D, Shi W, Hogue CW, Jr., et al. Clinical prediction rule for atrial fibrillation after coronary artery bypass grafting. J Am Coll Cardiol. 2004;44:1248–1253. doi: 10.1016/j.jacc.2004.05.078. [DOI] [PubMed] [Google Scholar]
- 12.Mathew JP, Fontes ML, Tudor IC, et al. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA. 2004;291:1720–1729. doi: 10.1001/jama.291.14.1720. [DOI] [PubMed] [Google Scholar]
- 13.Burgos LM, Seoane L, Parodi JB, et al. Postoperative atrial fibrillation is associated with higher scores on predictive indices. J Thorac Cardiovasc Surg. 2019;157:2279–2286. doi: 10.1016/j.jtcvs.2018.10.091. [DOI] [PubMed] [Google Scholar]
- 14.Pessoa-Amorim G, Mancio J, Vouga L, et al. Impaired left atrial strain as a predictor of new-onset atrial fibrillation after aortic valve replacement independently of left atrial size. Rev Esp Cardiol (Engl Ed) 2018;71:466–476. doi: 10.1016/j.rec.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 15.El-Chami MF, Kilgo PD, Elfstrom KM, et al. Prediction of new onset atrial fibrillation after cardiac revascularization surgery. Am J Cardiol. 2012;110:649–654. doi: 10.1016/j.amjcard.2012.04.048. [DOI] [PubMed] [Google Scholar]
- 16.Abbasi K, Karimi A, Abbasi SH, et al. Knowledge management in cardiac surgery: the second tehran heart center adult cardiac surgery database report. J Tehran Heart Cent. 2012;7:111–116. [PMC free article] [PubMed] [Google Scholar]
- 17.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Harrell F. Regression modeling strategies. Cham: Springer International Publishing. 2015:103–126. [Google Scholar]
- 19.Sullivan LM, Massaro JM, D'Agostino RB., Sr Presentation of multivariate data for clinical use: The Framingham Study risk score functions. Stat Med. 2004;23:1631–1660. doi: 10.1002/sim.1742. [DOI] [PubMed] [Google Scholar]
- 20.Dunning J, Treasure T, Versteegh M, et al. Guidelines on the prevention and management of de novo atrial fibrillation after cardiac and thoracic surgery. Eur J Cardiothorac Surg. 2006;30:852–872. doi: 10.1016/j.ejcts.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Camm AJ, Kirchhof P, Lip GY, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 2010;31:2369–2429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 22.Kashani RG, Sareh S, Genovese B, et al. Predicting postoperative atrial fibrillation using CHA 2 DS 2-VASc scores. J Surg Res. 2015;198:267–272. doi: 10.1016/j.jss.2015.04.047. [DOI] [PubMed] [Google Scholar]
- 23.Chua SK, Shyu KG, Lu MJ, et al. Clinical utility of CHADS2 and CHA2DS2-VASc scoring systems for predicting postoperative atrial fibrillation after cardiac surgery. J Thorac Cardiovasc Surg. 2013;146:919–926. doi: 10.1016/j.jtcvs.2013.03.040. [DOI] [PubMed] [Google Scholar]
- 24.Borde D, Gandhe U, Hargave N, et al. Prediction of postoperative atrial fibrillation after coronary artery bypass grafting surgery: is CHA2DS2-VASc score useful? Ann Card Anaesth. 2014;17:182–187. doi: 10.4103/0971-9784.135841. [DOI] [PubMed] [Google Scholar]
- 25.Güngör H, Zencir C, Akgüllü Ç, et al. CHADS2 and CHA2DS2-VASc scoring systems are not useful for predicting postoperative atrial fibrillation after coronary artery bypass graft surgery. J Am Coll Cardiol. 2013;62(18_S2):C101. [Google Scholar]
- 26.Silva RGd, Lima GGd, Guerra N, et al. Risk index proposal to predict atrial fibrillation after cardiac surgery. Rev Bras Cir Cardiovasc. 2010;25:183–189. doi: 10.1590/s0102-76382010000200009. [DOI] [PubMed] [Google Scholar]
- 27.Zacharias A, Schwann TA, Riordan CJ, et al. Obesity and risk of new-onset atrial fibrillation after cardiac surgery. Circulation. 2005;112:3247–3255. doi: 10.1161/CIRCULATIONAHA.105.553743. [DOI] [PubMed] [Google Scholar]
- 28.Haghjoo M, Basiri H, Salek M, et al. Predictors of postoperative atrial fibrillation after coronary artery bypass graft surgery. Indian Pacing Electrophysiol J. 2008;8:94–101. [PMC free article] [PubMed] [Google Scholar]
- 29.Thoren E, Hellgren L, Jideus L, et al. Prediction of postoperative atrial fibrillation in a large coronary artery bypass grafting cohort. Interact Cardiovasc Thorac Surg. 2012;14:588–593. doi: 10.1093/icvts/ivr162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsang TS, Abhayaratna WP, Barnes ME, et al. Prediction of cardiovascular outcomes with left atrial size: is volume superior to area or diameter? J Am Coll Cardiol. 2006;47:1018–1023. doi: 10.1016/j.jacc.2005.08.077. [DOI] [PubMed] [Google Scholar]


