Abstract
Background
Premature ventricular complexes (PVC) may cause ventricular dyssynchrony and lead to left atrium and ventricle mechanical abnormalities. Although ventricular cardiomyopathy due to PVCs has been well studied, little is known about atrial adaptation to PVCs.
Objectives
To assess atrial and ventricular responses to PVC therapy.
Methods
All patients with PVC burden > 5000 beats/day on Holter monitoring were enrolled. Baseline demographics, comorbidities, social habits, Holter parameters, and echocardiography profiles were recorded. Follow-up Holter electrocardiography (ECG) and echocardiography data were compared between PVC-treated and non-treated patients.
Results
Two hundred and eighty-six patients were enrolled, of whom 139 received PVC treatment. Among the treated patients, 125 who underwent follow up Holter ECG or echocardiography were included in the final analysis. The mean follow-up times of Holter ECG and echocardiography were 9.40 ± 6.70 and 9.40 ± 5.52 months, respectively. Ventricular arrhythmic burden was significantly reduced in the treatment group (16.46% vs. 13.41%, p = 0.041) but was significantly increased in the observation group (7.58% vs. 14.95%, p = 0.032). A significant increase in left atrial (LA) diameter (36.94 mm vs. 39.46 mm, p = 0.025) and reduction in left ventricular ejection fraction (LVEF) (57.26% vs. 53.8%, p = 0.040) were noted in the observation group. There were no significant differences in supraventricular arrhythmic burden in the observation group and LA diameter and LVEF in the treatment group.
Conclusions
PVC therapy effectively reduced ventricular arrhythmic burden in the treatment group on follow-up. Our data suggest that PVC treatment may prevent LA dilation and LVEF decline.
Keywords: Antiarrhythmic agent, Cardiomyopathy, Left atrium, Left ventricle, Premature ventricular complex, Radiofrequency catheter ablation
INTRODUCTION
Premature ventricular complexes (PVCs) are common arrhythmias that become more prevalent with older age and in people with comorbidities.1 In patients with underlying cardiac disease, the presence of PVCs is a well-established negative prognostic factor,2 even in the context of antiarrhythmic therapy.3 Although idiopathic PVCs are typically regarded as being benign, they have been associated with cardiovascular disease and sudden death.4-6
Frequent ventricular dyssynchrony due to PVCs causes dilated cardiomyopathy and impaired LV function.7,8 The relationship between PVCs and cardiomyopathy is a reciprocal one — PVCs can lead to cardiomyopathy, which, in turn, can exacerbate PVCs.9 In addition to ventricular cardiomyopathy, there is a higher incidence of ischemic stroke in patients with frequent PVCs.10,11 Ventricular dyssynchrony can result in left atrial mechanical abnormalities and reduce left atrial appendage flow velocity.12-14 However, a definitive association between new-onset atrial fibrillation and PVCs has not been demonstrated.11
Recent studies have shown that a reduction in PVC burden can reverse cardiomyopathy and lead to improved function.15-17 Catheter ablation is an effective method to eliminate PVCs,8,17,18 and antiarrhythmic agents can suppress PVCs and relieve symptoms.3,19-21 Although PVC-induced ventricular cardiomyopathy has been well described, little is known about atrial adaptation to PVCs or the response to treatment. The aim of this study was to assess atrial and ventricular responses to PVC therapy.
METHODS
Study population
Following Institutional Review Board approval, we performed a retrospective review of patients with PVC burden. Because prior studies have demonstrated a cutoff range of PVC-induced cardiomyopathy with a PVC burden ranging from 4% to 33%, we enrolled patients with a PVC burden of > 5000 beats on 24-hour Holter electrocardiography (ECG) monitoring in order to ensure complete inclusion.22,23 The study population included 286 consecutive patients between January 2017 and December 2017 at China Medical University Hospital. Patients with or without structural heart diseases, defined as heart failure, coronary heart disease, and valvular heart disease, were enrolled in this study. Baseline demographics, comorbidities, social habits, clinical symptoms, 12-lead ECG, Holter parameters, and echocardiography profiles were recorded. Holter ECG and echocardiography data were obtained at baseline and on follow-up. Follow-up Holter ECG and echocardiography data in the treatment group were compared to the non-treated observation group. PVC treatment included radiofrequency catheter ablation (RFCA) or antiarrhythmic therapy of at least 3 months duration. The antiarrhythmic agents used in this study were beta-blockers (Bisoprolol, Propranolol, Atenolol, Carvedilol), calcium channel blockers (Verapamil, Diltiazem), Class Ib (Mexiletine) and Class III (Amiodarone) antiarrhythmic agents. Written informed consent was obtained for all procedures.
Statistical analysis
Continuous data are expressed as mean ± standard deviation (SD) or median [Q1, Q3]. Differences between baseline and endpoints within each group were analyzed using a paired student T test or two-tailed Mann-Whitney test. A p value of < 0.05 was considered to be statistically significant.
RESULTS
Baseline demographics, PVCs, and echocardiographic characteristics
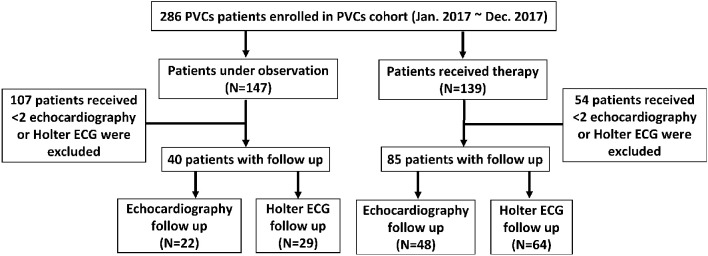
Two hundred and eighty-six patients were enrolled at a single institution between January 2017 and December 2017. Among these patients, 139 (48.6%) received PVC treatment (treatment group), and 147 (51.4%) did not receive treatment (observation group). Eighty-five patients in the treatment group and 40 patients in the observation group underwent baseline and follow-up Holter ECG and echocardiography monitoring (Figure 1). In the treatment group, 72 patients were treated with antiarrhythmic agents, and 13 patients received RFCA. The mean follow-up times between the baseline and endpoint Holter EKG and echocardiography studies were 9.40 ± 6.70 and 9.40 ± 5.52 months, respectively.
Figure 1.
Study design and patient flow. ECG, electrocardiography; PVCs, premature ventricular complexes.
The mean age was 62.25 ± 20.82 years in the observation group and 57.16 ± 15.90 years in the treatment group. Both groups were predominantly male. Nearly half of the patients had hypertension and 20-25% of the patients had hyperlipidemia and heart failure. The most common presenting symptoms were palpitations and chest tightness/pain. In both groups, around 15% of the patients developed exertional dyspnea, and 10% of the patients had syncopal or near-syncopal episodes. A significantly higher proportion of the patients in the observation group were asymptomatic. Twelve-lead ECG analysis showed that the duration of PVCs were both 171.4 [142.9, 185.7] ms in the observation group and treatment group. Using standard stepwise ECG algorithms to determine the location of PVCs,24 the most common origin was the ventricular outflow tract in both groups. There were no significant differences in burden of ventricular arrhythmias (12.98 [8.41, 22.28]% vs. 8.46 [4.85, 19.39]%, p = 0.079), premature atrial complexes (PACs) (0.01 [0, 0.07]% vs. 0.04 [0, 0.29]%, p = 0.026), and supraventricular arrhythmia (SVTs) (0 [0, 0]% vs. 0 [0, 0]%, p = 0.424) between the treatment group and observation group. On echocardiography, the left atrial (LA) diameter (36.91 [32.29, 43.39] mm vs. 35.66 [30.89, 40.73] mm, p = 0.128), left ventricular (LV) diameter (52.28 [45.97, 58.49] mm vs. 51.15 [46.96, 56.96] mm, p = 0.435), and LV ejection fraction (55.95 [48.40, 61.98]% vs. 59.85 [54.83, 63.15]%, p = 0.063) were similar in both groups (Table 1).
Table 1. Baseline characteristics of the 2 study groups.
| Characteristics | Observation (n = 40) | Treatment (n = 85) | p |
| Gender, n (%) | 0.24 | ||
| Female | 19 (47.5) | 31 (36.5) | |
| Male | 21 (52.5) | 54 (63.5) | |
| Age | 62.25 ± 20.82 | 57.16 ± 15.90 | 0.18 |
| Comorbidities, n (%) | |||
| Hypertension | 21 (52.5) | 41 (48.2) | 0.66 |
| Diabetes mellitus | 7 (17.5) | 13 (15.3) | 0.75 |
| Hyperlipidemia | 8 (20.0) | 22 (25.9) | 0.47 |
| Stroke | 5 (12.5) | 4 (4.7) | 0.14 |
| Coronary artery disease | 5 (12.5) | 18 (21.2) | 0.24 |
| Valvular heart disease | 2 (5.0) | 5 (5.9) | 1.00 |
| Heart failure | 8 (20). | 22 (25.9) | 0.47 |
| Thyroid diseases | 3 (7.5) | 7 (8.2) | 1.00 |
| Pulmonary disease | 5 (12.5) | 5 (5.9) | 0.29 |
| Chronic kidney disease | 9 (22.5) | 9 (10.6) | 0.08 |
| Malignancy | 2 (5) | 7 (8.2) | 0.72 |
| Symptoms, n (%) | |||
| Palpitation | 16 (40) | 28 (32.9) | 0.30 |
| Chest tightness/pain | 10 (25) | 29 (34.1) | 0.16 |
| Exertional dyspnea | 6 (15) | 12 (14.1) | 0.86 |
| Near-syncope/syncope | 4 (10) | 9 (10.6) | 0.89 |
| Asymptomatic | 19 (47.5) | 24 (28.2) | < 0.01 |
| PVC characteristics | |||
| QRS Duration (ms) | 171.4 [142.9, 185.7] | 171.4 [142.9, 185.7] | 0.77 |
| Location | 0.23 | ||
| RVOT | 12 (30) | 22 (25.9) | |
| LVOT | 11 (27.5) | 36 (42.4) | |
| RV | 4 (10) | 9 (11) | |
| LV | 6 (15) | 9 (11) | |
| Inadequate PVC information | 7 (17.5) | 9 (11) | |
| Holter ECG | |||
| Ventricular burden (%) | 8.46 [4.85, 19.39] | 12.98 [8.41, 22.28] | 0.08 |
| Total PVCs | 569 [45.75, 1705] | 942 [391, 1824] | 0.12 |
| Couplet (episodes) | 3 [0, 104.5] | 28 [1, 273] | 0.06 |
| Bigeminy (episodes) | 215.5 [1.75, 836.3] | 270 [13.5, 757] | 0.68 |
| Trigeminy (episodes) | 34.5 [0, 456.5] | 161.0 [17, 606.5] | 0.05 |
| NSVTs (episodes) | 0 [0, 1.75] | 0 [0, 7] | 0.04 |
| VTs (episodes) | 0 [0, 0] | 0 [0, 0] | 0.50 |
| Atrial burden (%) | 0.04[0, 0.29] | 0.01 [0, 0.07] | 0.03 |
| SVTs (episodes) | 0 [0, 0] | 0[0, 0] | 0.42 |
| Echocardiography | |||
| LA diameter (mm) | 35.66 [30.89, 40.73] | 36.91 [32.29, 43.39] | 0.13 |
| LV diameter (mm) | 51.15 [46.96, 56.96] | 52.28 [45.97, 58.49] | 0.44 |
| LVEF (%) | 59.85 [54.83, 63.15] | 55.95 [48.40, 61.98] | 0.06 |
| Radiofrequency ablation, n (%) | 13 (15.3) | ||
| Pharmacologic agents, n (%) | 72 (84.7) | ||
| Single therapy | 57 (67.1) | ||
| Beta-blocker | 46 (54.1) | ||
| Calcium-channel blocker | 1 (1.2) | ||
| MexIletine | 7(8.2) | ||
| Amiodarone | 3(3.5) | ||
| Dual therapy | 14(16.5) | ||
| Triple therapy | 1(1.2) |
Data are presented as mean ± SD or median [Q1, Q3] for continuous variables, and number (%) for categorical variables.
ECG, electrocardiography; LA, left atrium; LV, left ventricle; LVEF, left ventricular ejection fraction; LVOT, left ventricular outflow tract; NSVTs, non-sustained ventricular tachycardia; PVC, premature ventricular complex; RV, right ventricle; RVOT, right ventricular outflow tract; SD, standard deviation; SVT, supraventricular tachycardia; VT, ventricular tachycardia.
* Supraventricular tachycardia included non-sustained supraventricular tachycardia, atrial fibrillation, and atrial flutter.
Of the PVC-treated patients, 84.7% received pharmacologic therapy and 15.3% underwent successful RFCA. The medications for PVC control included beta-blockers (80.7%), amiodarone (5.3%), calcium channel blockers (1.8%), and mexiletine (12.3%). A small number of patients received dual therapy (16.5%) and triple therapy (1.2%).
Response to PVC therapy
Follow-up Holter ECG monitoring was performed to evaluate the anti-arrhythmic therapy. The ventricular arrhythmic burden decreased significantly after therapy in the treatment group (p = 0.041), while the burden of ventricular arrhythmias increased by more than 7% in the observation group (p = 0.032) (Figure 2). There were no significant differences in the frequency of PACs or SVTs in each group (Table 2).
Figure 2.
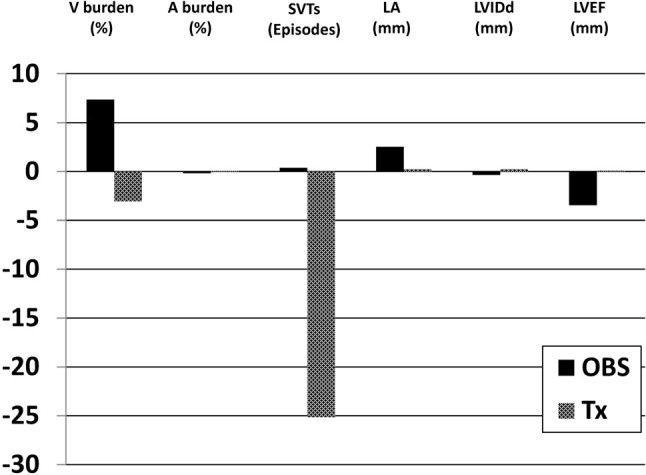
Response to PVC therapy in Holter ECG monitoring and echocardiography. A, premature atrial contraction; ECG, electrocardiogram; LA, left atrium; LVEF, left ventricular ejection fraction; LVIDd, left ventricular diastolic inner dimension; OBS, observation; PVC, premature ventricular contraction; SVT, supraventricular tachycardia; Tx, treatment; V, premature ventricular contraction.
Table 2. Outcome of PVC therapy for ventricular (V) burden, atrial (A) burden, and SVTs in Holter ECG group, and LA diameter, LVIDd, and LVEF in echocardiography group.
| Holter ECG | Echocardiography | |||||||||||
| V burden (%) | A burden (%) | SVTs (episodes) | LA diameter (mm) | LVIDd (mm) | LVEF (%) | |||||||
| OBS | Tx | OBS | Tx | OBS | Tx | OBS | Tx | OBS | Tx | OBS | Tx | |
| N | 22 | 64 | 22 | 64 | 22 | 64 | 29 | 48 | 29 | 48 | 29 | 48 |
| Unadjusted | ||||||||||||
| Baseline mean (SE) | 7.58 (1.79) | 16.46 (1.27) | 0.37 (0.24) | 0.19 (0.07) | 0.27 (0.16) | 33.08 (25.41) | 36.94 (1.62) | 40.45 (1.05) | 51.59 (1.54) | 55.85 (1.23) | 57.26 (2.08) | 50.64 (2.19) |
| End point mean (SE) | 14.95 (2.53) | 13.41 (1.27) | 0.19 (0.11) | 0.26 (0.10) | 0.64 (0.30) | 7.94 (3.92) | 39.46 (1.93) | 40.69 (1.21) | 51.24 (1.75) | 56.08 (1.29) | 53.80 (1.89) | 50.73 (1.95) |
| Difference between baseline and end point mean (SE) | 7.36 (3.22) | -3.05 (1.46) | -0.18 (0.27) | 0.07 (0.06) | 0.36 (0.19) | -25.14 (25.35) | 2.53 (1.06) | 0.24 (0.79) | -0.35 (1.30) | 0.23 (0.89) | -3.46 (1.61) | 0.10 (1.44) |
| p-value | 0.03 | 0.04 | 0.51 | 0.25 | 0.07 | 0.33 | 0.02 | 0.76 | 0.79 | 0.80 | 0.04 | 0.95 |
| Idiopathic heart disease | ||||||||||||
| Baseline mean (SE) | 6.50 (1.85) | 16.66 (1.51) | 0.40 (0.30) | 0.20 (0.09) | 0.18 (0.13) | 10.55 (7.36) | 35.54 (1.74) | 39.12 (1.52) | 49.27 (1.64) | 53.71 (1.75) | 61.00 (1.35) | 58.44 (2.64) |
| End point mean (SE) | 16.04 (3.14) | 12.87 (1.53) | 0.02 (0.01) | 0.25 (0.12) | 0.29 (0.17) | 5.53 (3.81) | 38.34 (2.30) | 38.95 (1.80) | 48.12 (1.69) | 54.24 (1.92) | 56.61 (1.20) | 57.38 (1.87) |
| p-value | 0.03 | 0.03 | 0.23 | 0.24 | 0.43 | 0.28 | 0.03 | 0.81 | 0.43 | 0.62 | 0.03 | 0.53 |
| Structural heart disease | ||||||||||||
| Baseline mean (SE) | 11.27 (4.84) | 15.90 (2.40) | 0.25 (0.23) | 0.14 (0.07) | 0.60 (0.60) | 95.35 (93.86) | 39.35 (3.13) | 41.68 (1.44) | 57.68 (2.60) | 57.82 (1.80) | 47.44 (5.49) | 43.46 (3.06) |
| End point mean (SE) | 11.23 (3.07) | 14.90 (2.25) | 0.74 (0.43) | 0.28 (0.20) | 1.80 (1.11) | 14.59 (10.4) | 42.40 (3.49) | 42.30 (1.59) | 59.41 (3.09) | 57.77 (1.87) | 46.41 (5.50) | 44.62 (2.84) |
| p-value | 0.99 | 0.73 | 0.42 | 0.53 | 0.11 | 0.41 | 0.16 | 0.65 | 0.56 | 0.98 | 0.75 | 0.62 |
The patients also underwent echocardiographic evaluations to assess remodeling of cardiac structures after PVC treatment. Follow-up LA diameter increased by 2.53 mm (p = 0.025) and left ventricular ejection fraction (LVEF) decreased by 3.46% (p = 0.04) in the observation group (Figure 2). In contrast, there was no deterioration in LA size (p = 0.763) or LV function (p = 0.947) in the treatment group. There were no significant differences in LV diameter during follow-up in either group (Table 2).
Result of PVC therapy in subgroup analysis
A preexisting history of structural heart disease was used in our subgroup analysis. The patients with idiopathic heart disease had a significant reduction in ventricular arrhythmic burden after therapy (p = 0.032), while those in the observation group developed increased ventricular arrhythmias by more than 9% (p = 0.028). Follow-up LA diameter increased by 2.80 mm (p = 0.032) and LVEF decreased by 4.39% (p = 0.030) in the observation group. However, no deterioration in LA size (p = 0.810) or LV function (p = 0.534) was detected in the treatment group. On the other hand, in patients with structural heart diseases, PVC therapy did not significantly reduce the ventricular arrhythmic burden (p = 0.730). In both treatment and observation groups, there were no significant changes in LA diameter, LV chamber size, or ventricular function during follow-up (Table 2).
DISCUSSION
Our study demonstrated that PVC treatment reduced ventricular arrhythmic burden, with no deterioration in atrial and ventricular diameters or LVEF in patients with > 5000 PVCs on Holter monitoring. Non-treated patients, however, experienced a significantly increased ventricular arrhythmic burden, significantly increased LA diameter, and significantly reduced LVEF. There was a consistent, beneficial tendency toward the treatment group.
PVCs are a known cause of cardiomyopathy and are associated with new-onset heart failure, sudden cardiac death, and cardiac-related hospitalizations.11,25 The pathophysiology of PVC-induced cardiomyopathy is not well defined. However, several studies (focusing predominantly on the ventricles) have offered some insight. PVCs may lead to slower and dyssynchronous ventricular contraction followed by a compensatory pause, which results in elevated diastolic filling pressures, increased wall stress, and ultimately LV remodeling and dysfunction.8,9,18,26 Furthermore, high PVC frequency, epicardial origin, duration of PVC exposure, increased QRS width, interpolated PVCs, male gender, absence of circadian fluctuation of PVC burden, and asymptomatic status have been shown to predict the presence of PVC-induced cardiomyopathy.9,23,26 Our study is consistent with previous reports. We found that non-treated patients had worse LV systolic function after approximately 9 months of follow-up, and around half of the patients in this group did not have PVC-related symptoms. An asymptomatic presentation may not only lead to a delayed diagnosis, but also lower response to treatment.1,27
In addition to ventricular cardiomyopathy, patients with PVCs can develop subclinical LA remodeling,28 LA enlargement,13 and decreased emptying velocity of the LA appendage.12 PVC-related atrioventricular dyssynchrony and elevated diastolic filling pressures is attrivuted to this undesired mechanical and electrical remodeling. It remains unknown, however, whether PVCs cause atrial fibrillation.13,14,29 Nevertheless, many studies have reported a higher incidence of stroke and stroke-like symptoms in patients with frequent PVCs.10,11,30,31 Since atrial fibrillation is usually underdiagnosed,32 we believe that PVC-related LA remodeling and dilation play an important role in the etiology of stroke. This highlights the importance of PVC treatment, which, as our study suggests, can protect against an increase in LA diameter.
PVC-induced ventricular cardiomyopathy is reversible after pharmacologic treatment or RFCA, especially in patients without underlying structural heart diseases.8,17,21 A meta-analysis of 15 studies with a total of 792 patients showed that successful RFCA increased the mean LVEF by 7.7% and reduced the mean LV diameter by 4.6 mm.33 Higher baseline PVC burden,17 effective PVC reduction,34 better baseline LVEF,34 and absence of preexisting structural diseases34 have been reported to be positive predictors of LV function improvement after PVC treatment. The response of patients with atrial cardiomyopathy to PVC treatment remains unknown. In the current study, PVC burden was significantly reduced with therapy, with no changes in LA and LV size or LVEF. Unlike prior studies, the patients enrolled in our study had relatively normal LA and LV dimensions and LVEF at baseline, which may account for the preventative, rather than reversal effect in this cohort. In addition, 20-25% of the patients in our cohort had structural heart diseases, rendering their cardiomyopathy and PVC burden more resistant to reversal with PVC therapy.17,35 Finally, RFCA has been shown to reduce PVC burden more effectively than anti-arrhythmic agents.8,21 Only 15% of the patients in our treatment group received RFCA, which may also explain the absence of beneficial reverse remodeling. Nevertheless, to the best of our knowledge, this is the first report of a protective atrial response to PVC treatment.
Previous studies have reported that antiarrhythmic agents not only failed to improve cardiovascular outcomes but elicited pro-arrhythmic effects in patients with underlying heart diseases.3 Thus, antiarrhythmic therapy is not routinely recommended to treat organic ventricular arrhythmias. Indeed, the mechanism of PVC arrhythmogenesis may be different between structural and non-structural heart diseases. However, we believe that a higher burden of and longer exposure to PVCs may lead to progressive cardiac remodeling, development of de novo cardiomyopathy, or exacerbation of existing cardiomyopathy, regardless of the presence of underlying cardiac diseases. We enrolled patients with a PVC burden of > 5000, and 20-25% had structural heart diseases. Our subgroup analysis of patients without structural heart diseases showed a consistent beneficial trend of PVC therapy. While among the patients with structural heart diseases, there were no increases in atrial or ventricular arrhythmias, worsened cardiac structure or function.
Limitations
The limitations of this study include its retrospective design, heterogeneous baseline parameters, and small sample size. As a result of the retrospective analysis, the study may have selection bias, based on unaccounted differences that initially segregated the patients in the treatment and observation groups. In addition, because some patients received Holter ECG monitoring at other institutions, these patients were not included in our study. Finally, while this study assessed the aggregate response to both pharmacologic therapy and RFCA, the actual efficacy varies with different PVC treatment. Therefore, the benefits of PVC treatment in our study may be underestimated, given the relatively low proportion of patients who received the more effective therapy, RFCA.
CONCLUSIONS
Our results showed that PVC therapy was associated with reduced ventricular arrhythmic burden, reduced LA dilation, and preserved LVEF. Further randomized, prospective studies are warranted to better define the therapeutic benefits of PVC therapy.
Acknowledgments
This work was supported by the China Medical University Hospital [DMR-105-015], the Ministry of Health and Welfare, Taiwan [MOHW107-TDU-B-212-123004], China Medical University Hospital, Academia Sinica Stroke Biosignature Project [BM10701010021], MOST Clinical Trial Consortium for Stroke [MOST 106-2321-B-039-005], Tseng-Lien Lin Foundation, Taichung, Taiwan, and Katsuzo and Kiyo Aoshima Memorial Funds, Japan.
CONFLICT OF INTEREST
All the authors declare no conflict of interest.
REFERENCES
- 1.Panizo JG, Barra S, Mellor G, et al. Premature ventricular complex-induced cardiomyopathy. Arrhythm Electrophysiol Rev. 2018;7:128–134. doi: 10.15420/aer.2018.23.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lown B, Fakhro AM, Hood WB, Jr., et al. The coronary care unit. New perspectives and directions. JAMA. 1967;199:188–198. [PubMed] [Google Scholar]
- 3.Echt DS, Liebson PR, Mitchell LB, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991;324:781–788. doi: 10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]
- 4.AlMahameed ST, Ziv O. Ventricular arrhythmias. Med Clin North Am. 2019;103:881–895. doi: 10.1016/j.mcna.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 5.Li Z, Zhao S, Chen K, et al. Prognostic significance of frequent premature ventricular complex early after implantation among patients with implantable cardioverter defibrillator. J Electrocardiol. 2018;51:898–905. doi: 10.1016/j.jelectrocard.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Kang JW, Yang WH, Chi JE, et al. Higher ventricular premature complex burden is associated with lower systolic blood pressure response. Acta Cardiol Sin. 2018;34:152–158. doi: 10.6515/ACS.201803_34(2).20171117A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spragg DD, Kass DA. Pathobiology of left ventricular dyssynchrony and resynchronization. Prog Cardiovasc Dis. 2006;49:26–41. doi: 10.1016/j.pcad.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Zhong L, Lee YH, Huang XM, et al. Relative efficacy of catheter ablation vs antiarrhythmic drugs in treating premature ventricular contractions: a single-center retrospective study. Heart Rhythm. 2014;11:187–193. doi: 10.1016/j.hrthm.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 9.Yalin K, Gölcük E. Frequent premature ventricular contractions and cardiomyopathy, chicken and egg situation. J Atr Fibrillation. 2017;10:1674. doi: 10.4022/jafib.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ofoma U, He F, Shaffer ML, et al. Premature cardiac contractions and risk of incident ischemic stroke. J Am Heart Assoc. 2012;1:e002519. doi: 10.1161/JAHA.112.002519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin CY, Chang SL, Chung FP, et al. Long-term outcome of non-sustained ventricular tachycardia in structurally normal hearts. PloS One. 2016;11:e0160181. doi: 10.1371/journal.pone.0160181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alizadeh A, Maleki M, Bassiri H, et al. Evaluation of atrial thrombus formation and atrial appendage function in patients with pacemaker by transesophageal echocardiography. Pacing Clin Electrophysiol. 2006;29:1251–1254. doi: 10.1111/j.1540-8159.2006.00520.x. [DOI] [PubMed] [Google Scholar]
- 13.Barutçu A, Gazi E, Temiz A, et al. Assessment of left-atrial strain parameters in patients with frequent ventricular ectopic beats without structural heart disease. Int J Cardiovasc Imaging. 2014;30:1027–1036. doi: 10.1007/s10554-014-0423-y. [DOI] [PubMed] [Google Scholar]
- 14.Park Y, Kim S, Shin J, et al. Frequent premature ventricular complex is associated with left atrial enlargement in patients with normal left ventricular ejection fraction. Pacing Clin Electrophysiol. 2014;37:1455–1461. doi: 10.1111/pace.12447. [DOI] [PubMed] [Google Scholar]
- 15.Yarlagadda RK, Iwai S, Stein KM, et al. Reversal of cardiomyopathy in patients with repetitive monomorphic ventricular ectopy originating from the right ventricular outflow tract. Circulation. 2005;112:1092–1097. doi: 10.1161/CIRCULATIONAHA.105.546432. [DOI] [PubMed] [Google Scholar]
- 16.Ogunbayo GO, Charnigo R, Darrat Y, et al. Comparison of complications of catheter ablation for ventricular arrhythmias in adults with versus without structural heart disease. Am J Cardiol. 2018;122:1345–1351. doi: 10.1016/j.amjcard.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 17.Cronin EM, Bogun FM, Maury P, et al. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias. J Arrhythm. 2019;35:323–484. doi: 10.1002/joa3.12185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wojdyła-Hordyńska A, Kowalski O, Hordyński GJ, et al. The effect of radiofrequency catheter ablation of frequent premature ventricular complexes and arrhythmia burden on left ventricular function. Kardiol Pol. 2017;75:698–704. doi: 10.5603/KP.a2017.0058. [DOI] [PubMed] [Google Scholar]
- 19.Morganroth J, Goin JE. Quinidine-related mortality in the short-to-medium-term treatment of ventricular arrhythmias. A meta-analysis. Circulation. 1991;84:1977–1983. doi: 10.1161/01.cir.84.5.1977. [DOI] [PubMed] [Google Scholar]
- 20.Waldo AL, Camm AJ, deRuyter H, et al. Effect of d-sotalol on mortality in patients with left ventricular dysfunction after recent and remote myocardial infarction. The SWORD Investigators. Survival With Oral d-Sotalol. Lancet. 1996;348:7–12. doi: 10.1016/s0140-6736(96)02149-6. [DOI] [PubMed] [Google Scholar]
- 21.Ling Z, Liu Z, Su L, et al. Radiofrequency ablation versus antiarrhythmic medication for treatment of ventricular premature beats from the right ventricular outflow tract: prospective randomized study. Circ Arrhythm Electrophysiol. 2014;7:237–243. doi: 10.1161/CIRCEP.113.000805. [DOI] [PubMed] [Google Scholar]
- 22.Shanmugam N, Chua TP, Ward D. ‘Frequent’ ventricular bigeminy--a reversible cause of dilated cardiomyopathy. How frequent is ‘frequent’? Eur J Heart Fail. 2006;8:869–873. doi: 10.1016/j.ejheart.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 23.Latchamsetty R, Bogun F. Premature ventricular complex-induced cardiomyopathy. JACC Clin Electrophysiol. 2019;5:537–550. doi: 10.1016/j.jacep.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 24.Ito S, Tada H, Naito S, et al. Development and validation of an ECG algorithm for identifying the optimal ablation site for idiopathic ventricular outflow tract tachycardia. J Cardiovasc Electrophysiol. 2003;14:1280–1286. doi: 10.1046/j.1540-8167.2003.03211.x. [DOI] [PubMed] [Google Scholar]
- 25.Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS Guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Circulation. 2018;138:e272–e391. doi: 10.1161/CIR.0000000000000549. [DOI] [PubMed] [Google Scholar]
- 26.Latchamsetty R, Bogun F. Premature ventricular complex ablation in structural heart disease. Card Electrophysiol Clin. 2017;9:133–140. doi: 10.1016/j.ccep.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 27.Yokokawa M, Kim HM, Good E, et al. Relation of symptoms and symptom duration to premature ventricular complex-induced cardiomyopathy. Heart Rhythm. 2012;9:92–95. doi: 10.1016/j.hrthm.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 28.Cozma D, Streian CG, Petrescu L, et al. Subclinical left atrium remodelling in patients with frequent premature ventricular contractions. Kardiol Pol. 2014;72:1141–1147. doi: 10.5603/KP.a2014.0133. [DOI] [PubMed] [Google Scholar]
- 29.Aizawa Y, Watanabe H, Okumura K. Electrocardiogram (ECG) for the prediction of incident atrial fibrillation: an overview. J Atr Fibrillation. 2017;10:1724. doi: 10.4022/jafib.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Im SI, Kim SH, Kim BJ, et al. Association of frequent premature ventricular complex > 10% and stroke-like symptoms without a prior diagnosis of stroke or transient ischemic attack. Int J Cardiol Heart Vasc. 2018;19:58–62. doi: 10.1016/j.ijcha.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agarwal SK, Heiss G, Rautaharju PM, et al. Premature ventricular complexes and the risk of incident stroke: the Atherosclerosis Risk In Communities (ARIC) Study. Stroke. 2010;41:588–593. doi: 10.1161/STROKEAHA.109.567800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanna T, Diener HC, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370:2478–2486. doi: 10.1056/NEJMoa1313600. [DOI] [PubMed] [Google Scholar]
- 33.Zang M, Zhang T, Mao J, et al. Beneficial effects of catheter ablation of frequent premature ventricular complexes on left ventricular function. Heart. 2014;100:787–793. doi: 10.1136/heartjnl-2013-305175. [DOI] [PubMed] [Google Scholar]
- 34.Mountantonakis SE, Frankel DS, Gerstenfeld EP, et al. Reversal of outflow tract ventricular premature depolarization-induced cardiomyopathy with ablation: effect of residual arrhythmia burden and preexisting cardiomyopathy on outcome. Heart Rhythm. 2011;8:1608–1614. doi: 10.1016/j.hrthm.2011.04.026. [DOI] [PubMed] [Google Scholar]
- 35.Penela D, Van Huls Van Taxis C, Van Huls Vans Taxis C, et al. Neurohormonal, structural, and functional recovery pattern after premature ventricular complex ablation is independent of structural heart disease status in patients with depressed left ventricular ejection fraction: a prospective multicenter study. J Am Coll Cardiol. 2013;62:1195–1202. doi: 10.1016/j.jacc.2013.06.012. [DOI] [PubMed] [Google Scholar]