INTRODUCTION
Deep vein thrombosis (DVT) is a common disorder but rarely involves the upper extremities. Predisposing causes include neoplasm, central venous catheter or pacemaker lead placement, hematological disease, and aneurysm.1-5 A recent study showed that the prevalence of patients with DVT in the upper extremities (UEDVT) was 3.0%1 and another study suggested 4-10%.6 Of these patients, approximately 50% and 40%, respectively, were reported to have had prior central catheter placements and cancer.1 Hematological disease is extremely rare as the cause of UEDVT. We report a 50-year-old man with UEDVT as the primary presentation of protein C deficiency.
CASE REPORT
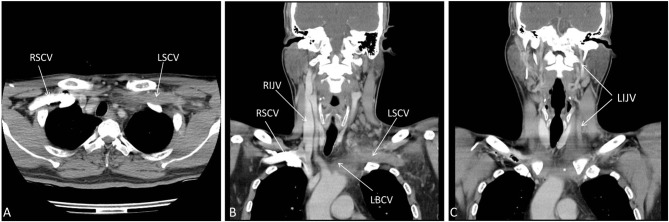
A 50-year-old man was admitted to the hospital due to painful swelling in his left neck for 4 days. He had no dizziness, headache, or any neurological symptom. There was no edema in any extremity. He had no medical or surgical history nor neck trauma. No invasive treatment such as central catheter placement had ever been done. Contrast-enhanced computed tomography (CT) showed thrombosis and total occlusion of the left internal jugular vein, left subclavian vein, and left brachiocephalic vein (Figure 1). The right internal jugular vein was significantly larger than the left one (Figure 1B, 1C). Laboratory tests revealed: hemoglobin: 12.1 g/dL; white blood cell count: 13,000/μL; C-reactive protein: 5.34 mg/dL; carcinoembryonic antigen: 2.07 ng/mL; alpha-fetoprotein: 2.7 ng/mL; antinuclear factor: negative; anti-ds-DNA < 1:10; C3: 85.8 mg/dL; C4: 23.6 mg/dL; and lupus anticoagulant: negative. Serum protein S and antithrombin-3 levels were normal, but the serum protein C level was 67.5% (normal range, 70.0-140.0). Enoxaparin was administered subcutaneously for 4 days. Apixaban 5 mg twice daily was prescribed on the fifth hospital day. The swelling decreased gradually. The patient continued to take apixaban at the same dosage for 3 months. A follow-up CT showed partial regression of the thrombus in the left subclavian vein and left internal jugular vein, but the veins were still totally occluded. The patient continued to take apixaban at the same dosage for another 3 months. Another follow-up CT showed further regression of the thrombus, but the vessels were still totally occluded (Figure 2). Nonetheless, the patient refused further anticoagulation therapy and endovascular intervention. There was no evidence of other hematological diseases, infection, or neoplasm during the 6-month treatment period.
Figure 1.
Contrast CT showed thrombosis and total occlusion of the left subclavian vein (A, B), left brachiocephalic vein (B), and left internal jugular vein (C). CT, computed tomography; LBCV, left brachiocephalic vein; LIJV, left internal jugular vein; LSCV, left subclavian vein; RIJV, right internal jugular vein; RSCV, right subclavian vein.
Figure 2.
Follow-up contrast CT approximately 6 months after the initial diagnosis showed further regression of the thrombus in the left subclavian vein (A, B) and left brachiocephalic vein (C). CT, computed tomography; LBCV, left brachiocephalic vein; LIJV, left internal jugular vein; LSCV, left subclavian vein; RSCV, right subclavian vein; Tb, thrombus.
DISCUSSION
Protein C deficiency is a rare autosomal recessive disorder. The coagulopathy is caused by impaired inactivation of factors Va and VIIIa by activated protein C after the propagation phase of coagulation activation.7,8 Acquired factors for low protein C activity include using vitamin K antagonists (eg, warfarin), vitamin K deficiency, recent thrombosis, recent invasive surgery and disseminated intravascular coagulation. If there is no possible assay interference, protein C activity assay is not necessarily to be rechecked and protein C antigen measurement is optional.8 Molecular testing for protein C deficiency is available in a few research laboratories and is not routinely used in clinical practice.8 The patient’s sample sent for measurement of serum protein C level was analyzed using a Sysmex CS-5100 coagulation analyzer, the accuracy of which has been validated.9
Heterozygous protein C deficiency is often asymptomatic but may involve venous thromboembolic events. Long term management in severe protein C deficiency involves anticoagulation with or without a protein C replacement regimen.7,8
The prevalence of patients with UEDVT was 3.0%1 and another study showed 4-10%.6 Most of them were reported to have had prior central catheter placements and cancer.1 Symptoms included neck pain, swelling of the neck or upper limbs and shortness of breath.3,10 Some of them might be asymptomatic.5 The thrombosis can be confirmed by ultrasound, contrast-enhanced CT or contrast venography.1
UEDVT could have many complications including pulmonary embolism, superior vena cava syndrome, infection, postthrombotic syndrome, loss of vascular access, necrotizing fasciitis, ovarian hyperstimulation syndrome and neurological disorders.1,2,3,6,10,11 The optimal management of these conditions remains unknown because there are no randomized controlled trials to evaluate the treatment of UEDVT.
The American College of Chest Physicians recommends the same initial and long-term treatment as for patients with lower extremity DVT.12 The initial parenteral anticoagulant therapy is strongly recommended. Anticoagulant therapy should be taken for at least 3-6 months in patients with active cancer and UEDVT. Additionally, prolonging anticoagulant therapy should be considered when the cancer is not yet cured, or the patient is still receiving cancer treatment. Bleker et al. showed that the median time to recurrence was 53 days during anticoagulant therapy and 417 days after cessation of anticoagulant treatment for patients with UEDVT who received anticoagulation therapy.6
After 6 months of anticoagulation therapy, the involved vessels of this patient remained entirely occluded. However, the extent of the thrombosis appeared smaller. It is possible that the smaller size of those thrombosed vein was associated with the chronicity of the occlusion. However, true regression of the thrombosis should have been occurred in this patient since the swelling decreased gradually after the administration of apixaban. Had the patient continued therapy, prolonged anticoagulant therapy might have worked to open the occluded vessels. Endovascular interventions including percutaneous mechanical thrombectomy, catheter-directed thrombolysis, or venous balloon dilatation followed by stent implantation were other treatments under consideration; however, he declined further treatment.
LEARNING POINTS
Protein C deficiency may be associated with UEDVT. The optimal management remains unknown. Anticoagulation therapy should be given for at least 3 to 6 months. Endovascular inventions can be considered in the case of treatment failure.
REFERENCES
- 1.Yamashita Y, Morimoto T, Amano H, et al. Deep vein thrombosis in upper extremities: clinical characteristics, management strategies and long-term outcomes from the COMMAND VTE Registry. Thromb Res. 2019;177:1–9. doi: 10.1016/j.thromres.2019.02.029. [DOI] [PubMed] [Google Scholar]
- 2.Lennep BW, Skelton TN, Tanawuttiwat T. Follow the lead: internal jugular vein thrombosis. Am J Med. 2018;131:1055–1057. doi: 10.1016/j.amjmed.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Al-Zoubi NA. Spontaneous internal jugular vein thrombosis as primary presentation of antiphospholipid syndrome: case report. Vasc Health Risk Manag. 2018;14:153–155. doi: 10.2147/VHRM.S170140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mnejja M, Hammami B, Bougacha L, et al. Internal jugular vein thrombosis by protein C activated resistance. Eur Ann Otorhinolaryngol Head Neck Dis. 2011;128:269–271. doi: 10.1016/j.anorl.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Shimizu R, Yasu T, Uema A, et al. Right internal jugular vein thrombosis caused by aneurysm of right-sided aortic arch. J Cardiol Cases. 2018;17:220–222. doi: 10.1016/j.jccase.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bleker SM, van Es N, Kleinjan A, et al. Current management strategies and long-term clinical outcomes of upper extremity venous thrombosis. J Thromb Haemost. 2016;14:973–981. doi: 10.1111/jth.13291. [DOI] [PubMed] [Google Scholar]
- 7.Goldenberg NA, Manco-Johnson MJ. Protein C deficiency. Haemophilia. 2008;14:1214–1221. doi: 10.1111/j.1365-2516.2008.01838.x. [DOI] [PubMed] [Google Scholar]
- 8.Dinarvand P, Moser KA. Protein C deficiency. Arch Pathol Lab Med. 2019;143:1281–1285. doi: 10.5858/arpa.2017-0403-RS. [DOI] [PubMed] [Google Scholar]
- 9.Flieder T, Gripp T, Knabbe C, et al. The Sysmex CS-5100 coagulation analyzer offers comparable analytical performance and excellent throughput capabilities. Pract Lab Med. 2016;6:38–47. doi: 10.1016/j.plabm.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang WH, Qian WT, Shi HM, et al. Internal jugular vein thrombosis with serious cervical necrotizing fasciitis. J Cranio Surg. 2019 doi: 10.1097/SCS.0000000000005359. [DOI] [PubMed] [Google Scholar]
- 11.Lee M, Heo Y, Kim T. Vernet’s syndrome associated with internal jugular vein thrombosis. J Stroke Cerebrovasc Dis. 2019;28:344–346. doi: 10.1016/j.jstrokecerebrovasdis.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:e419S–e494S. doi: 10.1378/chest.11-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]