Clinical Implications.
-
•
We describe 3 patients with X-linked agammaglobulinemia with coronavirus disease 2019 who failed supportive treatment but recovered after receiving convalescent plasma.
The coronavirus disease 2019 (COVID-19) pandemic has presented a global challenge. The pathogenesis of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is complex, and effective therapy is currently lacking. Convalescent plasma transfusion is safe and under investigation for effectiveness.1, 2, 3, 4, 5, 6
We report 3 hospitalized patients (see Table E1 and Figure E1 in this article's Online Repository at www.jaci-inpractice.org) with X-linked agammaglobulinemia (XLA) who experienced protracted courses with minimal improvement on supportive therapies, but demonstrated clinical improvement soon after transfusion with unmixed ABO-compatible donor convalescent plasma containing anti–spike protein titer of greater than or equal to 1:320 from the New York Blood Center.
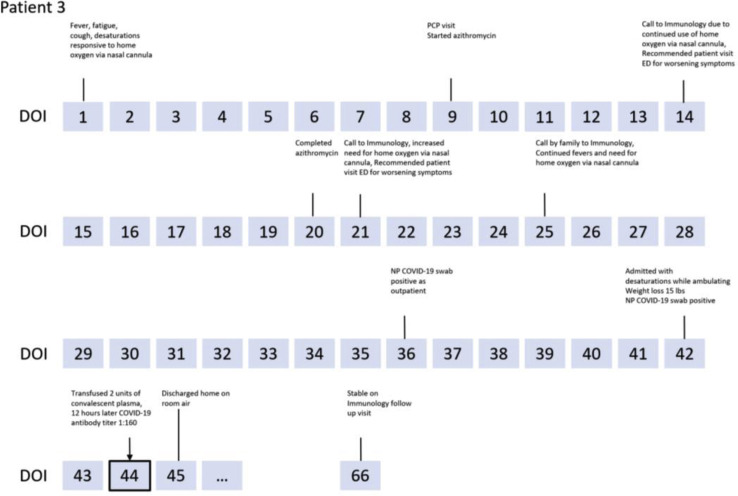
Figure E1.
Clinical course of patients. BAL, Bronchoalveolar lavage; C diff, Clostridium difficile; CXR, chest X-ray; ECHO, echocardiogram; ED, emergency department; GGO, ground-glass opacities; LLL, left lower lobe; LMCA, left main coronary artery; MRI, magnetic resonance imaging; NP, nasopharyngeal; PCP, primary care physician; pRBC, packed red blood cell; resp., respiratory; RLL, right lower lobe; RML, right middle lobe; SMX-TMP, sulfamethoxazole-trimethoprim; US, ultrasound.
Case 1 is a 10 year-old boy with a history of hereditary spherocytosis and XLA receiving subcutaneous immunoglobulin every other week with 2 pneumonia hospitalizations in the previous year. He was admitted for 10 days of fever, cough, bilateral chest pain, and lack of improvement on oral antibiotics. A chest X-ray suggested right middle and lower lobe infiltrates. On presentation, he was febrile, tachycardic, and tachypnic, and had scleral icterus, pallor, 2/6 systolic murmur, and splenomegaly. Two nasopharyngeal SARS-CoV-2 real RT-PCR test results were negative. Respiratory PCR panel and bacterial blood cultures were negative. He had leukopenia and thrombocytopenia, atypical lymphocytosis, hemolytic anemia, and elevated inflammatory markers (Table I ).
Table I.
Laboratory values
| CBC | Normal pediatric | Patient 1 |
Normal adults | Patient 2 |
Patient 3 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Admit | Peak | Discharge | Admit | Peak | Discharge | Admit | Peak | Discharge | |||
| WBC | 4.5-11.4 × 103/μL | 3.2 | 14.3 | 14.3∗ | 4.5-11.0 × 103/μL | 4.2 | 9.2 | 4.4 | 6.4 | 6.4 | 5.3 |
| HGB | 10.6-14.4 g/dL | 6.6 | 10.2 | 10.0∗ | 13.9-16.3 g/dL | 10.8 | 10.9 | 10.8 | 12.6 | 12.6 | 11.3 |
| PLTS | 150-450 × 103/μL | 134 | 193 | 175∗ | 150-450 × 103/μL | 260 | 391 | 333 | 248 | 248 | 187 |
| Lymph % | 12.2%-48.4% | 31.7 | 34 | 23.0∗ | 12.2%-48.4% | 28.8 | 61.4 | 54.6 | 17.0 | 29.3 | 24.7 |
| Inflammatory markers | Normal pediatric | Admit | Peak | Discharge | Normal adults | Admit | Peak | Discharge | Admit | Peak | Discharge |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CRP | 0.0-5.0 mg/L | — | 22.4 | 6.7† | 0.0-5.0 mg/L | 64 | 64 | 18.4 | 15.2 | 16.4 | 13.4 |
| ESR | 0-10 mm/h | — | 35 | — | 0-15 mm/h | 89 | 89 | — | — | — | — |
| LDH | 150-260 U/L | — | 530 | — | 100-220 U/L | 214 | 289‡ | 210 | 183 | 183 | — |
| Ferritin | 20-200 ng/mL | — | 642 | 642† | 30-400 ng/mL | 123 | 185 | 166 | 967 | 967 | 775 |
| IL-1β§ | 0-5.0 pg/mL | — | <0.3 | — | 0-5.0 pg/mL | 8.6 | 8.6 | <0.3 | <0.3 | 0.5 | 0.5 |
| IL-6§ | 0-5.0 pg/mL | — | 11.1 | — | 0-5.0 pg/mL | 20.5 | 20.5 | 3.8 | 14.1 | 15.1 | 15.1 |
| IL-8§ | 0-5.0 pg/mL | — | 12.7 | — | 0-5.0 pg/mL | 27.3 | 27.3 | 21.4 | 6.7 | 8.5 | 8.5 |
| TNF-α§ | 0-22.0 pg/mL | — | 19.8 | — | 0-22.0 pg/mL | 18.0 | 18.1 | 18.1 | 15.3 | 15.3 | 13.7 |
| Coagulation studies | Normal pediatric | Admit | Peak | Discharge | Normal adults | Admit | Peak | Discharge | Admit | Peak | Discharge |
|---|---|---|---|---|---|---|---|---|---|---|---|
| INR | 0.8-1.2 | — | 1.3 | — | 0.8-1.2 | 1.1 | 1.1 | 1.0 | 0.9 | 1.0 | 1.0 |
| D-Dimer | 0.00-0.50 μg/mL | — | 1.23 | 0.30∗ | 0.00-0.50 μg/mL | 0.67 | 1.04 | 0.28 | 0.45 | 0.45 | — |
| Immune regulation | Normal pediatric | Admit | Peak | Discharge | Normal adults | Admit | Peak | Discharge | Admit | Peak | Discharge |
|---|---|---|---|---|---|---|---|---|---|---|---|
| IgG quantitative | 698-1560 mg/dL | 560 | — | — | 700-1600 mg/dL | 821‖ | — | — | 1057 | — | — |
| Absolute B- cell count | 432-3345/mm3 | 13¶ | — | 25-335/mm3 | 1¶ | — | — | 3¶ | — | — |
ESR, Erythrocyte sedimentation rate; HGB, hemoglobin; INR, international normalized ratio; LDH, lactate dehydrogenase; Lymph%, lymphocyte percentage; PLTS, platelets; WBC, white blood cell count.
Bolded numbers are abnormal lab values. Pediatric ranges are given for patient 1 and adult ranges for patients 2 and 3.
These values are from 1 d before discharge.
These values are from 3 d before discharge.
A value of more than 10 times this is the recorded peak; however, it does fit with the remainder of the patient's clinical data.
For patients with COVID-19 aged 10-40 y at our institution, the first to third quartile ranges are 0.1-0.7 pg/mL for IL-1β, 9.9-70.8 pg/mL for IL-6, 13.3-44.3 pg/mL for IL-8, and 11.6-28.0 pg/mL for TNF-α.9
Before IVIG; later increased to 1021 on readmission.
At diagnosis.
In the first 2 weeks of hospitalization, he received a red blood cell transfusion, broad-spectrum antibiotics, oxygen supplementation, and albuterol treatments, and a dose of scheduled intravenous immunoglobulin (IVIG). However, his condition failed to improve, with further increases in C-reactive protein (CRB) and erythrocyte sedimentation rate. On day 16, he experienced episodes of oxygen desaturation to 89%, dyspnea, increased oxygen demand, and fatigue. Chest computed tomography (CT) scan on day 17 showed multiple peripherally distributed and predominant lower lobe ground-glass opacities bilaterally with total atelectasis of the right middle lobe (Figure 1 ). He was placed on enoxaparin. Bronchoalveolar lavage on day 19 was RT-PCR positive for SARS-CoV-2. Soon after diagnosis, the patient was started on a 10-day course of remdesivir, and 2 units of 200 mL of convalescent plasma were infused on days 22 and 23. One day later, the patient was afebrile for the first time in 3 weeks and had improved energy. He was weaned off oxygen support and discharged on day 29. The patient's SARS-CoV-2 antibody titer before convalescent plasma was undetectable; 3 days after infusion, the antibody titer was 1:80.
Figure 1.
CT Chest images for (A) patient 1, (B) 2, and (C) 3, respectively, showing diffuse bilateral ground-glass opacities.
Case 2 is a 24-year-old man with XLA receiving IVIG every 3 weeks with a history of chronic sinusitis, bronchiectasis, recurrent Clostridium difficile colitis, and Helicobacter skin infections; he was initially admitted for 5 days of febrile illness with chills, cough, and myalgia. Left lower lobe consolidation was noted on chest X-ray. Two nasopharyngeal and 1 rectal SARS-CoV-2 RT-PCR swabs were negative. He was discharged on day 8 after receiving broad-spectrum antibiotics and his scheduled dose of IVIG. The patient was readmitted on day 13 of illness because of fatigue, cough, shortness of breath, left-sided chest pain, diarrhea, myalgia, and headaches. Chest X-ray showed worsening pneumonia, and he was tachycardic, with an oxygen saturation of 93% on room air. Chest CT scan showed diffuse multifocal ground-glass and patchy airspace opacities throughout the lungs (Figure 1). Initial laboratory studies showed leukopenia, reduced hemoglobin, elevated CRP, d-dimer, and inflammatory cytokines (Table I). Nasopharyngeal respiratory panel and SARS-CoV-2 RT-PCR swab were negative; however, oropharyngeal SARS-CoV-2 swab was positive. Patient 2 was started on subcutaneous heparin and oral azithromycin. Patient 2 received 2 units of 200 mL convalescent plasma on day 16. His temperature rose to 38.1°C after infusion, but he defervesced within hours. Chest pain resolved and he tolerated room air. His inflammatory markers decreased, and he was discharged on day 19.
Case 3 is 40-year-old man with XLA receiving IVIG every 3 weeks with a history of chronic sinusitis. He had 7 weeks of fatigue, recurrent fevers and chills, cough, dyspnea, and 15-lb weight loss, with oxygen saturation of 90% requiring 2 to 3 L of oxygen at home. He completed a 12-day course of azithromycin with little improvement. He tested positive for COVID-19 by nasopharyngeal swab as an outpatient. With continued cough, dyspnea, and oxygen dependence, he was admitted on day 42. His oxygen saturation was 95% with otherwise-normal vital signs and physical examination findings. Laboratory results showed an elevated CRP, IL-6, IL-8, and ferritin (Table I). Chest CT scan showed irregular peripheral ground-glass opacities seen predominantly in the lower lobes (Figure 1). Two units of 200 mL convalescent plasma were infused on day 44 of illness. He was discharged the following day, tolerating room air. His d-dimer, fibrinogen, CRP, and ferritin were decreased. The patient's SARS-CoV-2 antibody titer was undetectable before transfusion and increased to 1:160 12 hours after infusion.
Patients with congenital immune defects are presumed to be at risk for more severe courses in the setting of COVID-19 infection, but data on these subjects are limited. Two recent articles described 4 patients with agammaglobulinemia with COVID-19, one of who had an autosomal-recessive form of agammaglobulinemia. He was asymptomatic. The patients with XLA endured mild short courses.7 , 8 The positive outcome of these cases led to a hypothesis that humoral immunity might not be essential to overcome COVID-19.
Our patients displayed strong proinflammatory responses in the absence of B-cell signaling, but have impaired abilities to control COVID-19, leading to prolonged courses. This highlights the importance of antibody in viral removal. The rapid response to convalescent plasma in these patients is somewhat unusual and the mechanism remains unclear. Whether B cells, as antigen-presenting cells, are important in T-cell activation in COVID-19 is unknown. We acknowledge the presence of multiple factors and different therapies in the treatment course of our patients. Although antivirals, such as remdesivir, may aid in limiting viral replication, convalescent plasma may help neutralize virus and bridge the gap from adaptive immunity and shorten the duration of illness, even in the later stages of COVID-19. We report 3 cases, but it raises possibilities regarding the role of B cells and antibodies in patients with XLA with COVID-19. Future investigations would be needed to draw more definitive conclusions.
Acknowledgments
We thank all front-line providers and consultants at Kravis Children's Hospital and Mount Sinai Hospital, Dr Jeffrey Gumprecht for assistance with the case management, Ms Denise Rodriguez for assistance in obtaining Emergency Investigational New Drug approval from the Food and Drug Administration, and Dr Christine Quake, Dr Nazifa Rahman, Dr Zoe Shtasel Gottlieb, Dr Karen Wilson, and Dr Prantik Saha for ensuring transfusion on the floor. We thank Dr Sacha Gnjatic and Ms Diane M. Del Valle for providing the cytokine reference range of patients at age of 10 to 40 years with COVID-19 between March and June 2020 in Mount Sinai Health System.
Footnotes
C.C.-R. received support from the National Institutes of Health (grant nos. AI 101093, AI-086037, and AI-48693) and the David S. Gottesman Immunology Chair.
Conflicts of interest: C. Cunningham-Rundles has received consulting fees from CSL Behring, Momenta, Atara, Pharming, and UBC; served on boards for CSL Behring and Takeda Pharmaceutical Company Limited; and serves on the Scientific Advisory Board of the Immune Deficiency Foundation.
Contributor Information
The Mount Sinai Health System Convalescent Plasma Team:
Sean T.H. Liu, Hung-Mo Lin, Alexandra Abrams-Downey, Krystal P. Cascetta, Aaron E. Glatt, Sanjana C. Koshy, Erna Kojic, Dana S. Mazo, David Perlman, Steven Rudolph, Jason Steinberg, Thomas Schneider, Ian Baine, Ania Wajnberg, Jeffrey P. Gumprecht, Farah Rahman, Denise Rodriguez, Charles Sanky, Amy Dupper, Deena R. Altman, Florian Krammer, Damodara Rao Mendu, Adolfo Firpo-Betancourt, Carlos Cordon-Cardo, Jeffrey S. Jhang, Suzanne A. Arinsberg, David L. Reich, Judith A. Aberg, and Nicole M. Bouvier
Online Repository
Table E1.
Demographic characteristics and clinical summary
| Patient | Age (y) | Sex | BMI (kg/m2) | Race | Comorbidities | Symptoms on admission | DOI on admission | Treatments received | ICU stay | Oxygen support | DOI on discharge |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 10 | M | 16.74 | White (non-Hispanic) | Hereditary spherocytosis | Fever Cough Chest pain |
11 | Amoxicillin Azithromycin Ceftriaxone Cefepime Sulfamethoxazole-trimethoprim Vancomycin Remdesivir Enoxaparin IVIG Convalescent plasma |
No | Nasal cannula | 29 |
| 2 | 24 | M | 21.74 | White (Hispanic) | Chronic sinusitis Bronchiectasis Left hip dysplasia Recurrent C difficile colitis Recurrent Helicobacter skin infections |
Fever Chills Cough Chest pain Bodyaches Fatigue Diarrhea |
13 | Doxycycline Ertapenem Vancomycin Heparin IVIG Convalescent plasma |
No | Room air | 19 |
| 3 | 40 | M | 22.7 | White (non-Hispanic) | Chronic sinusitis | Fever Coughs Dyspnea on exertion Chills Weight loss |
42 | Azithromycin IVIG Convalescent plasma |
No | Nasal cannula | 45 |
BMI, Body mass index; DOI, day of illness; ICU, intensive care unit.
The demographic characteristics, BMI, past medical history, symptoms on presentation, length of illness before presentation, therapies in addition to convalescent plasma, length of illness to discharge, ICU stay, and day of illness on discharge are summarized above.
References
- 1.Bloch E.M., Shoham S., Casadevall A., Sachais B.S., Shaz B., Winters J.L. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest. 2020;130:2757–2765. doi: 10.1172/JCI138745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joyner M.J., Wright R.S., Fairweather D., Senefeld J.W., Bruno K.A., Klassen S.A. Early safety indicators of COVID-19 convalescent plasma in 5,000 patients. J Clin Invest. 2020;130:4791–4797. doi: 10.1172/JCI140200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shen C., Wang Z., Zhao F., Yang Y., Li J., Yuan J. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323:1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duan K., Liu B., Li C., Zhang H., Yu T., Qu J. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A. 2020;117:9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ye M., Fu D., Ren Y., Wang F., Wang D., Zhang F. Treatment with convalescent plasma for COVID-19 patients in Wuhan, China. J Med Virol. 2020;92:1890–1901. doi: 10.1002/jmv.25882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu STH, Lin H-M, Baine I, Wajnberg A, Gumprecht JP, Rahman F, et al. Convalescent plasma treatment of severe COVID-19: a propensity score–matched control study [published online ahead of print]. Nat Med. 10.1038/s41591-020-1088-9. [DOI] [PubMed]
- 7.Quinti I., Lougaris V., Milito C., Cinetto F., Pecoraro A., Mezzaroma I. A possible role for B cells in COVID-19? Lesson from patients with agammaglobulinemia. J Allergy Clin Immunol. 2020;146:211–213. doi: 10.1016/j.jaci.2020.04.013. .e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soresina A, Moratto D, Chiarini M, Paolillo C, Baresi G, Foca E, et al. Two X-linked agammaglobulinemia patients develop pneumonia as COVID-19 manifestation but recover [published online ahead of print April 22, 2020]. Pediatr Allergy Immunol. 10.1111/pai.13263. [DOI] [PMC free article] [PubMed]
- 9.Del Valle D.M., Kim-Schulze S., Huang H.H., Beckmann N.D., Nirenberg S., Wang B. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26:1636–1643. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]